Abstract
The neurofibromatosis type 2 (NF2) tumor suppressor, Merlin, is a FERM (Four point one, Ezrin, Radixin, Moesin) domain-containing protein whose loss results in defective morphogenesis and tumorigenesis in multiple tissues. Like the closely related ERM proteins (Ezrin, Radixin and Moesin), Merlin may organize the plasma membrane by assembling membrane protein complexes and linking them to the cortical actin cytoskeleton. We previously found that Merlin is a critical mediator of contact-dependent inhibition of proliferation and is required for the establishment of stable adherens junctions (AJs) in cultured cells. Here we delineate the molecular function of Merlin in AJ establishment in epidermal keratinocytes in vitro and confirm that a role in AJ establishment is an essential function of Merlin in vivo. Our studies reveal that Merlin can associate directly with α-catenin and link it to Par3, thereby providing an essential link between the AJ and the Par3 polarity complex during junctional maturation.
Introduction
The establishment of polarity enables the compartmentalization of functions in single cells or within a multicellular tissue. Central to the establishment of cortical polarity is the ability of membrane-derived cues to effect local changes in the cortical cytoskeleton and in the targeting and retention of membrane proteins. Epithelial cells can use cadherin-mediated adhesion to establish apical and basolateral membrane compartments; this is followed by the stratification of specialized cell junctions that define the functional organization of an epithelium. The coordination of cell polarity and cell:cell communication is central to epithelial morphogenesis across metazoans; moreover, concomitant loss of cell adhesion and polarity leads to loss of proliferation control and tumorigenesis. However, the molecular mechanism(s) by which cells integrate cell adhesion and polarity cues remain poorly understood.
In mammalian epithelia, the mature apical junctional region consists of the adherens junction (AJ), which represents a major mechanical connection between cells and the more apical tight junction (TJ) that provides a barrier to the transepithelial passage of solutes (Bauer et al., 2010; Harris and Tepass, 2010; Nishimura and Takeichi, 2009; Steed et al., 2010). Increasing evidence suggests that a poorly understood process of junctional maturation involves a transition from an immature junction that contains both AJ and TJ components into discrete AJ and TJ structures (Gumbiner, 2005; Hartsock and Nelson, 2008; Itoh et al., 1997). The invariable final placement of the TJ apical to the AJ suggests that the immature junction is or becomes polarized. Indeed, the polarity proteins Par3 and aPKC localize to the apical junction and are necessary both for the establishment of polarity and the formation of TJs (Goldstein and Macara, 2007; Helfrich et al., 2007; Suzuki et al., 2002; Suzuki and Ohno, 2006). However, the functional and physical links between the AJ and Par3/aPKC complexes and their role in junctional maturation have not been delineated.
Members of the FERM (Four point one, Ezrin, Radixin, Moesin)-domain family of proteins organize the cell cortex by assembling membrane complexes and linking them directly or indirectly to the cortical actin cytoskeleton (Fehon et al., 2010; McClatchey and Fehon, 2009; Tepass, 2009). FERM domain-containing proteins have well-established roles in organizing membrane appendages and signaling platforms (Fehon et al., 2010; McClatchey and Fehon, 2009); however, recent studies in Drosophila reveal that they can also physically and functionally interact with proteins that have known roles in establishing cell polarity, suggesting that they may also participate in fundamental mechanisms by which cells establish membrane asymmetry (Laprise et al., 2006; Laprise et al., 2009; Ling et al., 2010; Robinson et al., 2010). The neurofibromatosis type 2 (NF2)-encoded tumor suppressor Merlin is a FERM domain-containing protein that is closely related to Ezrin, Radixin and Moesin (the ERM proteins) (Rouleau et al., 1993; Trofatter et al., 1993) and has been broadly linked to development and tumorigenesis in many tissues (McClatchey and Giovannini, 2005). Merlin is necessary for establishing stable adherens junctions (AJs) and for contact-dependent inhibition of proliferation in several types of cultured mammalian cells (Johnson et al., 2002; Lallemand et al., 2003; McClatchey and Giovannini, 2005; McLaughlin et al., 2007; Morrison et al., 2001; Okada et al., 2005). In addition to decorating the cortical cytoskeleton, Merlin localizes to AJs and associates with the core AJ complex containing E-cadherin, β-catenin and α-catenin but it is not clear how Merlin communicates with the AJ or controls AJ establishment (Cole et al., 2008; Lallemand et al., 2003).
Basal epidermal keratinocytes have been widely utilized to study AJ establishment in vitro and in vivo (Adams and Watt, 1991; Lien et al., 2008b; Mertens et al., 2005; Michels et al., 2009; Vasioukhin et al., 2001; Vasioukhin et al., 2000). In culture, keratinocytes enact a defined program of AJ establishment and maturation upon stimulation with media containing high levels of calcium (Vasioukhin et al., 2000; Yuki et al., 2007). In the mammalian skin, basal keratinocytes form an undifferentiated monolayer at the base of the stratified epidermis and enact a vertical program of junctional maturation that is driven in part, by asymmetric (perpendicular) division, yielding differentiated supra-basal daughter cells and epidermal stratification (Fuchs, 2009; Jensen et al., 1999; Lechler and Fuchs, 2005). In addition to hemidesmosomes and desmosomes, basal cells form AJs and associated primordial TJ structures (Schluter et al., 2007); in contrast, supra-basal daughters and overlying cornified epithelial cells develop discrete AJs and an extensive network of TJs that creates an intact barrier preventing water loss and protecting the organism from the external environment (Jamora and Fuchs, 2002). The organization and maturation of cell junctions is therefore central to the function and homeostasis of the mammalian epidermis.
Here we have used the keratinocyte as a well-studied system for defining the function of Merlin in AJ establishment. We identify Merlin as a partner for α-catenin and a critical component of the early AJ in vitro and in vivo. Indeed, the phenotypic consequences of eliminating Merlin function in the mouse epidermis are markedly similar to that of eliminating either cadherin or α-catenin, but not other AJ-associated proteins (Tinkle et al., 2008; Tunggal et al., 2005; Vasioukhin et al., 2001). Our data further suggest that Merlin is specifically required for the coordination of adhesion and polarity during junctional maturation; indeed, we demonstrate that Merlin can physically link α-catenin to Par3. In the absence of Merlin, the AJ and Par3 complexes do not associate and basal keratinocytes do not polarize or develop functional TJs, which, in turn, results in an aberrant program of asymmetric divisions and a severe and fatal defect in epidermal barrier formation in vivo. These studies expand the repertoire of FERM domain proteins that have fundamental roles in establishing cortical polarity and suggest that Merlin is a member of a growing list of proteins that have dual functions in controlling polarity and cell proliferation.
Results
Merlin interacts with α-catenin and is required for junctional maturation
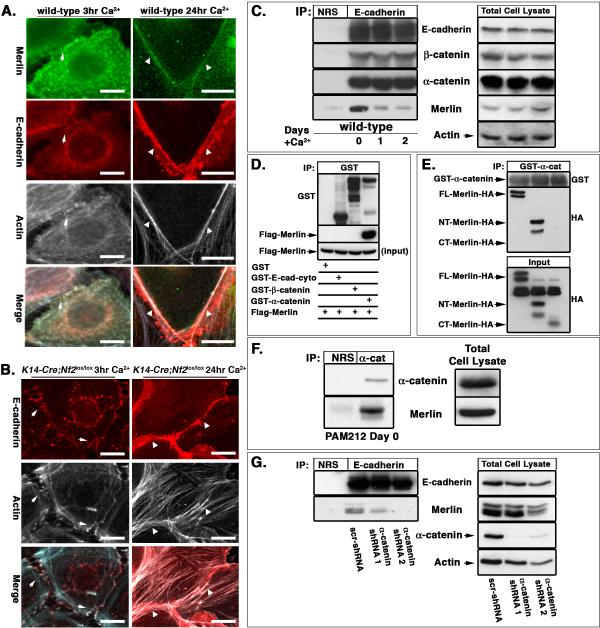
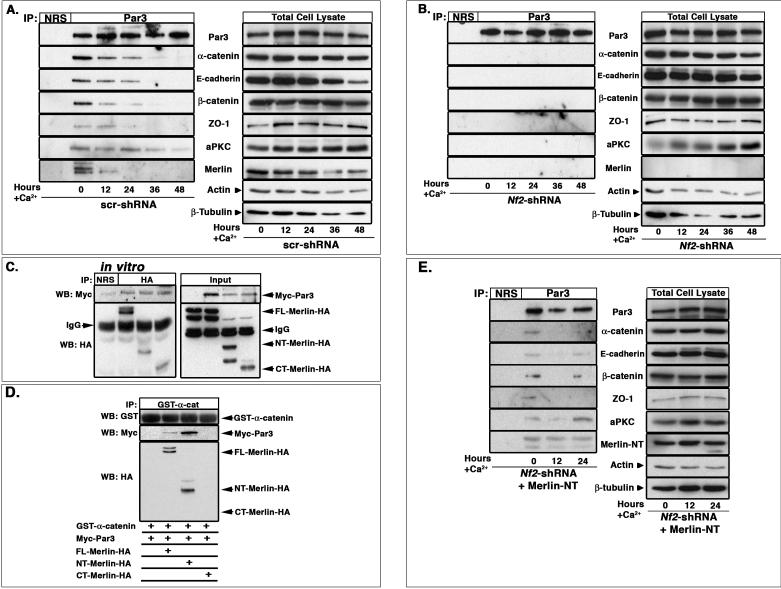
We previously found that Merlin localizes to AJs and is required for the establishment of mature AJs in mesenchymal cells and primary keratinocytes (Lallemand et al., 2003). However, the molecular role of Merlin in AJ establishment was unclear. In primary keratinocytes, Merlin localizes to E-cadherin and actin-containing punctae known as primordial adhesions (PAs) during the earliest stages of AJ formation and remains localized to the mature junctional belt after calcium-stimulated AJ maturation (Figure 1A). In the absence of Merlin, E-cadherin-and actin-containing PAs do form; however, in contrast to the mature and discrete junctional belts formed by wild-type keratinocytes after 24 hours of calcium stimulation, Nf2−/− keratinocytes form discontinuous and ragged rims of E-cadherin with little evidence of a cortical actin ring (Figure 1B; (Lallemand et al., 2003). Similar defects in AJ maturation and in AJ function were seen in immortalized keratinocytes expressing shRNAs directed against Nf2 (Figure S1A–E). In fact, Merlin associates with the core AJ complex prior to calcium-stimulation in wild-type immortalized keratinocytes when PAs predominate and mature junctions are not present, but the physical association of Merlin with the AJ complex decreases as the junctions mature (Figure 1C).
Figure 1. Merlin binds α-catenin and is required for the formation of mature junctional belts in keratinocytes.
(A,B) Primary wild-type (A) or K14-Cre;Nf2lox/lox (B) keratinocytes were cultured in media containing high calcium for the indicated times. Immunostaining revealed that Merlin (green) colocalizes with E-cadherin (red) and actin (white) in both immature PAs (arrows) and in the mature junctional belt (arrowheads) in wild-type cells. K14-Cre;Nf2lox/lox keratinocytes form PAs but after 24 hours actin remains disorganized and a mature junctional belt does not form. Bars, 5 μm.
(C) Immunoprecipitation of endogenous E-cadherin from immortalized wild-type PAM212 keratinocytes revealed that endogenous Merlin associates with the E-cadherin complex prior to calcium stimulation when only PAs are present (day 0). Although Merlin localizes to the mature junctional belt (A), the levels of Merlin that physically associate with the core cadherin complex diminish after calcium stimulation and junctional maturation. NRS, normal rabbit serum.
(D) Flag-Merlin produced in vitro in Sf9 cells associates with bacterially produced GST-α-catenin but not with GST-E-cad-cyto or GST-β-catenin. Input equals 10% of the Flag-Merlin used in the binding assay.
(E) Full-length (FL)-Merlin-HA, N-terminal (NT)-Merlin-HA and C-terminal (CT)-Merlin-HA were produced by in vitro transcription and translation (IVTT) and incubated with GST-α-catenin. Isolation of GST complexes and immunoblotting with anti-HA antibodies revealed that FL- and NT-Merlin-HA but not CT-Merlin-HA associated with GST-α-catenin in vitro. Input equals 10% of the IVTT products.
(F) Immunoprecipitation of endogenous α-catenin from PAM212 keratinocytes cultured in calcium-depleted media (day 0) revealed the association of endogenous Merlin with endogenous α-catenin.
(G) PAM212 keratinocytes were cultured as in (F) and infected with lentiviruses either expressing a scrambled shRNA (scr-shRNA) or two independent α-catenin-directed shRNAs. Immunoprecipitation of E-cadherin revealed that the association of Merlin with the AJ complex is nearly eliminated by the loss of α-catenin. See also Figures S1 and S2.
The levels and composition of the core AJ complex are unaffected by the absence of Merlin (Figure 1C, S1F), suggesting that rather than being involved in the assembly or stability of this complex, Merlin may link it to other membrane and/or cytoskeletal proteins. To determine whether Merlin communicates directly with the AJ complex, we tested its ability to interact with recombinant versions of each component in vitro. We found that although Merlin did not associate with the cytoplasmic domain of E-cadherin or with β-catenin, Merlin could directly associate with α-catenin (Figure 1D). Additional binding studies revealed that Merlin associates with the N-terminal VH1 domain of α-catenin in vitro (Figure S2A). Conversely, α-catenin associates only with the FERM domain-containing N-terminal portion of Merlin, and not with the C-terminal portion (Figure 1E). The specificity of this interaction is highlighted by the inability of α-catenin to interact with Ezrin, which has a very closely related FERM domain, even when the C-terminal residues of Ezrin have been removed, which abrogates self-association and exposes interacting sites within the FERM domain (Figure S2B). Consistent with their ability to associate in vitro, endogenous Merlin and α-catenin also associate with each other in immortalized keratinocytes (Figure 1F). Importantly, shRNA-mediated knockdown of α-catenin expression eliminated the association of Merlin with E-cadherin (Figure 1G). These data identify Merlin as a partner for α-catenin and suggest that Merlin can associate directly with the core AJ complex via α-catenin; this association appears to be critical for the transition from PAs to a mature AJ belt.
Merlin-deficiency mimics the loss of core AJ proteins in vivo
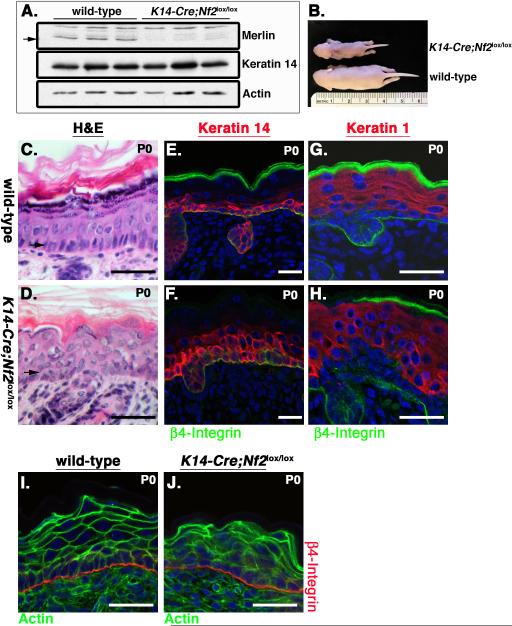
If Merlin is a critical AJ component, the consequences of Merlin-deficiency should mirror the loss of core AJ proteins in vivo. The phenotypic consequences of eliminating specific AJ proteins in the mouse epidermis have been described. To determine whether Merlin-deficiency yields similar phenotypes, we deleted Nf2 in the mouse epidermis by generating K14-Cre;Nf2lox/lox mice in which the Cre recombinase is expressed in basal cells under the control of the keratin 14 promoter (Vasioukhin et al., 1999) (Figure 2A). Indeed, deletion of Nf2 yielded striking macroscopic and microscopic similarities to the deletion of α-catenin, E-cadherin, or concomitant elimination of E-cadherin and P-cadherin using the same K14-Cre transgene (K14-Cre;α-E-catlox/lox, K14-Cre;Ecadlox/− or K14-Cre;Ecadlox/lox/K14-PcadRNAi) (Tinkle et al., 2008; Tunggal et al., 2005; Vasioukhin et al., 2001). Like K14-Cre;α-E-catlox/lox, K14-Cre;Ecadlox/− or K14-Cre;Ecadlox/lox/K14-PcadRNAi mice, K14-Cre;Nf2lox/lox mice died within a few days of birth with a runted appearance and markedly dry, flaky skin (Figure 2B). The newborn K14-Cre;Nf2lox/lox epidermis also exhibited histological similarities to that of the cadherin- or α-catenin-deficient epidermis, including disorganization and thickening of the K14-expressing basal layer and a failure of suprabasal cells to uniformly adopt a flattened squamous morphology despite their ability to differentiate normally. This was assessed by the expression of markers such as keratin 1, keratin 6, involucrin and filaggrin and by the appearance of a well-developed stratum corneum (Figure 2G–H; data not shown). As in both the cadherin- and α-catenin-deficient skin, Nf2−/− epidermal cells exhibited markedly aberrant organization of actin (Figure 2I, J).
Figure 2. Merlin is required for epidermal architecture and function in vivo.
(A) Immunoblotting of total epidermal lysates from three wild-type or K14-Cre;Nf2lox/lox neonates reveals the loss of Merlin protein (arrow).
(B) K14-Cre;Nf2lox/lox early postnatal (P4) mice are markedly runted and exhibit dry flaky skin.
(C–F) Hematoxylin and eosin (H&E) staining of neonatal (P0) wild-type (C) or K14-Cre;Nf2lox/lox (D) skin reveals that although the general epidermal organization is preserved in the absence of Merlin, the basal cell layer is expanded and often multilayered (arrows). This is highlighted by immunostaining for the basal cell marker Keratin 14 (red; E, F).
(G, H) The suprabasal marker Keratin 1 (red) stains all cells distal to K14-expressing cells in both the wild-type and K14-Cre;Nf2lox/lox epidermis. β4-integrin (green) labels the basement membrane for reference in E–H.
(I, J) Marked disorganization of actin (green) is apparent in the early postnatal (P0) K14-Cre;Nf2lox/lox skin. β4-integrin (red) labels the basement membrane. Bars, 40 μm.
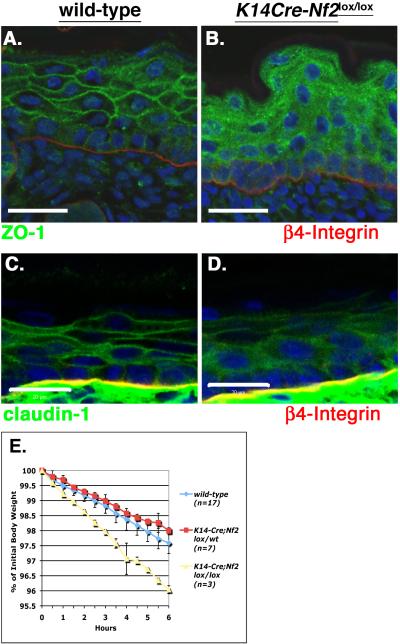
In the absence of α-catenin or cadherin the appearance of hemidesmosomes at the dermal:epidermal boundary and desmosomes throughout the epidermis appears normal, but markers of tight junctions (TJs) are mislocalized and functional TJs do not form, consistent with the notion that AJs are necessary for the establishment of TJs (Tinkle et al., 2008; Tunggal et al., 2005; Vasioukhin et al., 2001). Indeed, ultrastructural examination revealed that the appearance and distribution of desmosomes and hemidesmosomes were not affected by the loss of Merlin but TJs were not distinguishable (Figure S3D–G). In contrast to the discrete localization of TJ markers (ZO-1, Claudin-1, ZO-2) to cell:cell boundaries throughout the suprabasal layers of the wild-type epidermis, TJ proteins exhibited a diffuse and granular cytoplasmic localization in the absence of Merlin (Figure 3A–D; Figure S3A) (Morita et al., 1998). Finally, the measured decline in body weight due to water loss, together with the results of functional barrier assays, suggested that K14-Cre;Nf2lox/lox neonates have a poorly developed epidermal barrier, explaining their dry flaky skin and runted appearance within the first days after birth (Figure 3E; Figure S3B, C) (Hardman et al., 1998; Segre et al., 1999). Collectively, these data indicate that Merlin, like cadherin and α-catenin, is required for the formation of functional TJs in the epidermis in vivo.
Figure 3. Defective TJ formation and epidermal barrier function in the absence of Merlin in vivo.
(A–D) Immunostaining revealed the localization of both ZO-1 (green; A,B) and Claudin-1 (green; C,D) to cell:cell boundaries in suprabasal layers of the wild-type epidermis (A,C); in contrast, ZO-1 and Claudin-1 exhibited a diffuse and granular localization throughout the K14-Cre;Nf2lox/lox epidermis (B,D). Co-staining with anti-β4-integrin (red) antibodies and DAPI (blue) highlight the basement membrane and nuclei respectively. Bars, 40 μm (A and B), 20 μm (C and D).
(E) Body weight decrease due to water loss declined more rapidly in K14-Cre;Nf2lox/lox (yellow) neonatal mice relative to wild-type (blue) or K14-Cre;Nf2lox/wt (red) neonates, consistent with a defective inside-out epidermal barrier. Values = mean +/− SD, also see Figure S3.
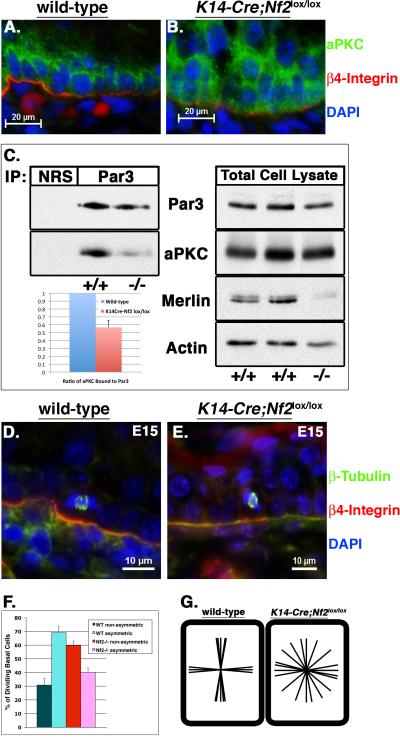
Cadherin-mediated cell adhesion is also necessary for epithelial cell polarity (Harris and Tepass, 2010; Macara, 2004; St Johnston and Ahringer, 2010). Indeed, elimination of cadherin or α-catenin from the epidermis yields mislocalization of aPKC, which is normally concentrated apically in basal cells at the basal:suprabasal interface, and is therefore a marker of epidermal basal cell polarity (Lechler and Fuchs, 2005; Tinkle et al., 2008; Tunggal et al., 2005). We found that elimination of Merlin also yields aPKC mislocalization (Figure 4A, B); in fact, very little aPKC coimmunoprecipitates with Par3 in the Nf2−/− skin, suggesting that Merlin is necessary for Par3/aPKC complex formation (Figure 4C). It has been argued that apical aPKC localization and activation is necessary for a program of oriented basal cell divisions that drives the process of epidermal stratification during development. Thus, prior to embryonic day 14.5 (E14.5), most epidermal basal cells divide symmetrically, parallel to the underlying basement membrane. At E14.5–15.5 a dramatic switch occurs, and most basal cells begin to divide asymmetrically, perpendicular to the basement membrane. Notably, this coincides with the onset of multilayering and disorganization of the basal cell layer in K14-Cre;Nf2lox/lox embryos, a phenotype that occurs without excess proliferation (data not shown). We monitored spindle orientation at E15 and found that, in excellent agreement with previous reports, 70% of wild-type basal cells undergo asymmetric/perpendicular divisions while 30% undergo symmetric/parallel divisions (Lechler and Fuchs, 2005) (Figure 4D, F, G). In contrast, like α-catenin-deficient basal cells (Lechler and Fuchs, 2005), Nf2-deficient basal cells exhibit a completely random spindle orientation (Figure 4E, F, G).
Figure 4. Loss of basal cell polarity in K14-Cre;Nf2lox/lox embryonic skin.
(A, B) In contrast to the apical concentration of aPKC (green) at the basal:suprabasal interface in the wild-type E15 epidermis, aPKC is diffusely localized throughout basal cells of the K14-Cre;Nf2lox/lox epidermis. DAPI (blue), and anti-β4-integrin (red) were used to label nuclei and basal lamina. Bars, 20 μm.
(C) Despite similar total levels of aPKC (right), very little aPKC immunoprecipitated with Par3 in the K14-Cre;Nf2lox/lox neonatal epidermis relative to wild-type. Quantitation of three independent experiments using ImageJ software is shown on the bottom left. Values = mean +/− SD.
(D, E) Immunostaining with an anti-β-tubulin antibody (green) shows representative spindle orientations in wild-type and K14-Cre;Nf2lox/lox basal cells. Co-staining with DAPI (blue) and anti-β4-integrin antibodies (red) labels nuclei and basal lamina, respectively. Bars, 10 μm.
(F) Calculation of the percentage of basal cells undergoing asymmetric division (mitotic spindles at a 90° angle relative to the basement membrane), compared to those undergoing non-asymmetric divisions (less than a 90° angle relative to the basement membrane). Values = mean +/− SD; n = >300 mitoses/genotype.
(G) Plotting of individual mitotic spindle orientations revealed that they were either parallel (symmetric) or perpendicular (asymmetric) in wild-type basal cells, as expected. In contrast, mitotic spindles were randomly oriented in K14-Cre;Nf2lox/lox embryonic epidermal basal cells.
Taken together, these data reveal striking similarities between the Merlin-deficient epidermis and that lacking either cadherin or α-catenin. In contrast, loss of Merlin does not elicit marked inflammation or major defects in hair follicle development, key features of p120- and β-catenin-deficiency, respectively (data not shown) (Huelsken et al., 2001; Perez-Moreno et al., 2008). Nevertheless, despite similar changes in actin organization, there is no epidermal blistering and/or obvious loss of mechanical integrity in the absence of Merlin, unlike the cadherin- and α-catenin-deficient epidermis (Tinkle et al., 2008; Vasioukhin et al., 2001). Indeed, as in cultured keratinocytes, we see no major difference in the localization, levels or stoichiometry of the core AJ complex in the Nf2-deficient epidermis (Figure S3H, data not shown). However, Merlin is required for basal cell polarity and for the establishment of TJs (Figure 3, 4), both of which depend upon AJ formation (Suzuki and Ohno, 2006). Together with our observation that cultured Nf2−/− keratinocytes form PAs that do not develop into a mature cortical junctional belt, these in vivo data suggest that Merlin is specifically necessary for the incompletely understood process of junctional maturation.
Merlin is required for junctional maturation
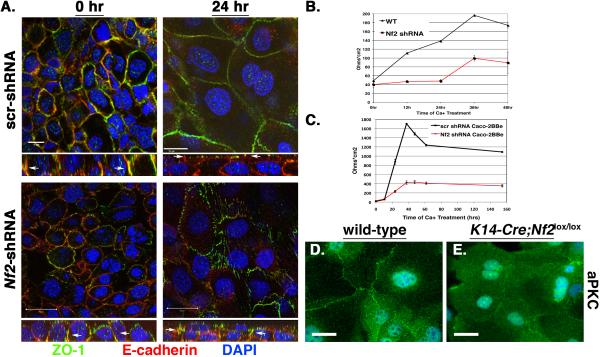
Next we confirmed that Merlin is required cell autonomously for TJ formation and polarity in cultured keratinocytes, which provide a system in which molecular studies of junctional maturation can be carried out. In calcium-stimulated keratinocytes, junctional maturation involves the coalescence of punctae that contain AJ and TJ proteins (PAs) to form a stratified, actin-associated junctional belt that contains AJs and apically positioned TJs (Vasioukhin et al., 2000; Yuki et al., 2007). In the absence of calcium, E-cadherin and the TJ marker ZO-1 extensively co-localize to PAs in immortalized control (scr-shRNA-expressing) or Nf2-shRNA-expressing keratinocytes (Figure 5A, left panels). In control cells, calcium stimulation prompts E-cadherin/ZO-1-containing punctae to coalesce, forming a thin continuous band in which ZO-1 and E-cadherin segregate apically and basally, respectively (Figure 5A, top right panel). In contrast, ZO-1 and E-cadherin remained colocalized in punctae following calcium stimulation of Nf2-shRNA expressing cells (Figure 5A, bottom right panel). Functional studies confirmed the requirement for Merlin in TJ establishment; thus, although control keratinocytes develop transepithelial resistance (TER) over time, Nf2-shRNA expressing keratinocytes exhibited only a modest increase in TER that was markedly delayed and never achieved wild-type levels (Figure 5B). Notably, we found that Nf2-shRNA expressing Caco-2BBe intestinal epithelial cells were also unable to form an electrically resistant barrier, indicating that Merlin is necessary for functional TJ formation in both stratified and columnar epithelial cells (Figure 5C).
Figure 5. Cell autonomous loss of TJ function and aPKC localization in the absence of Merlin in vitro.
(A) Confocal imaging showing the colocalization of ZO-1 and E-cadherin to PAs in both scr-shRNA and Nf2-shRNA-expressing PAM212 keratinocytes prior to calcium stimulation. After calcium stimulation ZO-1 and E-cadherin localize to distinct apical junctional rings. In contrast, ZO-1 and E-cadherin continue to colocalize to punctate structures in calcium-stimulated Nf2-shRNA-expressing cells. XZ sections are shown at the bottom of each panel. Nuclei labeled with DAPI (blue). Bars, 20 μm.
(B, C) Trans-epithelial resistance (TER) was measured across confluent control (black) or Nf2-shRNA-expressing (red) PAM212 keratinocyte (B) or Caco-2BBe intestinal epithelial cell (C) monolayers that had been stimulated with calcium for the indicated times. Values = mean +/− SD.
(D, E) Immunostaining reveals that aPKC (green) localizes primarily to cell:cell boundaries in calcium-stimulated wild-type keratinocytes but not in K14-Cre;Nf2lox/lox keratinocytes. Nuclei are stained with DAPI (blue). Bars, 15 μm.
In primary calcium-stimulated wild-type keratinocytes, aPKC localizes to cell:cell boundaries as expected (Figure 5D). However, as in the Nf2−/− epidermis, aPKC exhibited a diffuse cytoplasmic localization in cultured Nf2−/− keratinocytes (Figure 5E). Taken together, these data confirm that Merlin is required cell-autonomously for both TJ formation and polarity in vitro and in vivo.
Merlin links the AJ and Par3 complexes
The positioning of the TJ apical to the AJ in the mature junctional belt, together with the requirement for Par3/aPKC in TJ formation, suggests that recruitment of Par3 and/or aPKC to the early AJ may provide a critical apical cue for junctional stratification and polarization (Michels et al., 2009; Mizuno et al., 2003; Suzuki et al., 2002). Several studies conclude that Par3 co-localizes with AJ proteins during early stages of junctional establishment, and that this is followed by the recruitment of aPKC (Harris and Peifer, 2004, 2005, 2007; Ooshio et al., 2007; Suzuki et al., 2001; Yamanaka et al., 2001). However, it is not clear how communication between the AJ and Par3 is achieved. We found that in addition to co-localizing, endogenous Par3 associated with AJ proteins during early stages of junctional maturation in Nf2-expressing keratinocytes (Figure 6A). However, Par3 did not associate with AJ proteins in the absence of Merlin (Figure 6B). Par3 also failed to recruit either aPKC or TJ proteins in the absence of Merlin, consistent with our in vivo observations. These data are consistent with a model wherein Merlin is required for the physical association between Par3 and the AJ complex, which is in turn, a prerequisite for the recruitment of both aPKC and TJ proteins.
Figure 6. Merlin links the AJ and Par3 complexes in vivo and in vitro.
(A,B) Par3 (180kd isoform) was immunoprecipitated from (A) scr-shRNA- or (B) Nf2-shRNA-expressing PAM212 keratinocytes that had been stimulated with calcium for the indicated times. Immunoblotting revealed that Par3 associates with AJ and TJ proteins in the presence but not absence of endogenous Merlin. NRS, normal rabbit serum.
(C) FL-, NT- and CT-Merlin-HA produced by IVTT were mixed with Myc-Par3 produced in Sf9 cells. Immunoprecipitation using an anti-HA antibody and immunoblotting revealed that FL-, NT- and CT-Merlin-HA all associated with Par3 in vitro. 10% of the input mixture is shown in the right panel.
(D) Myc-Par3 produced in Sf9 cells was mixed with IVTT-produced FL-, NT- or CT-Merlin-HA and GST-α-catenin. Complexes were isolated using GST beads; immunoblotting with anti-GST, -Myc, or -HA antibodies revealed that FL- or NT-Merlin-HA, which bind to GST-α-catenin in vitro, but not CT-Merlin-HA, is sufficient to promote Myc-Par3:α-catenin association in vitro.
(E) Par3 was immunoprecipitated from Nf2-shRNA-expressing PAM212 keratinocytes that had been infected with a Merlin-NT-expressing adenovirus and stimulated with calcium for the indicated times. Immunoblotting revealed that reintroduction of Merlin-NT rescues the association of Par3 with AJ and TJ proteins in keratinocytes. NRS, normal rabbit serum; also see Figure S4.
Several FERM domain proteins have been shown to bind directly to other membrane-associated polarity proteins (Laprise et al., 2006; Laprise et al., 2009; Ling et al., 2010; Robinson et al., 2010). Therefore we asked whether Merlin associates with Par3 directly or indirectly. We found that full-length Merlin could associate with Par3 in vitro in the absence of AJ proteins (Figure 6C). Both the N- and C-terminal portions of Merlin associated with Par3 in vitro and in cultured cells, suggesting that Par3 interacts with the central linker region or that a stable interaction involves elements of both the N- and C-terminal portions (Figure 6C, S4A). Conversely, mapping studies suggested that Merlin associates with a central region of Par3 that contains the third PDZ domain - a region known to be required for TJ formation (Figure S4B) (Chen and Macara, 2005). Notably, this region of Par3 can also interact with Tiam-1, an activator of the small GTPases Rac and Cdc42 that has been implicated in TJ formation in keratinocytes (Chen and Macara, 2005; Mertens et al., 2005). However, although our primary and immortalized keratinocytes express Tiam-1 at low levels, we have not detected the coimmunoprecipitation of endogenous Tiam-1 with Par3 in our cells (not shown); in contrast, endogenous Merlin is readily detected (Figure 6A).
VE-cadherin can associate directly with Par3 in endothelial cells (Iden et al., 2006). However, while the E-cadherin-containing AJ complex does coimmunoprecipitate with Par3 in wild-type cultured keratinocytes (Figure 6A), the individual AJ proteins did not associate with Par3 on their own in vitro (Figure S4C). The association of endogenous Merlin with both Par3 and α-catenin in keratinocytes (Figure S4D), together with its ability to interact directly with both Par3 and α-catenin in vitro suggests that Merlin could link these two complexes. Indeed, the addition of either full-length or N-terminal Merlin was sufficient to promote the association between Par3 and α-catenin in vitro, while the C-terminal half of Merlin, which does not bind α-catenin, was not sufficient (Figure 6D). Moreover, expression of the N-terminal portion of Merlin in Nf2-deficient keratinocytes was sufficient to rescue both the association between Par3 and AJ proteins and the recruitment of aPKC and TJ proteins to Par3 (Figure 6E). These data suggest that Merlin directly links the AJ and Par3 complexes during early junctional maturation.
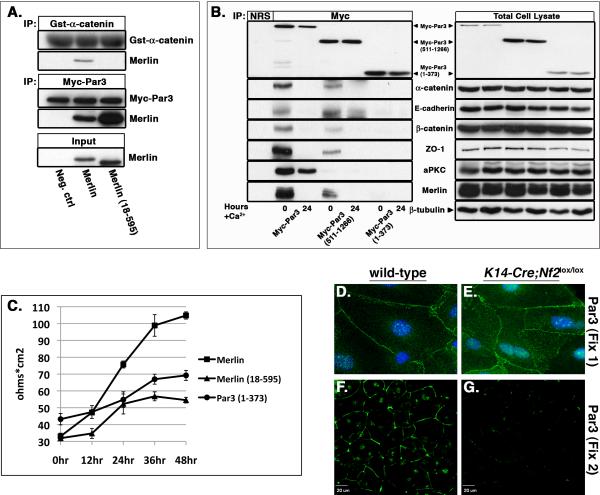
Is Merlin's ability to link the AJ and Par3 complexes necessary for functional junction maturation in cells? We previously found that a version of Merlin lacking the extreme N-terminal 17 amino acids (Nf218-595) fails to stably localize to cell:cell boundaries or to associate with the AJ complex (Cole et al., 2008). Indeed, Nf218-595 cannot associate with α-catenin in vitro (Figure 7A). However, we found that Nf218-595 could still interact with Par3 (Figure 7A), raising the possibility that this mutant could dominantly interfere with junctional maturation. Consistent with this hypothesis, expression of Nf218-595 in wild-type immortalized keratinocytes interfered with the establishment of functional TJs as measured by TER (Figure 7C). As would be expected, this mutant also could not restore TER to Nf2-shRNA-expressing cells (not shown). Similarly, in contrast to full-length Myc-Par3 and Myc-Par3511-1266 that associate with Merlin and with AJ and TJ proteins (Figure 7B, S4B), a mutant version of Par3 (Myc-Par31-373) that does not bind to Merlin fails to associate with AJ or TJ proteins (Figure 7B) and also dominantly interferes with the establishment of TER (Figure 7C). Taken together, these data provide functional evidence that Merlin-mediated tethering of Par3 to the AJ is necessary for junctional maturation. In final support of this hypothesis, we found that, despite its apparently normal localization in both primary and immortalized Nf2-deficient keratinocytes (Figure 7D, E, data not shown), Par3, but not α-catenin, is completely eliminated under more stringent fixation/detergent extraction conditions (Fix 2; see Methods) in the absence of Merlin (Figure 7G, Figure S5). In contrast, Par3 remains localized to cell:cell boundaries under the same conditions in the presence of Merlin (Figure 7F, Figure S5).
Figure 7. The interaction of Merlin with both β-catenin and Par3 is required for the establishment of functional junctions.
(A) IVTT-produced Merlin or Merlin18-595 was mixed with either GST-β-catenin (top) or Myc-Par3 (middle). Complexes were isolated using either GST beads (top panel) or an anti-Myc antibody (middle panel) and immunoblotted with anti-GST, -Myc, or -Nf2 antibodies. Merlin18-595 can readily bind Myc-Par3 but not GST-β-catenin in vitro. 10% of the input is shown in the bottom panel.
(B) PAM212 keratinocytes were transfected with Myc-Par3, Myc-Par3511-1266 or Myc-Par31-373 and cultured in calcium-containing media for the indicated times. Immunoprecipitation of Par3 followed by immunoblotting with Myc-, AJ- or TJ-specific antibodies revealed that full-length Myc-Par3 and Myc-Par3511-1266 can associate with Merlin and with AJ and TJ proteins. In contrast, Myc-Par31-373 does not associate with Merlin or with AJ and TJ proteins. Note that Myc-Par3511-1266 and Myc-Par31-373 are produced from a Par3 splice variant that does not contain the aPKC-binding site. NRS, normal rabbit serum.
(C) TER was measured across calcium-stimulated PAM212 keratinocyte monolayers that stably express Merlin, Merlin18-595, or Myc-Par31-373. Note that both Merlin18-595 and Myc-Par31-373 dominantly interfered with the establishment of TER. Values = mean +/− SD.
(D–G) Primary wild-type (D and F) or K14-Cre;Nf2lox/lox (E and G) keratinocytes were stimulated with calcium-containing media for 8 hours, and either processed for indirect immunofluorescence (Fix 1, D and E) or subject to a more stringent in situ extraction (Fix 2, F and G). Immunostaining revealed normal localization of Par3 (green) at cell-cell boundaries in K14-Cre;Nf2lox/lox cells that were processed via mild fixation/extraction (Fix 1; D and E). However, Par3 staining in these cells is completely eliminated by more stringent fixation/extraction (Fix 2). In contrast, Par3 localization to cell-cell boundaries is maintained in wild-type keratinocytes in Fix 2. See also Figure S5.
Discussion
Mounting evidence indicates that FERM domain containing proteins play fundamental roles in organizing the cell cortex. Our studies reveal that the FERM-domain containing NF2 tumor suppressor, Merlin, coordinates cell adhesion and polarity in the mammalian epidermis by promoting an association between Par3 and the AJ. Merlin is not required to recruit Par3 to the membrane; indeed, Par3 can associate directly with membrane phospholipids via its second PDZ domain (Wu et al., 2007). Instead, our data suggest that Merlin directly links Par3 to α-catenin, consistent with the abnormal sensitivity of Par3 to detergent extraction in the absence of Merlin (see Figure 7D–G and Figure S7). This physical interaction is required during early stages of junction formation for subsequent maturation steps, including the establishment of a cortical actin ring and segregation of TJs; it is also required for the recruitment of aPKC and establishment of a program of oriented cell divisions within the basal epidermis. Our data are consistent with studies in fly and mammalian epithelial cells that conclude that the localization of cadherin and Par3 to nascent cell junctions precedes the recruitment of aPKC to Par3 (Harris and Peifer, 2005; Ooshio et al., 2007; Suzuki et al., 2002; Suzuki et al., 2001). Our work further suggests that Par3 is unable to bind aPKC in the absence of Merlin. It is not yet clear how Merlin promotes Par3:aPKC interaction; Merlin-binding could cause a conformational change in Par3 that permits aPKC association or facilitate Par3 posttranslational modification, by directly or indirectly (via the AJ) recruiting additional proteins. Notably, the association of Merlin with both Par3 and the AJ diminishes after calcium stimulation at approximately the same time that TJs are apically segregated (see Figure 6A, not shown). Perhaps the regulated release of Merlin-mediated AJ:Par3 association represents a subsequent step in junctional maturation.
Increasing evidence supports the idea that FERM-domain containing proteins such as Band 4.1 and the ERM proteins, can simultaneously assemble protein complexes at the membrane and locally stabilize the cortical cytoskeleton (McClatchey and Fehon, 2009). Primary Nf2−/− keratinocytes fail to establish a mature AJ belt and associated cortical actin ring, suggesting that Merlin may similarly stabilize the interaction between the AJ and cytoskeleton. In fact, more actin associates with the AJ complex in the presence of Merlin than in its absence (see Figure S1F). This provides a compelling parallel to the closely related ERM proteins that are known to both stabilize the membrane:cortical actin interface and assemble membrane protein complexes (Fehon et al., 2010).
Merlin can decorate and affect the cortical actin cytoskeleton but lacks the C-terminal actin-binding domain found in the ERM proteins (Fehon et al., 2010). We found that Merlin can directly interact with α-catenin, which has long been thought to statically link the AJ to the actin cytoskeleton. However, recent studies challenge this notion and suggest that α-catenin may dynamically regulate the actin cytoskeleton at the AJ while multiple proteins establish and maintain this interface, particularly during morphogenetic processes that require junctional remodeling (Drees et al., 2005; Yamada et al., 2005). Indeed, mounting evidence suggests that the AJ itself is a heterogeneous, dynamic structure (Pilot et al., 2006). We found that the first 17 amino acids of Merlin are required for its association with α-catenin in vitro, consistent with our previous observation that these residues are required for Merlin localization to cell:cell boundaries and association with the AJ complex (Cole et al., 2008). Although it remains unclear whether these residues are sufficient to interact with α-catenin, it is interesting to note that they are also required for Merlin to decorate the cortical actin cytoskeleton away from cell:cell boundaries (Cole et al., 2008). Given the increasing suggestion that α-catenin may have junction-independent functions (Benjamin et al., 2010; Lien et al., 2008a), it is tempting to speculate that Merlin functionally interacts with α-catenin both at the AJ and across the cell cortex. The ability of Merlin to negatively regulate signaling downstream of the small GTPase Rac may also contribute to Merlin-dependent changes in the keratinocyte cortical cytoskeleton (Kissil et al., 2003; Shaw et al., 2001).
Individual FERM domain-containing proteins can likely assemble multiple different complexes even within a given cell. We previously found that Merlin promotes the contact-dependent association between the AJ complex and EGFR (via the adapter NHERF-1) in liver epithelial cells (Curto et al., 2007). Here we found that Merlin can link the AJ complex to Par3 in keratinocytes. It is not yet clear whether these complexes are mutually exclusive; however, in both cases, Merlin seems to coordinate the sensing and establishment of cell:cell contact with the positioning and function of another membrane protein (Par3 or EGFR). Merlin is also thought to cooperate with the FERM domain protein Expanded to negatively regulate the Hpo/Wts/Yki pathway that controls organ size in Drosophila and in some mammalian tissues, but the membrane-derived signals that direct Merlin and/or Expanded to control this pathway have not been well-defined (Genevet et al., 2009; Hamaratoglu et al., 2009; Hamaratoglu et al., 2006; Ling et al., 2010; Lu et al., 2010; Song et al., 2010; Zhang et al., 2010; Zhou et al., 2009). The recent identification of the apical polarity protein Crumbs as a physical and functional regulator of Expanded, but not Merlin, suggests an intriguing possibility: Although we have not detected Merlin-dependent changes in the analogous mammalian Mst/Lats/Yap pathway in keratinocytes (data not shown), our discovery that Merlin can physically and functionally interact with Par3, together with the known functional interdependence of Crumbs and Par3, suggests a mechanism whereby these proteins could cooperate to regulate this pathway in certain cells.
Experimental Procedures
Mouse strains and cell culture
K14-Cre;Nf2lox/lox mice were generated by crossing Nf2lox/lox (Giovannini et al., 2000) and K14-Cre mice (Vasioukhin et al., 1999) and genotyped as described. Epidermal water loss assays were performed as described (Segre et al., 1999). All experiments involving animals were approved by the MGH Subcommittee on Research Animal Care.
Keratinocytes and epidermal tissue were isolated from wild-type or K14-Cre;Nf2lox/lox neonates and floated in EDTA-free 0.25% trypsin overnight at 4°C to separate the dermis from the epidermis. The isolated tissue was minced and cell suspensions filtered and seeded onto collagen I-coated plates. Keratinocytes were cultured in calcium-depleted DMEM (Dulbecco's Modified Eagle Medium, GIBCO) containing 10% fetal calf serum (FCS), L-glutamine, sodium pyruvate, hydrocortizone, cholera toxin and 10 ng/ml EGF. Immortalized murine PAM212 keratinocytes (ATCC) were cultured in calcium-depleted DMEM containing 10% FCS, L-glutamine and sodium pyruvate. For junctional maturation keratinocytes were switched to DMEM containing 5% FCS, 1.5 mM CaCl2, for the indicated times. HEK293T cells were cultured in DMEM containing 10% FCS.
Lentiviruses expressing control (scrambled), α-catenin- or Nf2-directed shRNAs were obtained from Open Biosystems (Thermo Scientific). Plasmids expressing Nf2wt, Nf21-341, Nf2344-595, Nf218-595, Myc-Par3, Myc-Par3511-1266 and Myc-Par31-373 have been described (Chen and Macara, 2005; Cole et al., 2008) and were transfected into either HEK293T or PAM212 cells using Lipofectamine Plus (Invitrogen). Full-length Merlin, Merlin18-595 or Myc-Par31-373, were cloned into pBabe-puro and stably expressed in PAM212 cells via retroviral infection followed by selection in 6 uM puromycin.
Histology and immunohistochemistry
Tissues were fixed overnight in 3.7% buffered formalin and processed for paraffin embedding, sectioning and hematoxylin and eosin staining. For cryosectioning tissues were fixed in 4% paraformaldehyde overnight, dehydrated through increasing concentrations of sucrose and embedded in Optimal Cutting Temperature compound (OCT). Sections were stained with the following primary antibodies: ZO-1 (1:100, Z-R1, Zymed), Claudin-1 (1:100, 2H10D10, Zymed), ZO-2 (1:100, Z54.PL, Invitrogen), α-catenin (1:100, αCAT-7A4, Zymed), β4-integrin (1:200, 346-11A, Pharmingen), Keratin 14 (1:1000, AF64, Covance), Keratin 1 (1:1000, AF87, Covance), Keratin 6 (1:500, PRB-169P, Covance), Filaggrin (1:1000, PRB-417P, Covance), Involucrin (1:1000, PRB-140C, Covance), β-tubulin (1:250, SAP 4G5, Sigma), PKC-ζ (1:250, C-20, Santa Cruz). Secondary antibodies used (1:200) were: anti-rabbit or anti-mouse Alexa Fluor 488 (Invitrogen); anti-rat, anti-mouse or anti-rabbit Cy3 or Cy5 (Jackson ImmunoResearch). Nuclei were stained with 4'6'-diamidino-2-phenylindole (DAPI).
Indirect immunofluorescence and in situ extraction
For immunofluorescence primary keratinocytes or PAM212 cells were plated on collagen I coated coverslips in calcium-depleted media, grown to confluence and either collected or switched to calcium-containing medium (1.5 mM) for specified times. Coverslips were washed in PBS, fixed in 4% paraformaldehyde for 10 min, permeabilized in PBS containing 0.5% Triton X-100 for 10 min, blocked in 10% goat serum and stained with the following primary antibodies: NF2 (1:500, A-19 and C-18, Santa Cruz), E-cadherin (1:2000, #36, BD), Actin (1:2000, AC40, Sigma), ZO-1 (1:500, Z-R1, Zymed), aPKC (1:500, C-20, Santa Cruz), Par3 (1:100, 07-330, Zymed) and α-catenin (1:100, α-cat-7A4, Zymed), followed by secondary antibodies as described above. In situ extraction (Fix 2) of primary or immortalized keratinocytes was performed as described (Cole et al., 2008). Briefly, cells were simultaneously fixed and permeabilized in 1% formaldehyde and 2% Triton X-100 in CB (CB; 10 mM 2-(N-morpholino)-ethanesulfonic acid sodium salt (MES), pH 6.1, 138 mM KCl, 3 mM MgCl2, and 2 mM EGTA) for 15 min.
Transepithelial resistance (TER) measurement
Keratinocytes were grown on polycarbonate Transwell filters (0.4 μM pore size, 12 mm diameter; Corning) in calcium-depleted media until confluent and then switched to media containing 1.5 mM CaCl2. TER was measured with an epithelial voltmeter (Millipore) and values expressed in ohm × cm2 were calculated by subtracting the values of the blank filter with medium. Results are from five independent experiments and each reading reflects the average of three measurements at the indicated times.
Western blot and immunoprecipitation (IP)
Total protein extracts from epidermal tissue were prepared using RIPA lysis buffer (50 mM Tris pH 7.4, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 1 mM Na3VO4, 10 mM sodium fluoride, 10 mM β-glycerophosphate, 1 mg/ml aprotinin and 1 mg/ml leupeptin). For total Triton soluble cell lysates, samples were resuspended in Triton lysis buffer (50 mM Tris pH 7.4, 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 1 mM Na3VO4, 10 mM sodium fluoride, 10 mM β-glycerophosphate, 1 mg/ml aprotinin and 1 mg/ml leupeptin), followed by a brief sonication. Cell debris was cleared by centrifugation at 14,000 rpm (10 min, 4°C) and protein lysates were quantitated by DC protein assay (Bio-Rad). Protein samples were separated by SDS-PAGE, transferred to PVDF, and blocked with 5% non-fat dried milk.
For IP, protein lysates were diluted in Tween 20 IP buffer (50 mM HEPES pH 8, 150 mM NaCl, 2.5 mM EGTA, 2.5 mM EDTA, 0.1% Tween 20, 1mM PMSF, 1 mM Na3VO4, 10 mM sodium fluoride, 10 mM β-glycerophosphate, 1 mg/ml aprotinin and 1 mg/ml leupeptin) and incubated with Protein A sepharose beads (GE Healthcare) and the indicated antibodies overnight at 4°C. IP'd complexes were washed 4× with Tween 20 IP buffer, resuspended in protein loading buffer, boiled and loaded onto SDS-PAGE gels. For IP and western blot the following antibodies were used: NF2 (1:1000, A-19 and C-18, Santa Cruz), Keratin 14 (1:5000, AF64, Covance), Actin (1:1000, AC40, Sigma), β-tubulin (1:1000, SAP4G5, Sigma), Par3 (1:500, 07-330, Upstate), aPKC (1:500, C-20, Santa Cruz), HA (1:1000, clone 12CA5), Myc (1:1000, 9E10, Santa Cruz), ZO-1 (1:500, Z-R1, Zymed), E-cadherin (1:2000, #36, Pharmingen), β-catenin (1:2000, #14, Pharmingen), and α-catenin (1:1000, #5, Pharmingen).
In vitro binding
GST-E-cad-cyto, GST-β-catenin and GST-α-catenin were produced in bacteria as previously described (Yamada et al., 2005). Nf2wt, Nf21-341, Nf2344-595, Nf218-595, Flag-Ezrin and Flag-EzrinΔactin were produced by in vitro transcription and translation from the pCDNA3 plasmids (Cole et al., 2008). Flag-EzrinΔactin, a version of Ezrin in which residues 553–586 have been deleted, adopts a constitutively open conformation, exposing the FERM domain for interaction with other proteins (Speck et al., 2003). Myc-Par3 and Flag-Merlin were purified from Sf9 lysate. For in vitro binding assays, proteins were mixed together in Tween 20 IP buffer at room temperature for 3 hrs; GST-bound proteins were washed 5× with Tween 20 IP buffer, eluted with loading buffer and separated by gel electrophoresis.
Supplementary Material
Acknowledgments
We thank members of the McClatchey laboratory for helpful discussions and Bruce Morgan for comments on the manuscript. Par3 expression plasmids were graciously provided by Ian Macara (University of Virginia). We thank Marco Giovannini (House Ear Institute) for the Nf2lox/lox mice, Tony Pawson (Mount Sinai Hospital, Toronto) for the aPKC expression plasmid, William Weis (Stanford University) for GST AJ constructs and Rick Fehon (University of Chicago) for Ez and EzΔactin expression plasmids. The work was supported by an NRSA fellowship (5F32CA124030) awarded to A.B.G and by grants from the NIH (R01 CA113733), DOD (W81XWH-05-1-0189) and O'Brien Trust awarded to A.I.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional materials and methods are described in the supplemental experimental procedures.
References
- Adams JC, Watt FM. Expression of beta 1, beta 3, beta 4, and beta 5 integrins by human epidermal keratinocytes and non-differentiating keratinocytes. J Cell Biol. 1991;115:829–841. doi: 10.1083/jcb.115.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H, Zweimueller-Mayer J, Steinbacher P, Lametschwandtner A, Bauer HC. The dual role of zonula occludens (ZO) proteins. J Biomed Biotechnol. 2010;2010:402593. doi: 10.1155/2010/402593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin JM, Kwiatkowski AV, Yang C, Korobova F, Pokutta S, Svitkina T, Weis WI, Nelson WJ. AlphaE-catenin regulates actin dynamics independently of cadherin-mediated cell-cell adhesion. J Cell Biol. 2010;189:339–352. doi: 10.1083/jcb.200910041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005;7:262–269. doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- Cole BK, Curto M, Chan AW, McClatchey AI. Localization to the cortical cytoskeleton is necessary for Nf2/merlin-dependent epidermal growth factor receptor silencing. Mol Cell Biol. 2008;28:1274–1284. doi: 10.1128/MCB.01139-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Finding one's niche in the skin. Cell Stem Cell. 2009;4:499–502. doi: 10.1016/j.stem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevet A, Polesello C, Blight K, Robertson F, Collinson LM, Pichaud F, Tapon N. The Hippo pathway regulates apical-domain size independently of its growth-control function. J Cell Sci. 2009;122:2360–2370. doi: 10.1242/jcs.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L, Woodruff JM, Berns A, Thomas G. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14:1617–1630. [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, Gajewski K, Sansores-Garcia L, Morrison C, Tao C, Halder G. The Hippo tumor-suppressor pathway regulates apical-domain size in parallel to tissue growth. J Cell Sci. 2009;122:2351–2359. doi: 10.1242/jcs.046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Hardman MJ, Sisi P, Banbury DN, Byrne C. Patterned acquisition of skin barrier function during development. Development. 1998;125:1541–1552. doi: 10.1242/dev.125.8.1541. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J Cell Biol. 2004;167:135–147. doi: 10.1083/jcb.200406024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J Cell Biol. 2005;170:813–823. doi: 10.1083/jcb.200505127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Peifer M. aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development. Dev Cell. 2007;12:727–738. doi: 10.1016/j.devcel.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich I, Schmitz A, Zigrino P, Michels C, Haase I, le Bivic A, Leitges M, Niessen CM. Role of aPKC isoforms and their binding partners Par3 and Par6 in epidermal barrier formation. J Invest Dermatol. 2007;127:782–791. doi: 10.1038/sj.jid.5700621. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Iden S, Rehder D, August B, Suzuki A, Wolburg-Buchholz K, Wolburg H, Ohno S, Behrens J, Vestweber D, Ebnet K. A distinct PAR complex associates physically with VE-cadherin in vertebrate endothelial cells. EMBO Rep. 2006;7:1239–1246. doi: 10.1038/sj.embor.7400819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101–108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- Jensen UB, Lowell S, Watt FM. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole-mount labelling and lineage analysis. Development. 1999;126:2409–2418. doi: 10.1242/dev.126.11.2409. [DOI] [PubMed] [Google Scholar]
- Johnson KC, Kissil JL, Fry JL, Jacks T. Cellular transformation by a FERM domain mutant of the Nf2 tumor suppressor gene. Oncogene. 2002;21:5990–5997. doi: 10.1038/sj.onc.1205693. [DOI] [PubMed] [Google Scholar]
- Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003;12:841–849. doi: 10.1016/s1097-2765(03)00382-4. [DOI] [PubMed] [Google Scholar]
- Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17:1090–1100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise P, Beronja S, Silva-Gagliardi NF, Pellikka M, Jensen AM, McGlade CJ, Tepass U. The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev Cell. 2006;11:363–374. doi: 10.1016/j.devcel.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise P, Lau KM, Harris KP, Silva-Gagliardi NF, Paul SM, Beronja S, Beitel GJ, McGlade CJ, Tepass U. Yurt, Coracle, Neurexin IV and the Na(+),K(+)-ATPase form a novel group of epithelial polarity proteins. Nature. 2009;459:1141–1145. doi: 10.1038/nature08067. [DOI] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Gelfand VI, Vasioukhin V. Alpha-E-catenin binds to dynamitin and regulates dynactin-mediated intracellular traffic. J Cell Biol. 2008a;183:989–997. doi: 10.1083/jcb.200805041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Stepniak E, Vasioukhin V. Dissecting the role of cadherin-catenin proteins in mammalian epidermis. Proc Natl Acad Sci U S A. 2008b;105:15225–15226. doi: 10.1073/pnas.0808458105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, Wu S, Pan D. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci U S A. 2010;107:10532–10537. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara IG. Parsing the polarity code. Nat Rev Mol Cell Biol. 2004;5:220–231. doi: 10.1038/nrm1332. [DOI] [PubMed] [Google Scholar]
- McClatchey AI, Fehon RG. Merlin and the ERM proteins--regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 2009;19:198–206. doi: 10.1016/j.tcb.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey AI, Giovannini M. Membrane organization and tumorigenesis--the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–2277. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- McLaughlin ME, Kruger GM, Slocum KL, Crowley D, Michaud NA, Huang J, Magendantz M, Jacks T. The Nf2 tumor suppressor regulates cell-cell adhesion during tissue fusion. Proc Natl Acad Sci U S A. 2007;104:3261–3266. doi: 10.1073/pnas.0700044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens AE, Rygiel TP, Olivo C, van der Kammen R, Collard JG. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol. 2005;170:1029–1037. doi: 10.1083/jcb.200502129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels C, Aghdam SY, Niessen CM. Cadherin-mediated regulation of tight junctions in stratifying epithelia. Ann N Y Acad Sci. 2009;1165:163–168. doi: 10.1111/j.1749-6632.2009.04443.x. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Suzuki A, Hirose T, Kitamura K, Kutsuzawa K, Futaki M, Amano Y, Ohno S. Self-association of PAR-3-mediated by the conserved N-terminal domain contributes to the development of epithelial tight junctions. J Biol Chem. 2003;278:31240–31250. doi: 10.1074/jbc.M303593200. [DOI] [PubMed] [Google Scholar]
- Morita K, Itoh M, Saitou M, Ando-Akatsuka Y, Furuse M, Yoneda K, Imamura S, Fujimoto K, Tsukita S. Subcellular distribution of tight junction-associated proteins (occludin, ZO-1, ZO-2) in rodent skin. J Invest Dermatol. 1998;110:862–866. doi: 10.1046/j.1523-1747.1998.00209.x. [DOI] [PubMed] [Google Scholar]
- Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA, Gutmann DH, Ponta H, Herrlich P. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–980. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Takeichi M. Remodeling of the adherens junctions during morphogenesis. Curr Top Dev Biol. 2009;89:33–54. doi: 10.1016/S0070-2153(09)89002-9. [DOI] [PubMed] [Google Scholar]
- Okada T, Lopez-Lago M, Giancotti FG. Merlin/NF-2 mediates contact inhibition of growth by suppressing recruitment of Rac to the plasma membrane. J Cell Biol. 2005;171:361–371. doi: 10.1083/jcb.200503165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooshio T, Fujita N, Yamada A, Sato T, Kitagawa Y, Okamoto R, Nakata S, Miki A, Irie K, Takai Y. Cooperative roles of Par-3 and afadin in the formation of adherens and tight junctions. J Cell Sci. 2007;120:2352–2365. doi: 10.1242/jcs.03470. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Song W, Pasolli HA, Williams SE, Fuchs E. Loss of p120 catenin and links to mitotic alterations, inflammation, and skin cancer. Proc Natl Acad Sci U S A. 2008;105:15399–15404. doi: 10.1073/pnas.0807301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot F, Philippe JM, Lemmers C, Lecuit T. Spatial control of actin organization at adherens junctions by a synaptotagmin-like protein Btsz. Nature. 2006;442:580–584. doi: 10.1038/nature04935. [DOI] [PubMed] [Google Scholar]
- Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr Biol. 2010;20:582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang-Xuan K, Demczuk S, Desmaze C, Plougastel B, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- Schluter H, Moll I, Wolburg H, Franke WW. The different structures containing tight junction proteins in epidermal and other stratified epithelial cells, including squamous cell metaplasia. Eur J Cell Biol. 2007;86:645–655. doi: 10.1016/j.ejcb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, O'Bryan JP, Gupta V, Ratner N, Der CJ, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck O, Hughes SC, Noren NK, Kulikauskas RM, Fehon RG. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature. 2003;421:83–87. doi: 10.1038/nature01295. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141:757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010;20:142–149. doi: 10.1016/j.tcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, Ohno S. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J Cell Sci. 2002;115:3565–3573. doi: 10.1242/jcs.00032. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol. 2001;152:1183–1196. doi: 10.1083/jcb.152.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U. FERM proteins in animal morphogenesis. Curr Opin Genet Dev. 2009;19:357–367. doi: 10.1016/j.gde.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Tinkle CL, Pasolli HA, Stokes N, Fuchs E. New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proc Natl Acad Sci U S A. 2008;105:15405–15410. doi: 10.1073/pnas.0807374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, Eldridge R, Kley N, Menon AG, Pulaski K, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;75:826. doi: 10.1016/0092-8674(93)90501-g. [DOI] [PubMed] [Google Scholar]
- Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, Kemler R, Krieg T, Niessen CM. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. Embo J. 2005;24:1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci U S A. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Feng W, Chen J, Chan LN, Huang S, Zhang M. PDZ domains of Par-3 as potential phosphoinositide signaling integrators. Mol Cell. 2007;28:886–898. doi: 10.1016/j.molcel.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, Nagai Y, Yamashita A, Hirose T, Ishikawa H, et al. PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes Cells. 2001;6:721–731. doi: 10.1046/j.1365-2443.2001.00453.x. [DOI] [PubMed] [Google Scholar]
- Yuki T, Haratake A, Koishikawa H, Morita K, Miyachi Y, Inoue S. Tight junction proteins in keratinocytes: localization and contribution to barrier function. Exp Dermatol. 2007;16:324–330. doi: 10.1111/j.1600-0625.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.