Abstract
Stress is known to elicit pain relief, a phenomenon referred to as stress-induced analgesia. Based on stress parameters, opioid and non-opioid intrinsic pain inhibitory systems can be activated. In the present study, we assessed whether changing the duration of stress would affect the involvement of endogenous opioids in antinociception elicited by swim in warm water (32°C), known to be opioid-mediated. Using mice lacking beta-endorphin, enkephalins or dynorphins and their respective wild-type littermates, we assessed the role of each opioid peptide in antinociception induced by a short (3 min) vs. long (15 min) swim. Mice were tested for baseline hot plate latency, exposed to swim (3 or 15 min) in warm water (32°C) and then tested for antinociception at 5, 15 and 30 min. Our results revealed that both swim paradigms induced significant antinociception in wild-type mice. However, the short swim failed to induce antinociception in beta-endorphin-deficient mice, illustrating that beta-endorphin is important in this form of stress-induced antinociception. On the other hand, antinociception elicited by the long swim was only slightly reduced in beta-endorphin-deficient mice despite pretreatment with naloxone, a non-selective opioid receptor antagonist, significantly attenuated the antinociception elicited by the long swim. Nevertheless, a delayed hyperalgesic response developed in mice lacking beta-endorphin following exposure to either swim paradigm. On the other hand, mice lacking enkephalins or dynorphins and their respective wild-type littermates expressed a comparable antinociceptive response and did not exhibit the delayed hyperalgesic response. Together, our results suggest that the endogenous opioid peptide beta-endorphin not only mediates antinociception induced by the short swim but also prevents the delayed hyperalgesic response elicited by either swim paradigm.
Keywords: Stress-induced analgesia, Endogenous opioid peptides, Knockout mouse, Swim duration, Hot plate test
1. Introduction
The endogenous pain inhibitory systems can be activated by fear, anxiety, stress, etc. (Akil et al., 1976; Bodnar et al., 1978a; Butler and Finn, 2009; Lewis et al., 1981; Mayer et al., 1971; Terman et al., 1984). It is well documented that stress activates different intrinsic pain inhibitory mechanisms, leading to analgesia in humans and antinociception in rodents, a phenomenon referred to as stress-induced analgesia/antinociception (Butler and Finn, 2009). Depending on the nature of stressors, different pain inhibitory mechanisms can be activated. In general, less severe stressors are thought to activate the endogenous opioid system and elicit the opioid-mediated form of stress-induced analgesia. On the other hand, more severe stressors recruit pain inhibitory mechanisms that are independent of the endogenous opioid system (Akil et al., 1986; Amit and Galina, 1986; Butler and Finn, 2009; Mogil et al., 1996). For example, a 3-min swim in warm water (32°C) elicits antinociception that is mediated by the endogenous opioid system because this form of antinociception is blocked by naloxone, a non-selective opioid receptor antagonist (Butler and Finn, 2009; Madden et al., 1977; Marek et al., 1992; Mogil et al., 1996). Furthermore, tolerance develops to this form of stress-induced antinociception and animals tolerant to this form of stress-induced antinociception display cross-tolerance to morphine (Christie et al., 1982; Girardot and Holloway, 1984; Lewis et al., 1981; Maude-Griffin and Tiffany, 1989; Terman and Liebeskind, 1986). On the other hand, swim in cold water (2°C for 3.5 min) elicits antinociception that is not affected by low doses of naloxone that inhibit morphine-induced antinociception (Bodnar et al., 1978a). Furthermore, this form of stress-induced antinociception does not display cross-tolerance to morphine (Bodnar et al., 1978b). However, it is sensitive to neuroendocrine manipulations, such as hypophysectomy (Bodnar et al., 1979) and blockade by MK-801, a non-competitive NMDA receptor antagonist (Marek et al., 1992) as well as agents affecting several other neurotransmitter systems, such as endocannabinoids, GABA, etc. (Butler and Finn, 2009).
The duration of stress is also a determinant factor in eliciting opioid or non-opioid mediated forms of stress-induced antinociception. For example, intermittent swimming in cold water induces antinociception that is mediated by the endogenous opioid system. On the other hand, longer swim in cold water elicits antinociception that is not mediated by the opioid system (Girardot and Holloway, 1984). A similar phenomenon can be observed if foot shock is used as the source of stress (Akil et al., 1985). Previous studies have shown that a short (3 min) swim in warm water (32°C) elicits antinociception that is mediated by the endogenous opioid peptide (Marek et al., 1992) and beta-endorphin has been implicated in this response (Rubinstein et al., 1996). However, the role of the endogenous opioid system in antinociception induced by a longer swim in warm water is not fully characterized although a long swim (7 min) in warm water has been shown to induce a naloxone-insensitive antinociceptive effect (Mogil et al., 1996). Nevertheless, there is evidence implicating dynorphins in antinociception elicited by a swim in warm water for 15 min (McLaughlin et al., 2003). Accordingly, in the current study, we assessed whether antinociception induced by the long swim (15 min) would be sensitive to blockade by naloxone and whether endogenous beta-endorphin, enkephalins or dynorphins would play a functional role in this form of stress-induced antinociception. For comparison, we also examined the role of opioid peptides in antinociception elicited by the short (3 min) swim, known to be mediated via the opioid system (Marek et al., 1992; Rubinstein et al., 1996).
2. Material and Methods
2.1.Subjects
We originally obtained the fully backcrossed breeding pairs of the beta-endorphin null (Rubinstein et al., 1996) and proenkephalin null (Konig et al., 1996) mice from Jackson Laboratory (Bar Harbor, Main, USA). The prodynorphin null mice (Sharifi et al., 2001) were generously supplied by Dr. Ute Hochgeschwender (Oklahoma Medical Research Foundation, Oklahoma, USA). We have backcrossed these mice on C57BL/6J background and bred them in house from the fully backcrossed heterozygous breeding pairs of each line. Female mice (aged between 8–12 weeks) were used for all experiments. Mice were housed 2–4 per cage under a 12-h light/ 12-h dark cycle. The food and water were available ad libitum. All experiments were conducted according to the National Institute of Health guideline for the proper use of animals in research and approved by the Institutional Animal Care and Use Committee at Western University of Health Sciences (Pomona, California, USA).
2.2. Experimental procedures
The hot plate test was used to measure changes in nociceptive response prior to and at different time points following a short (3 min) or long (15 min) swim exposure. We used the hot plate test because it measures nociceptive responses at supra-spinal sites, where stress could cause the release of opioid peptides (Akil et al., 1985; Madden et al., 1977). Mice lacking beta-endorphin, enkephalins or dynorphins and their respective wild-type littermates were first tested for baseline hot plate (52°C) latency, in which mice were placed in the middle of a Plexiglas cylinder and the amount of time that elapsed for each mouse to lick or vigorously shake, whichever response occurred first, one of the hindpaws was recorded. A cut-off time of 40 sec was used to prevent tissue damage. Mice were then left undisturbed for 60 min and then forced to swim in a beaker (1.8 L) containing 1.4 L lukewarm water (32°C) for 3 or 15 min. Mice were then placed in a clean cage containing towel papers to dry for 2 min, and then tested for changes in hot plate latency at 5, 15 and 30 min after the onset of the swim.
2.3. Effects of naloxone on antinociception induced by the long swim in mice
Female C57BL/6J mice (Jackson Laboratories, inc.; Bar Harbor, Main, USA) were tested for baseline hot plate latency and 1 h later injected with saline or naloxone (10 mg/kg, s.c.). Fifteen min later, mice were exposed to swim in warm water for 15 min and tested for hot plate latency at 5, 15 and 30 min post-swim.
2.4. Data Analysis
Data represent mean (±S.E.M.) hot plate latency (sec) before and at different time points post-swim and were analyzed by a two-factor repeated-measure analysis of variance (ANOVA). The post-hoc Newman-Keuls test was used to reveal significant differences between the groups. P<0.05 was considered significant.
3. Results
3.1. Exposure to the long swim (15 min) compared to short swim (3 min) induced a greater antinociceptive response
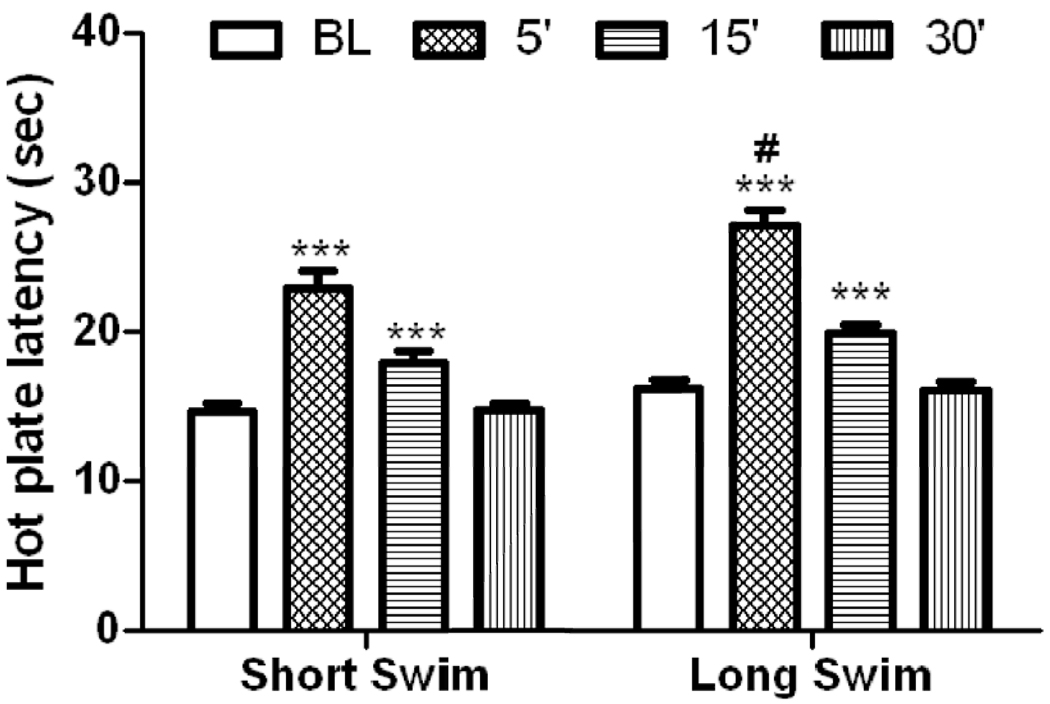
We assessed antinociception induced by the long versus short swim after combining the data of wild-type mice of all three genotypes (Fig. 1). Two-way repeated measures ANOVA of the data revealed a significant effect of hot plate latency before and after swim exposure (F3,171 = 96.51; P<0.0001), a significant effect of swim duration (F1,171 = 9.53; P<0.005) but no significant interaction between the two factors (F3,171 = 1.92; P>0.05), suggesting that both stressors induced antinociception in a time-dependent manner. However, the magnitude of this response was significantly greater at 5 min post swim in mice exposed to the long as compared to short swim.
Figure 1.
Hot plate latency before and at 5, 15 and 30 min following a 3-min (short) or 15-min (long) swim in warm (32°C) water in wild-type mice of all three genotypes combined. Data represent mean (±S.E.M.) of 29–30 mice per swim paradigm. ***significantly different from its baseline value (P<0.001); #significantly different from mice exposed to swim for 3 min at the given time point (P<0.01)
3.2. Antinociception elicited by the short (3 min) swim was blunted in beta-endorphin null mice
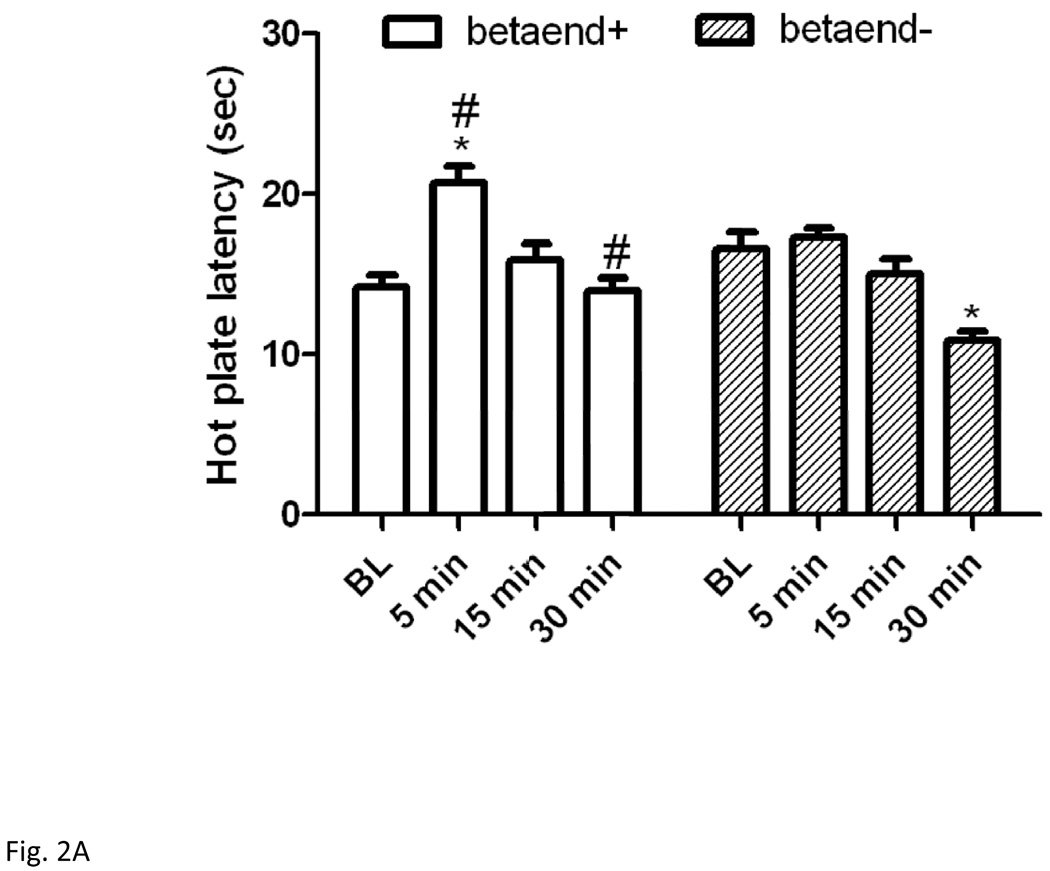
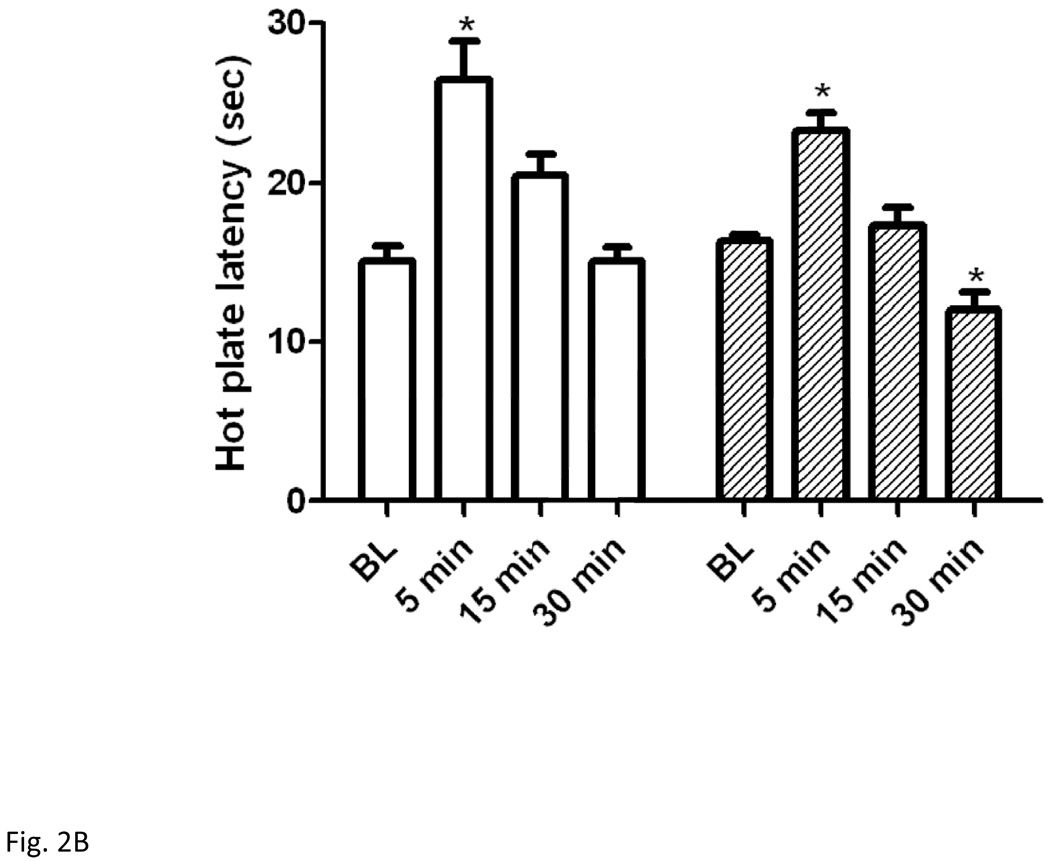
Figure 2 illustrates hot plate latency prior to and following the short (2A) or long (2B) swim in mice lacking beta-endorphin and their wild-type littermates. Two-way repeated measures ANOVA revealed no significant effect of genotype (F1,60 = 2.22; P>0.05), but there was a significant effect of hot plate latency (F3,60 = 28.97; P<0.001) and a significant interaction between the two factors (F3,60 = 7.24; P<0.001). Post-hoc analysis showed that there was no difference in baseline hot plate latency between the two genotypes (P>0.05). The 3-min swim paradigm prolonged hot plate latency in wild-type mice but not in beta-endorphin deficient mice (P<0.05; compare hot plate latency at 5-min post-swim in both genotypes). However, a hyperalgesic response was observed in beta-endorphin null mice at 30 min post-swim (Fig. 2A; compare the latency at 30 min post-swim in null mice to their baseline latency or the latency of wild-type mice at this time point).
Figure 2.
Hot plate latency before and at 5, 15 and 30 min following a 3-min (A) or 15 min (B) swim in warm (32°C) water in mice lacking beta-endorphin and their wild-type littermates. Data represent mean (±S.E.M.) of 7–11 mice per genotype per swim paradigm. *significantly different from its baseline value (P<0.05); #significantly different from beta-endorphin deficient mice at the given time point (P<0.05)
Antinociception elicited by the long (15 min) swim was slightly reduced in beta-endorphin null mice (Fig. 2B). Two-way repeated measures ANOVA revealed a significant effect of hot plate latency (F3,36 = 41.92; P<0.001), but no significant effect of genotype (F1,36 = 2.39; P>0.05) and no significant interaction between hot plate latency and genotype (F3,36 = 2.22; P>0.05). Once again, there was no difference in baseline hot plate latency between the two genotypes (P>0.05). The 15-min swimming paradigm increased hot plate latency in both wild-type and beta-endorphin-deficient mice, but there was no significant difference between the two genotypes at 5 min post-swim, when the response was maximal (P<0.05; compare latency at 5 min versus all other time points for each genotype). However, a delayed hyperalgesic response was observed in beta-endorphin null mice at 30 min time point following the long swim exposure (P<0.05; compare the 30-min time point to its baseline latency).
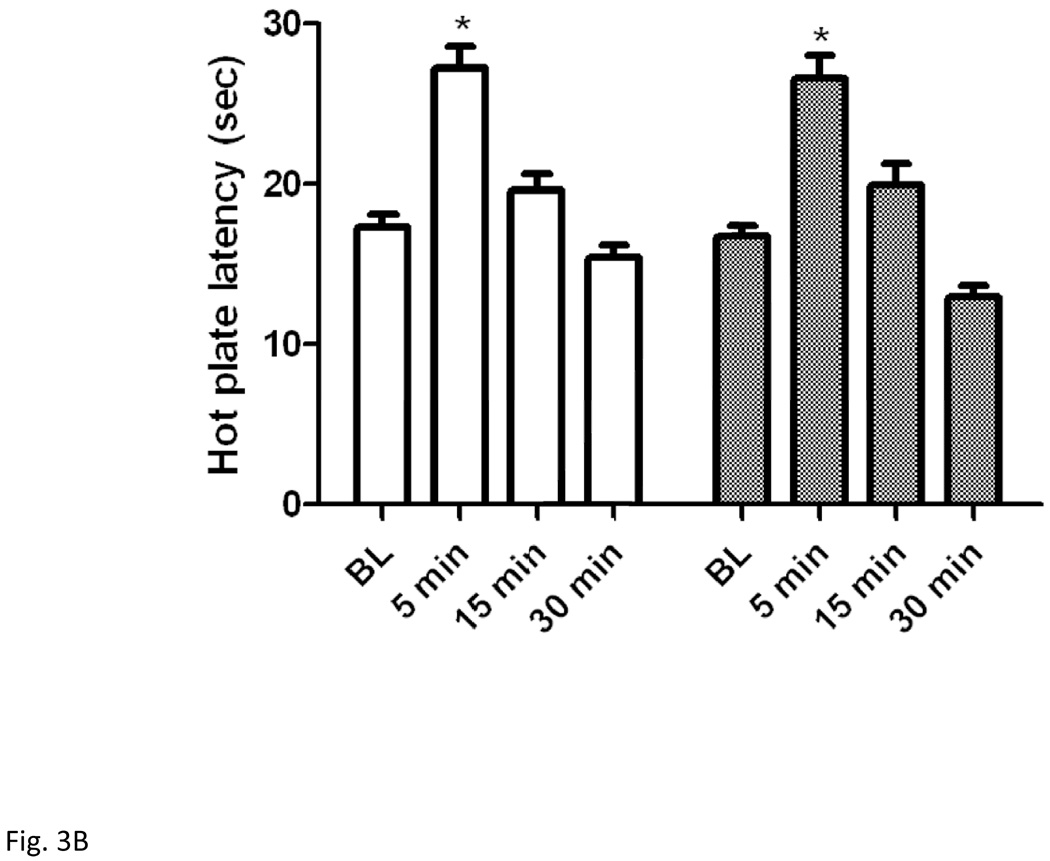
3.3. Mice lacking endogenous enkephalins and their wild-type littermates displayed comparable antinociception after exposure to either swim paradigm
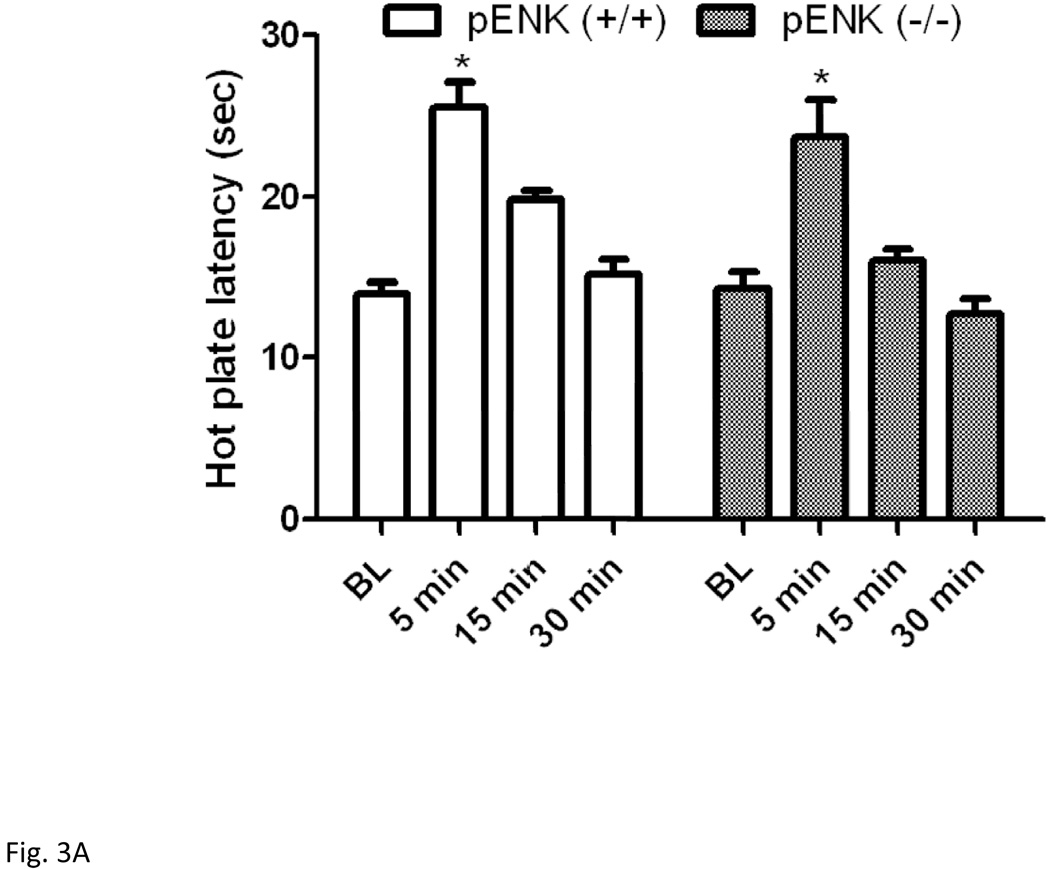
Figure 3 illustrates hot plate latency before and after the short (Fig. 3A) or long (Fig. 3B) swim in wild-type and enkephalin null mice. Two-way repeated measures ANOVA revealed a significant effect of hot plate latency (F3,42 = 36.07; P<0.001), a significant effect of genotype (F1,42 = 4.91; P<0.05) but no significant interaction between the two factors (F3,42 = 1.05; P>0.05) for the short swim paradigm. Post-hoc analysis demonstrated that there was no significant difference in hot plate latency before or at 5-min post swim between enkephalin-deficient mice and their wild-type littermates (P>0.05).
Figure 3.
Hot plate latency before and at 5, 15 and 30 min after a 3-min (A) or 15-min (B) swim in warm (32°C) water in mice lacking enkephalins and their wild-type littermates. Data represent mean (±S.E.M.) of 7–10 mice per genotype for each swim paradigm. *significantly different from their baseline value (P<0.05)
Two-way repeated measures ANOVA resulted in a significant effect of hot plate latency (F3,51 = 58.91; P<0.001), but no significant effect of genotype (F1,51 = 0.89; P>0.05) and no significant interaction between the two factors (F3,51 = 0.65; P>0.05) for the 15-min swim. Post-hoc analysis showed that swimming in warm water for 15 min induced antinociception which was not different between enkephalin-deficient mice and their wild-type littermates at 5-min post swim, when the antinociception induced by the long swim was maximal.
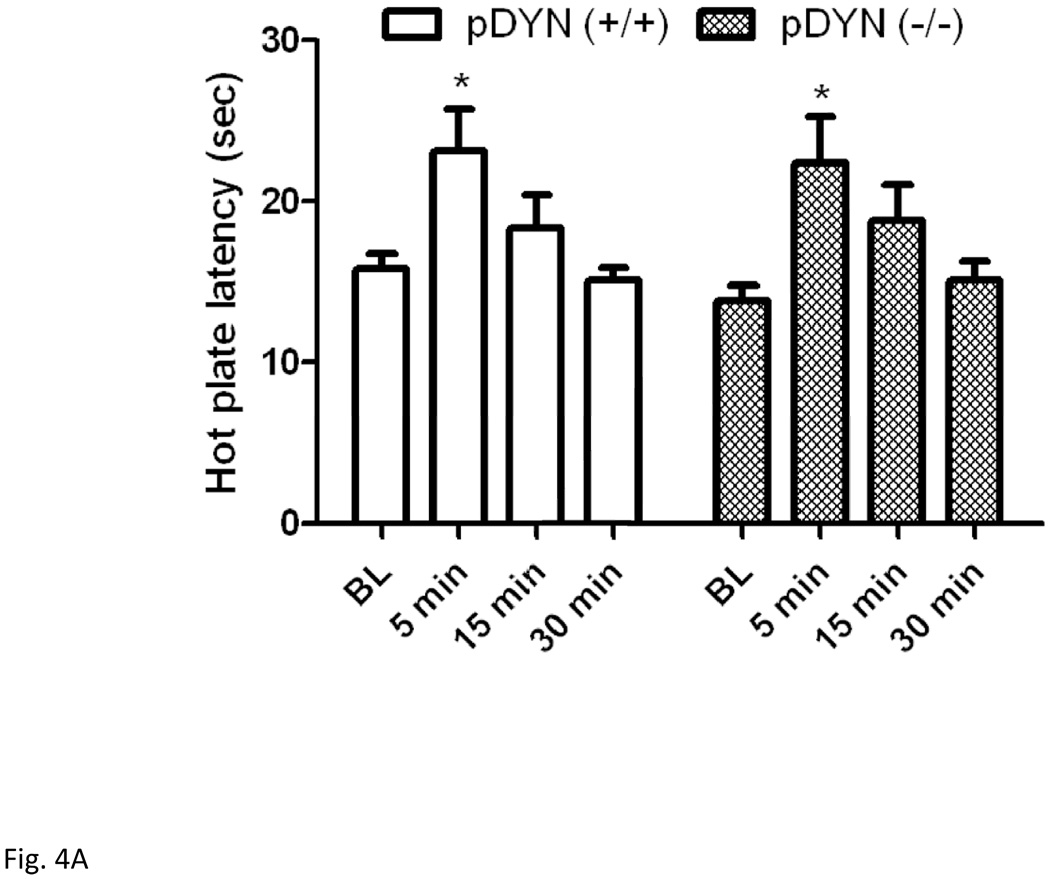
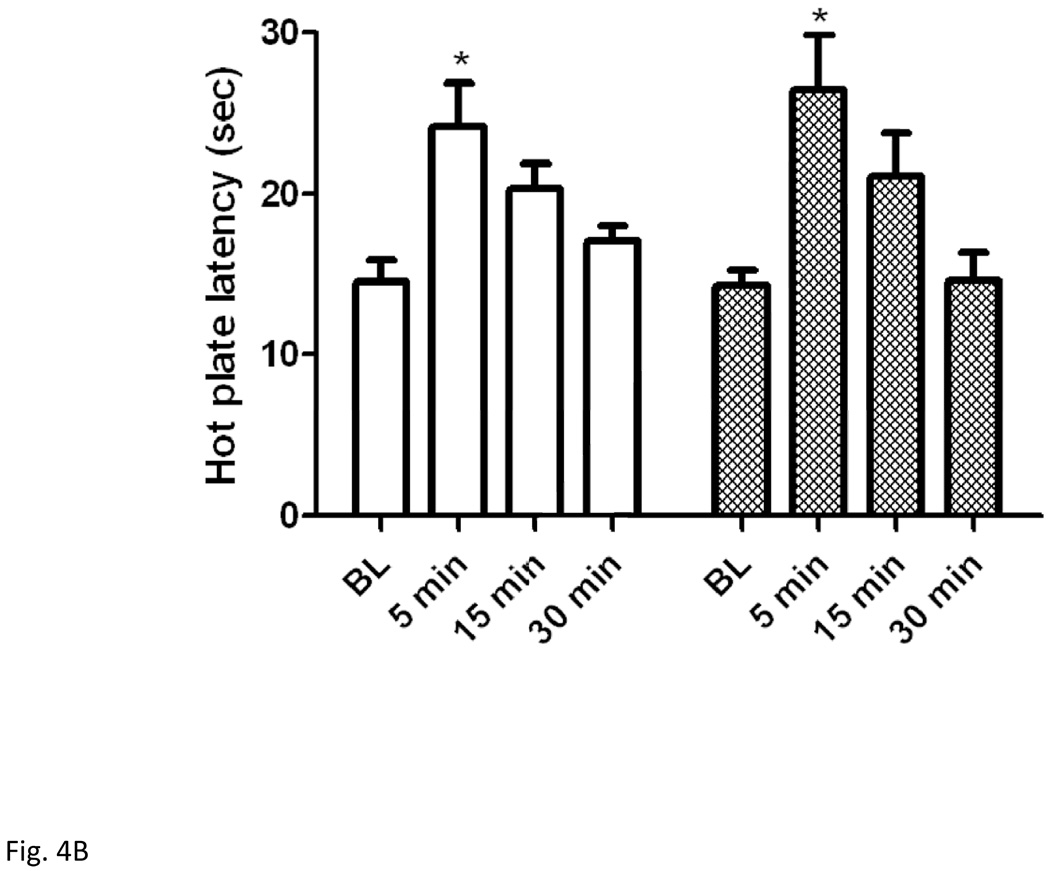
3.4. Mice lacking dynorphins and their wild-type littermates expressed comparable antinociception following either swim paradigm
Figure 4 depicts hot plate latency before and after the short (Fig. 4A) or long (Fig. 4B) swim in mice lacking the prodynorphin gene and their wild-type littermates. Two-way repeated measures ANOVA revealed a significant effect of hot plate latency (F3,54 = 13.34; P<0.001) but no significant effect of genotype (F1,54 = 0.07; P>0.05) and no significant interaction between the two factors (F3,54 = 0.29; P>0.05) for the short swim paradigm. Post-hoc analysis showed no significant difference in hot plate latency between the wild-type and dynorphin null mice at any time point following swim exposure (P>0.05).
Figure 4.
Hot plate latency prior to and at 5, 15 and 30 min following a 3-min (A) or 15-min (B) swim in warm (32°C) water in mice lacking dynorphin and their wild-type littermates. Data represent mean (±S.E.M.) of 7–10 mice per genotype for each swim paradigm. *significantly different from their baseline value (P<0.05)
Two-factor repeated measures ANOVA revealed a significant effect of hot plate latency (F3,39 = 14.74; P<0.001) but no significant effect of genotype (F1,39 = 0.01; P>0.05) and no significant interaction between the two factors (F3,39 = 0.61; P>0.05) for the 15-min swim paradigm. Posthoc analysis revealed no significant difference in antinociception elicited by the 15- min swimming paradigm between mice lacking dynorphins and their wild-type littermates (Fig. 4B).
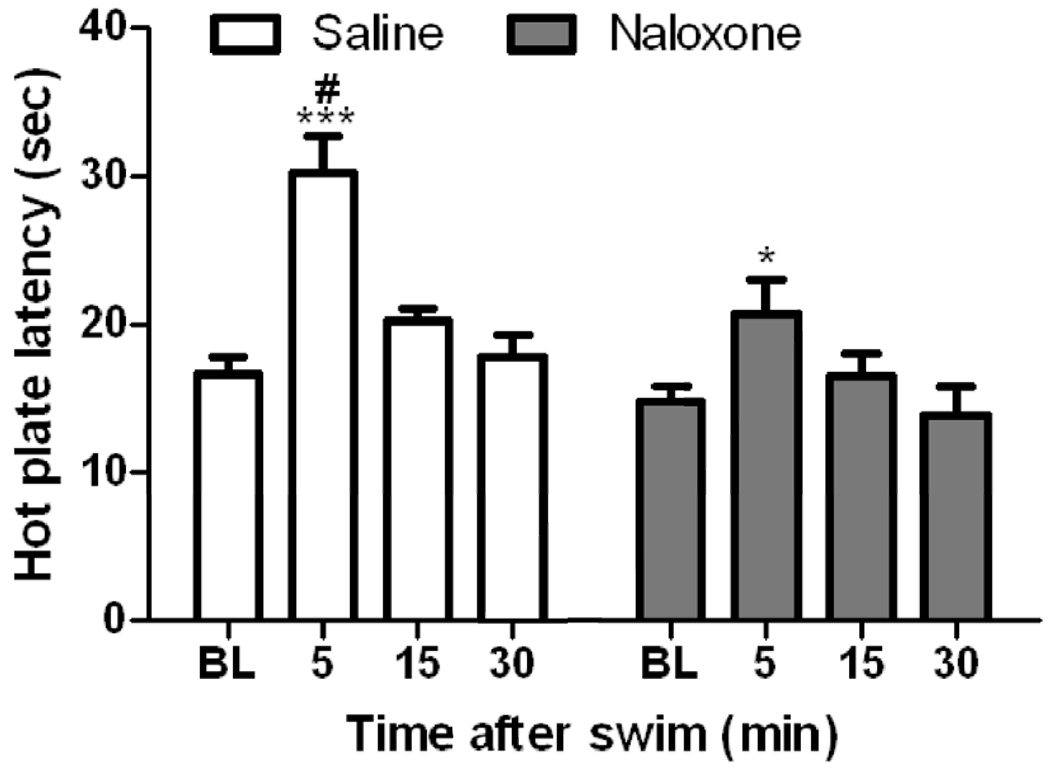
3.5. Naloxone significantly reduced antinociception induced by the long swim in C57BL/6J mice
Figure 5 illustrates hot plate latency prior to and after swim exposure in mice treated with saline or naloxone (10 mg/kg). Two-way repeated measures ANOVA revealed a significant effect of treatment (F1,39 = 8.44; P<0.05), a significant effect of hot plate latency (F3,39 = 21.18; P<0.001) and a significant interaction between the two factors (F3,39 = 2.80; P<0.05). The poshoc analysis of the data illustrated that swim induced antinociception in both groups but the magnitude of this response was significantly reduced in naloxone-treated mice compared to their saline-treated at the time of its peak effect. This result suggests that naloxone reduced the antinociceptive response elicited by this form of stress and implicates the endogenous opioid system in this response.
Fig. 5.
Hot plate latency prior to and at 5, 15 and 30 min following a 15-min swim in warm (32°C) water in C57BL/6J mice treated with saline or naloxone (10 mg/kg, s.c.) 10 min prior to swim. Data represent mean (±S.E.M.) of 7–8 mice per treatment. *significantly different from its respective baseline value (P<0.05); ***significantly different from its respective baseline value (P<0.001); #significantly different from saline-treated controls at the given time point (P<0.05)
4. Discussion
The results of the present study illustrate that antinociception elicited by the short, but not long swim, was blunted in mice lacking beta-endorphin compared to their wild-type littermates. However, a delayed hyperalgesic response was observed in null mice following either swim paradigm. On the other hand, mice lacking enkephalins or dynorphins and their respective wild-type littermates displayed comparable response following either swim paradigm. Together, the current results suggest that beta-endorphin is important in mediating antinociception elicited by the short swim as well as in preventing the delayed hyperalgesic response. However, neither enkephalins nor dynorphins play a major role in antinociception elicited by either swim paradigm.
Both swim paradigms induced a significant antinociceptive response which was maximal at 5 min post-swim and declined thereafter in a time-dependent manner. However, exposure to the long swim compared to short swim induced a significantly greater response when wild-type mice of all genotypes were combined (Fig. 1), suggesting that the duration of swim affects the magnitude of stress-induced antinociception. Although at the present time the underlying mechanisms of enhanced antinociception following the long swim compared to short swim is not clear, one possibility could be the contribution of non-opioid mediated mechanisms (see also below). The short swim has been reported to induce antinociception via the endogenous opioid system (Marek et al., 1992) and beta-endorphin has been implicated in this response (Rubinstein et al., 1996). However, the role of the endogenous opioid system in antinociception induced by more severe stress is not well studied. While Mogil and colleagues found that antinociception induced by swim in warm water for 7 min was not blocked by naloxone, McLaughlin and colleagues (2003) have implicated dynorphins in antinociception induced by swim in warm water for 15 min. Thus, using naloxone, a non-opioid receptor antagonist, we determined the sensitivity of the antinociception induced by the long swim (15 min) to naloxone. Our results revealed that swim in warm water for 15 min induced antinociception which was blocked by naloxone with a small naloxone-insensitive component, implicating both opioid and non-opioid mechanisms in this response. Although the reason for the difference between our result and that of Mogil et al. (1996) is not clear, one possible explanation would be the use of different experimental paradigms in the two studies. We used a longer (15 min vs. 7 min) swim duration and a different nociceptive test (hot plate vs. tail withdrawal assay). The residual antinociception observed in naloxone-treated mice most likely mediated by non-opioid mechanisms and may explain the enhanced antinociception induced by the long swim as compared to short swim in wild-type mice (Fig. 1). Overall, our results illustrate that a major component of the antinociception induced by the long swim is opioid-mediated because naloxone significantly reduced this form of swim-induced antinociception. Additionally, we found that antinociception induced by the short swim was blunted in beta-endorphin deficient mice (Fig. 2A), suggesting that beta-endorphin is the primary neural substrate mediating this form of stress-induced antinociception. Surprisingly, despite antinociception induced by the 15-min swimming paradigm was significantly reduced by naloxone, the antinociceptive response induced by the long swim was only slightly reduced in mice lacking beta-endorphin compared to their wild-type littermates (Fig. 2B). However, antinociception elicited by swimming in warm water for 15 min may not require the opioid peptide beta-endorphin or the response may be mediated by other endogenous opioid peptides, such as enkephalins or dynorphins (see below). Interestingly, following either swim paradigm, a delayed (30 min post-swim) hyperalgesic response was observed in beta-endorphin null mice but not in their wild-type littermates. Although hyperalgesia following either single or repeated stress has been reported previously and different neurotransmitter/neuropeptide systems have been implicated in this response (Imbe et al., 2006; Khasar et al., 2008; Quintero et al., 2000; Suarez-Roca et al., 2006; Vidal and Jacob, 1982), our result are novel and showing that beta-endorphin is important not only in mediating antinociception elicited by a short swim, but also in preventing the development of a delayed hyperalgesic response. The hyperalgesic response could have been due an imbalance between the nociceptive inhibitory and excitatory mechanisms following repeated hot plate measurements. For example, this hyperalgesic response could have been due the release of some facilitatory nociceptive neurochemical(s) in response to swim exposure and/ or repeated exposure to the hot plate that would have remained unopposed in the absence of beta-endorphin. We are hoping to elucidate the mechanisms of this hyperalgesic response in future studies.
Previous studies have excluded endogenous enkephalins as mediators of antinociception induced by the short swim in warm water as no difference in hot plate latency was observed between mice lacking enkephalins and their wild-type littermates following exposure to short swim (Konig et al., 1996). We assessed the role of enkephalins in antinociception induced by the long swim paradigm and compared it to the response elicited by the short swim. Although our data analysis revealed a significant effect of genotype, the post-hoc analysis of the data yielded no significant difference between the wild-type and null mice at each time point post swim exposure (p>0.05), confirming the results of Konig and colleagues (1996) and illustrating that a comparable antinociceptive response was observed in mice lacking enkephalins and their wild-type littermates following the short swim in the hot plate test. Here, we also report that antinociception induced by the long swim was not different between mice lacking enkephalins and their wild-type littermates, suggesting that endogenous enkephalins play minimal functional role in analgesia elicited by the short or long swim in mice.
Earlier reports have linked endogenous dynorphins to antinociception elicited by the long swim in warm water (McLaughlin et al., 2003; Starec et al., 1996). However, our result revealed no difference in stress-induced antinociception elicited by either swim paradigm between mice lacking dynorphins and their wild-type littermates. Nevertheless, it needs to be noted that we measured nociceptive responses immediately following a single swim exposure, whereas McLaughlin and colleagues (2003) examined stress-induced antinociception after several swim exposures (one 15-min swim on day 1 followed by 4×6 min on day 2). Furthermore, the tail withdrawal assay as compared to the hot plate latency was used to measure nociceptive response in the previous study (McLaughlin et al., 2003). It is particularly important to note that the tail withdrawal assay is used to assess spinal mechanisms of thermal nociceptive responses while the hot plate test is reported to study supraspinally-mediated components of nociceptive processing. Thus, the inconsistency between the current and previous study could have been due to differences in experimental paradigms.
5. Conclusion
The current results suggest that beta-endorphin plays a functional role in antinociception induced by the short, but not long, swim. The opioid peptide beta-endorphin also plays a functional role in preventing the development of a delayed hyperalgesic response. The endogenous opioid peptides enkephalins and dynorphins do not seem to contribute significantly to either form of stress-induced antinociception under the current experimental paradigm. Given that naloxone, a non-selective opioid receptor antagonist, reduced the antinociceptive responses elicited by the long swim, it is tempting to hypothesize that the antinociceptive response elicited by long swim may be mediated by more than one opioid peptide. Thus, further studies using double and triple opioid peptide knockout mice are needed to shed light on this issue. It is also possible that other endogenous opioid peptides, such as endomorphins (Zadina et al., 1997) mediate this form of stress-induced antinociception. However, at the present time, mice lacking endomorphins are not available to test this possibility.
Acknowledgments
The present study was supported in part by MIDARP grant # R24 DA017298 to TCF and in part by the NIDA Grant # R01 DA016682 to KL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akil H, Mayer DJ, Liebeskind JC. Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science. 1976;191:961–962. doi: 10.1126/science.1251210. [DOI] [PubMed] [Google Scholar]
- Akil H, Shiomi H, Matthews J. Induction of the intermediate pituitary by stress: synthesis and release of a nonopioid form of beta-endorphin. Science. 1985;227:424–426. doi: 10.1126/science.3155575. [DOI] [PubMed] [Google Scholar]
- Akil H, Young E, Walker JM, Watson SJ. The many possible roles of opioids and related peptides in stress-induced analgesia. Ann N Y Acad Sci. 1986;467:140–153. doi: 10.1111/j.1749-6632.1986.tb14625.x. [DOI] [PubMed] [Google Scholar]
- Amit Z, Galina ZH. Stress-induced analgesia: adaptive pain suppression. Physiol Rev. 1986;66:1091–1120. doi: 10.1152/physrev.1986.66.4.1091. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Glusman M, Brutus M, Spiaggia A, Kelly DD. Analgesia induced by cold-water stress: attenuation following hypophysectomy. Physiol Behav. 1979;23:53–62. doi: 10.1016/0031-9384(79)90122-7. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Kelly DD, Spiaggia A, Ehrenberg C, Glusman M. Dose-dependent reductions by naloxone of analgesia induced by cold-water stress. Pharmacol Biochem Behav. 1978a;8:667–672. doi: 10.1016/0091-3057(78)90264-2. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Kelly DD, Steiner SS, Glusman M. Stress-produced analgesia and morphine-produced analgesia: lack of cross-tolerance. Pharmacol Biochem Behav. 1978b;8:661–666. doi: 10.1016/0091-3057(78)90263-0. [DOI] [PubMed] [Google Scholar]
- Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Trisdikoon P, Chesher GB. Tolerance and cross tolerance with morphine resulting from physiological release of endogenous opiates. Life Sci. 1982;31:839–845. doi: 10.1016/0024-3205(82)90538-0. [DOI] [PubMed] [Google Scholar]
- Girardot MN, Holloway FA. Intermittent cold water stress-analgesia in rats: cross-tolerance to morphine. Pharmacol Biochem Behav. 1984;20:631–633. doi: 10.1016/0091-3057(84)90315-0. [DOI] [PubMed] [Google Scholar]
- Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci. 2006;11:2179–2192. doi: 10.2741/1960. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Sherman JE, Liebeskind JC. Opioid and non-opioid stress analgesia: assessment of tolerance and cross-tolerance with morphine. J Neurosci. 1981;1:358–363. doi: 10.1523/JNEUROSCI.01-04-00358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden Jt, Akil H, Patrick RL, Barchas JD. Stress-induced parallel changes in central opioid levels and pain responsiveness in the rat. Nature. 1977;265:358–360. doi: 10.1038/265358a0. [DOI] [PubMed] [Google Scholar]
- Marek P, Mogil JS, Sternberg WF, Panocka I, Liebeskind JC. N-methyl-D-aspartic acid (NMDA) receptor antagonist MK-801 blocks non-opioid stress-induced analgesia. II. Comparison across three swim-stress paradigms in selectively bred mice. Brain Res. 1992;578:197–203. doi: 10.1016/0006-8993(92)90248-8. [DOI] [PubMed] [Google Scholar]
- Maude-Griffin PM, Tiffany ST. Associative morphine tolerance in the rat: examinations of compensatory responding and cross-tolerance with stress-induced analgesia. Behav Neural Biol. 1989;51:11–33. doi: 10.1016/s0163-1047(89)90621-3. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Wolfle TL, Akil H, Carder B, Liebeskind JC. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174:1351–1354. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Sternberg WF, Marek P, Sadowski B, Belknap JK, Liebeskind JC. The genetics of pain and pain inhibition. Proc Natl Acad Sci U S A. 1996;93:3048–3055. doi: 10.1073/pnas.93.7.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero L, Moreno M, Avila C, Arcaya J, Maixner W, Suarez-Roca H. Long-lasting delayed hyperalgesia after subchronic swim stress. Pharmacol Biochem Behav. 2000;67:449–458. doi: 10.1016/s0091-3057(00)00374-9. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Mogil JS, Japon M, Chan EC, Allen RG, Low MJ. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1996;93:3995–4000. doi: 10.1073/pnas.93.9.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi N, Diehl N, Yaswen L, Brennan MB, Hochgeschwender U. Generation of dynorphin knockout mice. Brain Res Mol Brain Res. 2001;86:70–75. doi: 10.1016/s0169-328x(00)00264-3. [DOI] [PubMed] [Google Scholar]
- Starec M, Rosina J, Malek J, Krsiak M. Influence of dynorphin A (1–13) and dynorphin A (1–10) amide on stress-induced analgesia. Physiol Res. 1996;45:433–438. [PubMed] [Google Scholar]
- Suarez-Roca H, Silva JA, Arcaya JL, Quintero L, Maixner W, Pinerua-Shuhaibar L. Role of mu-opioid and NMDA receptors in the development and maintenance of repeated swim stress-induced thermal hyperalgesia. Behav Brain Res. 2006;167:205–211. doi: 10.1016/j.bbr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Terman GW, Liebeskind JC. Relation of stress-induced analgesia to stimulation-produced analgesia. Ann N Y Acad Sci. 1986;467:300–308. doi: 10.1111/j.1749-6632.1986.tb14636.x. [DOI] [PubMed] [Google Scholar]
- Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebeskind JC. Intrinsic mechanisms of pain inhibition: activation by stress. Science. 1984;226:1270–1277. doi: 10.1126/science.6505691. [DOI] [PubMed] [Google Scholar]
- Vidal C, Jacob J. Hyperalgesia induced by non-noxious stress in the rat. Neurosci Lett. 1982;32:75–80. doi: 10.1016/0304-3940(82)90232-4. [DOI] [PubMed] [Google Scholar]