Abstract
Our objectives were to evaluate kinase insert domain protein receptor (KDR)-β-galactosidase (LacZ) expression as a marker for vascular development during gonadal morphogenesis and to determine whether any novel non-angiogenic KDR-LacZ expression was present in mouse testes or ovaries. Gonads were collected from mice expressing LacZ driven by the Kdr promoter (KDR-LacZ) from embryonic day 11 (E11) through postnatal day 60 (P60). At E11.5, mesonephric cells expressing KDR-LacZ seemed to migrate into the developing testis and surrounded developing seminiferous cords. Cells expressing KDR-LacZ appeared in the ovary with no apparent migration from the adjacent mesonephros, suggesting a different origin of endothelial cells. Testis organ cultures from E11 mice were treated with 8 μM VEGFR-TKI, a vascular endothelial growth factor A signal transduction inhibitor; subsequently, the amount of KDR-LacZ staining was reduced by 66%-99% (P<0.002), and the ability of KDR-expressing cells to form a densely organized vascular network was inhibited. Novel non-angiogenic KDR-LacZ staining was detected in the testis on specific subsets of germ cells at E16, E17, P4, P20, P30, and P60. In ovaries, staining was present on oocytes within oocyte cysts at E17 and within late secondary follicles of postnatal mice. Thus, KDR is an excellent marker for analyzing vascular development in the gonads. Inhibition of VEGFA signal transduction prevents the development of testis-specific vasculature. Furthermore, non-vascular KDR-LacZ staining suggests that KDR directly affects both spermatogenesis and somatic-oocyte interactions during gametogenesis.
Keywords: Vascular endothelial growth factor, Ovary, Gonadal development, Tyrosine kinase inhibitor, Mouse
Introduction
Formation of the seminiferous cords and testis-specific vasculature are the earliest events that occur to establish testis morphology. Expression of Sry by Sertoli cells triggers migration of mesonephric cells and expression of testis-specific genes in a time-dependent fashion (Martineau et al. 1997). In normal development, these mesonephric endothelial-type cells surround the Sertoli and germ cell aggregates, thus forming two hallmarks of testis morphogenesis: seminiferous cords and testis-specific vasculature (Buehr et al. 1993). The timing of cell migration is critical to the development of seminiferous cords. Mesonephric cell migration must occur around 11.5-12 days post coitus (dpc or embryonic day 11.5-12: E11.5-12) in mice in order to establish cord formation in XY gonads (Tilmann and Capel 1999). Previously, researchers believed that both pre-peritubular and endothelial cells migrated into the developing gonad to form testicular cords (Buehr et al. 1993). However, recent data suggest that the only mesonephric cells that migrate and are responsible for testis morphogenesis are those cells that are positive for endothelial cell markers (Combes et al. 2009; Cool et al. 2008).
Contrary to what was once believed, early ovarian morphology does not occur as a default pathway. The expression of genes, such as Rspo1 (Chassot et al. 2008; Parma et al. 2006; Tevosian and Manuylov 2008; Tomizuka et al. 2008) and Wnt4 (Berta et al. 1990), directs the differentiation of ovarian somatic cells. However, the development of morphological structures during ovarian morphogenesis is delayed slightly from the onset of testis differentiation. The first ovarian-specific structures to form are oocyte cysts. These cysts contain clusters of primordial germ cells connected by cytoplasmic bridges and develop in the ovarian cortex as early as E12-13.5 in some lines of mice (Loffler and Koopman 2002). Oocyte cysts break apart to allow for the formation of individual primordial follicles around postnatal day 0 (P0) to P3 during early perinatal development (Pepling and Spradling 1998, 2001). Consequently, the majority of ovarian morphological development occurs between E17 and P3.
The formation of gonadal vasculature occurs in a sex-specific pattern. Although a similar primitive vasculature is seen in XX and XY bipotential gonads, previous research has suggested that mesonephric endothelial cells migrate into the developing testis to establish the coelomic vessel and vasculature between seminiferous cords after 11.5 dpc in the mouse (Brennan et al. 2002; Buehr et al. 1993). Specifically, the available endothelial cells that result from the breakdown of the mesonephric vasculature migrate into the testis and reaggregate to form the coelomic vessel, between 11.5-12.5 dpc. During this process, these endothelial cells only migrate between the regions in which testis cords are forming. In contrast, vascular development in the ovary at this same developmental stage occurs completely independently of mesonephric contributions. Mesonephric vasculature remains intact, and mesonephric cells do not migrate into the ovary; no obvious vascular pattern has been detected in the ovary (Coveney et al. 2008).
Vascular endothelial growth factor A (VEGFA), a potent mitogen originally thought to be specific to endothelial cells (Ferrara and Henzel 1989; Keck et al. 1989; Leung et al. 1989), induces vascular leakage as a mechanism to form new vascular networks (Ferrara and Davis-Smyth 1997). Two predominant receptors bind to VEGFA: fms-related tyrosine kinase 1 (FLT1; also known as VEGFR1) and kinase insert domain protein receptor (KDR; also known as FLK1 or VEGFR2). Endothelial cells and their precursors express KDR and are important in the establishment of initial vasculature (vasculogenesis) and the development of new vasculature from existing blood vessels (angiogenesis; Bott et al. 2006; Yamaguchi et al. 1993). Binding of VEGFA to KDR promotes endothelial cell survival, differentiation, and migration (Claesson-Welsh 2003).
Previous experiments from our laboratory have yielded data demonstrating that VEGFA is expressed in Sertoli cells during morphological development of the rat testis (Bott et al. 2006). Additionally, inhibition of VEGFA signal transduction reduces vascular density by 90% in our in vitro testis organ culture system and severely inhibits the formation of seminiferous cords (Bott et al. 2006). We have attributed these actions of VEGFA to be primarily regulated through the KDR receptor, since it is the only receptor expressed during the time of seminiferous cord formation, and since the inhibition of KDR-specific signal transduction inhibits seminiferous cord formation (Bott et al. 2006).
In similar experiments on rat ovaries, VEGFA has been shown to be expressed in pre-granulosa and granulosa cells of all follicle stages and in theca cells of advanced stage follicles (McFee et al. 2009). Inhibition of VEGFA signal transduction reduces vascular density by 94% in ovarian organ cultures; however, treatment with a KDR-specific antagonist does not reduce vascular density in cultured ovaries (McFee et al. 2009). Treatment with VEGFA inhibitors also alters the progression of follicles from primordial to developing stages (McFee et al. 2009). The results from these studies have led us to the conclusion that VEGFA plays a critical role in angiogenesis of developing gonads and is required, at least indirectly, for normal morphological development of the testis and follicular progression in the developing ovary.
The first objective in the current study was to evaluate KDR-β-galactosidase (LacZ) expression as a marker for analyzing patterns of vascular development during gonadal morphogenesis. Furthermore, we sought to determine whether any novel non-angiogenic KDR-LacZ expression was present in mouse testes or ovaries.
Materials and methods
The KDR-LacZ mice used as the model in the current studies (B6.129-Kdrtm1Jrt/J) were obtained from Jackson Laboratories (Bar Harbor, Me., USA). In this strain of mice, the insertion of an in-frame LacZ gene in place of the translated region of the first Kdr coding exon and the proximal portion of the next intron results in LacZ being under the transcriptional control of the endogenous Kdr promoter. Cells expressing KDR protein in these mice produce LacZ and stain blue for β-galactosidase. Homozygotes for the Kdrtm1Jrt-targeted mutation fail to form organized blood vessels and die around E8.5-9.5 (Shalaby et al. 1995). Mice were time-bred, and mid-day on the plug date was considered to be E0.5 (0.5 dpc). Tail somites were counted to confirm the ages of early embryos (E10.5≈8 somites, E11.5≈18 somites, E12.5≈30 somites) according to reported methods (Hacker et al. 1995; Karl and Capel 1998). Gonads were collected at various developmental time points, and conventional polymerase chain reaction for Sry was utilized to confirm the sex of early embryonic gonads as previously described (Cupp et al. 2000). All animal procedures were approved by the University of Nebraska Animal Care and Use Committee.
Staining, whole-mount, and histological preparation of gonads
Following dissection, organs were fixed in 4% paraformaldehyde for 1-2 h, depending on organ size. Tissues were then stained for β-galactosidase according to the manufacturer’s protocol (Specialty Media, Phillipsburg, N.J., USA) by using 1 mg/ml of x-gal. The x-gal was diluted in staining solution (Specialty Media), and gonads were incubated overnight at 37°C. The following morning, organs were post-fixed in neutral buffered formalin for 1 h. Tissues were dehydrated and stored in 70% ethanol. For imaging, organs were either hydrated and mounted whole in Gel/Mount (Biomedia, Foster City, Calif., USA) or were paraffin-embedded for histology or immunohistochemistry (see below) according to standard procedures (Itoh et al. 1998). Paraffin-embedded gonads were sectioned (5 μm), deparaffinized, and rehydrated. Tissue sections were then stained with a 25% eosin solution (diluted in 70% ethanol with 0.5% glacial acetic acid), dehydrated, and mounted in Permount (Fisher Scientific, Fairlawn, N.J., USA). All tissues were viewed on an Olympus BX51 brightfield microscope (Olympus America, Center Valley, Pa., USA) and imaged with Windows Spot Advanced imaging software (Diagnostic Instruments, Sterling Heights, Mich., USA).
Mouse organ cultures
Indifferent testis and mesonephros pairs were dissected from E11 embryos (14-18 tail somites). Gonads were cultured in droplets of medium on 0.4-μm Millicell-CM filters (Millipore, Bedford, Mass., USA) floating on the surface of 0.4 ml medium in 4-well plates. The medium consisted of high-glucose Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, Calif., USA) supplemented with ampicillin (5 μg/mL) and 10% fetal bovine serum (VWR International, West Chester, Pa., USA). Organs were cultured for 3 days at 37°C in a humidified atmosphere with 5% CO2. One testis and mesonephros pair from each animal was designated as a control, while the other pair was subjected to treatment with a VEGFA receptor signal transduction inhibitor (VEGFR-TKI; Calbiochem, La Jolla, Calif., USA). We utilized a dose of 8 μM VEGFR-TKI in our organ culture system, because we had demonstrated that this dose affected testis morphogenesis and vascular development in rat testis organ cultures but did not affect germ cell mitosis (Bott et al. 2006). VEGFR-TKI diluted in dimethyl sulfoxide (DMSO) was added directly to the culture medium of the treated wells daily, while a similar dose of DMSO was added to the control wells. The medium was changed after every 2 days of culture.
Organs were mounted whole and imaged (as described above) to evaluate patterns of KDR-LacZ expression after 1, 2, or 3 days of culture. A minimum of three organ pairs were imaged after each day of culture. In order to compare the amount of KDR-LacZ-positive staining between control and treated testes, the β-galactosidase staining index (SI) was quantified with the NIH Scion image program (Scion Image, Frederick, Md., USA). Densitometry was performed on each testis at 100× magnification. Within each field, the SI was defined as the number of pixels exceeding an arbitrary grayscale value. The SI for each control testis was set to 100%, and the SI of each treated testis was calculated as a percentage of its paired control. The β-galactosidase SI was determined for 3-5 testis pairs after each day of culture.
Immunohistochemical staining of gonads
To characterize KDR-LacZ staining further, immunohistochemistry was performed for β-galactosidase, platelet endothelial cell adhesion molecule 1 (PECAM1), and cadherin 5 (CDH5, also known as vascular endothelial cadherin). Sectioned (5 μm), deparaffinized, and rehydrated tissue sections were microwaved in 0.01 M sodium citrate to boiling point for 5 min. After this antigen-retrieval step, sections were cooled for 1-2 h and then incubated in blocking buffer (10% goat serum in phosphate-buffered saline [PBS]) for 30 min at room temperature. Tissue sections were incubated overnight at 4°C with primary antibody (1:200 dilution in 10% goat serum for β-galactosidase, 1:500 dilution for PECAM1 and CDH5) and then washed with washing buffer (PBS). Sections were then incubated for 30 min at room temperature with the secondary antibody (1:300 dilution in 10% goat serum) and washed with washing buffer. The primary antibodies for β-galactosidase (catalog number: ab616; Abcam, Cambridge, Mass., USA), PECAM1 (catalog number: ab28364; Abcam), and CDH5 (catalog number: ab33168; Abcam) were all rabbit polyclonal antibodies. The secondary antibody was a fluorescein-isothiocyanate-conjugated goat anti-rabbit IgG (H+L; catalog number: 816111; Zymed, South San Francisco, Calif., USA). As a negative control, serial sections were processed without primary antibody. Images were collected with an Olympus BX51 microscope (Olympus America) and Windows Spot Advanced imaging software (Diagnostic Instruments).
Statistical analysis
Data were analyzed by one-way analysis of variance with JMP software (SAS Institute, Cary, N.C., USA). A Student t-test and a Dunnett test were used to compare the β-galactosidase SI between control and treated organs. Differences in data were considered to be statistically significant at a P value of <0.05, unless otherwise stated.
Results
Whole-mount analysis of KDR-LacZ expression during early embryonic development
Distinct patterns of KDR-LacZ expression were easily identified in whole-mount organs (Fig. 1). The bipotential gonad did not appear to have any cells expressing KDR-LacZ at E10, but networks of vasculature stained for KDR-LacZ within the mesonephros (data not shown). By E11, the vasculature within the mesonephros had prominent KDR-LacZ staining (Fig. 1a, b), but only a few cells in the testis (Fig. 1a) and ovary (Fig. 1b) stained as intensely. At E12, no differences were apparent in KDR-LacZ staining or organ size between the testis (Fig. 1c) and ovary (Fig. 1d). However, by E12.5, the testis (Fig. 1e) had surpassed the ovary (Fig. 1f) in size, and the vascular pattern appeared different in the testis, with vasculature surrounding the seminiferous cords and with the formation of a coelomic vessel (Fig. 1e). By E13 in the male, the mesonephros had maintained its levels of KDR-LacZ cell staining, the testis was larger and more rounded in shape, a more defined vasculature was present around the seminiferous cords, and a distinct coelomic vessel could be seen (Fig. 1g). In contrast, the vasculature within the ovary and mesonephros of the E13 female was much more densely organized than that in the male (Fig. 1h).
Fig. 1.
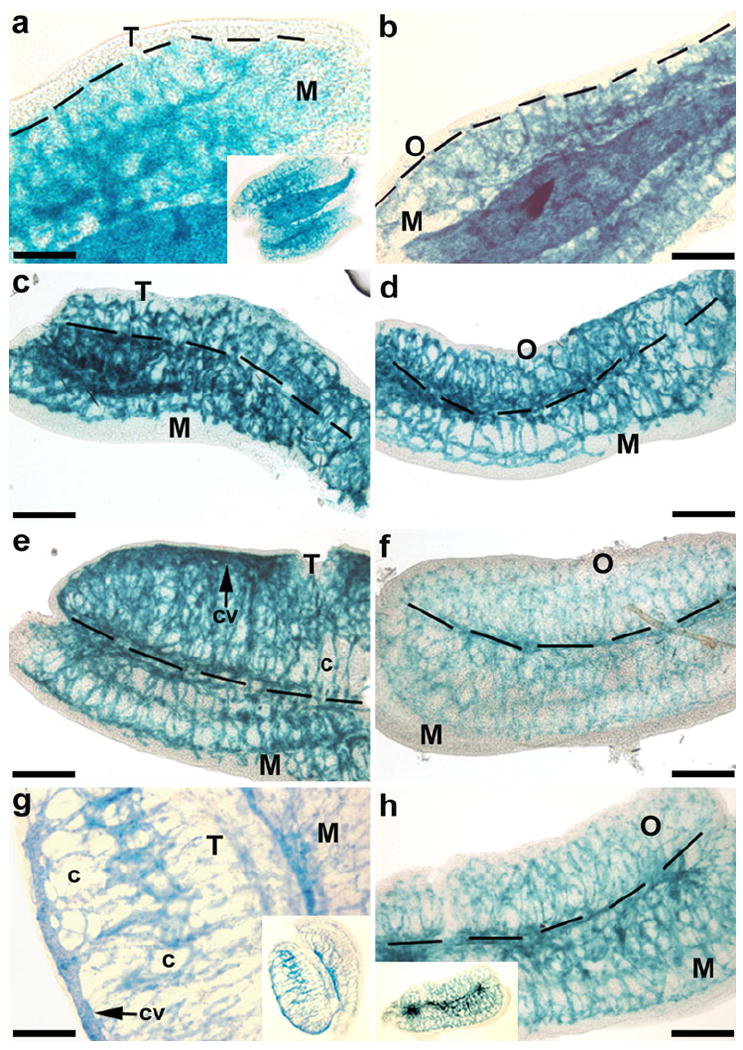
Whole-mount images of gonads from embryonic day 11-13 (E11-13) kinase insert domain protein receptor (KDR)-LacZ mice. In the genital ridges from E11 male (a) and female (b) mice, KDR-LacZ staining was located predominately within the vasculature of the mesonephros (M). Staining patterns and organ sizes were similar between testes (T) and ovaries (O) of E12 mice (c, d). By E12.5, the testis (e) had surpassed the ovary (f) in size, and the vascular pattern began to become distinct in the testis with the formation of a coelomic vessel (cv) and vasculature surrounding the seminiferous cords (c). At E13, the testis (g) was larger and more rounded in shape with more defined vasculature around the seminiferous cords, while the vasculature within the ovary and mesonephros of the female (h) was much more densely organized than in the male. Insets Low-power views. Bars 200 μm
Expression of KDR-LacZ during early embryonic development in histological sections
The presence of KDR-LacZ was detected in the mesonephros and gonads of E11.5-13 mice in histological sections of x-gal-stained tissue (Fig. 2). In the E11.5 male, an abundance of KDR-LacZ-expressing cells was present throughout the mesonephros, and a small subset of cells expressed KDR-LacZ within the testis (Fig. 2a,c). By E12.5, the coelomic vessel had developed, and KDR-LacZ staining surrounded the newly formed seminiferous cords (Fig. 2e, g). At E13.5, KDR-LacZ staining continued to identify the coelomic vessel and the interstitium around the cords (Fig. 2i).
Fig. 2.
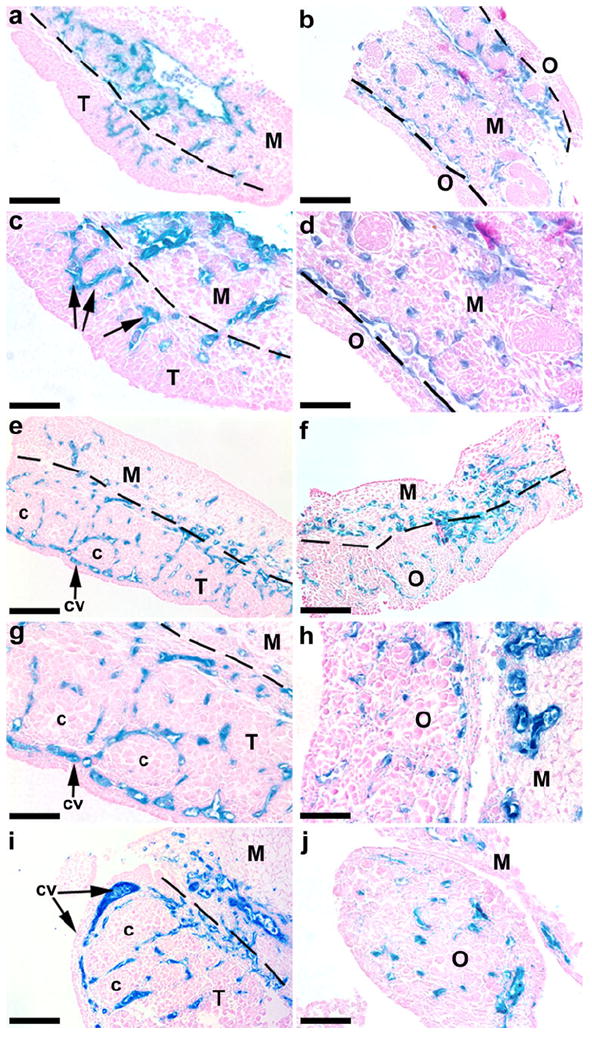
Expression of KDR-LacZ in the mesonephroi and gonads from E11.5-13.5 mice. In E11.5 testes (a, c), small populations of KDR-expressing cells (arrows) appeared in the testis (T), in close proximity to the mesonephros (M). By E12.5 (e, g) and E13.5 (i, cross section), the coelomic vessel (cv) had developed in the testis, and KDR-expressing cells surrounded the seminiferous cords (c). In E11.5 females (b, d), KDR-LacZ staining was located predominately within the vasculature of the mesonephros. Vascular development was evident at E12.5 (f) in the ovary (O). At E13.5 (h, j, cross sections), KDR-LacZ staining was still present throughout the ovary. Bars 100 μm (a-b, e-f), 50 μm (c-d, g-j)
KDR-LacZ expression was detected in the mesonephroi of E11.5 female mice (Fig. 2b, d), and vascular development was present within the ovary by E12.5 (Fig. 2f). Ovarian KDR-LacZ expression was maintained in the E13.5 ovary (Fig. 2h, j).
Effects of VEGFA signal transduction inhibitor, VEGFR-TKI, on mouse testis cultures
In prior experiments in our laboratory, VEGFR-TKI treatment of testis organ cultures reduced vascular density by 90%, while also severely perturbing the formation of seminiferous cords (Bott et al. 2006). To determine whether mesonephric cells positive for KDR-LacZ were affected by VEGFA signal transduction inhibition, E11 KDR-LacZ mouse testis organ cultures were treated with VEGFR-TKI, a VEGFA receptor tyrosine kinase antagonist, which inhibits both KDR and FLT1. Organs that were imaged on day zero (DO), prior to culture or treatment, exhibited KDR-LacZ expression in cells localized predominantly within the mesonephros (Fig. 3a, b). A small number of these cells were also detected within the testis in close proximity to the mesonephros (Fig. 3a, b).
Fig. 3.
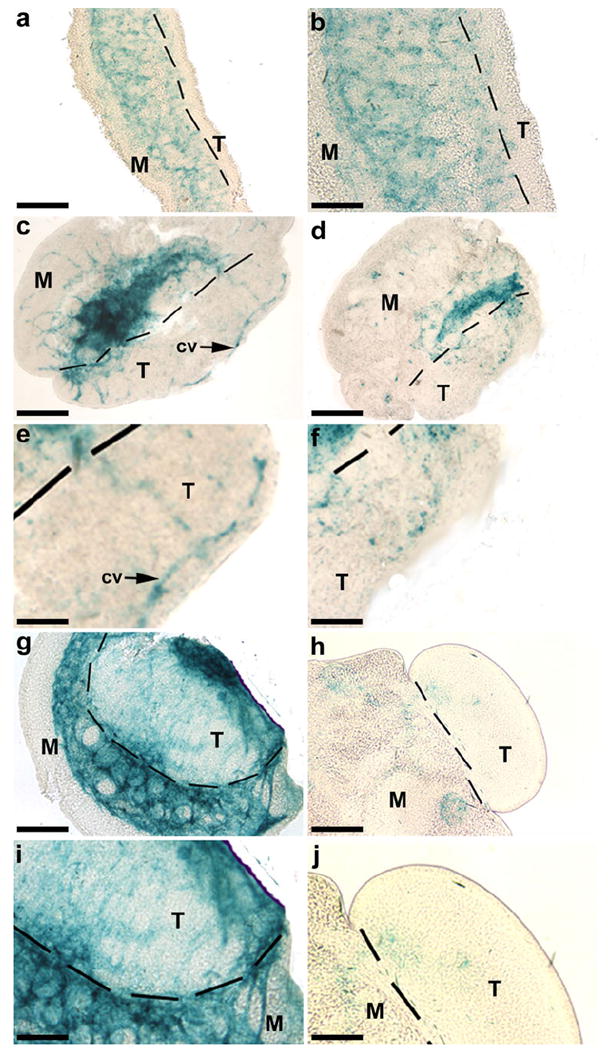
Effects on KDR-LacZ expression in E11 mouse organ cultures after treatment with a vascular endothelial growth factor A (VEGFA) receptor tyrosine kinase antagonist (VEGFR-TKI). Immediately before culture (a, b), KDR-LacZ staining was located predominantly in the mesonephros (M) with only a few KDR-expressing cells in the testis (T). After 1 day in culture (D1), endothelial cells had established vascular patterns, including a distinct coelomic vessel (cv), within the control testis (c, e). Treated testes contained KDR-expressing cells on D1, but in fewer numbers and without distinct patterns (d, f). By D3 of culture, KDR-LacZ was still present in control testes (g, i), whereas treated testes (h, j) had little apparent staining. Bars 200 μm (a, c-d), 100 μm (b, e-f, i-j), 50 μm (g-h)
Cells positive for KDR-LacZ were detected throughout the mesonephros and testis in both treated (Fig. 3d, f, h, j) and control (Fig. 3c, e, g, i) organs at D1-3 of culture. On D1, KDR-LacZ expression was located within the developing vasculature of control testes, most notably in the developing coelomic vessel (Fig. 3c, e). Testes that were treated with VEGFR-TKI had reduced numbers of β-galactosidase-positive cells and less distinct vascular patterns compared with their paired control organs (Fig. 3d, f). By D2, the number of stained cells had increased in control organs but decreased in treated organs (data not shown). After the final day of culture (D3), a drastic decline occurred in KDR-expressing cells in the testis and mesonephros of treated organs (Fig. 3h, j) compared with control organs (Fig. 3g, i).
Since treatment with VEGFR-TKI appeared to alter the amount of KDR-LacZ-positive cells within the developing testis, densitometry was utilized to quantify this difference. After 1 day of culture, β-galactosidase staining was reduced by 66% in treated testes (P<0.002; Fig. 4). After subsequent days of culture, KDR-LacZ staining was even more drastically reduced. Treated testes had 90% and 99% less staining than control testes after days 2 and 3 of culture, respectively (P<0.0002; Fig. 4).
Fig. 4.
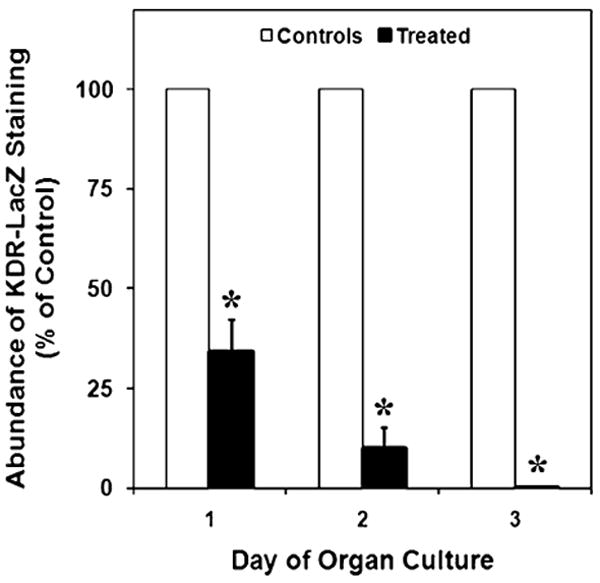
Effects of VEGFR-TKI treatment on KDR-LacZ staining in cultured E11 testes, expressed as a percentage of control organs. After days 1, 2, and 3 of culture, β-galactosidase staining was reduced by 66%, 90%, and 99%, respectively, in treated testes. The mean areas of staining (±SEM) are presented. *Statistically significant difference compared with the means of controls at P<0.002
Whole-mount analysis of KDR-LacZ expression in late embryonic and perinatal development
Extensive KDR-LacZ staining was evident in late embryonic and perinatal testes and ovaries (Fig. 5). A distinct vascular pattern was present in E16 testes, with a prominent coelomic vessel and defined vasculature surrounding the seminiferous cords (Fig. 5a). Similar vascular patterns were also evident in P0 (Fig. 5c) and P3 (Fig. 5e) testes. Vasculature with intense KDR-LacZ staining was evident throughout ovaries from E16 (Fig. 5b), P0 (Fig. 5d), and P3 (Fig. 5f) mice. Cells expressing KDR were also present within the developing vasculature of the epididymis (Fig. 5c) and oviduct (Fig. 5d).
Fig. 5.
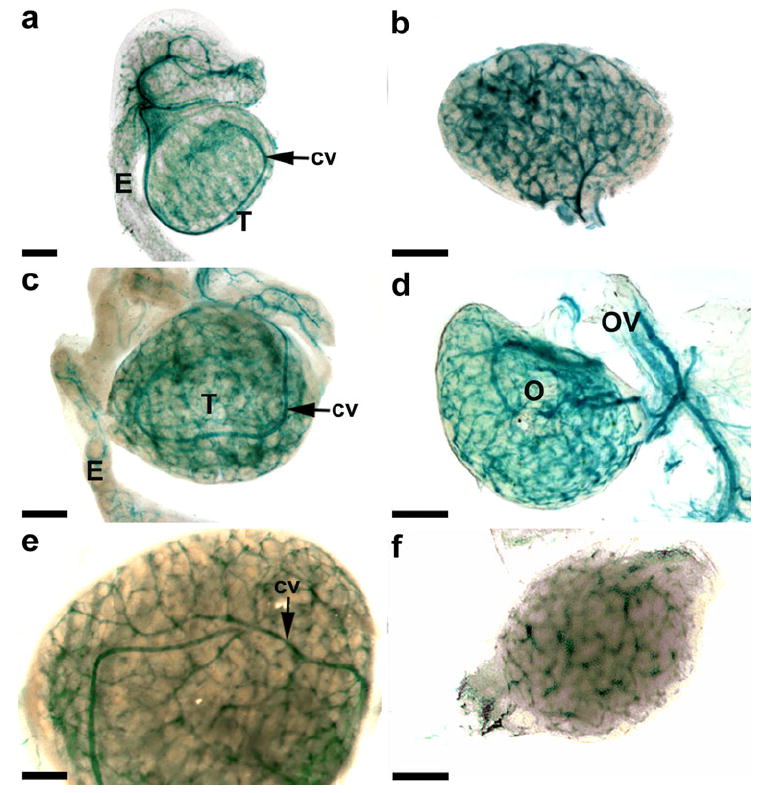
Whole-mount images of gonads from late embryonic and early postnatal KDR-LacZ mice. Testes (T) at E16 (a) had a defined vascular pattern, with a prominent coelomic vessel (cv) and vasculature surrounding the seminiferous cords. Similar vascular patterns were also evident in postnatal day 0 (P0; c) and P3 (e) testes. Extensive vasculature with KDR-LacZ staining was evident throughout ovaries (O) from E16 (b), P0 (d), and P3 (f) mice. Cells expressing KDR (c, d) were also present within the developing vasculature of the epididymis (E) and oviduct (OV). Bars 200 μm
Expression of KDR-LacZ in late embryonic and postnatal testes in histological sections
As the testis continued to develop through E17, the amount of stained cells within the mesonephros had begun to decline (data not shown). Cells expressing KDR were also located throughout the interstitium of the E17 testis, with intense expression around the seminiferous cords (Fig. 6a). Early in perinatal development, KDR-LacZ expression patterns were well established throughout the interstitium of the P0 (Fig. 6b) and P3 (data not shown) testis.
Fig. 6.
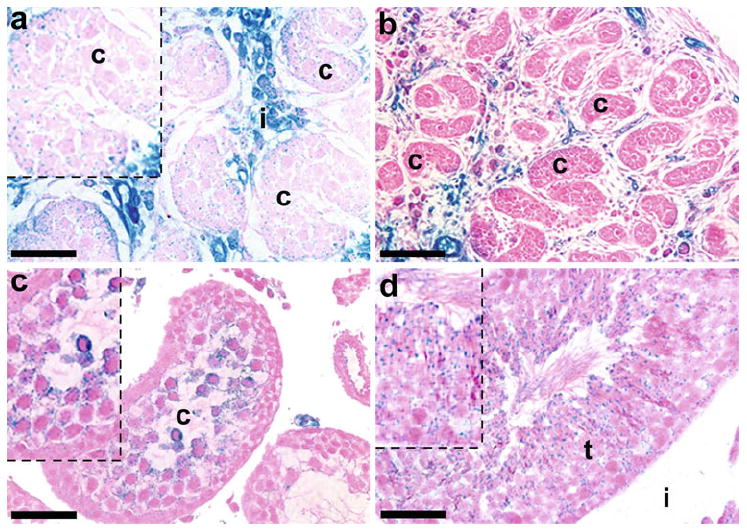
Expression of KDR-LacZ in late embryonic and postnatal mouse testes (T). In E17 mice (a), intense KDR-LacZ staining was still present in the interstitium (i) surrounding the seminiferous cords, but the amount of staining within the mesonephros (M) had diminished. Staining was also localized to Sertoli cells and early stage germ cells within the seminiferous cords (c). In P0 testes (b), KDR expression continued to be strongly present within interstitial cells. At P20 (c), KDR-LacZ expression was present in round spermatids, and KDR-LacZ was located in round spermatids, elongating spermatids, and Sertoli cell cytoplasm within the seminiferous tubules (t) in P60 testes (d). Insets Higher-power views. Bars 100 μm (b), 50 μm (a, c-d)
Expression of KDR was detected for the first time in Sertoli cells and early stage germ cells within the seminiferous cords in approximately 50% of E16 males and 70% of E17 males (Fig. 6a). Expression within cords was not detected again until P4 when small numbers of Sertoli cells and gonocytes expressed KDR (data not shown). Staining for KDR-LacZ was present in round spermatids at P20 (Fig. 6c) and P30 (data not shown). By P60, KDR-LacZ was detected in round spermatids, elongating, or elongated spermatids, and Sertoli cell cytoplasm (Fig. 6d).
Expression of KDR-LacZ in late embryonic and postnatal ovaries in histological sections
By E17, the developing ovary had developed a more rounded form, and numerous KDR-LacZ expressing cells were present in the highly vascularized medulla and between oocyte cysts in the cortex (Fig. 7a, b). However, unlike the male, mesonephric expression of KDR-LacZ was maintained and strongly detected in mesonephric vessels (Fig. 7a). Furthermore, KDR-expressing cells were present near oocyte cysts in the ovarian cortex of E14-E18 mice (Fig. 7a, b). Light KDR-LacZ staining was also present on oocytes within oocyte cysts of E17 ovaries (Fig. 7b).
Fig. 7.
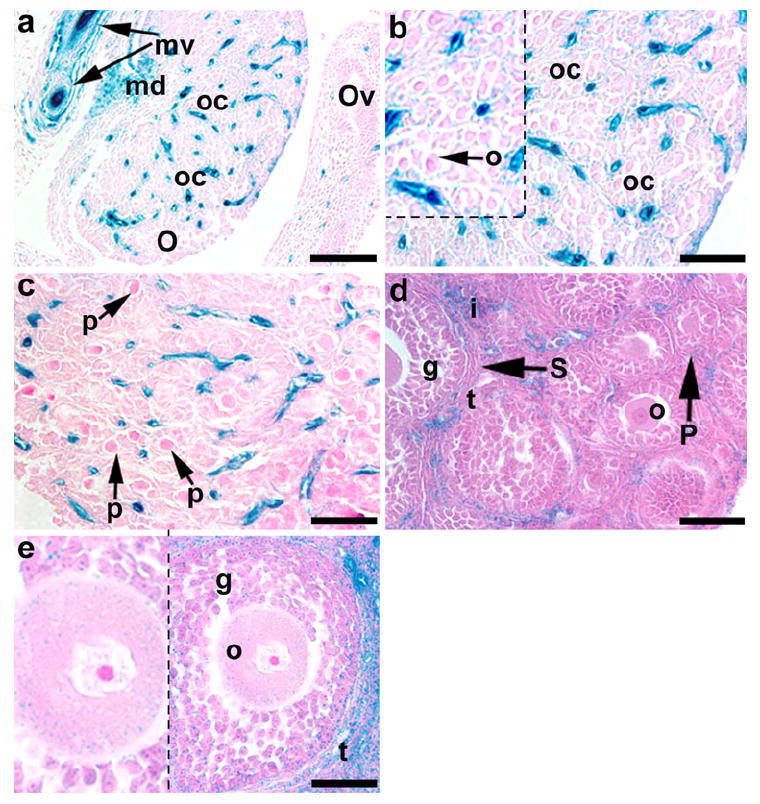
Expression of KDR-LacZ in late embryonic and postnatal mouse ovaries (O). Intense KDR-LacZ staining was evident in the mesonephric vessels (mv) with some staining being located in the oviducts (OV) of E17 mice (a). In E17 ovaries (a, b), prominent KDR-LacZ staining was present in the medulla (md) and between oocyte cysts (oc) in the cortex; faint staining was also evident on oocytes (o) within the oocyte cysts. In P0 mice (c), primordial follicles (p) had developed between KDR-expressing cells. Several primary (P) and secondary (S) follicles were present in P15 (d) and P30 (e) ovaries, and KDR-LacZ staining was most abundant in theca (t) and interstitial (i) cells surrounding these follicles; note the light staining in granulosa cells (g) and the cytoplasm of oocytes (o) in late secondary follicles. Bars 100 μm (a), 50 μm (b-e)
In the perinatal period, rapid morphological changes occurred in the ovary. Primordial and primary follicles began to develop in P0 (Fig. 7c) and P3 (data not shown) ovaries, respectively, and were surrounded by KDR-LacZ-expressing cells. Several primary and secondary follicles were present in P15 (Fig. 7d) and P30 (Fig. 7e) ovaries, and the most abundant KDR expression was located in the interstitium surrounding these follicles. Prominent KDR-LacZ staining was also evident in theca cells associated with secondary follicles (Fig. 7d, e). Light staining was evident in granulosa cells associated with primary and secondary follicles and the cytoplasm of oocytes in late secondary follicles (Fig. 7e).
Immunohistochemical staining for β-galactosidase
Immunohistochemistry was performed to localize further the expression of β-galactosidase and thus, the expression of KDR (Fig. 8). In E17 testes (Fig. 8a), β-galactosidase-positive staining was detected around germ cells within the seminiferous cords, and intense staining was also localized to interstitial cells (Leydig cells). Expression of β-galactosidase was detected in Leydig cells and in round spermatids at P20 (Fig. 8b). In P60 testes (Fig. 8c, d), positive staining was also seen in elongating and elongated spermatids. For ovaries, positive β-galactosidase staining was localized around oocytes within oocyte cysts at E17 (Fig. 8e). No staining was identified in tissue sections that were processed without primary antibody (Fig. 8f).
Fig. 8.
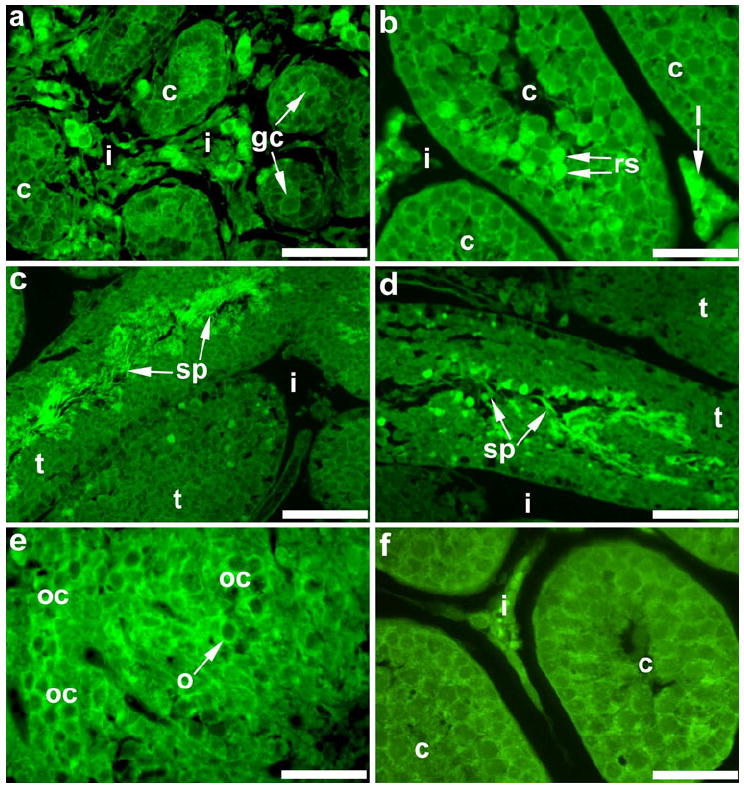
Immunohistochemical staining for β-galactosidase in embryonic and postnatal mouse gonads. In E17 testes (a), staining was detectable around germ cells (gc) within the seminiferous cords (c), and intense staining was present in the interstitium (i). In the postnatal testis, β-galactosidase was localized to Leydig cells (l) in the interstitium and round spermatids (rs) within cords at P20 (b) and in sperm (sp) within seminiferous tubules (t) at P60 (c, d). Immunohistochemistry for β-galactosidase in the E17 ovary (e) revealed positive staining around oocytes within oocyte cysts (oc). Gonadal sections with no primary antibody served as negative controls; a P20 testis section is shown (f). Bars 100 μm (c), 50 μm (a, b, d-f)
Immunohistochemical staining for PECAM1 and CDH5
Immunohistochemistry was performed for the endothelial cell markers, PECAM1 (constitutively expressed on the surface of endothelial cells) and CDH5 (found at intercellular junctions of endothelial cells), to compare staining for endothelial cells with that for KDR-LacZ (Fig. 9). In E17 testis sections stained for KDR-LacZ (Fig. 9a, d), staining was identified around germ cells within the seminiferous cords and in Leydig cells within the interstitium. Immunohistochemical staining for PECAM1 (Fig. 9b) and CDH5 (Fig. 9e) was only present on interstitial cells. Superimposition of the images for the different stains (Fig. 9c, f) revealed that only KDR-LacZ staining was localized within the seminiferous cords. No staining was detected in immunohistochemical sections processed without primary antibody (Fig. 9g).
Fig. 9.
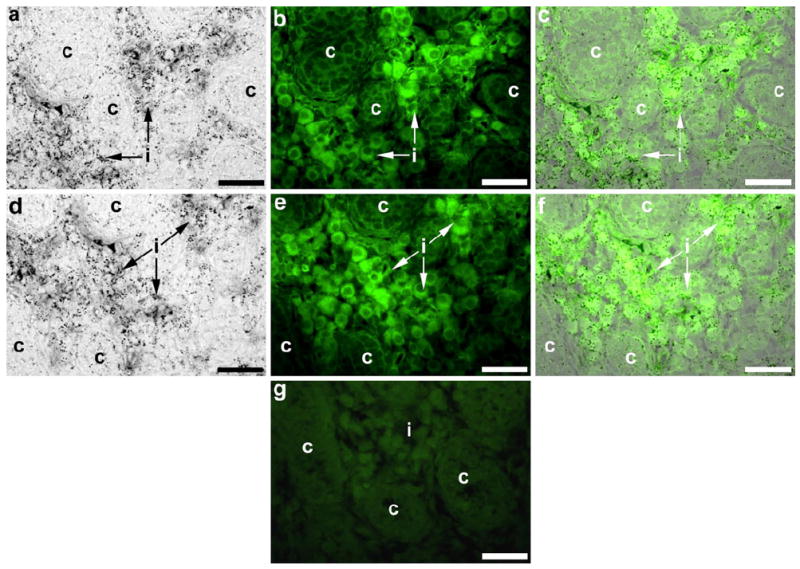
Immunhistochemical staining for the endothelial cell markers, PECAM1 (b, c) and CDH5 (e, f), in embryonic testes to reveal possible co-localization (c, f) with staining for KDR-LacZ (a, c, d, f). In E17 testes, KDR-LacZ staining (a, d) was detected within the seminiferous cords (c) and the interstitium (i), whereas PECAM1 (b) and CDH5 (e) staining was present in the interstitium. Superimposition of the different stains (c, f) confirmed that only KDR-LacZ staining was localized within the seminiferous cords. Sections processed without primary antibody served as immunohistochemistry negative controls; a E16 testis section is shown (g). Bars 40 μm
Discussion
The current study provides evidence that KDR is an excellent marker to study the sex-specific development of the vasculature within the testis and the ovary during gonadal morphogenesis. We have used the KDR-LacZ mice in these experiments because we can accurately identify KDR protein expression.
During development, VEGFA is first detected in the mouse in the primitive endoderm of the blastocyst at E4 and in most developing organs by E14 (Miquerol et al. 1999). Inhibition of VEGFA during embryogenesis results in impaired body growth and increased mortality (Carmeliet et al. 1996; Gerber et al. 1999). Furthermore, the loss of even a single Vegfa allele results in embryonic death by E9 (Carmeliet et al. 1996; Ferrara et al. 1996). Overexpression of Vegfa in the testis and epididymis causes infertility in adult male mice because of spermatogenic arrest and epithelial hyperplasia in the reproductive tract (Korpelainen et al. 1998). These data indicate that VEGFA signal transduction is important in the gonad at various developmental time points and must be carefully regulated.
Previous experiments have demonstrated a potential mechanism for VEGFA in arterial-specific differentiation of endothelial cells (Lawson et al. 2002). At E11.5, prior to structural development, markers for both arterial and venous vasculature can be found in the testis and ovary. However, during seminiferous cord formation, a shift occurs toward predominantly arterial development within the testis (Brennan et al. 2002). Currently, the way that the sex-specific patterns of vasculature develop is unknown, but arterial development has been suggested to follow the innervation of organs in other systems (Weinstein 2005). More specifically, VEGFA is involved in vascular patterning around nerves (Rosenkilde and Schwartz 2004; Schwarz et al. 2004). Previous studies have confirmed the importance of the nervous system in seminiferous cord formation by identifying the roles of neurotropins and their receptors during testis morphogenesis (Cupp et al. 2000, 2003; Levine et al. 2000). In the current study, we have demonstrated that KDR is present in the sex-specific patterns of vascular development, which arise during gonadal morphogenesis. We hypothesize that VEGFA, working through KDR, promotes this shift of endothelial cells toward arterial differentiation within the testis and elicits differences in vascular organization by following nerve patterns within the testis.
During seminiferous cord formation, VEGFA has been detected in Sertoli cells (Gerber et al. 1999). The presumable function of VEGFA at this time is to induce endothelial cell migration in order to establish a vascular network around the forming seminiferous cords. In the current study, KDR-LacZ testis organ cultures that were treated with a VEGFA signal transduction inhibitor, VEGFR-TKI, had reduced numbers of KDR-LacZ stained cell in developing testes; this indicates decreased migration of KDR-expressing endothelial cells from the mesonephros to the testis. Over the duration of the organ cultures, overall KDR-LacZ cell numbers were reduced, which may have been attributable to decreased endothelial cell proliferation and survival. Consequently, inhibition of VEGFA signal transduction appears to impair the migration of endothelial cells and to reduce their viability in culture.
Traditionally, KDR has been found to be localized to endothelial cells and progenitor endothelial cells (Shalaby et al. 1995), whereas VEGFA and its receptors have been well characterized in vascular development (Ferrara and Davis-Smyth 1997). Based on the presence of KDR-LacZ staining in non-vascular cells, we propose an additional non-angiogenic role for VEGFA in the ovary and testis. Prior experiments in our laboratory had localized KDR in ovaries to oocytes and granulosa cells (McFee et al. 2009). In our present KDR-LacZ experiments, KDR expression was seen in oocytes within oocytes cysts and within secondary follicles. Granulosa cells also displayed KDR-LacZ staining in primary and secondary follicles. Treatment of ovarian organ cultures with a KDR signal transduction inhibitor alters follicle development in perinatal rat ovaries (McFee et al. 2009). Taken together, these results indicate that VEGFA and KDR are involved in interactions between oocytes, follicular cells, and/or vascular cells, potentially promoting oocyte cyst breakdown and follicular development.
In the current and previous studies (Nalbandian et al. 2003), KDR was detected within the seminiferous cords and tubules in subsets of germ cells. Specifically, in the current study, KDR-LacZ was expressed in germ cells at E16, E17, P4, and after P20. The occurrence of the initial KDR expression in the seminiferous cords is concomitant with a period of increased steroidogenesis. Around E16-17 in the mouse, the expression of steroidogenic enzymes and production of steroid hormones occurs (El-Gehani et al. 1998). VEGFA and its receptor KDR may be involved in these events to ensure appropriate germ cell maturation and survival.
KDR expression has been shown to recur in type A spermatagonia in the P6 mouse testis, whereas the other principal VEGFA receptor, FLT1, is expressed in later stage germ cells, including spermatids and spermatozoa (Nalbandian et al. 2003). Consequently, VEGFA may have a function in the development of mitotic stage germ cells during early maturation through KDR and in later germ cell stages via FLT1. Expression of FLT1 has been demonstrated in mid-pachytene spermatocytes and in round spermatids of adult wild-type mice (Korpelainen et al. 1998). When VEGFA is overexpressed in mice, FLT1 is detectable in additional germ cells stages, and both FLT1 and KDR are upregulated in Leydig cells and interstitial vasculature (Korpelainen et al. 1998). These data suggest that VEGFA potentiates the expression of FLT1 and KDR on specific germ and testicular cells, and that FLT1 and KDR, in response to VEGFA, are involved in spermatogenesis and steroidogenesis. We suggest that VEGFA signaling through these receptors mediates sperm cell cycle progression in response to various stimuli, such as increased steroidogenesis at the time of initial KDR expression in germ cells.
In the current study, the greatest increase in KDR-LacZ expressing cells occurs at time points associated with rapid morphological development in both the testis and ovary, indicating that vascular development and gonadal morphogenesis are closely related. In summary, KDR-LacZ expression patterns illustrate that (1) KDR is an excellent marker for vascular development in both the testis and the ovary; (2) VEGFA and its receptor, KDR, are involved in vascular development in both the testis and ovary; (3) VEGFA and KDR may be involved in somatic-oocyte interactions during ovarian and follicular development; and (4) VEGFA actions have a potential role through KDR in embryonic and postnatal testicular germ cell maturation and/or survival.
Acknowledgments
The authors are grateful to Vanessa Brauer, Robin Artac, Michelle Baltes-Breitwisch, Stephanie Glanz, Kylie Benton, Robyn Longfellow Smith, and Natalie Hart for their contributions to this manuscript and to Dr. Derek McLean for his help in identifying KDR expression on specific cell types within seminiferous cords.
This research was supported by grants HD41546, HD045250, and HD051979 to Andrea S. Cupp from NIH/NICHD.
References
- Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, Fellous M. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990;348:448–450. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- Bott RC, McFee RM, Clopton DT, Toombs C, Cupp AS. Vascular endothelial growth factor and kinase domain region receptor are involved in both seminiferous cord formation and vascular development during testis morphogenesis in the rat. Biol Reprod. 2006;75:56–67. doi: 10.1095/biolreprod.105.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J, Karl J, Capel B. Divergent vascular mechanisms downstream of Sry establish the arterial system in the XY gonad. Dev Biol. 2002;244:418–428. doi: 10.1006/dbio.2002.0578. [DOI] [PubMed] [Google Scholar]
- Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development. 1993;117:273–281. doi: 10.1242/dev.117.1.273. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Chassot AA, Gregoire EP, Magliano M, Lavery R, Chaboissier MC. Genetics of ovarian differentiation: Rspo1, a major player. Sex Dev. 2008;1:219–227. doi: 10.1159/000152038. [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem Soc Trans. 2003;31:20–24. doi: 10.1042/bst0310020. [DOI] [PubMed] [Google Scholar]
- Combes AN, Wilhelm D, Davidson T, Dejana E, Harley V, Sinclair A, Koopman P. Endothelial cell migration directs testis cord formation. Dev Biol. 2009;326:112–120. doi: 10.1016/j.ydbio.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Cool J, Carmona FD, Szucsik JC, Capel B. Peritubular myoid cells are not the migrating population required for testis cord formation in the XY gonad. Sex Dev. 2008;2:128–133. doi: 10.1159/000143430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coveney D, Cool J, Oliver T, Capel B. Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc Natl Acad Sci USA. 2008;105:7212–7217. doi: 10.1073/pnas.0707674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupp AS, Kim GH, Skinner MK. Expression and action of neurotropin-3 and nerve growth factor in embryonic and early postnatal rat testis development. Biol Reprod. 2000;63:1617–1628. doi: 10.1095/biolreprod63.6.1617. [DOI] [PubMed] [Google Scholar]
- Cupp AS, Uzumcu M, Skinner MK. Chemotactic role of neurotropin 3 in the embryonic testis that facilitates male sex determination. Biol Reprod. 2003;68:2033–2037. doi: 10.1095/biolreprod.102.012617. [DOI] [PubMed] [Google Scholar]
- El-Gehani F, Zhang FP, Pakarinen P, Rannikko A, Huhtaniemi I. Gonadotropin-independent regulation of steroidogenesis in the fetal rat testis. Biol Reprod. 1998;58:116–123. doi: 10.1095/biolreprod58.1.116. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Hilan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- Itoh N, Patel U, Cupp AS, Skinner MK. Developmental and hormonal regulation of transforming growth factor-beta1 (TGFbeta1), -2, and -3 gene expression in isolated prostatic epithelial and stromal cells: epidermal growth factor and TGFbeta interactions. Endocrinology. 1998;139:1378–1388. doi: 10.1210/endo.139.3.5787. [DOI] [PubMed] [Google Scholar]
- Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Korpelainen EI, Karkkainen MJ, Tenhunen A, Lakso M, Rauvala H, Vierula M, Parvinen M, Alitalo K. Overexpression of VEGF in testis and epididymis causes infertility in transgenic mice: evidence for nonendothelial targets for VEGF. J Cell Biol. 1998;143:1705–1712. doi: 10.1083/jcb.143.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Levine E, Cupp AS, Skinner MK. Role of neurotropins in rat embryonic testis morphogenesis (cord formation) Biol Reprod. 2000;62:132–142. doi: 10.1095/biolreprod62.1.132. [DOI] [PubMed] [Google Scholar]
- Loffler KA, Koopman P. Charting the course of ovarian development in vertebrates. Int J Dev Biol. 2002;46:503–510. [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Curr Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- McFee RM, Artac RA, McFee RM, Clopton DT, Smith RA, Rozell TG, Cupp AS. Inhibition of vascular endothelial growth factor receptor signal transduction blocks follicle progression but does not necessarily disrupt vascular development in perinatal rat ovaries. Biol Reprod. 2009;81:966–977. doi: 10.1095/biolreprod.109.078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol. 1999;212:307–322. doi: 10.1006/dbio.1999.9355. [DOI] [PubMed] [Google Scholar]
- Nalbandian A, Dettin L, Dym M, Ravindranath N. Expression of vascular endothelial growth factor receptors during male germ cell differentiation in the mouse. Biol Reprod. 2003;69:985–994. doi: 10.1095/biolreprod.102.013581. [DOI] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–1309. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125:3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Schwartz TW. The chemokine system—a major regulator of angiogenesis in health and disease. APMIS. 2004;112:481–495. doi: 10.1111/j.1600-0463.2004.apm11207-0808.x. [DOI] [PubMed] [Google Scholar]
- Schwarz MA, Wan Z, Liu J, Lee MK. Epithelial-mesenchymal interactions are linked to neovascularization. Am J Respir Cell Mol Biol. 2004;30:784–792. doi: 10.1165/rcmb.2003-0145OC. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Manuylov NL. To beta or not to beta: canonical beta-catenin signaling pathway and ovarian development. Dev Dyn. 2008;237:3672–3680. doi: 10.1002/dvdy.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilmann C, Capel B. Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development. 1999;126:2883–2890. doi: 10.1242/dev.126.13.2883. [DOI] [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T, Kakitani M. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet. 2008;17:1278–1291. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- Weinstein BM. Vessels and nerves: marching to the same tune. Cell. 2005;120:299–302. doi: 10.1016/j.cell.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]


