Abstract
It has recently been demonstrated that motoneurons in neonatal rodents release an excitatory amino acid, in addition to acetylcholine, from their central terminals onto Renshaw cells. Although the function of this amino acid release is not understood, it may mediate the excitatory actions of motor axon stimulation on spinal motor networks. Stimulation of motor axons in the ventral roots or muscle nerves can activate the locomotor central pattern generator or entrain bursting in the disinhibited cord. Both of these effects persist in the presence of cholinergic antagonists and are abolished or diminished by ionotropic and metabotropic glutamate antagonists.
Calcium imaging in the disinhibited cord shows that a ventral root stimulus evokes ventrolateral activity initially which subsequently propagates to the rest of the cord. This finding suggests that excitatory interneurons excited by motoneuron recurrent collaterals are located in this region. However, motoneurons do not exhibit short latency excitatory potentials in response to ventral root stimulation indicating that the excitatory effects are mediated polysynaptically. The significance of these findings is discussed.
Keywords: calcium imaging, motoneuron, recurrent excitation, spinal cord
Introduction
The mammalian spinal cord contains the circuitry for several rhythmic motor behaviors. It is generally assumed that such behaviors are produced by networks of spinal interneurons and that motoneurons do not contribute causally to their generation1. However, recent work in the developing spinal cord suggests that motoneurons might play a more important role in rhythmogenesis than had been previously thought. In the Xenopus tadpole, for example, that motoneurons are coupled to each other through cholinergic and electrical synapses and also to some of the interneurons involved in the generation of swimming2. Moreover, in the developing spinal cord of the chick3,4 and mouse embryo5 stimulation of motor axons can trigger bursting of spinal motor circuits. In the early embryonic spinal cord, network excitation by motoneuron stimulation has been related to the depolarizing actions of GABA and glycine. However, the reversal potential of inhibitory synapses becomes more hyperpolarized in late embryonic and neonatal motoneurons6 precluding the possibility that the excitatory effects of motoneuron stimulation are mediated through the depolarizing effects of GABA and glycine. Nevertheless, ventral root stimulation can synaptically excite motoneurons in the spinal cords of neonatal rats and mice7,8, and has been reported to evoke glutamatergic EPSPs in neonatal rat motoneurons9. In this review, we discuss our recent work on the excitatory actions of motoneurons in the neonatal spinal cord of the mouse and consider the possible role of motoneurons in locomotor rhythmogenesis.
Motoneurons release an excitatory amino acid at their central terminals onto Renshaw cells
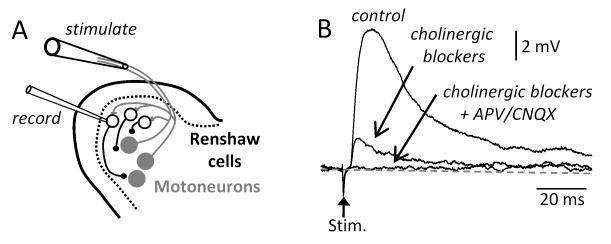
A single stimulus applied to a ventral root evokes a monosynaptic excitatory potential in Renshaw cells in the neonatal mouse spinal cord7,10. This potential can be reduced in amplitude, but not abolished, by a combination of bath applied antagonists to acetylcholine (fig.1B). The remaining non-cholinergic components of the potential are abolished by a combination of the NMDA receptor antagonist APV and the non-NMDA antagonists CNQX7,10 or NBQX11.
Figure 1. Motoneurons release an excitatory amino acid from their terminals onto Renshaw cells in addition to acetylcholine.
(A) Schematic showing a whole cell recording from an identified Renshaw cell (blue filled circles) and a suction electrode on the ventral root to antidromically stimulate motoneurons (grey filled circles) in the isolated spinal cord in vitro. (B) Synaptic responses in response to a single antidromic stimulus applied to the ipsilateral ventral root. Addition of cholinergic blockers reduced but did not abolish the response. The response was abolished by the addition of the glutamatergic antagonists APV and CNQX. The data in B were modified from ref 10.
It has not been possible to identify the released excitatory amino acid as glutamate because motoneuron synaptic terminals on Renshaw cells do not contain immunocytochemically detectable levels any of any of the known vesicular glutamate transporters10,12. While this result may mean that a different, as yet unknown, vesicular glutamate transporter (VGLUT) is present on motoneuron terminals it is also possible that the motoneurons release significant amounts of aspartate rather than glutamate. Given that NMDA receptors are gated by both aspartate and glutamate, while AMPA receptors preferentially bind glutamate13, the presence of significant aspartate release together with the lack of an efficient synaptic vesicle packaging mechanism for glutamate could partly explain why NMDA components at this synapse are frequently larger and less variable than AMPA components11. VGLUTs do not transport aspartate 14-17 therefore aspartate synaptic release must be VGLUT-independent. A recent report suggested that sialin (SLC17A5) packages aspartate and glutamate into hippocampal synaptic vesicles18, but results from our laboratories indicate that this transporter is not located at the intraspinal terminals of motor axon collaterals. Therefore, the release of an excitatory amino acid from motoneuron terminals must operate through a vesicular transport mechanism that is distinct from the currently known VGLUTs or sialin. Unfortunately, it is not possible to reliably distinguish between aspartate and glutamate release pharmacologically and for this reason electron microscopy studies are under way to analyze the relative enrichments of aspartate and glutamate inside motor axon synapses on Renshaw cells. Whatever the exact mechanism of release, or the particular excitatory amino acid being released, it is now well-established that the intraspinal recurrent collaterals of motor axons can activate NMDA and AMPA receptors postsynaptically.
Ventral root stimulation can activate locomotor-like activity and entrain disinhibited bursting
Stimulation of motor axons in the ventral root or the sciatic nerve can trigger episodes of locomotor-like activity in the isolated neonatal mouse spinal cord10 or can entrain the spontaneous bursting that occurs in the presence of bicuculline and strychnine 8,19. Surprisingly neither of these excitatory effects is blocked by bath application of a cocktail of muscarinic and nicotinic cholinergic antagonists 10,19 (fig.2). Rather we found that the excitatory effects of ventral root stimulation could be reversibly abolished by ionotropic glutamatergic antagonists (fig.2C,D).
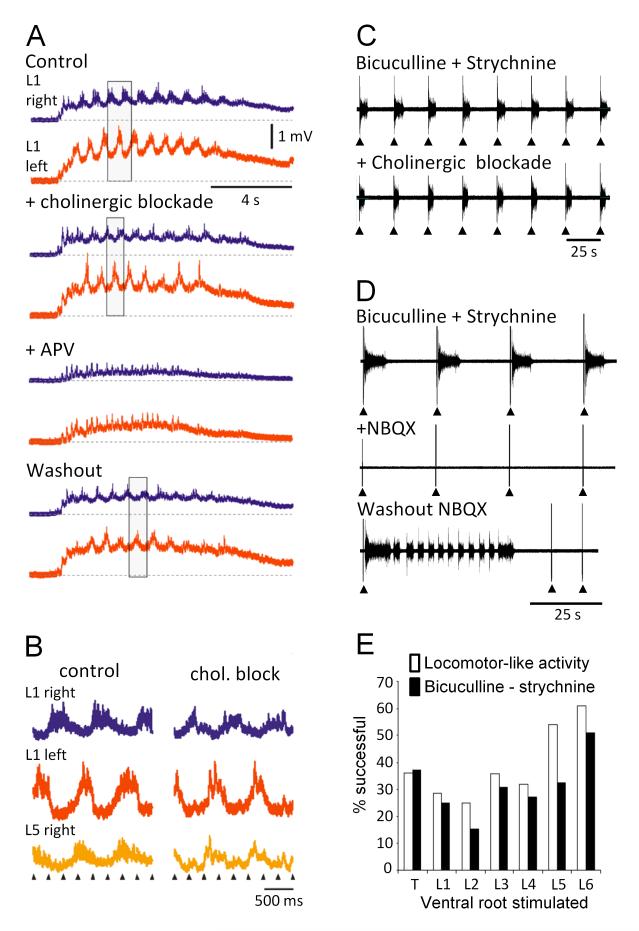
Figure 2. Locomotor-like activity and disinhibited bursting evoked by stimulation of motor axons exhibit similar properties in the neonatal spinal cord of the mouse.
(A) Locomotor-like activity evoked by a train of stimuli applied to the sciatic nerve. To ensure exclusive stimulation of motor axons, the ipsilateral dorsal roots were cut. The records are direct coupled (DC) recordings of the electrical activity recorded from the left (red traces) and right (blue traces) lumbar 1 (L1) ventral roots. The rectangle in this and the subsequent panels highlights the alternation between the activity on the left and right sides. The top set of traces (control) show the activity induced by sciatic nerve stimulation in the absence of drugs. The next traces show that the locomotor-like activity persists in the presence of a cocktail of cholinergic antagonists (50 μM mecamylamine, 50μM dihydro-β-erythroidine, and 5 μM atropine), although the discharge has been reduced. In the presence of the NMDA antagonist APV, the locomotor-like activity is abolished and it recovers when the drugs are washed out (Washout). (B) The phase relations between the activity recorded from the left and right L1 and the right L5 ventral roots under control conditions (control) are unchanged in the presence of cholinergic blockade (chol. block). (C) Spontaneous bursting in the presence of the inhibitory antagonists bicuculline and strychnine can be entrained by a brief train of 5 stimuli (20 Hz, arrowheads) applied to an adjacent ventral root. The effect is maintained in the presence of cholinergic blockade but abolished in the presence of the AMPA/kainate antagonist CNQX (panel D). (E) Histogram comparing the efficacy of ventral root stimulation at evoking locomotor-like activity (open bars) or the entrainment of disinhibited bursting (filled bars). The ordinate shows the percentage of experiments in which the ventral root stimulus produced locomotor-like activity or entrained disinhibited bursting. Modified from refs 10 (A and B) and 19 (C-E).
One unusual feature of the excitatory effects of ventral root stimulation was their lability and rostrocaudal variation in efficacy19. This is shown in fig.2E which displays the percentage of experiments in which a particular ventral root was effective at evoking locomotor-like activity or entraining disinhibited bursting. We found that even the most effective roots were capable of evoking excitatory actions in only ~60-70% of experiments, while the efficacy of the least effective roots was substantially lower (~30% of experiments). We do not know the reason for this lability. It suggests that some aspect of spinal cord function varies from animal to animal and from segment to segment in the isolated spinal cord preparation. These rostrocaudal variations do not appear to correlate with spinal segment size or the number of myelinated and unmyelinated axons in the roots. We found that L5 was more effective than L2 at evoking excitatory effects even though it is larger. Thus, we can eliminate the possibility that the lability is associated with the extent of tissue oxygenation which is presumably poorer in the largest segments. Similarly, there was no correlation between the efficacy of a particular root and the number of myelinated or unmyelinated axons it contains in the adult mouse19,20. For example, the thinner L6 ventral root elicited excitatory effects as effectively as the much larger L4 and L5 ventral roots.
Ventral root evoked locomotor-like activity and the entrainment of disinhibited bursting exhibited similar pharmacological properties and rostrocaudal variations in efficacy, suggesting that the mechanisms underlying these excitatory effects are similar21. As a result, the disinhibited cord can be used as a model for investigating the excitatory effects of ventral root stimulation. This is advantageous experimentally because the synaptic output of Renshaw cells is blocked so that their activation cannot mediate the excitatory actions of ventral root stimulation. Although Renshaw cells are known to be inhibitory in the adult animal, during the neonatal period it is possible that some of their synaptic targets within the spinal cord might have an elevated chloride equilibrium potential rendering the synaptic actions of GABA and glycine excitatory22. In addition, the spontaneous bursting that occurs in the disinhibited cord is highly synchronized among spinal neurons, thereby facilitating calcium imaging of neuronal activity (see below).
Ventral root activation of spinal networks is blocked or abolished by bath application of the mGluR1 antagonist CPCCOEt
Locomotor-like activity evoked by ventral root stimulation usually appears after 2 to 5 stimuli (fig.3A). These initial stimuli, prior to the onset of locomotor bursting, evoke long latency responses that can be recorded from individual motoneurons or from the ventral roots (fig.3A, right hand panel). These long-latency ventral root potentials are abolished in the presence of bicuculline and strychnine19. However, it is unlikely that they are depolarizing inhibitory potentials because the chloride equilibrium potential in neonatal mouse motoneurons is at, or just below, the resting membrane potential6. Why these potentials should disappear in the presence of inhibitory antagonists is unclear unless their expression in motoneurons is somehow facilitated by the presence of depolarizing IPSPs in interneurons. The absence of short latency responses in motoneurons (fig.3A, right hand panel) in response to ventral root stimulation indicates that motoneuron axon collaterals do not contact an excitatory interneuron that projects back onto motoneurons in a similar manner to Renshaw cells.
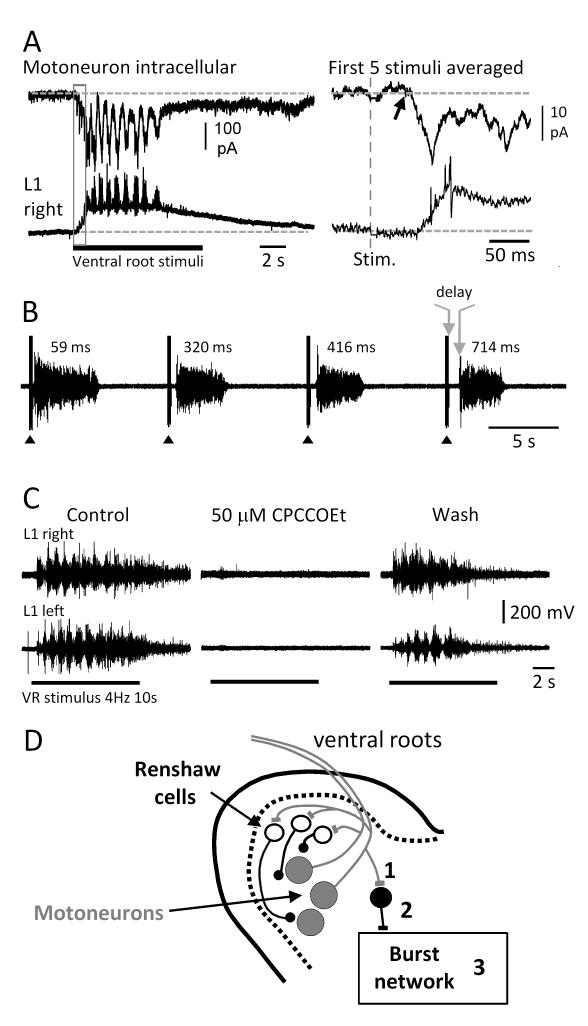
Figure 3. Ventral root responses evoked in motoneurons exhibit prolonged delays that may reflect metabotropic glutamate receptor activation.
(A) Whole cell voltage clamp recording from an identified motoneuron together with the DC extracellular signal recorded from the right L1 ventral root during an episode of locomotor-like activity evoked by a train (4Hz, 10s) of ventral root stimuli. The responses evoked by the first 5 stimuli (delineated by the grey rectangle) were averaged and are displayed in the expanded traces on the right. The arrow marks the onset of the evoked inward current with a latency of 50ms from the ventral root stimulus (red dotted line). (B) During disinhibited bursting entrained by a brief train of stimuli (5 stimuli at 20Hz) applied to an adjacent ventral root the delay between the last stimulus in the train and the onset of the evoked burst (arrows and delay on 4th burst) progressively increases. The delays are indicated over the individual bursts. (C) Locomotor-like activity evoked by a ventral root stimulus train is reversibly abolished in the presence of the mGluR1 receptor antagonist CPCCOEt. The bars below the records indicate the duration of the stimulus train. (D) Schematic of the ventral part of the cord showing the possible loci of action of CPCCOEt. It is assumed that motoneuron collaterals release glutamate at an excitatory interneuron that is distinct from Renshaw cells. This interneuron in turn projects to the burst generating network. CPCCOEt could act presynaptically on motoneuron synaptic terminals (1), pre- and/or postsynaptically on the excitatory interneuron (2) or within the burst network itself (3). Parts of the figure (B and C) were modified from ref. 19.
The bursts evoked by ventral root stimulation in the disinhibited cord were also characterized by a long-latency after the stimulus (fig.3B). Furthermore, the time from the stimulus train to the onset of the ventral root burst progressively lengthened with successive stimuli. As shown in figure 3B, the first burst evoked by the ventral root stimulus exhibited a delay from the last stimulus in the train (5 pulses at 20Hz) of ~60 ms. This delay progressively lengthened with successive bursts so that by the fourth burst the delay was ~700 ms.
These surprisingly long delays raised the possibility that the excitatory effects of ventral root stimulation might be mediated, at least in part, by metabotropic glutamate receptor activation which is known to be slow. Consistent with this idea, we found that the mGluR1 antagonist CPCCOEt reduced the ability of a ventral root stimulus to entrain disinhibited bursting and reversibly blocked the locomotor-like activity evoked by ventral root stimulation19 (fig.3C). The sensitivity of locomotor-like activity and disinhibited bursting to mGluR1 antagonists is consistent with previous work showing that mGluR1 activation can accelerate the locomotor rhythm in the lamprey23 and in the rat spinal cords24.
The most parsimonious explanation to account for the excitatory effects of ventral root stimulation is that an excitatory amino acid released from motoneuron terminals acts on metabotropic glutamate receptors on interneurons other than Renshaw cells (2 in fig.3D). Alternatively, a glutamatergic interneuron activated by motoneuron collaterals might be responsible for activating the metabotropic receptors within the burst generating network (3 in fig.3D). The location and identity of such cells is currently unknown, so we used calcium imaging in the disinhibited cord to identify the origin of the earliest optical activity following stimuli applied to the ventral roots.
Calcium imaging reveals the existence of a ventro-laterally located network activated by ventral root stimulation
We used calcium imaging of either the lateral aspect of the spinal cord or the cut face (rostral L5) to identify the locus of the initial optical activity following a ventral root stimulus. As illustrated in figure 4, the earliest optical activity appeared in the ventrolateral area from where it spread to encompass the rest of the cord. In ~40% of spontaneously occurring bursts a similar pattern of propagation was observed19. The fact that this pattern of propagation can be observed in spontaneous bursts is significant, because it suggests the existence of a functionally discrete ventrolateral network that can be recruited either spontaneously or in response to ventral root stimulation.
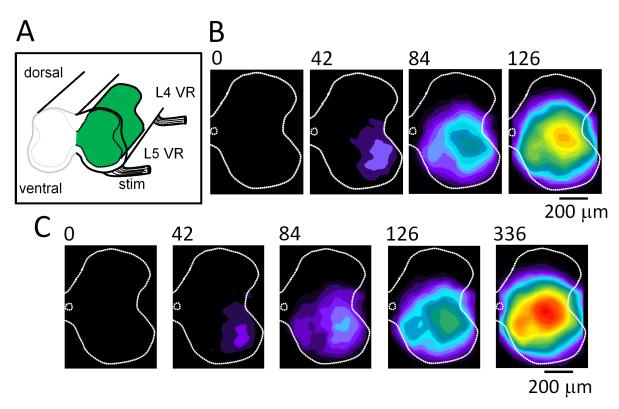
Figure 4. Calcium imaging of the cut face of the disinhibited spinal cord (rostral L5) showing the ventrolateral origin of optical activity following a ventral root stimulus or at the onset of some spontaneous bursts.
(A) Schematic of the spinal cord preparation showing the cut face of the rostral L5 segment. One hemi-segment was injected with the calcium-sensitive dye fluo-3 (green region). (B) Series of images showing the ventrolateral origin of activity following a brief stimulus train (5 stimuli, 20Hz) applied to the ipsilateral L5 ventral root. Each image is a difference image obtained by subtracting a pre-stimulus control image from the active frame. The time of acquisition of the frame is indicated above the images. (C) A similar pattern of activation can be observed during ~40% of the spontaneously occurring bursts. Modified from ref. 19.
However, as pointed out earlier, the maturation of chloride equilibrium potentials makes it highly unlikely that the Renshaw cell circuit is responsible for the propagation of ventral root antidromic activity into the spinal network of neonatal mice. Moreover, because the output of Renshaw cells is blocked in the disinhibited mouse spinal cord, the excitatory actions of ventral root stimulation are probably mediated through activation of excitatory interneurons by the ventral root stimulus. A hint to the mechanism of propagation can be inferred from the velocity of the ventro-dorsal wave of excitation. This is approximately 10μm/ms which is much too slow to be accounted for by conduction delays. The most likely explanation is that it represents the sequential synaptic activation of groups of neurons coupled by short range excitatory synaptic connections.
Concluding Remarks
The existence of recurrent excitatory pathways from motoneurons into spinal cord networks may have gone undetected for so long because the pathways are labile, operate through a non-cholinergic mechanism and are not easily activated in the neonatal spinal cord. This is consistent with the suggestion of Machacek and Hochman8 that the excitatory circuit activated by motoneurons might be under noradrenergic or some other form of neuromodulatory control, which may vary from animal to animal. It is also possible that this pathway is only functional in the neonatal animal and is ‘deselected’ as the animal matures. A precedent for such de-selection is observed in the gradual weakening of primary afferent input onto Renshaw cells in the mouse25.
The functional role of this pathway in locomotion is unclear. Mice do not begin weight bearing locomotion until the second week postnatally. It is possible that the interneuronal circuitry responsible for the locomotor drive to motoneurons is immature at this stage so that the recurrent excitation derived from motoneurons is necessary to maintain adequate motoneuronal drive to muscle. As the interneuronal circuitry matures the contribution of motoneurons to the excitatory drive would decrease. Alternatively, recurrent excitatory motoneuron circuits may persist into adulthood. Ultimately, these issues can only be resolved by identifying the interneurons responsible for transmitting ventral root excitation into spinal networks.
Acknowledgement
This work was supported by the intramural program of NINDS.
REFERENCES
- 1.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 2.Perrins R, Roberts A. Cholinergic contribution to excitation in a spinal locomotor central pattern generator in Xenopus embryos. J Neurophysiol. 1995;73:1013–1019. doi: 10.1152/jn.1995.73.3.1013. [DOI] [PubMed] [Google Scholar]
- 3.Wenner P, O’Donovan MJ. Identification of an interneuronal population that mediates recurrent inhibition of motoneurons in the developing spinal cord. J. Neuroscience. 1999;19:7557–7567. doi: 10.1523/JNEUROSCI.19-17-07557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenner P, O’Donovan MJ. Mechanisms that initiate spontaneous network activity in the developing chick spinal cord. J Neurophysiol. 2001;86:1481–1498. doi: 10.1152/jn.2001.86.3.1481. [DOI] [PubMed] [Google Scholar]
- 5.Hanson MG, Landmesser LT. Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J Neurosci. 2003;23:587–600. doi: 10.1523/JNEUROSCI.23-02-00587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delpy A, Allain AE, Meyrand P, Branchereau P. NKCC1 cotransporter inactivation underlies embryonic development of chloride-mediated inhibition in mouse spinal motoneuron. J Physiol. 2008;586:1059–1075. doi: 10.1113/jphysiol.2007.146993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses 2005. Proc Natl Acad Sci USA. 2005;102:5245–5249. doi: 10.1073/pnas.0501331102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machacek DW, Hochman S. Noradrenaline unmasks novel self-reinforcing motor circuits within the mammalian spinal cord. J Neurosci. 2006;26:5920–5928. doi: 10.1523/JNEUROSCI.4623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang ZG, Shen E, Wang MY, Dun NJ. Excitatory postsynaptic potentials evoked by ventral root stimulation in neonate rat motoneurons in vitro. J Neurophysiol. 1991;65:57–65. doi: 10.1152/jn.1991.65.1.57. [DOI] [PubMed] [Google Scholar]
- 10.Mentis GZ, Alvarez FJ, Bonnot A, Richards DS, Gonzalez-Forero D, Zerda R, O’Donovan MJ. Non-cholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc Natl Acad Sci USA. 2005;102:7344–7349. doi: 10.1073/pnas.0502788102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.d’Incamps B Lamotte, Ascher P. Four excitatory postsynaptic ionotropic receptors coactivated at the motoneuron-Renshaw cell synapse. J Neurosci. 2008;28:14121–14131. doi: 10.1523/JNEUROSCI.3311-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu TT, Bannatyne BA, Jankowska E, Maxwell DJ. Cholinergic terminals in the ventral horn of adult rat and cat: evidence that glutamate is a cotransmitter at putative interneuron synapses but not at central synapses of motoneurons. Neuroscience. 2009;161:111–22. doi: 10.1016/j.neuroscience.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curras MC, Dingledine R. Selectivity of amino acid transmitters acting at N-methyl-D-aspartate and amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors. Mol Pharmacol. 1992;41:520–6. [PubMed] [Google Scholar]
- 14.Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–60. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- 15.Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21:RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99:14488–93. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varoqui H, Schäfer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–55. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyaji T, Echigo N, Hiasa M, Senoh S, Omote H, Moriyama Y. Identification of a vesicular aspartate transporter. Proc Natl Acad Sci U S A. 2008;105:11720–4. doi: 10.1073/pnas.0804015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnot A, Chub N, Pujala A, O’Donovan MJ. Excitatory actions of ventral root stimulation during network activity generated by the disinhibited neonatal mouse spinal cord. J Neurophysiol. 2009;101:2995–3011. doi: 10.1152/jn.90740.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biscoe TJ, Nickels SM, Stirling CA. Numbers and sizes of nerve fibres in mouse spinal roots. Q J Exp Physiol. 1982;67:473–494. doi: 10.1113/expphysiol.1982.sp002663. [DOI] [PubMed] [Google Scholar]
- 21.Beato M, Nistri A. Interaction between disinhibited bursting and fictive locomotor patterns in the rat isolated spinal cord. J Neurophysiol. 1999;82:2029–2038. doi: 10.1152/jn.1999.82.5.2029. [DOI] [PubMed] [Google Scholar]
- 22.Chub N, O’Donovan MJ. Post-episode depression of GABAergic transmission in spinal neurons of the chick embryo. J Neurophysiol. 2001;85:2166–76. doi: 10.1152/jn.2001.85.5.2166. [DOI] [PubMed] [Google Scholar]
- 23.Krieger P, Grillner S, El Manira A. Endogenous activation of metabotropic glutamate receptors contributes to burst frequency regulation in the lamprey locomotor network. Eur J Neurosci. 1998;10:3333–3342. doi: 10.1046/j.1460-9568.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 24.Taccola G, Marchetti C, Nistri A. Modulation of rhythmic patterns and cumulative depolarization by group I metabotropic glutamate receptors in the neonatal rat spinal cord in vitro. Eur J Neurosci. 2004;19:533–541. doi: 10.1111/j.0953-816x.2003.03148.x. [DOI] [PubMed] [Google Scholar]
- 25.Mentis GZ, Siembab VC, Zerda R, O’Donovan MJ, Alvarez FJ. Primary afferent synapses on developing Renshaw cells. J. Neurosci. 2006;26:13297–13310. doi: 10.1523/JNEUROSCI.2945-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]