Abstract
Background
Methamphetamine (MA) use among pregnant women is an increasing problem in the United States. The impact of prenatal MA exposure on development in childhood is unknown.
Objective
To examine the effects of prenatal MA exposure on motor and cognitive development in children at 1, 2, and 3 years of age.
Design/Methods
IDEAL enrolled 412 mother-infant pairs at four sites (Tulsa OK, Des Moines IA, Los Angeles CA, and Honolulu HI). MA subjects (n=204) were identified by self-report or GC/MS confirmation of amphetamine and metabolites in infant meconium. Comparison subjects (n=208) were matched (race, birth weight, maternal education, type of insurance), denied amphetamine use, and had a negative meconium screen. Both groups included prenatal alcohol, tobacco and marijuana use, but excluded use of opiates, lysergic acid diethylamide, phencyclidine or cocaine only. The Peabody Developmental Motor Scales (PDMS-2) were administered to the infants at the 1 and 3 year visits. This analysis includes a subsample (n=350) of the IDEAL study with completed 1 and/or 3 year visits (n= 330 and 281, respectively). At each annual visit we also conducted the Bayley Scales of Infant Development (BSID-II) as a general evaluation of mental and motor development. The BSID-II analysis includes a subsample (n=356) of the IDEAL study with completed 1, 2, and/or 3 year visits (n= 331, 288, and 278 respectively). GLM analysis conducted on the PDMS-2 and BSID-II examined the effects of MA exposure and heavy MA exposure (≥3 days of use/week), with and without covariates. Longitudinal analyses were used to examine the effects of MA exposure on changes in motor and cognitive performance over time.
Results
Heavy MA exposure was associated with significantly lower grasping scores than some and no use at 1 year (P = 0.018). In longitudinal analysis, lower grasping scores associated with any MA exposure and heavy exposure persisted to 3 years. There were no effects of MA exposure, including heavy exposure, on the Bayley Mental Development Index (MDI) or Psychomotor Development Index (PDI) at any or across age.
Conclusions
There were no differences in cognition as assessed by the BSID-II between the groups. There was a subtle MA exposure effect on fine motor performance at 1 year with the poorest performance observed in the most heavily exposed children. By 3 years, no differences in fine motor performance were observed. These findings suggest MA exposure has modest motor effects at 1 year that are mostly resolved by 3 years.
Keywords: prenatal exposure, neurodevelopment, Bayley, Peabody
1. Introduction
Methamphetamine (MA) use continues to be a significant public health problem in the United States. In 1999, the Substance Abuse and Mental Health Services Administration (SAMHSA) reported the number of Americans who had tried MA in their lifetime was 9.4 million(1), double from what it was in 1994(2). By 2004, the number had reached nearly 12 million(3). Data from the Treatment Episode Data Set (TEDS), a national database obtained from admissions to substance abuse treatment centers, recorded a three fold increase in admissions due to MA between 1993 and 2003(4). There is little information about MA use by pregnant women, but available data suggest substance abuse by pregnant women continues to be a significant public health problem. TEDS data reported that 45% of patients treated for MA abuse were women(5) with a prevalence rate for pregnant MA users of 6.4%. The 2007 SAMHSA report found 5.1% of pregnant women aged 15-44 years used illicit drugs during pregnancy with an estimated 5% of persons ages 12 and older having used MA at least once in their lifetime(6).
Because of the significant use of MA in the United States, understanding the effects of prenatal exposure on the developing child is essential. In the rat model, prenatal administration of MA caused abnormal spatial learning(7;8) and in mice, prenatal MA is associated with dopaminergic nerve terminal degeneration and long term motor deficits in offspring(9). Adult MA users have dopamine transmitter loss associated with reduced motor speed and impaired verbal learning(10). To date, however, little is known about the potential neurotoxic effects of prenatal MA exposure on the development of children.
The most extensive follow-up data regarding amphetamine exposed children are from a series of reports from Sweden by Billing and colleagues who have followed a group of amphetamine exposed children from birth to age 14. Among children exposed to amphetamine continuously throughout pregnancy, emotional signs of autism, speech problems and signs of wariness of strangers were noted by age one(11). By age 4, IQ was lower than a normative group of Swedish children(12) and at age 8 prenatal exposure predicted aggressive behavior and problems with peers(13). At age 14, the children showed problems with advancement in school due to delays in math and language and had difficulties with physical fitness activities(14). Other investigators have reported poorer visual recognition memory, a measure correlated with subsequent IQ in MA and cocaine exposed newborns(15). A neuroimaging study of 26 MA exposed and non-exposed children(16) found neurocognitive deficits in visual motor integration, sustained attention, verbal memory and long term spatial memory in the MA-exposed children. The deficits in sustained attention and verbal memory were correlated with reduced volume in targeted brain structures(17). The limitations of many of these reports include the lack of a control group, small sample size and confounds with other prenatal drug use. Though these findings are limited, they suggest these children are at risk for poor child outcome due to both drug and psychosocial risk factors including poverty and family stress.
The Infant Development, Environment, and Lifestyle (IDEAL) study was funded to conduct a large, controlled longitudinal study of MA exposed children in diverse populations and geographic locations. We have previously reported preliminary results from the first year of recruitment that prenatal MA exposure is associated with poor quality of movement, low arousal and increased stress signs in the newborn period(18). This study addresses the effects of prenatal MA exposure on motor and cognitive development in children 1, 2, and 3 years of age.
2. Methods
Study Design
The IDEAL study is a multi-site, longitudinal study investigating the effects of prenatal MA exposure on child outcome. Detailed recruitment methods for the IDEAL study have been reported previously(19). Briefly, between September 2002 - November 2004, subjects in this sample were recruited at the time of delivery from seven hospitals in four geographically diverse, collaborating centers in the following cities: Los Angeles, CA; Des Moines, IA; Tulsa, OK; and Honolulu, HI. All women delivering at each of the four clinical sites were approached, screened for eligibility, and consented to participate in this three-year study.
A postpartum mother was excluded if she was: <18 years of age, using opiate, lysergic acid diethylamide, phencyclidine or cocaine only during her pregnancy, institutionalized for retardation or emotional disorders, low cognitive functioning (did not understand consent after third explanation attempt or could not answer basic demographic information about self or baby), overtly psychotic or a documented history of psychosis, or non-English speaking. Exclusion criteria for the infants were: critically ill and unlikely to survive, multiple birth, major life threatening congenital anomaly, documented chromosomal abnormality associated with mental or neurological deficiency, overt clinical evidence of an intrauterine infection, and sibling previously enrolled in the IDEAL study.
The study was approved by the Institutional Review Boards at all participating sites and signed informed consent was obtained from all subjects. A National Institute on Drug Abuse Certificate of Confidentiality was obtained for the project that assured confidentiality of information regarding the mothers' drug use, superseding mandatory reporting of illegal substance use. The certificate was explained to the mother during the recruitment and informed consent process, including the condition that the certificate did not exclude reporting of evidence of child abuse or neglect. Re-training of staff occurred annually to maintain standardization of the data collection procedures.
At recruitment, sociodemographic and prenatal substance use information was collected by interview from each subject. All interview questions were read to the mother to ensure standardization. A meconium sample was collected from each infant and shipped to a central laboratory (United States Drug Testing Laboratories) for analysis of drug metabolites.
MA exposure was determined by self-reported MA use during this pregnancy and/or a positive meconium screen and gas chromatography/mass spectroscopy confirmation. Meconium toxicology results were required to be enrolled in the study. Of the 204 subjects in the exposed group, eight subjects denied MA use but were identified as exposed by toxicology only; 196 subjects reported amphetamine use with 146 by self report only (toxicology was negative) and 50 by self-report and positive toxicology.
The recruitment sample included 204 MA exposed and 3,701 unexposed subjects who represent the general population of the site. This sample provides the prevalence of MA exposure as well as maternal and newborn characteristics associated with MA exposure(20). The longitudinal follow up sample included all the MA exposed infants and mothers (n=204) and comparisons dyads (n=208) who denied MA use during this pregnancy and had a negative meconium screen for MA. The exposed and comparison groups were matched on maternal race, birth weight category (<1500 g, 1500-2500 g, >2500 g), private versus public insurance, and education (high school education completed versus not completed). Comparison dyads with characteristics that were difficult-to-match were enrolled before a matching exposed dyad, leading to slightly different sample sizes in the two groups. Prenatal exposure to alcohol, tobacco and marijuana existed in both groups and were considered background variables.
The longitudinal phase of this study included visits when the child was 1, 12, 24, 30 and 36 months of age. The present study evaluates the impact of prenatal MA exposure on infant motor and cognitive development assessed at the 1, 2, and/or 3 year study visits.
Subjects
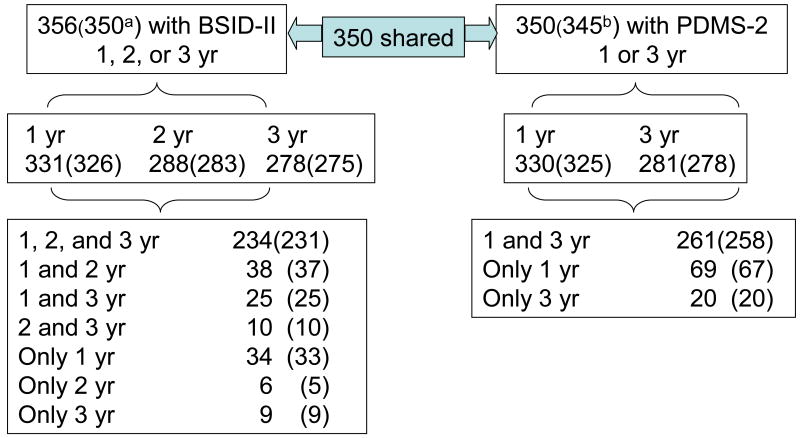
All subjects in the follow up sample who were evaluated at 1, 2 or 3 years on either cognitive or motor assessments were included. There were 356 subjects overall (n=179 MA exposed and n=177 comparison). Figure 1 is a flowchart of the number of subjects evaluated at each visit including the pattern of missing visits.
Fig. 1.
Flow chart of cohort (n=412) with follow up 1 to 3 years (yr). Number of subjects included in heavy use analysis in parentheses.
aN =350 included in analyses of heavy MA use; 6 cases identified as exposed by toxicology only.
bN =345 included in analyses of heavy MA use; 5 cases identified as exposed by toxicology only.
Selective attrition
Analyses of selective attrition compared maternal and newborn characteristics of subjects included in the study versus those excluded because they missed the follow up visit. Comparison of the 284 included in 3 year evaluation of BSID-II and/or PDMS-2 to the 128 excluded (Table 1) showed higher gestational age at the 1st prenatal visit. Analyses of selective attrition at 1 year showed that the 331 included subjects were more likely to be low SES than the 81 subjects who missed the visit (32% vs. 21%, P = 0.034). At 2 years, there were no significant differences on any characteristic between the included (n=288) and excluded (n=124) subjects. Of special note, there were no differences between included and excluded subjects on the prevalence of MA exposure including heavy MA use at any age point.
Table 1.
Comparison of dyads included and not included at the 3 year evaluation.a
| n(%) or Mean (SD) | Included N=284 |
Excluded N=128 |
p-value |
|---|---|---|---|
| MATERNAL/DEMOGRAPHIC CHARACTERISTICS | |||
| Race | 0.639 | ||
| White | 106 (37.3%) | 54 (42.2%) | |
| Hispanic | 66 (23.2%) | 26 (20.3%) | |
| Pacific Islander | 50 (17.6%) | 21 (16.4%) | |
| Asian | 36 (12.7%) | 21 (16.4%) | |
| Black | 17 (6.0%) | 4 (3.1%) | |
| American Indian | 8 (2.8%) | 2 (1.6%) | |
| Other | 1 (0.4%) | 0 (0%) | |
| Low SES | 60 (21.1%) | 33 (26.2%) | 0.259 |
| Public insurance | 247 (87.0%) | 116 (90.6%) | 0.484 |
| Partner at birth | 160 (56.3%) | 67 (52.3%) | 0.451 |
| Education <12 years | 113 (39.8%) | 59 (46.8%) | 0.183 |
| Maternal age (yr) | 25.2 (5.7) | 25.2 (5.6) | 0.971 |
| GA at 1st prenatal visit | 11.2 (7.0) | 13.8 (7.9) | 0.001 |
| Prenatal care | 271 (95.4%) | 120 (93.8%) | 0.475 |
| Prenatal methamphetamine (MA) use | 139 (48.9%) | 65 (50.8%) | 0.730 |
| Heavy MA use (≥ 3 days/wk across pregnancy) | 27 (9.6%) | 9 (7.3%) | 0.719 |
| Prenatal tobacco use | 144 (50.7%) | 74 (57.8%) | 0.181 |
| Average number of cigarettes/day across pregnancy | 6.4 (10.0) | 8.1 (11.4) | 0.136 |
| Prenatal alcohol use | 70 (24.6%) | 36 (28.1%) | 0.455 |
| Average oz. absolute alcohol/day across pregnancy | 0.18 (0.75) | 0.28 (0.72) | 0.183 |
| Prenatal marijuana use | 55 (19.4%) | 21 (16.4%) | 0.473 |
| Average number of joints/day across pregnancy | 0.13 (0.43) | 0.07 (0.20) | 0.108 |
| NEONATAL CHARACTERISTICS | |||
| Gender (boy) | 149 (52.5%) | 71 (55.5%) | 0.572 |
| Birth Weight (g) | 3,262 (622) | 3,215 (546) | 0.454 |
| Length (cm) | 50.5 (3.5) | 50.2 (2.9) | 0.338 |
| Head Circumference (cm) | 33.9 (1.9) | 33.9 (1.7) | 0.880 |
| Gestational Age (weeks) | 38.6 (2.2) | 38.7 (1.8) | 0.929 |
| Small for Gestational Age | 41 (14.4%) | 14 (10.9%) | 0.334 |
| Low Birth (<2500) | 34 (12.0%) | 13 (10.2%) | 0.592 |
1 year visit: 331 participants included were less likely to be low SES than 81 excluded (21% vs. 32%, P = 0.034). 2 year visit: No significant differences for 288 vs. 124 excluded (P > 0.05).
Covariates
Once consented, each subject was administered the Lifestyle Interview which collected the following information: 1) number of prenatal visits, 2) demographics including age, education, occupation, race, marital status, type of insurance, and socioeconomic status (SES), calculated using the four-factor Hollingshead Index which has been adapted to single parent and non-nuclear families(21;22), 3) licit and illicit drug use during pregnancy including tobacco, alcohol and marijuana use.
The Substance Use Inventory (SUI)(23) was administered to all subjects who admitted use of a licit or illicit drug in the Lifestyle Interview. This questionnaire asked detailed questions about the frequency and quantity of MA use during four time periods: 3 months prior to pregnancy, first, second and third trimester of pregnancy. Consistent with published studies of MA exposure(18) and similar studies of cocaine exposure(24;25), heavy MA use was defined as ≥3 days per week across pregnancy. Some use was any use not meeting the criterion for heavy use. Of the MA users, 36 (18%) met criterion for heavy use.
The Peabody Picture Vocabulary Test-III (PPVT-III) was administered to the child's mother or primary caretaker at the 30 month home visit. The PPVT-III is a standardized assessment of receptive vocabulary that serves as a proxy measure of the caretaker's IQ(26). The standard score based on the number of correct answers is used in this study. The Home Observation for Measurement of the Environment (HOME) Inventory was also completed at the 30-month home visit. It measures the quality of the home environment including social-emotional and cognitive support available in the home (27). Items are scored on the basis of family interview and direct observations made by the interviewer. The overall summary score for the total quality of home is used in this study.
Outcome measures
Examiners masked to exposure status were trained and certified in the administration and scoring of the motor and cognitive assessments. Age of administration was corrected for prematurity for infants born <37 weeks gestation. The Peabody Developmental Motor Scales (PDMS-2) were administered when the infant was 12 ± 0.5 and 36 ± 1.5 months of age. The PDMS-2 measures gross and fine motor skills. The gross motor scale is comprised of three subtests: stationary or body control and equilibrium, locomotion, and object manipulation. The fine motor scale is comprised of two subtests: grasping, and visual-motor integration. The PDMS-2 was conducted on infants (n=350) who were evaluated at the 1 or 3 year visits (n= 330 and 281, respectively with n=261 at both visits).
The Bayley Scales of Infant Development II (BSID-II) were administered when the infants were 12 ± 0.5, 24 ± 1, and 36 ± 1.5 months of age. The BSID-II is a standardized assessment of developmental functioning of infants including mental and motor scales (28). The Mental Scale includes items that assess memory, problem solving, early number concepts, generalization and vocalizations. From these items, the standard score, Mental Development Index (MDI) is generated. The Motor Scales assess control of gross and fine muscle groups and results in the Psychomotor Development Index (PDI). Both indices have a mean of 100 with a standard deviation of 16. The BSID-II analysis includes a subsample (n=356) of the IDEAL study with completed 1, 2, and/or 3 year visits (n= 331, 288, and 278 respectively, with 234 at all visits).
Statistical analysis
Maternal and neonatal characteristics were assessed using one-way analysis of variance (ANOVA) for continuous measures or chi-square for categorical measures.
Cross sectional analyses
One-way ANOVA tested the effects of MA exposure and heavy MA use on motor and cognitive outcomes as measured by the PDMS-2 at 1 and 3 years of age and the BSID-II at 1, 2, and 3 years of age. All subtests of the PDMS-2 were initially evaluated by MA exposure, but only subtests with significant effects were analyzed further using General Linear Modeling (GLM). GLM tested the effects of MA exposure and heavy MA use with covariates on BSID-II and PDMS-2 outcomes. The level of MA use was recoded into heavy use versus some and no use to test whether heavy use had a greater effect on developmental outcomes over and above some use and no use. Significant PDMS-2 subtest findings were followed by a Bonferroni correction to minimize type I errors due to multiple comparison tests.
Longitudinal analyses
General linear mixed models (GLMM) were used to test longitudinal effects of prenatal MA exposure and heavy prenatal MA exposure on cognitive and motor outcomes, after controlling for potential covariates. GLMM are able to accommodate missing data and unbalanced designs, which are issues in the current study. Separate models were conducted for BSID-II MDI and PDI scores at 1, 2, and 3 years and PDMS-2 at 1 and 3 years, using PROC MIXED procedure in SAS for Windows 9.13 (SAS Institute, Cary, NC). Continuous covariates (e.g., birth weight) were grand mean centered. Site was included to address the nesting structure of children in study sites. Significant PDMS-2 subtest findings were followed by a Bonferroni correction to minimize type I errors due to multiple comparison tests.
Imputation of missing values
There were 73 (20.5%) and 62 (17.4%) missing values for the HOME and PPVT scores, respectively, in our sample for longitudinal analyses of the BSID-II. We used multiple imputation as implemented in Proc MI of SAS for BSID-II MDI and PDI separately. Twenty imputed data sets were generated for each analysis. The results of each were combined for the estimation of regression parameters using Proc MIANALYZE. Sensitivity analyses were performed on the data with and without the imputed values for PPVT and HOME scores. The results were very similar. Thus, we report the results from analyses with imputed data to retain the full sample.
In all analyses, significance was accepted at P < 0.05. Data were analyzed using SAS for Windows (version 9.1.3; SAS Institute, Cary, NC) and SPSS for Windows (Rel. 17.0.0 2008 Chicago: SPSS Inc.).
Covariate selection
Covariates were selected based on conceptual reasons, published literature, and maternal and neonatal characteristics that differed between MA exposure groups (P < 0.05) if not highly (r < 0.7) correlated with other covariates. Prenatal exposures to alcohol, tobacco and marijuana, socioeconomic status (SES), birth weight and study site were included a priori as covariates in all analyses based on literature as well as study design. Further, the quality of home from the HOME scale, maternal IQ from the PPVT-III, and gender were included a priori as covariates in analyses the BSID-II analyses because they have been previously shown to be associated with cognitive development in children (35). Maternal characteristics during the neonatal period that differed by exposure status (Table 2) were examined as potential covariates, but these factors were unrelated to cognitive or motor outcomes and not included as covariates. Although gestational age, birth length, and birth head circumference (Table 3) differed by exposure status, they were highly associated with birth weight, which was already selected as a covariate. Thus, the covariates included in all cross sectional and longitudinal models were any prenatal exposures to tobacco, marijuana, and alcohol, low SES, gender, and birth weight. Gender was included in all cross sectional and longitudinal models for BSID-II outcomes. The quality of the home (HOME) and maternal IQ (PPVT) were included in longitudinal analyses of BSID-II outcomes, but not cross sectional analyses as two of three ages points occurred prior to their measurement. Subjects were nested in site in longitudinal analyses. For cross sectional analyses, the correlation between site and prenatal MA exposure was tested for each outcome. There was no significant association between site and MA exposure or heavy use. Thus, site was included as a control variable. Finally, covariate analyses were repeated while excluding birth weight to test for the possibility that birth weight was mediating the impact of MA exposure.
Table 2. Maternal characteristics by MA exposure.
| Number (Percent)a/ Mean (SD) | P-Value | ||
|---|---|---|---|
| Exposed (N = 179) | Comparison (N= 177) | ||
| Race | 0.871 | ||
| White | 69 (38.5%) | 70 (39.5%) | |
| Hispanic | 41 (22.9%) | 38 (21.5%) | |
| Pacific Islander | 30 (16.8%) | 32 (18.1%) | |
| Asian | 25 (14.0%) | 23 (13.0%) | |
| Black | 8 (4.5%) | 10 (5.6%) | |
| American Indian | 6 (3.4%) | 3 (1.7%) | |
| Other | 0 (0%) | 1 (0.6%) | |
| Low socioeconomic status | 59 (33.1%) | 18 (10.2%) | <0.001 |
| Public insurance | 160 (89.4%) | 151 (85.3%) | 0.072 |
| Partner at birth | 83 (46.4%) | 117 (66.1%) | <0.001 |
| Education <12 years | 84 (47.2%) | 66 (37.5%) | 0.065 |
| Maternal age (yr) | 25.5 (5.7) | 24.6 (5.5) | 0.109 |
| GA at 1st prenatal visit | 14.5 (8.3) | 9.4 (5.7) | <0.001 |
| Prenatal care | 165 (92.2%) | 174 (98.3%) | 0.007 |
| Prenatal tobacco use | 143 (79.9%) | 46 (26.0%) | <0.001 |
| Average number of cigarettes/day across pregnancy | 10.8 (11.3) | 2.9 (7.2) | <0.001 |
| Prenatal alcohol use | 66 (36.9%) | 23 (13.0%) | <0.001 |
| Average oz. absolute alcohol/day across pregnancy | 0.37 (1.0) | 0.04 (0.22) | <0.001 |
| Prenatal marijuana use | 58 (32.4%) | 7 (4.0%) | <0.001 |
| Average number of joints/day across pregnancy | 0.23 (0.52) | 0.02 (0.15) | <0.001 |
The number of participants includes BSID-II or PDMS-2 evaluation at any visit.
Table 3. Neonatal characteristics by MA exposure.
| Number (Percent)a/ Mean (SD) | P-Value | ||
|---|---|---|---|
| Exposed (N = 179) |
Comparison (N= 177) |
||
| Gender (boy) | 97 (54.2%) | 93 (52.5%) | 0.755 |
| Birth Weight (g) | 3186 (620) | 3293 (569) | 0.090 |
| Length (cm) | 49.8 (3.6) | 51.0 (3.0) | 0.001 |
| Head Circumference (cm) | 33.7 (1.8) | 34.1 (1.8) | 0.028 |
| Gestational Age (weeks) | 38.3 (2.3) | 39.0 (1.7) | 0.001 |
| Small for Gestational Age | 28 (15.6%) | 21 (11.9%) | 0.301 |
| Low Birth (<2500) | 22 (12.3%) | 21 (11.9%) | 0.902 |
The number of participants includes BSID-II or PDMS-2 evaluation at any visit.
3. Results
Characteristics of the sample
Demographic and neonatal characteristics were examined between the exposed and comparison groups in 356 subjects who were evaluated at 1, 2 or 3 years on cognitive or motor assessments. As presented in Table 2, there were no differences in race, type of insurance, education level or maternal age between the groups. Women in the exposed group were more likely to have a lower SES, be without a partner at the time of delivery, obtain prenatal care later in gestation and less likely to have attended prenatal care visits than the comparison group. Further, mothers in the exposed group were more likely to use more tobacco, alcohol and marijuana during pregnancy. Newborn demographics of the study subjects are presented in Table 3. There were no differences in gender, birth weight or incidence of small for gestational age or low birth weight by exposure group. The MA group had decreased gestational age, length and head circumference at birth.
We also measured caretaker IQ (Exp n=147; Comp n=147) and the quality of the home (Exp n=141; Comp n=142) at 30 months. There were no effects of MA exposure on the quality of the home (Exp 34.01 SD 4.08 versus Comp 34.25 SD 3.83, P = 0.612). Further, there were no significant differences in IQ between caretakers in the exposed and comparison groups (Exp 91.88 SD11.18 versus Comp 90.82 SD 14.25, P = 0.475). Due to mandatory reporting of illicit drug use during pregnancy to child protective services, 65 children in the exposed group and 4 in the comparison group were not living with their biological mother at 30 months of age. Non-maternal caretakers for the exposed group were as follows: 31 relatives, 26 adoptive parents, 5 foster parents and 3 non-relatives. Non-maternal caregivers in the comparison group were as follows: 3 relatives, 1 adoptive parent.
Maternal drug use
The study criterion for heavy use is based on the average frequency of MA use across pregnancy. Heavy use is defined as ≥3 days per week and some use has < 3 days per week. Table 4 shows the average pattern of reported use by trimester for heavy and some users. Overall, the pattern is toward declining MA use over the course of the pregnancy with 28 (16%) abstaining in the 1st trimester, 79 (56%) abstaining in the 2nd trimester and 102 (59%) abstaining or quitting in the 3rd trimester. Further, 67 (47%) quit MA use by the end of the 1st trimester. We compared the pattern of declining use and quitting between mothers designated as heavy or some MA users. The decline in MA use from the 1st to 2nd trimester was greater (P = 0.001) in the some use (68%) versus the heavy use group (36%), but the decline from 2nd to 3rd trimester was greater in the heavy use group (64%) than the some use group (31%, P < 0.001). No heavy users quit at the end of the 1st trimester compared to 67 (47%) in the some use group. Eleven (31%) of heavy users quit MA use by the end of the 2nd trimester compared to 91 (64%) in the some use group (P < 0.001). These findings suggest that the study designation for heavy use not only reflects greater frequency of use across pregnancy, but also distinguishes two key components of the pattern of MA use in this sample, declining use and quitting.
Table 4.
Frequency of self-reported methamphetamine use by trimester of pregnancy.a
| MA use | Heavy MA Use (N=31) | Some MA Use (N=142) | ||||
|---|---|---|---|---|---|---|
| Trimester | ||||||
| First | Second | Third | First | Second | Third | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Daily | 18 (58.1%) | 14 (45.2%) | 4 (12.9%) | 13 (9.2%) | 1 (0.7%) | 1 (0.7%) |
| 3-6 days/wk. | 12 (38.7%) | 15 (48.4%) | 10 (32.3%) | 36 (25.4%) | 9 (6.3%) | 1 (0.7%) |
| 1-2 days/wk. | 0 (0%) | 2 (6.5%) | 2 (6.5%) | 24 (16.9%) | 15 (10.6%) | 8 (5.6%) |
| 1-3 days/mo. | 0 (0%) | 0 (0%) | 1 (3.2%) | 16 (11.3%) | 17 (12.0%) | 13 (9.2%) |
| 1-2 days/3 mos. | 0 (0%) | 0 (0%) | 2 (6.5%) | 24 (16.9%) | 21 (14.8%) | 28 (19.7%) |
| Not at all | 1 (3.2%) b | 0 (0%) | 11 (35.5%) | 27 (19.0%) | 79 (55.6%) | 91 (64.1%) |
| Days/week (mean, SD) | 5.87 (1.70) | 5.15 (1.89) | 2.59 (2.69) | 1.98 (2.22) | 0.50 (1.07) | 0.21 (0.73) |
6 of the 179 MA users in this study were identified as exposed by toxicology only
1 case classified as a heavy user who abstained in the 1st trimester: 2nd trimester use-3.5 days/wk; 3rd trimester use-5.5 days/wk
The predominant routes of administration are smoking (74.9%), sniffing/snorting (27.4%), ingestion (4.5%), or injection (10.1%). Most MA users used only one route of administration (77%). To address the effects of route of administration, we recoded the data into smoking versus all else. No group differences were observed on any cognitive or motor outcome at any age (P > 0.05).
Cross- sectional analyses of cognitive and motor outcomes
On the PDMS-2, MA exposure was associated with lower scores on the grasping subtest at 1 year relative to the comparison group (Table 5) after controlling for covariates. The grasping score assesses the fine motor ability of the hands and fingers; a lower grasping score (P = 0.027) indicates a specific deficit in fine motor coordination. However, when corrected for multiple comparison tests, the results was marginally significant (P = 0.054). There were no effects of grasp at 3 years. Heavy MA exposure was associated with significantly lower grasp scores (Table 6) than some MA exposed and no use (P = 0.024) after adjusting for covariates, and the effect was maintained after correction for multiple comparison tests (P = 0.048). These results suggest a dose-response effect. No effects were observed at 3 years, however there was a trend for lower grasping scores (P = 0.099) in the heavy MA use group. There were no significant effects of MA exposure or heavy exposure on fine or gross motor scores at 1 or 3 years (P > 0.05 in all cases).
Table 5. PDMS-2 by MA exposure.
| Quotient/Subscales | Mean ± (SD) | |||
|---|---|---|---|---|
| Exposed | Comparison | Unadjusted P-Value | aAdjusted P-Value | |
| 1 year | (N = 160) | (N= 168) | ||
| Gross Motor Quotient | 103.82 ± 5.79 | 103.64 ± 7.10 | 0.806 | 0.630 |
| Stationary | 10.70 ± 1.09 | 10.63 ± 1.29 | 0.603 | 0.412 |
| Locomotion | 10.97 ± 1.68 | 10.83 ± 2.00 | 0.490 | 0.763 |
| Object Manipulation | 10.34 ± 1.33 | 10.48 ± 1.46 | 0.486 | 0.262 |
| (N = 161) | (N= 169) | |||
| Fine Motor Quotient | 104.94 ± 9.80 | 106.16 ± 10.46 | 0.277 | 0.240 |
| Grasping | 10.24 ± 1.89 | 10.61 ± 2.01 | 0.083 | 0.027b |
| Visual-Motor Integration | 11.42 ± 2.05 | 11.43 ± 2.12 | 0.933 | 0.761 |
| 3 years | (N = 132) | (N=130) | ||
| Gross Motor Quotient | 95.26 ± 10.71 | 94.76 ± 7.54 | 0.666 | 0.492 |
| Stationary | 9.49 ±1.83 | 9.38 ± 1.81 | 0.630 | 0.481 |
| Locomotion | 9.12 ± 1.67 | 8.93 ± 1.64 | 0.337 | 0.249 |
| Object Manipulation | 9.21 ± 1.74 | 9.10 ± 1.66 | 0.578 | 0.756 |
| (N = 139) | (N=142) | |||
| Fine Motor Quotient | 98.78 ± 11.75 | 98.83 ± 11.99 | 0.974 | 0.697 |
| Grasping | 9.80 ± 2.37 | 10.22 ± 2.45 | 0.146 | 0.205 |
| Visual-Motor Integration | 9.81 ± 1.81 | 9.63 ± 1.55 | 0.375 | 0.100 |
Adjusted for study site, birth weight, prenatal drug exposures, and socioeconomic status.
Correction for multiple comparison tests P = 0.054
Table 6. Heavy MA use and PDMS-2 Gross, Fine and Total Motor Scores.
| Mean ± (SD) | ||||
|---|---|---|---|---|
| Heavy Use >=3 days per week | Some Use/No Use | Unadjusted P-Value | aAdjusted P-Value | |
| 1 year | (N = 30) | (N=293) | ||
| Gross motor Quotient | 103.63 ± 5.41 | 103.75 ± 6.60 | 0.916 | 0.651 |
| (N = 30) | (N=295) | |||
| Fine motor Quotient | 104.07 ± 9.98 | 105.87 ± 10.11 | 0.353 | 0.122 |
| Grasping | 9.83 ± 1.66 | 10.51 ± 1.97 | 0.072 | 0.024b |
| 3 years | (N=26) | (N=234) | ||
| Gross motor Quotient | 94.27 ± 8.75 | 95.02 ± 9.32 | 0.695 | 0.832 |
| (N=27) | (N=251) | |||
| Fine motor Quotient | 96.44 ± 7.16 | 98.94 ± 12.17 | 0.297 | 0.436 |
| Grasping | 9.37 ± 1.67 | 10.06 ± 2.48 | 0.158 | 0.099 |
Adjusted for study site, birth weight, prenatal drug exposures, and socioeconomic status.
Correction for multiple comparison tests P = 0.048.
The BSID-II MDI and PDI data for the 1, 2 and 3 year visits are presented in Table 7. There were no differences in the MDI or PDI scores between the comparison and MA groups. All covariates were entered into an initial model with MA exposure. After excluding nonsignificant covariates, the covariates included in the final analysis for the MDI and PDI at 1, 2 and 3 years were the a priori covariates, study site, birth weight, gender, prenatal drug exposures, and SES. In both unadjusted and adjusted models, there were no effects of MA exposure on MDI and PDI at any age (P > 0.05 in all cases). Similarly, there were no effects of heavy MA exposure on the MDI and PDI at any age (P > 0.05 in all cases).
Table 7. BSID-II by MA exposure.
| Standard scores | Mean ± (SD) | Unadjusted P-Value | aAdjusted P-Value | |
|---|---|---|---|---|
| Exposed | Comparison | |||
| 12 months | (N = 162) | (N= 169) | ||
| MDI | 94.23 ± 9.56 | 94.28 ± 10.64 | 0.964 | 0.785 |
| PDI | 93.86 ± 11.32 | 93.24 ± 12.60 | 0.638 | 0.964 |
| 24 months | (N = 147) | (N= 139) | ||
| MDIb | 84.51 ± 12.03 | 83.53 ± 13.36 | 0.515 | 0.216 |
| (N = 147) | (N= 137) | |||
| PDIc | 94.71 ± 13.32 | 93.45 ± 12.38 | 0.412 | 0.549 |
| 36 months | (N = 135) | (N= 141) | ||
| MDIb | 88.90 ±11.11 | 87.76 ± 15.27 | 0.481 | 0.240 |
| (N = 133) | (N= 140) | |||
| PDIc | 92.79 ± 11.06 | 92.43 ±11.23 | 0.789 | 0.230 |
Adjusted for study site, birth weight, gender, prenatal drug exposures, and socioeconomic status.
MDI: 2 cases with missing values at 24 months; 2 cases with missing values at 36 months.
PDI: 4 cases with missing values at 24 months; 5 cases with missing values at 36 months.
Further, MA exposure effects were unchanged across all cross sectional analyses of BSID-II and PDMS-2 outcomes when birth weight was removed indicating that birth weight was not mediating the impact of MA exposure.
Longitudinal analyses of motor and cognitive outcomes
In longitudinal analyses (Table 8), there were 347 subjects with gross motor scores. There were 350 subjects with fine motor and grasping scores at 1or 3 years or both. After adjustments for birth weight, prenatal drug (alcohol, marijuana and tobacco) exposure and SES, grasping scores were lower (P = 0.021) in children with prenatal MA exposure (9.96±0.15) than those in the comparison group (10.51±0.15). Further, children with heavy MA exposure had lower grasping scores than children who had some or no exposure to MA (9.49±0.31 versus 10.32±0.10, respectively, P < 0.02).
Table 8.
Selected repeated measures coefficients from fixed-effect mixed models.
| Outcome | Parameter | Any Use | Heavy vs. Some/ No Use | ||||
|---|---|---|---|---|---|---|---|
| Estimate | SE | p | Estimate | SE | P | ||
| PDMS-2 | |||||||
| Gross motor | Prenatal MA | 0.20 | 0.87 | 0.819 | -0.55 | 1.18 | 0.643 |
| Birth weight a | 0.14 | 0.06 | 0.013 | 0.14 | 0.06 | 0.012 | |
| Fine motor | Prenatal MA | -0.58 | 1.16 | 0.616 | -2.49 | 1.59 | 0.118 |
| Low SES | -2.99 | 1.19 | 0.013 | -2.84 | 1.18 | 0.017 | |
| Grasping | Prenatal MA | -0.55 | 0.24 | 0.021 | -0.83 | 0.32 | 0.011 |
| BSID-II | |||||||
| MDI | Prenatal MA | 0.57 | 1.22 | 0.639 | -1.57 | 1.69 | 0.354 |
| Maternal IQ | 0.13 | 0.04 | 0.003 | 0.14 | 0.04 | 0.002 | |
| Quality of home | 0.48 | 0.14 | 0.001 | 0.49 | 0.14 | <0.001 | |
| Gender (Boy) | -5.38 | 0.95 | <.001 | -5.26 | 0.95 | <.0001 | |
| PDI | Prenatal MA | 0.87 | 1.2 | 0.469 | -0.06 | 1.66 | 0.969 |
| Birth weight a | 0.27 | 0.08 | <0.001 | 0.27 | 0.08 | 0.001 | |
| Gender (Boy) | -3.14 | 0.94 | 0.001 | -3.08 | 0.94 | 0.001 | |
The increment of birth weight for parameter estimates is 100 g.
Notes: Non-significant findings for prenatal exposure to alcohol, tobacco and marijuana, low socioeconomic status, birth weight, IQ, and quality of home are not presented. Time trends were significantly decreasing in all analyses except not significant in analyses of PDI. Site effects were mostly not significant.
There were no significant differences in fine motor scores between MA exposed and comparison groups (P = 0.616) as well as between heavy MA exposure versus some and no exposure groups (P = 0.118). However low SES was associated with lower fine motor scores (MA exposure P = 0.013; heavy MA exposure P = 0.017). There were also no significant differences in gross motor scores by either any MA exposure (P = 0.819) or heavy MA use vs. combined some and non-use (P = 0.643). In gross motor analyses, birth weight was a significant predictor (MA exposure P = 0.013; heavy MA exposure P = 0.012).
In longitudinal analyses, there were 356 subjects with MDI and PDI scores. There were no significant differences in MDI and PDI scores between MA exposed and comparison groups (P = 0.639 for MDI and P = 0.469 for PDI) as well as between heavy MA exposure versus some and no exposure groups (P = 0.354 for MDI and P = 0.969 for PDI). Birth weight was not significantly associated with MDI, but was associated with PDI. For each 100 g increment in birth weight, PDI increases by 0.27. Maternal IQ and quality of home were associated with MDI, but neither factor was significantly associated with PDI. For both MDI and PDI, boys scored significantly lower than girls.
In all longitudinal analyses, we examined the interaction of MA exposure and time of evaluation and found no significant interactions (P >0.05). Birth weight was also tested, but there were no significant mediating effects (P > 0.05).
4. Discussion
The effects of prenatal MA exposure on child development are not clearly defined. Previous studies have been unable to determine the direct toxic effects of MA versus the multiple risk factors associated with drug use during pregnancy. To our knowledge, the IDEAL study is the only prospective longitudinal study of prenatal MA exposure and child outcome that has the power to control for multiple covariates. We found poorer fine motor performance at 1 year in children exposed to prenatal MA exposure with the poorest performance observed in the most heavily exposed children. By 3 years, no differences in fine motor performance were observed between the groups. There were no differences noted in the Bayley cognitive or behavioral performances. These findings suggest MA exposure has modest motor effects at 1 year that are mostly resolved by 3 years with no evidence of cognitive dysfunction noted in children MA-exposed. Motor development during infancy is associated with visual perceptual and spatial skills(29;30). Because future visual perceptual processing may be adversely affected, these children may be at risk for poorer performance in tasks requiring coordination of movements such as bicycle riding and other physical fitness activities.
Our findings of poorer motor performance in the exposed group are consistent with previous findings in exposed children and adult MA users. Neuroimaging studies in adult MA users have found reduced motor speed associated with dopamine transmitter loss(10). A study of school aged children exposed to MA in utero found poorer visual motor integration in association with smaller globus pallidus volumes(17). In addition, lower thalamic myoinositol levels are associated with poor visual motor performance in MA exposed children ages 3-4 years(31). We previously reported that MA exposed newborns enrolled in the IDEAL study demonstrated poorer quality of movement on the NICU Network Neurobehavioral Scale relative to the comparison group (18). These motor findings are consistent with previous work in amphetamine exposed children reporting hypertonia in the newborn period, with poorer performance in physical fitness activities by age 14 (32;33).
The finding of subtle motor effects in MA exposed children is consistent with research in children exposed to prenatal cocaine(34-36). Several have reported cocaine associated effects on motor development including effects on the Bayley PDI at 3 months(37), 6 and 18 months(38), on the Movement Assessment Inventory at 4 months(39;40) and on the Alberta Infant Motor Scales at 7 months(39). Similar to our findings in MA exposed newborns, Miller-Loncar reported cocaine-exposed one month old infants had poorer motor performance. Poorer performance was linked to the infants most heavily exposed(41), though no differences in motor performance was found in these children by 18 months.
There are numerous possible etiologies for the poorer motor performance in the MA exposed children. Administration of MA to laboratory animals results in profound and long lasting toxicity to the brain. In rodents, MA is toxic to dopaminergic and serotonergic neurons(42;43) and neurotoxic effects on serotogenetic neurons produce neurochemical alternations in the central nervous system(44;45). Prenatal MA exposure in 3 week old rats (equivalent to approximately age 5 years) demonstrate impaired postural motor movements(46) and these abnormal postural movements can still be noted in the second generation of rats exposed to MA prenatally(47). Administration of MA to laboratory animals also results in delayed motor development(48;49) and in pregnant mice, MA administration to the mother leads to dopaminergic nerve terminal degeneration and long term motor deficits in the exposed offspring(50).
It is possible the environment of the exposed children contributed to our motor findings. In the rodent model, cross fostering MA exposed offspring with non exposed dams resulted in improvement in motor function relative to non-cross fostered MA exposed rats(51). Mayes found decreased motor performance with increasing age from 3 to 24 months of age(52) in a group of cocaine-exposed and non-exposed children matched for socioeconomic status, suggesting high-risk environments may influence motor performance. However, we did adjust for environmental influences and continued to find differences in fine motor function at 1 year of age. In addition, no differences were noted on the Bayley examination between the groups which tests for global development. These measures of cognition and behavior are typically more sensitive to environmental influences than fine motor performance(34). Though we adjusted for prenatal nicotine exposure, the incidence of smoking during pregnancy in our sample population was higher than the national average. Given the increased incidence of motor tone abnormalities in 6 week old infants exposed to nicotine(53), the high rate of maternal smoking may have contributed to the motor findings observed at 1 year in this study.
We found no differences in the MA exposed group with performance on the Bayley exam at age 1, 2 and 3 years. However, long term follow-up of these MA exposed children is critical, because the Bayley assessment may not be able to ascertain subtle drug associated effects of higher order cognitive and executive functions at this young age. Studies in cocaine-exposed children have yielded conflicting findings with some reporting cocaine associated effects on the Bayley MDI(32;37;40) and attention(54),(25), whereas multiple longitudinal studies have found no differences in cognitive function up to the age of 3 years (36;55;56). A meta-analysis of studies controlling for risk factors such as poverty found subtle decreases in IQ scores, and receptive and expressive language skills in cocaine exposed children at school age (57). These effects, though subtle, have significant impact from a public health perspective based on the costs of special education services(57).
A limitation of this report is that the exposed group is primarily determined by self report. Because meconium production begins at 14-16 weeks' gestation, meconium testing primarily reflects maternal drug use during the second and third trimester(58). Thus, information regarding drug use in the first trimester was ascertained only by self report. However, given that data from a national surveillance study report usage patterns consistent with our findings(4), and only eight subjects were ascertained by GC/MS without also having self reported, the exposure group is likely reliable. Another limitation is that there was attrition over the course of the three year study period. However, analyses of selective attrition found no differences between included and excluded subjects on the prevalence of methamphetamine exposure including heavy MA use at any age studied.
5. Conclusion
In summary, we report no significant differences in cognitive function in MA exposed children relative to the comparison group. The MA exposed children had fine motor deficits at age 1 year that were no longer appreciated at age 3. Longitudinal follow-up is critical to determine if these drug associated effects lead to motor and/or cognitive impairments as the children progress to school age.
Acknowledgments
This study is part of the Infant Development, Environment and Lifestyle (IDEAL) Study, which was conducted with support from the National Institute on Drug Abuse (NIDA), R01DA021757 (Linda LaGasse, PhD.) and R01DA014948 (Barry Lester, Ph.D.) and in part by the National Center on Research Resources, Grant # 3 M01 RR00425 and P20 RR11091.
The authors thank Vince Smeriglio, Ph.D. for his commitment to drug abuse research and specifically for the vision he had to establish Community Research Partnerships (CRN). The CRN has facilitated the career development of many of the clinical investigators serving as coauthors on this manuscript. His honesty, integrity and humanity set an excellent example for those committing their career to drug abuse research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Substance Abuse and Mental Health Services Administration. Summary of findings from the 1999-2000 National Household Survey on Drug Abuse. 2002 http://www.samhsa.gov/oas/oasftp.htm.
- 2.Office Applied Studies. National Household Survey on Drug Abuse #18. 1995 [Google Scholar]
- 3.Office of Applied Studies. Results from the 2004 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2005. [Google Scholar]
- 4.Treatment Episode Data Set (TEDS) 1993-2003. National Admissions to Substance Abuse Treatment Services for the Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, MD: 2005. [Google Scholar]
- 5.O.o A.S Substance Abuse and Mental Health Services Administration. Treatment Episode Data Set (TEDS). Highlights - 2003. National Admissions to Substance Abuse Treatment Services; Rockville, MD: 2005. [Google Scholar]
- 6.Substance Abuse and Mental Health Services Admistrations (SAMHSA) Results from the 2008 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; 2008. [Google Scholar]
- 7.Slamberova R, Pometlova M, Syllabova L, Mancuskova M. Learning in the Place navigation task, not the New-learning task, is altered by prenatal methamphetamine exposure. Brain Res Dev Brain Res. 2005 Jun 30;157(2):217–9. doi: 10.1016/j.devbrainres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, Gudelsky GA, Vorhees CV. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Brain Res Dev Brain Res. 2003 Dec 30;147(1-2):163–75. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Jeng W, Wong AW, Ting AK, Wells PG. Methamphetamine-enhanced embryonic oxidative DNA damage and neurodevelopmental deficits. Free Radic Biol Med. 2005 Aug 1;39(3):317–26. doi: 10.1016/j.freeradbiomed.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Volkow N, Chang L, Wang G, Fowler J, Leonido-Yee M, Franceschi D, et al. Dopamine trasporter losses in methamphetamine abusers are associated with psychomotor impairment. Am J Psychiatry. 2001;158:377–82. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 11.Billing L, Eriksson M, Larsson G, Zetterstrom R. Amphetamine addiction and pregnancy. III. One year follow-up of the children. Psychosocial and pediatric aspects. Acta Paediatr Scand. 1980 Sep;69(5):675–80. doi: 10.1111/j.1651-2227.1980.tb07342.x. [DOI] [PubMed] [Google Scholar]
- 12.Billing L, Eriksson M, Steneroth G, Zetterstrom R. Predictive indicators for adjustment in 4-year-old children whose mothers used amphetamine during pregnancy. Child Abuse Negl. 1988;12(4):503–7. doi: 10.1016/0145-2134(88)90067-1. [DOI] [PubMed] [Google Scholar]
- 13.Billing L, Eriksson M, Jonsson B, Steneroth G, Zatterstrom R. The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse and Neglect. 1994;18:3–9. doi: 10.1016/0145-2134(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 14.Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterstrom R. Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatr. 1996 Feb;85(2):204–8. doi: 10.1111/j.1651-2227.1996.tb13993.x. [DOI] [PubMed] [Google Scholar]
- 15.Hansen RL, Struthers JM, Gospe SM., Jr Visual evoked potentials and visual processing in stimulant drug-exposed infants. Dev Med Child Neurol. 1993 Sep;35(9):798–805. doi: 10.1111/j.1469-8749.1993.tb11731.x. [DOI] [PubMed] [Google Scholar]
- 16.Smith LM, Chang L, Yonekura ML, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001 Jul 24;57(2):255–60. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- 17.Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004 Dec 15;132(2):95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008 Jan;30(1):20–8. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, et al. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006 Sep;118(3):1149–56. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen D, Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, et al. Intrautertine Growth of Infants Exposed to Prenatal Methamphetamine: Results from the Infant Development, Environment, and Lifestyle (IDEAL) Study. The Journal of Pediatrics. 2010 doi: 10.1016/j.jpeds.2010.04.024. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollingshead AB. Four factor index of social status. New Haven, CT: Department of Sociology, Yale University; 1975. [Google Scholar]
- 22.LaGasse L, Seifer R, Wright LL, Lester BM, Tronick EZ, Bauer CR, et al. The Maternal Lifestyle Study (MLS): The caretaking environment of infants exposed to cocaine/opiates. Pediatric Research. 1999;45:247A. [Google Scholar]
- 23.Della GS, LaGasse LL, Arria AM, Derauf C, Grant P, Smith LM, et al. Patterns of Methamphetamine Use During Pregnancy: Results from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Matern Child Health J. 2009 Jun 30; doi: 10.1007/s10995-009-0491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002 Dec;110(6):1182–92. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM. New evidence for neurobehavioral effects of in utero cocaine exposure. J Pediatr. 1996 Oct;129(4):581–90. doi: 10.1016/s0022-3476(96)70124-5. [DOI] [PubMed] [Google Scholar]
- 26.Dunn LM. Peabody picture vocabulary test. Minneapolis: American Guidance Service; 1959. [Google Scholar]
- 27.Caldwell BM, Bradley RH. Administration Manual (Revised Edition): Home Observation for Measurement of the Environment. Little Rock: University of Arkansas; 1984. [Google Scholar]
- 28.Bayley N. Bayley Scales of Infant Development, Second Edition. New York, NY: Psychological Corporation; 1993. [Google Scholar]
- 29.Bai DL, Bertenthal BI. Locomotor status and the development of spatial search skills. Child Dev. 1992 Feb;63(1):215–26. [PubMed] [Google Scholar]
- 30.Bushnell EW, Boudreau JP. Motor development and the mind: the potential role of motor abilities as a determinant of aspects of perceptual development. Child Dev. 1993 Aug;64(4):1005–21. [PubMed] [Google Scholar]
- 31.Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S, et al. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009 Nov 1;48(2):391–7. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J Pediatr. 1987 Oct;111(4):571–8. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- 33.Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterstrom R. Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatr. 1996 Feb;85(2):204–8. doi: 10.1111/j.1651-2227.1996.tb13993.x. [DOI] [PubMed] [Google Scholar]
- 34.Arendt R, Angelopoulos J, Salvator A, Singer L. Motor development of cocaine-exposed children at age two years. Pediatrics. 1999 Jan;103(1):86–92. doi: 10.1542/peds.103.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilbride H, Castor C, Hoffman E, Fuger KL. Thirty-six-month outcome of prenatal cocaine exposure for term or near-term infants: impact of early case management. J Dev Behav Pediatr. 2000 Feb;21(1):19–26. doi: 10.1097/00004703-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Messinger DS, Bauer CR, Das A, Seifer R, Lester BM, LaGasse LL, et al. The maternal lifestyle study: cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics. 2004 Jun;113(6):1677–85. doi: 10.1542/peds.113.6.1677. [DOI] [PubMed] [Google Scholar]
- 37.Mayes LC, Bornstein MH, Chawarska K, Granger RH. Information processing and developmental assessments in 3-month-old infants exposed prenatally to cocaine. Pediatrics. 1995 Apr;95(4):539–45. [PubMed] [Google Scholar]
- 38.Singer L, Arendt R, Song LY, Warshawsky E, Kliegman R. Direct and indirect interactions of cocaine with childbirth outcomes. Arch Pediatr Adolesc Med. 1994 Sep;148(9):959–64. doi: 10.1001/archpedi.1994.02170090073014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fetters L, Tronick EZ. Neuromotor development of cocaine-exposed and control infants from birth through 15 months: poor and poorer performance. Pediatrics. 1996 Nov;98(5):938–43. [PubMed] [Google Scholar]
- 40.Schneider JW, Chasnoff IJ. Motor assessment of cocaine/polydrug exposed infants at age 4 months. Neurotoxicol Teratol. 1992 Mar;14(2):97–101. doi: 10.1016/0892-0362(92)90057-h. [DOI] [PubMed] [Google Scholar]
- 41.Miller-Loncar C, Lester BM, Seifer R, LaGasse LL, Bauer CR, Shankaran S, et al. Predictors of motor development in children prenatally exposed to cocaine. Neurotoxicol Teratol. 2005 Mar;27(2):213–20. doi: 10.1016/j.ntt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Fuller R, Hemrick-Luecke S. Further studies on the long-term depletion of striatal dopamine in iprindole-treated rats by amphetamine. Neuropharmacology. 1992;21(5):433–8. doi: 10.1016/0028-3908(82)90027-2. [DOI] [PubMed] [Google Scholar]
- 43.Pu C, Vorhees CV. Developmental dissociation of methamphetamine-induced depletion of dopaminergic terminals and astrocyte reaction in rat striatum. Brain Res Dev Brain Res. 1993 Apr 16;72(2):325–8. doi: 10.1016/0165-3806(93)90201-k. [DOI] [PubMed] [Google Scholar]
- 44.Cabrera TM, Levy AD, Li Q, Van de Kar LD, Battaglia G. Prenatal methamphetamine attenuates serotonin mediated renin secretion in male and female rat progeny: evidence for selective long-term dysfunction of serotonin pathways in brain. Synapse. 1993 Nov;15(3):198–208. doi: 10.1002/syn.890150305. [DOI] [PubMed] [Google Scholar]
- 45.Weissman AD, Caldecott-Hazard S. Developmental neurotoxicity to methamphetamines. Clin Exp Pharmacol Physiol. 1995 May;22(5):372–4. doi: 10.1111/j.1440-1681.1995.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 46.Slamberova R, Pometlova M, Charousova P. Postnatal development of rat pups is altered by prenatal methamphetamine exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2006 Jan;30(1):82–8. doi: 10.1016/j.pnpbp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Slamberova R, Pometlova M, Rokyta R. Effect of methamphetamine exposure during prenatal and preweaning periods lasts for generations in rats. Dev Psychobiol. 2007 Apr;49(3):312–22. doi: 10.1002/dev.20203. [DOI] [PubMed] [Google Scholar]
- 48.Acuff-Smith KD, Schilling MA, Fisher JE, Vorhees CV. Stage-specific effects of prenatal d-methamphetamine exposure on behavioral and eye development in rats. Neurotoxicol Teratol. 1996 Mar;18(2):199–215. doi: 10.1016/0892-0362(95)02015-2. [DOI] [PubMed] [Google Scholar]
- 49.Cho DH, Lyu HM, Lee HB, Kim PY, Chin K. Behavioral teratogenicity of methamphetamine. J Toxicol Sci. 1991 Feb;16 1:37–49. doi: 10.2131/jts.16.supplementi_37. [DOI] [PubMed] [Google Scholar]
- 50.Jeng W, Wong AW, Ting AK, Wells PG. Methamphetamine-enhanced embryonic oxidative DNA damage and neurodevelopmental deficits. Free Radic Biol Med. 2005 Aug 1;39(3):317–26. doi: 10.1016/j.freeradbiomed.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Hruba L, Schutova B, Slamberova R, Pometlova M, Rokyta R. Effect of methamphetamine exposure and cross-fostering on sensorimotor development of male and female rat pups. Dev Psychobiol. 2009 Jan;51(1):73–83. doi: 10.1002/dev.20346. [DOI] [PubMed] [Google Scholar]
- 52.Mayes LC, Cicchetti D, Acharyya S, Zhang H. Developmental trajectories of cocaine-and-other-drug-exposed and non-cocaine-exposed children. J Dev Behav Pediatr. 2003 Oct;24(5):323–35. doi: 10.1097/00004703-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Dempsey DA, Hajnal BL, Partridge JC, Jacobson SN, Good W, Jones RT, et al. Tone abnormalities are associated with maternal cigarette smoking during pregnancy in in utero cocaine-exposed infants. Pediatrics. 2000 Jul;106(1 Pt 1):79–85. doi: 10.1542/peds.106.1.79. [DOI] [PubMed] [Google Scholar]
- 54.Struthers JM, Hansen RL. Visual recognition memory in drug-exposed infants. J Dev Behav Pediatr. 1992 Apr;13(2):108–11. doi: 10.1097/00004703-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Alessandri SM, Bendersky M, Lewis M. Cognitive functioning in 8- to 18-month-old drug-exposed infants. Dev Psychol. 1998 May;34(3):565–73. doi: 10.1037//0012-1649.34.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frank DA, Jacobs RR, Beeghly M, Augustyn M, Bellinger D, Cabral H, et al. Level of prenatal cocaine exposure and scores on the Bayley Scales of Infant Development: modifying effects of caregiver, early intervention, and birth weight. Pediatrics. 2002 Dec;110(6):1143–52. doi: 10.1542/peds.110.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lester BM, LaGasse LL, Seifer R. Cocaine exposure and children: the meaning of subtle effects. Science. 1998 Oct 23;282(5389):633–4. doi: 10.1126/science.282.5389.633. [DOI] [PubMed] [Google Scholar]
- 58.Bar-Oz B, Klein J, Karaskov T, Koren G. Comparison of meconium and neonatal hair analysis for detection of gestational exposure to drugs of abuse. Arch Dis Child Fetal Neonatal Ed. 2005 Mar;88(2):F98–F100. doi: 10.1136/fn.88.2.F98. [DOI] [PMC free article] [PubMed] [Google Scholar]