Abstract
Potential treatments for ovarian cancers that have become resistant to standard chemotherapies include modulators of tumor cell survival, such as endothelin receptor (ETR) antagonist. We investigated the therapeutic efficacy of the dual ETR antagonist, macitentan, on human ovarian cancer cells, SKOV3ip1 and IGROV1, growing orthotopically in nude mice. Mice with established disease were treated with vehicle (control), paclitaxel (weekly, intraperitoneal injections), macitentan (daily oral administrations), or a combination of paclitaxel and macitentan. Treatment with paclitaxel decreased tumor weight and volume of ascites. Combination therapy with macitentan and paclitaxel reduced tumor incidence and further reduced tumor weight and volume of ascites when compared with paclitaxel alone. Macitentan alone occasionally reduced tumor weight but alone had no effect on tumor incidence or ascites. Immunohistochemical analyses revealed that treatment with macitentan and macitentan plus paclitaxel inhibited the phosphorylation of ETRs and suppressed the survival pathways of tumor cells by decreasing the levels of pVEGFR2, pAkt, and pMAPK. The dose of macitentan necessary for inhibition of phosphorylation correlated with the dose required to increase antitumor efficacy of paclitaxel. Treatment with macitentan enhanced the cytotoxicity mediated by paclitaxel as measured by the degree of apoptosis in tumor cells and tumor-associated endothelial cells. Collectively, these results show that administration of macitentan in combination with paclitaxel prevents the progression of ovarian cancer in the peritoneal cavity of nude mice in part by inhibiting survival pathways of both tumor cells and tumor-associated endothelial cells.
Introduction
In 2009, ovarian cancer was the leading cause of death from gynecologic cancer in the United States [1]. Despite initial response rates that can exceed 80% [2,3], most patients with advanced ovarian cancer ultimately relapse with drug-resistant disease [4,5]. Because the response rate to second-line agents is approximately 15% to 20% [6], new therapeutic regimens are urgently needed for this devastating cancer. Recent approaches to overcoming tumor resistance to chemotherapy include modulation of cell signaling pathways involved in tumor cell growth and survival and of the interaction of tumor cells with the organ microenvironment [7,8].
One intriguing therapeutic possibility is based on antagonists of the endothelin (ET) family of small peptides consisting of ET-1, -2, and -3 [9,10]. ETs share structural homology and initiate signaling by binding to G protein-coupled receptors ETAR and ETBR [11–14]. ETs, initially defined as potent vasoconstrictors and non-peptidic small molecule ET receptor (ETR) antagonists [14,15], were developed to treat cardiovascular diseases. For example, the dual ETAR and ETBR inhibitor, bosentan, is now used to treat pulmonary arterial hypertension [15–17].
It is important to note that ETs, beyond inducing vasoconstriction, act as paracrine or autocrine tissue factors to regulate biologic processes such as tissue remodeling and repair [18], smooth muscle cell proliferation [19], and inflammation [20]. ETs and their receptors are expressed in many tumor types [21] and are involved in tumor cell proliferation, migration, invasiveness, and vascular differentiation [21–25]. In addition, activated ETRs have also been reported to be involved in inhibition of apoptosis, matrix remodeling, and bone deposition [10,11,13,14,16,21,26], in prostate cancer [27,28], lung cancer [29,30], colon cancer [31,32], renal cancer [33], cervical cancer [34,35], brain tumors [36–38], ovarian cancer [25,39–48], and other tumors [49,50]. ET production has been demonstrated in many human tumor cell lines and human tumors. In the tumor vasculature, the endothelium is the main source of ET [49–51]. In most carcinoma cells, the dominating receptor is the ETAR receptor [50], whereas in melanoma cells and glioblastoma cells, the ETBR receptor is highly expressed [50,52]. Vascular endothelial cells express high levels of ETBR receptors [53], and ETBR receptor signaling has been associated with endothelial cell proliferation, migration, differentiation, and vascular endothelial growth factor (VEGF) induction [43,54–57].
The ET axis in ovarian cancer has been broadly studied [25,44–46,58]. Examination of clinical specimens revealed increased expression of ET-1 and ETAR in ovarian cancer cells, indicating their involvement in an autocrine loop [44]. In another study using primary and metastatic ovarian carcinomas, ETAR receptors were localized to carcinoma cells and intratumoral vessels, whereas ETBR were mainly found in the endothelial cells. VEGF production from cancer cells was found to be stimulated by ET in vitro through induction of hypoxia-inducible factor-1α [59], leading to VEGF-mediated neovascularization in vivo [44,54,56]. The level of ET-1 is also increased in peritoneal ascitic fluid of patients with ovarian carcinoma, suggesting a correlation with the level of VEGF, whereas effusions from patients without cytologic disease had low or undetectable levels of ET-1 [43]. Collectively, these data indicate that the expression of ET-1 and ETAR correlates with advanced stages of the disease [24,58,60].
In the present study, we determined whether the daily oral administration of the dual ETR receptor antagonist macitentan [61], combined with once-weekly intraperitoneal injections of paclitaxel, produced significant therapeutic effects in intraperitoneally transplanted human ovarian tumor xenografts in nude mice. Therapeutic effects were correlated with induction of apoptosis in both tumor cells and tumor-associated endothelial cells and the inhibition of phosphorylation of proteins involved in signal transduction and cell survival.
Materials and Methods
Human Ovarian Cancer Cell Lines
Highly metastatic human ovarian cancer cell lines, SKOV3ip1 [61] and IGROV1 [62, 63], were maintained as monolayer cultures in Eagle minimal essential medium supplemented with 10% fetal bovine serum (Life Technologies, Inc, Grand Island, NY), l-glutamine, pyruvate, non-essential amino acids, two-fold vitamins, and penicillin-streptomycin (Invitrogen, Carlsbad, CA) and incubated at 37°C in 5% CO2 and 95% air. Immorto mouse lung endothelial cells were incubated at 33°C [64]. All reagents used for tissue culture were free of endotoxin, Mycoplasma, and viral pathogens, reovirus type 3; pneumonia virus; K virus; Theiler's encephalitis virus; Sendai virus; min virus; mouse adenovirus; mouse hepatitis virus; lymphocytic choriomeningitis virus; ectromelia virus; and lactate dehydrogenase virus (assayed by M. A. Bioproducts, Walkersville, MD).
Reagents
Macitentan, also called ACT-064992 or (N-[5-(4-bromophenyl)-6-(2-(5-bromopyrimidin-2-yloxy)ethoxy)pyrimidin-4-yl]-N′-propylaminosulfonamide), was provided by Actelion Pharmaceuticals, Ltd (Allschwil, Switzerland) as powder. For oral administration, macitentan was reconstituted in 0.05% (wt/wt) methylcellulose solution containing 0.05% (vol/vol) Tween 80 and diluted to different concentrations in 200 µl of vehicle before use. Paclitaxel (Taxol), purchased from Bristol-Myers Squibb (Princeton, NJ), was diluted in distilled water for intraperitoneal injection.
Animals
Female athymic nude mice (NCI-nu) were purchased from the Animal Production Area of the National Cancer Institute — Frederick Cancer Research Facility (Frederick, MD). The mice were housed and maintained in specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with all current regulations and standards of the US Department of Agriculture, the US Department of Health and Human Services, and the National Institutes of Health. The mice were used in these experiments in accordance with institutional guidelines when they were 8 to 12 weeks old.
Orthotopic Implantation of Ovarian Cancer in Animal Models
To produce tumors, SKOV3ip1 and IGROV1 cells were harvested from subconfluent cultures by a brief exposure to 0.25% trypsin and 0.02% EDTA. Trypsinization was stopped by replacing the trypsin-EDTA with medium containing 10% fetal bovine serum, and the cells were washed once in serum-free medium and resuspended in Ca2+-/Mg2+-free Hank's balanced salt solution. Cell viability was determined by trypan blue exclusion, and only single-cell suspensions of more than 95% viability were used for injection. In a preliminary experiment, 1 x 106 cells in 200 µl of Ca2+-/Mg2+-free Hank's balanced salt solution were injected into the peritoneal cavity of female nude mice. Ten days after the injection, three mice were randomly selected and examined by necropsy to confirm development of tumors (Figure W1).
Therapy Experiments
To induce peritoneal tumors, SKOV3ip1 (1 x 106) or IGROV1 (1 x 106) cells were injected into the peritoneal cavity of female nude mice. Ten days later, the mice were randomized into treatment groups (n = 10). In the first set of experiments, we investigated the therapeutic effect of macitentan. Mice were implanted with SKOV3ip1 and randomized into the following treatment groups: 1) daily oral administration and weekly intraperitoneal injection of vehicle (control), 2) weekly intraperitoneal injections of paclitaxel (5 mg/kg) and daily oral administration of vehicle, 3) daily oral administration of macitentan (100 mg/kg) and weekly intraperitoneal injection of vehicle, and 4) daily oral administration of macitentan (100 mg/kg) and weekly intraperitoneal injection of paclitaxel (5 mg/kg). These treatments were continued for 4 weeks.
In this first set of experiments, the therapeutic effect of macitentan was confirmed, but serious toxicities, such as weight loss, poor skin turgor, dehydration, diarrhea, and sluggishness, were observed. Therefore, in the second set of experiments, we determined the biologic optimal dose of macitentan. Ten days after the intraperitoneal implantation of SKOV3ip1 (1 x 106), mice were randomized into five groups (n = 10): 1) daily oral administration and weekly intraperitoneal injection of vehicle (control), 2) weekly intraperitoneal injection of paclitaxel (5 mg/kg) and daily oral administration of vehicle, 3) weekly intraperitoneal injection of paclitaxel (5 mg/kg) and daily oral administration of macitentan (30 mg/kg), 4) weekly intraperitoneal injection of paclitaxel (5 mg/kg) and daily oral administration of macitentan (10 mg/kg), and 5) weekly intraperitoneal injection of paclitaxel (5 mg/kg) and daily oral administration of macitentan (3 mg/kg).
In the third set of experiments, we determined whether blockade of the ET axis with the ET antagonist macitentan could increase the sensitivity of cancer cells to lower doses of paclitaxel. Ten days after the intraperitoneal implantation of SKOV3ip1 (1 x 106), mice were randomized into six groups (n = 10): 1) daily oral administration and weekly intraperitoneal injections of vehicle (control), 2) weekly intraperitoneal injections of nontherapeutic dose of paclitaxel (2 mg/kg) and daily oral administration of vehicle, 3) weekly intraperitoneal injections of therapeutic dose of paclitaxel (5 mg/kg) and daily oral administration of vehicle, 4) daily oral administration of macitentan (50 mg/kg) and weekly intraperitoneal injections of vehicle, 5) daily oral administration of macitentan (50 mg/kg) and weekly intraperitoneal injections of nontherapeutic dose of paclitaxel (2 mg/kg), and 6) daily oral administration of macitentan (50 mg/kg) and weekly intraperitoneal injections of therapeutic dose of paclitaxel (5 mg/kg). These treatments were continued for 4 weeks.
From these three sets of experiments, we concluded that 50 mg/kg of macitentan is a biologically optimal dose, and so further therapy experiments were repeated with the 50-mg/kg dose. To determine whether therapeutic effects were not limited to SKOV3ip1, we did experiments using an additional human ovarian cancer cell line, IGROV1.
Necropsy Procedures and Preparation of Tissues
After 4 weeks of treatment, mice were injected in the tail vein with 100 µl of BrdU (Sigma; 25 mg/ml). Two hours later, the mice were killed by intramuscular injection of Nembutal (1 g/kg) and examined by necropsy. Tumor incidence, tumor weight, and volume of ascites were recorded (Figure W2). Tumor tissues were embedded in OCT compound (Miles, Inc, Elkhart, IN) and rapidly frozen in liquid nitrogen or fixed in 10% buffered formalin for 24 hours and processed for paraffin block.
Immunohistochemical Analyses and TUNEL Assay
Primary antibodies used in this study were: mouse anti-BrdU monoclonal antibody (1:400; Becton Dickinson, San Jose, CA), rat antimouse CD31 monoclonal antibody (1:600; BD Pharmingen, San Diego, CA), goat antihuman ET-1 polyclonal antibody (1:200; Santa Cruz Biotechnology, Inc, Santa Cruz, CA), goat antihuman ET-2 polyclonal antibody (1:200; Santa Cruz Biotechnology, Inc), goat antihuman ETAR polyclonal antibody (1:200, immunohistochemistry experiments; Santa Cruz Biotechnology, Inc), rabbit anti-ETAR (1:200, immunohistochemistry experiments; Acris, Herford, Germany), goat antihuman ETBR polyclonal antibody (1:200, immunofluorescence experiments; Santa Cruz Biotechnology, Inc), rabbit anti-ETBR (1:200, immunohistochemistry experiments; Acris), rabbit antimouse phosphorylated Akt monoclonal antibody (1:200; Cell Signaling Technology, Boston, MA), rabbit antihuman phosphorylated-p44/42 MAP kinase (Thr202/Tyr204) polyclonal antibody (1:200; Cell Signaling Technology), and rabbit antihuman phosphorylated VEGF receptor 2 (VEGFR2) monoclonal antibody (1:200; Cell Signaling Technology). HRP-conjugated donkey antigoat IgG (1:400; Santa Cruz Biotechnology), goat antirat Alexa 594 IgG (1:400; Invitrogen), rabbit antigoat Alexa 488 IgG (1:400; Invitrogen), rabbit antigoat FITC IgG (1:400; Jackson Immunoresearch Laboratories, West Grove, PA), and goat antirabbit Alexa 488 IgG (1:400; Invitrogen) were purchased for use as secondary antibodies. TUNEL assay was performed using a commercial apoptosis detection kit (Promega Corp, Madison, WI) with modification [9]. For color reaction of HRP-conjugated secondary antibodies, stable 3′,3-diaminobenzidine (Research Genetics, Huntsville, AL) was used.
Immunofluorescence Staining
To determine the expression of ET-1, ET-2, ETAR, ETBR, phosphorylated VEGFR2 (pVEGFR2), phosphorylated Akt (pAkt), and phosphorylated MAP kinase (pMAPK) on tumor cells and/or tumor-associated endothelial cells, tissues were costained with anti-CD31 antibody and anti-ET-1, anti-ET-2, anti-ETAR, anti-ETBR, anti-pVEGFR2, or anti-pAkt antibodies. In brief, frozen slides were air-dried and fixed in acetone. After washing with phosphate-buffered saline (PBS), blocking for nonspecific protein was done with 4% fish gel. The slides were incubated with primary antibodies for 18 hours, washed with PBS three times, and incubated with compatible secondary antibodies for 2 hours at room temperature. During incubation, the slides were shielded from ambient light. For examination of the endothelium, colocalization staining with CD31 was performed followed by staining with the second primary antibodies as described previously [8].
Proliferative and apoptotic indices of tumor-associated endothelial cells as well as tumor cells were determined by colocalization of CD31 and anti-BrdU or TUNEL staining. Images were captured by an Olympus BX-51 microscope (Olympus America, Inc, Center Valley, PA).
Double Immunofluorescence Staining for ETRs and Phospho-Serine in Tumor Tissues
Because specific antibodies to detect activated, serine/threonine-phosphorylated ETRs were not available, we used double immunofluorescence staining with anti-ETAR or ETBR and anti-phospho-serine antibodies. Tissue sections (6–8 µm) of frozen samples were mounted on positively charged slides and air-dried for 30 minutes. The sections were fixed in cold acetone for 10 minutes, washed three times with PBS for 3 minutes, and incubated with protein blocking solution containing 5% normal horse serum and 1% normal goat serum in PBS for 20 minutes at room temperature. The slides were incubated with a primary antibody against ETAR (1:100, 12977; Abcam, Cambridge, MA) or ETBR (1:100, 65972; Abcam) at 4°C overnight, washed three times with PBS, and then incubated with AlexaFluor 594 goat anti-rabbit secondary antibody (1:600 dilution, A11037; Invitrogen). The slides were then incubated with the blocking solution at room temperature for 30 minutes and then incubated at 4°C overnight with primary antibody against phospho-serine (1:100, sc-81514; Santa Cruz Biotechnology). The slides were then washed three times with PBS for 3 minutes and incubated at room temperature for 1 hour with Alexa Fluor 488 goat antimouse secondary antibody (1:600 dilutions, A11029; Invitrogen). All slides were rinsed, incubated with Hoechst 33342 (H3570; Invitrogen) to visualize nuclei, and then mounted with a glycerol/PBS solution containing 0.1 M propyl gallate to minimize fluorescent bleaching. All images were captured with an Olympus microscope (BX-51) with an attached DP71 digital camera and processed with DP Controller and DP Manager software (Olympus).
Quantification of Proliferating Cells and Apoptotic Cells
To quantify proliferating cells and apoptotic cells, BrdU-positive cells or TUNEL-positive cells in 10 random 0.159-mm2 fields of the tumors were counted at 100x magnification as described previously [8].
Radioligand Binding Assay
Radioligand binding assays on cell lines were performed by diluting cells in Dulbecco modified Eagle medium, 25 mM HEPES, pH 7.4, and 0.1% bovine serum albumin (Sigma). Cells were then incubated with 125I-labeled ET-1 in the presence or absence of competing ligands on ice for 4 hours in a total volume of 250 µl in 96-well microtiter plates. BQ-123 (10 µM final concentration) (Sigma) and Sarafotoxin 6c (1 µM final concentration; Sigma) were used as competitive ligands for ETAR- and ETBR-specific binding, respectively. The bound ligand was separated from free ligand by filtration through GF/C filters pre-soaked with binding buffer by cell harvester (Filtermate; Packard Bioscience, Meriden, CT). After the addition of ReadySafe (Beckman, Fullerton, CA) scintillation fluid, bound radioactivity was quantitated using the TopCountNXT (Packard).
Radioligand binding assays were performed on tumor sections by equilibrating slides to room temperature before blocking twice for 10 minutes each in assay buffer (50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 5% skimmed milk). Slides were then briefly dipped in distilled water and dried under a stream of cold air for 2 minutes. Sections were incubated for 4 hours at room temperature with 100 pM 125I-ET-1 in assay in the presence or absence of competitive ligands. BQ-123 (50 µM final; Sigma) and Sarafotoxin 6c (10 µM final concentration; Sigma) were used as competitive ligands for ETAR- and ETBR-specific binding, respectively. After incubation, sections were washed four times for 2 minutes in ice-cold wash buffer (50 mM Tris-HCl, pH 7.5, 5 mM MgCl2), briefly dipped in distilled water at 4°C, and dried under a stream of cold air. Sections were exposed to BAS2500 imaging plate (Fuji Photo Film, Kanazawa, Japan) overnight and then Kodak Biomax MS film (Rochester, NY) for 2 days.
ET Secretion
ET levels were quantified using the QuantiGlo ET-1 Immunoassay (R&D Systems, Abingdon, UK) according to the manufacturer's instructions. For quantification of ET produced by cell lines, 50,000 cells were seeded per well of a 24-well plate and ET levels measured 48 hours later.
Statistical Analyses
Tumor incidence, the incidence of ascites (χ2 test), tumor weight, ascites volume (Mann-Whitney t test), and the number of BrdU-positive and the number of TUNEL-positive cells (unpaired Student t test) were compared across the treatment groups.
Results
ETR and ET Expression in Ovarian Cancer Cell Lines
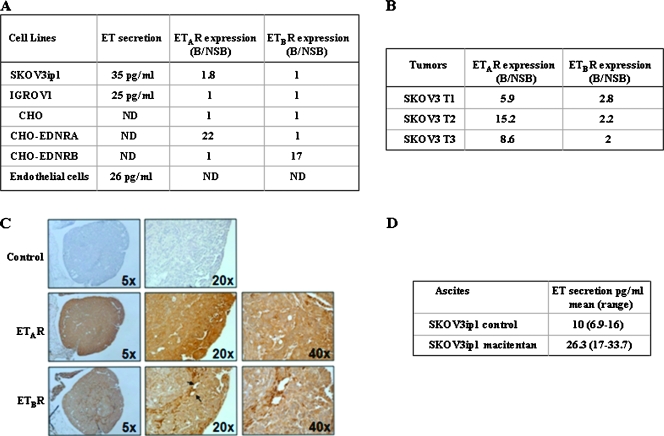
We first assessed the expression level of ETAR and ETBR and the level of secreted ETs in two ovarian cancer cell lines, SKOV3ip1 and IGROV1. In both an ETAR- and an ETBR-specific radioligand assay, ETBR was not expressed at the cell surface of either cell line (Figure 1A). In contrast, ETAR was weakly expressed in SKOV3ip1 cells (ratio of specific to nonspecific binding, 1.8) but absent in IGROV1. CHO cells overexpressing ETAR or ETBR were used as a positive control (ratio of specific binding to nonspecific binding, 17 to 22), and CHO mock-transfected cells were used as a negative control. Next, we determined the level of ETR expression in SKOV3ip1 tumors growing in the peritoneal cavity of nude mice using ETAR- and ETBR-specific radioligand assays (Figure 1B). ETAR was highly expressed in the three tumors (ratio of specific to nonspecific binding, 5.9 to 15.2). ETBR was also expressed in tumors although at a lower level (ratio of specific to nonspecific binding, 2 to 2.8). The expression of the ETRs in SKOV3ip1 tumors was also investigated using immunohistochemistry (Figure 1C). Both receptors were detected in the cytoplasm and/or plasma membranes of tumor cells but not in the nucleus. Consistent with the radioligand assay, both tumor cells and endothelial cells stained positive for ETAR. In contrast, expression of ETBR was weak and heterogeneous in tumor cells and more pronounced in tumor-associated endothelial cells and possibly in infiltrating leukocytes [65]. ETAR and ETBR were also found to be expressed in tumors derived from IGROV1 cells growing in the peritoneal cavity of nude mice as determined by immunofluorescence (data not shown). Collectively, these results indicate that the expression of ETRs was stronger in ovarian cancer cells growing in vivo than in vitro.
Figure 1.
Expression level of ETAR, ETBR, and secreted ETs by SKOV3ip1 and IGROV1 cells growing in vitro and in vivo. (A) ET secretion was determined by ELISA in culture supernatant of SKOV3ip1, IGROV1, and mouse lung endothelial cells in vitro 48 hours after seeding. ETAR and ETBR expression was determined using a radioligand binding method. Nonspecific binding (NSB) was determined by incubating cells with BQ-123 or Sarafotoxin 6c. The ratio binding-B/NSB indicates the specific binding. ETAR and ETBR expression was measured by radioligand binding (B) and immunohistochemistry (C) in SKOV3ip1 tumors growing in the peritoneal cavity of nude mice (arrows indicating endothelial cells). (D) ET secretion was measured by ELISA in ascites from three control mice and three mice injected intraperitoneally with SKOV3ip1 cells and treated with macitentan (50 mg/kg).
We next determined the level of ET secretion by the cell lines growing in vitro. As shown in Figure 1A, SKOV3ip1 and IGROV1 cells expressed levels of ET (35 and 25 pg/ml, respectively) that were similar to those measured in immorto mouse lung endothelial cells (26 pg/ml). The level of ET secretion in SKOV3ip1 tumors was determined by measuring the level of ETs in ascitic fluids (Figure 1D). ETs could be detected in ascites of the three mice tested with levels ranging from 6.9 to 16 pg/ml.
Therapy for SKOV3ip1 and IGORV1 with Macitentan and Paclitaxel
In the first set of therapy experiments, treatment of SKOV3ip1 with paclitaxel significantly decreased tumor weight as compared with the control group (Table 1A; median [range]: 0.4 g [0.1–0.5 g] vs 1.1 g [0–1.8 g], P < .05). Tumor weight in mice treated with only macitentan was not reduced. The combination of paclitaxel and macitentan further decreased tumor weight (0.1 g [0–0.3 g], P < .01). Treatment with a combination of paclitaxel and macitentan also significantly decreased tumor incidence as compared with the control group (5/9 vs 8/10, P < .05). The incidence and volume of ascites were significantly decreased by the treatment with paclitaxel (4/9 vs 8/10, P < .05; and median [range] of 0.4 ml [0–0.9 ml] vs 0.1 ml [0–0.2 ml], P < .01, respectively) and the combination therapy completely inhibited development of ascites (0/9 vs 8/10, P < .01). The combination of macitentan with paclitaxel significantly enhanced the therapeutic efficacy of paclitaxel as measured by tumor weight (0.4 g [0.1–0.5 g] vs 0.1 g [0–0.3 g], P < .01), tumor incidence (9/9 vs 5/9, P < .05), and incidence of ascites (4/9 vs 0.9, P < .05).
Table 1.
Treatment of Human Ovarian Cancers SKOV3ip1 (A) and IGROV1 (B) Growing in the Peritoneal Cavity of Female Nude Mice with Macitentan and Paclitaxel.
| (A) | ||||
| Treatment Group | Tumor Incidence | Tumor Weight, Median (Range), g | Ascite Incidence | Ascites, Median (Range), ml |
| Control | 8/10 | 1.1 (0–1.8) | 8/10 | 0.4 (0–0.9) |
| Paclitaxel, 5 mg/kg | 9/9 | 0.4 (0.1–0.5)* | 4/9* | 0.1 (0–0.2)† |
| Macitentan, 100 mg/kg | 7/10 | 2.3 (0–4.6) | 7/10 | 0.4 (0–4.7) |
| Paclitaxel + macitentan | 5/9*,‡ | 0.1 (0–0.3)†,‡ | 0/9†,‡ | 0† |
| (B) | ||
| Treatment Group | Tumor Incidence | Tumor Weight, Median (Range), g |
| Control | 10/10 | 1.1 (0.4–2.1) |
| Paclitaxel, 5 mg/kg | 9/10 | 0.5 (0–0.9)* |
| Macitentan, 50 mg/kg | 10/10 | 0.7 (0.4–1.3) |
| Paclitaxel + macitentan | 9/9 | 0.3 (0–0.6)†,‡ |
Mice were injected intraperitoneally with 1 x 106 SKOV3ip1 or IGROV1 cells. Ten days later, treatment began with vehicle, paclitaxel, macitentan, or a combination of macitentan and paclitaxel. Treatment continued for 4 weeks when mice were examined by necropsy. Tumor incidence, weight, incidence, and volume of ascites were recorded.
Statistically significant compared with the control group, P < .05.
Statistically significant compared with the control group, P < .01.
Statistically significant compared with the paclitaxel group, P < .05.
In a repeated therapy experiment using 50 mg/kg macitentan, similar therapeutic effects were found in mice treated with the combination of paclitaxel and macitentan (data not shown).
In mice bearing IGROV1 tumors in the peritoneal cavity, treatment with paclitaxel significantly decreased tumor weight as compared with control mice (0.5 g [0–0.9 g] vs 1.1 g [0.4–2.1 g], P < .05; Table 1B). Treatment with macitentan alone did not produce a significant therapeutic effect. The combination of macitentan (50 mg/kg) and paclitaxel (5 mg/kg) further decreased the weight of the tumors (0.3 g [0–0.6 g] vs 1.1 g [0.4–2.1 g], P < .01; Table 1B). Macitentan combined with paclitaxel significantly enhanced the therapeutic efficacy of paclitaxel as measured by tumor weight (0.5 g [0–0.9 g] vs 0.3 g [0–0.6 g], P < .05). Mice treated with macitentan at 100 mg/kg exhibited poor skin turgor, dehydration, diarrhea, and sluggishness. For this reason, in the next set of experiments, we determined the biologically optimal dose of macitentan. Treatment with paclitaxel (5 mg/kg) and 3 or 10 mg/kg macitentan did not produce enhanced therapeutic effects (data not shown). In contrast, treatment with 5 mg/kg paclitaxel combined with 30 mg of macitentan significantly reduced tumor weight but not tumor incidence (data not shown). For these reasons, all future experiments used a macitentan dose of 50 mg/kg administered daily to treat SKOV3ip1 and IGROV.
Sensitization or Ovarian Cancer Cells to Paclitaxel with Macitentan
Mice were implanted with 1 x 106 SKOV3ip1 cells, and 10 days later when tumors were established, treatment began either with non-therapeutic (2 mg/kg) or therapeutic (5 mg/kg) doses of paclitaxel (Table 2). Nontherapeutic doses of paclitaxel (2 mg/kg) did not decrease the weight of the tumors (2.5 g [1.2–3.3 g]) compared with the control group (1.9 g [0.5–4.8 g], P > .05). However, the combination of macitentan (50 mg/kg) with a nontherapeutic dose of paclitaxel (2 mg/kg) significantly decreased the tumor weight (0.3 g [0–0.7 g], P < .001) compared with control mice or mice treated with only a nontherapeutic dose of paclitaxel (2.5 g [1.2–3.3 g], P < .001; Table 2). Tumor incidence (5/10 vs 10/10) and incidence of ascites (4/10 vs 10/10) were also significantly decreased (P < .05) implying that administration of macitentan at 50 mg/kg increases the sensitivity of tumor cells to paclitaxel. Interestingly, whereas treatment with 100 mg/kg of macitentan as a single agent did not show therapeutic benefits (Table 1), mice treated with a nontoxic dose of 50 mg/kg macitentan as a single agent had a decrease in tumor weight (0.6 g [0–1.1 g] vs 1.9 g [0.5–4.8 g], P < .05). The full therapeutic efficacy in this study, however, was produced by combining 5 mg/kg paclitaxel with 50 mg/kg macitentan (0.1 g [0–0.5 g], P < .001).
Table 2.
Treatment ofHuman Ovarian Cancer, SKOV3ip1, with Macitentan and PaclitaxelGrowing in the Peritoneal Cavity of Female Nude Mice.
| Treatment Group | Tumor Incidence | Tumor Weight, Median (Range), g | Ascite Incidence |
| Vehicle | 10/10 | 1.9 (0.5–4.8) | 10/10 |
| Paclitaxel, 2 mg/kg | 10/10 | 2.5 (1.2–3.3) | 10/10 |
| Paclitaxel, 5 mg/kg | 10/10 | 0.4 (0.2–1.3)* | 5/10† |
| Macitentan, 50 mg/kg | 9/10 | 0.6 (0–1.1)† | 7/10 |
| Paclitaxel, 2 mg/kg + macitentan | 5/10†,‡ | 0.3 (0–0.7)†,§ | 4/10†,‡ |
| Paclitaxel, 5 mg/kg + macitentan | 5/10†,‡ | 0.05 (0–0.5)*,§ | 0/10†,‡ |
Mice were injected intraperitoneally with 1 x 106 SKOV3ip1 cells. Ten days later, treatment with vehicle, paclitaxel, macitentan, or macitentan and paclitaxel began and continued for 4 weeks. At the end of the experiments, the mice were examined by necropsy. Tumor incidence, weight, and incidence and volume of ascites were recorded.
Statistically significant compared with the control group, P < .01.
Statistically significant compared with the control group, P < .05.
Statistically significant compared with the 2-mg/kg paclitaxel-treated group and 5-mg/kg paclitaxel-treated group, respectively, P < .05.
Statistically significant compared with the 2-mg/kg paclitaxel-treated group and 5-mg/kg paclitaxel-treated group, respectively, P < .01.
From these three sets of experiments, we conclude that 50 mg/kg macitentan administered once a day is the biologic optimal dose, and less than 10 mg/kg is an ineffective dose in the SKOV3ip1 tumor. The 50-mg/kg dose of macitentan did not produce toxicity.
Immunohistochemical Analyses of the ET Axis and Signaling Molecules in SKOV3ip1 and IGROV1
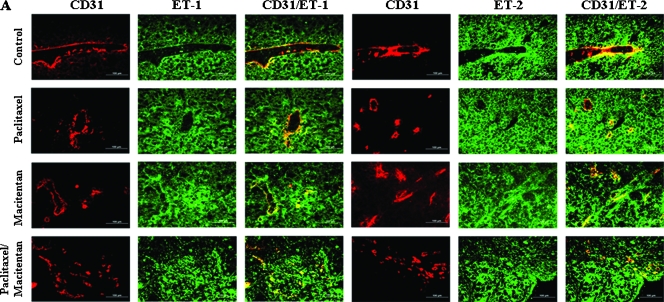
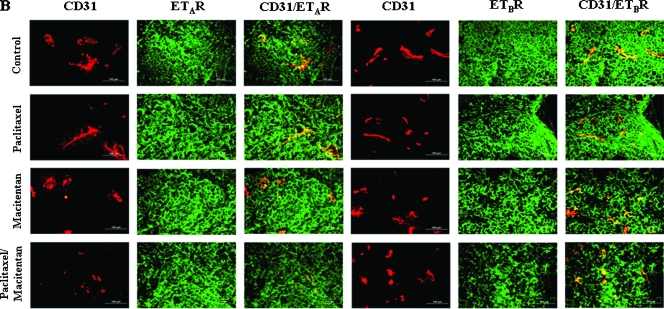
Tumor cells and tumor associated endothelial cells of SKOV3ip1 (Figure 2) and IGROV1 (not shown) peritoneal tumors were analyzed for expression of ET-1, ET-2, ETAR, and ETBR. Treatment with paclitaxel (5 mg/kg), macitentan (100 mg/kg), or a combination of paclitaxel and macitentan did not alter the expression of the ligands or the receptors in both tumor cells and tumor-associated endothelial cells (Figure 2, A and B). ET-1, ET-2, ETAR, and ETBR produced green fluorescence, and endothelial cells (CD31) produced red fluorescence. Thus, colocalization of ET-1, ET-2, ETAR, or ETBR with CD31 produced a yellow stain (Figure 2, A and B). The ET receptor antagonist, macitentan, did not affect the expression of ET-1, ET-2, ETAR, or ETBR on the tumor cells or tumor-associated endothelial cells.
Figure 2.
Immunohistochemical analyses of human ovarian carcinoma SKOV3ip1 growing in the peritoneal cavity of nude mice treated with macitentan and paclitaxel. SKOV3ip1 tumor tissues were harvested and processed for the frozen sections. Tissues were stained with anti-CD31 antibody (red) and anti-ET-1, ET-2, ETAR, or ETBR antibody (green). Expression of ET-1, ET-2, ETAR, or ETBR on tumor-associated endothelial cells yielded yellow signal by colocalization of red and green. Experimental conditions; see Table 1A. (A) ET-1 and ET-2 were expressed on tumor cells (green) as well as tumor-associated endothelial cells (yellow) and treatment with ETR antagonist, macitentan, with or without paclitaxel, did not affect the expression of ET-1 or ET-2. (B) ETAR and ETBR were expressed on tumor cells (green) as well as tumor-associated endothelial cells (yellow), and treatment with ETR antagonist, macitentan, with or without paclitaxel, did not affect the expression of ETAR or ETBR.
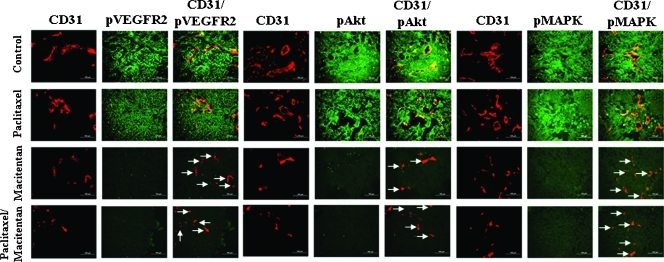
Treatment with macitentan alone (50 mg/kg) or treatment with a combination of macitentan and paclitaxel (5 mg/kg) significantly decreased the levels of pVEGFR2, pAkt, and pMAPK in tumor cells and in tumor-associated ET cells (note decreased yellow signal as compared with the control group) (IGROV1; Figure 3).
Figure 3.
Immunohistochemical analyses of IGROV1 growing in the peritoneal cavity of nude mice treated with macitentan and paclitaxel. IGROV1 tumor tissues were harvested and processed for the frozen section. Tissues were stained with anti-CD31 antibody (red) and anti-pVEGFR2, pAkt, or pMAPK antibody (green). Expression of pVEGFR2, pAkt, or pMAPK on tumor-associated endothelial cells yielded a yellow signal by colocalization of red and green. Treatment with macitentan significantly decreased the expression of pVEGFR2, pAkt, and pMAPK on tumor cells as well as tumor-associated endothelial cells (arrows). Treatment with paclitaxel did not show a significant change in expression of pVEGFR2, pAkt, or pMAPK. Experimental conditions; see Table 1B.
When pVEGFR and pAkt were analyzed in SKOV3ip1 tumors from mice treated with different doses of macitentan, the 10 mg/kg administered daily did not inhibit Akt and VEGFR phosphorylation, whereas the 30 mg/kg administered daily strongly inhibited Akt phosphorylation and, to a lesser extent, VEGFR phosphorylation. When macitentan was administered at 100 mg/kg, both pAkt and pVEGFR were completely inhibited, and this correlated with the stronger efficacy in terms of tumor incidence (data not shown).
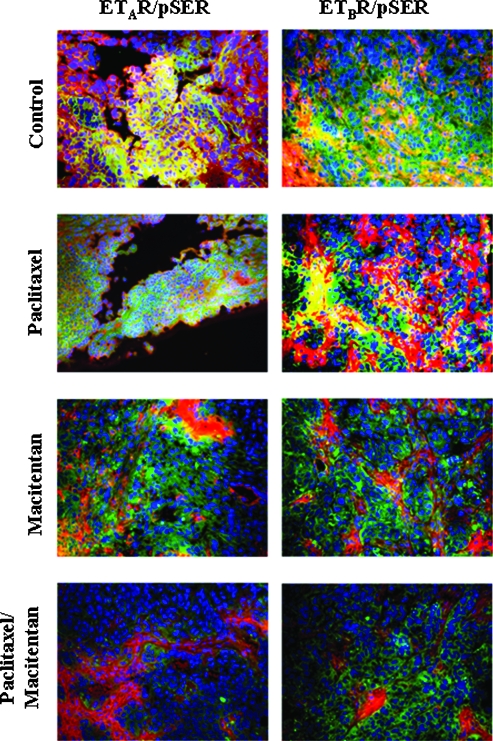
Because β-arrestin is recruited to the ETRs [48] via binding to serine/threonine phosphorylated sites at the cytoplasmic C-terminus of the receptor proteins, we applied double immunofluorescence staining for phosphorylated serine and ETRs to tumor tissues to probe the activation status of the receptors by assessing colocalization of antibody binding.
Phosphorylated serine was captured as green, and ETAR and ETBR were captured as red. Colocalization of phosphorylated serine and ETAR or ETBR (phosphorylated ETRs) yielded yellow. Colocalization was significantly decreased in a dose-dependent manner (Figure W3). Treatment of mice with 50 mg/kg macitentan significantly inhibited colocalization (yellow) of phospho-serine (green) and of ETAR or ETBR (red), indicating inhibition of ETR phosphorylation. The nuclei were stained with Hoechst 33342 (blue) (Figure 4).
Figure 4.
Double immunofluorescence staining for phosphorylated ETRs. The SKOV3ip1 tumors in control mice and paclitaxel-treated mice expressed phosphorylated ETAR and ETBR (yellow color derived from dual colocalization of red anti-ETR, and green anti-phospho-serine). Treatment with macitentan alone (50 mg/kg once a day) or macitentan plus paclitaxel (5 gm/kg once a week) suppressed phosphorylation of the ETRs (red signal equal to nonphosphorylated receptors). Size of tumor cells is variable, depending on the harvest site.
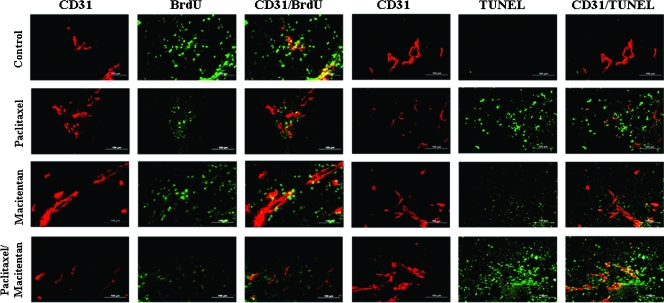
Proliferation and Apoptosis of Cells in Tumors
Treatment of SKOV3 tumors with paclitaxel alone at 5 mg/kg significantly decreased the number of BrdU-positive proliferating cells (green tumor cells and yellow endothelial cells) and increased the number of apoptotic tumor cells (TUNEL-positive, green) and TUNEL-positive endothelial cells (TUNEL-positive, yellow) (SKOV3ip1; Table 3A and Figure 5). Treatment with macitentan (50 or 100 mg/kg) alone also increased the number of apoptotic tumor cells and endothelial cells but did not decrease the number of proliferating tumor cells (Figure 5). Combination therapy with macitentan and paclitaxel significantly increased the number of apoptotic tumor cells compared with paclitaxel or macitentan alone. Furthermore, the combination therapy also increased apoptosis in tumor-associated endothelial cells (Figure 5). Proliferating and apoptotic cells were quantified by counting BrdU-positive cells and TUNEL-positive cells, respectively, in 10 random 0.159-mm2 fields at 100x magnification (Table 3). In SKOV3ip1 tumors, paclitaxel (5 mg/kg) significantly increased the number of TUNEL-positive cells compared with control treatment. Treatment with macitentan (50 mg/kg) also significantly increased the number of apoptotic cells. The combination of paclitaxel and macitentan yielded the highest level of apoptosis (with statistical significance compared with the paclitaxel group [195.3 ± 42.6 vs 150.0 ± 38.3], P < .01). The number of proliferating cells was significantly decreased by treatment with paclitaxel; however, treatment with macitentan alone did not significantly decrease the number of proliferating cells. The combination of paclitaxel and macitentan further decreased the number of proliferating cells, but it was not statistically significant compared with tumors treated with only paclitaxel (18.5 ± 11.1 vs 30.8 ± 14.9, P < .051; Table 3A).
Table 3.
Quantitative Analysis of Dividing and Apoptotic Cells in Human Ovarian Cancers SKOV3ip1 (A) and IGROV1 (B) Growing in the Peritoneal Cavity of Nude Mice.
| Treatment Group | BrdU* | TUNEL* |
| (A) | ||
| Control | 64.8 ± 20.0 | 6.5 ± 3.2 |
| Paclitaxel, 5 mg/kg | 30.8 ± 14.9† | 150.0 ± 38.3† |
| Macitentan, 50 mg/kg | 48.7 ± 15.1 | 54.7 ± 17.9† |
| Paclitaxel + macitentan | 18.5 ± 11.1† | 195.3 ± 42.6†,‡ |
| (B) | ||
| Control | 55.2 ± 13.9 | 5.7 ± 3.3 |
| Paclitaxel, 5 mg/kg | 28.2 ± 10.0‡ | 139.0 ± 29.7† |
| Macitentan, 50 mg/kg | 45.9 ± 13.4 | 69.0 ± 13.1† |
| Paclitaxel + macitentan | 19.6 ± 8.8† | 181.4 ± 32.9†,‡ |
Number of cells in 0.159-mm2 field.
Statistically significant compared with the control group (P < .05).
Statistically significant compared with the paclitaxel group (P < .01).
Figure 5.
Cell proliferation and apoptosis in control and macitentan-treated SKOV3ip1 tumors growing in the peritoneal cavity of nude mice. SKOV3ip1 tumor tissues were harvested and processed for the frozen section. To detect proliferating or apoptotic cells, tissues were stained with anti-CD31 antibody (red) and anti-BrdU antibody or TUNEL staining (green), respectively. Colocalization of BrdU- or TUNEL-positive cells with CD31 yielded yellow signals. Treatment with paclitaxel significantly decreased the number of proliferating cells (BrdU-positive cells) but macitentan alone did not significantly decrease the number of BrdU-positive cells. Combination of macitentan and paclitaxel did not induce significant additive effects over the effects of paclitaxel on proliferating cells. Treatment with macitentan alone significantly increased the number of apoptotic cells (TUNEL-positive), and the combination treatment significantly enhanced the effects of paclitaxel on tumor cells and associated endothelial cells (yellow). Experimental conditions; see Table 1A.
In IGROV1 tumors (Table 3B), treatment with paclitaxel (5 mg/kg) significantly increased the number of apoptotic cells, and treatment with macitentan (50 mg/kg) alone also significantly increased the apoptotic cells (69 ± 13.1 vs 5.7 ± 3.3, P < .05) compared with the control group. The combination of paclitaxel and macitentan yielded the best outcome compared with paclitaxel-treated mice (181.4 ± 32.9 vs 139.0 ± 29.7, P < .05). The number of proliferating cells was significantly decreased by the treatment with paclitaxel compared with the control group. Treatment with macitentan alone did not induce a significant decrease in the number of proliferating cells. Treatment with both paclitaxel and macitentan further decreased cell proliferation as compared with the paclitaxel group, but the decrease was not statistically significant (19.3 ± 8.8 vs 28.2 ± 10.0, P = .057; Table 3B).
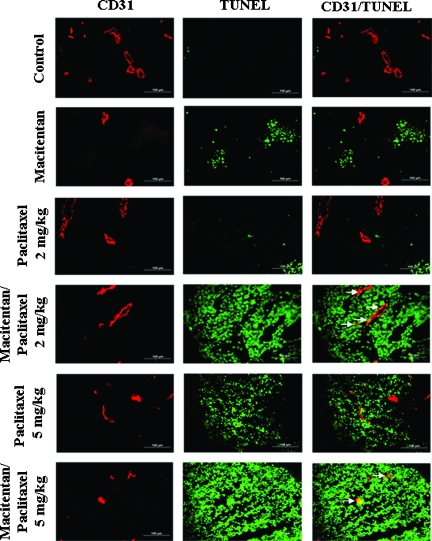
In the third set of experiments, treatment with the nontherapeutic dose (2 mg/kg) of paclitaxel did not induce significant apoptosis of tumor cells (green) or tumor-associated endothelial cells (yellow), but the combination of macitentan (50 mg/kg) with 2 mg/kg paclitaxel induced significant apoptosis in both the tumor cells and tumor-associated endothelial cells (Figure 6). The number of apoptotic cells induced by the therapeutic dose of paclitaxel (5 mg/kg) was increased by combining the paclitaxel with macitentan (Figure 6).
Figure 6.
Induction of apoptosis in SKOV3ip1 tumors by paclitaxel and macitentan. SKOV3ip1 tumor tissues were harvested and processed for frozen section. Tissues were stained with CD31 and TUNEL to detect the apoptotic cells. Treatment with nontherapeutic dose (2 mg/kg) of paclitaxel did not induce apoptosis of tumor cells or tumor-associated endothelial cells, but its combination with macitentan made cells sensitive to this low dose of paclitaxel, which was clearly demonstrated by induction of significant apoptosis of tumor cells as well as tumor-associated endothelial cells (arrows). Macitentan significantly further enhanced the effect of the therapeutic dose (5 mg/kg) of paclitaxel. Experimental conditions; see Table 2.
Discussion
The purpose of the present study was to determine whether blockade of ETRs by macitentan, coupled with administration of paclitaxel, decreases the progressive growth of human ovarian cancer cells implanted into the peritoneal cavity of nude mice and to identify potential pharmacodynamic markers, which could be useful in dose finding and therapy. Multiple studies indicate that ET and ETRs contribute to the progressive growth of ovarian cancer [25,39–48] and expression of the ETAR has been shown to correlate with advanced stages of ovarian cancer in patients [39,66,67]. Gene expression profiles of advanced ovarian cancer further indicate that ETAR expression is associated with cell migration and invasion [66,67]. ET-1 has also been shown to promote survival of cells through Bcl-2-dependent mechanisms and inhibit paclitaxel-mediated apoptosis [39]. In the current study, we demonstrated, using two ovarian cancer cell lines grown in vitro and in vivo, that ETRs were preferentially expressed when tumors were grown in vivo. Both tumors produced ET in vitro and in vivo in ascites (SKOV3ip1). Immunohistochemistry confirmed the expression of ETRs on tumor cells (ETAR) and on tumor-associated vascular endothelial cells (ETBR). As demonstrated in therapy experiments, inhibition of ET signaling sensitized not only tumor cells but also tumor-associated endothelial cells to killing by paclitaxel.
Results obtained in preclinical cancer models [49,68] and in analyzing clinical tumor specimens led to clinical studies of ETR antagonists, mainly using ETAR-selective antagonists in hormone-refractory and metastatic prostate cancer [49,69,70]. Clinical data obtained with ETAR selective antagonists in prostate cancer patients, in particular with zibotentan, justify additional studies of these antagonists, both in prostate cancer and in other tumors [70,71]. The dual receptor antagonist, bosentan, has been studied in melanoma patients in a phase 2 trial [72] based on data that indicate a fundamental role of the ETBR in melanocyte physiology [73] and a correlation of ETBR overexpression in melanoma with tumor progression [74]. The single agent phase 2 study suggested that additional studies of the agent in combination with other anticancer drugs were warranted.
A double-blind, placebo-controlled study of high-dose bosentan, in combination with dacarbazine, was performed in patients with stage IV metastatic melanoma. In this study, the addition of bosentan had no effect on time to tumor progression or other measures of efficacy [75]. No pharmacodynamic markers were applied to determine the drug action in tumors. The inability of this drug to affect response to dacarbazine may be due to poor tissue penetration by bosentan because ET and ETRs are expressed in cancer cells and tumor stroma. Macitentan was selected for our study because of its high tissue targeting properties and for targeting both the ETARs and ETBRs [61].
In pathologic situations, both ETA and ETB receptors have been shown to mediate vasoconstriction [76], smooth muscle proliferation [77], and inflammation [78]. These findings, together with those we describe regarding the highly expressed ET axis in tumors, make further evaluation of dual ET receptor antagonists in oncology warranted.
The efficacy of ETAR-selective inhibitors have been tested in ovarian cancer xenografts. ABT-627 (atrasentan) has shown antitumor efficacy in subcutaneously transplanted HEY ovarian tumors alone and in combination with Taxol. The antitumor effect was accompanied with reduced production of VEGF, reduced expression of matrix metalloproteinase 2, and an increase in the number of apoptotic tumor cells [79]. In other studies using the HEY tumor model, ABT-627 efficacy was confirmed, and in one case, the antitumor effect was associated with the reversal of markers typical for the epithelial-to-mesenchymal transition [80] and in another case with the reduction of integrin-linked kinase expression and reduced AKT and GSK-3β phosphorylation [81]. The ETAR selective antagonist ZD4054 (zibotentan) showed antitumor activity in subcutaneous HEY ovarian tumor xenografts alone and in combination with paclitaxel [65] and the epidermal growth factor receptor kinase inhibitor gefitinib [40]. Administration of ZD4054 reduced VEGF expression and phosphorylation of p42/44 MAP kinase and inhibited growth of metastatic nodules after intraperitoneal transplantation of HEY ovarian cancer cells [48]. In this case, the metastatic phenotype was linked to ETAR-mediated β-arrestin signaling and activation of the wnt pathway.
In the present study, we show that blockade of the ET axis by the dual ETR antagonist macitentan inhibits the growth of human ovarian cancer cells, SKOV3ip1 and IGROV1, in the peritoneal cavity of nude mice (orthotopic site). SKOV3ip1 and IGROV1 cells were both sensitive to paclitaxel and highly express ETRs in vivo. The SKOV3ip1 tumor also produces ET in ascites fluid. Treatment with macitentan as a single agent did not produce significant therapeutic effects, but combining paclitaxel with macitentan did. The blockage of the ETBR was demonstrated by the increased level of ET in ascites in the macitentan groups (Figure 1D). Immunohistochemical analyses demonstrated that, although treatment with macitentan did not change the expression levels of ET-1, ET-2, ETAR, and ETBR in tumor cells, it significantly decreased the phosphorylation of ETRs and such cell survival pathway markers as pVEGFR2, pAkt, and pMAPK [59,82,83]. These data extend those in earlier reports [39,40,79,81]. Treatment with macitentan alone increased the number of apoptotic cells, and its combination with paclitaxel significantly enhanced apoptosis in tumor cells and the tumor-associated endothelial cells compared to each agent alone, indicating loss of blood vessels. Similarly, paclitaxel and the drug combination significantly decreased the number of proliferating cells, whereas macitentan alone did not. Collectively, these results suggest that blockade of the ET axis may suppress antiapoptotic mechanisms in tumor cells and associated endothelial cells by affecting survival pathways through the inhibition of phosphorylation of pAkt, pMAPK, and pVEGFR2. Thus, treatment with macitentan rendered tumor cells and tumor-associated endothelial cells more sensitive to paclitaxel, even when paclitaxel was administered at the dose of 2 mg/kg, which is less than half of its therapeutic dose (5 mg/kg). This suggests that the combination of the ET antagonist macitentan with a suboptimal dose of the chemotherapeutic agent may be just as effective as paclitaxel alone at the higher therapeutic dose.
The efficacy of macitentan was correlated with inhibition of protein phosphorylation in a dose-response study. Doses of macitentan that did not increase the efficacy of paclitaxel did not downregulate expression of pAkt, pMAPK, or pVEGFR. These markers can therefore be used as pharmacodynamic biomarkers to determine the minimal effective dose in other animal models or patients. Importantly, a direct measurement of the activation state of the ETRs would be a most desirable biomarker. It has recently been shown that ETRs also signal through the β-arrestin pathway [48]. β-Arrestin binding to an activated G protein-coupled receptor requires phosphorylation of the C-terminal intracellular part of the receptor at the amino acids serine or threonine by G protein-coupled receptor kinases [84]. Because antibodies that are able to detect phospho-serine or phospho-threonine in an ETR-specific peptide sequence are not available, we determined the phosphorylation of ETRs by colocalization of ETRs and phosphorylated serine using anti-ETR and nonspecific anti-phospho-serine antibodies. Colocalized signals of ETRs and phosphorylated serine were decreased in a dose-dependent manner in tumors of animals treated with efficacious doses of macitentan, indicating blockage of ETR activation by the receptor antagonist. This colocalization assay is highly dependent on the specificity of the antibodies. To increase the link of this assay with the activation status of ETR, in vitro cell culture experiments with SKOV3ip1 cells were performed. In these experiments, concentrations as low as 100 nM macitentan, which has been shown to functionally inhibit ETR signaling in cellular assays [61], were able to block ET-1-induced colocalization of the antibodies (data not shown). Preliminary determination of macitentan level in the plasma correlated with the localization of ETR-pSer in the tumors. These measurements may therefore serve as biomarkers most closely linked to ETR activation.
Our present study clearly demonstrates that treatment of mice with macitentan and paclitaxel inhibited phosphorylation of ETRs in tumor cells and tumor-associated vascular endothelial cells and produced apoptosis in both cell types. Previous data from our laboratory demonstrated that therapy for ovarian cancer [62], colon cancer [85], and prostate cancer [86,87] can be mediated by targeting the tumor vasculature.
In summary, this study confirms the efficacy of inhibiting cell signaling pathways and tumor growth by ETR antagonists in combination with paclitaxel in orthotopic models of ovarian cancer. Of importance, macitentan decreased the effective dose of paclitaxel. Efficacy is associated not only with tumor cell killing but also with destruction of tumor-associated vascular endothelial cells. The contribution to paclitaxel efficacy of the tissue-targeting dual ETR antagonist macitentan was correlated with inhibition of phosphorylation of signaling and survival proteins. Therefore, the degree of protein phosphorylation might be used as a pharmacodynamic biomarker in future studies and supports the finding of the biologically active therapeutic dose.
Supplementary Material
Acknowledgments
The authors thank Anna Stalder, Panja Strickner, and Celine Mangold for technical assistance; Walter Pagel for critical editorial comments; and Arminda Martinez and Lola Lüpez for expert assistance in the preparation of this article.
Abbreviations
- ET
endothelin
- ETR
endothelin receptor
- ETAR
endothelin A receptor
- ETBR
endothelin B receptor
- PBS
phosphate-buffered saline
- VEGFR
vascular endothelial growth factor receptor
Footnotes
This work was supported in part by Cancer Center Support Core grant CA16672 and grant 1U54CA143837-01 from the National Cancer Institute, National Institutes of Health, and by Actelion Pharmaceuticals, Ltd.
This article refers to supplementary materials, which are designated by Figures W1 to W3 and are available online at www.neoplasia.com.
References
- 1.Jemal A, Siegal R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra SA. Cancer of the ovary. N Engl J Med. 1993;329:1550–1559. doi: 10.1056/NEJM199311183292108. [DOI] [PubMed] [Google Scholar]
- 3.du Bois A, Lück HJ, Meier W, Adams HP, Möbus V, Costa S, Bauknecht T, Richter B, Warm M, Schröder W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;9:1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 4.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 5.Bookman MA, McGuire WP, III, Kilpatrick D, Keenan E, Hogan WM, Johnson SW, O'Dwyer P, Rowinsky E, Gallion HH, Ozols RF. Carboplatin and paclitaxel in ovarian carcinoma: a phase I study of the Gynecologic Oncology Group. J Clin Oncol. 1996;14:1895–1902. doi: 10.1200/JCO.1996.14.6.1895. [DOI] [PubMed] [Google Scholar]
- 6.Gore ME. American Society of Clinical Oncology 2001 Education Book. Alexandria, VA: American Society of Clinical Oncology; 2001. Treatment of relapsed epithelial ovarian cancer; pp. 468–476. [Google Scholar]
- 7.Fidler IJ. Modulation of the organ microenvironment for the treatment of cancer metastasis. J Natl Cancer Inst. 1995;87:1588–1592. doi: 10.1093/jnci/87.21.1588. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Uehara H, Karashima T, Sheperd DL, Killion JJ, Fidler IJ. Blockade of epidermal growth factor receptor signaling in tumor cells and tumor-associated endothelial cells for therapy of androgen-independent human prostate cancer growing in the bone of nude mice. Clin Cancer Res. 2003;9:1200–1210. [PubMed] [Google Scholar]
- 9.Levin ER. Endothelins. N Engl J Med. 1995;333:356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- 10.Masaki T. The endothelin family: an overview. J Cardiovasc Pharmacol. 2000;35:S3–S5. doi: 10.1097/00005344-200000002-00002. [DOI] [PubMed] [Google Scholar]
- 11.Bloch KD, Friedrich SP, Lee ME, Eddy RL, Shows TB, Quertermous T. Structural organization and chromosomal assignment of the gene encoding endothelin. J Biol Chem. 1989;264:10851–10857. [PubMed] [Google Scholar]
- 12.Battistini B, Chailler P, D'Orleans-Juste P, Briere N, Sirois P. Growth regulatory properties of endothelins. Peptides. 1993;14:385–399. doi: 10.1016/0196-9781(93)90057-n. [DOI] [PubMed] [Google Scholar]
- 13.Goldie RG. Endothelins in health and disease: an overview. Clin Exp Pharmacol Physiol. 1999;26:145–148. doi: 10.1046/j.1440-1681.1999.03014.x. [DOI] [PubMed] [Google Scholar]
- 14.Remuzzi G, Perico N, Benigni A. New therapeutics that antagonize endothelin: promises and frustrations. Nat Rev Drug Discov. 2002;1:986–1000. doi: 10.1038/nrd962. [DOI] [PubMed] [Google Scholar]
- 15.Opgenorth TJ. Endothelin receptor antagonism. Adv Pharmacol. 1995;33:1–65. doi: 10.1016/s1054-3589(08)60665-1. [DOI] [PubMed] [Google Scholar]
- 16.Lusher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class cardiovascular drugs. Circulation. 2000;102:1434–1440. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- 17.Clozel M. Endothelin receptor antagonists: current status and perspectives. J Cardiovasc Pharmacol. 2000;35:S65–S68. doi: 10.1097/00005344-200000002-00015. [DOI] [PubMed] [Google Scholar]
- 18.Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, et al. Endothelin-1 promotes myofibroblasts induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15:2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Krasnici N, Lüscher TF. Endothelin-1 potentiates human smooth muscle cell growth to PDGF: effects of ETA and ETB receptor blockade. Circulation. 1999;100:5–8. doi: 10.1161/01.cir.100.1.5. [DOI] [PubMed] [Google Scholar]
- 20.Hocher B, Schwarz A, Fagan KA, Thöne-Reineke C, El-Hag K, Kusserow H, Elitok S, Bauer C, Neumayer H-H, Rodman DM, et al. Pulmonary fibrosis and chronic lung inflammation in ET-1 transgenic mice. Am J Respir Cell Mol Biol. 2000;23:19–26. doi: 10.1165/ajrcmb.23.1.4030. [DOI] [PubMed] [Google Scholar]
- 21.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nature Rev Cancer. 2003;3:110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 22.Spinella F, Garrafa E, DiDi Castro V, Rosano L, Nicotra MR, Caruso A, Natali PG, Bagnato A. Endothelin-1 stimulates lymphatic endothelial cells and lymphatic vessels to grow and invade. Cancer Res. 2009;69:2669–2676. doi: 10.1158/0008-5472.CAN-08-1879. [DOI] [PubMed] [Google Scholar]
- 23.Sonveaux P, Dessy C, Martinive P, Havaux X, Jordan BF, Gallez B, Grégoire V, Balligand J-L, Feron O. Endothelin-1 is a critical mediator of mitogenic tone in tumor arterioles: implications for cancer treatment. Cancer Res. 2004;64:3209–3214. doi: 10.1158/0008-5472.can-03-1291. [DOI] [PubMed] [Google Scholar]
- 24.Rosanò L, Spinella F, Di Castro V, Nicotra MR, Dedhar S, de Herreros AG, Natali PG, Bagnato A. Endothelin-1 promotes epithelial-to-mesenchymal transition in human ovarian cancer cells. Cancer Res. 2005;65:11649–11657. doi: 10.1158/0008-5472.CAN-05-2123. [DOI] [PubMed] [Google Scholar]
- 25.Rosanò L, Varmi M, Salani D, Di Castro V, Spinella F, Natali PG, Bagnato A. Endothelin-1 induces tumor proteinase activation and invasiveness of ovarian carcinoma cells. Cancer Res. 2001;61:8340–8346. [PubMed] [Google Scholar]
- 26.Kulasekaran P, Scavone CA, Rogers DS, Arenberg DA, Thannickal VJ, Horowitz JC. Endothelin-1 and transforming growth factor-β1 independently induce fibroblast resistance to apoptosis via AKT activation. Am J Respir Cell Mol Biol. 2009;41:484–493. doi: 10.1165/rcmb.2008-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pristivishalil G, Nelson JB. Endothelium-derived factors as paracrine mediators of prostate cancer progression. Prostate. 2000;44:44–77. doi: 10.1002/1097-0045(20000615)44:1<77::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 28.Nelson JB, Chan-Tack K, Hedican SP, Magnuson SR, Opgenorth TJ, Bova GS, Simons JW. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996;56:663–668. [PubMed] [Google Scholar]
- 29.Ahmed SI, Thompson J, Coulson JM, Woll PJ. Studies on the expression of endothelin, its receptor subtypes, and converting enzymes in lung cancer and in human bronchial epithelium. Am J Respir Cell Mol Biol. 2000;22:422–431. doi: 10.1165/ajrcmb.22.4.3795. [DOI] [PubMed] [Google Scholar]
- 30.Zhao YD, Springall DR, Hamid Q, Levene M, Polak JM. Localization and characterization of endothelin-1 receptor binding in the blood vessels of human pulmonary tumors. J Cardiovasc Pharmacol. 1995;26(suppl 3):S341–S345. [PubMed] [Google Scholar]
- 31.Asham E, Shankar A, Loizidou M, Fredericks S, Miller K, Boulos PB, Burnstock G, Taylor I. Increased endothelin-1 in colorectal cancer and reduction of tumour growth by ET(A) receptor antagonism. Br J Cancer. 2001;85:1759–1763. doi: 10.1054/bjoc.2001.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eberl LP, Valdenaire O, Saintgiorgio V, Jeannin JF, Juillerat-Jeanneret L. Endothelin receptor blockade potentiates FasL-induced apoptosis in rat colon carcinoma cells. Int J Cancer. 2000;86:182–187. doi: 10.1002/(sici)1097-0215(20000415)86:2<182::aid-ijc6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 33.Pflug BR, Zheng H, Udan MS, D'Antonio JM, Marshall FF, Brooks JD, Nelson JB. Endothelin-1 promotes cell survival in renal cell carcinoma through the ET(A) receptor. Cancer Lett. 2007;246:139–148. doi: 10.1016/j.canlet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Venuti A, Salani D, Manni V, Poggiali F, Bagnato A. Expression of endothelin 1 and endothelin A receptor in HPV-associated cervical carcinoma: new potential targets for anticancer therapy. FASEB J. 2000;14:2277–2283. doi: 10.1096/fj.00-0024com. [DOI] [PubMed] [Google Scholar]
- 35.Bagnato A, Cirilli A, Salani D, Simeone P, Muller A, Nicotra MR, Natali PG, Venuti A. Growth inhibition of cervix carcinoma cells in vivo by endothelin A receptor blockade. Cancer Res. 2002;62:6381–6384. [PubMed] [Google Scholar]
- 36.Pagotto U, Arzberger T, Hopfner U, Sauer J, Renner U, Newton CJ, Lange M, Uhl E, Weindl A, Stalla GK. Expression and localization of endothelin-1 and endothelin receptors in human meningiomas. Evidence for a role in tumoral growth. J Clin Invest. 1995;96:2017–2025. doi: 10.1172/JCI118249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harland SP, Kuc RE, Pickard JD, Davenport AP. Expression of endothelin( A) receptors in human gliomas and meningiomas, with high affinity for the selective antagonist PD156707. Neurosurgery. 1998;43:890–898. doi: 10.1097/00006123-199810000-00097. discussion 898–899. [DOI] [PubMed] [Google Scholar]
- 38.Egidy G, Eberl LP, Valdenaire OV, Irmler M, Majdi R, Diserens A-C, Fontana A, Janzer R-C, Pinet F, Juillerat-Jeanneret L. The endothelin system in human glioblastoma. Lab Invest. 2000;80:1681–1689. doi: 10.1038/labinvest.3780178. [DOI] [PubMed] [Google Scholar]
- 39.Rosanò L, Di Castro V, Spinella F, Nicotra MR, Natali PG, Bagnato A. ZD4054, a specific antagonist of the endothelin A receptor, inhibits tumor growth and enhances paclitaxel activity in human ovarian carcinoma in vitro and in vivo. Mol Cancer Ther. 2007;6:2003–2011. doi: 10.1158/1535-7163.MCT-07-0151. [DOI] [PubMed] [Google Scholar]
- 40.Rosanò L, Di Castro V, Spinella F, Tortora G, Nicotra MR, Natali PG, Bagnato A. Combined targeting of endothelin A receptor and epidermal growth factor receptor in ovarian cancer shows enhanced antitumor activity. Cancer Res. 2007;67:6351–6359. doi: 10.1158/0008-5472.CAN-07-0883. [DOI] [PubMed] [Google Scholar]
- 41.Vacca F, Bagnato A, Catt KJ, Tecce R. Transactivation of the epidermal growth factor receptor in endothelin-1-induced mitogenic signaling in human ovarian carcinoma cells. Cancer Res. 2000;60:5310–5317. [PubMed] [Google Scholar]
- 42.Rayhman O, Klipper E, Muller L, Davidson B, Reich R, Meidan R. Small interfering RNA molecules targeting endothelin-converting enzyme-1 inhibit endothelin-1 synthesis and the invasive phenotype of ovarian carcinoma cells. Cancer Res. 2008;68:9265–9273. doi: 10.1158/0008-5472.CAN-08-2093. [DOI] [PubMed] [Google Scholar]
- 43.Salani D, Di Castro V, Nicotra MR, Rosanò L, Tecce R, Venuti A, Natali PG, Bagnato A. Role of endothelin-1 in neovascularization of ovarian carcinoma. Am J Pathol. 2000;157:1537–1547. doi: 10.1016/S0002-9440(10)64791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagnato A, Salani D, Di Castro V, Wu-Wong JR, Tecce R, Nicotra MR, Venuti A, Natali PG. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res. 1999;59:720–727. [PubMed] [Google Scholar]
- 45.Bagnato A, Tecce R, Moretti C, Di Castro V, Spergel D, Catt KJ. Autocrine actions of endothelin-1 as a growth factor in human ovarian carcinoma cells. Clin Cancer Res. 1995;1:1059–1066. [PubMed] [Google Scholar]
- 46.Moraitis S, Langdon SP, Miller WR. Endothelin expression and responsiveness in human ovarian carcinoma cell lines. Eur J Cancer. 1997;33:661–668. doi: 10.1016/s0959-8049(97)00012-9. [DOI] [PubMed] [Google Scholar]
- 47.Moraitis S, Miller WR, Smyth JF, Langdon SP. Paracrine regulation of ovarian cancer by endothelin. Eur J Cancer. 1999;35:1381–1387. doi: 10.1016/s0959-8049(99)00131-8. [DOI] [PubMed] [Google Scholar]
- 48.Rosanò L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG, Bagnato A. β-Arrestin links endothelin A receptor to β-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci USA. 2009;106:2806–2811. doi: 10.1073/pnas.0807158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lalich M, McNeel DG, Wilding G, Liu G. Endothelin receptor antagonists in cancer therapy. Cancer Invest. 2007;25:785–794. doi: 10.1080/07357900701522588. [DOI] [PubMed] [Google Scholar]
- 50.Bagnato A, Rosanò L. The endothelin axis in cancer. Int J Biochem Cell Biol. 2008;40:1443–1451. doi: 10.1016/j.biocel.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 51.Kusuhara M, Yamaguchi K, Nagasaki K, Hayashi C, Suzaki A, Hori S, Handa S, Nakamura Y, Abe K. Production of endothelin in human cancer cell lines. Cancer Res. 1990;50:3257–3261. [PubMed] [Google Scholar]
- 52.Lahav R. Endothelin receptor B is required for the expansion of melanocyte precursors and malignant melanoma. Int J Dev Biol. 2005;49:173–180. doi: 10.1387/ijdb.041951rl. [DOI] [PubMed] [Google Scholar]
- 53.Morbidelli L, Orlando C, Maggi CA, Ledda F, Ziche M. Proliferation and migration of endothelial cells is promoted by endothelins via activation of ETB receptors. Am J Physiol. 1995;269(2 pt 2):H686–H695. doi: 10.1152/ajpheart.1995.269.2.H686. [DOI] [PubMed] [Google Scholar]
- 54.Ziche M, Morbidelli L, Donnini S, Ledda F. ETB receptors promote proliferation and migration of endothelial cells. J Cardiovasc Pharmacol. 1995;26(suppl 3):S284–S286. [PubMed] [Google Scholar]
- 55.Knowles J, Loizidou M, Taylor I. Endothelin-1 and angiogenesis in cancer. Curr Vasc Pharmacol. 2005;3:309–314. doi: 10.2174/157016105774329462. [DOI] [PubMed] [Google Scholar]
- 56.Salani D, Taraboletti G, Rosanò L, Di Castro V, Borsotti P, Giavazzi R, Bagnato A. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol. 2000;157:1703–1711. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egidy G, Juillerat-Jeanneret L, Jeannin J-F, Korth P, Bosman FT, Pinet F. Modulation of human colon tumor-stromal interactions by the endothelin system. Am J Pathol. 2000;157:1863–1874. doi: 10.1016/S0002-9440(10)64825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bagnato A, Spinella F, Rosanò L. Emerging role of the endothelin axis in ovarian tumor progression. Endocr Relat Cancer. 2005;12:761–772. doi: 10.1677/erc.1.01077. [DOI] [PubMed] [Google Scholar]
- 59.Spinella F, Rosanò L, DiDi Castro V, Natali PG, Bagnato A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1α in ovarian carcinoma cells. J Biol Chem. 2002;277:27850–27855. doi: 10.1074/jbc.M202421200. [DOI] [PubMed] [Google Scholar]
- 60.Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- 61.Iglarz M, Binkert C, Morrison K, Fischli W, Gatfield J, Treiber A, Weller T, Bolli MH, Boss C, Buchmann S, et al. Pharmacology of macitentan, an orally active tissue targeting dual endothelin receptor antagonist. J Pharmacol Exp Ther. 2008;327:736–745. doi: 10.1124/jpet.108.142976. [DOI] [PubMed] [Google Scholar]
- 62.Thaker PH, Yazici S, Nilsson MB, Yokoi K, Tsan RZ, He J, Kim SJ, Fidler IJ, Sood AK. Antivascular therapy for orthotopic human ovarian carcinoma through blockade of the vascular endothelial growth factor and epidermal growth factor receptors. Clin Cancer Res. 2005;11:4923–4933. doi: 10.1158/1078-0432.CCR-04-2060. [DOI] [PubMed] [Google Scholar]
- 63.Bénard J, Da Silva J, De Blois MC, Boyer P, Duvillard P, Chiric E, Riou G. Characterization of a human ovarian adenocarcinoma line, IGROV1, in tissue culture and in nude mice. Cancer Res. 1985;45:4970–4979. [PubMed] [Google Scholar]
- 64.Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ. Tissue-specific microvascular endothelial cell lines from H-2K(b)-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res. 2003;63:2971–2976. [PubMed] [Google Scholar]
- 65.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O'Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 66.Donninger H, Bonome T, Radonovich M, Pise-Masison CA, Brady J, Shih JH, Barrett JC, Birrer MJ. Whole genome expression profiling of advance stage papillary serous ovarian cancer reveals activated pathways. Oncogene. 2004;23:8065–8077. doi: 10.1038/sj.onc.1207959. [DOI] [PubMed] [Google Scholar]
- 67.Bignotti E, Tassi RA, Calza S, Ravaggi A, Bandiera E, Rossi E, Donzelli C, Pasinetti B, Pecorelli S, Santin AD. Gene expression profile of ovarian serous papillary carcinomas: identification of metastasis-associated genes. Am J Obstet Gynecol. 2007;196:245.e1–245.e11. doi: 10.1016/j.ajog.2006.10.874. [DOI] [PubMed] [Google Scholar]
- 68.Akhavan A, McHugh KH, Guruli G, Bies RR, Zamboni WC, Strychor S, Nelson JB, Pflug BR. Endothelin receptor A blockade enhances taxane effects in prostate cancer. Neoplasia. 2006;8:725–732. doi: 10.1593/neo.06388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aubert JD, Juillerat-Jeanneret L. Therapeutic potential of endothelin receptor modulators: lessons from human clinical trials. Expert Opin Ther Targets. 2009;13:1069–1084. doi: 10.1517/14728220903074570. [DOI] [PubMed] [Google Scholar]
- 70.Thakkar SG, Chouveiri TK, Garcia JA. Endothelin receptor antagonists: rationale, clinical development, and role in prostate cancer therapeutics. Curr Oncol Rep. 2006;8:108–113. doi: 10.1007/s11912-006-0045-1. [DOI] [PubMed] [Google Scholar]
- 71.James ND, Caty A, Borre M, Zonnenberg BA, Beuzeboc P, Morris T, Phung D, Dawson NA. Safety and efficacy of the specific endothelin-A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: a double-blind, placebo-controlled, randomized, phase 2 trial. Eur Urol. 2009;55:1112–1123. doi: 10.1016/j.eururo.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Kefford R, Beith JM, Van Hazel GA, Millward M, Trotter JM, Wyld DK, Kusic R, Shreeniwas R, Morganti A, Ballmer A, et al. A phase II study of Bosentan, a dual endothelin receptor antagonist, as monotherapy in patients with stage IV metastatic melanoma. Invest New Drugs. 2007;25:247–252. doi: 10.1007/s10637-006-9014-7. [DOI] [PubMed] [Google Scholar]
- 73.Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons: missense mutation of endothelin-3 gene in lethal spotting mice. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 74.Demunter A, de Wolf-Peters C, Degreef H, Stas M, van den Oord JJ. Expression of the endothelin-B receptor in pigment cell lesions of the skin: evidence for its role as tumor progression marker in malignant melanoma. Virchows Arch. 2001;438:485–491. doi: 10.1007/s004280000362. [DOI] [PubMed] [Google Scholar]
- 75.Kefford R, Clingan PR, Brady B, Ballmer A, Morganti A, Hersey P. A randomized, double-blind, placebo-controlled study of high-dose bosentan in patients with stage IV metastatic melanoma receiving first-line dacarbazine chemotherapy. Mol Cancer. 2010;9:69. doi: 10.1186/1476-4598-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sauvageau S, Thorin E, Caron A, Dupuis J. Endothelin-1-induced pulmonary vasoreactivity is regulated by ETA and ETB receptor interactions. J Vasc Res. 2007;44:375–381. doi: 10.1159/000102534. [DOI] [PubMed] [Google Scholar]
- 77.Porter KE, Olojugba DH, Masood I, Pemberton M, Bell PRF, London NJM. Endothelin-B receptors mediate intimal hyperplasia in an organ culture of human saphenous vein. J Vasc Surg. 1998;28:695–701. doi: 10.1016/s0741-5214(98)70096-5. [DOI] [PubMed] [Google Scholar]
- 78.Muller DN, Mervaala EMA, Schmidt F, Park JK, Dechend R, Genersch E, Breu V, Löffler BM, Ganten D, Schneider W, et al. Effect of bosentan on NF-κB, inflammation, and tissue factor in angiotensin II-induced end-organ damage. Hypertension. 2000;36:282–290. doi: 10.1161/01.hyp.36.2.282. [DOI] [PubMed] [Google Scholar]
- 79.Rosanò L, Di Castro V, Spinella F, Decandia S, Natali PG, Bagnato A. ZD4054, a potent endothelin receptor A antagonist, inhibits ovarian carcinoma cell proliferation. Exp Biol Med. 2006;231:1132–1135. [PubMed] [Google Scholar]
- 80.Smollich M, Götte M, Fischgräbe J, Macedo LF, Brodie A, Chen S, Radke I, Kiesel L, Wülfing P. ETAR antagonist ZD4054 exhibits additive effects with aromatase inhibitors and fulvestrant in breast cancer therapy and improves in vivo efficacy of anastrozole. Breast Cancer Res Treat. 2010;123:345–357. doi: 10.1007/s10549-009-0644-2. [DOI] [PubMed] [Google Scholar]
- 81.Rosanò L, Spinella F, Di Castro V, Dedhar S, Nicotra MR, Natali PG, Bagnato A. Integrin-linked kinase functions as a downstream mediator of endothelin-1 to promote invasive behavior in ovarian carcinoma. Mol Cancer Ther. 2006;5:833–843. doi: 10.1158/1535-7163.MCT-05-0523. [DOI] [PubMed] [Google Scholar]
- 82.Bagnato A, Tecce R, DiCastro V, Catt KJ. Activation of mitogenic signaling by endothelin 1 in ovarian carcinoma cells. Cancer Res. 1997;57:1306–13011. [PubMed] [Google Scholar]
- 83.Del Bufalo D, Di Castro V, Biroccio A, Salani D, Rosanò L, Spinella F, Bagnato A. Endothelin-1 acts as a survival factor in ovarian carcinoma cells. Clin Sci (Lond) 2002;(suppl 48):302S–305S. doi: 10.1042/CS103S302S. [DOI] [PubMed] [Google Scholar]
- 84.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestin. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 85.Yokoi K, Kim SJ, Thaker P, Yazici S, Nam DH, He J, Chiao PJ, Sclabas GM, Abbruzzese JL, Hamilton SR, et al. Induction of apoptosis in tumorassociated endothelial cells and therapy of orthotopic human pancreatic carcinoma in nude mice. Neoplasia. 2005;7:696–704. doi: 10.1593/neo.05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yazici S, Kim SJ, Busby JE, He J, Thaker P, Yokoi K, Fan D, Fidler IJ. Dual inhibition of the epidermal growth factor receptor and vascular endothelial growth factor phosphorylation for antivascular therapy of human prostate cancer in the prostate of nude mice. Prostate. 2005;65:203–215. doi: 10.1002/pros.20283. [DOI] [PubMed] [Google Scholar]
- 87.Kim SJ, Uehara H, Yazici S, Busby JE, He J, Maya M, Logothetis CJ, Mathew P, Wang X, Do KA, et al. Targeting platelet-derived growth factor receptor on endothelial cells of multidrug resistant prostate cancer. J Natl Cancer Inst. 2006;98:783–793. doi: 10.1093/jnci/djj211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.