Abstract
Polar replication fork barriers (RFBs) near the 3′ end of the rRNA transcriptional unit are a conserved feature of ribosomal DNA (rDNA) replication in eukaryotes. In the mouse, in vivo studies indicate that the cis-acting Sal boxes required for rRNA transcription termination are also involved in replication fork blockage. On the contrary, in the budding yeast Saccharomyces cerevisiae, the rRNA transcription termination factors are not required for RFBs. Here we characterized the rDNA RFBs in the fission yeast Schizosaccharomyces pombe. S. pombe rDNA contains three closely spaced polar replication barriers named RFB1, RFB2, and RFB3 in the 3′ to 5′ order. The transcription termination protein reb1 and its two binding sites, present at the 3′ end of the coding region, were required for fork arrest at RFB2 and RFB3 in vivo. On the other hand, fork arrest at the strongest RFB1 barrier was independent of the above transcription termination factors. Therefore, RFB2 and RFB3 resemble the barriers present in the mouse rDNA, whereas RFB1 is similar to the budding yeast RFBs. These results suggest that during evolution, cis- and trans-acting factors required for rRNA transcription termination became involved in replication fork blockage also. S. pombe is suggested to be a transitional species in which both mechanisms coexist.
During eukaryotic ribosomal DNA (rDNA) replication, the fork moving opposite to transcription is arrested at replication fork barriers (RFBs) close to the 3′ end of the coding region (3, 13, 24-27, 42, 43). RFBs must play a relevant biological role, since they are highly conserved in eukaryotes. Due to the polar nature of RFBs, rDNA is replicated mainly in a unidirectional mode cooriented with transcription. Thus, one possible role for the RFB may be to prevent the deleterious effects of head-on collisions between replication and transcription machineries (32). Since the DNA sequence at the RFB is not sufficient per se to stall replication (4, 28), fork arrest must be induced by a protein factor(s) bound to the rDNA at the barrier.
In Saccharomyces cerevisiae rDNA, protein Fob1 is required for RFB activity (19), although it is still unknown whether it arrests rDNA replication by binding to the RFB sites or through a different mechanism. Functional RFBs are required for HOT1 recombination, contraction and expansion of the rDNA repeat number, and the formation of extrachromosomal ribosomal circles (17-20), suggesting that RFB activity stimulates recombination occurring at the rDNA locus in this budding yeast (2). On the other hand, it has been recently shown that RFBs and HOT1 recombination are independent activities although they share cis-acting sequences (41).
In mouse rDNA, replication forks stall at the rRNA transcriptional terminator elements known as Sal boxes (27), which are the specific binding sites for transcription termination factor mTTF-1 (12, 21). This protein was able to arrest replication forks in an in vitro replication assay (10, 35). These in vivo and in vitro results suggest that Sal boxes and mTTF-1 block replication forks with the opposite polarity as they direct transcription termination. A protein factor that specifically binds to 27-bp repeated sequences located at the barrier has been proposed also to be involved in the RFBs of pea rDNA (28).
Contrary to what happens in the mouse, neither the rRNA transcription termination factor Reb1p nor its rDNA binding sequence seems to be involved in the RFB of S. cerevisiae (4, 41). These observations suggest that the molecular mechanism that regulates rDNA replication arrest diverged through evolution. In the present work, we found three independent closely spaced RFBs in the fission yeast rDNA. Two of these RFBs required both the transcription termination factor reb1p and its two binding sites near the 3′ end of the 25S gene, whereas the other RFB functioned in the absence of these cis- and trans-acting factors. Therefore, Schizosaccharomyces pombe could be a transitional species in which the mechanisms operating in budding yeast and mammals coexist.
MATERIALS AND METHODS
Yeast strains and growth conditions.
The S. pombe strains used were 972 h− (wt h−), 35 (h− leu1-32), 117 (h− leu1-32 ura4-d18 ade6-M210), 118 (h+ leu1-32 ura4-d18 ade6-M216), 117×118 (h−/h+ leu1-32/leu1-32 ura4-d18/ura4-d18 ade6-M210/ade6-M216), D7 (h− leu1-32 ura4-d18 ade6-M210 reb1Δ::kanMX6+), and D8 (h− leu1-32 ura4-d18 ade6-M210 reb1+). Media and growth conditions used were as previously described (31). All pIRT2-derived plasmids containing S. pombe rDNA fragments were transformed by electroporation (34). Escherichia coli strain DH5α was used for recombinant DNA procedures.
Construction of plasmids containing rDNA sequences.
Plasmid pBL1263, containing a complete repeat of S. pombe rDNA (23), was the source of the sequences analyzed for RFB activity. Autonomously replicating plasmid pIRT2 (14) was used as a vector. For convenience, the HindIII insert in pBL1263 was first cloned into the HindIII site of pUC18 generating plasmid pHH10.4. Plasmids pIRT1.6(+) and pIRT1.6(−) were obtained by inserting the blunt-ended 1.6-kb XhoI-HindIII rDNA fragment from pHH10.4 into the SmaI site of pIRT2. The (+) plasmids contained the insert in the orientation expected to block fork progression. Construction of plasmid pRebs was carried out as follows. A PCR product was obtained using primer 1 (5′-CCCCTGCAGTTTTGAAGAGATAAAAGG-3′) and primer 2 (5′-CCCGGATCCTTTTACTAGGATTTGTGC-3′) on pIRT1.6(+). Each primer contained 18 nucleotides (underlined) that annealed a few nucleotides upstream from the 5′ reb1p binding sequence (primer 1) or downstream from the 3′ reb1p binding sequence (primer 2), a PstI (primer 1) or BamHI (primer 2) restriction site and a CCC tail. This fragment was PstI+BamHI digested and inserted into the polycloning site of pIRT2 near ars1. Thus, the 229-bp fragment cloned in pRebs contained, in addition to both reb1p binding sites and the sequence between them, 15 bp upstream from the 5′ binding sequence and 14 bp downstream from the 3′ binding sequence. For construction of plasmids pRebBS(+) and pRebBS(−), a double-stranded oligonucleotide containing the 17-bp reb1p binding site (5′-AGGTAAGGGTAATGCAC-3′) (44) was inserted in both orientations into the PstI and BamHI sites of the pIRT2 polycloning site. To direct the ligation to the desired orientation, the oligonucleotide contained PstI and BamHI sticky ends. Appropriate insertion was checked by sequencing. For the construction of plasmid pRebsΔBS, a PCR product was obtained by using oligonucleotides 5′-AAGGCCTAAATCCTAGTAAAAGGATC-3′ and 5′-AAGGCCTTTTCCCTTCAAAAAG-3′. These primers annealed divergently next to each side of the reb1p binding sequence closer to ars1 in pRebs (underlined nucleotides) and contained a StuI site at their 5′ ends (boldface nucleotides). After digestion with StuI, the PCR product was ligase-mediated circularized. For the construction of pRebsSEP, first a StuI site was created by PCR between both reb1p binding sequences of plasmid pRebs. This StuI site was located 60 bp from the binding site closer to ars1 and at 106 bp from the other binding site. Then, the 787-bp EcoRV-NruI fragment from the pBR322 tetracycline resistance gene was cloned into this StuI site.
Deletion of reb1+ gene.
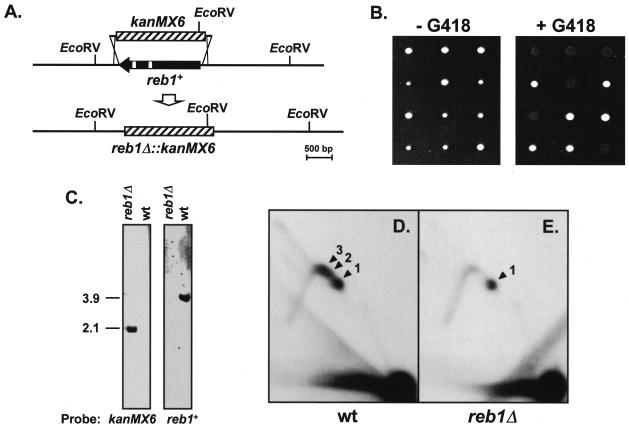
Deletion of the reb1+ gene was achieved by PCR-mediated replacement of the complete open reading frame by the kanMX6+ gene (1). PCRs were performed with two 84-nucleotide-long primers (5′-GATATTAGCG ATTGATAAGT TGAAGTGATT ACTCAATTAT AGTACTTCAA AAATATAATC CGCCAGGGTT TTCCCAGTCA CGAC-3′ and 5′-ATTGTAAGGA CGTCAATTGG AGAATCCAGA AAGTACCACT TTAAAGTCAT CAATGGCTGA AGCGGATAAC AATTTCACAC AGGA-3′), where the first 60 nucleotides (underlined) corresponded to sequences flanking the reb1+ open reading frame. The remaining 24 nucleotides of each primer corresponded to sequences located at either side of the pBluescript SK(+) polycloning site, where kanMX6+ was cloned (a gift from S. Moreno). Transformation of S. pombe 117×118 diploid strain with the PCR fragment and selection of G418-resistant diploids were performed as described previously (1). Genomic DNA from selected transformants was digested with EcoRV, electrophoresed, and hybridized with a probe specific for kanMX6+ gene to confirm its integration. reb1+/reb1Δ diploids were induced to form spores to allow further analysis by tetrad separation. The four spores from selected asci were again checked for integration of the kanMX6+ gene as described above (see Fig. 5C).
FIG. 5.
Deletion of the reb1+ gene. (A) Diagrammatic representation of the reb1+ gene deletion by PCR-mediated disruption. One allele of the reb1+/reb1+ diploid strain 117×118 was replaced by the G418-resistant gene kanMX6+ (hatched box). Open small boxes within reb1+ represent introns. The locations of two EcoRV restriction sites flanking reb1+ and an additional one within kanMX6+ are indicated. (B) Tetrad analysis of three selected heterozygous diploids. Spores from three asci were separated and grown in yeast extract with supplements without G418 (left panel). Colonies were then replicated onto a new plate containing 100 μg of G418/ml (right panel) to determine the segregation of the alleles. (C) Southern blot verifying the deletion of reb1+ gene by replacement with kanMX6+. EcoRV-digested DNA from reb1Δ and wild-type haploid cells was hybridized with a probe specific for kanMX6+ (left panel) or reb1+ (right panel). The kanMX6+ probe hybridized to the expected 2.1-kb restriction fragment from reb1Δ DNA, containing most of the kanMX6+ gene (see bottom map in panel A) and did not hybridize to the DNA from wild-type cells. As expected, the reb1+ probe did not hybridize to the DNA from reb1Δ cells but detected the 3.9-kb fragment containing reb1+ in the DNA from wild-type cells. (D and E) 2D gels of plasmid pIRT1.6(+) replicating in wild-type (wt) and reb1Δ cells, respectively. The restriction fragment analyzed was the same as in Fig. 2C. Arrowheads labeled 1 to 3 point to the spots of accumulated replication intermediates induced by the three barriers in wt cells. Note that in reb1Δ cells only the spot generated by RFB1 remained. The probe used in these autoradiograms was the same as for Fig. 2.
Two-dimensional gel electrophoresis.
Genomic and plasmid DNAs for two-dimensional (2D) gel analysis were isolated from asynchronous log phase cultures by using the procedures described by Caddle and Calos (5) (for plasmid analysis) or by Huberman and coworkers (15) (for genomic rDNA analysis). Electrophoresis conditions were as described in reference 9.
RESULTS
S. pombe rDNA contains three closely spaced RFBs.
The S. pombe genome contains ∼100 copies of rRNA genes organized in two arrays near both ends of chromosome III (29, 33). Two 17-bp binding sequences for the transcription termination factor reb1p, separated by 166 bp, are present in the nontranscribed spacer (NTS) close to the 3′ end of the 25S gene (Fig. 1) (44). Replication of S. pombe rDNA was analyzed by 2D agarose gel electrophoresis. DNA from exponentially growing strain 972 h− cells was digested with the restriction enzymes indicated, separated in 2D gels, transferred, and hybridized with probes specific for a series of overlapping fragments covering the rDNA repeat (Fig. 1, fragments A through E). Analysis of fragment A showed a spot corresponding to an accumulated Y-shaped replication intermediate (Fig. 1A, arrow), confirming previous observations of a replication barrier in S. pombe rDNA (26, 37). However, the elongated appearance of this signal suggested that forks stalled at several sites rather than at a single site. 2D gel analysis of the overlapping fragment B supported this possibility. Three independent spots were identified on the descending portion of the simple-Y arc (Fig. 1B), indicating that replication stalled at three alternative sites, herein called RFB1, RFB2, and RFB3. Equivalent spots on the ascending portion of the arc were absent, indicating that, as in other species, these three RFBs were polar, arresting only forks moving against the direction of transcription (leftwards in the map of Fig. 1). The intensities of the spots of accumulated replication intermediates were clearly different. The strongest signal corresponded to the first pausing site that leftward moving forks encounter (RFB1). The middle site, RFB2, gave the weakest signal, and RFB3 produced a signal of intermediate intensity. Replication analysis of fragment C (HindIII-SalI) showed no spots of accumulated intermediates (Fig. 1C), indicating that DNA sequences involved in fork stalling were located to the left of the HindIII site. Two additional fragments, corresponding to the coding region, revealed no replication impediment, as uniform simple-Y arcs were observed (Fig. 1D and E).
FIG. 1.
Analysis of S. pombe chromosomal rDNA replication by 2D agarose gel electrophoresis. The upper diagram represents an rDNA repeat unit, where the regions coding for the mature rRNAs (black boxes), the NTS, and the 17-bp reb1p binding sequences (striped boxes) are indicated. Restriction site abbreviations: B, BamHI; BII, BglII; H, HindIII; K, KpnI; S, SalI; X, XhoI. Horizontal lines labeled A through E represent the restriction fragments analyzed in the panels below. The arrow in panel A points to the elongated signal of accumulated replication intermediates. Arrowheads in panel B indicate three spots of accumulated intermediates labeled 1, 2, and 3, which correspond to barriers RFB1, RFB2, and RFB3, respectively. The open arrowhead in panel C points to the signal corresponding to replication intermediates containing a growing bubble. The probe used for 2D gels in panels A, B, and C was the 3.0-kb HindIII-KpnI fragment spanning most of the NTS and the 5′ end of the transcription unit. The probe used for 2D gels in panels D and E was the 3.5-kb SalI-BglII fragment within the coding region.
A bubble arc was also visible in the 2D gel corresponding to fragment C (Fig. 1C), indicating that a replication origin located within this fragment fires in a fraction of rDNA repeats. This observation is in agreement with the previous identification of an autonomously replicating sequence (ARS) (ars3001) in the NTS of S. pombe rDNA (37).
All three S. pombe RFBs are active in autonomously replicating plasmids with the same polarity and relative efficiency as in the chromosome.
As described above, S. pombe RFBs are located in the NTS, 5′ to the HindIII site. Therefore, the 1.6-kb XhoI-HindIII restriction fragment of S. pombe rDNA (Fig. 2A) was cloned in both orientations close to the ars1 replication origin of vector pIRT2 (Fig. 2B). This fragment contained the NTS portion lying to the left of the HindIII site and the 3′ end of the 25S gene. In pIRT1.6(+) the inserted sequence is replicated in the direction in which the barriers are active in the chromosome, whereas in pIRT1.6(−) the insert is replicated in the opposite direction. Replication of these plasmids in strain 35 was analyzed by 2D gel electrophoresis after double digestion with PvuII and EcoRV, using a specific probe to detect the fragment containing the insert. Locating the insert close to ars1 ensured that the clockwise-moving fork would reach the RFBs before the fork moving counterclockwise entered the fragment analyzed. If the RFBs were active, simple-Y-shaped intermediates of this fragment would accumulate while the counterclockwise fork replicated the other fragment. The results obtained are shown in Fig. 2C and D. All three RFBs observed in the chromosomal context were also detected in pIRT1.6(+), visualized as three spots of accumulated intermediates on the Y arc (Fig. 2C). The relative intensities of these spots fitted well with those observed in the chromosome. No arrest sites were detected in pIRT1.6(−) (Fig. 2D), indicating that all three RFBs retained the same polarity as in the chromosomal context. No additional barriers were detected within the rightward HindIII-BamHI fragment of the NTS (not shown).
FIG. 2.
All three S. pombe RFBs are active in autonomously replicating plasmids with the same polarity as in their chromosomal context. (A) Detail of the rDNA NTS. The horizontal line represents the 1.6-kb XhoI-HindIII restriction fragment analyzed below, which includes both 17-bp reb1p binding sequences (striped boxes). (B) Diagram of plasmids pIRT1.6(+) and pIRT1.6(−) obtained by inserting the XhoI-HindIII fragment (internal arc) in both orientations into pIRT2. The ars1-containing fragment (large striped box) and the site where replication initiates (black rhomb) (11) are included. (C) 2D gel analyzing replication of the PvuII-EcoRV fragment of pIRT1.6(+) containing the rDNA insert. As in Fig. 1B, arrowheads labeled 1, 2, and 3 point to the spots of accumulated replication intermediates induced by RFB1, RFB2, and RFB3, respectively. (C') A longer exposure of the autoradiogram shown in panel C in which the signals corresponding to bubble-containing intermediates (open arrowhead) and double Ys (arrow) are visible. (D) 2D gel analyzing replication of the same fragment as in panel C but from pIRT1.6(−), in which no accumulated intermediates were detected. The probe used in both autoradiograms was the PvuII-EcoRV fragment from vector pIRT2.
A longer exposure of the same gel as that shown in Fig. 2C allowed detection of a partial bubble arc (Fig. 2C'), generated upon bidirectional replication from ars1. In addition, a straight line emerged from the spots of accumulated intermediates extending upward and to the left in a diagonal fashion (Fig. 2C'). This signal corresponded to double-Y intermediates generated when the counterclockwise-advancing fork entered the fragment until it encountered the clockwise fork arrested at the barriers. These observations showed that in a significant fraction of the plasmid molecules replication termination occurred at the barriers.
Location of RFB1.
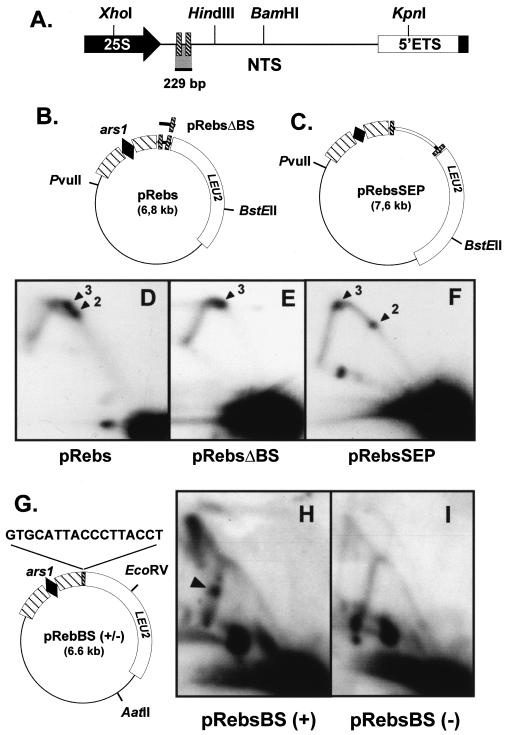
The spots of accumulated intermediates showed in Fig. 2 appeared on the simple-Y arc to the right of the inflexion point. This indicated that RFBs were located closer to the HindIII site. Therefore, to locate the DNA sequence required for RFB1, we analyzed a fragment spanning 383 bp next to the HindIII site (Fig. 3A). This fragment was cloned into pIRT2 and two restriction fragments of the resulting plasmid (p3′Rebs) (Fig. 3B) were analyzed. 2D gels showed a single strong spot of a specific Y-shaped intermediate corresponding to RFB1 (Fig. 3C and D). Replication termination structures were also visible (Fig. 3C and D), indicating termination at the barrier.
FIG. 3.
Location of barrier RFB1. (A) NTS of S. pombe rDNA where the location of the 383-bp sequence analyzed below is indicated. (B) Diagram of plasmid p3′Rebs obtained upon insertion of the aforementioned 383-bp sequence (internal arc) between ars1 and LEU2 of pIRT2. (C and D) 2D gels of the PvuII-BstEII and AatII-EcoRV fragments of p3′Rebs, respectively. Black arrowhead points to the spot of accumulated replication intermediates induced by RFB1. The open arrowhead indicates the arc of bubble-containing intermediates. The arrow points to the signal generated by replication termination structures due to fork meeting at the barrier. The probe used was the same as that for Fig. 2.
The 17-bp binding sequence for the rRNA transcription terminator factor reb1p is required and sufficient to induce replication fork arrest at RFB2 and RFB3.
As mentioned before, S. pombe rDNA contains two identical 17-bp binding sequences for the transcription termination protein reb1p (30, 44). Both termination signals are included in the fragment where RFBs were mapped (Fig. 2). To address if these binding sequences are also DNA cis-acting elements for the remaining barriers RFB2 and RFB3, we tested the capacity of a 229-bp fragment, containing both reb1p binding sites (Fig. 4A), to arrest replication forks. This fragment was cloned in the proper orientation into pIRT2 (pRebs) (Fig. 4B). The 2D gel of the PvuII-BstEII fragment containing the insert showed two signals of accumulated intermediates at the expected positions on the simple-Y arc (Fig. 4D), indicating that this fragment contains the cis-acting signals required for RFB2 and RFB3. We removed one of the 17-bp binding sequences (the one closer to ars1) from this plasmid (the modified insert is shown in Fig. 4B). As a consequence of this deletion, one of the spots was missing in the 2D gel of the resulting plasmid pRebsΔBS and only the spot closer to the inflexion of the Y arc remained (Fig. 4E). These results indicated that reb1p binding sites are essential cis-acting signals for RFB2 and RFB3 and that they function independently. When both binding sites were placed further apart by inserting a 787-bp sequence between them (Fig. 4C), the two fork arrest positions also appeared to be separated from each other as indicated by the new relative locations of the spots in the corresponding 2D gel (compare panels F and D of Fig. 4). In addition, we found that the 17-bp sequence was not only necessary but also sufficient for fork arrest. A synthetic sequence identical to the reb1p binding site inserted into pIRT2 (Fig. 4G) induced fork arrest in the (+) orientation (Fig. 4H) and had no effect in the opposite (−) orientation (Fig. 4I).
FIG. 4.
Characterization of barriers RFB2 and RFB3. (A) Detail of the NTS indicating the location of the 229-bp sequence analyzed below. This sequence contains both 17-bp reb1p binding sites (striped boxes). (B) Diagram of plasmid pRebs obtained upon insertion of the above-mentioned 229-bp sequence between ars1 and LEU2 of pIRT2. The modified insert in pRebsΔBS, where one of the reb1p binding sites was removed, is also shown. (C) Diagram of pRebs-derived pRebsSEP plasmid, in which both reb1p binding sites were separated further by inserting a 787-bp sequence between them (open box). (D through F) 2D gel analyses of the PvuII-BstEII fragment from pRebs, pRebsΔBS, and pRebsSEP, respectively. Arrowheads point to the spots of accumulated replication intermediates induced by barriers RFB2 and RFB3. (G) Diagram of plasmids pRebBS(+) and pRebBS(−), where the 17-bp reb1p binding sequence was inserted in both orientations into pIRT2. Only the (+) orientation of the sequence is indicated above the diagram. (H and I) 2D gels of the AatII-EcoRV fragment of pRebBS(+) and pRebBS(−), respectively. The arrowhead in panel H points to the spot of the accumulated replication intermediate induced by the reb1p binding sequence only in the (+) orientation. The probe used in these autoradiograms was the same as that used for Fig. 2.
Deletion of reb1+ abolishes fork arrest at RFB2 and RFB3.
Since the reb1p binding sequence was necessary and sufficient to induce polar replication fork arrest, we regarded this transcription termination protein as a candidate to be involved in RFB2 and RFB3, even though the S. cerevisiae homologue Reb1p is not involved in rDNA RFBs (41).
To analyze the function of S. pombe reb1p in RFB activity, we constructed a heterozygous reb1+/reb1Δ diploid strain. The null allele was obtained by PCR-mediated gene replacement of reb1+ by the selectable gene marker kanMX6+, which confers resistance to the antibiotic G418 (Fig. 5A). Dissections of three representative tetrads of the G418-resistant reb1+/reb1Δ diploids are shown in Fig. 5B. In the absence of G418 all four spores were viable, indicating that reb1+ is not an essential gene in S. pombe (Fig. 5B, left panel). However, two spores of each tetrad gave colonies slightly smaller than the other two. In all cases the smaller colonies were those generated by reb1Δ::kanMX6+ spores, as they grew in the presence of G418 (Fig. 5B, right panel). Replacement of reb1+ by kanMX6+ was confirmed by Southern blotting (Fig. 5C) and PCR analysis (not shown). Therefore, deletion of reb1+ gene was not lethal, but mutant cells grew somewhat more slowly than wild-type cells. This small effect of reb1+ deletion on cell growth was confirmed by dilution assays (data not shown).
To address the involvement of reb1p in the barriers, replication of pIRT1.6(+) containing all three RFBs was analyzed in isogenic reb1+ and reb1Δ haploid strains. As shown in Fig. 5D and E, in the reb1Δ strain the spots corresponding to RFB2 and RFB3 disappeared and only the one generated by RFB1 remained. Similar results were obtained upon analysis of plasmids pRebs and pRebBS(+) (data not shown). This observation demonstrates that the transcription termination factor reb1p is required for RFB2 and RFB3.
DISCUSSION
In S. pombe, the rRNA transcription termination factor reb1p has been recently identified, showing sequence similarity with S. cerevisiae Reb1p and mouse m-TTF1 (44). These three termination factors share myb-like DNA binding domains. S. pombe reb1p has two identical 17-bp binding sites that block read-through transcription in vitro (44). In addition, reb1p also causes in vitro 3′-end RNA formation at two sites of S. pombe rDNA that correspond to the transcription termination sites determined in vivo (38, 44).
In this work we have identified three RFBs in the S. pombe rDNA. Fork arrest at two of these RFBs (RFB2 and RFB3) is produced upon binding of the transcription termination protein reb1, in a fashion similar to what has been proposed to occur in mouse (27, 35). Removal of the reb1p binding sequence or deletion of reb1+ completely abolished replication blockage at RFB2 and RFB3 (Fig. 4E and 5E). Therefore, upon binding to its cognate sequence, reb1p inhibits both rRNA transcription and rDNA replication occurring with opposite directions, thus preventing head-on collision of both machineries. It cannot be ruled out that other factor(s) besides reb1p are also required for fork arrest at RFB2 and RFB3. On the other hand, fork arrest at RFB1 is independent of this transcription termination factor, since it remains active in a reb1Δ mutant strain (Fig. 5E). Moreover, we have found a reb1p-unrelated protein that specifically binds to a short sequence within the RFB1-containing fragment analyzed in Fig. 3 (unpublished data). Thus, S. pombe RFB1 seems to be similar to the two barriers found in budding yeast, as both of them are normal in strains with a temperature-sensitive allele of REB1 growing at restrictive temperature (41).
Therefore, two different and independent mechanisms operate in S. pombe rDNA to arrest replication forks. Remarkably, although different trans- and cis-acting factors are involved in these two mechanisms, both of them block replication progression in a polar fashion. This indicates that polarity of rDNA barriers is an essential feature in accomplishing their biological role, which may be to avoid or regulate head-on collision between transcription and replication.
Besides the RFBs present in the rDNA, another barrier, named RTS1, has been described for S. pombe. RTS1 is involved in the mating-type switching by determining the direction of replication at the mat1 locus (6, 7). As in the case of rDNA RFBs, RTS1 is a polar barrier that contains a cluster of three full-length and one truncated ∼60-bp imperfect direct repeats (7). Interestingly, each of these repeats includes a sequence that shows homology to the 17-bp reb1p binding sequence required for RFB2 and RFB3. Moreover, according to our findings, the orientation of these homologous sequences is in agreement with the reported polarity of RTS1 (7). This observation raises the possibility that reb1p also plays a role in fork arrest at the mat1 locus.
It is interesting that whereas REB1 is an essential gene in S. cerevisiae (16), deletion of reb1+ in S. pombe has only a weak effect on cell growth (Fig. 5B and D). Besides its function in RNA polymerase I transcription termination (22, 36), S. cerevisiae Reb1p regulates the expression of several unrelated RNA polymerase II transcribed genes (see reference 40 and references therein). In addition, Reb1p binding sites are also present in the subtelomeric X and Y′ regions in budding yeast (8). Thus, the essential nature of Reb1p in S. cerevisiae could be due to the function(s) that this protein performs at these additional sites, but not at the rDNA locus. In agreement with this hypothesis, it was recently shown that deletion of the Reb1p binding site in all rDNA chromosomal repeats of S. cerevisiae has no effect on cell growth or rRNA synthesis (39). Altogether, these results suggest that, at least in these two species, in the absence of the cis- or trans-acting factors currently known to be involved in rRNA transcription termination, rRNA transcripts are terminated by an alternative unknown pathway and processed properly to form functional ribosomes. It would be worthy to investigate if factors involved in S. pombe RFB1 play a role in this alternative pathway.
Acknowledgments
We are grateful to S. Moreno and members of his group for their invaluable help in deleting the reb1+ gene. We are also indebted to F. Antequera and his group for their support in Salamanca. We thank E. Mejía, A. Benguria, L. Olavarrieta, and R. Torres for stimulating discussions and help throughout the course of this study, B. Lapeyre for providing plasmid pBL1263, J. Jiménez for the S. pombe strain 35, and M. Martínez-Robles and P. Robles for technical assistance.
This work was partially supported by Spanish grants 99/0850 from the Fondo de Investigación Sanitaria (Ministerio de Sanidad y Consumo), SAF2001-1740 from the Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica (Ministerio de Ciencia y Tecnología), PB98-048 from the Comisión Interministerial de Ciencia y Tecnología (CICYT), and a fellowship from the Consejería de Educación de la Comunidad de Madrid-Fondo Social Europeo to A.S.-G.
REFERENCES
- 1.Bähler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie III, A. B. Steever, A. Wach, P. Philippsen, and J. R. Pringle. 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14:943-951. [DOI] [PubMed] [Google Scholar]
- 2.Benguría, A., P. Hernández, D. B. Krimer, and J. B. Schvartzman. 2003. Sir2p suppresses recombination of replication forks stalled at the replication fork barrier of ribosomal DNA in Saccharomyces cerevisiae. Nucleic Acids Res. 31:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewer, B. J., and W. L. Fangman. 1988. A replication fork barrier at the 3′ end of yeast ribosomal RNA genes. Cell 55:637-643. [DOI] [PubMed] [Google Scholar]
- 4.Brewer, B. J., D. Lockshon, and W. L. Fangman. 1992. The arrest of replication forks in the rDNA of yeast occurs independently of transcription. Cell 71:267-276. [DOI] [PubMed] [Google Scholar]
- 5.Caddle, M. S., and M. P. Calos. 1994. Specific initiation at an origin of replication from Schizosaccharomyces pombe. Mol. Cell. Biol. 14:1796-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalgaard, J. Z., and A. J. S. Klar. 2000. swi1 and swi3 perform imprinting, pausing, and termination of DNA replication in S. pombe. Cell 102:745-751. [DOI] [PubMed] [Google Scholar]
- 7.Dalgaard, J. Z., and A. J. S. Klar. 2001. A DNA replication-arrest site RTS1 regulates imprinting by determining the direction of replication at mat1 in S. pombe. Genes Dev. 15:2060-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fourel, G., E. Revardel, C. E. Koering, and E. Gilson. 1999. Cohabitation of insulators and silencing elements in yeast subtelomeric regions. EMBO J. 18:2522-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman, K. L., and B. J. Brewer. 1994. The analysis of replication intermediates by two-dimensional agarose gel electrophoresis. Methods Enzymol. 262:613-627. [DOI] [PubMed] [Google Scholar]
- 10.Gerber, J.-K., E. Göegel, C. Berger, M. Wallisch, F. Müller, I. Grummt, and F. Grummt. 1997. Termination of mammalian rDNA replication: polar arrest of replication fork movement by transcription termination factor TTF-I. Cell 90:559-567. [DOI] [PubMed] [Google Scholar]
- 11.Gomez, M., and F. Antequera. 1999. Organization of DNA replication origins in the fission yeast genome. EMBO J. 18:5683-5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grummt, I., U. Maier, A. Ohrlein, N. Hassouna, and J. P. Bachellerie. 1985. Transcription of mouse rDNA terminates downstream of the 3′ end of 28S RNA and involves interaction of factors with repeated sequences in the 3′ spacer. Cell 43:801-810. [DOI] [PubMed] [Google Scholar]
- 13.Hernández, P., L. Martín-Parras, M. L. Martínez-Robles, and J. B. Schvartzman. 1993. Conserved features in the mode of replication of eukaryotic ribosomal RNA genes. EMBO J. 12:1475-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hindley, J., G. Phear, M. Stein, and D. Beach. 1987. Suc11encodes a predicted 13-kilodalton protein that is essential for cell viability and is directly involved in the division cycle of Schizosaccharomyces pombe. Mol. Cell. Biol. 7:504-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huberman, J. A., L. D. Spotila, K. A. Nawotka, S. M. El-Assouli, and L. R. Davis. 1987. The in vivo replication origin of the yeast 2 microns plasmid. Cell 51:473-481. [DOI] [PubMed] [Google Scholar]
- 16.Ju, Q. D., B. E. Morrow, and J. R. Warner. 1990. REB1, a yeast DNA-binding protein with many targets, is essential for growth and bears some resemblance to the oncogene myb. Mol. Cell. Biol. 10:5226-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaeberlein, M., M. McVey, and L. Guarente. 1999. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13:2570-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi, T., D. J. Heck, M. Nomura, and T. Horiuchi. 1998. Expansion and contraction of ribosomal DNA repeats in Saccharomyces cerevisiae: requirement of replication fork blocking (Fob1) protein and the role of RNA polymerase I. Genes Dev. 12:3821-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi, T., and T. Horiuchi. 1996. A yeast gene product, Fob1 protein, required for both replication fork blocking and recombinational hotspot activities. Genes Cells 1:465-474. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi, T., M. Nomura, and T. Horiuchi. 2001. Identification of DNA cis elements essential for expansion of ribosomal DNA repeats in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:136-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhn, A., I. Bartsch, and I. Grummt. 1990. Specific interaction of the murine transcription termination factor TTF I with class-I RNA polymerases. Nature 344:559-562. [DOI] [PubMed] [Google Scholar]
- 22.Lang, W. H., and R. H. Reeder. 1993. The REB1 site is an essential component of a terminator for RNA polymerase I in Saccharomyces cerevisiae. Mol. Cell. Biol. 13:649-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapeyre, B., B. Michot, J. Feliu, and J. P. Bachellerie. 1993. Nucleotide sequence of the Schizosaccharomyces pombe 25S ribosomal RNA and its phylogenetic implications. Nucleic Acids Res. 21:3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linskens, M. H. K., and J. A. Huberman. 1988. Organization of replication of ribosomal DNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 8:4927-4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little, R. D., T. H. K. Platt, and C. L. Schildkraut. 1993. Initiation and termination of DNA replication in human rRNA genes. Mol. Cell. Biol. 13:6600-6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López-Estraño, C., J. B. Schvartzman, and P. Hernández. 1997. The replication of ribosomal RNA genes in eukaryotes. Chromosomes Today 12:149-169. [Google Scholar]
- 27.López-Estraño, C., J. B. Schvartzman, D. B. Krimer, and P. Hernández. 1998. Co-localization of polar replication fork barriers and rRNA transcription terminators in mouse rDNA. J. Mol. Biol. 277:249-256. [DOI] [PubMed] [Google Scholar]
- 28.López-Estraño, C., J. B. Schvartzman, D. B. Krimer, and P. Hernández. 1999. Characterization of the pea rDNA replication fork barrier: putative cis-acting and trans-acting factors. Plant Mol. Biol. 40:99-110. [DOI] [PubMed] [Google Scholar]
- 29.Maleszka, R., and G. D. Clark-Walker. 1993. Yeasts have a four-fold variation in ribosomal DNA copy number. Yeast 9:53-58. [DOI] [PubMed] [Google Scholar]
- 30.Melekhovets, Y. F., P. S. Shwed, and R. N. Nazar. 1997. In vivo analyses of RNA polymerase I termination in Schizosaccharomyces pombe. Nucleic Acids Res. 25:5103-5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795-823. [DOI] [PubMed] [Google Scholar]
- 32.Olavarrieta, L., P. Hernández, D. B. Krimer, and J. B. Schvartzman. 2002. DNA knotting caused by head-on collision of transcription and replication. J. Mol. Biol. 322:1-6. [DOI] [PubMed] [Google Scholar]
- 33.Pasero, P., and M. Marilley. 1993. Size variation of rDNA clusters in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Mol. Gen. Genet. 236:448-452. [DOI] [PubMed] [Google Scholar]
- 34.Prentice, H. L. 1992. High efficiency transformation of Schizosaccharomyces pombe by electroporation. Nucleic Acids Res. 20:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pütter, V., and F. Grummt. 2002. Transcription termination factor TTF-I exhibits contrahelicase activity during DNA replication. EMBO Rep. 3:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeder, R. H., P. Guevara, and J. G. Roan. 1999. Saccharomyces cerevisiae RNA polymerase I terminates transcription at the Reb1 terminator in vivo. Mol. Cell. Biol. 19:7369-7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez, J. A., S. M. Kim, and J. A. Huberman. 1998. Ribosomal DNA replication in the fission yeast, Schizosaccharomyces pombe. Exp. Cell Res. 238:220-230. [DOI] [PubMed] [Google Scholar]
- 38.Shwed, P. S., and R. N. Nazar. 1999. Terminator element mutations affect both the efficiency and position of RNA polymerase I termination in Schizosaccharomyces pombe. Nucleic Acids Res. 27:2883-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wai, H., K. Johzuka, L. Vu, K. Eliason, T. Kobayashi, T. Horiuchi, and M. Nomura. 2001. Yeast RNA polymerase I enhancer is dispensable for transcription of the chromosomal rRNA gene and cell growth, and its apparent transcription enhancement from ectopic promoters requires Fob1 protein. Mol. Cell. Biol. 21:5541-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, K. L., and J. R. Warner. 1998. Positive and negative autoregulation of REB1 transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:4368-4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward, T. R., M. L. Hoang, R. Prusty, C. K. Lau, R. L. Keil, W. L. Fangman, and B. J. Brewer. 2000. Ribosomal DNA replication fork barrier and HOT1 recombination hot spot: shared sequences but independent activities. Mol. Cell. Biol. 20:4948-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiesendanger, B., R. Lucchini, T. Koller, and J. M. Sogo. 1994. Replication fork barriers in the Xenopus rDNA. Nucleic Acids Res. 22:5038-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, Z., D. M. Macalpine, and G. M. Kapler. 1997. Developmental regulation of DNA replication: replication fork barriers and programmed gene amplification in Tetrahymena thermophila. Mol. Cell. Biol. 17:6147-6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao, A., A. Guo, Z. Liu, and L. Pape. 1997. Molecular cloning and analysis of Schizosaccharomyces pombe Reb1p: sequence-specific recognition of two sites in the far upstream rDNA intergenic spacer. Nucleic Acids Res. 25:904-910. [DOI] [PMC free article] [PubMed] [Google Scholar]