Abstract
Background:
Fast ripples (FR, 250-500 Hz) detected with chronic intracranial electrodes are proposed biomarkers of epileptogenesis. This study determined whether resection of FR-containing neocortex recorded during intraoperative electrocorticography (ECoG) was associated with postoperative seizure freedom in pediatric patients with mostly extratemporal lesions.
Methods:
FRs were retrospectively reviewed in 30 consecutive pediatric cases. ECoGs were recorded at 2,000 Hz sampling rate and visually inspected for FR, with reviewer blinded to the resection and outcome.
Results:
Average age at surgery was 9.1 ± 6.7 years, ECoG duration was 11.8 ± 8.1 minutes, and postoperative follow-up was 27 ± 4 months. FRs were undetected in 6 ECoGs with remote or extensive lesions. FR episodes (n = 273) were identified in ECoGs from 24 patients, and in 64% FRs were independent of spikes, sharp waves, voltage attenuation, and paroxysmal fast activity. Of these 24 children, FR-containing cortex was removed in 19 and all became seizure-free, including 1 child after a second surgery. The remaining 5 children had incomplete FR resection and all continued with seizures postoperatively. In 2 ECoGs, the location of electrographic seizures matched FR location. FR-containing cortex was found outside of MRI and FDG-PET abnormalities in 6 children.
Conclusion:
FRs were detected during intraoperative ECoG in 80% of pediatric epilepsy cases, and complete resection of FR cortex correlated with postoperative seizure freedom. These findings support the view that interictal FRs are excellent surrogate markers of epileptogenesis, can be recorded during brief ECoG, and could be used to guide future surgical resections in children.
GLOSSARY
- AED
= antiepileptic drug;
- ANOVA
= analysis of variance;
- CD
= cortical dysplasia;
- ECoG
= electrocorticography;
- FDG-PET
= 18fluoro-deoxyglucose PET;
- FR
= fast ripples;
- HFO
= high-frequency oscillation;
- TSC
= tuberous sclerosis complex;
- UCLA
= University of California, Los Angeles.
High-frequency oscillations (HFO) termed ripples (80-250 Hz) and fast ripples (FRs; 250-500 Hz) may be normal and physiologic in memory1 and somatosensory recordings.2 More recently, HFO has been identified in experimental models of temporal lobe epilepsy3–5 and subsequently in mesial temporal lobe epilepsy.6–11 FRs are hypothesized to be pathologic and a possible surrogate EEG cortical marker of epileptogenesis.3 Initially recorded with chronic extraoperative intracranial monitoring with microelectrodes, HFOs have been recorded with macroelectrodes during long-term invasive EEG monitoring.12,13 Other studies have shown that HFOs are not limited to mesial temporal lobe epilepsy, but can be neocortical and extratemporal in origin.14–16
The aims of this study were to investigate if FRs could be recorded with macroelectrodes during short intraoperative electrocorticography (ECoG) in pediatric epilepsy surgery patients. The other aim was to examine whether complete resection of FR-containing neocortex was associated with postoperative seizure freedom in children, with the operative team blinded to FR location at the time of surgical resection.
METHODS
Cohort.
Children with medically refractory epilepsy who underwent surgical resections at the University of California, Los Angeles (UCLA) Pediatric Epilepsy Surgery Program from August 2007 to September 2008 were consecutively and prospectively studied. Refractory epilepsy was defined as monthly or greater seizure frequency, and failure of a minimum of 3 first-line antiepileptic drugs.17
Standard protocol approvals, registrations, and patient consents.
The institutional review board at UCLA approved the use of human subjects and waived the need for written informed consent, as all testing was deemed clinically relevant for patient care. This study is not a clinical trial, and it is not registered in any public registry.
Patient evaluation.
All children with medically refractory epilepsy referred during the study period underwent a standardized presurgical evaluation, which consisted of inpatient video-EEG monitoring, high-resolution (1.5-Tesla) brain MRI, and 18fluoro-deoxyglucose PET (FDG-PET). When appropriate, magnetic source imaging, FDG-PET/MRI coregistration, neuropsychological assessment, intracarotid amobarbital test (Wada), and functional MRI were also obtained.18,19
Decisions for surgical candidacy and the resection zones were made by group consensus consisting of a case conference attended by adult and pediatric epileptologists, adult and pediatric epilepsy neurosurgeons, a neuroradiologist, and neuropsychologists. Surgical decisions were based on all presurgical test results for each patient. Two or more tests localizing to a region or hemisphere were typically necessary to propose that a patient was a surgical candidate, balanced between the probable success of epilepsy surgery and the potential loss of motor, sensory, visual, and language functions.
The clinical characteristics of these children were abstracted from the medical record. These included gender, age at seizure onset, age at surgery, seizure duration (time period between seizure onset and surgery), history of infantile spasms, active infantile spasms at the time of surgery, and the number of antiepileptic drugs (AEDs) at the time of surgery.19,20
ECoG recording.
All surgical resections had intraoperative ECoG recorded with macroelectrodes (AdTech, Racine, WI). Every ECoG was recorded at 2,000 Hz sampling rate with no low-pass or high-pass filter setting on the Harmonie long-term monitoring system (Alpine Biomed; Montreal, Canada). Intraoperative ECoG was performed using a standard surgical and anesthesia protocol whereby sevoflurane was reduced to 0.1% or less recorded end-tidal either at, or achieved shortly after, the start of the ECoG recording, so as to minimize potential interference from anesthesia.21,22 Patients were kept on nitrous oxide and narcotics with muscle paralysis during the ECoG, and incision and Mayfield pin sites were injected with local anesthesia (0.25% Marcaine with epinephrine). In addition, the patient's core body temperature was maintained above 35.5 °C. After the dura was opened, combinations of grids (4 × 5) and strips (1 × 4 or 1 × 8) were used, with 4-mm diameter electrode contact separated from adjacent electrodes by 10 mm from center to center. The findings on ECoG pertinent to the final resection zone included interictal spikes and sharp waves, interictal paroxysmal fast activity, continuous epileptiform discharges, focal slowing, and focal background voltage attenuation. In 2 cases, electrographic seizures were captured during ECoG. The final area of resection was determined by combining the video-EEG, neuroimaging, and MRI localization with the ECoG data and did not consider the location of FR, as this was recorded and reviewed separately (see below). To avoid ECoG sampling bias, at least 1 noncontiguous “control” region not suspected to be epileptogenic from presurgical testing was recorded, with a minimum of 2 lobes sampled for each ECoG. No child in this cohort underwent extraoperative intracranial recording.
FR identification.
Methods of FR review and FR identification were guided by Dr. Jean Gotman's group in Montreal, Canada (see Acknowledgment). Each ECoG was reviewed twice in a blinded fashion, such that the reviewer had no knowledge of the type and location of resection, the type and location of neuroimaging findings, and the location of the “control” region not suspected to be epileptogenic. The ECoG sample was first reviewed in its entirety with a referential montage (reference electrode on the contralateral frontal scalp), with a high-pass 240-Hz filter and a low-pass 500-Hz filter, at a maximally allowed time scale of 338 mm/s, to visually identify and mark FR-like episodes defined as 250- to 500-Hz events. This prevented potential bias of viewing epileptiform discharges and therefore selecting FR events occurring with these discharges. The entire record was reviewed a second time by a vertically split screen, with the above settings on the right screen, and no filters and standard time scale of 30 mm/s on the left screen to distinguish FR from artifacts. Events marked as FR were excluded if they were found to correspond to artifacts on the second review. FR events co-occurring with epileptiform discharges and voltage attenuation were noted during this second review. In both reviews, the maximum resolution corresponded to 1.6 seconds/screen on a 21-inch-wide screen, a finite impulse response filter was used to avoid ringing, and high-frequency oscillations were required to contain at least 4 consecutive peaks clearly visible above the background signal to be considered FR.13
The features for FR events were recorded for analysis in this study. This included the frequency (Hz) and voltage (μV) of each FR event, number of channels demonstrating independent FR events, the area (total number of channels) each independent FR spanned, the duration of each event (milliseconds), whether the FR event occurred before or after the anesthetic agent sevoflurane was lowered to the standard 0.1%, and whether each FR event co-occurred with spikes, sharp waves, paroxysmal fast activity, background voltage attenuation, or occurred independently. Total number of FR events was tallied for each ECoG, and whether the surgical resection included all, some, or none of the FR events. We assessed retrospectively whether the resection area would have been different if FR location was known prior to resection. In addition, whether FR was located inside or outside the MRI lesion, inside or outside the FDG-PET hypometabolism, or both, was also assessed.
Postoperative assessment.
Follow-up after surgical resection occurred at 6 months, 12 months, and 24 months after surgery. Seizure outcome was noted at each of these visits, and medications were adjusted when appropriate. Histopathologic diagnosis from each of the surgical resections was noted.
Data analysis.
Statistical results were obtained with the Statview software, version 5.0.1 for Windows (SAS Institute Inc., Cary, NC). A priori, significant results were considered at p < 0.05.
RESULTS
Cohort characteristics.
A total of 30 children with intractable epilepsy had their intraoperative ECoG assessed for FR consecutively from August 2007 to September 2008 (table). Average age (±SD) at the time of ECoG was 9.1 ± 6.7 years (range 8 months-20 years), with 15 female patients. The mean age at seizure onset was 4.2 ± 4.8 years (range birth-13.5 years), the mean seizure duration was 4.3 ± 3.3 years (range 7 months-12.5 years), and the mean number of AEDs at the time of surgery was 2.0 ± 1.0 (range 1-4). Six children had a history of infantile spasms, and 2 children had active infantile spasms at the time of surgery.
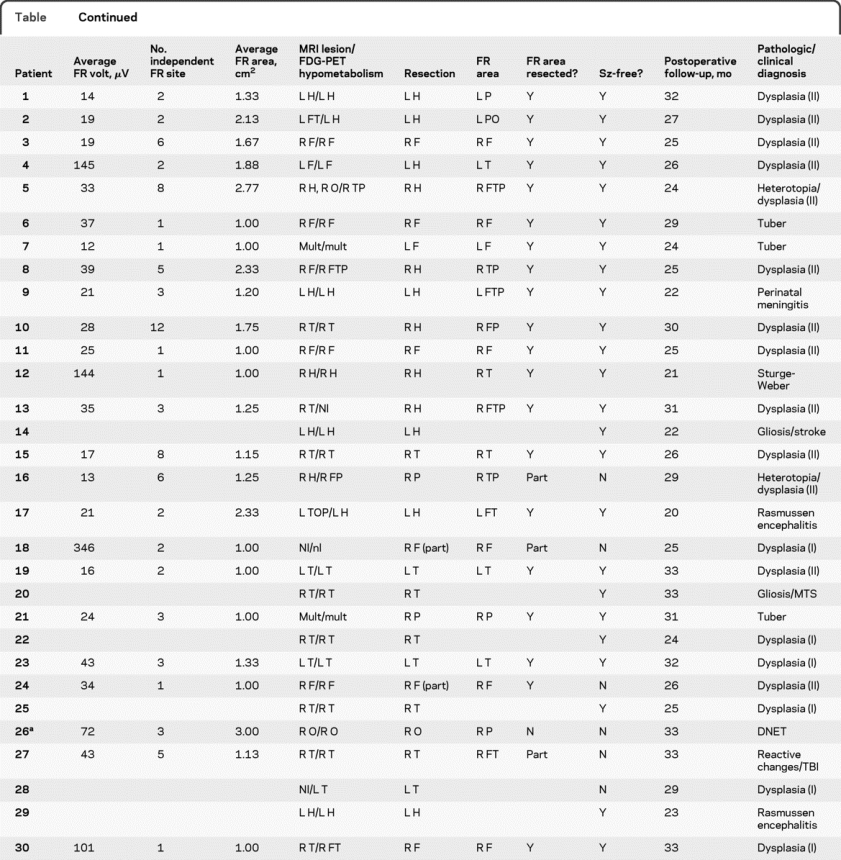
Table Clinical and ECoG parameters
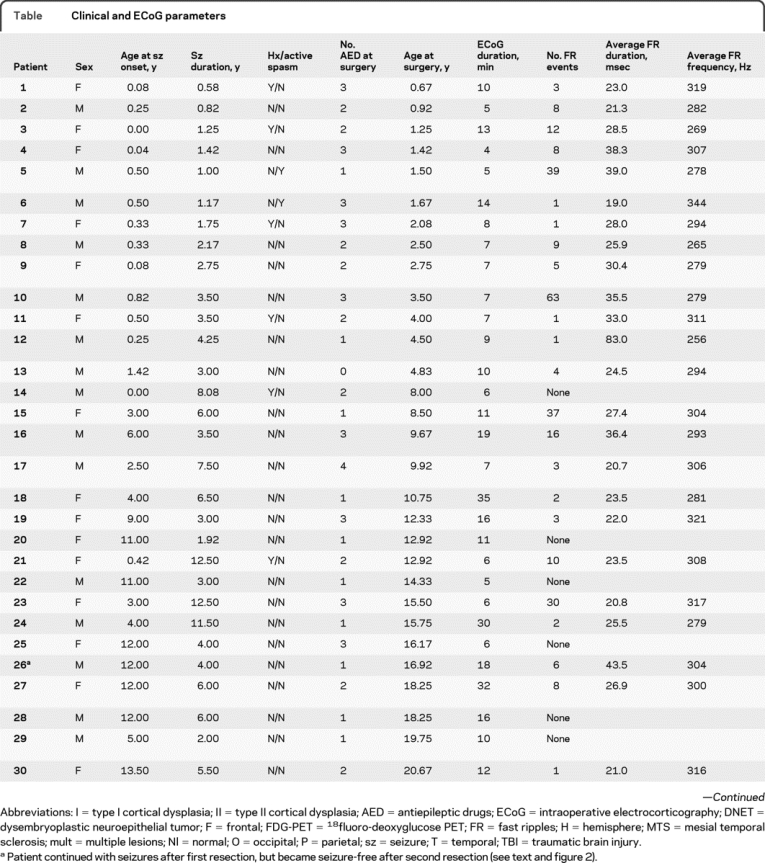
Table Continued
Type of surgery.
Twelve children had hemispherectomy, and 18 had lobar/focal resection (7 frontal, 8 temporal, 2 parietal, and 1 occipital). Most children in this series had extratemporal operations (table). As might be expected, children undergoing cerebral hemispherectomy were younger at age at seizure onset (0.9 ± 1.5 years) and surgery (5.0 ± 5.4 years) compared with children undergoing lobar/focal resections (seizure onset 6.3 ± 5.1 years, p = 0.0014; surgery 11.8 ± 6.1 years, p = 0.0044). The type of surgery was not associated with differences in seizure duration (p = 0.88).
ECoG sampling and FR findings.
Of the 30 ECoGs, 2 lobes were sampled in 10, 3 lobes were sampled in 18, and 4 lobes were sampled in 2 patients. The average ECoG duration was 11.7 ± 8.1 minutes (range 4-35 minutes). FR were not identified in ECoGs from 6 patients, while 273 distinct FR episodes were found in the remaining 24 ECoGs (range 1-63 FR per ECoG, average 11.4 ± 15.5). Of the 6 ECoGs that did not detect FR, 4 were children with anterior mesial temporal lesions (3 with cortical dysplasia and 1 case of hippocampal sclerosis) and grid and strip placement did not sample the mesial structures of neuroimaging abnormality; and 2 children had significant preexisting tissue loss at the time of surgery (1 was a completion hemispherectomy with Rasmussen encephalitis, and 1 a large perinatal MCA infarct).
When recorded, interictal FR events (figure 1) seemed to be markers of epileptogenic cortex. Spatially, all FR events were found in or near the suspected epileptogenic cortex based on neuroimaging, and no FR were found in the noncontiguous “control” cortical regions. FRs were accompanied by spikes in 59 (22%), by sharp waves in 29 (11%), by background voltage attenuation in 6 (2%), by paroxysmal fast activity in 3 (1%), and occurred independently of other EEG findings in 182 (64%) recorded events. Of the 2 intraoperative ECoGs in which seizures were captured, the location of FR recorded before the seizure matched the location of seizure onset by electrode location and number of electrodes. The number of FR events did not depend on whether the anesthetic agent sevoflurane was already below 0.1% or still slightly above but being reduced to 0.1% (p = 0.19, paired t test). The number of FR events did not correlate with the duration of ECoG recording (p = 0.43, paired t test). The average duration of FR events was 30 ± 13.1 msec (range, 19-83). The average amplitude was 54.2 ± 72.5 μV (range 12-346). The average frequency was 296 ± 21 Hz (range 256-344). The number of channels recording independent FR events ranged from 1 to 12. The area of electrode contact containing FR events averaged 1.5 ± 0.6 cm2 (range 1-8).
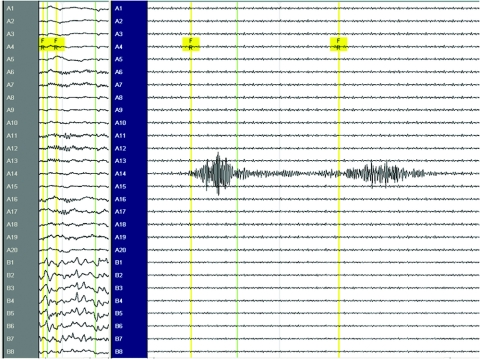
Figure 1 Example of interictal fast ripples (FR) during intraoperative electrocorticography (patient 17)
Note the split screen on the left with no filter settings at 30 mm/s paper speed, and on the right with FR filter settings of 240 and 500 Hz at the maximally allowed 338 mm/s paper speed. The 2 events of interictal FR measured 338 and 342 Hz at electrode 14 on the grid (A14), occurred independently of spikes and sharp waves on the strip (B1-8), and appeared adjacent to paroxysmal fast activity elsewhere on the grid (A1-20).
Correlations of FR and clinical characteristics.
When the interaction of age at surgery and type of resection (hemispherectomy vs lobar/focal resection) was taken into statistical consideration, the presence or absence of FR did not correlate with age at surgery, age at seizure onset, or type of resection (p > 0.12, logistic regression). The presence or absence of FR did not correlate with seizure duration (p = 0.88), gender (p = 0.36), history of infantile spasms (p = 0.82), active spasms at the time of surgery (p = 0.46), or the number of AEDs at surgery (p = 0.19).
Postoperative outcome.
The average postoperative follow-up period for this cohort was 27.3 ± 4.1 months (range 20-33). Seizure freedom was achieved for 24 of the 30 (80%) children in this surgical series similar to previous studies from our institution (table).19 The chance of achieving seizure freedom did not correlate with seizure duration (p = 0.12), number of AEDs (p = 0.19), the length of postoperative follow-up (p = 0.21), or the type of surgery (hemispherectomy vs lobar/sublobar resection; p = 0.11). When the interaction with type of resection was considered (logistic regression), seizure freedom did not correlate with age at surgery (p = 0.99) or age at seizure onset (p = 0.85).
Seizure control after surgery was linked with resection of FR-containing neocortex. Of the 24 children whose ECoG revealed FRs, 19 became seizure-free and all had their FR-containing cortex removed (p = 0.0001, χ2). This included one child whose FR-containing cortex was not removed in the first surgery, but who became seizure-free with a second resection, in which FR was completely resected (figure 2). Of the 5 children whose ECoG revealed FR and who continued with their seizures postoperatively, 4 had incomplete resection of the FR region and 1 had insular involvement not accessible for ECoG recording.
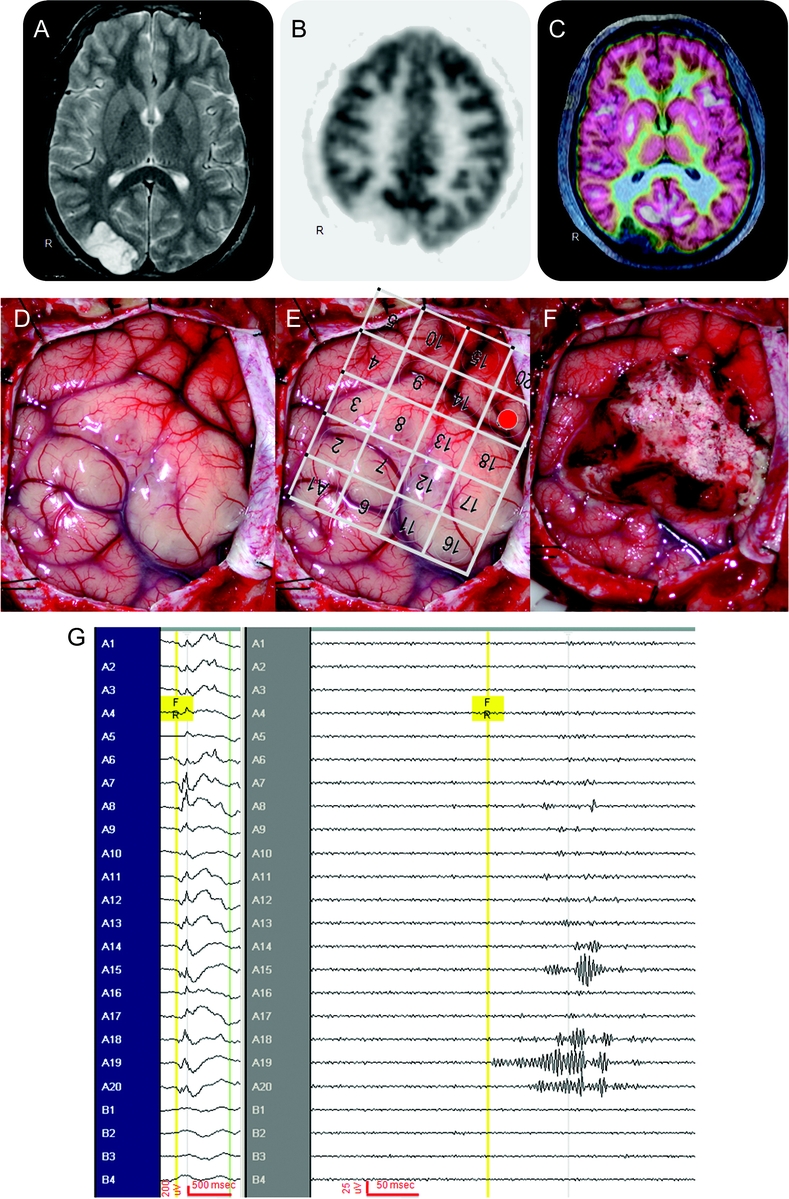
Figure 2 An illustrative case (patient 26)
This is a 16-year-old patient with 4 years of intractable epilepsy described as visual blurring and distortion. His video-EEG did not show clear electrographic onset during all stereotypical events (not shown), and his head MRI (A) showed a lesion over the right occipital region. His 18fluoro-deoxyglucose PET (FDG-PET) (B) showed hypometabolism over the right occipital region, and the coregistration of MRI and FDG-PET (C) demonstrated hypometabolism limited to the lesion. At surgery, the occipital lesion is seen in the surgical field (D). A 4 × 5 macroelectrode grid was placed over the surgical field (E), with a 1 × 4 macroelectrode strip off the surgical field (not shown) in the right frontal region as “control.” Intraoperative electrocorticography showed intermittent slowing, attenuation, and sharp waves superimposed with paroxysmal fast activity (G, left screen, no filters, no notch filter, time scale 30 mm/s, referential montage to contralateral frontal scalp electrode). After the lesion was resected (F), interictal fast ripples (FRs) (G, right screen, 240-Hz high-pass filter and 500-Hz low-pass filter, time scale 338 mm/s, referential montage to contralateral frontal scalp electrode) were seen first over A19 (E, shaded red) in the parietal region, with quick propagation to adjacent A20 and A15 superiorly, and A18 inferiorly, and importantly not found in the “control” frontal lobe. Thus the occipital lesion was resected but the parietal region containing FR remained. The patient's seizures returned in 3 months with similar semiology of visual distortion and blurring, again without electrographic changes in a second video-EEG. A second surgery 10 months after the first resection revealed similar electrocorticography, and FR was again found in the right parietal region. This FR-containing region was resected in the second surgery, and patient has been seizure-free for 20 months since the second resection, now off all antiepileptic drugs. Final pathology showed dysembryoplastic neuroepithelial tumor in the first resected specimen, and residual tumor in the second resected specimen.
Correlation with histopathology.
The most common histopathologic abnormality was cortical dysplasia (CD; n = 19), followed by tuberous sclerosis complex (TSC; n = 3), stroke, and Rasmussen encephalitis (2 each), along with postinfectious, posttraumatic, Sturge-Weber syndrome, and tumor (1 each). The type of pathologic diagnosis, divided into developmental (CD and TSC) and acquired (all other etiologies), did not correlate with the presence or absence of FR (p = 0.10, χ2), postoperative seizure freedom (p = 0.16), amplitude of FR (p = 0.66, analysis of variance [ANOVA]), duration of FR (p = 0.95, ANOVA), frequency of FR (p = 0.91, ANOVA), number of FR events (p = 0.22, ANOVA), number of primary channels (p = 0.88, ANOVA), or the area FR spanned (p = 0.12, ANOVA).
FR location and neuroimaging.
The location of the MRI lesion and the location of the hypometabolic region on interictal FDG-PET study were assessed relative to the location of FR events recorded with ECoG. For the 24 children whose ECoG demonstrated FR, events were partially or entirely located outside of the MRI lesion in 10 children (42%), outside of the FDG-PET hypometabolic region in 8 children (33%), and outside of both MRI lesion and FDG-PET hypometabolism in 6 children. Thus MRI and FDG-PET, individually and combined, would not necessarily have included the cortical region containing FR in 25% of children.
FR and surgical planning.
We assessed whether resections would have been different, compared to our conventional ECoG and neuroimaging directed resections, if interictal FR location were known and incorporated in the decision-making at the time of cortical resection. In 4 children, the resection area would have been expanded to include the FR region, and one would have avoided a second surgery in which FR region was excised and seizure freedom was achieved. Two children would have received a hemispherectomy based on our conventional ECoG reading, but had frontal resections instead due to parental request to spare motor function. Both children are seizure-free, and FR events were found in only the frontal areas of the resection and were absent elsewhere over the hemisphere. Hence, of the 24 children whose ECoG demonstrated FRs, 6 (25%) would have had a resection different from that of our conventional approach if interictal FR location had been known at the time of surgery.
DISCUSSION
The findings from this study support the notion from prior experimental and human studies that interictal FRs are spatially restricted surrogate markers of the epileptogenic zone. This study extends that concept to pediatric patients by the findings that complete removal of FR-containing cortex by ECoG was associated with postoperative seizure freedom. In addition, the detection of FRs in short intraoperative ECoG makes it possible that the localization of epileptogenic cortex can be achieved without the need for extended extraoperative intracranial EEG monitoring.
The finding that FR predominantly occurred without other interictal electrographic signatures in this study is consistent with previous findings that FR is an independent and unique marker of the epileptogenic zone.12 This concept is further supported by the finding that 25% of the children in our surgical series would have had a different resection if FR information were known and incorporated into the decision-making process at the time of the surgery. Such findings suggest that the conventional ECoG reading based on other interictal electrographic signatures localizes somewhat differently than ECoG based on FR localization.
It is of interest to note that the mesial temporal lobe, and insular region, may have been difficult for surface grids and strips to evaluate and properly assess for the presence of FR, especially in the anterior mesial and basal portions. Such findings support the spatial restriction of FR events, but also emphasize that better positioning of grids and strips for best access may help improve the detection of FR in these regions for future intraoperative ECoGs. Alternatively, macroelectrodes may not be as well-suited as depth electrodes for FR recording in these regions.
The finding that FRs do not always overlap with neuroimaging findings is consistent with a recent study that interictal FRs localize with the epileptogenic cortex but not necessarily with MRI-detected lesions.15 That FR was found with a variety of pathologic diagnoses in this study is consistent with the same study supporting the idea that FRs are a ubiquitous indicator of the epileptogenic zone, regardless of seizure etiology.
Our results should be considered preliminary due to the relatively small cohort of children and the fact that ECoGs were examined retrospectively. Follow-up was also relatively short and thus a longer period is needed. Furthermore, cortical regions which were difficult to access may not be as amenable to macroelectrode recording for FRs. Despite these limitations, this study demonstrates that FRs are able to be detected and localized on brief intraoperative ECoG in the majority of children in this series with predominantly neocortical epilepsy. The finding that complete removal of FR was associated with seizure freedom suggests that incorporation of interictal FR localization at the time of intraoperative ECoG (i.e., “live” read in the operating room) may be warranted for the best seizure outcome, but technical challenges need to be overcome so that the “live” read can be done quickly and accurately without exposing the patients to unnecessary risks.
For the practicing neurologist, our study indicates that the presence and localization of FRs recorded at operative ECoG may be a new approach to improve postoperative seizure outcome without the need for chronic intracranial EEG recordings. This approach may be particularly applicable with macroelectrodes in the pediatric population because the epileptogenic zones are mostly neocortical, extratemporal, and close to the cortical surface. Because interictal FR localizes with the epileptogenic zone, rather than neuroimaging abnormalities, this surrogate marker may be especially helpful when MRI is nonlesional or demonstrates multiple lesions such as TSC, which comprise 2 of the most challenging subpopulations of pediatric epilepsy surgery.
ACKNOWLEDGMENT
The authors thank Drs. Julia Jacobs and Jean Gotman of Montreal, Canada, for their assistance and expertise in teaching the filter and time scale setting, sampling rate determination, and fast ripple identification and review for FRs.
DISCLOSURE
Dr. Wu serves on the professional advisory board for the Tuberous Sclerosis Alliance and receives research support from the Tuberous Sclerosis Alliance, Novartis, and the NIH (NINDS K23 NS051637 [PI] and NIMH R34 MH089299 [coinvestigator]). Dr. Sankar serves on scientific advisory boards for and has received funding for travel from Ortho-McNeil-Janssen Pharmaceuticals, Inc., NeuroTherapeutics Pharma, Inc., King Pharmaceuticals, and Valeant Pharmaceuticals International; receives royalties from the publication of Pediatric Neurology, 3rd ed. (Demos Publishing, 2008); serves on speakers' bureaus for and has received speaker honoraria from Ortho-McNeil-Janssen Pharmaceuticals, Inc., Valeant Pharmaceuticals International, UCB, Eisai Inc., GlaxoSmithKline, Lundbeck Inc., and Cyberonics, Inc.; and has received research support from Valeant Pharmaceuticals International, Marinus Pharmaceuticals, Inc., the NIH (NS046516 [PI], NS045911 [co-PI], NS059505 [co-I], and MH079933 [coinvestigator]), and the Epilepsy Foundation of America. Dr. Lerner receives research support from the Thrasher Research Fund and Lundbeck Inc. (formerly Ovation Pharmaceuticals, Inc.). Dr. Matsumoto reports no disclosures. Dr. Vinters serves on the editorial boards of the Journal of Neuroscience Research, Neuropathology & Applied Neurobiology, Korean Journal of Pathology, and Neuropathology (journal of the Japanese Society of Neuropathology); and holds stock in Minnesota Mining & Manufacturing, Teva Pharmaceutical Industries Ltd., GlaxoSmithKline, and Pfizer Inc. Dr. Mathern serves on the editorial boards of Neurology®, Epileptic Disorders, Journal of Neuropathology & Experimental Neurology, Epilepsy & Seizures, Surgical Neurology International, and Epilepsy Research and on the Data Management Committee of Neuropace, Inc.; and received research support from NIH (R01 NS38992 [PI] and R21 NS606075 [PI]).
Address correspondence and reprint requests to Dr. Joyce Y. Wu, 22-474 MDCC, Division of Pediatric Neurology, Mattel Children's Hospital at UCLA, David Geffen School of Medicine, Los Angeles, CA 90095-1752 joycewu@mednet.ucla.edu
Editorial, page 1666
e-Pub ahead of print on October 6, 2010, at www.neurology.org.
Disclosure: Author disclosures are provided at the end of the article.
Received February 6, 2010. Accepted in final form June 29, 2010.
REFERENCES
- 1.Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science 1992;256:1025–1027. [DOI] [PubMed] [Google Scholar]
- 2.Curio G. Linking 600-Hz “spikelike” EEG/MEG wavelets (“sigma-bursts”) to cellular substrates: concepts and caveats J Clin Neurophysiol 2000;17:377–396. [DOI] [PubMed] [Google Scholar]
- 3.Bragin A, Engel J Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100-500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia 1999;40:127–137. [DOI] [PubMed] [Google Scholar]
- 4.Bragin A, Mody I, Wilson CL, Engel J Jr. Local generation of fast ripples in epileptic brain. J Neurosci 2002;22:2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bragin A, Wilson CL, Engel J. Spatial stability over time of brain areas generating fast ripples in the epileptic rat. Epilepsia 2003;44:1233–1237. [DOI] [PubMed] [Google Scholar]
- 6.Bragin A, Engel J Jr, Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus 1999;9:137–142. [DOI] [PubMed] [Google Scholar]
- 7.Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J Jr. Interictal high-frequency oscillations (80-500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol 2002;52:407–415. [DOI] [PubMed] [Google Scholar]
- 8.Staba RJ, Wilson CL, Bragin A, Fried I, Engel J Jr. Quantitative analysis of high-frequency oscillations (80-500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol 2002;88:1743–1752. [DOI] [PubMed] [Google Scholar]
- 9.Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J Jr. High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol 2004;56:108–115. [DOI] [PubMed] [Google Scholar]
- 10.Staba RJ, Frighetto L, Behnke EJ, et al. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia 2007;48:2130–2138. [DOI] [PubMed] [Google Scholar]
- 11.Le Van Quyen M, Bragin A, Staba R, Crépon B, Wilson CL, Engel J Jr. Cell type-specific firing during ripple oscillations in the hippocampal formation of humans. J Neurosci 2008;28:6104–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80-500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia 2008;49:1893–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagshaw AP, Jacobs J, LeVan P, Dubeau F, Gotman J. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia 2009;50:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology 2009;72:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs J, Levan P, Châtillon CE, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain 2009;132:1022–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain 2004;127:1496–1506. [DOI] [PubMed] [Google Scholar]
- 17.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia Epub 2009 Nov 3. [DOI] [PubMed]
- 18.Salamon N, Kung J, Shaw SJ, et al. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology 2008;71:1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemb M, Velasco TR, Parnes MS, et al. Improved outcomes in pediatric epilepsy surgery: the UCLA experience, 1986-2008. Neurology Epub 2010 Apr 28. [DOI] [PMC free article] [PubMed]
- 20.Wu JY, Salamon N, Kirsch HE, et al. Noninvasive testing, early surgery, and seizure freedom in tuberous sclerosis complex. Neurology 2010;74:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cepeda C, André VM, Flores-Hernández J, et al. Pediatric cortical dysplasia: correlations between neuroimaging, electrophysiology and location of cytomegalic neurons and balloon cells and glutamate/GABA synaptic circuits. Dev Neurosci 2005;27:59–76. [DOI] [PubMed] [Google Scholar]
- 22.Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Paediatr Anaesth 2005;15:266–274. [DOI] [PubMed] [Google Scholar]