Abstract
Objective:
Some patients with Parkinson disease (PD) develop pathological gambling when treated with dopamine agonists (DAs). However, little is known about DA-induced changes in neuronal networks that may underpin this drug-induced change in behavior in vulnerable individuals. In this case-control study, we aimed to investigate DA-induced changes in brain activity that may differentiate patients with PD with DA-induced pathological gambling (gamblers) from patients with PD without such a history (controls).
Methods:
Following overnight withdrawal of antiparkinsonian medication, patients were studied with H2 15O PET before and after administration of DA (3 mg apomorphine) to measure changes in regional cerebral blood flow as an index of regional brain activity during a card selection game with probabilistic feedback.
Results:
We observed that the direction of DA-related activity change in brain areas that are implicated in impulse control and response inhibition (lateral orbitofrontal cortex, rostral cingulate zone, amygdala, external pallidum) distinguished gamblers from controls. DA significantly increased activity in these areas in controls, while gamblers showed a significant DA-induced reduction of activity.
Conclusions:
We propose that in vulnerable patients with PD, DAs produce an abnormal neuronal pattern that resembles those found in nonparkinsonian pathological gambling and drug addiction. DA-induced disruption of inhibitory key functions—outcome monitoring (rostral cingulate zone), acquisition and retention of negative action-outcome associations (amygdala and lateral orbitofrontal cortex)—together with restricted access of those areas to executive control (external pallidum)—may well explain loss of impulse control and response inhibition in vulnerable patients with PD, thereby fostering the development of pathological gambling.
GLOSSARY
- ANOVA
= analysis of variance;
- DA
= dopamine agonist;
- G-SAS
= Gambling Symptom Assessment Scale;
- GPe
= external pallidum;
- MNI
= Montréal Neurological Institute;
- OFC
= orbitofrontal cortex;
- PD
= Parkinson disease;
- rCBF
= regional cerebral blood flow;
- RCZ
= rostral cingulated zone;
- UPDRS
= Unified Parkinson's Disease Rating Scale.
Impulse control disorders, such as pathological gambling, may develop in patients with Parkinson disease (PD) as a nonmotor complication of dopamine replacement therapy, specifically of dopamine agonists (DAs).1 It has recently been shown that an impairment of learning from negative outcomes is a general neurobehavioral effect of DAs, which may render all DA-treated patients with PD at risk to develop pathological gambling.2,3 Yet the majority of patients seem to overcome this drug-induced susceptibility, since pathological gambling only occurs in a minority of medicated patients.1 Therefore, we reasoned that DAs interact with an intrinsic trait in vulnerable patients. The impact of such an interaction may be a functional deviance in crucial brain areas that fosters the development of impulse control disorders.
Pathological gambling is often described as a “behavioral addiction,” underlining the phenomenological overlap with drug addiction, which includes features such as tolerance, withdrawal, and preoccupation. In nonparkinsonian participants with drug addictions or pathological gambling, considerable evidence points toward a disrupted function of frontolimbic and ventral frontostriatal areas—the lateral orbitofrontal cortex (OFC) and the rostral cingulate zone (RCZ), among others—that are believed to exert inhibitory control during decision-making and action monitoring.4,5
This study focuses on the specific impact of DAs in vulnerable individuals by studying DA-driven effects on regional cerebral blood flow (rCBF) that identify patients with PD with DA-induced pathological gambling in comparison to patients with PD without such a history. Based on previous findings,4,5 we reasoned that in contrast to controls, gamblers may show DA-induced hypoactivity of inhibitory frontolimbic networks encompassing the lateral OFC and the RCZ.
METHODS
Patients.
Seven patients with PD (Queen's Square Brain Bank Criteria) with DA-induced pathological gambling (hereafter referred to as “gamblers”) and 7 patients with PD without such a history (hereafter referred to as “controls”) participated in the study. The 2 patient groups were matched for dopaminergic medication, age, and disease duration and severity. All gamblers developed pathological gambling on exposure to DAs independent of the time of initiation of levodopa therapy. Controls had no history of any impulse control disorder (for further information, see appendix e-1, and specifically table e-1, on the Neurology® Web site at www.neurology.org).
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Research Ethics Committees for the Centre for Addiction and Mental Health and the University Health Network of the University of Toronto. After complete description of the study to the participants, written informed consent was obtained.
DA-related increase of gambling.
In order to acquire an estimate of the specific influence of DA therapy on gambling symptoms in the individual patient, we assessed gambling severity in both groups by using the Gambling Symptom Assessment Scale (G-SAS), which has been validated for detecting changes in gambling symptoms.6 Patients were asked to fill out the questionnaires twice. Once with regard to their recollection of gambling symptoms in the “worst week” prior to treatment initiation with DAs and once according to their recollection of gambling symptoms in the “worst week” on DA treatment.
Activation tasks.
During H2O15 PET, patients played a computerized card selection game wearing video eyewear (VR920; Vuzix Corporation, NY) and providing responses with their right hand on a 4-button keyboard. In every trial, 4 horizontally arranged cards were presented facedown in the center of the visual field. Participants had to select 1 card that would be flipped. Subjects were told that in each trial only 1 card contained a feedback and that the others were blank. Consistently, a feedback-displaying card occurred in 25% of the trials together with a yellow smiley face on the top of the screen. In the other 75% of the trials the flipped card was blank and there was no smiley face. There were 2 variants of the game: In the “financial” variant, the feedback was monetary (±1, 3, 5 $) and the smiley face commented losses with a frown and wins with a smile. In the “neutral” variant, the feedback was a neutral symbol (#) and the smiley face had a neutral expression.
PET scanning, image transformation, and statistical analysis.
General PET scanning and image transformation procedures were identical to our previous studies (see appendix e-1). Subjects were studied after overnight withdrawal (12–18 hours) of their antiparkinsonian medications with H2 15O PET to measure changes in rCBF. The experiment consisted of 12 emission scans and was divided in 2 sessions. The first session was performed without antiparkinsonian medication (OFF) and the second session following subcutaneous administration of 3 mg apomorphine (ON) and an interval of 30 minutes. Patients were examined with the motor section of the Unified Parkinson's Disease Rating Scale (UPDRS) to ensure treatment response. Each PET session consisted of 6 PET scans (3 scans for each task variant).
In a first step, we performed individual one-way repeated-measures analyses of variance (ANOVA) in all 14 subjects to obtain mean individual effects (β images) of each of the conditions (OFFfinancial, OFFneutral, ONfinancial, ONneutral). For a post hoc analysis, we additionally obtained the mean individual effect of medication (ON relative to OFF). In a second step, β images were entered in a multifactorial ANOVA with the factors group (gamblers, controls), medication (OFF, ON), and task variant (financial, neutral).
Results were thresholded at a level of p < 0.001 uncorrected with an extent threshold of at least 25 contiguous voxels. Regions were considered significant at the voxel-level threshold of p < 0.05 after correction for multiple comparisons (false discovery rate).7 All coordinates are reported in Montréal Neurological Institute (MNI) space.
RESULTS
Gamblers had significantly higher gambling scores than controls before and after DA initiation as well as significantly higher DA-related increase in gambling severity (controls: G-SAS before DA 4 ± 3; G-SAS after DA initiation 6 ± 5; gamblers: G-SAS before DA 13 ± 6; G-SAS after DA initiation 20 ± 4). Motor scores of the UPDRS improved after apomorphine injection in both groups to a similar degree (paired t tests: gamblers −8.1, p = 0.005; controls −6.4, p = 0.006; 2-factorial ANOVA: F1,25 = 0.003, p = 0.95).
Multifactorial ANOVA revealed that the variant of the task (i.e., financial/neutral) did not significantly influence rCBF (no effect of factor task variant, no interaction with either of the other factors, no 3-way interaction). No voxel exceeded the liberal threshold of p < 0.001, uncorrected. The effects of the factors group and medication are summarized in appendix e-1 and tables e-2 and e-3.
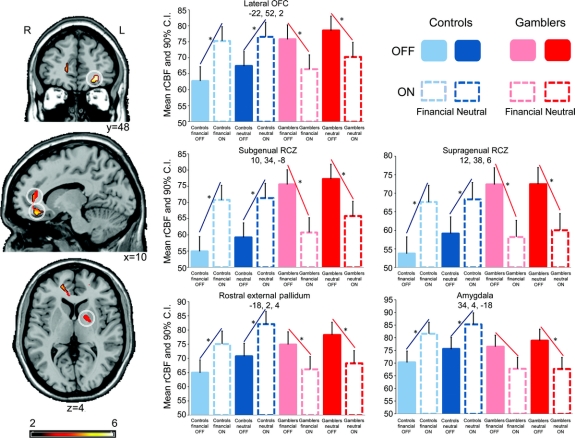
The differential effect of medication in the 2 patient groups constituted our main research question, since the behavioral response to DAs is evidently different in gamblers and controls. Therefore, we were most interested in the interaction between the factors medication and group. Here, multifactorial ANOVA revealed a strong interaction, showing that the effect of medication in one group was the inverse of the effect in the other. In controls, DA significantly increased activity in the left lateral OFC, right RCZ, right amygdala, and left ventral anterior external pallidum (GPe), while gamblers showed a significant DA-induced reduction of neuronal activity in these regions (table e-4 and figure 1).
Figure 1 Differential effect of medication on brain activity in the 2 patient groups
In brain areas that are implicated in impulse control and response inhibition (lateral orbitofrontal cortex [OFC], rostral cingulate zone [RCZ], amygdala, external pallidum), controls significantly increased activity in response to dopamine agonist (DA), while gamblers showed a significant DA-induced reduction of activity. Projections of statistical parametric maps superimposed on a standardized MRI template. The bar graphs show regional cerebral blood flow (rCBF) in the peak voxel of the significant cluster. Stereotactic coordinates (x, y, z) are given in mm. *Significant differences in rCBF. CI = confidence interval.
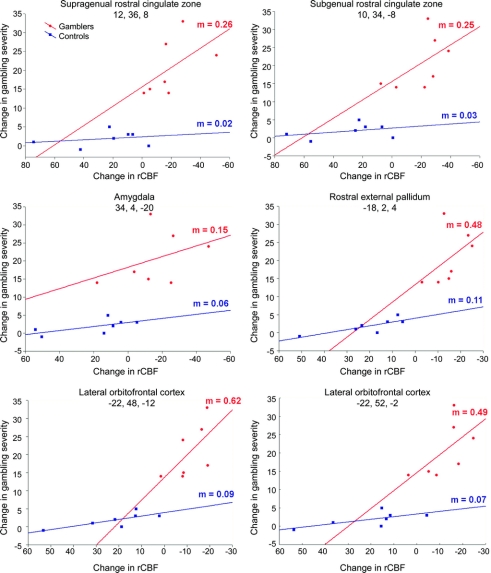
In a post hoc analysis, we aimed to characterize the relationship between DA-induced rCBF changes in these areas and DA-induced changes in gambling symptoms. To this end, we explored this relationship in 2-dimensional scatterplots, calculating linear regressions for both groups using the individual values for DA-induced rCBF change in a specific area (mean value of a 10-mm sphere centered at the peak maximum of the second level ANOVA and extracted from the comparison ON vs OFF in the individual analyses) and the individual DA-related change in gambling score (according to the G-SAS questionnaire) (figure 2). Only in gamblers, DA-induced rCBF change showed a strong positive relationship with DA-induced change in gambling severity.
Figure 2 Relationship between regional cerebral blood flow (rCBF) change and changes in gambling symptoms
Dopamine agonist (DA)-induced change in gambling severity plotted against DA-induced change in brain activity in gamblers (red) and controls (blue). Only in gamblers, DA-induced rCBF change showed a strong positive relationship with DA-induced change in gambling severity. m = Slope of linear regression (change in gambling severity/change in rCBF). Stereotactic coordinates (x, y, z) are given in mm.
DISCUSSION
This study identified DA-induced changes in brain function that are specific to patients with PD with DA-induced pathological gambling. In contrast to PD controls, gamblers showed a significant DA-induced reduction of rCBF in brain areas that are implicated in impulse control and response inhibition (lateral OFC, RCZ, amygdala, GPe). Moreover, this DA-induced change showed a positive relationship with DA-induced change in gambling severity.
What is the behavioral significance of these rCBF changes? Hypoactivity of the lateral OFC has previously been reported in drug addiction and pathological gambling in non-PD subjects.5,8 The OFC is highly interconnected with subcortical structures involved in affective processing such as the amygdala and the ventral striatum,9 and is thought to play a crucial role in assigning subjective value to actions by encoding and updating expectations of future rewards or punishments.10,11 Further evidence points toward a functional dissociation between medial and lateral parts of the OFC. The medial OFC appears engaged in reward-based decision-making, whereas the lateral part plays a greater role in punishment-based decision-making.10 Interestingly, there appears to be a specific role for the lateral OFC in suppressing previously rewarded behavior.11 It could be argued that this function has great potential to prevent uncontrolled gambling behavior. Similar to the lateral OFC, impaired RCZ function has been suggested to foster addictions.4,5 The RCZ is thought to be engaged in monitoring functions that are involved in preventing negative consequences.12 In cocaine abusers, relative hypoactivity of the RCZ was found to be associated with impaired suppression of impulsive behavior.13 We also found the ventral anterior GPe to be deactivated by DA only in gamblers. The GPe is in a key position to regulate the thalamo-cortical output. The impact of dysfunction or a lesion of the ventral anterior GPe, which is thought to be its limbic part, is not well-known in humans. In monkeys, however, chemical lesioning of the limbic GPe produces stereotyped complex movements resembling compulsive behaviors.14 Pathological gambling is also felt to lie within an impulsive-compulsive behavioral spectrum and may likewise evolve with chronic, drug-induced, deactivation of the GPe. We observed that in clear contrast to gamblers, controls showed increased activity in these inhibitory networks in response to DA. Control patients received the same medication as gamblers and were therefore subject to the same general drug-induced susceptibility for addiction (i.e., impaired negative feedback processing). Therefore, we speculate that this increase may enable controls to overcome pharmacologically driven impairment of negative feedback processing.
Reduced striatal D2 receptor availability might represent a critical vulnerability that promotes DA-induced impairment of these inhibitory networks. It has been shown that nonparkinsonian drug abusers have impaired OFC and RCZ metabolism in addition to decreased D2 receptor availability, with functionality of the OFC and the RCZ and D2 receptor availability showing a strong positive relationship.15 Likewise, we found markedly reduced striatal D2 receptor availability in gamblers with PD in comparison to control patients with PD in a recent PET study.16 We would assume a similar difference between gamblers and controls in the present study, because the majority of the patients also participated in the earlier study (only 2 out of each group did not).
Although this study generally corroborates the role of the RCZ and the lateral OFC in addiction, the relatively small sample size may limit generalizability. Furthermore, future studies will have to refine our understanding of pharmacodynamic interactions using different dopaminergic agents (e.g., levodopa) and time courses (e.g., acute vs chronic DA stimulation).
DISCLOSURE
Dr. van Eimeren and Dr. Pellecchia report no disclosures. Dr. Cilia receives research support from Fondazione Grigioni per il Morbo di Parkinson. Dr. Ballanger and Dr. Steeves report no disclosures. Dr. Houle is listed as author on a patent re: A PET radiochemistry technique to label radiotracers with carbon-11 and has received license fee payments from Bioscan for use of patent related to a radiolabeling method; and receives research support from the Ontario Ministry of Research and Innovation and the Canada Foundation for Innovation. Dr. Zurowski receives research support from the CIHR. Dr. Miyasaki has served on a scientific advisory board for Teva Pharmaceutical Industries Ltd.; serves on the editorial board of Movement Disorders; has received speaker honoraria from Biovail Corporation and Teva Pharmaceutical Industries Ltd.; serves/has served as a consultant to Janssen-Ortho, Inc., Merz Pharmaceuticals GmbH, Schering-Plough Corp., the NIH (Independent Medical Monitor), Ontario Drug Benefits, and Common Drug Review, Canada; and receives research support from Teva Pharmaceutical Industries Ltd., Boehringer Ingelheim, Solvay Pharmaceuticals, Inc., Solstice Neurosciences, Inc., Impax Laboratories, Neurogen, Medivation, Inc., the National Parkinson Foundation, the Parkinson Society Canada, the Michael J. Fox Foundation, and the Huntington Study Group. Dr. Lang has served on scientific advisory boards for Allon Therapeutics, Inc., Biovail Corporation, Boehringer Ingelheim, Cephalon, Inc., Ceregene, Eisai Inc., Medtronic, Inc., Lundbeck Inc., NeuroMolecular Pharmaceuticals, Novartis, Merck Serono, Solvay Pharmaceuticals, Inc., TaroPharma, and Teva Pharmaceutical Industries Ltd.; received speaker honoraria from GlaxoSmithKline and UCB; has received research support from Taro Pharma, the CIHR, the Dystonia Medical Research Foundation, the Michael J. Fox Foundation, the National Parkinson Foundation, the Ontario Problem Gambling Research Centre, and the Parkinson's Disease Foundation; and has participated in legal proceedings involving welding rod companies. Dr. Strafella receives research support from the Ontario Problem Gambling Research Centre.
Supplementary Material
Address correspondence and reprint requests to Dr. A.P. Strafella, Toronto Western Hospital and Research Institute and CAMH-PET Centre, Toronto, ON, Canada antonio.strafella@uhnres.utoronto.ca
Editorial, page 1668
Supplemental data at www.neurology.org
e-Pub ahead of print on October 6, 2010, at www.neurology.org.
Study funding: Supported by the Ontario Problem Gambling Research Centre and Canadian Institutes of Health Research (CIHR) (MOP-64423 to A.P.S.). A.P.S. is supported by the CIHR New Investigator Research Award.
Disclosure: Author disclosures are provided at the end of the article.
Received April 2, 2010. Accepted in final form July 19, 2010.
REFERENCES
- 1.Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 2010;67:589–595. [DOI] [PubMed] [Google Scholar]
- 2.Bodi N, Keri S, Nagy H, et al. Reward-learning and the novelty-seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson's patients. Brain 2009;132:2385–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Eimeren T, Ballanger B, Pellecchia G, Miyasaki JM, Lang AE, Strafella AP. Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: a trigger for pathological gambling in Parkinson's disease? Neuropsychopharmacology 2009;34:2758–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med 2006;12:559–566. [DOI] [PubMed] [Google Scholar]
- 5.Potenza MN. Review: the neurobiology of pathological gambling and drug addiction: an overview and new findings. Philos Trans R Soc Lond B Biol Sci 2008;363:3181–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SW, Grant JE, Potenza MN, Blanco C, Hollander E. The Gambling Symptom Assessment Scale (G-SAS): a reliability and validity study. Psychiatry Res 2009;166:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 2002;15:870–878. [DOI] [PubMed] [Google Scholar]
- 8.Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology 2004;47(suppl 1):3–13. [DOI] [PubMed] [Google Scholar]
- 9.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 1995;363:615–641. [DOI] [PubMed] [Google Scholar]
- 10.O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci 2001;4:95–102. [DOI] [PubMed] [Google Scholar]
- 11.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex 2000;10:284–294. [DOI] [PubMed] [Google Scholar]
- 12.Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M. Genetically determined differences in learning from errors. Science 2007;318:1642–1645. [DOI] [PubMed] [Google Scholar]
- 13.Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci 2004;24:11017–11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabli D, McCairn K, Hirsch EC, et al. Behavioural disorders induced by external globus pallidus dysfunction in primates: I: behavioural study. Brain 2004;127:2039–2054. [DOI] [PubMed] [Google Scholar]
- 15.Volkow ND, Chang L, Wang GJ, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 2001;158:2015–2021. [DOI] [PubMed] [Google Scholar]
- 16.Steeves TD, Miyasaki J, Zurowski M, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain 2009;132:1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.