Abstract
Histone levels are a key factor in several nuclear processes, including transcription and chromosome segregation. Previous studies have demonstrated that Spt10 and Spt21 are required for the normal transcription of a subset of the histone genes in Saccharomyces cerevisiae, and sequence analysis has suggested that Spt10 is an acetyltransferase. We have now characterized several aspects of transcriptional activation of histone genes by Spt10 in vivo. Our results show that activation by Spt10 is dependent on its acetyltransferase domain. At HTA2-HTB2, the histone locus whose transcription is most strongly dependent on Spt10, Spt10 is physically recruited to the promoter in an Spt21-dependent and a cell cycle-dependent manner. Furthermore, Spt10 and Spt21 directly interact. These results, taken together with the identification of spt10 mutations that suppress an spt21Δ mutation, suggest a model for transcriptional activation by Spt10 and Spt21.
Regulation of histone levels plays critical roles in cell growth and division. In Saccharomyces cerevisiae, altered histone gene dosage has been shown to affect chromosome segregation, transcription, and other processes (21). The S. cerevisiae genome contains four loci that encode pairs of core histones; two encode histones H2A and H2B, and the other two encode histones H3 and H4 (9, 28). The transcription of all of the histone genes is cell cycle regulated, with the peak of expression in S phase (21). In spite of this similar regulation of histone gene transcription, the promoters at the four histone loci are highly divergent and are controlled by distinct sets of transcription factors (8, 10, 26, 27, 29, 37).
In this study we have focused on two factors, Spt10 and Spt21, known to be required for the transcription of two of the four histone loci in S. cerevisiae (8). Previous work has shown that spt10 and spt21 mutations alter normal transcription of several genes in addition to those encoding histones (18, 25, 26, 38). Further studies have shown that spt10 and spt21 mutations suppress the loss of some upstream activating sequences and some transcriptional activators (7, 22, 38). With respect to the histone genes and phenotypes tested, spt10 and spt21 mutants are similar. However, some differences in phenotypes have been observed, including greater transcriptional effects and significantly poorer growth by an spt10Δ mutant compared to an spt21Δ mutant (7, 19). Overall, these phenotypes suggest broad and significant roles for Spt10 in transcriptional regulation, with Spt21 required for a subset of these roles.
Previous analyses of Spt10 and Spt21 have suggested a possible mechanism for their activation of histone gene transcription. First, Spt10 has motifs found in acetyltransferases, including the histone acetyltransferase (HAT) Gcn5 (20). Acetyltransferases have been shown to play critical roles in transcription through their ability to acetylate both histones and other transcription factors (31). Consistent with a role for Spt10 in acetylation of histones, recent work has shown that the transcriptional induction of the CUP1 gene is defective in spt10 mutants and that this defect correlates with a decreased level of acetylation of histones H3 and H4 at the CUP1 promoter (25). Second, SPT21 mRNA levels are cell cycle controlled, with a peak at the G1/S transition, consistent with a role in controlling histone gene transcription (5, 30). Thus, at the time when histone genes are transcribed, Spt21 may play a role in activating Spt10 to enable it to activate transcription of particular histone genes through its putative acetyltransferase activity.
In this work we have addressed several issues concerning the roles of Spt10 and Spt21 in the control of histone gene expression. First, our results demonstrate that activation by Spt10 is dependent on the Spt10 acetyltransferase domain. We also demonstrate that both Spt10 and Spt21 are physically associated with the promoters of histone genes, strongly suggesting a direct role for these factors in transcriptional control. Furthermore, our results show that Spt21 is required for the association of Spt10 with histone promoters at S phase and that Spt21 directly interacts with Spt10. Finally, we describe spt10 mutations that suppress an spt21Δ mutation. Taken together, these results provide new insights into the functions of Spt10 and Spt21 and suggest a model for their mechanism of transcriptional activation.
MATERIALS AND METHODS
Yeast strains and genetic methods.
All S. cerevisiae strains used in this study (Table 1) are descended from a GAL2+ derivative of S288C (35). Standard methods for mating, sporulation, transformation, and tetrad analysis were used, and all media were prepared as previously described (23). The spt10 null mutant allele, spt10Δ101::HIS3, and the spt21 null mutant allele, spt21Δ201::HIS3, were constructed by standard methods (2, 16). We constructed derivatives of SPT10 fused to sequences encoding either the hemagglutinin (HA) or Myc epitope tags as previously described (15, 24). The spt10-3 allele encodes three copies of the HA epitope fused to the amino terminus. The chromatin immunoprecipitation (ChIP) experiments used derivatives of Spt10 and Spt21 fused to 13 copies of the Myc epitope at their carboxy termini. Studies of the Spt10 acetyltransferase domain mutants were performed using strains FY2190 and FY2191. These strains contain both the designated spt10 mutation on a LEU2-marked plasmid and a wild-type copy of SPT10 on a URA3-marked plasmid (pFW217) (19). Prior to experiments to test mutant phenotypes, these strains were grown on media containing 5-fluoroorotic acid (5FOA) (3) and lacking leucine to select for cells that contain only the mutant spt10 gene. To examine cells at specific stages of the cell cycle, cells were arrested in liquid media with α-factor as previously described (4). The time zero release point was used for cells denoted G1 phase, and the 30-min time point following release was used for cells in S phase.
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Genotype |
|---|---|
| FY2017 | MATaspt10Δ201::HIS3 spt21Δ201::HIS3 his3Δ200 leu2Δ0 ura3Δ0 lys2-128δ (pFW217) |
| FY2190 | MATaspt10Δ201::HIS3 ade2 ade3 his3Δ200 leu2Δ1 ura3-52 lys2-128δ (pFW217) |
| FY2191 | MATaspt10Δ201::HIS3 his3Δ200 leu2Δ1 ura3-52 lys2-128δ (pFW217) |
| FY2192 | MATaura3-52::LexA-LacZ reporter::URA3 his3Δ200 leu2Δ1 lys2-128δ trp1Δ63 |
| FY2193 | MATaura3-52::LexA-LacZ reporter::URA3 spt21Δ201::HIS3 his3Δ200 leu2Δ1 lys2-128δ trp1Δ63 |
| FY2194 | MATaSPT21-MYC::kanMX his3Δ200 leu2Δ0 ura3Δ0 lys2-128δ trp1Δ63 |
| FY2195 | MATaSPT10-MYC::kanMX his3Δ200 leu2Δ0 ura3Δ0 lys2-128δ trp1Δ63 |
| FY2196 | MATaSPT10-MYC::kanMX spt21Δ201::HIS3 his3Δ200 leu2Δ0 ura3Δ0 lys2-128δ trp1Δ63 |
| FY2197 | MATaspt10-3 his3Δ200 ura3Δ0 lys2-128δ |
| FY2198 | MATaspt10-3 spt21Δ201::HIS3 his3Δ200 ura3Δ0 lys2-128δ |
| FY2199 | MATaspt21Δ201::HIS3 his3Δ200 leu2Δ0 ura3Δ0 lys2-128δ |
| FY2200 | MATahis3Δ200 leu2Δ0 ura3Δ0 lys2-128δ |
Plasmids.
Plasmids were constructed by standard methods and maintained in Escherichia coli strain DH5α (1). Details of the plasmid constructions are available upon request. Restriction enzymes, T4 DNA ligase, and Taq polymerase were purchased from New England Biolabs and Gibco BRL. pCWD29 encodes Spt10 amino acids 15 to 641 fused at the amino terminus to a six-His tag subcloned into the expression vector pET28a (Novagen). pLBS27 encodes full-length Spt10 fused at the carboxy terminus to a six-His tag subcloned into pET28a. pLBS39 contains the full-length untagged Spt21 coding sequence subcloned into pET17b (Novagen). pLBS41 encodes both Spt10 with a carboxy-terminal six-His tag and untagged Spt21. pLBS42 encodes both Spt10 with an amino-terminal tag and untagged Spt21.
The site-directed mutations in SPT10 that alter the Spt10 acetyltransferase domain were constructed with the QuikChange system (Stratagene Inc.). All mutations were verified by DNA sequencing. In our experiments, we used the following plasmids: pDH17 (spt10-199 LEU2 CEN), pDH63 (spt10-199 LEU2 2μm), pDH62 (spt10-122 LEU2 CEN), and pDH64 (spt10-122 LEU2 2μm).
The plasmids that encode the LexA-Spt10 fusions were constructed from pBTM116-ADE2 (32). pWDC32 contained a PstI fragment of SPT10, which encodes amino acids 15 to 641 cloned into pBTM116-ADE2 following fill-ins of EcoRI and BamHI sites to set the appropriate reading frame. pWDC35 contained a PCR fragment of SPT10 which encodes amino acids 1 to 330 cloned into the BamHI and SalI sites of pBTM116-ADE2. pDH21 encodes amino acids 15 to 641 of spt10-199 and was created by subcloning the PstI fragment from pDH17 into pWDC32. pDH76 encodes amino acids 15 to 641 of spt10-122 and was created by subcloning the PstI fragment from pDH62 into pWDC32.
RNA isolation and reverse transcription-PCR (RT-PCR) analysis.
RNA isolation was performed as previously described (1, 33). cDNA synthesis was performed with Superscript II RNase H- reverse transcriptase (Gibco BRL). Briefly, 1 μg of RNA was mixed with 500 ng of oligo(dT)12-18 and 1 μl of 10 mM deoxynucleoside triphosphates in a total volume of 12 μl. The mixture was heated to 65°C for 5 min and then chilled briefly on ice and briefly centrifuged to collect the contents of the tube. Four microliters of 5× first-strand buffer, 2 μl of 0.1 M dithiothreitol, and 1 μl of RNaseOUT (40 U/μl) were added. The contents were mixed and placed at 42°C for 2 min, and then 1 μl of Superscript II was added. The mixture was mixed, incubated at 42°C for 50 min, and heat inactivated at 70°C for 15 min. PCR was performed under standard conditions, and dilutions of the cDNA were examined to ensure linearity. cDNA preparations were diluted such that the levels of ACT1 amplification from all samples were equivalent. These normalized cDNA concentrations were then used for amplification by the experimental primer sets. In cases where bands were quantitated with National Institutes of Health (NIH) Image software, the HTB2/ACT1 ratio of the wild type was set to 100.
β-Galactosidase assays.
Strain FY2192 contains an integrated copy of pSH1834Δspe (provided by R. Brent), which contains a lacZ reporter with six LexA operator sites upstream of a minimal promoter. FY2192 transformants containing LexA-Spt10 fusions were grown to 1 × 107 to 2 × 107 cells/ml in synthetic complete (SC) medium lacking Trp. β-Galactosidase assays were performed as described previously (23). Protein concentrations were determined by Bradford protein assays (Bio-Rad, Richmond, Calif.). The average β-galactosidase units for each strain are presented as normalized to the wild-type LexA-Spt10 fusion plus or minus the standard error.
ChIP.
Chromatin immunoprecipitations were performed as previously described (17). Cross-linked chromatin was sonicated to an average length of 500 bp. Spt10-Myc and Spt21-Myc were immunoprecipitated from 1/10 of the cross-linked chromatin by a two-step method using a rabbit polyclonal anti-Myc A14 antibody (Santa Cruz Biotechnology) followed by immunoglobulin G-Sepharose beads (Pharmacia). Dilutions of input DNA and immunoprecipitated DNA were subjected to quantitative radioactive PCR as described previously (14). Specific binding of Spt10-Myc and Spt21-Myc to DNA amplified by each primer set was evaluated by calculating the ratio of the percent immunoprecipitation (IP) of the primer set to the percent IP of a control region of the genome (12). The control region used amplifies bp 9716 to 9863 of chromosome V, a region devoid of transcription by RNA polymerase II (12). The regions amplified for ChIP analysis of the histone gene promoters were chosen as likely regulatory regions based on the positions relative to the TATA elements. DNA sequences within these regions are conserved among related yeasts and are candidates to be regulatory sites (6, 11).
Isolation of SPT10-11 as a suppressor of spt21Δ.
To screen for mutations in SPT10 that can suppress spt21Δ, plasmid pGN1101 (SPT10 LEU2) (19) was first mutagenized by PCR. The mutagenized plasmid was then used to transform strain FY2017. Leu+ transformants were replica plated to medium containing 5FOA and lacking leucine. These plates were then replica plated to media lacking leucine and lysine to screen for suppressors of the spt21Δ Spt− (Lys+) phenotype with respect to the lys2-128δ mutation. Candidates were retested by isolation of the plasmid and retransformation. The mutant allele isolated, SPT10-11, was sequenced and shown to encode a protein with a single amino acid change, S508F.
Spt10 and Spt21 recombinant expression and copurification.
Proteins were expressed in E. coli BL21(DE3) Codon Plus, which was induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 6 h at room temperature. They were affinity purified with His-Bind resin (Novagen). The purified proteins were separated on a sodium dodecyl sulfate-10% polyacrylamide (SDS-10% PAGE) gel.
Western analysis.
Whole-cell extracts were prepared in extract buffer (40 mM HEPES [pH 7.4], 350 mM NaCl, 10% glycerol, 0.1% Tween, 1 mM phenylmethylsulfonyl fluoride) by bead beating in a minibeadbeater (36). Cellular debris was removed by spinning extracts at 10,000 × g for 10 min. Protein concentrations were determined by Bradford assay (Bio-Rad). Equal amounts of whole-cell extracts were loaded on SDS-PAGE gels. Transfer was performed as described previously (34). The membranes were probed with the A14 antibody (Santa Cruz Biotechnology) at a 1:2,500 dilution.
RESULTS
Transcriptional dependence of the histone loci on Spt10.
Previous studies demonstrated that transcription at two of the four histone loci, HTA2-HTB2 and HHT2-HHF2, is dependent on Spt10 and Spt21 (8). However, in those studies, expression of only a subset of the eight histone genes was examined. To understand fully the role of Spt10 in histone gene expression, we examined the mRNA levels for all eight histone-encoding genes in wild-type and spt10Δ strains. In this analysis, we used RT-PCR to circumvent the cross-hybridization that occurs between duplicate histone pairs (see Materials and Methods). As previously shown (8), both genes at the HTA2-HTB2 locus are highly dependent on Spt10 for normal mRNA levels (Fig. 1, lanes 1 to 6). At the HHT2-HHF2 locus, one of the two genes, HHF2, is clearly dependent on Spt10. The dependence of the second gene at this locus, HHT2, appears to be more modest. At the other two histone loci, none of the other four histone genes were affected by the spt10Δ mutation. These results show that Spt10 is required for normal mRNA levels for a subset of the eight histone genes.
FIG. 1.
Particular histone mRNA levels are reduced in spt10 mutants. LEU2-marked plasmids which contained the indicated allele of SPT10 were used to transform strain FY2190 (spt10Δ ura3-52 leu2Δ0 lys2-128δ). Each allele of SPT10 tested was expressed from both a low-copy-number plasmid (CEN) and a high-copy-number plasmid (2μm). Lanes 5 and 6 represent strain FY2190 transformed with an empty LEU2 vector. Representative RT-PCR analysis is shown for the indicated spt10 strains. For each sample, two dilutions of cDNA template (1× and 4×) were used to show linearity.
The Spt10 acetyltransferase domain is required for Spt10 function.
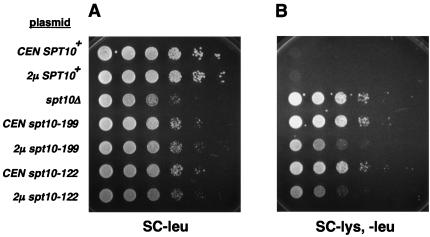
Spt10 contains a region conserved with HATs (20). To test the requirement for this Spt10 domain in vivo, mutant alleles that encode amino acid changes at residues highly conserved among HATs were constructed. The first allele, spt10-199, encodes the changes G199A and G201A and was designed based on previous identification of an equivalent mutant Gcn5 that abolished catalytic activity (13). The second mutant allele, spt10-122, encodes the change D122A. D122 is conserved and is located in a region of Spt10 that is shared by a subset of acetyltransferases (20). When these spt10 alleles were expressed from low-copy-number plasmids, the mutant Spt10 proteins were present at decreased levels compared to wild-type Spt10. To ensure that any mutant phenotypes were not merely the result of reduced protein levels, we included strains in our analyses in which the mutants were expressed from high-copy-number plasmids. This resulted in mutant protein levels comparable to the level of Spt10 in a wild-type strain (D. Hess and F. Winston, unpublished data). For the initial analysis of these spt10 mutants we tested growth and suppression of the transcriptional defect of the lys2-128δ insertion mutation (Spt− phenotype). Our results showed that both acetyltransferase mutants have a slow-growth phenotype, albeit not as dramatic as that of an spt10Δ mutant (Fig. 2A). In addition, both acetyltransferase mutants have an Spt− phenotype, almost as severe as that of an spt10Δ mutant (Fig. 2B). These data show that the Spt10 acetyltransferase domain is important for the normal function of Spt10 in vivo.
FIG. 2.
Phenotypes of spt10 mutants. The phenotype of each strain was assessed by spotting a dilution series of cells (from 108 to 103 cells/ml) on either SC medium lacking Leu (SC-Leu medium) to test for growth defects or on SC-Leu-Lys medium to test for Spt− phenotypes. (A) Growth of strains after 2 days of incubation at 30°C. (B) The Spt− phenotype of each strain was determined by testing for suppression of lys2-128δ. In an otherwise wild-type background, this insertion mutation confers a Lys− phenotype (Spt+ phenotype). In the spt10 mutants, lys2-128δ is suppressed and the strains are Lys+ (Spt− phenotype).
To test if the Spt10 acetyltransferase domain is required for the transcription of histone genes, we measured the mRNA levels for all eight histone genes in spt10-199 and spt10-122 strains (Fig. 1, lanes 7 to 14). Our results demonstrate that these spt10 mutants had virtually the same defect as an spt10Δ mutant (Fig. 1, compare lanes 7 to 14 to lanes 5 and 6). We conclude from these data that the Spt10 acetyltransferase domain is of primary importance in the ability of Spt10 to activate histone gene transcription.
Spt10 and Spt21 are recruited to the promoters of the histone loci.
Previous studies demonstrated that, in addition to Spt10, Spt21 is required for normal transcription of particular histone genes (8). To test whether Spt10 and Spt21 directly regulate histone gene transcription, we used ChIP to examine the possible physical association of Spt10 and Spt21 with histone gene promoters. Because histone gene transcription is cell cycle regulated, we performed the experiments using synchronized cells, taking samples in G1, when histone gene transcription is low, and in S phase, when it is at its peak. To immunoprecipitate Spt10 or Spt21 in these experiments, we constructed separate strains that encode either Spt10 or Spt21 fused to 13 copies of the Myc epitope. Each construct encodes a functional protein that was wild type for all tested phenotypes, including the growth and Spt phenotype (D. Hess and F. Winston, unpublished data).
First, at HTA2-HTB2, the locus most strongly dependent on Spt10 and Spt21, our results (Fig. 3A and B) demonstrate that Spt10 and Spt21 are physically associated with the promoter region. This association was cell cycle dependent, as Spt10 and Spt21 were associated during S phase, when histone gene transcription is maximal, but they are not significantly associated in G1, when histone gene transcription is low. To localize the binding of Spt10 and Spt21 within the HTA2-HTB2 promoter, we examined three regions by ChIP. We found that both Spt10 and Spt21 binding was maximal over the upstream regulatory sites for the HTA2-HTB2 locus (Fig. 3C), suggesting that Spt10 and Spt21 bind either directly or indirectly via the promoter regulatory elements. These results strongly suggest that Spt10 and Spt21 act directly at the HTA2-HTB2 promoter to activate transcription.
FIG. 3.
Spt10 and Spt21 are recruited to the HTA2-HTB2 promoter. ChIPs were performed on wild-type strains expressing either Spt10-Myc or Spt21-Myc. (A) Representative PCRs for Spt10 ChIP (left) and Spt21 ChIP (right). PCR products synthesized from total input chromatin (in) and from immunoprecipitated chromatin (ip) are shown. Twofold dilutions of the template were used to ensure linearity of the PCR. The HTA2-HTB2 promoter region and a negative control region are shown. (B) Quantitation of Spt10 and Spt21 binding at the HTA2-HTB2 promoter at both G1 (white bars) and S (gray bars) phases. Values were calculated from the results of three independent experiments and are reported as the increase in enrichment over the negative control (see Materials and Methods). (C) Localization of Spt10 binding at the HTA2-HTB2 promoter. Three regions of the HTA2-HTB2 promoter were tested for Spt10 and Spt21 binding by ChIP (top). Quantitation of three independent experiments is shown (bottom). The values are the increase in enrichment over that for a control region (see Materials and Methods).
When ChIP analysis was performed at the three other histone loci, we found that Spt10 and Spt21 were associated with all three of these promoters as well (Fig. 4). These results were unexpected since Spt10 and Spt21 are not required for normal transcription at two of these loci, HTA1-HTB1 and HHT1-HHF1. The association of Spt10 at these three loci differed from its association at HTA2-HTB2, as it was present in both G1 and S phases. In contrast, the association of Spt21 showed the same S phase dependence at all four loci, consistent with the S-phase-specific expression of SPT21. As a control, we also performed ChIP experiments to examine the association of Spt10 with two nonhistone promoters, ACT1 and YER083C. In neither case did we observe significant association (1.7 ± 0.4-fold enrichment at ACT1 and 1.1 ± 0.2-fold enrichment at YER083C). These results suggest that Spt10 and Spt21 are specifically associated with the promoter regions at the four histone loci.
FIG. 4.
Spt10 and Spt21 are recruited to all four histone promoters. (A) Quantitation of Spt10 binding at all four histone promoters at both G1 (white bars) and S (gray bars) phases. Values were calculated from the results of three independent experiments and are reported as the increase in enrichment over the negative control (see Materials and Methods). (B) Quantitation of Spt21 binding at all four histone promoters, displayed and calculated as described for panel A.
To determine if Spt21 is required for the association of Spt10 with histone promoters, we performed ChIP experiments on Spt10 in an spt21Δ mutant. Our results demonstrate that, in S phase, Spt10 association at all four histone promoters is significantly reduced in an spt21Δ mutant (Fig. 5A and D). In addition, there was no change in Spt10 protein levels in an spt21Δ mutant (Fig. 5B). Therefore, Spt21 is important for Spt10 binding to histone promoters when histone genes are transcribed. We also analyzed Spt10 binding in an spt21Δ mutant in G1 (Fig. 5C). At HTA2-HTB2 we observed the expected low level of Spt10 association. At the other three histone loci, consistent with our unexpected finding that Spt10 binds to these three promoters in an SPT21+ strain in G1, we found that this binding is largely Spt21 independent. In summary, at the HTA2-HTB2 promoter, Spt10 binds in a cell cycle-dependent and Spt21-dependent fashion. At the other three histone promoters, Spt10 binds in an Spt21-independent fashion in G1, but in an Spt21-dependent fashion in S, suggesting dynamic changes at these promoters during the cell cycle.
FIG. 5.
Spt10 recruitment requires Spt21. ChIPs were performed on spt21Δ strains expressing Spt10-Myc. (A) Representative results with twofold dilutions of the template. The HTA2-HTB2 promoter region and a negative control region are shown. (B) Western analysis of Spt10 protein levels in wild-type and spt21Δ strains. Numbers on the left represent the positions of protein size markers in kilodaltons (Gibco BRL). (C) Quantitation of Spt10 binding at all four histone promoters during G1 in a wild-type background (white bars) or spt21Δ background (gray bars). Values are from three independent experiments. (D) Quantitation of Spt10 binding at all four histone promoters in wild-type and spt21Δ backgrounds during S phase, arranged as in panel C.
LexA-Spt10 requires the Spt10 acetyltransferase domain and Spt21 for full activity.
Our ChIP experiments showed that Spt10 fails to bind to the HTA2-HTB2 promoter in an spt21Δ mutant, strongly suggesting that one key role of Spt21 in activation of histone gene transcription is by the recruitment of Spt10. We also wanted to test whether Spt21 might play an additional role in transcriptional activation, after Spt10 is recruited to DNA. To do this, we used LexA-Spt10 fusions and an integrated lacZ reporter with six LexA operator sites upstream of a minimal promoter. The wild-type LexA-Spt10 fusion was a strong activator of transcription, approximately 50% of the level for LexA-Gal4 (Fig. 6). However, the wild-type LexA-Spt10 fusion showed a strong dependence on Spt21 for activation. In addition, a LexA-Spt10 fusion that contains only the amino-terminal half of Spt10, including the acetyltransferase domain, showed complete dependence on Spt21 for activation. We also tested the two Spt10 acetyltransferase domain mutants and found that they are defective for activation. Western blot analysis showed that all LexA fusion proteins were present at similar levels (D. Hess and F. Winston, unpublished data). These results, then, provide evidence that Spt21 is also required for activation by Spt10 at a step after its recruitment to the promoter and provide additional support for the idea that the Spt10 acetyltransferase domain is necessary for activation by Spt10 in an Spt21-dependent fashion.
FIG. 6.
β-Galactosidase levels in strains expressing Spt10-LexA fusions. The values are normalized to the full-length wild-type fusion in the wild-type strain. All values were determined from at least three independent experiments and are reported with standard errors. The value for the wild-type LexA-Spt10 fusion was 1,288 ± 168 U compared to 2,480 ± 57 U for LexA-Gal. Wild-type and mutant fusion proteins were expressed at comparable levels as assayed by Western analysis (D. Hess and F. Winston, unpublished data). Gray box, HAT domain; X, point mutation. Mutant proteins fused to LexA included the products of the spt10-122 and spt10-199 alleles and a carboxy-terminal truncation that consisted of the first 330 amino acids of Spt10.
Spt10 and Spt21 physically interact.
The strong genetic and molecular interactions between Spt10 and Spt21 suggest that they might physically interact. To test for a direct interaction, we expressed both proteins in E. coli using a derivative of Spt10 fused to a six-histidine tag. Purification of Spt10 resulted in copurification of Spt21 in nearly stoichiometric amounts (Fig. 7). When this interaction was tested in yeast, we also observed a reproducible physical interaction, albeit at significantly lower levels, even in S phase or when Spt21 was overproduced (D. Hess and F. Winston, unpublished data).
FIG. 7.
Spt10 physically interacts with Spt21. Plasmids that expressed either tagged Spt10, untagged Spt21, or both were induced in E. coli and purified (see Materials and Methods). The protein products of the purified plasmids were separated on an SDS-PAGE gel and Coomassie stained. The proteins present in each E. coli strain are indicated above the gel. N, amino-terminal six-His tag on Spt10; C, carboxy-terminal six-His tag.
Identification of spt10 mutations that suppress spt21Δ.
During the course of testing the phenotypes of strains in which different epitope tags were fused to Spt10, we discovered that an allele of spt10 that encoded a triple-HA tag fused to the N terminus of Spt10 (hereafter called spt10-3) confers suppression of the spt21Δ Spt− phenotype (Fig. 8A). spt10-3 did not produce any other detectable mutant phenotypes. Based on this finding, we screened for additional spt10 mutations that could suppress the spt21Δ Spt− phenotype and identified one mutation (Fig. 8B). This allele, SPT10-11, was sequenced and found to encode an S508F change in Spt10. This change does not fall within any known motif in Spt10. Both spt10-3 and SPT10-11 were tested for dominance. Surprisingly, spt10-3 was found to be recessive (D. Hess and F. Winston, unpublished data), while SPT10-11 was dominant (Fig. 8).
FIG. 8.
Two spt10 mutations that suppress spt21Δ. (A) Suppression by spt10-3. (Left) Yeast strains tested for an Spt− phenotype with respect to suppression of lys2-128δ. The third row demonstrates suppression of spt21Δ by spt10-3. Strains FY2197 and FY2198 have integrated copies of the spt10-3 allele. (Right) RT-PCR analysis of histone mRNA levels. The levels are presented as a twofold dilution series from 1× to 8× cDNA template. Bands were quantitated with National Institutes of Health Image software from three independent cDNA sets. The HTB2/ACT1 ratio was designated a value of 100 for the wild type. (B) Suppression by SPT10-11. The same tests are shown as for the spt10-3 suppressor mutation except that the SPT10 alleles are expressed from plasmids. Furthermore, we tested the dominance of SPT10-11 by expressing it in FY2199, which has a wild-type copy of SPT10 in the genome (bottom patch). SPT10-11 was also dominant in diploids (D. Hess and F. Winston, unpublished data).
To test whether the suppression of spt21Δ by these spt10 mutations extended to the control of histone gene transcription, we measured histone mRNA levels (Fig. 8). Our results show that both spt10 suppressor alleles partially suppress the transcriptional defect at HTB2 caused by spt21Δ. The ability of Spt10 mutants to partially bypass loss of Spt21 suggests that Spt21 plays an auxiliary role in the activation of transcription by Spt10.
DISCUSSION
Previous work had demonstrated that Spt10 and Spt21 are required for normal transcription of a subset of histone genes (8). We have now elucidated several previously unknown aspects concerning transcriptional control of histone genes by Spt10. First, we have shown that the Spt10 acetyltransferase domain is required for this expression. Second, both Spt10 and Spt21 are physically associated with the regulatory regions of all four histone gene pairs, and in S phase the association of Spt10 is dependent on Spt21. Third, analysis of LexA-Spt10 activation suggests that Spt21 is also required at a postrecruitment step. Fourth, Spt10 and Spt21 physically interact. Finally, mutations in SPT10 can bypass the requirement for Spt21. Taken together these data suggest a model for Spt10 and Spt21 transcriptional activation, discussed later in this section.
The association of Spt10 and Spt21 at histone promoters.
The physical association of Spt10 and Spt21 at the HTA2-HTB2 promoter, at which both proteins are strongly required for transcription, correlates well with expression of the two histone genes, as both Spt10 and Spt21 are present at this promoter at a high level during S phase and at a low level in G1. This pattern is logically explained by the S-phase-specific expression of Spt21 (5, 30) and the dependence of Spt10 on Spt21 for association with this promoter. (We were unable to determine if Spt21 association is dependent on Spt10, as the poor growth of spt10Δ mutants prevents ChIP analysis of these strains [D. Hess and F. Winston, unpublished data]). These data strongly suggest that Spt10 and Spt21 control transcription initiation directly at the HTA2-HTB2 promoter. The requirement for the Spt10 acetyltransferase domain suggests that a key component of this activation is acetylation.
The finding that Spt10 and Spt21 are associated with the promoters at all four histone loci was surprising for two reasons. First, at two of these loci (HTA1-HTB1 and HHF1-HHT1) there is no apparent requirement for Spt10 and Spt21. Perhaps at these loci they are redundant with other transcriptional activators or they are required for some other aspect of transcriptional control. Previous studies have shown that Spt10 and Spt21 are required for repression of HTA1-HTB1 upon treatment of cells with hydroxyurea (26), and this effect may require the association of Spt10 and Spt21 at this locus. Second, the binding of Spt10 at three of the loci (HTA1-HTB1, HHF1-HHT1, and HHF2-HHT2) is complex, as it depends on Spt21 in S phase but not in G1. This finding suggests that there are dynamic changes at these promoters during the cell cycle and that the association of Spt10 may occur via distinct factors in G1 and S.
The Spt10 acetyltransferase domain.
Our results have established that the Spt10 acetyltransferase domain is required for Spt10 function. While most proteins with this domain have been shown to acetylate histones, we have not been able to detect HAT activity for Spt10. Several approaches have been taken to test for an Spt10 HAT activity, using preparations from either S. cerevisiae cells or E. coli cells, in the presence or absence of Spt21. These experiments have not shown acetyltransferase activity using a variety of histone substrates (D. Hess, B. Liu, R. Sternglanz, and F. Winston, unpublished data). If histones are a substrate for Spt10 in vivo, there may be a condition or a cofactor lacking from our experiments that is necessary to observe the activity in vitro.
There is mixed evidence with respect to a role for Spt10 in histone acetylation. Recent data have implicated Spt10 as being required for H3 and H4 acetylation at the CUP1 promoter upon transcriptional induction (25), although those studies did not determine whether this effect was direct or indirect. In our studies, we examined H3 and H4 acetylation levels at the HTA2-HTB2 promoter during the cell cycle using ChIP analysis. We detected an increase in the level of H4 acetylation in S phase compared to G1. However, this increase was unaffected by an spt21Δ mutation (D. Hess and F. Winston, unpublished data), a condition in which Spt10 is not recruited to the HTA2-HTB2 promoter. Thus, at HTA2-HTB2, there is no evidence that Spt10 acetylates histones as part of its role in transcriptional activation. One intriguing possibility is that the substrate for an Spt10 acetyltransferase activity is a nonhistone protein. While the demonstration of an Spt10 acetyltransferase activity has remained elusive, it is clear that this domain of Spt10 is crucial for its ability to activate transcription.
A model for Spt10 and Spt21 transcriptional activation.
Our results suggest a model for the roles of Spt10 and Spt21 in transcriptional activation at the HTA2-HTB2 locus (Fig. 9). In this model, Spt10 activates transcription in a manner dependent on its acetyltransferase domain and on Spt21, which is present only in S phase. As neither Spt10 nor Spt21 has known DNA-binding motifs, we suggest that they are recruited to the HTA2-HTB2 promoter via interaction with a DNA binding factor. Two possibilities for such a factor include MBF and SBF, each of which, it has been suggested, binds to the HTA2-HTB2 promoter in a cell cycle-dependent fashion (10). Our results suggest that Spt21 is required for Spt10 activity both at the level of recruitment of Spt10 to the HTA2-HTB2 promoter and in a postrecruitment step in transcriptional activation. As diagrammed, the direct interaction of Spt21 with Spt10 might cause a conformational change in Spt10 that stimulates both of these activities. By this model, the two Spt10 mutants that we identified that are partially independent of Spt21 might be constitutively in such an active conformation. Future experiments will be aimed at testing this model, including the identification of the target of the putative Spt10 acetyltransferase activity.
FIG. 9.
A model for Spt10 and Spt21 in activation of HTA2-HTB2. In G1 (top), Spt10 is incapable of interacting with the HTA2-HTB2 promoter because Spt21 is not present and, perhaps, because an unidentified site-specific DNA-binding factor is not present. During S phase, Spt21 is present (left) and interacts with Spt10, thereby helping it bind to the HTA2-HTB2 promoter. Spt21 may be necessary for Spt10 binding either by a modification of Spt10, such as by a conformational change, or by contributing directly to interactions with Spt10 and with other factors, such as a DNA-binding factor, at the HTA2-HTB2 promoter. If Spt21 is not present (right), then Spt10 is not recruited to the HTA2-HTB2 promoter.
Acknowledgments
We thank Krista Dobi and Andrea Duina for helpful comments on the manuscript. We thank Natalie Watson for editing and formatting assistance. We are grateful to Weidong Chen for help in construction of the lexA-SPT10 fusions.
This work was supported by NIH grants GM55641 to R.S. and GM32967 to F.W.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1988. Current protocols in molecular biology. Greene Publishing Associates/Wiley Interscience, New York, N.Y.
- 2.Baudin, A., O. Ozier-Kalogeropoulos, A. Denouel, F. Lacroute, and C. Cullin. 1993. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 21:3329-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeke, J. D., F. LaCroute, and G. R. Fink. 1984. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197:345-346. [DOI] [PubMed] [Google Scholar]
- 4.Burke, D., D. Dawson, and T. Stearns. 2000. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 5.Cho, R. J., M. J. Campbell, E. A. Winzeler, L. Steinmetz, A. Conway, L. Wodicka, T. G. Wolfsberg, A. E. Gabrielian, D. Landsman, D. J. Lockhart, and R. W. Davis. 1998. A genome-wide transcriptional analysis of the mitotic cell cycle. Mol. Cell 2:65-73. [DOI] [PubMed] [Google Scholar]
- 6.Cliften, P., P. Sudarsanam, A. Desikan, L. Fulton, B. Fulton, J. Majors, R. Waterston, B. A. Cohen, and M. Johnston. 2003. Finding functional features in Saccharomyces genomes by phylogenetic footprinting. Science 301:71-76. [DOI] [PubMed] [Google Scholar]
- 7.Denis, C. L., and T. Malvar. 1990. The CCR4 gene from Saccharomyces cerevisiae is required for both nonfermentative and spt-mediated gene expression. Genetics 124:283-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dollard, C., S. L. Ricupero-Hovasse, G. Natsoulis, J. D. Boeke, and F. Winston. 1994. SPT10 and SPT21 are required for transcription of particular histone genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 14:5223-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hereford, L., K. Fahrner, J. Woolford, Jr., M. Rosbash, and D. B. Kaback. 1979. Isolation of yeast histone genes H2A and H2B. Cell 18:1261-1271. [DOI] [PubMed] [Google Scholar]
- 10.Iyer, V. R., C. E. Horak, C. S. Scafe, D. Botstein, M. Snyder, and P. O. Brown. 2001. Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature 409:533-538. [DOI] [PubMed] [Google Scholar]
- 11.Kellis, M., N. Patterson, M. Endrizzi, B. Birren, and E. S. Lander. 2003. Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 423:241-254. [DOI] [PubMed] [Google Scholar]
- 12.Komarnitsky, P., E. J. Cho, and S. Buratowski. 2000. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 14:2452-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo, M. H., J. Zhou, P. Jambeck, M. E. Churchill, and C. D. Allis. 1998. Histone acetyltransferase activity of yeast Gcn5p is required for the activation of target genes in vivo. Genes Dev. 12:627-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larschan, E., and F. Winston. 2001. The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4. Genes Dev. 15:1946-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz, M. C., R. S. Muir, E. Lim, J. McElver, S. C. Weber, and J. Heitman. 1995. Gene disruption with PCR products in Saccharomyces cerevisiae. Gene 158:113-117. [DOI] [PubMed] [Google Scholar]
- 17.Martens, J. A., and F. Winston. 2002. Evidence that Swi/Snf directly represses transcription in S. cerevisiae. Genes Dev. 16:2231-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natsoulis, G., C. Dollard, F. Winston, and J. D. Boeke. 1991. The products of the SPT10 and SPT21 genes of Saccharomyces cerevisiae increase the amplitude of transcriptional regulation at a large number of unlinked loci. New Biol. 3:1249-1259. [PubMed] [Google Scholar]
- 19.Natsoulis, G., F. Winston, and J. D. Boeke. 1994. The SPT10 and SPT21 genes of Saccharomyces cerevisiae. Genetics 136:93-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuwald, A. F., and D. Landsman. 1997. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem. Sci. 22:154-155. [DOI] [PubMed] [Google Scholar]
- 21.Osley, M. A. 1991. The regulation of histone synthesis in the cell cycle. Annu. Rev. Biochem. 60:827-861. [DOI] [PubMed] [Google Scholar]
- 22.Prelich, G., and F. Winston. 1993. Mutations that suppress the deletion of an upstream activating sequence in yeast: involvement of a protein kinase and histone H3 in repressing transcription in vivo. Genetics 135:665-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose, M. D., F. Winston, and P. Hieter. 1990. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Schneider, B. L., B. Steiner, W. Seufert, and A. B. Futcher. 1996. pMPY-ZAP: a reusable polymerase chain reaction-directed gene disruption cassette for Saccharomyces cerevisiae. Yeast 12:129-134. [DOI] [PubMed] [Google Scholar]
- 25.Shen, C. H., B. P. Leblanc, C. Neal, R. Akhavan, and D. J. Clark. 2002. Targeted histone acetylation at the yeast CUP1 promoter requires the transcriptional activator, the TATA boxes, and the putative histone acetylase encoded by SPT10. Mol. Cell. Biol. 22:6406-6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherwood, P. W., and M. A. Osley. 1991. Histone regulatory (hir) mutations suppress delta insertion alleles in Saccharomyces cerevisiae. Genetics 128:729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon, I., J. Barnett, N. Hannett, C. T. Harbison, N. J. Rinaldi, T. L. Volkert, J. J. Wyrick, J. Zeitlinger, D. K. Gifford, T. S. Jaakkola, and R. A. Young. 2001. Serial regulation of transcriptional regulators in the yeast cell cycle. Cell 106:697-708. [DOI] [PubMed] [Google Scholar]
- 28.Smith, M. M., and K. Murray. 1983. Yeast H3 and H4 histone messenger RNAs are transcribed from two non-allelic gene sets. J. Mol. Biol. 169:641-661. [DOI] [PubMed] [Google Scholar]
- 29.Spector, M. S., and M. A. Osley. 1993. The HIR4-1 mutation defines a new class of histone regulatory genes in Saccharomyces cerevisiae. Genetics 135:25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spellman, P. T., G. Sherlock, M. Q. Zhang, V. R. Iyer, K. Anders, M. B. Eisen, P. O. Brown, D. Botstein, and B. Futcher. 1998. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol. Biol. Cell 9:3273-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutton, A., R. C. Heller, J. Landry, J. S. Choy, A. Sirko, and R. Sternglanz. 2001. A novel form of transcriptional silencing by Sum1-1 requires Hst1 and the origin recognition complex. Mol. Cell. Biol. 21:3514-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swanson, M. S., E. A. Malone, and F. Winston. 1991. SPT5, an essential gene important for normal transcription in Saccharomyces cerevisiae, encodes an acidic nuclear protein with a carboxy-terminal repeat. Mol. Cell. Biol. 11:4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swanson, M. S., and F. Winston. 1992. SPT4, SPT5 and SPT6 interactions: effects on transcription and viability in Saccharomyces cerevisiae. Genetics 132:325-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winston, F., C. Dollard, and S. L. Ricupero-Hovasse. 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11:53-55. [DOI] [PubMed] [Google Scholar]
- 36.Wu, P. Y., and F. Winston. 2002. Analysis of Spt7 function in the Saccharomyces cerevisiae SAGA coactivator complex. Mol. Cell. Biol. 22:5367-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu, H., U. J. Kim, T. Schuster, and M. Grunstein. 1992. Identification of a new set of cell cycle-regulatory genes that regulate S-phase transcription of histone genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 12:5249-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamashita, I. 1993. Isolation and characterization of the SUD1 gene, which encodes a global repressor of core promoter activity in Saccharomyces cerevisiae. Mol. Gen. Genet. 241:616-626. [DOI] [PubMed] [Google Scholar]