Abstract
The detailed mechanisms of DNA interstrand cross-link (ICL) repair and the involvement of the Fanconi anemia (FA)/BRCA pathway in this process are not known. Present models suggest that recognition and repair of ICL in human cells occur primarily during the S phase. Here we provide evidence for a refined model in which ICLs are recognized and are rapidly incised by ERCC1/XPF independent of DNA replication. However, the incised ICLs are then processed further and DNA double-strand breaks (DSB) form exclusively in the S phase. FA cells are fully proficient in the sensing and incision of ICL as well as in the subsequent formation of DSB, suggesting a role of the FA/BRCA pathway downstream in ICL repair. In fact, activation of FANCD2 occurs slowly after ICL treatment and correlates with the appearance of DSB in the S phase. In contrast, activation is rapid after ionizing radiation, indicating that the FA/BRCA pathway is specifically activated upon DSB formation. Furthermore, the formation of FANCD2 foci is restricted to a subpopulation of cells, which can be labeled by bromodeoxyuridine incorporation. We therefore conclude that the FA/BRCA pathway, while being dispensable for the early events in ICL repair, is activated in S-phase cells after DSB have formed.
DNA interstrand cross-links (ICL) are among the most cytotoxic of all DNA lesions, and the presence of a single unrepaired ICL is sufficient to kill repair-deficient bacteria and yeast (27, 29). ICL effectively prevent the separation of DNA strands and therefore block essential cellular processes, e.g., transcription and DNA replication and recombination. DNA ICL agents such as mitomycin C, cisplatin and photoactivated psoralens are widely used as potent antitumor drugs (33). The importance of understanding details of ICL repair pathways is also highlighted by the hypersensitivity to ICL-inducing agents in the human genetic disease Fanconi anemia (FA) and in cells mutant in the breast cancer genes BRCA1 and -2 (34, 46, 58).
Despite the medical relevance of this class of DNA damage, details of ICL repair in human cells remain largely unknown. Because both strands of the DNA are simultaneously affected and because no undamaged strand is available as template, ICL repair is thought to be complex and appears to involve several different pathways (for a recent review see reference 15). We have previously shown that psoralen-treated primary fibroblasts arrest in the late S phase regardless of when the damage was introduced during the cell cycle (2). FA mutant cells displayed a significantly prolonged S-phase arrest, suggesting that the FA pathway acts in an S-phase-specific damage response during ICL repair (1). This hypothesis was strengthened by the recent discovery that BRCA2, known to control homologous recombination (HR) of DNA double-strand breaks (DSB) (26, 35), is mutated in the FA complementation groups B and D1 (21). Accordingly, a model for ICL repair in human cells has been proposed, in which repair events are restricted to the S phase (30). The model predicts that, during DNA synthesis, a replication fork stalls and collapses after encountering an ICL. As a result of the recombinational restart of the replication fork, a DSB is generated. Incisions mediated by the excinuclease XPF/ERCC1 lead to an uncoupling of the ICL and a recombination event replaces the sequence across the gapped site. The remaining monoadduct is hypothesized to then be removed by a second incision event.
Although strong genetic and biochemical evidence exists for the involvement of both nucleotide excision repair (NER) and recombinational repair in the removal of mammalian ICL (15), the detailed sequence of events remains to be established. For example, the interdependence of ICL incision and DSB formation is not clear and it is not known whether early repair events can occur independently of DNA replication. In addition, the involvement of the FA complex in the initial steps in ICL repair in humans remains obscure. The FANCD2 protein is monoubiquitinated in response to ICL damage and also during the S phase. The dependence of this FANCD2 monoubiquitination on the wild-type function of the upstream FA genes (FANCA, FANCC, FANCE, FANCF, FANCG, and FANCL) suggests a potential role for the FA pathway in the recognition and signaling of ICL (17, 51).
In this study, we therefore set out to determine the detailed sequence of initial repair events in normal and FA cells. In order to minimize the potential experimental artifacts associated with extremely high doses of ICL or the use of plasmid substrates, we studied primary human cells with intact cell cycle checkpoints and used treatment with photoactivated 4′-hydroxymethyl-4,5′,8-trimethylpsoralen (HMT), a potent inducer of ICL (15). Earlier studies have shown that this regimen allows the study of ICL induction and repair without inducing major cell death (1, 2). The use of sensitive, single-cell based techniques for the detection of incision events and DSB formation further enabled us to investigate the fate of ICL under physiologic conditions, which are compatible with cell survival and repair. In contrast to the recently postulated model for ICL repair in humans (30), our results provide evidence for S-phase-independent initiation of ICL repair. However, DNA replication is indispensable for the further processing of the lesion. Furthermore, we show that the FA/BRCA pathway is not involved in these initial steps of ICL repair but in contrast is activated once DSB form at ICL during the S phase. We propose a model in which the FA/BRCA pathway acts downstream in ICL repair after DSB have formed.
MATERIALS AND METHODS
Cells and media.
The normal primary diploid fibroblast cell lines PD743.f and PD797.f were derived from human neonatal foreskin samples. FA cell lines (FA-A, PD720f; FA-C, PD331.f; and FA-D2, PD733.f) were provided by the Oregon Health & Science University Fanconi Anemia Cell Repository (Portland, Oreg.). Cells between passages 6 and 12 were maintained in α-modified Eagle medium (GIBCO/BRL) supplemented with 20% fetal calf serum (FCS) (Summit, Fort Collins, Colo.), 1× glutamine (GIBCO/BRL), and penicillin-streptomycin (GIBCO/BRL) at 37°C and 5% CO2. The Chinese hamster ovary (CHO)-derived ERCC1−/− cell line RPM41-77 sci α (RPM41-77) was a gift from J. Wilson (Baylor College, Houston, Tex.). RPM41-77 cells, CHO cells, HeLa cells, and XPG human fibroblasts (gift from A. D'Andrea, Harvard Medical School, Boston, Mass.) were grown in Dulbecco's modified medium (GIBCO/BRL) supplemented with 10% FCS, 1× glutamine, 1× nonessential amino acids (GIBCO/BRL), and penicillin-streptomycin at 37°C and 5% CO2.
Cell synchronization protocols.
For synchronization in G1, cells were incubated in 0.25% FCS-containing medium for 48 h (serum starvation). Cell synchronization was monitored by fluorescence-activated cell sorter (FACS) analysis. Briefly, trypsinized cells were fixed in ice-cold 70% ethanol, resuspended in phosphate-buffered saline (PBS) containing 0.5 mg of RNase A/ml and 50 μg of propidium iodide (Sigma)/ml, and monitored for DNA distribution by using a FACScalibur (Becton Dickinson, Mountain View, Calif.) with a laser setting of 495 nm.
Treatment with photoactivated psoralen (HMT plus UVA).
Cells in exponential growth phase were washed with Hanks balanced salt solution (GIBCO/BRL) and were treated with HMT (Sigma) in Hanks balanced salt solution and 2% FCS. After incubation for 10 min in the dark, cells were exposed to UVA light (365 nm) at a dose of 10 mW/cm2 for 20 min by using a transilluminator (Ultra-Lum, Paramount, Calif.). Subsequently, cells were washed twice with PBS at 15-min intervals and were reirradiated for 30 min. Following treatment, cells were allowed to recover at 37°C in complete medium. This protocol has been shown to result in an excessive formation of DNA ICL without inducing major acute cytotoxicity (1, 2). In all experiments, control cells were exposed to UVA but without the drug. In some experiments, the same protocol was used for treating cells with angelicin (Sigma) and UVA.
Determination of ICL induction and incision by single-cell gel electrophoresis (modified comet assay).
The comet assay was performed under alkaline conditions as previously reported (44) with minor modifications to detect the presence of ICL (16, 49). Briefly, cells treated with HMT plus UVA were washed and trypsinized either immediately or at various time points after the treatment to determine the initial induction of ICL and their uncoupling. About 104 cells in 10 μl were mixed with 120 μl of 0.5% low-melting-point agarose (Fisher) and were spread on a frosted microscope slide that had been precoated with agarose. Slides were placed in cold lysis solution for at least 1 h (2.5 M sodium chloride, 100 mM EDTA, 10 mM Tris [pH 10], 1% Triton X-100, and 10% dimethyl sulfoxide added freshly before use). After lysis, the slides were washed three times for 5 min each in PBS and were treated with 1 mg of proteinase K (Roche)/ml in dimethyl sulfoxide-free lysis solution for 2 h at 37°C in order to remove DNA-protein cross-links that might have been introduced by the treatment (32). After being washed, slides were incubated for 60 min in alkaline unwinding buffer (300 mM NaOH and 1 mM EDTA, pH > 13) in the dark at 4°C and were electrophoresed under the same conditions for 2 h (1 h for CHO and RMP41-77 cells) at 25 V (0.72 V/cm) and 300 mA. Slides were neutralized in 0.4 M Tris, pH 7.5, dried with 100% ethanol, and stained for analysis with ethidium bromide (20 μg/ml). Images of at least 50 cells per sample were captured by using a fluorescence microscope and Openlab software (Improvision, Lexington, Mass.). Necrotic and apoptotic cells can be identified by their microscopic appearance (i.e., comets with no heads and nearly all DNA in the tail) and were excluded from analysis (50). Individual comet images were evaluated by using Scion Image software (Scion, Frederick, Md.) combined with an additional comet macro (gift from A. Rapp, Jena, Germany). The mean of the tail moment (TM) (DNA migration × tail intensity) was calculated as a measure of DNA damage. The degree of cross-linking after treatment with HMT plus UVA was described by comparing the TM of the UVA-irradiated drug-treated samples (TMP) with that of the UVA-irradiated untreated control samples (TMC): % relative TM (RTM) = (TMP/TMC) × 100. Differences between mean values were tested for statistical significance (P < 0.05) by using Student's t test and one-way analysis of variance.
Immunofluorescence.
Cells were seeded onto 18-mm-diameter glass coverslips and were allowed to recover for 24 h before treatment with HMT plus UVA or ionizing radiation (IR). At various time points after the treatment, the cells were fixed in a 2% paraformaldehyde-buffered solution for 20 min and were permeabilized in 0.25% Triton X-100 for 15 min at room temperature. Cells were incubated with rabbit polyclonal anti-phospho H2AX (1:1,000 dilution; Upstate Biotech, Lake Placid, N.Y.) or rabbit polyclonal anti-FANCD2 (1:100 dilution; Novus Biologicals, Littleton, Colo.) for 1 h at room temperature and appropriately diluted fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulin G (Jackson Immunoresearch Laboratories) for 1 h at room temperature. The coverslips were mounted in 4′,6′-diamidino-2-phenylindole-containing antifade solution (Molecular Probes, Eugene, Oreg.) and were analyzed by fluorescence microscopy. Quantification of γH2AX-staining intensity was done as follows: at least 100 images per sample were captured with Openlab software. After transformation into grayscale images, the total fluorescence intensity (FI) was calculated with Scion Image software in combination with an additional macro. The results are expressed as the mean and 95% confidence interval of FI of HMT-plus-UVA-treated samples relative to those of UVA-treated controls (relative FI [RFI]).
For single-pulse experiments with bromodeoxyuridine (BrDU), asynchronously growing cells were incubated in the presence of 10 μM BrDU either for 30 min before treatment with IR or throughout the treatment and subsequent repair incubation in the case of HMT-plus-UVA treatment. Cells were then fixed in 2% paraformaldehyde and were processed for γH2AX immunostaining as described above. After a second brief fixation with 4% paraformaldehyde for 5 min, DNA was denatured with 4 N HCl for 10 min on ice. Mouse anti-BrDU monoclonal antibody (1:150 dilution; Chemicon, Temecula, Calif.) along with Cy3-labeled donkey-anti-mouse secondary antibody (1:500 dilution; Jackson Immunoresearch Laboratories) was used to label replication sites.
Immunoblotting.
Cells were scraped in lysis buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1% Nonidet NP-40, 0.5% sodium deoxycholate, 1 mM EDTA, and protease inhibitors) and were subjected to sodium dodecyl sulfate-5.5% polyacrylamide gel electrophoresis. After transfer to a polyvinyl difluoride membrane, the membrane was blocked with 5% nonfat dried milk in TBS-T (50 mM Tris HCl, pH 8.0, 150 mM NaCl, and 0.1% Tween 20) and was incubated with a 1:200 dilution of mouse monoclonal anti-FANCD2 Fl 17 (Santa Cruz Biotechnology, Santa Cruz, Calif.) overnight at 4C. After extensive washing, horseradish peroxidase-conjugated anti-mouse immunoglobulin G1 secondary antibody was added and chemiluminescence was used for detection. For quantification of UbFANCD2, films were scanned and signal intensity was quantified with multianalyst software (Bio-Rad, Hercules, Calif.).
RESULTS
Development of a modified comet assay to detect ICL formation and incision.
To study the processing of ICL in primary cells under physiologic conditions, a highly sensitive assay was needed. The detection limit of previously existing technology was >6,000 ICL/genome, an already highly cytotoxic level of lesions (2, 54). The comet assay allows the sensitive detection of DNA damage at the level of individual cells, but standard comet assay protocols (37) were not able to reliably detect small numbers of ICL. We therefore modified the technique such that cells exhibited a high degree of spontaneous DNA migration (see Materials and Methods for details), representing stretched DNA molecules (Fig. 1A). DNA ICL markedly inhibited the denaturation of DNA under alkaline conditions and therefore retarded the migration of DNA in the modified comet assay. We used this approach to quantify the induction and incision of ICL induced by photoactivated psoralen (HMT plus UVA). Analysis of primary wild-type fibroblasts immediately after the treatment showed a dose-dependent reduction in RTM compared to that found in control cells, which were only UVA irradiated (Fig. 1B). The detection limit of the assay was found to be 0.1 ng of HMT/ml plus UVA (P < 0.05 compared to UVA-treated cells; unpaired t test), which correlates to approximately 600 ICL/genome (2). Compared to previously reported techniques, the comet assay in our hands was therefore able to detect HMT-plus-UVA-induced ICL with ∼10-fold-higher sensitivity (2).
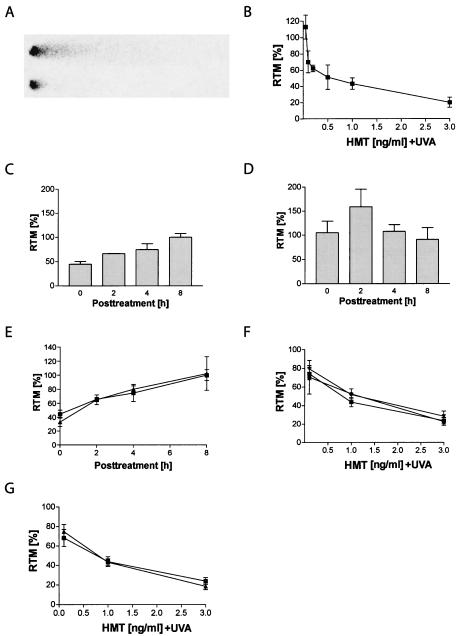
FIG. 1.
Validation of the alkaline comet assay. (A) Under the conditions used in this study, control cells show a pronounced spontaneous migration of DNA (top). In contrast, ICL induced by HMT plus UVA inhibit denaturation and cause a retardation of DNA migration (bottom). (B) Dose-dependent decrease in RTM compared to that found in UVA-irradiated controls in PD743.f cells analyzed immediately after treatment with HMT plus UVA. (C) Time-dependent restoration of RTM in PD743.f cells after treatment with 0.5 ng of HMT/ml plus UVA. Complete restoration of DNA migration was observed 8 h after treatment. (D) The psoralen analog angelicin induces non-cross-linkable monoadducts. Incisions generated after treatment with 5 ng of angelicin/ml caused an increase in RTM. (E) Comparison of the incision kinetics after treatment with 0.5 ng of HMT/ml plus UVA (▪) or with 0.5 ng of HMT/ml plus UVA in the presence of 5 ng of angelicin/ml (▴) showed that parallel repair of monoadducts did not bias the detection of ICL incisions. (F) Treatment with equimolar doses of HMT plus UVA resulted in a similar initial formation of ICL in wild-type PD743.f (▪), PD720.f (FA-A) (•), and PD733.f (FA-D2) (♦) cells as well as in asynchronously growing (▪) and G1-arrested (▴) PD743.f cells (G).
Incisions formed during ICL repair cause the formation of an excess of DNA strand breaks, which antagonize the migration-inhibiting effect of ICL in the comet assay. The quantification of restoration of DNA migration at various time points after the treatment thus allows an accurate description of the kinetics of ICL incision (Fig. 1C).
In order to prove that the observed restoration of DNA migration reflects incisions were specifically related to ICL and not psoralen-induced monoadducts, we compared the effects of a cotreatment of HMT plus UVA together with angelicin. Angelicin is a psoralen analog, which mainly forms monoadducts but hardly any ICL in DNA upon UVA irradiation (6) (Fig. 1D). Even in the presence of a 10-fold excess of angelicin, we observed the same incision kinetics as with HMT plus UVA alone (Fig. 1E), indicating that our results were not biased by unrelated incision events occurring during repair of monoadducts or by futile incision events (36).
Influence of the FA/BRCA pathway and cell cycle on ICL induction.
Having established a sensitive and reproducible protocol for the detection of HMT-plus-UVA-induced ICL, we next asked whether the hypersensitivity of FA cells to ICL is based on a higher initial induction of cross-links. We found no significant difference in the initial cross-linking effect of HMT plus UVA in two FA cell lines from that in wild-type cells (Fig. 1F), indicating that the initial formation of ICL at equimolar doses is not different in FA cells. In addition, the efficiency of HMT plus UVA to induce ICL was found to be independent of the cell cycle, since we could not observe significant differences between the cross-linking effect in asynchronously growing and G1-arrested cells (Fig. 1G). It is important, however, to mention that the comet assay detects ICL on the level of the whole genome; our results therefore do not exclude the possibility of gene-specific differences in ICL induction (23).
Influence of the FA/BRCA pathway and cell cycle on ICL incision.
The dose-dependent incision kinetics of HMT-plus-UVA-induced ICL were examined next. Unsynchronized wild-type cells restored DNA migration very rapidly and efficiently upon treatment with HMT plus UVA (Fig. 2A). The migration inhibition induced by a low number of ICL (0.1 ng of HMT/ml plus UVA or ∼600 ICL) was completely restored after 2 h (i.e., RTM ≥ 100%). With higher doses, a time-dependent increase in RTM was observed and complete restoration was measured after 8 h (1 ng of HMT/ml plus UVA) and 24 h (3 ng of HMT/ml plus UVA).
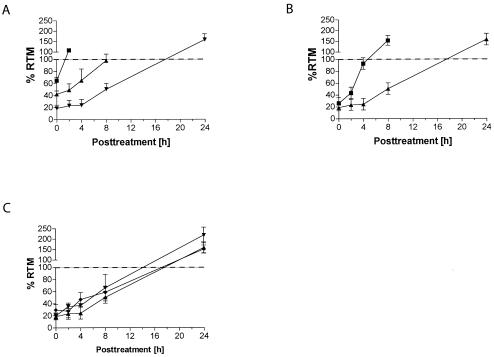
FIG. 2.
Incision kinetics of ICL in wild-type and FA cells. (A) Analysis of RTM in asynchronous PD743.f cells at different time points after treatment with HMT plus UVA showed a time-dependent restoration of DNA migration. Complete restoration of RTM (values ≥ 100%) was observed after 2 h (0.1 ng of HMT/ml plus UVA [▪]), 8 h (1 ng of HMT/ml plus UVA [▴]), and 24 h (3 ng of HMT/ml plus UVA [▾]), respectively. (B) Incision of ICL induced by 3 ng of HMT/ml plus UVA was significantly faster in G1-arrested cells (▪) than in asynchronous cells (▴). (C) ICL were incised with similar kinetics in asynchronously growing PD720.f (FA-A [▾]), PD733.f (FA-D2 [♦]), and wild-type PD743.f (▴) cells.
The observed rapid unhooking kinetics (Fig. 2A) already indicated that incisions during ICL repair did not occur only during the S phase, as recently proposed (30), but actually occur throughout the cell cycle. To corroborate that ICL incision happened independently of DNA replication, we determined the incision kinetics in G1-arrested cells. FACS analysis confirmed that the majority of cells (∼90%) were arrested in G1 during treatment and repair (data not shown). Importantly, cells arrested in G1 were at least as proficient in incising ICL as were asynchronously growing cells (Fig. 2B). The incision kinetics of ICL induced by 3 ng of HMT/ml were significantly faster in G1-arrested than in unsynchronized cells (P < 0.05; one-way analysis of variance). A similar trend was observed at lower HMT-plus-UVA doses (data not shown). Analysis of the distribution of individual TM values showed that this effect was not mediated by a subpopulation of cells (data not shown), which would be expected if only the remaining cycling cells incised ICL. Furthermore, the rapidity of RTM restoration makes the contribution of apoptosis-induced DNA strand breaks unlikely. Together our observations show that the incision of ICL is the initial event in ICL repair and is not restricted to the S phase but instead occurs without specificity for a cell cycle stage.
To investigate whether the extreme sensitivity of FA cells to cross-linking agents is due to a defect in the incision step of ICL repair, we compared the incision kinetics in two asynchronously growing FA cell lines (Fig. 2C). Both FA-A and FA-D2 cell lines showed rapid incision kinetics after treatment with 3 ng of HMT/ml plus UVA indistinguishable from those observed in wild-type cells. This was also true after treatment with 0.1 and 1 ng of HMT/ml plus UVA (data not shown). We therefore conclude that both the sensing of ICL and incision at sites of ICL are fully functional in FA cells and that the FA pathway is not involved in these initial steps of ICL repair. Interestingly, in all of our incision experiments, we observed that the restoration of DNA migration was increased compared to results for control cells (i.e., RTM ≫ 100%). The reason for this effect is not clear but most likely reflects additional strand breaks occurring after the initial incision.
Formation of DSB during ICL repair is dependent on DNA replication.
It has been shown that DSB are formed after ICL-inducing treatment in immortalized mammalian (13) and human (39) cell lines. However, these DSB were only detected under highly cytotoxic conditions and therefore these studies did not exclude the possibility that they were formed during cell death rather than DNA repair. To overcome this potential problem, we used a sensitive, indirect immunostaining approach to detect DSB. The histone variant H2AX becomes rapidly phosphorylated upon the induction of DSB (43) and appears as discrete nuclear foci (γH2AX foci). The quantification of γH2AX foci allows a very sensitive detection of DSB, since such foci can be induced by very few DSB (48). Furthermore, a recent study provided evidence for a linear relationship between the number of DSB and the number of γH2AX foci formed (45). We performed quantitative immunocytochemical analyses on HMT-plus-UVA-treated primary human fibroblasts by measuring the total FI of nuclei after incubation with fluorescein isothiocyanate-labeled anti-γH2AX antibody. The use of image analysis software (see Materials and Methods) to objectively measure FI eliminated observer bias in the scoring of foci and resulted in quantifiable and reproducible data. One day after ICL induction, a dose-dependent increase in γH2AX-staining intensity was observed in asynchronously growing wild-type cells (Fig. 3A and representative images in Fig. 3B). Cells treated with 1 ng of HMT/ml plus UVA (∼6,000 ICL/genome) revealed a three- to fourfold increase in RFI compared to results for the UVA-irradiated control cells. Concomitantly performed cell cycle analysis confirmed that fibroblasts were arrested in the late S phase with a nearly 4 N DNA content (2). The time course showed that γH2AX formation peaked at 48 h but was still clearly detectable 3 days posttreatment, indicating the presence of long-lived DSB. In contrast, γH2AX staining was minimal during the first 8 h posttreatment. Since the majority of ICL were already incised at these time points (Fig. 2A), we conclude that DSB formation occurred secondary to cross-link incision. Since DSB formation during ICL repair occurred concomitant with a cell cycle arrest in the late S phase, we then hypothesized that DSB arose during DNA replication, as shown in yeast and mammalian cells. In fact, γH2AX staining was almost absent in wild-type cells, which were synchronized in G1 of the cell cycle (∼90% of cells in G1) (Fig. 3C). In order to corroborate that DSB formation was dependent on DNA replication, we performed costaining experiments in unsynchronized primary fibroblasts with antibodies directed against γH2AX and BrDU (Fig. 3D). BrDU was present throughout ICL treatment and the entire 24-h repair incubation. Cells, which progressed through the S phase during this time, could be identified by their bright BrDU staining. An analysis of 100 cells showed that almost all (69 of 72 = 96%) BrDU-positive nuclei were also positive for γH2AX staining. Furthermore, all γH2AX-negative cells also were BrDU negative (28 of 28 = 100%), thus confirming that DNA replication is necessary to form DSB during ICL repair.
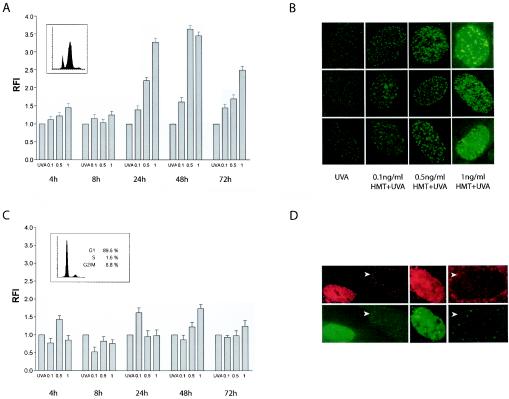
FIG. 3.
DSB formation during ICL repair is dependent on DNA replication. Asynchronously growing PD743.f cells were treated with HMT+UVA and were analyzed for γH2AX staining. (A) Quantification of the RFI (see Materials and Methods) showed a dose-dependent increase in γH2AX staining starting 24 h posttreatment. At this time point, cells were arrested with a nearly 4 N DNA content (small inset). RFI values further increased at 48 h and subsequently declined. In contrast, no significant increase in RFI was observed at 4 h and 8 h posttreatment. (B) Representative images of H2AX foci in PD743.f cells 24 h after treatment with UVA alone or different concentrations of HMT plus UVA. (C) PD743.f cells were arrested in G1 during treatment and repair (small inset) and were analyzed for γH2AX staining. No significant increases in RFI were measured throughout the time course. (D) Costaining experiments with BrDU (top panel) and γH2AX (bottom panel) confirmed that only cells positive for BrDU foci also showed γH2AX foci. In contrast, BrDU-negative cells (arrows) were also negative for γH2AX.
Incision of ICL is necessary for efficient DSB formation.
The interdependence of incision at sites of ICL and of formation of DSB has not been previously reported. We therefore asked whether cells, which are deficient in the incision step of ICL repair, are able to form DSB to the same extent as wild-type cells. The heterodimer ERCC1/XPF has been shown to be able to incise ICL in vitro (25). In accordance with a reduced incision activity (13), ERCC1 mutant cells showed a clear deficiency in the resolution of cross-link-induced migration inhibition compared to the parental CHO cell line (Fig. 4A). After treatment with 1 ng of HMT/ml plus UVA, ERCC1-deficient cells did not show significant uncoupling at cross-linked sites during the first 8 h after treatment. At this time point, wild-type CHO cells already completely restored DNA migration. Similar findings were obtained after treatment with 0.1 ng of HMT/ml (data not shown). In contrast, XPG mutant human fibroblasts, which are unable to form the 5′ gap (5) but possess the XPF/ERCC1 excinuclease, were fully proficient in incising ICL and showed incision kinetics similar to those of wild-type PD743.f cells.
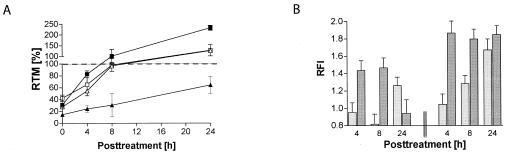
FIG. 4.
Initial incision is required for efficient DSB formation. (A) RMP41-77 cells (ERCC1−/− [▴]) show clearly delayed incision kinetics compared to parental CHO cells (▪) after treatment with 1 ng of HMT/ml plus UVA. In contrast, XPG-deficient cells (▵) are able to restore DNA migration similar to that of wild-type PD743.f cells (□). (B) γH2AX formation is similarly delayed in RMP41-77 cells (light bars) compared to CHO cells (dark bars) after treatment with 0.1 (left part) and 1 ng of HMT/ml plus UVA (right part), respectively.
Interestingly, ERCC1−/− cells were similarly delayed in γH2AX formation during ICL repair (Fig. 4B). At 4 h posttreatment, RFI was not significantly different from control cells treated with UVA alone. In contrast, control CHO cells treated in parallel showed a clear and dose-dependent increase in γH2AX staining at this time point. Cell cycle analysis confirmed that this difference was not due to different percentages of S-phase cells (4 h, 20.1 and 18.6% for CHO and ERCC1−/−, respectively; 8 h, 22.1 and 18.9%; and 24 h, 9 and 13.2%). γH2AX staining increased significantly only after 24 h in ERCC1 mutant cells. We therefore conclude that efficient DSB formation during ICL repair is dependent on preceding ERCC1-dependent incision events.
DSB formation is normal in FA cells.
Because recognition and incision of ICL are normal in FA cells, we wondered whether the ICL sensitivity of FA cells is due to defective DSB formation. However, neither FA-C (Fig. 5A) nor FA-D2 (Fig. 5B) cells showed differences in the time kinetics or the extent of γH2AX formation from those in wild-type cells. We therefore conclude that the FA pathway is also not involved in the formation of DSB during ICL repair.
FIG. 5.
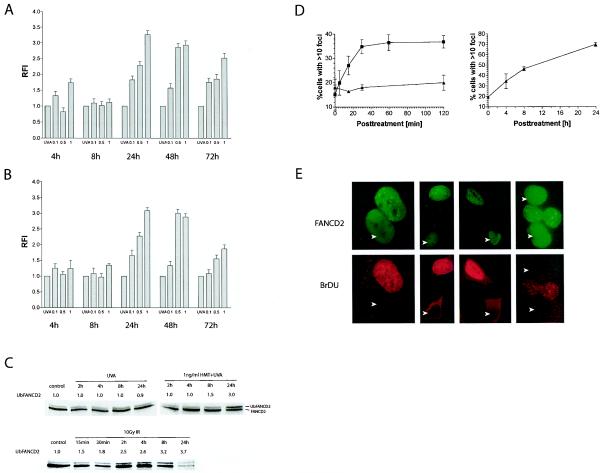
The FA/BRCA pathway is activated upon DSB formation in S-phase cells. (A) The kinetics of DSB formation is normal in both PD331.f (FA-C) and PD733.f (FA-D2) (B) cells. (C) Total cellular extracts of wild-type PD797.f cells were immunoblotted to detect both FANCD2 isoforms. Quantification of the fraction of Ub-FANCD2 shows a significant activation of FANCD2 only 24 h after treatment with HMT plus UVA. In contrast, activation is rapid after treatment with 10 Gy of IR. Note the clear decline in overall FANCD2 protein 24 h after IR. (D) Analysis of HeLa cells with FANCD2 foci (>10 foci per cell) confirms the rapid activation of FANCD2 after 10 Gy of IR (▪). In contrast, treatment with 1 ng of HMT/ml plus UVA (▴) does not cause activation of FANCD2-positive cells during this time (left panel). FANCD2-positve cells start to increase in a time-dependent manner only at 4 h after treatment with HMT plus UVA (right panel). (E) Costaining experiments with FANCD2 (top panel) and BrDU (bottom panel) show that only cells positive for BrDU foci also display FANCD2 foci. In contrast, BrDU-negative cells (arrows) are also negative for FANCD2 foci.
DSB-dependent activation of the FA/BRCA pathway during the S phase.
It has been established that several FA proteins, including A, C, E, F, G and L, form a constitutive nuclear complex in the nucleus that, in response to DNA damage or during the S phase of the cell cycle, mediates the monoubiquitination of FANCD2 (10, 31). Activated FANCD2 (Ub-FANCD2) accumulates in nuclear foci, which also contain the BRCA1 and BRCA2/FANCD1 proteins (10). Since we had observed that the initial processes in ICL repair (i.e., incision and DSB formation) were not altered in FA cells, we asked next at which time point during ICL repair the FA pathway is activated (i.e., ubiquitination of FANCD2) and whether this activation could be related to specific cellular repair processes. Western blot experiments revealed the presence of both isoforms, FANCD2 and Ub-FANCD2, in cell extracts from unsynchronized human fibroblasts (Fig. 5C), with approximately 10% of the total FANCD2 protein being monoubiquitinated in untreated control cells. Treatment with UVA alone did not lead to an increase in FAND2 monoubiquitination. Interestingly, this ratio did not increase until 4 h after treatment with 1 ng of HMT/ml plus UVA. UbFANCD2 started to increase at 8 h, and by 24 h a pronounced activation of the FA pathway was evident, causing a threefold increase in FANCD2 monoubiquitination. The FANCD2 activation kinetics therefore closely resembled the kinetics of DSB formation during ICL repair (Fig. 3A), suggesting that the FA/BRCA pathway may be activated by the induction of DSB. To further corroborate this hypothesis, we also investigated the kinetics of FANCD2 activation in cells treated with IR. In contrast to the activation kinetics after ICL treatment, irradiation with 10 Gy caused a rapid increase in UbFANCD2 formation. Only 30 min after irradiation, the ratio of UbFANCD2 was almost doubled from that in the untreated controls. FANCD2 activation further increased in a time-dependent manner, and at 8 h postirradiation, a threefold increase in UbFANCD2 was measured. Similar values were observed at 24 h, despite a significant reduction in total FANCD2 protein. Since FANCD2 expression is clearly reduced in G1-arrested cells (data not shown), we speculate that the reduction in total FANCD2 protein observed at 24 h was due to the IR-induced G1 cell cycle arrest. We next asked whether the activation of the FA/BRCA pathway occurred ubiquitously or whether it was restricted to a subpopulation of cells. For this, we compared the time course of FANCD2 focus formation in cells after treatment with HMT plus UVA and IR (Fig. 5D). Since the analysis of FANCD2 foci is difficult in primary fibroblasts, we used HeLa cells for this analysis. Analysis of FANCD2 foci after IR confirmed the rapid activation of the FA/BRCA pathway (Fig. 5E). Whereas only 15% of cells in an unirradiated culture showed 10 or more foci (focus positive), the percentage increased rapidly and reached its maximum already 30 min postirradiation (∼40%). Doubling of the IR dose (20 Gy) did not lead to an increase of the percentage of FANCD2-positive cells (data not shown), indicating that, at a given time point, only a distinct subpopulation of cells can form FANCD2 foci. The observed time-dependent increase in total FANCD2 monoubiquitination detected by Western blotting (Fig. 5C) therefore suggests that the degree of UbFANCD2 formation within a cell continues to increase, even though the fraction of activated cells becomes saturated. In contrast to ionizing radiation, treatment with HMT plus UVA did not lead to an increased formation of focus-positive cells until 4 h after the treatment at the earliest. This was consistent with the lack of elevated UbFANCD2 in immunoblots at this time point (Fig. 5C). However, formation of focus-positive cells started to increase, and at 24 h after the treatment, almost 80% of the cells showed FANCD2 foci.
Importantly, the percentage of FANCD2-positive cells after IR correlated very well with the proportion of BrDU-positive cells in the same cell population (38.5% ± 1.5%). We therefore asked whether FANCD2 focus formation after DSB induction was restricted to S-phase cells. In fact, coimmunostaining experiments with antibodies against FANCD2 and BrDU showed that almost all BrDU-positive cells also had FANCD2 foci (87 of 100), whereas no BrDU-negative cell stained positive for FANCD2 (0 of 105). Together we conclude that the FA/BRCA pathway is activated by DSB and that this activation only occurs in cells that are in the S phase.
DISCUSSION
Studies on mutant cell lines with extreme sensitivity to ICL-inducing agents have indicated that NER, HR, and postreplication/translesion bypass interact in order to repair ICL in mammalian cells (15), similar to the situation in Escherichia coli (3, 7) and yeast (19). However, the details of ICL repair in humans, in particular of the initial events as well as the involvement of the FA/BRCA pathway, are not clear.
ICL are efficiently incised independently of the S phase.
Previous reports have indicated that the unhooking of ICL occurs during the S phase, after a replication fork becomes arrested at the site of an ICL (12, 13). Here we have provided the first direct evidence for a cell cycle-independent incision of genomic ICL in primary human fibroblasts. Several lines of reasoning indicate that the modified comet assay described here detects incision events specifically related to ICL repair. Firstly, cotreatment with an excess of monoadduct-forming angelicin did not alter the incision kinetics of HMT-plus-UVA-induced ICL. Secondly, the kinetics of ICL incision showed a dose-dependent delay to restore DNA migration, which would not be observed if treatment-unrelated incisions were being detected. And finally, the fact that ERCC1-mutant cells displayed significantly reduced ICL incision under these conditions showed that the process observed by the comet assay is genetically controlled and is not due to random DNA nicks. Our results therefore indicate that HMT-plus-UVA-induced genomic ICL are rapidly detected and efficiently incised regardless of the cell cycle stage. Earlier studies using transformed cell lines already indicated that the majority of HMT-plus-UVA-induced ICL can be efficiently uncoupled (20, 54). However, the conditions used in these studies were not compatible with cellular survival and repair (2). Our study is therefore the first to detect psoralen-induced ICL incision in the genome of primary human fibroblasts under physiologic conditions.
Biochemical studies using defined cross-linked substrates have suggested two distinct mechanisms for ICL incision. The first is based on the observation that cells deficient for the excinuclease ERCC1/XPF are the only NER mutants, which are exceptionally sensitive to ICL-inducing agents (9, 13, 22, 55). In fact, biochemical studies showed that ERCC1/XPF possibly in combination with hMutSβ is able to incise ICL at both the 5′ and 3′ sides of the lesion, which leads to a release of one arm of the ICL (“uncoupling reaction”) (25, 59). In addition, a further, unusual incision reaction involving the entire NER machinery has been reported, where dual incisions are produced 5′ to the cross-linked base (5). The remaining gap is filled by futile repair synthesis but is inefficiently ligated, leaving the ICL unremoved but nicked at the 5′ side (36).
The relative importance of both types of incision in vivo is not clear. The comet assay used here is not able to discriminate between frequent incisions on one side or both sides of the ICL, since both events lead to a restoration of DNA migration. However, the clearly delayed incision kinetics in ERCC1 mutant cells and the fact that XPG mutant cells are fully proficient in incising ICL (this study and reference 13) indicate that futile incision reactions might only play a minor role in vivo.
The structure specificity of the ERCC1/XPF endonuclease suggests that incisions are generated at partially unwound structures consisting of a junction between a duplex and a single strand (25). Such Y shapes are expected to form frequently in vivo, e.g., by a stalled replication fork, but could theoretically also arise during transcription or be induced by a helicase. Accordingly, the incision action of the ERCC1/XPF exinuclease would also occur independent of the S phase and might therefore lead to an uncoupling of ICL during G1. In any case, independent of the mode of incision, the repair intermediate generated cannot be further degraded by NER but has to then be processed by using a different repair pathway.
DSB form exclusively in the S phase during ICL repair.
The ICL hypersensitivity of cells with defects in HR (8) suggests that DSB occur during ICL repair. In fact, by using pulsed-field gel electrophoresis, recent studies detected DSB in asynchronously growing human cells after treatment with high concentrations of mitomycin C (39). The use of a sensitive indirect immunofluorescent detection of DSB by measuring the formation of γH2AX in psoralen-treated primary fibroblasts allowed us to study the kinetics and cell cycle dependenc of DSB formation after induction of only a few ICL. DSB lead to the rapid phosphorylation of H2AX (γH2AX) at the site of the break, and the specificity of this reaction provides a reliable marker for DSB formation (42, 43). Several studies showed that DSB arising during cellular processes, such as replication, recombination, and apoptosis, cause an efficient formation of γH2AX (28, 38, 42, 56). Quantitative studies further indicated that extent of γH2AX formation is proportional to the number of DSB and that their appearance and disappearance, respectively, correlate very well with the kinetics of DSB repair (40, 41). Our results show that DSB formation during ICL repair is dependent on DNA replication, since γH2AX formation was readily detectable in asynchronously growing but not in G1-arrested and noncycling cells. This result confirms earlier work on hamster cells (13) and is consistent with the idea that DNA replication is necessary for triggering cellular responses to ICL (2). The comparison of the incision kinetics with the DSB formation kinetics in wild-type cells clearly shows that DSB form later than the incision of ICL. Since DSB occurred in parallel with a cell cycle arrest in the late S phase (2), we hypothesize that DSB are passively formed when a replication fork arrests at the site of an uncoupled/incised ICL. In fact, a recent study provided direct evidence for a replication-dependent formation of DSB at or near a nicked ICL site in vitro (4).
Furthermore, our results on incision-deficient ERCC1 mutant hamster cells show that DSB formation during the S phase is dependent on a preceding incision event. This finding is in contrast to previous work, where ERCC1 mutant cells were not found to be impaired in DSB formation (13). However, in this study, DSB induction was analyzed after treatment with highly cytotoxic doses of nitrogen mustard, which is known to induce ICL poorly (15). Since our findings are based on the analysis of viable cells, we conclude that ICL incision is in fact required for a subsequent DSB formation.
Primary FA cells are not defective in initial steps of ICL repair.
The precise role of the FA pathway in the repair of ICL is not clear (18). So far, eight distinct FA complementation groups have been identified (FANCA, -B, -C, -D2, -E, -F, -G, and -L and BRCA2) (10, 31). In response to DNA damage and during the S phase, the multiunit nuclear FA complex mediates the monoubiquitination of FANCD2 (17), suggesting a potential role of the pathway in the recognition and initial processing of ICL during the S phase. However, the results obtained here rule out this hypothesis. The kinetics of the initial incision or in the subsequent DSB formation were not altered in FA cells from those in two different complementation groups. Although an involvement of FA proteins in the detection and incision of ICL has been suggested (24), other studies confirm that the hypersensitivity of FA cells is not due to a defect in this initial step of ICL repair (39, 59). Furthermore, no difference in DSB formation between FA-C and wild-type cells was observed after mitomycin C treatment (39). Overall, our data clearly indicate that the FA complex is not necessary for the initial steps of ICL processing.
FA/BRCA pathway is activated upon DSB formation during the S phase.
In contrast, our results point to a role of the FA proteins further downstream in ICL repair, during the resolution of DSB in the S phase. Several indications are in favor of such a model. The activation of the FA/BRCA pathway both during ICL repair and after IR was closely connected to the appearance of DSB. Our analysis of FANCD2 focus formation showed that this activation only occurred in S-phase cells, consistent with an S-phase-specific DNA damage response of the FA pathway (2). It has been shown that FANCD2 monoubiquitination is highly regulated during the cell cycle and is significantly present only during the S phase (51). In contrast, FANCD2 protein is not expressed in G1-arrested cells (51) (data not shown), which also fail to form DSB during ICL repair. The appearance of UbFANCD2 during the normal S phase might reflect the formation of DSB at stalled replication forks, a situation that is drastically enhanced during ICL repair.
It remains to be determined how the FA/BRCA pathway specifically contributes to the repair of DSB. However, evidence is accumulating that suggests a function of the FA/BRCA pathway in the HR of DSB. FANCD2 has been shown to colocalize with the HR proteins Rad51 and BRCA1 during the S phase (51), and an attenuated focus formation upon DNA damage has been described in FA cells (14, 39). The recent discovery that BRCA2, known to play a role in HR (26, 35, 47, 53), is mutated in FA complementation group D1 (21) further strengthens the involvement of FA in the HR of DSB. Most importantly, a direct link to recombination comes from the recent observation that fancg DT40 mutant cells show a decreased HR capacity for repairing I-SceI-induced DSB (57). Finally, it has been shown that FA cells have an increased error-prone HR activity (52). This hyperrecombinogenic phenotype could also explain the occurrence of radial chromosome formation, i.e., aberrant recombination events between nonhomologous chromosomes observed after treatment with ICL-inducing agents in FA cells (11).
Model for initial events in ICL repair in humans.
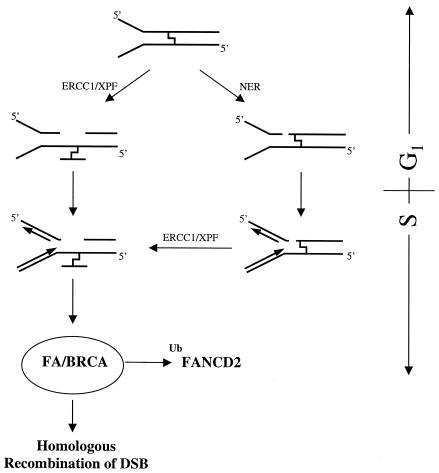
Based on our observations, we propose a model for the initial events of ICL repair in human cells (Fig. 6). This model is based on our experiments on psoralen-induced cross-links. Given that other ICL-inducing agents mediate different helical distortion and mediate different cell cycle checkpoints, it is possible that the cell cycle-dependent effects observed here may be different for different ICL-damaging agents. The exact nature of factors that are responsible for the sensing of psoralen-induced ICL remains to be identified, but our data indicate that this process occurs very rapidly. After the presence of ICL has been sensed, a structure is generated that allows the excinuclease ERCC1/XPF to perform dual incisions at both sides of the lesion, leading to an uncoupling of one arm of the cross-link. Such structures can arise independent of DNA replication, whenever chromatin is locally unwound and a nick is generated (during transcription or chromatin remodeling or by helicases or repair factors). In cells, which are in the S phase at the time ICL are induced, stalled replication forks provide incisable substrates for ERCC1/XPF. Alternatively, the NER machinery can lead to dual incisions 5′ of the ICL, which results in a nicked, but otherwise intact ICL. During progression through the S phase, replication forks stall at the incised ICL. This arrest directly generates a DSB at the site of incision. In case of the nicked, but otherwise intact ICL, a structure is generated that is unpaired at the 3′ side and therefore can be incised by ERCC1/XPF, leading to an uncoupling of the ICL. During the restart of the replication fork, DSB can occur; therefore, dependent of the situation (uni- versus bidirectional replication forks), up to four DSB can be formed at the site of an ICL (10). The generation of DSB triggers the activation of the FA/BRCA pathway leading to the monoubiquitination of FANCD2. We propose that the FA/BRCA pathway functions in the HR of DSB in the S phase.
FIG. 6.
Model for the initial events of ICL repair in human cells.
Acknowledgments
We thank J. Wilson for providing RMP41-77 cells, Alan D'Andrea for XPG cells, and Mark Foster for help with FACS analysis. We greatly acknowledge Alexander Rapp for providing the image analysis macro and Alisson Gontijo for valuable comments on the comet assay.
This study was supported by the Deutsche Forschungsgemeinschaft (A.R.) and NHLBI Program Project Grant 1PO1HL48546 (M.G.).
REFERENCES
- 1.Akkari, Y. M., R. L. Bateman, C. A. Reifsteck, A. D. D'Andrea, S. B. Olson, and M. Grompe. 2001. The 4N cell cycle delay in Fanconi anemia reflects growth arrest in late S phase. Mol. Genet. Metab. 74:403-412. [DOI] [PubMed] [Google Scholar]
- 2.Akkari, Y. M., R. L. Bateman, C. A. Reifsteck, S. B. Olson, and M. Grompe. 2000. DNA replication is required to elicit cellular responses to psoralen-induced DNA interstrand cross-links. Mol. Cell. Biol. 20:8283-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berardini, M., P. L. Foster, and E. L. Loechler. 1999. DNA polymerase II (polB) is involved in a new DNA repair pathway for DNA interstrand cross-links in Escherichia coli. J. Bacteriol. 181:2878-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bessho, T. 2003. Induction of DNA replication-mediated double strand breaks by psoralen DNA interstrand cross-links. J. Biol. Chem. 278:5250-5254. [DOI] [PubMed] [Google Scholar]
- 5.Bessho, T., D. Mu, and A. Sancar. 1997. Initiation of DNA interstrand cross-link repair in humans: the nucleotide excision repair system makes dual incisions 5′ to the cross-linked base and removes a 22- to 28-nucleotide-long damage-free strand. Mol. Cell. Biol. 17:6822-6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bordin, F., F. Dall'Acqua, and A. Guiotto. 1991. Angelicins, angular analogs of psoralens: chemistry, photochemical, photobiological and phototherapeutic properties. Pharmacol. Ther. 52:331-363. [DOI] [PubMed] [Google Scholar]
- 7.Cole, R. S. 1973. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc. Natl. Acad. Sci. USA 70:1064-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, A. R. 1993. Mutant rodent cell lines sensitive to ultraviolet light, ionizing radiation and cross-linking agents: a comprehensive survey of genetic and biochemical characteristics. Mutat. Res. 293:99-118. [DOI] [PubMed] [Google Scholar]
- 9.Damia, G., L. Imperatori, M. Stefanini, and M. D'Incalci. 1996. Sensitivity of CHO mutant cell lines with specific defects in nucleotide excision repair to different anti-cancer agents. Int. J. Cancer 66:779-783. [DOI] [PubMed] [Google Scholar]
- 10.D'Andrea, A. D., and M. Grompe. 2003. The Fanconi anaemia/BRCA pathway. Nat. Rev. Cancer 3:23-34. [DOI] [PubMed] [Google Scholar]
- 11.D'Andrea, A. D., and M. Grompe. 1997. Molecular biology of Fanconi anemia: implications for diagnosis and therapy. Blood 90:1725-1736. [PubMed] [Google Scholar]
- 12.De Silva, I. U., P. J. McHugh, P. H. Clingen, and J. A. Hartley. 2002. Defects in interstrand cross-link uncoupling do not account for the extreme sensitivity of ERCC1 and XPF cells to cisplatin. Nucleic Acids Res. 30:3848-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Silva, I. U., P. J. McHugh, P. H. Clingen, and J. A. Hartley. 2000. Defining the roles of nucleotide excision repair and recombination in the repair of DNA interstrand cross-links in mammalian cells. Mol. Cell. Biol. 20:7980-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Digweed, M., S. Rothe, I. Demuth, R. Scholz, D. Schindler, M. Stumm, M. Grompe, A. Jordan, and K. Sperling. 2002. Attenuation of the formation of DNA-repair foci containing RAD51 in Fanconi anaemia. Carcinogenesis 23:1121-1126. [DOI] [PubMed] [Google Scholar]
- 15.Dronkert, M. L., and R. Kanaar. 2001. Repair of DNA interstrand cross-links. Mutat. Res. 486:217-247. [DOI] [PubMed] [Google Scholar]
- 16.Fuscoe, J. C., A. J. Afshari, M. H. George, A. B. DeAngelo, R. R. Tice, T. Salman, and J. W. Allen. 1996. In vivo genotoxicity of dichloroacetic acid: evaluation with the mouse peripheral blood micronucleus assay and the single cell gel assay. Environ. Mol. Mutagen. 27:1-9. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Higuera, I., T. Taniguchi, S. Ganesan, M. S. Meyn, C. Timmers, J. Hejna, M. Grompe, and A. D. D'Andrea. 2001. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol. Cell 7:249-262. [DOI] [PubMed] [Google Scholar]
- 18.Grompe, M., and A. D'Andrea. 2001. Fanconi anemia and DNA repair. Hum. Mol. Genet. 10:2253-2259. [DOI] [PubMed] [Google Scholar]
- 19.Grossmann, K. F., A. M. Ward, M. E. Matkovic, A. E. Folias, and R. E. Moses. 2001. S. cerevisiae has three pathways for DNA interstrand crosslink repair. Mutat. Res. 487:73-83. [DOI] [PubMed] [Google Scholar]
- 20.Gruenert, D. C., and J. E. Cleaver. 1985. Repair of psoralen-induced cross-links and monoadducts in normal and repair-deficient human fibroblasts. Cancer Res. 45:5399-5404. [PubMed] [Google Scholar]
- 21.Howlett, N. G., T. Taniguchi, S. Olson, B. Cox, Q. Waisfisz, C. De Die-Smulders, N. Persky, M. Grompe, H. Joenje, G. Pals, H. Ikeda, E. A. Fox, and A. D. D'Andrea. 2002. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 297:606-609. [DOI] [PubMed] [Google Scholar]
- 22.Hoy, C. A., L. H. Thompson, C. L. Mooney, and E. P. Salazar. 1985. Defective DNA cross-link removal in Chinese hamster cell mutants hypersensitive to bifunctional alkylating agents. Cancer Res. 45:1737-1743. [PubMed] [Google Scholar]
- 23.Islas, A. L., J. M. Vos, and P. C. Hanawalt. 1991. Differential introduction and repair of psoralen photoadducts to DNA in specific human genes. Cancer Res. 51:2867-2873. [PubMed] [Google Scholar]
- 24.Kumaresan, K. R., and M. W. Lambert. 2000. Fanconi anemia, complementation group A, cells are defective in ability to produce incisions at sites of psoralen interstrand cross-links. Carcinogenesis 21:741-751. [DOI] [PubMed] [Google Scholar]
- 25.Kuraoka, I., W. R. Kobertz, R. R. Ariza, M. Biggerstaff, J. M. Essigmann, and R. D. Wood. 2000. Repair of an interstrand DNA cross-link initiated by ERCC1-XPF repair/recombination nuclease. J. Biol. Chem. 275:26632-26636. [DOI] [PubMed] [Google Scholar]
- 26.Larminat, F., M. Germanier, E. Papouli, and M. Defais. 2002. Deficiency in BRCA2 leads to increase in non-conservative homologous recombination. Oncogene 21:5188-5192. [DOI] [PubMed] [Google Scholar]
- 27.Lawley, P. D., and D. H. Phillips. 1996. DNA adducts from chemotherapeutic agents. Mutat. Res. 355:13-40. [DOI] [PubMed] [Google Scholar]
- 28.Limoli, C. L., E. Giedzinski, W. M. Bonner, and J. E. Cleaver. 2002. UV-induced replication arrest in the xeroderma pigmentosum variant leads to DNA double-strand breaks, gamma-H2AX formation, and Mre11 relocalization. Proc. Natl. Acad. Sci. USA 99:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magana-Schwencke, N., J. A. Henriques, R. Chanet, and E. Moustacchi. 1982. The fate of 8-methoxypsoralen photoinduced crosslinks in nuclear and mitochondrial yeast DNA: comparison of wild-type and repair-deficient strains. Proc. Natl. Acad. Sci. USA 79:1722-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McHugh, P. J., V. J. Spanswick, and J. A. Hartley. 2001. Repair of DNA interstrand crosslinks: molecular mechanisms and clinical relevance. Lancet Oncol. 2:483-490. [DOI] [PubMed] [Google Scholar]
- 31.Meetei, A. R., J. P. de Winter, A. L. Medhurst, M. Wallisch, Q. Waisfisz, H. J. van de Vrugt, A. B. Oostra, Z. Yan, C. Ling, C. E. Bishop, M. E. Hoatlin, H. Joenje, and W. Wang. 2003. A novel ubiquitin ligase is deficient in Fanconi anemia. Nat. Genet. 35:165-170. [DOI] [PubMed] [Google Scholar]
- 32.Merk, O., and G. Speit. 1999. Detection of crosslinks with the comet assay in relationship to genotoxicity and cytotoxicity. Environ. Mol. Mutagen. 33:167-172. [DOI] [PubMed] [Google Scholar]
- 33.Metzler, M. 1986. DNA adducts of medicinal drugs: some selected examples. J. Cancer Res. Clin. Oncol. 112:210-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moynahan, M. E., T. Y. Cui, and M. Jasin. 2001. Homology-directed dna repair, mitomycin-c resistance, and chromosome stability is restored with correction of a Brca1 mutation. Cancer Res. 61:4842-4850. [PubMed] [Google Scholar]
- 35.Moynahan, M. E., A. J. Pierce, and M. Jasin. 2001. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 7:263-272. [DOI] [PubMed] [Google Scholar]
- 36.Mu, D., T. Bessho, L. V. Nechev, D. J. Chen, T. M. Harris, J. E. Hearst, and A. Sancar. 2000. DNA interstrand cross-links induce futile repair synthesis in mammalian cell extracts. Mol. Cell. Biol. 20:2446-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olive, P. L. 2002. The comet assay. An overview of techniques. Methods Mol. Biol. 203:179-194. [DOI] [PubMed] [Google Scholar]
- 38.Paull, T. T., E. P. Rogakou, V. Yamazaki, C. U. Kirchgessner, M. Gellert, and W. M. Bonner. 2000. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10:886-895. [DOI] [PubMed] [Google Scholar]
- 39.Pichierri, P., D. Averbeck, and F. Rosselli. 2002. DNA cross-link-dependent RAD50/MRE11/NBS1 subnuclear assembly requires the Fanconi anemia C protein. Hum. Mol. Genet. 11:2531-2546. [DOI] [PubMed] [Google Scholar]
- 40.Redon, C., D. Pilch, E. Rogakou, O. Sedelnikova, K. Newrock, and W. Bonner. 2002. Histone H2A variants H2AX and H2AZ. Curr. Opin. Genet. Dev. 12:162-169. [DOI] [PubMed] [Google Scholar]
- 41.Rogakou, E. P., C. Boon, C. Redon, and W. M. Bonner. 1999. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 146:905-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogakou, E. P., W. Nieves-Neira, C. Boon, Y. Pommier, and W. M. Bonner. 2000. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J. Biol. Chem. 275:9390-9395. [DOI] [PubMed] [Google Scholar]
- 43.Rogakou, E. P., D. R. Pilch, A. H. Orr, V. S. Ivanova, and W. M. Bonner. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273:5858-5868. [DOI] [PubMed] [Google Scholar]
- 44.Rothfuss, A., P. Schutz, S. Bochum, T. Volm, E. Eberhardt, R. Kreienberg, W. Vogel, and G. Speit. 2000. Induced micronucleus frequencies in peripheral lymphocytes as a screening test for carriers of a BRCA1 mutation in breast cancer families. Cancer Res. 60:390-394. [PubMed] [Google Scholar]
- 45.Rothkamm, K., and M. Lobrich. 2003. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. USA 100:5057-5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki, M. S., and A. Tonomura. 1973. A high susceptibility of Fanconi's anemia to chromosome breakage by DNA cross-linking agents. Cancer Res. 33:1829-1836. [PubMed] [Google Scholar]
- 47.Scully, R., N. Puget, and K. Vlasakova. 2000. DNA polymerase stalling, sister chromatid recombination and the BRCA genes. Oncogene 19:6176-6183. [DOI] [PubMed] [Google Scholar]
- 48.Sedelnikova, O. A., E. P. Rogakou, I. G. Panyutin, and W. M. Bonner. 2002. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat. Res. 158:486-492. [DOI] [PubMed] [Google Scholar]
- 49.Singh, N. P., and R. E. Stephens. 1997. Microgel electrophoresis: sensitivity, mechanisms, and DNA electrostretching. Mutat. Res. 383:167-175. [DOI] [PubMed] [Google Scholar]
- 50.Speit, G. H., and A. Hartmann. 1999. The comet assay (single-cell gel test). A sensitive genotoxicity test for the detection of DNA damage and repair. Methods Mol. Biol. 113:203-212. [DOI] [PubMed] [Google Scholar]
- 51.Taniguchi, T., I. Garcia-Higuera, P. R. Andreassen, R. C. Gregory, M. Grompe, and A. D. D'Andrea. 2002. S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood 100:2414-2420. [DOI] [PubMed] [Google Scholar]
- 52.Thyagarajan, B., and C. Campbell. 1997. Elevated homologous recombination activity in fanconi anemia fibroblasts. J. Biol. Chem. 272:23328-23333. [DOI] [PubMed] [Google Scholar]
- 53.Tutt, A., D. Bertwistle, J. Valentine, A. Gabriel, S. Swift, G. Ross, C. Griffin, J. Thacker, and A. Ashworth. 2001. Mutation in Brca2 stimulates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequences. EMBO J. 20:4704-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vos, J. M., and P. C. Hanawalt. 1987. Processing of psoralen adducts in an active human gene: repair and replication of DNA containing monoadducts and interstrand cross-links. Cell 50:789-799. [DOI] [PubMed] [Google Scholar]
- 55.Wang, X., C. A. Peterson, H. Zheng, R. S. Nairn, R. J. Legerski, and L. Li. 2001. Involvement of nucleotide excision repair in a recombination-independent and error-prone pathway of DNA interstrand cross-link repair. Mol. Cell. Biol. 21:713-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward, I. M., and J. Chen. 2001. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276:47759-47762. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto, K., M. Ishiai, N. Matsushita, H. Arakawa, J. E. Lamerdin, J. M. Buerstedde, M. Tanimoto, M. Harada, L. H. Thompson, and M. Takata. 2003. Fanconi anemia FANCG protein in mitigating radiation- and enzyme-induced DNA double-strand breaks by homologous recombination in vertebrate cells. Mol. Cell. Biol. 23:5421-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu, V. P., M. Koehler, C. Steinlein, M. Schmid, L. A. Hanakahi, A. J. van Gool, S. C. West, and A. R. Venkitaraman. 2000. Gross chromosomal rearrangements and genetic exchange between nonhomologous chromosomes following BRCA2 inactivation. Genes Dev. 14:1400-1406. [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, N., X. Lu, X. Zhang, C. A. Peterson, and R. J. Legerski. 2002. hMutSβis required for the recognition and uncoupling of psoralen interstrand cross-links in vitro. Mol. Cell. Biol. 22:2388-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]