Abstract
Background
Obesity is associated with clinical depression among women. However, depressed women are often excluded from weight loss trials.
Purpose
This study examined treatment outcomes among women with comorbid obesity and depression.
Methods
Two hundred three (203) women were randomized to behavioral weight loss (n=102) or behavioral weight loss combined with cognitive-behavioral depression management (n=101).
Results
Average participant age was 52 years; mean baseline body mass index was 39 kg/m2. Mean Patient Health Questionnaire and Hopkins Symptom Checklist (SCL-20) scores indicated moderate to severe baseline depression. Weight loss and SCL-20 changes did not differ between groups at 6 or 12 months in intent-to-treat analyses (p=0.26 and 0.55 for weight, p=0.70 and 0.25 for depressive symptoms).
Conclusions
Depressed obese women lost weight and demonstrated improved mood in both treatment programs. Future weight loss trials are encouraged to enroll depressed women.
Keywords: Obesity, Depression, Comorbidity, Intervention, Women
Introduction
Obesity is associated with clinically significant depressive symptoms, particularly among women [1, 2]. Women are more likely to seek weight loss treatment on their own or be referred by their physicians for specialist weight loss treatment than men [3]. In addition, adults who seek weight loss treatment present with higher rates of depression than those seeking other medical treatment [4]. Adding to the burden of comorbidity between the two conditions, depression is associated with attrition from weight loss programs [5], poorer weight loss outcomes [6], and weight regain after successful losses [7]. Even though rates of clinically significant depression exceed 25% in obese women [2], data regarding treatment of co-occurring obesity and depression are limited. Women with significant depression are often excluded from trials of weight loss intervention because of untested assumptions that standard weight loss interventions would be ineffective or inappropriate [8, 9].
Experts in the fields of weight control and depression management have concluded independently that cognitive-behavioral treatment approaches that address behavior activation, problem solving, and reassessment of condition-specific beliefs are optimal ways to address each area of concern [10–14]. For both conditions, these treatments are effective when delivered in either individual or group formats [12, 14]. However, group cognitive-behavioral approaches that address both obesity and depression simultaneously have not been examined. To our knowledge, the only related study is a pending trial to treat depressive symptoms with a combination of individual and group visits prior to group weight loss treatment [15].
The purpose of the current study was to examine the effects on weight loss and depressive symptoms of standard behavior therapy for obesity with specialized treatment addressing both weight loss and depression simultaneously among women who were obese and had comorbid clinical depression. Women were randomly assigned either to behavioral weight loss treatment or to a combination of behavioral weight loss and cognitive-behavioral therapy for depression. To observe whether depression had a negative effect on motivation for study participation, participants were recruited for the study based solely on weight and depression status.
Methods
Study Recruitment
Recruitment took place from April 2003 to April 2005. Eligible individuals were enrolled in the Group Health Cooperative, a mixed-model prepaid health plan serving over 500,000 members in Washington and northern Idaho. The study was approved by the Group Health Cooperative Institutional Review Board.
Participants were recruited from a cohort of women in eight Group Health primary care clinics between the ages of 45 and 65 who had completed a population-based survey delivered over the telephone by trained study staff and designed to establish the prevalence of clinical depression and obesity [16]. Existing data from computerized administrative records and the Group Health Breast Cancer Screening Program were used to identify three strata for survey sampling based on self-reported height and weight, from which body mass index (BMI) status was calculated [BMI<30, BMI≥30, or BMI unknown (did not participate in screening program)]. Computerized administrative data were used to exclude those who were diagnosed with bipolar disorder or psychotic disorder in the last 2 years. Details of recruitment and procedures are described elsewhere [16].
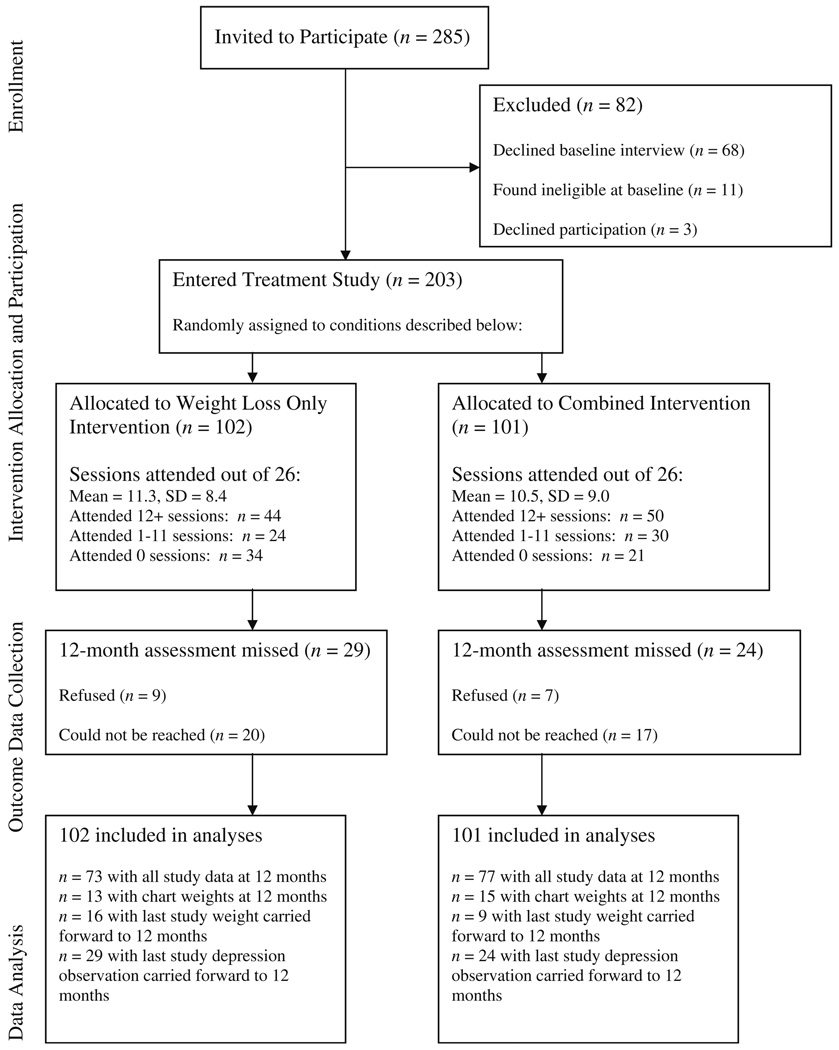
Women with BMI≥30 and who met depressive disorder criteria from the survey sample were invited to an in-person baseline interview to determine eligibility for the present study. Depression status was indicated by scores in the moderate range or higher (≥10) on the Patient Health Questionnaire-9 (PHQ-9) [17] administered as part of the epidemiological survey described above, which assesses depressive disorder via self-reported items based on criteria in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) [18]. Of the 285 depressed women (based on survey screening) invited to these baseline interviews, 68 declined the interview, 11 were ineligible for the treatment study, and three declined participation in the treatment study, for a final sample of 203 depressed and obese women randomized into this study (see Fig. 1). Randomization to the treatment conditions described here was conducted based on PHQ-9 depression status assessed during the epidemiological survey. All participants provided written informed consent prior to this baseline interview, which was conducted in person by trained survey interviewers. Women were paid $20 as compensation for completing the interview.
Fig. 1.
Study recruitment flow diagram
Measures
At baseline and 6 and 12 months, participants completed in-person interviews conducted by trained study interviewers who were blinded to participants’ treatment assignment, for collection of anthropometric and survey data. Each participant was encouraged to complete 6 and 12-month interviews regardless of her level of treatment participation. Participants were compensated $20 for completion of each interview. Baseline interviews took place between April 2003 and April 2005, 6-month interviews took place between October 2003 and November 2005, and 12-month interviews took place between May 2004 and April 2006. All measures are described as follows:
Demographic Characteristics
A brief questionnaire assessed age, race/ethnicity, marital status, and educational attainment at baseline.
Height and Weight
Height was measured by study staff at baseline using a wall-mounted stadiometer. Weight was assessed on a calibrated balance beam scale with women wearing light street clothes without shoes at baseline and 6 and 12 months. BMI (kilograms/m2) was calculated from measured height and weight.
Depression Diagnosis
The Structured Clinical Interview for DSM-IV or SCID [19] mood module was used to assess current depression diagnosis at the baseline interview, after randomization but before the start of treatment. The SCID is a semi-structured interview to diagnose major DSM-IV mental disorders and has been used in studies as the gold standard in determining the accuracy of clinical diagnoses [20]. A diagnosis of current major depression was conferred if a participant reported a major depressive episode during the previous 2 weeks (i.e., depressed mood for at least 2 weeks and/or loss of interest in typically enjoyable activities, plus at least four additional symptoms of depression such as feelings of worthlessness, fatigue, insomnia or hypersomnia, difficulty concentrating, psychomotor changes, appetite changes, unintended weight changes, or suicidal ideation, as specified in DSM-IV).
Depression Severity
Depressive symptom severity was assessed at baseline and 6 and 12 months using the Symptom Checklist-20 (SCL-20), a 20-item depression scale extracted from the SCL-90, scored on a 0 to 4 scale [21]. The SCL has been found to have high reliability and validity in multiple studies with medical patients and is sensitive to change in depressed primary care patients [21]. A score of 1.72 on the SCL-20 has been shown to have the highest positive predictive value for major depression [22]. Previous trials in primary care that have used the SCL depression scale score as an outcome measure support the following depression severity estimates: 0.75=remission, greater than 0.75 to 1.5=mild depression, greater than 1.5 to 2.0=moderate depression, and greater than 2.0=severe depression [23, 24].
Physical Activity
The Paffenbarger Activity Questionnaire or PAQ [25] was administered at baseline and 6 and 12 months to provide an estimate of calories expended per week in leisure time physical activities. It assessed activities performed in the previous week, including specific activities of stairs climbed and blocks walked and minutes of any other activities using a free recall format. Open-ended responses are scored by categorizing activities as light (5 kcal/min), medium (7.5 kcal/min), or high (10 kcal/min) intensity [26]. The PAQ has been shown to predict weight outcomes in previous weight loss intervention trials [27].
Dietary Intake
Dietary intake was assessed at baseline and 6 and 12 months using a food frequency questionnaire developed by the Nutrition Assessment Shared Resource of the Fred Hutchinson Cancer Research Center [28, 29]. Nutrient calculations were performed using the Nutrient Data System for Research software version 2005, developed by the Nutrition Coordinating Center at the University of Minnesota. Due to changes in the survey protocol during the epidemiological survey from which the current study participants were recruited [16], Nutrition Assessment Shared Resource dietary intake data are not available for 27 participants; missing data are distributed equally between treatment groups.
Antidepressant Use
Computerized pharmacy refill records were used to examine use of antidepressant medications during the 12-month intervention period.
Intervention Procedures
A computerized randomized selection process conducted by study programmers was employed to assign participants to groups; this process used a “biased coin” strategy (in which the randomization method applied a differential probability to the selection process depending on divergence of numbers of cases between groups) to ensure that assignments were equally distributed between the two groups [30, 31]. Assignments were computer generated after the baseline assessment; whereas participants and intervention staff were not blinded to group assignment, interviewers conducting evaluations for the study (as described in Measures above) were blinded to group assignment. Time between randomization and intervention start date was not normally distributed; the mean duration between randomization and group start date was 92 days for both intervention conditions (25th and 75th percentile values: 36 and 97 days for weight loss groups, 41 and 139 days for weight loss/depression groups). Women receiving treatment for weight loss or psychotherapy for depression were excluded from entering the study, but participants were not restricted from seeking depression or weight loss treatment outside the study protocol once enrolled. Women taking antidepressant medications were not excluded from the study.
Intervention groups met between June 2003 and April 2006. The two treatment groups were as follows:
Weight Loss Only Intervention
The behavioral weight loss intervention was adapted from programs developed at the University of Minnesota over the last 20 years. This program has been used in numerous randomized trials [32–35] and has consistently produced mean weight losses at least as good as other currently available non-medical treatments. Session content was centered on behavioral goal setting and self-monitoring of caloric intake, physical activity, and body weight. Each session contained a topically relevant quiz and motivational messages to enhance session content. Participants were given daily caloric intake goals of 1,200 or 1,500 kcal as appropriate to produce weight loss of 1 to 2 lb per week, based on initial body weight. Participants were asked to restrict fat intake to 20% of daily caloric intake. Physical activity goals were increased in biweekly increments of 500 kcal/week until the goal of 2,500 kcal/week was reached. Participants received structured meal plans and specific skills training in environmental stimulus control (e.g., not having high calorie snacks in the home). Some physical activity (e.g., group walks, light strength training) was directed during sessions with specific activity content; otherwise, participants were instructed to engage in activities of their choosing on their own or with partners outside of sessions, and motivational materials and relevant quizzes were distributed as part of session content. Participants were asked to keep food intake and physical activity diaries that were reviewed weekly by study interventionists. The program did not specifically address symptoms of depression. Treatment was delivered by trained weight loss counselors with prior experience in nutrition or exercise physiology.
Combined Weight Loss/Depression Intervention
The combined intervention integrated the behavioral weight loss program described above with essential elements of cognitive-behavioral treatment for depression, as drawn from the Coping with Depression manual [36]. Approximately 55% of didactic content in the combined program addressed treatment for depression, with the remaining 45% of the content addressing behavioral weight loss treatment; session content was interlaced, with order and content determined by experts on the research team who represented each discipline. In addition to completing behavioral goal setting and self-monitoring tasks for weight loss as described above, participants in the combined groups set additional behavioral and cognitive change goals to improve depressive symptoms and completed regular mood self-monitoring assignments as part of their weekly diaries. Combined groups were led by a licensed clinical psychologist who was cross-trained to deliver weight loss content by expert members of the research team; a second licensed clinical psychologist (also a member of the research team) was available as needed.
Intervention was delivered in 21 groups (13 weight loss only, eight combined) with 3–16 participants assigned to each group (3–11 for weight loss only; 4–16 for combined); variability of group size was driven by participant preferences for group times and locations. Groups were held during daytime and evening hours at three locations in the Seattle metropolitan area. Thirty-eight women (22 weight loss only, 16 combined) were not assigned to an intervention group because they were unable or unwilling to attend any of the 21 available groups (see Fig. 1 for study participant flow information). An additional 125 obese women who did not meet criteria for major depressive disorder were assigned to weight loss only groups; outcomes for depressed versus nondepressed women in weight loss only groups are described elsewhere [37].
The intervention, which took place in 26 sessions over 1 year, began with weekly sessions for 16 weeks, followed by four sessions every other week, then tapering to monthly maintenance sessions for 6 months; a summary of intervention group content is presented in the Appendix. Weight loss only groups lasted 90 min, and combined groups lasted 120 min. Each session included collection of self-monitoring logs and review of homework assignments, didactic instruction, small-group discussion, a quiz to reinforce session content, and specific skills training and practice. Participants received written summaries of material covered in each treatment session. To enhance treatment fidelity and to provide therapist feedback, all treatment delivery staff participated in weekly live telephone supervision sessions that included expert consultation with relevant members of the research team. During these weekly calls, therapists briefed the research team on the previous week’s sessions, discussed any challenges or questions that had been raised in sessions or other participant contacts, and were given the opportunity to consult directly with weight loss or depression experts on the study team. Experts also answered any questions about delivery of content for upcoming sessions to monitor ongoing comprehension of study materials and to recalibrate procedures as needed.
Data Analysis
All analyses were conducted using SAS Version 9.1. Initial comparisons of baseline measures between the two treatment groups were made using t tests for continuous variables and chi-square tests for categorical variables. No significant differences were observed between groups with regard to baseline demographic variables (age, race, marital status, education), body weight, or depressive symptoms (p=0.18–0.90). Primary analyses compared participants in terms of mean weight change and mean SCL-20 score change by group from baseline to 6- and 12-month assessments using general linear regression models. The study was powered to detect a mean 1.7 kg difference (SD± 5 kg) in weight and a mean 0.28-point difference (SD± 0.64 points) on the SCL-20 for depressed women in weight loss only versus the combined intervention, assuming 80% power and a group size of 90 participants in each condition at 12 months. Cohen’s d statistic [38] was calculated to determine effect sizes for weight and depression outcomes at 6 and 12 months. Secondary analyses compared the treatment groups in terms of participation in the initial weekly treatment sessions and attrition from treatment as well as changes in dietary intake or physical activity during treatment.
To reduce bias, missing weight data were replaced prior to analysis using information from two sources. The first source was the electronic medical record available for all Group Health members; the closest weight within 3 months of the scheduled interview date was extracted from the record. If electronic medical record data were unavailable, missing weights were substituted with the last study observation carried forward. To assess the accuracy of weights extracted from medical records as compared to measured study weights, medical record weights within 3 months of the baseline assessment were compared to measured weights at baseline; the resulting correlation was 0.98 [39]. Percentages of measured weight data, electronic medical record data, and last observation carried forward data at 6 and 12 months by treatment group are presented in Table 1. Across both treatment groups at 6 months, 79% of weight data were measured for the study, 13% were taken from electronic medical records, and 8% were taken from last observation carried forward; at 12 months, 74% of weight data were measured for the study, 14% were taken from electronic medical records, and 12% were taken from last observation carried forward. Rates of available data were greater for combined versus weight loss only participants at 6 months [86% combined versus 71% weight loss only, χ2(1)=6.45, p<0.05]; however, differences were absent at 12 months [76% combined versus 71% weight loss only, χ2(1)=0.57, p=0.44]. For depressive symptom data, missing SCL-20 scores at 6 and 12 months were replaced by last observation carried forward.
Table 1.
Sources of weight data at 6 and 12 months
| Weight loss only (n=102) | Combined (n=101) | |||
|---|---|---|---|---|
| 6 months n (%) |
12 months | 6 months | 12 months | |
| Measured weight | 73 (71) | 73 (71) | 87 (86) | 77 (76) |
| Chart weight | 20 (20) | 13 (13) | 6 (6) | 15 (15) |
| Last study observation carried forward | 9 (9) | 16 (16) | 8 (8) | 9 (9) |
Results
Participant Characteristics
Baseline participant characteristics are shown in Table 2. Women were 52 years of age on average, and approximately 50% were married. Over 75% of women were of white ethnicity, and nearly 90% had attained at least a high school education. Mean BMI was in the obese range and did not differ significantly by treatment group (39.5 kg/m2 for weight loss only, 38.6 kg/m2 for combined). Mean depression scores on the SCL-20, the primary means of assessing depressive symptoms at follow-up interviews, indicated that study participants were experiencing moderate depression at baseline; mean PHQ scores at the point of study randomization were comparable, with participants scoring in the low end of the moderate to severe range of depressive symptoms. Overall rates of antidepressant use were higher than past month prevalence rates of approximately 10% reported by comparable adults in NHANES survey data [40], with 77% of women having taken any antidepressant medication and 52% having used antidepressant medication for 180 days or more. Among the antidepressant medications used, participants were most often prescribed those associated with weight loss or no weight effect (e.g., 34% fluoxetine, 19% buproprion) rather than weight gain (e.g., 7% doxepin, 3% amitriptyline, 1% mirtazapine, 1% imipramine). Rates of antidepressant use did not differ significantly between treatment groups [75.4% weight loss only versus 78.2% combined, χ2(1)= 0.21, p=0.65] and were not considered as a confounder of weight or mood group effects over time in the study. No participants received additional formal weight loss treatment outside of the study protocol, and rates of psychotherapy outside of the study protocol were small and comparable across groups [9% weight loss only versus 7% combined, χ2(1)=0.25, p=0.62].
Table 2.
Baseline characteristics by random assignment to treatment group
| Weight loss only (n=102) | Combined (n=101) | |||
|---|---|---|---|---|
| Mean or percent | SD | Mean or percent | SD | |
| Age | 52.1 | 6.5 | 52.3 | 6.2 |
| Weight (kilograms) | 106.6 | 22.3 | 103.4 | 17.7 |
| Body mass index (kilograms/m2) | 39.5 | 7.7 | 38.6 | 6.8 |
| Any antidepressant medication | 75.4% | 78.2% | ||
| PHQ depression score from epidemiological survey (prior to treatment randomization) | 15.8 | 3.3 | 15.7 | 3.5 |
| SCL-20 depression score at intervention study baseline (prior to start of treatment) | 1.82 | 0.59 | 1.87 | 0.62 |
| Percent married | 45% | 54% | ||
| Percent white | 78% | 79% | ||
| Percent with high school education or greater | 86% | 89% | ||
| Dietary intake (kilocalories/day)a | 2,216 | 969 | 2,270 | 879 |
| Physical activity (kilocalories/week) | 506 | 772 | 549 | 789 |
There were no statistically significant differences in baseline characteristics between treatment groups
PHQ Patient Health Questionnaire, SCL-20 Symptom Checklist-20
Due to missing data, n=88 in weight loss only, 88 in combined
Treatment Session Participation
Session attendance rates by treatment group are presented in Fig. 1. Out of 26 possible sessions, approximately one half of participants attended at least 12 sessions (43% weight loss only, 50% combined), and an additional one quarter of participants (24% weight loss only, 30% combined) attended at least one session. The remaining participants (33% weight loss only, 20% combined) were assigned to treatment groups but did not attend any sessions. Attendance rates did not differ significantly by treatment group [χ2(2)=4.12, p=0.13] nor were there any differences in mean number of sessions attended by group [mean (standard deviation)=11.3 (8.4) versus 10.5 (9.0) sessions attended in weight loss only versus combined, t(201)=0.63, p=0.53]. No study-related adverse events were reported by participants in either treatment group.
Changes in Weight and Depression Outcomes by Treatment Group
Results for changes in weight and depression outcomes by treatment group are presented in Table 3. There was no statistically significant interaction between treatment group and session attendance in weight loss or depression models (data not shown). During the study, participants lost weight on average over time, with no statistically significant differences by treatment group at 6 or 12 months (mean changes at 6 months, −2.8 kg weight loss only versus −1.8 kg combined, p=0.26; 12 months, −3.1 kg weight loss only versus −2.3 kg combined, p= 0.55). Weight change results for those who were interviewed at 12-month follow-up (n=73 weight loss only, n= 77 combined; data not shown) did not differ from weight change outcomes for which missing data were imputed using a combination of medical chart weights and last observation carried forward, as described in the “Data Analysis” section above.
Table 3.
Weight and depressive symptom changes at 6 and 12 months by treatment group
| Weight loss only (n=102) | Combined (n=101) | p | d | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Mean | SD | 95% CI | |||
| Mean weight change from baseline (kilograms) | ||||||||
| 6 months | −2.8 | 6.6 | −4.1, −1.6 | −1.8 | 6.6 | −3.1, −0.5 | 0.26 | 0.16 |
| 12 months | −3.1 | 8.9 | −4.8, −1.3 | −2.3 | 8.9 | −4.1, −0.6 | 0.55 | 0.09 |
| Mean depressive symptom change from baseline (SCL-20) | ||||||||
| 6 months | −0.54 | 0.71 | −0.68, −0.40 | −0.50 | 0.70 | −0.64, −0.36 | 0.70 | 0.06 |
| 12 months | −0.53 | 0.81 | −0.68, −0.37 | −0.65 | 0.80 | −0.81, −0.50 | 0.25 | 0.16 |
SCL-20 Symptom Checklist-20
Depressed mood, as measured by the SCL-20, decreased over time as well, with scores falling from moderate levels (1.82 weight loss only, 1.87 combined at baseline) to mild levels by 6 months that were maintained at 12 months (6 months, 1.28 weight loss only, 1.36 combined; 12 months, 1.30 weight loss only, 1.22 combined). At baseline, 26% of participants in each treatment group rated their depressive symptoms in the nonclinical to mild range on the SCL-20 [weight loss only versus combined χ2(1)= 0.00, p=0.97]; by 12 months, 61% of those in weight loss only and 67% of those in combined groups rated their depressive symptoms as nonclinical to mild [weight loss only versus combined χ2(1)=0.94, p=0.33]. The change in proportion of those who reported nonclinical to mild symptoms at baseline versus 12 months was statistically significant in each group [weight loss only χ2(1)=35.28, combined χ2(1)=24.92, p values<0.0001]. There were no statistically significant differences in depressive mood changes by treatment group at 6 or 12 months in general linear models (mean changes at 6 months, −0.54 weight loss only versus −0.50 combined, p=0.70; 12 months, −0.53 weight loss only versus −0.65 combined, p=0.25). Depressive symptom change results for those who were interviewed at 12-month follow-up (n=73 weight loss only, n=77 combined; data not shown) did not differ from outcomes for which missing data were imputed by last observation carried forward as described in the “Data Analysis” section above.
Effect sizes, calculated as Cohen’s d statistic, were derived from the statistical models of weight changes and depressive symptom changes at 6 and 12 months. Effect sizes were quite small for both weight changes (d=0.16 at 6 months, 0.09 at 12 months) and depressive symptom changes (d=0.06 at 6 months, 0.16 at 12 months) at both assessment points.
Effects of Attendance on Weight Loss and Depression Outcomes
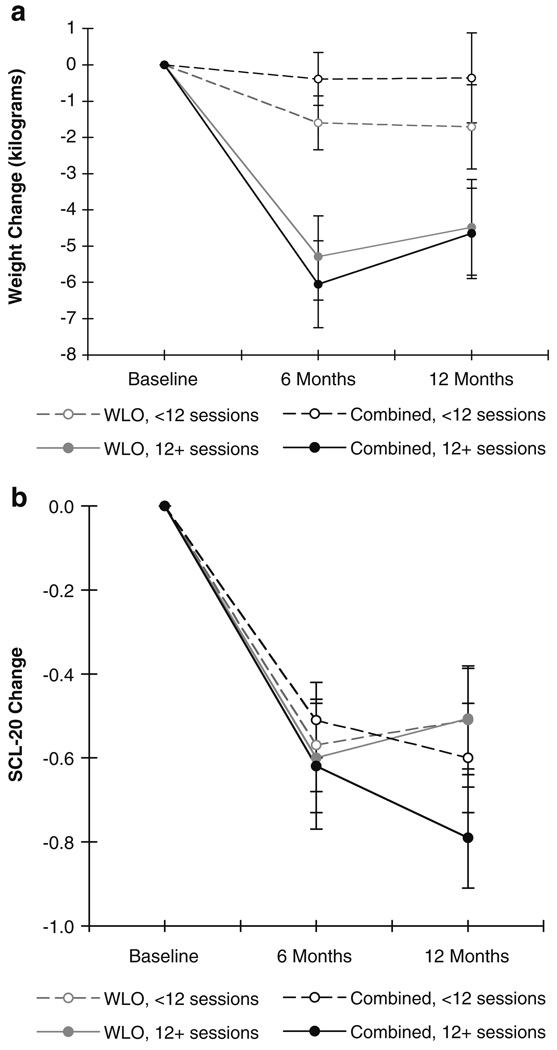
As described above and in Fig. 1, 43% of weight loss only participants and 50% of combined participants attended 12 or more sessions (i.e., were exposed to at least 75% of core content sessions). Therefore, weight change and depressive symptom change results were also examined by attendance status (i.e., attending 12 or more sessions versus fewer than 12 sessions) to determine the impact of attendance on outcomes. Results are presented in Fig. 2a, b.
Fig. 2.
a Means and standard errors of weight change (in kilograms) over time by treatment session attendance. b Means and standard errors of depressive symptom change over time by treatment session attendance
Attendance had a significant effect on weight change outcomes for both treatment groups (Fig. 2a), with those attending 12 or more sessions faring better in terms of weight loss than those attending fewer than 12 sessions (p<0.01, Cohen’s d=0.62 for weight loss only and p<0.001, Cohen’s d=0.79 for combined at 6 months; p=0.17, Cohen’s d=0.29 for weight loss only and p<0.05, Cohen’s d=0.50 for combined at 12 months). Regardless of treatment group, the weight change trajectory during the study for those who attended 12 or more sessions was statistically significant [time trend F(2, 91)=11.18, p<0.0001], whereas the weight change trajectory for those attending fewer than 12 sessions was marginally significant [time trend F(2,106)=2.40, p=0.095].
As presented in Fig. 2b, attendance did not have a significant effect on depressive symptom outcomes, as assessed by the SCL-20, for either treatment group (p=0.72 for weight loss only and p=0.47 for combined at 6 months, p=0.98 for weight loss only and p=0.37 for combined at 12 months, all Cohen’s d<0.21). That is, regardless of attendance status, all participants showed comparable and statistically significant changes in depressive symptoms over time [time trend F(2, 82)=36.64 for 12+ sessions, time trend F(2, 57)=20.20 for <12 sessions, p values<0.0001].
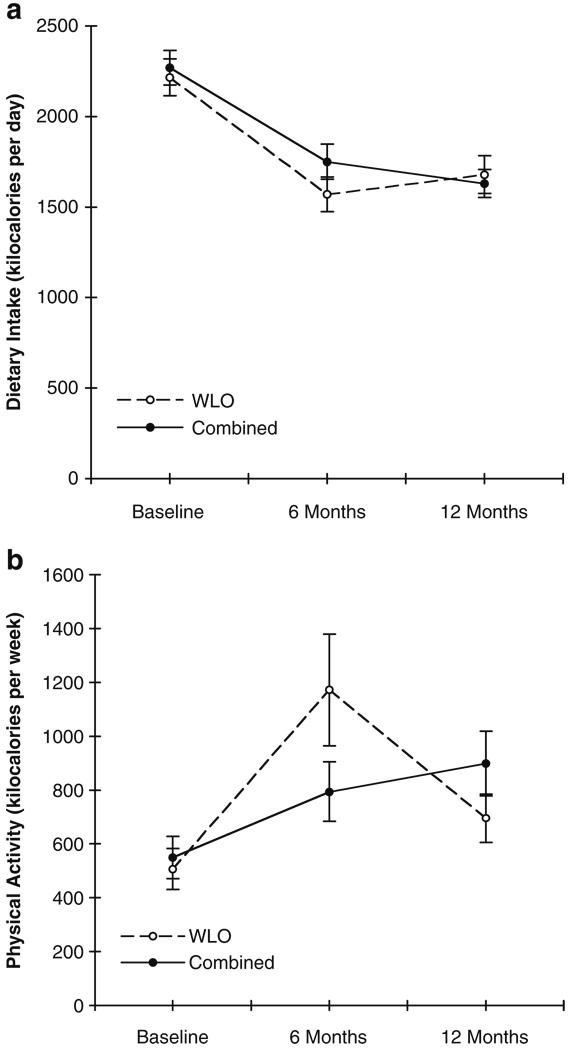
Changes in Dietary Intake and Physical Activity over Time
Patterns of dietary and physical activity changes in repeated measures analyses (depicted in Fig. 3a, b) were statistically comparable across treatment groups between baseline and 12 months. Women decreased their dietary intake and increased their physical activity significantly over time in both groups [dietary intake time trend F(2, 103)=29.77, physical activity time trend F(2, 128)=10.88, p values<0.0001]. With the exception of lower dietary intake for weight loss only versus combined treatment participants at 6 months among those who attended at least 12 treatment sessions (p=0.03, data not shown), attendance did not affect dietary or physical activity patterns during the weight loss trial.
Fig. 3.
a Means and standard errors of dietary intake changes by treatment group. b Means and standard errors of physical activity changes by treatment group
Discussion
This study examined the effects of group behavioral weight loss treatment versus a program of weight loss treatment combined with cognitive-behavioral therapy for depression for obese women with major depressive disorder. Results for both outcomes were comparable between the two intervention conditions. Study findings are indicative of modest reductions in weight and depressive symptoms for depressed women in either treatment. Although the changes were not of expected magnitude, these findings are encouraging in light of a prior population study that suggested poor health and treatment responses among those with significant obesity-depression comorbidity: the authors reported increased risk of suicidal ideation among those with comorbid obesity and major depression and suggested that this finding might have implications for obesity treatment, as those with depression tend to respond poorly to treatment and with reduced compliance [1]. Here, we found that obese, depressed women were able to lose weight and improve mood, whether or not they received specialized depression treatment as part of weight loss treatment. Weight losses among the depressed women in the present study were equal to or greater than those observed on average at 12 months (−2.28 kg) in a sample of nondepressed managed care organization members of similar age and BMI (mean age=51, mean BMI=34 kg/m2) who were randomized to either a telephone- or mail-based weight loss curriculum developed by members of the current study team [41].
Women in both treatment groups responded comparably in terms of study engagement (i.e., attendance at groups and follow-up interview visits over 12 months) and in terms of experiencing reductions in depressive symptoms over the course of the study, as well as comparable changes in weight over time. We do not exclude the possibility that depressive symptoms remitted spontaneously over time either during the waiting period before groups started or during the course of weight loss only treatment [42], but we are optimistic that this study, as with other promising trials such as the ongoing study of whether a pre-weight loss course of behavioral therapy for depression will improve weight loss outcomes in depressed obese women [15], will advance this field so that women with these comorbid conditions are better able to receive appropriate care.
Despite concerted efforts by study team experts to create a unique depression and weight loss management intervention (versus weight loss only) by augmenting basic nutrition and physical activity advice with additional cognitive-behavioral depression management strategies, commonalities between the two intervention programs may have led to comparable treatment outcomes for the women enrolled in this study. For example, though foci and behavioral targets (strictly weight loss versus a combination of weight loss and depression management) differed between intervention conditions, both programs involved behavioral activation, problem solving skills, cognitive restructuring, and social support. The extensive overlap between programs may have contributed to our inability to detect differences between group outcomes at 12 months as estimated by a priori power calculations for this study; however, the confidence intervals around the results presented in Table 3 for both outcomes include the expected differences from our initial power calculations. As we did not make any predictions regarding which program might provide greater benefit to participants, future studies may consider examining preferences for treatment focus (e.g., weight loss alone versus combined treatment or treating depression alone), as well as examination of results with other demographic groups (e.g., men or younger adults).
Study limitations include restrictions on demographic variability of the sample, particularly with respect to age, race/ethnicity, and gender. Although we were able to account for 13–14% of missing weight data at 6 and 12 months by using proximally measured chart weights from medical records, we relied on last observation carried forward as an imputation method for 8% of cases at 6 months and 12% of cases at 12 months; the last observation carried forward method of imputation is limited by the assumption that weight remained unchanged during the time period of the study and may represent an overly generous interpretation of the data.
As depressive symptom data (assessed via SCL-20) were collected rather than using repeated SCID administrations at study follow-up points, we are limited to commenting on symptom changes over the course of the study rather than on changes in DSM-IV diagnostic status. The mean lag time of approximately 90 days between baseline measurement of depression status (by SCL-20 and SCID) and the start of intervention groups, without ascertainment of depression status at the outset of treatment, does not allow us to account for potential remission of symptoms during the period of time prior to treatment start date. Due to higher rates of female recruitment in weight loss programs in general, much more is known about women and weight loss than about men and weight loss, and we are not able to generalize the present findings to men. Another limitation is that approximately three quarters of the participants were receiving antidepressant medication, which may have hindered our ability to detect differential changes in depression levels. In addition, because combined sessions were 30 min longer than weight loss only sessions (to accommodate added depression content and to extend homework review of additional self-monitoring tasks in the combined treatment), participants in the combined intervention were potentially exposed to an average of 5.25 more treatment hours than those in weight loss only (based on mean session attendance of 10.5 sessions for the combined group); the added contact may have affected outcomes for combined participants relative to those in weight loss only.
As in the companion study of the effects of major depression on behavioral weight loss treatment among obese women [37], one of the most significant limitations of this study was that rates of treatment participation were fairly low (one quarter of women assigned to treatment groups failed to attend a single session, and only half attended 12 or more of 26 sessions). The study design, in which women were screened for treatment eligibility following participation in an epidemiological survey, resulted in lag time between group assignment and group start dates, to allow for treatment groups of optimal size to be constructed at several study clinic locations. This procedure may have led to attrition prior to initial group sessions, either due to wait times or to failure to place all participants in groups at optimally convenient clinic locations.
Given the association between attendance and weight loss observed here, either program might have yielded more significant weight loss results had participant attendance been more robust. However, it is equally likely that greater weight loss success promoted better attendance over the course of the study; the correlational nature of our data limits our ability to draw causal inferences with regard to our findings. It is also possible that low attendance rates across both conditions may have masked differences between the two programs, given that relatively few participants attended the number of sessions considered to be an optimal dose of treatment. However, it is encouraging that attendance did not differ by treatment condition, suggesting that program content (addressing depressive symptoms and weight loss versus weight loss only) did not contribute to differential attendance rates among these depressed women.
Results for depression symptom change outcomes were not affected by attendance, which is consistent with observations of symptom remission during cognitive-behavioral therapy for depression in a dosage range of 8–12 sessions [43] nor was depression (assessed by PHQ-9 administered at pre-treatment study screening for randomization purposes and by SCL-20 administered at treatment study baseline and 6 and 12-month interviews to assess symptom severity during treatment) correlated significantly with attendance (r=−0.00 to −0.11, p=0.15 to 0.99). Depression, as assessed by the SCID prior to the start of treatment, was modestly correlated with total number of sessions attended (r=−0.14, p<0.05); however, this result appears to have been driven largely by lower attendance at the final maintenance session, rather than at active treatment sessions, as the correlations between baseline SCID diagnoses and attendance rates at 6 months (at which time 20 of 26 sessions had been held) or 12 months (at which time 25 of 26 sessions had been held) were nonsignificant (r values=−0.08 and −0.09, p values=0.29 and 0.23, respectively).
The current study has several important strengths, including examination of an understudied but prevalent clinical population, a relatively large sample, rigorous assessment of depression symptoms prior to study entry, evaluation of a novel intervention, and delivery of an intensive behavioral program to all participants. As all participants were invited to the intervention study after a larger-scale screening trial [16], the recruitment success of the current project has positive implications for public health agency or other outreach strategies to address obesity and depression in the community.
Overall, the present results suggest that obese women with depressive symptoms are able to adjust their dietary intake and physical activity frequency to achieve weight loss, regardless of whether depression is targeted specifically during intervention. Most importantly, comparable adherence to treatment recommendations and significant improvements in depressive mood may be achieved in programs that address weight loss only, as well as those that address both depressed mood and weight control. Future weight loss trials are encouraged to consider the option of enrolling women regardless of depression status to provide a more valid evaluation of program effectiveness.
Acknowledgments
This study was funded by National Institutes of Health Grant R01MH068127 (G. E. Simon, PI); ClinicalTrials.gov identifier: NCT00169273, “Epidemiology and Care of Comorbid Obesity and Depression.”
Appendix
Appendix.
Session topics: weight loss only and combined weight loss/depression group visits
| Session | Weight loss only (90-min sessions) | Combined weight loss/depression (120-min sessions) |
|---|---|---|
| 1 | Orientation: group norms, introduction to self-monitoring, behavior change processes | Orientation: group norms, introduction to self-monitoring, behavior change processes, relation between depression and weight |
| 2 | Energy balance and healthy food choices | Energy balance and healthy food choices |
| 3 | Diet quality: Fat and cholesterol | Pleasant activities and depression I |
| 4 | High fiber, low fat eating | Pleasant activities and depression II |
| 5 | Increasing physical activity | Increasing physical activity |
| 6 | Lifestyle exercise | Lifestyle exercise, barriers to exercise |
| 7 | Barriers to exercise | Relaxation training |
| 8 | Eating patterns | Problem solving I |
| 9 | Eating in social situations | Problem solving II |
| 10 | Eating in restaurants | Eating patterns, eating in social situations, eating in restaurants |
| 11 | Re-evaluating diet and exercise goals I | Re-evaluating diet and exercise goals I |
| 12 | Re-evaluating diet and exercise goals II | Re-evaluating diet and exercise goals II |
| 13 | Stress and eating | Cognitive goals: monitoring thinking |
| 14 | Fad diets, weight loss medications, surgery for weight loss | Cognitive techniques: what’s the evidence? |
| 15 | Cues for eating and exercise | Cognitive techniques: thought balancing |
| 16 | Advanced diet change | Advanced diet change |
| 17 | Advanced exercise change | Advanced exercise change |
| 18 | Assertion and eating | Social skills and assertiveness I |
| 19 | High risk situations | Social skills and assertiveness II |
| 20 | Managing slips and lapses | Managing slips and lapses |
| 21 | Summing up | Summing up |
| 22 | Long-term self-care plan | Long-term self-care plan |
| 23 | Monthly check-in: review and reinforcement (diet and exercise; goals and motivation; plans for next month), ad hoc session content determined by group members | Monthly check-in: review and reinforcement (diet, exercise,pleasant activities, and thought balancing; goals and motivation; plans for next month), ad hoc session content determined by group members |
| 24–26 | Same as session 23 | Same as session 23 |
For both interventions, sessions 1–16 were held weekly, sessions 17–22 were held biweekly, and sessions 23–26 were held monthly. Topics that are unique to the combined weight loss/depression program are highlighted in bold text
Footnotes
Conflict of Interest Statement The authors have no conflicts of interest to disclose.
Contributor Information
Jennifer A. Linde, Email: linde074@umn.edu, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Gregory E. Simon, Group Health Research Institute, Group Health Cooperative, Seattle, WA, USA
Evette J. Ludman, Group Health Research Institute, Group Health Cooperative, Seattle, WA, USA
Laura E. Ichikawa, Group Health Research Institute, Group Health Cooperative, Seattle, WA, USA
Belinda H. Operskalski, Group Health Research Institute, Group Health Cooperative, Seattle, WA, USA
David Arterburn, Group Health Research Institute, Group Health Cooperative, Seattle, WA, USA
Paul Rohde, Oregon Research Institute, Eugene, OR, USA
Emily A. Finch, Indiana University School of Medicine, Indianapolis, IN, USA
Robert W. Jeffery, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN, USA
References
- 1.Carpenter KM, Hasin DS, Allison DB, Faith MS. Relationships between obesity and DSM-IV major depressive disorder, suicidal ideation, and suicide attempts: Results from a general population survey. Am J Public Health. 2000;90:251–257. doi: 10.2105/ajph.90.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiat. 2006;63:824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thande NK, Hurstak EE, Sciacca RE, Giardina EGV. Management of obesity: A challenge for medical training and practice. Obes. 2008;17:107–113. doi: 10.1038/oby.2008.478. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein LT, Goldsmith SJ, Anger K, Leon AC. Psychiatric symptoms in clients presenting for commercial weight reduction treatment. Int J Eat Disord. 1996;20:191–197. doi: 10.1002/(SICI)1098-108X(199609)20:2<191::AID-EAT10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Clark MM, Niaura R, King TK, Pera V. Depression, smoking, activity level, and health status: Pretreatment predictors of attrition in obesity treatment. Addict Behav. 1996;21:509–313. doi: 10.1016/0306-4603(95)00081-x. [DOI] [PubMed] [Google Scholar]
- 6.Linde JA, Jeffery RW, Levy RL, et al. Binge eating disorder, weight control self-efficacy, and depression in overweight men and women. Int J Obes Relat Metab Disord. 2004;28:418–425. doi: 10.1038/sj.ijo.0802570. [DOI] [PubMed] [Google Scholar]
- 7.McGuire MT, Wing RR, Klem ML, Lang W, Hill JO. What predicts weight regain in a group of successful weight losers? J Consult Clin Psychol. 1999;67:177–185. doi: 10.1037//0022-006x.67.2.177. [DOI] [PubMed] [Google Scholar]
- 8.Anderson RE, Wadden TA, Bartlett SJ, Zemel B, Verde TJ, Franckowiak SC. Effects of lifestyle activity vs. structured aerobic exercise in obese women: A randomized trial. JAMA. 1999;281:335–340. doi: 10.1001/jama.281.4.335. [DOI] [PubMed] [Google Scholar]
- 9.Wirth A, Krause J. Long-term weight loss with sibutramine: A randomized controlled trial. JAMA. 2001;286:1331–1339. doi: 10.1001/jama.286.11.1331. [DOI] [PubMed] [Google Scholar]
- 10.Barlow DH, Allen LB, Choate ML. Toward a unified treatment for emotional disorders. Behav Therapy. 2004;35:205–230. doi: 10.1016/j.beth.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Cooper Z, Fairburn CG. A new cognitive behavioural approach to the treatment of obesity. Beh Res Therapy. 2001;39:499–511. doi: 10.1016/s0005-7967(00)00065-6. [DOI] [PubMed] [Google Scholar]
- 12.Craigle MA, Nathan P. A nonrandomized effectiveness comparison of broad-spectrum group CBT to individual CBT for depressed outpatients in a community mental health setting. Behav Therapy. 2009;40:302–314. doi: 10.1016/j.beth.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Wing RR. Behavioral approaches to the treatment of obesity. In: Bray GA, Bouchard C, James WPT, editors. Handbook of Obesity. New York: Marcel Dekker; 1998. pp. 855–878. [Google Scholar]
- 14.Wing RR, Jeffery RW. Benefits of recruiting participants with friends and increasing social support for weight loss and maintenance. J Consult Clin Psychol. 1999;67:132–138. doi: 10.1037//0022-006x.67.1.132. [DOI] [PubMed] [Google Scholar]
- 15.Schneider KL, Bodenlos JS, Ma Y, et al. Design and methods for a randomized clinical trial treating comorbid obesity and major depressive disorder. BMC Psychiatr. 2008;8:77. doi: 10.1186/1471-244X-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon GE, Ludman EJ, Linde JA, et al. Association between obesity and depression in middle-aged women. Gen Hosp Psychiat. 2008;30:32–39. doi: 10.1016/j.genhosppsych.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spitzer R, Kroenke K, Williams J. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 19.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version. Washington: American Psychiatric Press; 1997. [Google Scholar]
- 20.Shear MK, Greeno C, Kang J, et al. Diagnosis of nonpsychotic patients in community clinics. Am J Psychiatr. 2000;157:581–587. doi: 10.1176/appi.ajp.157.4.581. [DOI] [PubMed] [Google Scholar]
- 21.Derogatis L, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist: A measure of primary symptom dimensions. In: Pichot P, editor. Psychological Measurements in Psychopharmacology: Problems in Psychopharmacology. Basel, Switzerland: Kargerman; 1974. pp. 79–110. [Google Scholar]
- 22.Mulrow CD, Williams JW, Jr, Gerety MB, Ramirez G, Montiel OM, Kerber C. Case-finding instruments for depression in primary care settings. Ann Intern Med. 1995;122:913–921. doi: 10.7326/0003-4819-122-12-199506150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Katon W, Robinson P, Von Korff M, et al. A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatr. 1996;53:924–932. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- 24.Simon GE, Von Korff M, Rutter C, Wagner E. A randomized trial of monitoring, feedback, and management of care by telephone to improve depression treatment in primary care. BMJ. 2000;320:550–554. doi: 10.1136/bmj.320.7234.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paffenbarger R, Wing A, Hyde R. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of Physical Activities: An update of activity codes and MET intensities. Med Sci Sport Exer. 2000;32 Suppl:S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 27.Harris JK, French SA, Jeffery RW, McGovern PG, Wing RR. Dietary and physical activity correlates of long-term weight loss. Obes Res. 1994;2:307–313. doi: 10.1002/j.1550-8528.1994.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 28.Schakel SF. Procedures for estimating nutrient values for food composition databases. J Food Comp Anal. 1997;10:102–114. [Google Scholar]
- 29.Schakel SF. Maintaining a nutrient database in a changing marketplace: Keeping pace with changing food products—A research perspective. J Food Comp Anal. 2001;14:315–322. [Google Scholar]
- 30.Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58:403–417. [Google Scholar]
- 31.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 32.Jeffery RW, Wing RR, Thorson C, et al. Strengthening behavioral interventions for weight loss: A randomized trial of food provision and monetary incentives. J Consult Clin Psychol. 1993;61:1038–1045. doi: 10.1037//0022-006x.61.6.1038. [DOI] [PubMed] [Google Scholar]
- 33.Jeffery RW, Wing RR, Thorson C, Burton LR. Use of personal trainers and financial incentives to increase exercise in a behavioral weight-loss program. J Consult Clin Psychol. 1998;66:777–783. doi: 10.1037//0022-006x.66.5.777. [DOI] [PubMed] [Google Scholar]
- 34.Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: Does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78:684–689. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- 35.Wing RR, Jeffery RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs. structured meal plans in the behavioral treatment of obesity. Int J Obes. 1996;20:56–62. [PubMed] [Google Scholar]
- 36.Brown R, Lewinsohn P. A psychoeducational approach to the treatment of depression: Comparison of group, individual, and minimal contact procedures. J Consult Clin Psychol. 1984;52:774–783. doi: 10.1037//0022-006x.52.5.774. [DOI] [PubMed] [Google Scholar]
- 37.Ludman EJ, Simon GE, Ichikawa L, et al. Does depression reduce the effectiveness of behavioral weight loss treatment? Behav Med. 2009;35:126–134. doi: 10.1080/08964280903334527. [DOI] [PubMed] [Google Scholar]
- 38.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 39.Arterburn D, Ichikawa L, Ludman EJ, et al. Validity of clinical body weight measures as substitutes for missing data in a randomized trial. Obes Res Clin Pract. 2008;2:277–281. doi: 10.1016/j.orcp.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paulose-Ram R, Safran MA, Jonas BS, Gu Q, Orwig D. Trends in psychotropic medication use among U.S. adults. Pharmacoepidemiol Drug Saf. 2007;16:560–570. doi: 10.1002/pds.1367. [DOI] [PubMed] [Google Scholar]
- 41.Jeffery RW, Sherwood NE, Brelje K, et al. Mail and phone interventions for weight loss in a managed-care setting: Weigh-to-be one-year outcomes. Int J Obes. 2003;27:1584–1592. doi: 10.1038/sj.ijo.0802473. [DOI] [PubMed] [Google Scholar]
- 42.Andrews G. Placebo response in depression: Bane of research, boon to therapy. Br J Psychiatr. 2001;178:192–194. doi: 10.1192/bjp.178.3.192. [DOI] [PubMed] [Google Scholar]
- 43.Butler AC, Beck AT. Cognitive therapy for depression. Clin Psychol. 1995;48:3–5. [Google Scholar]