Abstract
Mitochondrial functions are dynamically regulated in the heart. In particular, protein phosphorylation has been shown to be a key mechanism modulating mitochondrial function in diverse cardiovascular phenotypes. However, site-specific phosphorylation information remains scarce for this organ. Accordingly, we performed a comprehensive characterization of murine cardiac mitochondrial phosphoproteome in the context of mitochondrial functional pathways. A platform using the complementary fragmentation technologies of collision-induced dissociation (CID) and electron transfer dissociation (ETD) demonstrated successful identification of a total of 236 phosphorylation sites in the murine heart; 210 of these sites were novel. These 236 sites were mapped to 181 phosphoproteins and 203 phosphopeptides. Among those identified, 45 phosphorylation sites were captured only by CID, whereas 185 phosphorylation sites, including a novel modification on ubiquinol-cytochrome c reductase protein 1 (Ser-212), were identified only by ETD, underscoring the advantage of a combined CID and ETD approach. The biological significance of the cardiac mitochondrial phosphoproteome was evaluated. Our investigations illustrated key regulatory sites in murine cardiac mitochondrial pathways as targets of phosphorylation regulation, including components of the electron transport chain (ETC) complexes and enzymes involved in metabolic pathways (e.g. tricarboxylic acid cycle). Furthermore, calcium overload injured cardiac mitochondrial ETC function, whereas enhanced phosphorylation of ETC via application of phosphatase inhibitors restored calcium-attenuated ETC complex I and complex III activities, demonstrating positive regulation of ETC function by phosphorylation. Moreover, in silico analyses of the identified phosphopeptide motifs illuminated the molecular nature of participating kinases, which included several known mitochondrial kinases (e.g. pyruvate dehydrogenase kinase) as well as kinases whose mitochondrial location was not previously appreciated (e.g. Src). In conclusion, the phosphorylation events defined herein advance our understanding of cardiac mitochondrial biology, facilitating the integration of the still fragmentary knowledge about mitochondrial signaling networks, metabolic pathways, and intrinsic mechanisms of functional regulation in the heart.
Mitochondria are the source of energy to sustain life. In addition to their evolutionary origin as an energy-producing organelle, their functionality has integrated into every aspect of life, including the cell cycle, ROS1 production, apoptosis, and ion balance (1, 2). Our understanding of mitochondrial biology is still growing. Several systems biology approaches have been dedicated to exploring the molecular infrastructure and dynamics of the functional versatility associated with this organelle (3–5).
To meet tissue-specific functional demands, mitochondria acquire heterogeneous properties in individual organs, a first statement of their plasticity in function and proteome composition (1, 6). The heterogeneity is evident even in an individual cardiomyocyte (7). A catalogue of the cardiac mitochondrial proteome is emerging via a joint effort (3–5). The dynamics of the mitochondrial proteome manifest at multiple levels, including post-translational modifications, such as phosphorylation. Our investigative goal is to decode this organellar proteome and its post-translational modification in a biological and functional context. In cardiomyocytes, mitochondria are also constantly exposed to fluctuation in energy demands and in ionic conditions. The capacity of mitochondria to cope with such a dynamic environment is essential for the functional role of mitochondria in normal and disease phenotypes (8–10). Unique protein features enabling the mitochondrial proteome to adapt to these biological changes can be interrogated by proteomics tools (10–12). Protein phosphorylation as a rapid and reversible chemical event is an integral component of these protein features (12–14).
It has been estimated that one-third of cellular proteins exist in a phosphorylated state at least one time in their lifetime (15). However, only a handful of phosphorylation events have been identified to tune mitochondrial functionality (13, 14, 16) despite the fact that the first demonstration of phosphorylation was reported on a mitochondrial protein more than 5 decades ago (17). Kinases and phosphatases comprise nearly 3% of the human genome (18, 19). In mitochondria, ∼30 kinases and phosphatases have been identified thus far within the expected organellar proteome of a few thousand (3–5, 16). The number of identified mitochondrial phosphoproteins is far below one-third of its proteome size (20). Thus, it appears that the current pool of reported phosphoproteins represents only a small fraction of the anticipated mitochondrial phosphoproteome. The seminal studies from several groups (12–14, 16) demonstrated the prevalence as well as the dynamic nature of phosphorylation in cardiac mitochondria, suggesting that obtaining a comprehensive map of the mitochondrial phosphoproteome is feasible.
In this study, we took a systematic approach to tackle the phosphorylation of murine cardiac mitochondrial pathways. We applied the unique strengths of both electron transfer dissociation (ETD) and collision-induced dissociation (CID) LC-MS/MS to screen phosphorylation events in a site-specific fashion. A total of 236 phosphorylation sites in 203 unique phosphopeptides were identified and mapped to 181 phosphoproteins. Novel phosphorylation modifications were discovered in diverse pathways of mitochondrial biology, including ion balance, proteolysis, and apoptosis. Consistent with the role of mitochondria as the major source of energy production under delicate control, metabolic pathways claimed one-third of phosphorylation sites captured in this analysis. To study molecular players steering mitochondrial phosphorylation, we probed the effects of calcium loading on phosphorylation. In addition, a number of kinases with previously unappreciated mitochondrial residence are suggested as potential players modulating mitochondrial pathways. Taken together, the cohort of novel phosphorylation events discovered in this study constitutes an essential step toward the full delineation of the cardiac mitochondrial phosphoproteome.
EXPERIMENTAL PROCEDURES
All procedures were performed in accordance with the Animal Research Committee guidelines at UCLA and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Materials
N-Dodecyl β-d-maltoside (Avanti Polar Lipids), protease inhibitor mixture (Roche Applied Science), fenvalerate and calyculin A (EMD Chemicals), sequencing grade modified trypsin (Promega), MonoTip TiO2 enrichment resin (GL Sciences) were obtained from the indicated sources. All other reagents were acquired from Sigma-Aldrich.
Purification of Mitochondria and Functional Validation
Mitochondria were freshly isolated and purified from murine hearts as described previously (5). Briefly, 45 mouse hearts (8–10 weeks old, ICR strain, male) were pooled immediately after collection and homogenized as a biological replicate (isolation buffer: 250 mm sucrose, 1 mm EGTA, 20 mm HEPES, pH 7.5, protease inhibitor mixture, and phosphatase inhibitor mixtures). After removal of the nuclear fraction and tissue debris, the crude mitochondrial fraction was collected by centrifugation at 4000 × g for 20min. The resultant pellet was then resuspended with 19% Percoll solution in isolation buffer and slowly layered on top of a preformed discontinuous Percoll gradient, 30 and 60% (v/v), respectively. After 15-min centrifugation at 10,000 × g, purified mitochondria were retrieved at the interface of the two layers. All procedures were performed at 4 °C. A total of nine biological replicates were prepared and examined independently. Four independent biological replicates were analyzed by ETD with three technical replicates for each biological replicate. Five independent biological replicates were analyzed by CID with one technical replicate each. The purity as well as structural/functional integrity of each preparation was validated as described previously (10).
Mitochondrial Electron Transport Chain (ETC) Activity Assay
To examine the functional consequence of phosphorylation, we subjected cardiac mitochondria to calcium loading and characterized the impact of phosphorylation on ETC complex activities. Phosphorylation was modulated via the application of three distinct phosphatase inhibitors: fenvalerate (primarily targeting against PP2B family), calyculin A (primarily targeting against PP2A and PP1), and okadaic acid (primarily targeting against PP2A and PP1). Freshly isolated mitochondria (100 μg) were resuspended in reaction buffer (120 mm KCl, 10 mm HEPES, pH 7.4, 2.5 mm potassium phosphate, 5 mm glutamate, 5 mm malate, 0.1 mm ADP, 2.5 mm MgCl2, and 0.5 mg/ml BSA) and subsequently treated for 5 min with Ca2+ at a concentration of 40 μm at ambient temperature. In parallel groups, mitochondria were treated with Ca2+ in the presence of phosphatase inhibitor fenvalerate (100 nm), calyculin A (40 nm), or okadaic acid (300 nm). Subsequently, mitochondria were lysed with a hypotonic buffer (5 mm Tris-HCl, pH 7.4) and then subjected to biochemical assays.
The specific activities of ETC complexes I (CI) and III (CIII) were measured in a 96-well microplate format (21, 22). Briefly, CI activity assays were initiated by mixing 100 μl of mitochondrial lysates (15 μg of protein) with 100 μl of CI assay buffer (5 mm Tris-HCl, pH 7.4, 0.2 mg/ml NADH, 2.0/ml nitro blue tetrazolium, 2 mm KCN, and 2 μm antimycin A). Assays were conducted at ambient temperature, and CI inhibitor (10 μm diphenyleneiodonium)-added groups served as negative controls to determine the inhibitor-insensitive background activity of the samples. The absorbance at 595 nm was monitored spectrometrically. Similarly, CIII activity assays were initiated by mixing mitochondrial lysates (20 μg of protein) with CIII assay buffer (50 mm sodium phosphate, pH 7.2, 50 μm cytochrome c, 0.1% BSA, 2 mm KCN, and 1 μg/ml rotenone) and 100 μm freshly reduced decylubiquinone (DBH2). CIII-specific inhibitor antimycin A (2 μm) was applied in negative control groups. CIII activity was monitored spectrometrically at 550 nm.
Preparative SDS-PAGE and In-gel Digestion
Purified cardiac mitochondria were lysed with 0.5% N-dodecyl β-d-maltoside in the isolation buffer on ice. After centrifugation at 13,000 × g for 30 min, mitochondrial proteins were collected from the supernatant and then incubated with Laemmli sample buffer for 30 min. Denatured proteins were resolved by 12.5% SDS-PAGE (23). Subsequently, the gels were fixed and stained with colloidal Coomassie Blue. A total of 21 gel slices were excised from each lane.
Gel slices were destained with 25% acetonitrile (ACN) in 50 mm ammonium bicarbonate (AmBC). Proteins were sequentially reduced with DTT and alkylated with iodoacetamide. Partial in-gel digestions (for ETD) were carried out with trypsin (1:100 ratio of trypsin versus protein in 50 mm AmBC, pH 8.0) at 37 °C for 3 h. For CID analysis, the proteins were digested overnight at 37 °C (1:50 ratio of trypsin versus protein). Peptides were extracted from gel slices with 50% ACN and 0.1% TFA and then with ACN only.
Phosphopeptide Enrichment
Dried peptides were reconstituted with 100 μl of 50% ACN containing 0.1% TFA (v/v). Phosphopeptides were enriched using a TiO2 MonoTip as described previously (24). Briefly, the TiO2 MonoTip was sequentially equilibrated with three solutions: Solution 1, 50 mm AmBC containing 25% ACN; Solution 2, 50% ACN solution containing 5% TFA (v/v); and Solution 3, 50% ACN solution containing 0.1% TFA (v/v). Subsequently, peptides were loaded onto the tip with 50 repetitions and then rinsed for 10 cycles with 100 μl of 50% ACN containing 0.1% TFA (v/v). Finally, the enriched phosphopeptides were eluted sequentially with three buffers: Buffer 1, 100 μl of 50% ACN containing 5% TFA (v/v); Buffer 2, 50 mm AmBC supplemented with 25% ACN (pH 10.5); and Buffer 3, 1% aqueous ammonia solution. All eluted fractions were dried and subjected to mass spectrometric analyses.
LC-MS/MS Operation
Enriched phosphopeptides were analyzed in parallel in both CID mode (LTQ-Orbitrap) and ETD mode (LTQ-XL-ETD). On-line peptide separation was achieved on a prepacked PicoFrit column (BioBasic C18, 75 μm × 10 cm, 5 μm, 300 Å; New Objective). The flow rate of chromatography was set to 5 μl/min for loading and 220 nl/min for separation (buffer A, 0.1% formic acid and 2% ACN; buffer B, 0.1% formic acid and 80% ACN). The resolving gradient was set as follows: 5–40% buffer B over 90 min to 100% buffer B over 10 min, maintained at 100% for 10 min, and then back to 0% buffer B. The mass spectrometer was operated in a data-dependent acquisition mode (25); one full MS scan was followed by five MS2 scans. Each biological sample was analyzed in three parallel technical replicates. In ETD mode, the default precursor charge state was set at 5+. The automatic gain control for fluoranthene was set to 4 × 105, and the ion/ion reaction time was set to a 100 ms. In CID mode, the LTQ-Orbitrap was operated in a data-dependent mode without using the FT module to assure the sensitivity and duty cycle of the analyses. An MS3 scan was triggered automatically when a neutral loss peak of 98, 49, or 32.7 m/z was detected within the top 10 most intense peaks in the MS2 spectrum.
Database Search and Data Analyses
For both ETD and CID analyses, Bioworks version 3.3.1 was used for the .dta file generation. The minimal ion threshold was set to 10, intensity threshold was 1000, precursor tolerance was 1.4 amu, and mass range was 400–4500 Da.
The ETD spectra were searched against the International Protein Index mouse database (version 3.34 with 51,424 entries) using the Open Mass Spectrometry Search Algorithm (OMSSA) (version 2.2.1) (26). The following search parameters were set: partial enzymatic digestion (trypsin) permitting three missed cleavages; fixed modification of cysteine carboxyamidomethylation (+57 Da); and dynamic modifications of methionine oxidation (+16 Da) and serine, threonine, and tyrosine phosphorylation (+80 Da). The precursor charge states were set between 1+ and 6+. Precursor ion mass tolerance was set at ±1.5 Da; product ion tolerance was set at ±0.5 Da. The cutoff value of the filter threshold (E-value) was set to be better than 0.1, and then peptide spectra were subjected to manual inspection with the following criteria: Criterion 1, the charge state of the precursor was confirmed by characteristic precursor signals generated by charge stripping (27); and Criterion 2, the mass spectrum was in agreement with the theoretical fragmentation profile obtained from ProteinProspector (28).
The CID MS2 and MS3 spectra were searched against the International Protein Index mouse database (version 3.34) with the SEQUEST algorithm (Bioworks version 3.3.1). For MS2 spectra, the following parameters were set: partial enzymatic digestion allowing for two missed cleavages; fixed modification of cysteine carboxyamidomethylation (+57 Da); variable modifications of methionine oxidation (+16 Da) and serine, threonine, and tyrosine phosphorylation (+80 Da). For MS3 spectra, the search parameters were set identically to that of MS2 except serine and threonine water loss (−18 Da) was added. The cutoff values of the filter threshold were set to pass a significant threshold of cross-correlation versus charge state: Xcorr > 1.9 (z = 1+), 2.5 (z = 2+), and 3.0 (z = 3+) (29). Manual inspection was conducted on all potential phosphopeptides to remove false positives. This two-step screening procedure for both ETD and CID analyses was implemented to minimize the bias of a particular search engine as well as to improve the confidence of identification.
Functional Annotation
Functional annotation was conducted with the aid of established bioinformatic tools. Gene ontology (GO) annotation was performed using g:Profiler (http://biit.cs.ut.ee/gprofiler/) (30). The GO terms corresponding to the identified phosphoproteins were filtered first with a p value threshold of <0.05 using a hypergeometric test. For multiple GO terms testing, the p values were adjusted with the Benjamini-Hochberg algorithm. The phosphoproteins that do not associate with a GO term via g:Profiler were manually annotated using the following knowledgebases: Gene Ontology Annotation database (http://www.ebi.ac.uk/GOA/) (31), UniProt Knowledgebase (www.uniport.org) (32), and LOCATE database (www.scivee.tv/node/1413) (33).
RESULTS
In this study, we performed a systematic investigation to characterize the mitochondrial phosphoproteome in the heart as summarized in supplemental Fig. S1. We combined TiO2 affinity chromatography and the complementary advantages of both ETD and CID mass spectrometry. An overview of our experimental strategy is presented in supplemental Fig. S2. A total of 236 phosphorylation sites were identified in 203 unique peptides that were mapped to 181 mitochondrial proteins. In addition, phosphorylation was shown to govern cardiac mitochondrial functions (supplemental Table S1). With the aid of bioinformatics algorithms, the mitochondrial phosphoproteome was delineated systematically in the context of mitochondrial pathways.
Identification of Novel Phosphorylation Events in Diverse Pathways of Murine Cardiac Mitochondria
Within the 236 identified phosphorylation sites, 210 were not previously reported by studies in mouse, and 202 were not previously reported in mammals. Within this data set, only a small fraction is of relative less confidence: three phosphorylation sites were captured at the termini of corresponding peptides, and six phosphopeptide spectra can be attributed to phosphorylation at two alternative sites within the same peptide (supplemental Table S2). Consistent with the major regulatory role of mitochondrial metabolism in the heart, a significant portion of phosphorylation events in the cardiac mitochondria are located to the metabolic pathways.
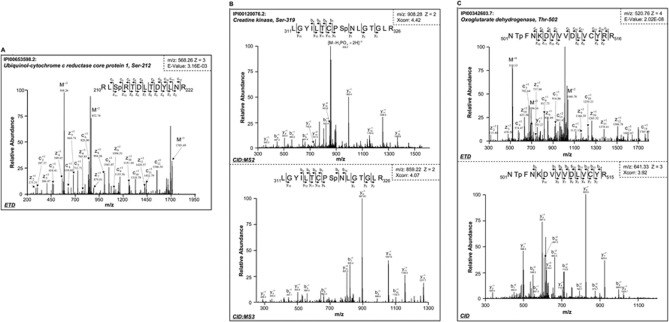
In the mitochondrial ETC, a novel phosphorylation site (serine 212) was identified in ubiquinol-cytochrome c reductase core protein 1 (QCR1), a subunit of the ETC complex III (Fig. 1A). The combination of limited trypsin digestion and ETD technology was essential to this identification. The phosphorylated serine 212 residue is coded within a cluster of arginine residues. Complete trypsin digestion will constrain serine 212 into a short peptide not suitable for LC-MS/MS analysis. QCR1 is one of two core subunits within the 11-mer ETC complex III. To date, the only reported form of post-translational modification on QCR1 was acetylation identified in liver mitochondria (34). The capture of this phosphorylation event sheds light on a novel regulatory mechanism tuning the throughput of ETC complexes.
Fig. 1.
Identification of novel phosphorylation events in diverse pathways of cardiac mitochondria. A, mass spectrum for ubiquinol-cytochrome c reductase core protein 1, a subunit of complex III in the mitochondrial electron transport chain. The peptide was phosphorylated at serine residue 212 (Sp) as detected by ETD analyses. B, creatine kinase, which is an essential energy metabolism protein in mitochondria, was shown by CID analysis to be phosphorylated at serine 319. A strong neutral loss signal was observed in the MS2 spectrum (top). The peptide precursors with phosphate neutral loss were isolated and further characterized by MS3 (bottom). C, oxoglutarate dehydrogenase, an important component of the mitochondrial tricarboxylic acid cycle, was identified by both ETD (top) and CID (bottom) to be phosphorylated at threonine residue 502.
With CID, a phosphorylation site was identified at serine 319 of mitochondrial creatine kinase (Fig. 1B), corroborating a recent report on this regulatory site in the porcine heart (13). A characteristic neutral loss of a phosphate group was observed in its MS2 spectrum with additional peptide fragments suggesting serine 319 as the site of modification. The MS3 spectrum confirmed this indication. In cardiomyocytes, the equilibrium between phosphocreatine and ATP serves as the essential molecular basis of instantaneous adaptation to fluctuation in energy demands. Under stressed conditions, the level of preserved energy determines life or death. Therefore, the function of creatine kinase impacts not only the overall cardiac homeostasis but also the pathogenesis of cardiac diseases. In addition to serine 319, another regulatory site was identified at serine 343 of creatine kinase using ETD (supplemental Fig. S3, Spectrum 41 and Spectrum 42).
The tricarboxylic acid (TCA) cycle is fundamental to both energy metabolism and metabolite biosynthesis. Among the array of enzymes within this functional module, oxoglutarate dehydrogenase serves as a key regulatory hub. Several parallel mechanisms of functional regulation have been reported, including substrate inhibition (35) and oxidative post-translational modifications (36). Pioneer studies by other investigators captured the phosphorylated form of oxoglutarate dehydrogenase with specific affinity probes (13, 14). In this study, a novel phosphorylation site was pinpointed to threonine 502 in the murine cardiac mitochondria by both ETD and CID (Fig. 1C).
Cardiac Mitochondrial Function Regulated by Reversible Phosphorylation
Calcium is a master regulator of cardiac cell function, playing a significant role in cardiac contractile function and an indispensable role in cardiac energy metabolism. The functional impact of free calcium toward mitochondrial metabolic pathways has been documented repeatedly (14, 37, 38). The potency of a few mitochondrial kinases and phosphatases is reported to be calcium-dependent, including pyruvate dehydrogenase phosphatase and protein kinase C (14). Calcium is also a major regulator of the ETC complexes, which are essential to oxidative phosphorylation and ROS production in cardiac mitochondria. To examine the molecular link between phosphorylation and mitochondrial function, we determined the impact of calcium regulation on activities of ETC complex I and complex III using phosphatase inhibitors.
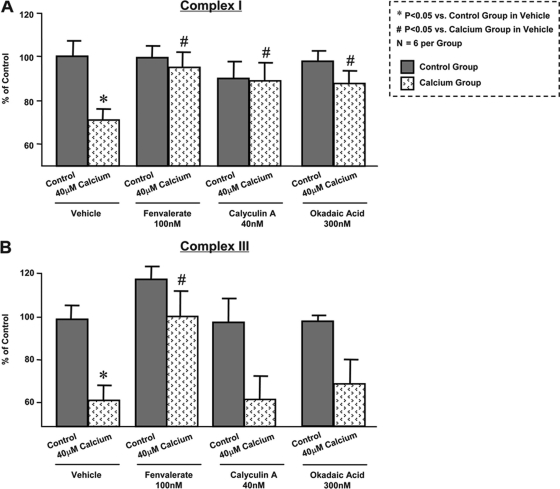
Calcium loading significantly decreased the activity of ETC complex I by ∼30% (Fig. 2A). Three distinct phosphatase inhibitors, fenvalerate, calyculin A, and okadaic acid, were used to evaluate the impact of phosphorylation to regulate ETC function. All three phosphatase inhibitors were sufficient to reverse calcium-induced suppression of complex I activity (Fig. 2A). In parallel experiments, calcium loading significantly attenuated ETC complex III activity. Interestingly, calcium-injured complex III activity was restored by the treatment of fenvalerate, whereas calyculin A and okadaic acid had a marginal impact (Fig. 2B). Taken together, the collective effects observed by the three phosphatase inhibitors suggest the involvement of multiple kinases and phosphatases in this process, demonstrating phosphorylation as an intrinsic molecular mechanism to regulate mitochondrial ETC function.
Fig. 2.
Phosphorylation plays essential role in mitochondrial complex I and III activity down-regulation induced by calcium loading. Mitochondrial complex I (A) and III (B) activities were suppressed by calcium treatment (40 μm). The calcium-sensitive inhibition of complex I activity was reversed independently with three phosphatase inhibitors, fenvalerate, calyculin A, and okadaic acid. The calcium-sensitive inhibition of complex III was reversed by fenvalerate, whereas calyculin A and okadaic acid showed marginal effects. *, p < 0.05 versus control + vehicle group; #, p < 0.05 versus calcium + vehicle group; n = 6 per group. The error bars represent standard deviation.
Mapping Identified Regulatory Sites into Major Mitochondrial Pathways
According to the mitochondrial phosphoproteome data set contributed by this study, there exist many regulatory sites in key mitochondrial pathways; mapping these phosphorylation events to the major mitochondrial pathways will facilitate the integration of proteomics data with biological function, therefore providing interpretation of the potential functional relevance of the identified phosphopeptides. Metabolism is a significant element of mitochondrial function; this functional category encompassed the most phosphorylation sites identified. Thus, an effort was devoted to dissect the regulation sites identified in each metabolic pathway (Fig. 3A).
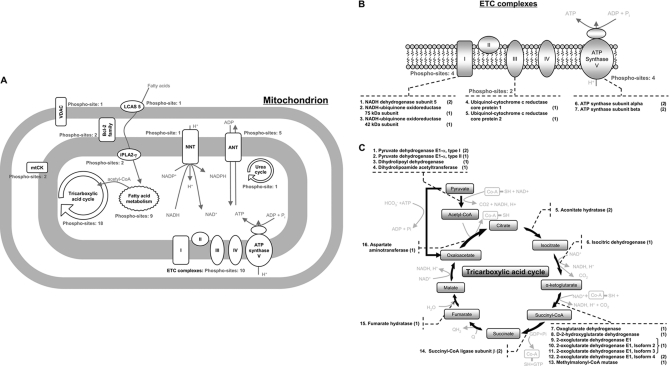
Fig. 3.
Working scheme to delineate phosphorylation events in diverse mitochondrial pathways. A, many of these regulatory sites are involved in the major mitochondrial pathways of metabolism and transport. By combining both ETD and CID technologies, it was possible to ascertain a total of 18 and 10 phosphorylation sites from the TCA cycle and ETC complexes, respectively. A further 12, two, and one phosphorylation sites were also identified for fatty acid metabolism, creatine kinase, and the urea cycle, respectively. A number of additional phosphorylation sites were identified in ANT (5), nicotinamide nucleotide transhydrogenase (NNT) (1), and voltage-dependent anion channel (VDAC) (1), which are major regulatory proteins involved in transport. The identified phosphoproteins involved in the ETC complexes and TCA cycle are illustrated in B and C, respectively. The identified phosphoproteins and the number of identified phosphorylation sites are shown. The number showing in parenthesis represents the number of phosphorylation sites identified for the listed protein. mtCK, mitochondrial creatine kinase.
A total of 10 phosphorylation sites were identified in ETC complexes with four in complex I, two in complex III, and four in complex V (Fig. 3B). Adenine nucleotide translocator (ANT) mediates the exchange of ADP and ATP across mitochondrial membrane; this protein is also thought to be essential to mitochondrial permeability transition (39). The biological significance of ANT phosphorylation was previously evaluated by site-directed mutagenesis (40). In this study, we identified five phosphorylation sites on ANT of which one was novel (supplemental Table S1). The nicotinamide nucleotide transhydrogenase, an inner mitochondrial membrane protein, catalyzes the conversion of NADH and NADPH and affects proton permeability. The metabolism of NADPH by NADPH dehydrogenase is another important source of ROS production in cardiomyocytes. One novel phosphorylation site was identified in nicotinamide nucleotide transhydrogenase by both ETD and CID (supplemental Fig. S3, Spectrum 12 and Spectrum 13). The primary energy substrate for healthy heart is fatty acid. A total of nine phosphorylation sites were identified in the fatty acid metabolic pathway, and an additional three phosphorylation sites were identified in fatty acid transporters.
The tricarboxylic acid cycle interfaces carbohydrate metabolism and fatty acid metabolism as well as energy metabolism and biogenesis. Arguably, it represents the most investigated territory of mitochondrial biology. In this study, a total of 18 phosphorylation sites were identified in the tricarboxylic acid cycle of which 15 were novel (Fig. 3C). Furthermore, a subset of these novel identifications were found in enzymes known to be regulated by phosphorylation (14).
Characterization of Cardiac Mitochondrial Phosphorylation Complemented by Both ETD and CID
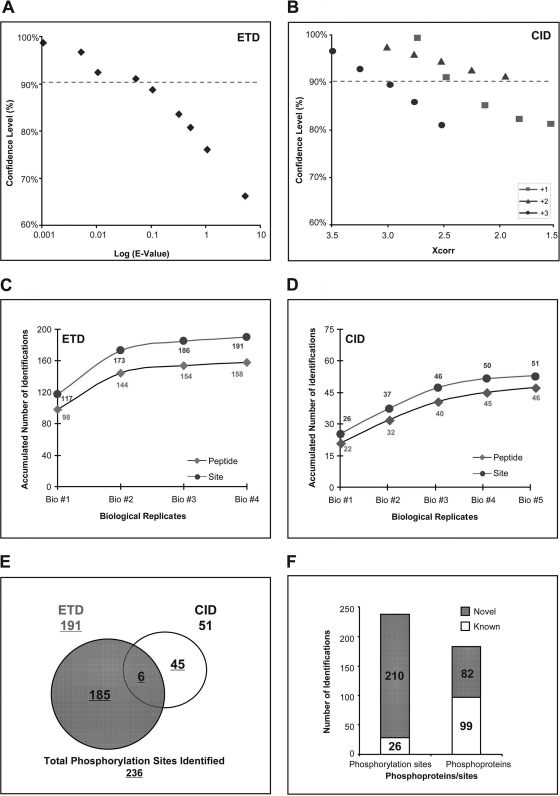
ETD and CID fragment phosphopeptides via different mechanisms, thus exhibiting distinct preferences toward unique peptides. In this study, the strengths of ETD and CID were combined to maximize the coverage of mitochondrial phosphoproteome. A two-phase validation procedure was carefully implemented to ensure confidence in phosphorylation identification via either ETD or CID. In the first phase, the calculation of the false positive rate (FPR) was conducted via decoy search using a sequence-reversed database. For ETD data analyses, the E-values assigned by OMSSA algorithm were plotted against the level of FPR (Fig. 4A), whereas for CID data analyses, Xcorr values assigned by the SEQUEST algorithm were plotted against FPR (Fig. 4B). The statistical thresholds for the first phase were set to a confidence level of no less than 90%. The second phase of the validation procedure involved manual spectrum inspection with the aid of ProteinProspector (28). Approximately 50% of the initial candidates from the first phase survived the second phase and are reported in the final data set (supplemental Table S1).
Fig. 4.
Characterization of murine mitochondrial phosphoproteome utilizing two types of fragmentation, namely ETD and CID. A, plot showing the correlation between the confidence level of ETD-based identifications and the E-value obtained from OMSSA. E-values were expressed to log10. The confidence level was examined by searching all the spectra against a sequence-reversed decoy database. B, plot examining the correlation between the confidence of CID-based identifications and the Xcorr values from three distinct charge states (z = 1+, z = 2+, and z = 3+). The confidence level was scrutinized in a similar way to the ETD analyses using a decoy database. C, the accumulated numbers of identified phosphopeptides and phosphorylation sites from the ETD analyses plotted against our number of biological replicates. No significant increases in identifications of phosphopeptides and phosphorylation sites were observed after using four biological replicates. D, the accumulated number of identifications plotted against the number of biological replicates for the CID analyses. No significant increases in identifications were observed after using five biological replicates. E, pie chart illustrating the complementary identifications of the mitochondrial phosphorylation sites by both ETD and CID. Each fragmentation process has a distinct preference in peptide sequencing, underlining the importance of our combined approach to increase the identification of mitochondrial phosphorylation sites. F, graph emphasizing the significant expansion in the mitochondrial phosphoproteome data set as identified in this study. 210 phosphorylation sites and 82 phosphoproteins were novel identifications.
The inclusion of sufficient biological and technical replications is an important strategy to overcome the variation in individual biological samples and insufficient analytical sampling. The number of replicates was plotted for each phosphorylation site identified (supplemental Table S3). The capacity of our ETD platform was saturated with four replicates (Fig. 4C), whereas the identification plateau of CID platform was reached with five replicates (Fig. 4D). The complementary strength of ETD and CID was evident: 191 identifications were obtained via ETD, and 51 were obtained via CID with six shared identifications (Fig. 4E). Among the 236 phosphorylation sites identified in this study, 26 sites have been reported previously, and 210 sites were novel identifications (Fig. 4F), which demonstrated the unique specificity and capacity of this technical platform. A complete list of identified phosphopeptides and their corresponding mass spectra is provided in supplemental Table S1 and supplemental Fig. S3, respectively. These phosphorylation sites were mapped to 181 phosphoproteins of which 82 were new discoveries.
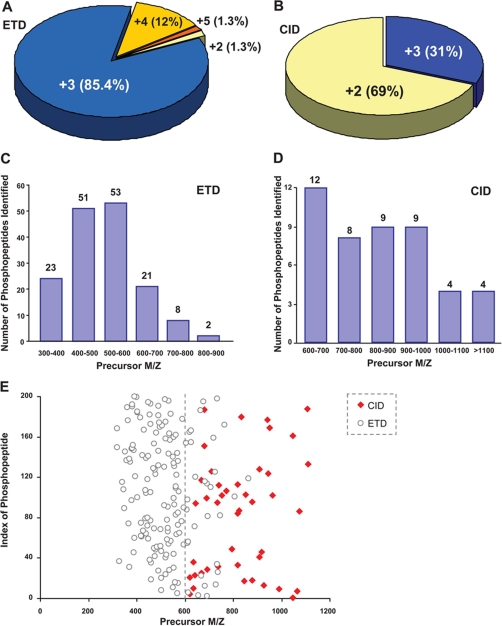
The distinct features of ETD and CID data sets are summarized in Fig. 5. More than 98% of the phosphopeptides identified by ETD had a charge state of 3+ or higher (Fig. 5A). The preference of ETD toward multiply charged (⩾3+) peptides is known, while a charge-stripping effect may dominate fragmentation kinetics for doubly charged peptides. To maximize the efficiency of ETD-based identification, limited trypsin digestion was used to improve the yield of multiply charged peptides. Such a procedure afforded additional advantages. A family of kinases, including protein kinase A (PKA) and PKC, favors arginine or lysine in the context of substrate consensus motifs (41). Complete trypsin digestion may put these consensus motifs into very short peptides, compromising their detection sensitivity and specificity by mass spectrometry. This scenario was evidenced by serine 212 in QCR1 (Fig. 1A). CID, on the other hand, identified 69% of phosphopeptides in their doubly charged state and 31% in their triply charged state (Fig. 5B). Therefore, the overall mass to charge ratio of phosphopeptides identified by ETD was significantly lower than that of CID (Fig. 5, C and D). In a scatter plot, this distinction is clearly illustrated (Fig. 5E). The unique features of either CID or ETD sequencing supported the complementary identification of mitochondrial phosphoproteome as evidenced in Fig. 4E and supplemental Table S1.
Fig. 5.
Performance characteristics of ETD/CID in this investigation. A, pie chart depicting the percentage of our identified phosphopeptides according to different charge states for the ETD analysis. ETD performed better for a 3+ charge state. B, pie chart depicting the percentage of the identified phosphopeptides according to different charge states for the CID analysis. As expected, CID performed better for doubly charged precursors. C, bar chart depicting the number of identified phosphopeptides as a function of precursor m/z for the ETD analysis. As can be seen, ETD performed better for precursors with an m/z of 600 or less. D, bar chart depicting the number of identified phosphopeptides as a function of precursor m/z for the CID analysis. As can be seen, CID performed better for precursors with an m/z of 600 or more. E, scatter plot comparing the distribution of precursor m/z of the identified phosphopeptides by both ETD and CID analyses.
Functional Annotation and Clustering of Cardiac Mitochondrial Phosphoproteome
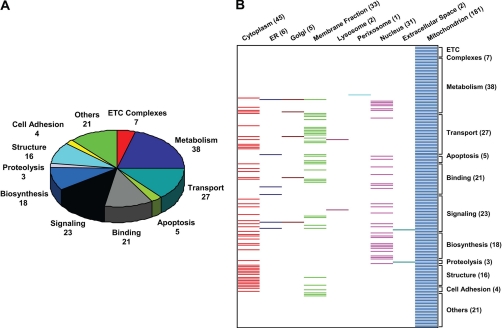
A systematic functional annotation of the identified mitochondrial phosphoproteins was conducted to align phosphoproteins in the context of their biological functions (Fig. 6A). Metabolism and oxidative phosphorylation together represented the largest functional category, consistent with the essential contribution of mitochondria toward energy production. Transporters and protein binding/folding were the next largest functional groups. In addition, a second dimension was added to coordinate these functional categories against the primary cellular residence of these phosphoproteins in the form of a “heat map” (Fig. 6B). Ostensibly, a significant portion of mitochondrial phosphoproteins had more than one subcellular destiny. The biological significance of this phenomenon remains to be discerned. One possible utility of multiresidential proteins is to support mitochondrial protein trafficking and communication with other organelles. A more detailed functional annotation of the identified mitochondrial phosphoproteins is provided in supplemental Table S2.
Fig. 6.
Functional annotation of identified mitochondrial phosphoproteins. A, pie chart showing the functional annotation of the 181 identified phosphoproteins based on their protein functions. Of the 181 annotated phosphoproteins, 38 were involved in critical metabolic mechanisms that are essential for healthy cardiac mitochondria, including the TCA cycle, fatty acid transport/oxidation, and nucleic acid metabolism. B, the identified phosphoproteins were annotated for both their cell locations (horizontal) and their protein function (vertical) in the form of a heat map. The number of mitochondrial phosphoproteins within each functional category is listed in parentheses. ER, endoplasmic reticulum.
The sequence consensus of phosphopeptide motifs reflected the kinase-specific regulation of mitochondrial proteins as well as the identity of the corresponding kinases. A list of consensus motifs is archived in NetPhosK (http://www.cbs.dtu.dk/services/NetPhosK/) (42) and Human Protein Reference Database (http://www.hprd.org/) (43), including several known mitochondrial kinases, e.g. pyruvate dehydrogenase kinase and oxoglutarate dehydrogenase kinase, as well as kinases whose mitochondrial location was not previously appreciated, e.g. PKA, Src kinase, and glycogen synthase kinase 3 (GSK3) (44) (supplemental Table S4). Indeed, a total of 29 phosphopeptides matched the consensus substrate motifs of eight kinases with known mitochondrion residence (Table I) (13, 16, 40, 45–49). As a full inventory of mitochondrial kinases has yet to be obtained, a comprehensive analysis is provided in supplemental Table S4 with 29 consensus matches.
Table I. Eight representative substrate kinase motifs matched to phosphopeptides identified in this study.
A summary of eight representative substrate kinase motifs predicted to be matched to the identified phosphopeptides is shown. A total of 29 identified phosphopeptides matched the consensus substrate motifs of these eight kinases. The name of each kinase and the name of the identified phosphoproteins (Kinase and phosphoprotein), the experimentally identified phosphopeptide sequence (Phosphopeptide sequence), function of the phosphoproteins (Function), peptide fragmentation methods (MS/MS), and the references of previously reported phosphorylation sites (Refs.) are listed. A more comprehensive overview is provided in supplemental Table S4 with 29 consensus matches. EGFR, EGF receptor. FYVE, Fablp, YoTB, Vaclp, and EEA domain-containing proteins. Lowercase letters in sequences indicate modified residues.
| Kinase and phosphoprotein | Phosphopeptide sequence | Function | MS/MS | Refs. |
|---|---|---|---|---|
| Pyruvate dehydrogenase kinase | ||||
| Pyruvate dehydrogenase E1-α | YHGHsMSDPGVSYR | TCA cycle | ETD | 16, 40 |
| Ubiquinol-cytochrome c reductase core protein 1 | RLsRTDLTDYLNR | ETC complex III | ETD | |
| Netrin receptor UNC5B | ICsSLDAPNSRGNDWR | Apoptosis | ETD | |
| Ras-related protein Rab-3B | MAsVtDGKTGIK | Transport | ETD | |
| Zinc finger protein 811 | EILRNVVsIGDIRK | Transcription | ETD | |
| Microtubule-associated protein 6 | AsGADERDTRR | Binding | ETD | |
| Hypothetical ATP/GTP-binding site motif A (P-loop)-containing protein | RQGsDVDVLK | Binding | ETD | |
| Src kinase | ||||
| Pyruvate dehydrogenase E1-α | KGGCAKGKGGSMHMyGK | TCA cycle | ETD | |
| Pantothenate kinase 1 | LVKDIyGGDyER | Biosynthesis | ETD | |
| Cytosol aminopeptidase | mtKGLVLGIyAKDK | Proteolysis | ETD | |
| Arginase-2, mitochondrial | NyDIQYFSMR | Urea cycle | ETD | |
| Methylmalonyl-CoA mutase | GyDSDNPRVRGDVG | TCA cycle | CID | |
| Zymogen granule membrane protein 16 | TSSYSGEyGGKGGKR | Other | ETD | |
| Src family kinase | ||||
| Retinaldehyde-binding protein 1 | LQyPELFDsLSMEALR | Transport | ETD | |
| Transmembrane channel-like protein 2 | LTVSDmLVTyLTILVGDFL | Other | CID | |
| Branched-chain α-ketoacid dehydrogenase | ||||
| 2-Oxoisovalerate dehydrogenase subunit α, mitochondrial | IGHHSTSDDSSAYRsVDEVNYWDKQDHPISR | Metabolism | ETD | 16, 40, 45, 46 |
| GSK3, ERK1, ERK2, CDK5 | ||||
| Dihydrolipoyllysine-residue acetyltransferase component of pyruvate dehydrogenase complex | EGPGAPCGsPR | TCA cycle | ETD | |
| Apolipoprotein B100 receptor | AEGSItPmETEGLLR | Metabolism, transport | ETD | |
| 40 S ribosomal protein S20 | RLIDLHsPSEIVK | RNA binding | ETD | |
| Junctophilin-2 | GLGTGLPERPREsPQLHER | Protein binding | ETD | |
| FYVE and coiled coil domain-containing protein 1 | AEKGEKtPPDTELHQEPVPADLVLK | Transport | ETD | 46 |
| Bcl-2-like 13 protein | AEKTsPTPSVFVELGEEELEAVTARPEAVER | Apoptosis | ETD | 16 |
| Disks large-associated protein 5 | ELGNIHETSQDLsPEK | Signaling | ETD | 47, 48 |
| Coiled coil-helix-coiled coil-helix domain-containing protein 2 | RAPAAQPPAAAAPSAVGsPAAAPR | Biosynthesis | ETD | 16 |
| PKCϵ kinase | ||||
| Zinc finger, CCHC domain-containing 5 | EPREPsAIsELR | Binding | ETD | |
| Cardiac phospholamban | RAsTIEMPQQAR | Transport | CID | 13, 49 |
| EGFR kinase | ||||
| Pantothenate kinase 1 | LVKDIyGGDyER | Biosynthesis | ETD | |
| Zymogen granule membrane protein 16 | TSSYSGEyGGKGGKR | Exocytosis | ETD | |
| GSK3 kinase | ||||
| Regulating synaptic membrane exocytosis protein 2 | sMQRsQSR | Exocytosis | ETD |
DISCUSSION
This investigation systematically characterized the mitochondrial phosphoproteome in the heart. The delineation of regulatory sites in diverse mitochondrial pathways was supported by the combination of phosphopeptide enrichment procedures and complementary proteomics technologies of ETD and CID. Via this approach, we identified 236 phosphorylation sites with 210 novel identifications. These regulatory sites were mapped to 181 distinct proteins, covering a wide range of mitochondrial functions from metabolism to apoptosis. The contribution of reversible phosphorylation toward mitochondrial function regulation was illustrated with calcium loading and phosphatase inhibitors. The phosphorylation events captured in this study facilitate the integration of yet fragmentary knowledge on the mitochondrial proteome in a functional and biological context.
Integration of Proteomics Platform to Characterize Cardiac Mitochondrial Phosphoproteome
Proteomics technologies have advanced considerably in systematical characterization of phosphorylation events. The technical challenges of phosphoproteome analysis include a limited dynamic detection range for low abundance phosphopeptides and the fragile nature of the phosphoester bond during the collision process in mass spectrometers. The sensitivity for phosphopeptide detection has been extended significantly with an array of enrichment resins, including IMAC, TiO2, etc. Individual affinity resins show modest variation with enrichment preferences, which are further influenced by the choice of application procedures (50, 51). A pioneering study by Lee et al. (16) identified 84 phosphorylation sites in 62 phosphoproteins with IMAC in murine liver mitochondria. With TiO2, a total of 56 phosphorylation sites were reported in porcine cardiac mitochondria (13). The application of an enrichment procedure may also extend our scope toward the mitochondrial proteome, leading to the capture of proteins with low abundance that otherwise escape detection in mitochondria.
CID and ETD both have wide applications in proteomics. In a phosphoproteome analysis, the neutral loss of a phosphate group usually associated with CID serves as a specificity checkpoint at the expense of detection sensitivity. The maturation of ETD technology aids the preservation of the labile phosphate moiety (25, 52). The distinct advantages of CID and ETD have been observed repeatedly for analyses of phosphopeptides and non-phosphopeptides (25, 53); thus, the combination of both warrants unbiased investigation.
In this investigation, we integrated TiO2 enrichment with complementary ETD and CID proteomics to characterize the mitochondrial phosphoproteome in murine hearts (supplemental Fig. S2). The unique features of ETD and CID data sets are summarized in Fig. 5. ETD favored multiply charged (⩾3+) phosphopeptides, while doubly charged peptides were fragmented less efficiently due to a charge-stripping effect. CID identified phosphopeptides in their doubly charged state or triply charged state (Fig. 5B). This phenomenon results from the composite effects of the scan range of a mass spectrometer, the length of fully digested tryptic peptides, and the pH value of the chromatography mobile phase. According to the mobile proton mechanism, complete trypsin digestion is ideal for CID (24). Otherwise, the fragmentation pattern may become less random as peptides acquire more than two charges, which compromises the efficiency of peptide sequencing. However, for ETD analyses, the trypsin digestion efficiency was intentionally reduced to generate multiple charged peptides. Therefore, the mass to charge ratio of phosphopeptides identified by ETD showed a different trend compared with that of CID (Fig. 5, C–E). The application of partial trypsin digestion contributed to the generation of peptides compatible with ETD analysis and maximal coverage of the mitochondrial phosphoproteome. In addition, it supported the identification of phosphorylation sites governed by unique sets of kinases (e.g. PKA and PKC), which have lysine or arginine residues adjacent to phosphorylation sites. Thus, partial trypsin digestion supported an unbiased survey of the cardiac mitochondrial phosphoproteome. Alternatively, the amount of peptides suitable for ETD may be increased by conducting digestion with multiple endoproteases independently. Such a procedure will be favorable for targeted quantitative analyses as well as to provide independent evidence for phosphopeptides with phosphorylation sites identified at their termini.
Several distinct proteomics platforms have been organized to probe the mitochondrial phosphoproteome (12–14, 16). Each study identified a unique set of phosphoproteins, an indication that a comprehensive portrait of the mitochondrial phosphoproteome has still yet to be obtained. In this investigation, sufficient replications were included to reach the saturation points of both the ETD and CID procedures.
Cardiac Mitochondrial Phosphoproteome and Functional Regulation
With the proteomics platform described above, 236 phosphorylation sites were identified of which 210 were novel. Besides integration of multiple technical approaches, the animal species, tissue sample collections, and biological functional stage of the samples all may have contributed to the identifications of additional novel sites. Diverse bioinformatics tools were used to facilitate the interpretation of this data set. The largest functional cluster was metabolism-related proteins. This corroborated with the consensus that a dynamic balance exists between cardiac output and metabolic rates. A total of 61 phosphorylation events were identified in the major known mitochondrial metabolic pathways, including 10 for the electron transport chain complexes, 18 for the TCA cycle, 12 for fatty acid metabolism, and a further 21 for other metabolic pathways, including the nucleic acid metabolic pathway, the amino acid metabolic pathway, and the urea cycle.
A systematic portrait of the mitochondrial signaling network requires delineation of mitochondrial kinases and phosphatases. Despite the various choices of phosphoprotein or phosphopeptide enrichment procedures, the identification of low abundance kinases and phosphatases still represents a significant technical challenge. The BLAST search of identified phosphopeptide sequences against known substrate consensus sequences suggested that at least 29 distinct kinases may contribute to the dynamics of the mitochondrial phosphoproteome.
The functional assays of mitochondrial ETC complexes I and III with calcium loading provided evidence that murine cardiac mitochondrial function may be acutely regulated by phosphorylation. With three distinct phosphatase inhibitors, reversible phosphorylation was shown to impact mitochondrial function significantly. The most intriguing observation was that at least a third of the mitochondrial energy production rate was modulated by phosphorylation. The drastic effects induced by fenvalerate, calyculin A, and okadaic acid suggested involvement of multiple kinases and phosphatases in the functional regulation of mitochondrial ETC complexes. Moreover, a discrepancy was observed with respect to the sensitivity of complex I and complex III activities toward calyculin A and okadaic acid. Given the critical contribution to ROS production by complex I and complex III, these data indicated that phosphorylation events might also tune ROS release from cardiac mitochondria. An accurate assay of the dynamics in the mitochondrial phosphoproteome is a necessary next step toward the thorough delineation of molecular regulation mechanisms. Quantitative proteomics with a stable isotope-coded internal standard would aid such an investigation in a site-specific fashion.
Taken together, phosphorylation is an essential element of mitochondrial functional regulation. The technical platform applied in this study provided novel information regarding phosphorylation of mitochondrial pathways, contributing to a knowledgebase of mitochondrial proteome biology in the heart.
Footnotes
* This work was supported, in whole or in part, by National Institutes of Health Grants P01 HL80111 and R01 HL80691 (to P. P.).
 This article contains supplemental Figs. S1–S3 and Tables S1–S4.
This article contains supplemental Figs. S1–S3 and Tables S1–S4.
1 The abbreviations used are:
- ROS
- reactive oxygen species
- AmBC
- ammonium bicarbonate
- ANT
- adenine nucleotide translocator
- ETC
- electron transport chain
- ETD
- electron transfer dissociation
- QCR1
- ubiquinol-cytochrome c reductase core protein 1
- CI
- complex I
- CIII
- complex III
- OMSSA
- Open Mass Spectrometry Search Algorithm
- GO
- gene ontology
- FPR
- false positive rate
- GSK3
- glycogen synthase kinase 3.
REFERENCES
- 1. Johnson D. T., Harris R. A., Blair P. V., Balaban R. S. (2007) Functional consequences of mitochondrial proteome heterogeneity. Am. J. Physiol. Cell Physiol. 292, C698–C707 [DOI] [PubMed] [Google Scholar]
- 2. McBride H. M., Neuspiel M., Wasiak S. (2006) Mitochondria: more than just a powerhouse. Curr. Biol. 16, R551–R560 [DOI] [PubMed] [Google Scholar]
- 3. Kislinger T., Cox B., Kannan A., Chung C., Hu P., Ignatchenko A., Scott M. S., Gramolini A. O., Morris Q., Hallett M. T., Rossant J., Hughes T. R., Frey B., Emili A. (2006) Global survey of organ and organelle protein expression in mouse: combined proteomic and transcriptomic profiling. Cell 125, 173–186 [DOI] [PubMed] [Google Scholar]
- 4. Pagliarini D. J., Calvo S. E., Chang B., Sheth S. A., Vafai S. B., Ong S. E., Walford G. A., Sugiana C., Boneh A., Chen W. K., Hill D. E., Vidal M., Evans J. G., Thorburn D. R., Carr S. A., Mootha V. K. (2008) A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J., Li X., Mueller M., Wang Y., Zong C., Deng N., Vondriska T. M., Liem D. A., Yang J. I., Korge P., Honda H., Weiss J. N., Apweiler R., Ping P. (2008) Systematic characterization of the murine mitochondrial proteome using functionally validated cardiac mitochondria. Proteomics 8, 1564–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mootha V. K., Bunkenborg J., Olsen J. V., Hjerrild M., Wisniewski J. R., Stahl E., Bolouri M. S., Ray H. N., Sihag S., Kamal M., Patterson N., Lander E. S., Mann M. (2003) Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 115, 629–640 [DOI] [PubMed] [Google Scholar]
- 7. Riva A., Tandler B., Loffredo F., Vazquez E., Hoppel C. (2005) Structural differences in two biochemically defined populations of cardiac mitochondria. Am. J. Physiol. Heart Circ. Physiol. 289, H868–H872 [DOI] [PubMed] [Google Scholar]
- 8. Foster D. B., O'Rourke B., Van Eyk J. E. (2008) What can mitochondrial proteomics tell us about cardioprotection afforded by preconditioning? Expert Rev. Proteomics 5, 633–636 [DOI] [PubMed] [Google Scholar]
- 9. Weiss J. N., Korge P., Honda H. M., Ping P. (2003) Role of the mitochondrial permeability transition in myocardial disease. Circ. Res. 93, 292–301 [DOI] [PubMed] [Google Scholar]
- 10. Zhang J., Liem D. A., Mueller M., Wang Y., Zong C., Deng N., Vondriska T. M., Korge P., Drews O., Maclellan W. R., Honda H., Weiss J. N., Apweiler R., Ping P. (2008) Altered proteome biology of cardiac mitochondria under stress conditions. J. Proteome Res. 7, 2204–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mayr M., Liem D., Zhang J., Li X., Avliyakulov N. K., Yang J. I., Young G., Vondriska T. M., Ladroue C., Madhu B., Griffiths J. R., Gomes A., Xu Q., Ping P. (2009) Proteomic and metabolomic analysis of cardioprotection: Interplay between protein kinase C epsilon and delta in regulating glucose metabolism of murine hearts. J. Mol. Cell. Cardiol. 46, 268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arrell D. K., Elliott S. T., Kane L. A., Guo Y., Ko Y. H., Pedersen P. L., Robinson J., Murata M., Murphy A. M., Marbán E., Van Eyk J. E. (2006) Proteomic analysis of pharmacological preconditioning: novel protein targets converge to mitochondrial metabolism pathways. Circ. Res. 99, 706–714 [DOI] [PubMed] [Google Scholar]
- 13. Boja E. S., Phillips D., French S. A., Harris R. A., Balaban R. S. (2009) Quantitative mitochondrial phosphoproteomics using iTRAQ on an LTQ-Orbitrap with high energy collision dissociation. J. Proteome Res. 8, 4665–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hopper R. K., Carroll S., Aponte A. M., Johnson D. T., French S., Shen R. F., Witzmann F. A., Harris R. A., Balaban R. S. (2006) Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry 45, 2524–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hunter T. (1995) Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell 80, 225–236 [DOI] [PubMed] [Google Scholar]
- 16. Lee J., Xu Y., Chen Y., Sprung R., Kim S. C., Xie S., Zhao Y. (2007) Mitochondrial phosphoproteome revealed by an improved IMAC method and MS/MS/MS. Mol. Cell. Proteomics 6, 669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burnett G., Kennedy E. P. (1954) The enzymatic phosphorylation of proteins. J. Biol. Chem. 211, 969–980 [PubMed] [Google Scholar]
- 18. Alonso A., Sasin J., Bottini N., Friedberg I., Friedberg I., Osterman A., Godzik A., Hunter T., Dixon J., Mustelin T. (2004) Protein tyrosine phosphatases in the human genome. Cell 117, 699–711 [DOI] [PubMed] [Google Scholar]
- 19. Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. (2002) The protein kinase complement of the human genome. Science 298, 1912–1934 [DOI] [PubMed] [Google Scholar]
- 20. Pagliarini D. J., Dixon J. E. (2006) Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem. Sci. 31, 26–34 [DOI] [PubMed] [Google Scholar]
- 21. Wittig I., Braun H. P., Schägger H. (2006) Blue native PAGE. Nat. Protoc. 1, 418–428 [DOI] [PubMed] [Google Scholar]
- 22. Zerbetto E., Vergani L., Dabbeni-Sala F. (1997) Quantification of muscle mitochondrial oxidative phosphorylation enzymes via histochemical staining of blue native polyacrylamide gels. Electrophoresis 18, 2059–2064 [DOI] [PubMed] [Google Scholar]
- 23. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 24. Pinkse M. W., Uitto P. M., Hilhorst M. J., Ooms B., Heck A. J. (2004) Selective isolation at the femtomole level of phosphopeptides from proteolytic digests using 2D-NanoLC-ESI-MS/MS and titanium oxide precolumns. Anal. Chem. 76, 3935–3943 [DOI] [PubMed] [Google Scholar]
- 25. Good D. M., Wirtala M., McAlister G. C., Coon J. J. (2007) Performance characteristics of electron transfer dissociation mass spectrometry. Mol. Cell. Proteomics 6, 1942–1951 [DOI] [PubMed] [Google Scholar]
- 26. Chi A., Huttenhower C., Geer L. Y., Coon J. J., Syka J. E., Bai D. L., Shabanowitz J., Burke D. J., Troyanskaya O. G., Hunt D. F. (2007) Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 104, 2193–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molina H., Horn D. M., Tang N., Mathivanan S., Pandey A. (2007) Global proteomic profiling of phosphopeptides using electron transfer dissociation tandem mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 104, 2199–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clauser K. R., Baker P., Burlingame A. L. (1999) Role of accurate mass measurement (+/−10 ppm) in protein identification strategies employing MS or MS/MS and database searching. Anal. Chem. 71, 2871–2882 [DOI] [PubMed] [Google Scholar]
- 29. Cantin G. T., Venable J. D., Cociorva D., Yates J. R., 3rd (2006) Quantitative phosphoproteomic analysis of the tumor necrosis factor pathway. J. Proteome Res. 5, 127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reimand J., Kull M., Peterson H., Hansen J., Vilo J. (2007) g:Profiler—a web-based toolset for functional profiling of gene lists from large-scale experiments. Nucleic Acids Res. 35, W193–W200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Camon E., Magrane M., Barrell D., Lee V., Dimmer E., Maslen J., Binns D., Harte N., Lopez R., Apweiler R. (2004) The Gene Ontology Annotation (GOA) Database: sharing knowledge in UniProt with Gene Ontology. Nucleic Acids Res. 32, D262–D266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu C. H., Apweiler R., Bairoch A., Natale D. A., Barker W. C., Boeckmann B., Ferro S., Gasteiger E., Huang H., Lopez R., Magrane M., Martin M. J., Mazumder R., O'Donovan C., Redaschi N., Suzek B. (2006) The Universal Protein Resource (UniProt): an expanding universe of protein information. Nucleic Acids Res. 34, D187–D191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Plymate S. R., Haugk K. H., Sprenger C. C., Nelson P. S., Tennant M. K., Zhang Y., Oberley L. W., Zhong W., Drivdahl R., Oberley T. D. (2003) Increased manganese superoxide dismutase (SOD-2) is part of the mechanism for prostate tumor suppression by Mac25/insulin-like growth factor binding-protein-related protein-1. Oncogene 22, 1024–1034 [DOI] [PubMed] [Google Scholar]
- 34. Kim S. C., Sprung R., Chen Y., Xu Y., Ball H., Pei J., Cheng T., Kho Y., Xiao H., Xiao L., Grishin N. V., White M., Yang X. J., Zhao Y. (2006) Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell 23, 607–618 [DOI] [PubMed] [Google Scholar]
- 35. Rokutan K., Kawai K., Asada K. (1987) Inactivation of 2-oxoglutarate dehydrogenase in rat liver mitochondria by its substrate and t-butyl hydroperoxide. J. Biochem. 101, 415–422 [DOI] [PubMed] [Google Scholar]
- 36. Applegate M. A., Humphries K. M., Szweda L. I. (2008) Reversible inhibition of alpha-ketoglutarate dehydrogenase by hydrogen peroxide: glutathionylation and protection of lipoic acid. Biochemistry 47, 473–478 [DOI] [PubMed] [Google Scholar]
- 37. Cortassa S., Aon M. A., Marbán E., Winslow R. L., O'Rourke B. (2003) An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys. J. 84, 2734–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Terrand J., Papageorgiou I., Rosenblatt-Velin N., Lerch R. (2001) Calcium-mediated activation of pyruvate dehydrogenase in severely injured postischemic myocardium. Am. J. Physiol. Heart Circ. Physiol. 281, H722–H730 [DOI] [PubMed] [Google Scholar]
- 39. Murphy E., Steenbergen C. (2007) Preconditioning: the mitochondrial connection. Annu. Rev. Physiol. 69, 51–67 [DOI] [PubMed] [Google Scholar]
- 40. Feng J., Zhu M., Schaub M. C., Gehrig P., Roschitzki B., Lucchinetti E., Zaugg M. (2008) Phosphoproteome analysis of isoflurane-protected heart mitochondria: phosphorylation of adenine nucleotide translocator-1 on Tyr194 regulates mitochondrial function. Cardiovasc. Res. 80, 20–29 [DOI] [PubMed] [Google Scholar]
- 41. Pearson R. B., Kemp B. E. (1991) Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 200, 62–81 [DOI] [PubMed] [Google Scholar]
- 42. Blom N., Sicheritz-Pontén T., Gupta R., Gammeltoft S., Brunak S. (2004) Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4, 1633–1649 [DOI] [PubMed] [Google Scholar]
- 43. Keshava Prasad T. S., Goel R., Kandasamy K., Keerthikumar S., Kumar S., Mathivanan S., Telikicherla D., Raju R., Shafreen B., Venugopal A., Balakrishnan L., Marimuthu A., Banerjee S., Somanathan D. S., Sebastian A., Rani S., Ray S., Harrys Kishore C. J., Kanth S., Ahmed M., Kashyap M. K., Mohmood R., Ramachandra Y. L., Krishna V., Rahiman B. A., Mohan S., Ranganathan P., Ramabadran S., Chaerkady R., Pandey A. (2009) Human Protein Reference Database—2009 update. Nucleic Acids Res. 37, D767–D772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Das S., Wong R., Rajapakse N., Murphy E., Steenbergen C. (2008) Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ. Res. 103, 983–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dai J., Jin W. H., Sheng Q. H., Shieh C. H., Wu J. R., Zeng R. (2007) Protein phosphorylation and expression profiling by Yin-yang multidimensional liquid chromatography (Yin-yang MDLC) mass spectrometry. J. Proteome Res. 6, 250–262 [DOI] [PubMed] [Google Scholar]
- 46. Villén J., Beausoleil S. A., Gerber S. A., Gygi S. P. (2007) Large-scale phosphorylation analysis of mouse liver. Proc. Natl. Acad. Sci. U.S.A. 104, 1488–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li H., Xing X., Ding G., Li Q., Wang C., Xie L., Zeng R., Li Y. (2009) SysPTM: a systematic resource for proteomic research on post-translational modifications. Mol. Cell. Proteomics 8, 1839–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pan C., Gnad F., Olsen J. V., Mann M. (2008) Quantitative phosphoproteome analysis of a mouse liver cell line reveals specificity of phosphatase inhibitors. Proteomics 8, 4534–4546 [DOI] [PubMed] [Google Scholar]
- 49. Mohamed T. M., Oceandy D., Prehar S., Alatwi N., Hegab Z., Baudoin F. M., Pickard A., Zaki A. O., Nadif R., Cartwright E. J., Neyses L. (2009) Specific role of neuronal nitric-oxide synthase when tethered to the plasma membrane calcium pump in regulating the beta-adrenergic signal in the myocardium. J. Biol. Chem. 284, 12091–12098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liang X., Fonnum G., Hajivandi M., Stene T., Kjus N. H., Ragnhildstveit E., Amshey J. W., Predki P., Pope R. M. (2007) Quantitative comparison of IMAC and TiO2 surfaces used in the study of regulated, dynamic protein phosphorylation. J. Am. Soc. Mass Spectrom. 18, 1932–1944 [DOI] [PubMed] [Google Scholar]
- 51. Thingholm T. E., Jensen O. N., Larsen M. R. (2009) Analytical strategies for phosphoproteomics. Proteomics 9, 1451–1468 [DOI] [PubMed] [Google Scholar]
- 52. Syka J. E., Coon J. J., Schroeder M. J., Shabanowitz J., Hunt D. F. (2004) Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 101, 9528–9533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lu H., Zong C., Wang Y., Young G. W., Deng N., Souda P., Li X., Whitelegge J., Drews O., Yang P. Y., Ping P. (2008) Revealing the dynamics of the 20 S proteasome phosphoproteome: a combined CID and electron transfer dissociation approach. Mol. Cell. Proteomics 7, 2073–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]