Abstract
Lactobacillus rhamnosus GG (GG) is a widely used and intensively studied probiotic bacterium. Although the health benefits of strain GG are well documented, the systematic exploration of mechanisms by which this strain exerts probiotic effects in the host has only recently been initiated. The ability to survive the harsh conditions of the gastrointestinal tract, including gastric juice containing bile salts, is one of the vital characteristics that enables a probiotic bacterium to transiently colonize the host. Here we used gene expression profiling at the transcriptome and proteome levels to investigate the cellular response of strain GG toward bile under defined bioreactor conditions. The analyses revealed that in response to growth of strain GG in the presence of 0.2% ox gall the transcript levels of 316 genes changed significantly (p < 0.01, t test), and 42 proteins, including both intracellular and surface-exposed proteins (i.e. surfome), were differentially abundant (p < 0.01, t test in total proteome analysis; p < 0.05, t test in surfome analysis). Protein abundance changes correlated with transcriptome level changes for 14 of these proteins. The identified proteins suggest diverse and specific changes in general stress responses as well as in cell envelope-related functions, including in pathways affecting fatty acid composition, cell surface charge, and thickness of the exopolysaccharide layer. These changes are likely to strengthen the cell envelope against bile-induced stress and signal the GG cells of gut entrance. Notably, the surfome analyses demonstrated significant reduction in the abundance of a protein catalyzing the synthesis of exopolysaccharides, whereas a protein dedicated for active removal of bile compounds from the cells was up-regulated. These findings suggest a role for these proteins in facilitating the well founded interaction of strain GG with the host mucus in the presence of sublethal doses of bile. The significance of these findings in terms of the functionality of a probiotic bacterium is discussed.
The human gastrointestinal tract (GIT)1 is estimated to harbor several hundreds of bacterial species, some of which are natural inhabitants of the intestinal tract and some of which originate from food (1, 2). Certain bacterial strains, through their interactions with their hosts, are reported to benefit the health of the host. These health-promoting (i.e. probiotic) bacteria can, for example, stimulate the host immune system, protect the host from invading bacteria and viruses (3), and aid digestion (4). The in vivo responses after consumption of probiotic bacteria appear to be strongly affected by the state (e.g. growth phase) of the consumed probiotic preparation (5). One probiotic bacterium is the widely studied Lactobacillus rhamnosus GG (GG), which was originally isolated from human intestinal microbiota by Goldin and Gorbach in 1985 (6). The discovered health-promoting effects associated with the consumption of strain GG include reduced treatment days and lowered risk for acute diarrhea in children (7), reduced risk for atopic diseases in infants (8), relief for milk allergy/atopic dermatitis in infants (9–11), reduced risk for respiratory infections (12, 13), and reduced risk of occurrence of dental caries (14). Although the health benefits conferred by GG are well documented, the systematic exploration of the host interaction mechanism between this probiotic strain and human has only recently been initiated (15, 16). Di Caro et al. (16) pioneered the study of the effects of GG administration on human mRNA expression in small bowel mucosa and found in a limited number of subjects a complex genetic response, including up-regulation of genes involved in pathways such as the immune response, inflammation, and apoptosis. Kankainen et al. (15) compared the genome of GG with its close relative L. rhamnosus LC705, which differs from strain GG by its dairy origin and reduced binding to mucus, and demonstrated that the GG genome encodes a unique pilus structure involved in the adhesion of GG to human mucus.
Use of lactobacilli in dairy processes and as probiotics implies that these bacteria are exposed to several environmental stress conditions. Because it is essential to know which mechanisms permit survival and probiotic activity under given conditions, application of genome-scale analyses and functional genomics approaches is increasing in the research of Lactobacillus spp. (17–19). The ability to persist in the harsh conditions of the GIT is one of the vital characteristics that enables a probiotic bacterium to survive and transiently colonize the host during passage through the GIT. Exposure to bile is a serious challenge to the viability of probiotics because human liver has been shown to secrete as much as a liter of bile daily into the small intestine; the concentration of bile acids typically varies between 0.2 and 2% following food ingestion (20, 21). Bile is known to function as a biological detergent emulsifying and solubilizing lipids, thus playing an essential role in digestion of fat, whereas the detergent property of bile can also contribute to antimicrobial activity (21, 22). When challenged with bile, bacteria are known to modify their cell envelope properties such as cell membrane fatty acid composition, peptidoglycan composition, and membrane charge (23, 24). Bile stress can also cause deleterious effects, including protein misfolding and denaturation, DNA damage, secondary structure formation in RNA, and intracellular acidification (21, 23, 25, 26).
Probiotic bacterial species differ from each other in their resistances to bile salts, but within one species, the strain-specific variation in bile tolerance is remarkable (21). Thus, lactobacilli are also very heterogeneous in terms of their intrinsic resistance to bile salts, varying from highly sensitive to resistant strains (27–29). Accordingly, global proteome and/or transcriptome analyses to assess the effect of bile on different probiotic lactobacilli suggest that the strains of Lactobacillus species have evolved a complex network of global regulatory systems to cope with the toxic effects of bile (26, 30–34). A proteomics study on the Lactobacillus reuteri ATCC 23272 strain has revealed that proteins involved in carbohydrate metabolism, transcription-translation, nucleotide metabolism, amino acid biosynthesis, pH homeostasis, general stress responses, and oxidation-reduction reactions were differentially expressed after exposure to bile (31). In contrast, bile-responsive expression of genes involved in cell envelope stress, protein denaturation, and DNA damage has been demonstrated by a transcriptome level study conducted on another L. reuteri strain (26). In Lactobacillus acidophilus NCFM, bile exposure was found to result in up-regulation of genes involved in signal transduction, carbohydrate metabolism, transport, and oxidation-reduction reactions (32). The transcriptional profiling of Lactobacillus plantarum WCFS1 revealed several bile-responsive genes encoding proteins located in the cell envelope as well as proteins involved in tolerance against oxidative and acid stress (30). In Lactobacillus delbrueckii subsp. lactis 200 and its bile-resistant derivative, the abundance of proteins involved in energy metabolism, translation, stress response, lipid metabolism, and exopolysaccharide synthesis was shown to be affected by bile stress (33). A proteomics study on a bile-tolerant Lactobacillus casei strain revealed that functions involved in bile response included cell protection, modifications in cell membranes, and key components of central metabolism (34). A recent comparative transcriptome study indicates that in the human intestine probiotic L. plantarum strain 299v specifically modifies its metabolic capacity for carbohydrate acquisition and expression of exopolysaccharides and cell surface proteins (35). These findings indicate that bile shock and adaptation responses to GIT conditions are necessary to maintain bile tolerance in different Lactobacillus strains, but the bile resistance mechanisms are not well understood.
Functional genomics studies aiming to identify probiotic mechanisms exploited by GG have recently been initiated (15, 36). This strain has been shown to tolerate bile to some extent through experiments that demonstrated that it survives in MRS broth containing 0.3% ox gall for several hours, but it is not able to replicate under such conditions (29). However, the cellular and molecular responses toward bile have not yet been thoroughly explored in this bacterium. The present study represents an essential first step toward a global molecular characterization of the cellular response to bile exposure and provides information necessary for future examination of stress-linked gene regulatory networks in this probiotic bacterium. To our knowledge, this is the first quantitative study of bile-induced global mRNA and protein level expression changes in a probiotic bacterium growing under strictly controlled bioreactor conditions.
EXPERIMENTAL PROCEDURES
Growth Conditions and Bile Treatment
GG (ATCC 53103) was preserved in a laboratory culture collection as a glycerol stock at −70 °C and propagated at 37 °C in MRS broth (Labema). After 12 h of cultivation, these MRS cultures were used to inoculate (2% (v/v) inoculum) four Biostat Q fermentors (B. Braun Biotech International) containing 750 ml of MRS broth. The bioreactor cultures were grown at 37 °C and constantly stirred (150 rpm/min). pH was maintained at 6.0 by automatic titration with 5% (v/v) ammonia. Cells were grown to an A600 between 0.7 and 0.8, which represents midlogarithmic growth phase, and then challenged with bile by adding ox gall solution (B3883, Sigma-Aldrich) to a final concentration of 0.2%. Cell samples for transcriptomics were harvested right before (time point 0 min) and 10, 30, and 120 min after the addition of bile, whereas samples for proteomics were collected at two time points, 0 and 60 min. Cells from the 1.5–2-ml proteomic samples were harvested by centrifuging at +4 °C; washed twice with ice-cold 50 mm Tris-HCl, pH 8 (Sigma-Aldrich); and stored at −20 °C. For the surface-exposed proteome (i.e. surfome) analysis, cells withdrawn at time points 0 and 60 min were washed and used directly for CyDye labeling as detailed below (under “Protein Extraction and CyDye Labeling”).
Transcriptomics
Experimental Design, RNA Methods, cDNA Synthesis, and Labeling
The RNA samples from four independent biological replicates (i.e. from four cultivations) at each time point were hybridized to microarrays using the sample retrieved at time point 0 min as a reference sample. A total of 12 hybridizations were performed using a balanced dye swap design. Dyes were balanced between compared sample pairs and between biological replicates.
One to three milliliters of bacterial culture were mixed with 2–6 ml of RNAprotect Bacteria reagent (Qiagen) and handled according to the manufacturer's instructions. The cell pellets were stored at −70 °C for subsequent RNA extraction. Cells were lysed with 10 mg/ml lysozyme (Amresco), 3 mg/ml proteinase K (Sigma-Aldrich), and 100 units of mutanolysin (Sigma-Aldrich) at 37 °C for 30 min. The suspension was supplemented with 1 ml of preheated (65 °C) TRIzol reagent (Invitrogen) and vortexed for 3 min. After incubation at RT for 5 min, the cell lysate was homogenized in a MagNA Lyser instrument (Roche Applied Science) with <106-μm glass beads (Sigma-Aldrich) for four 30-s cycles at 6000 rpm. Between each cycle, the cells were chilled on ice for 1 min. Cell debris were removed by centrifugation (12,000 × g at 4 °C for 15 min), and the lysate was extracted with 200 μl of chloroform by vortexing for 15 s. After incubation at RT for 3 min, the phases were separated by centrifugation (12,000 × g at 4 °C for 15 min). The aqueous phase was mixed with 500 μl of 80% ethanol for total RNA purification with an RNeasy Mini kit (Qiagen). During RNA purification, DNA was removed using RNase-free DNase (Qiagen) as described in the manufacturer's manual. The concentration and purity of the RNA samples were determined using both the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc.) and denaturing agarose gel electrophoresis.
Five micrograms of RNA were reverse transcribed to cDNA with the SuperScript Indirect cDNA Labeling System (Invitrogen) according to the manufacturer's protocol except that 6 μg of random primers (Invitrogen, 48190-011) were used instead of anchored oligo(dT)20 primers and random hexamers. The cDNA was fluorescently labeled using Cy3 or Cy5 monoreactive dyes (Amersham Biosciences) and purified with a column included in the SuperScript Indirect cDNA Labeling System kit. Labeling efficiency was assessed with the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc.).
Array Design
Probes for 2820 target ORFs and 1072 intergenic regions of GG were designed with Agilent eArray software. No probes were designed for intergenic regions shorter than 150 bp. Regions longer than 300 bp were divided into shorter fragments (minimum 150 bp) resulting in 2182 intergenic sequence fragments in total. Of 2820 target ORFs, eArray was able to design unique probes for 2783 sequences for both sense and antisense orientations. Thirty-seven target sequences were too repetitive or similar with some other targets, so it was not possible to obtain unique probes. Probes for all 2182 intergenic target regions were successfully generated. The total number of probes for all designs was 20,964. Probe sequences were remapped to the updated version of the genome to obtain gene expression ratios from the most recent set of genes. In the remapping, 699 probe sequences matched several genomic regions, 183 probe sequences matched the borders of the coding regions, 13 did not have a perfect match against the genome, and 11,971 and 8098 probe sequences matched a unique intergenic or coding region, respectively.
Hybridization, Image Analysis, and Normalization
The labeled cDNA samples were hybridized to microarrays following Agilent's procedure titled “Two-Color Microarray-Based Gene Expression Analysis.” Microarrays were scanned at 5-μm resolution with a GenePix 4200 AL scanner (Axon Instruments). The fluorescence intensities were quantified and addressed to genomic ORFs with GenePix Pro 6.0 software (Axon Instruments/Molecular Devices Corp.). Microarray image analysis and feature detection were performed using GenePix Pro 6.0 software with default parameters, and results were further improved manually.
Data analysis was performed using Bioconductor for the R statistical software (37). Background correction and normalization were done using the limma package (38), and the statistical significance was assessed using CyberT (39). The data set contained 12 two-color microarrays with each condition measured four times and was analyzed as an entity. The foreground and background median intensity estimates were used, and the data were background-corrected using the normexp-function (with offset set to 50) (40), normalized within arrays using loess (100 iterations, suspicious spots and probe sequences deleted, matching multiple hits or borders of the coding regions down-weighted to 0, and intergenic probe sequences down-weighted to 0.1) (38), and normalized between arrays using quantile normalization (41). Expression ratios for genes were obtained by taking the averages of the log2-transformed expression ratios of probes describing the same gene and matching a single genetic coding region locus. The gene expression ratios were calculated for 2798 genes of the total 2944 (95% coverage) genes in the genome.
The statistical significance of the expression ratio of a gene between two conditions was analyzed using a paired t test method implemented in CyberT (Bayesian prior estimate of within-treatment variance was set to 5, and the window size was set to 101) (39). p values were Bonferroni adjusted by the number of performed t tests in total (8394). The analysis showed that 248, 133, and nine genes had significant differences in expression between the 0- and 10-min conditions, the 0- and 30-min conditions, and 0- and 120-min conditions, respectively, when using 0.01 as the threshold for statistical significance and when requiring at least 2-fold changes in expression ratio. The microarray data discussed in this publication have been deposited in the NCBI Gene Expression Omnibus (42) and are accessible through GEO Series accession number GSE22536 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE22536).
Proteomics
Protein Extraction and CyDye Labeling
Four biological replicate samples representing independent bioreactor cultures at time points 0 and 60 min (samples taken right before and 60 min after the bile challenge) were included in the total proteome (i) and surfome (ii) experiments.
(i) For total proteome analysis, cells were broken with glass beads, and the proteins were extracted as described previously (36). Proteins were purified using the 2-D Clean-Up kit (GE Healthcare) and solubilized in 10–20 μl of 7 m urea (Sigma-Aldrich), 2 m thiourea (Sigma-Aldrich), 4% CHAPS (Sigma-Aldrich), and 30 mm Tris (Bio-Rad). The protein concentration was determined using the 2-D Quant kit (GE Healthcare) according to the manufacturer's protocol. Prior to CyDye labeling, the pH of each protein sample was adjusted to 8.5 by the addition of 2 m Tris. The samples were then labeled using Cy2, Cy3, or Cy5 dye (CyDye DIGE Fluor minimal dyes, GE Healthcare) according to the EttanTM two-dimensional difference gel electrophoresis (DIGE) protocol. Briefly, 32 μg of protein from each of the control and bile-treated cells were labeled with 256 pmol of the Cy3 and Cy5 dyes. As an internal standard, aliquots from each sample were combined and labeled with Cy2 dye. To exclude dye-specific effects, Cy3 and Cy5 were used interchangeably according to a dye-swapping approach (supplemental Table S1). The labeling mixtures were incubated on ice in the dark for 30 min, and the reactions were quenched with 1 mm lysine (Sigma-Aldrich) followed by incubation on ice for 10 min. The labeled samples were pooled and separated by two-dimensional gel electrophoresis as detailed below.
(ii) For surfome analysis (i.e. CyDye labeling of intact cells, which covers the external exposed cell envelope proteins and possibly also includes proteins in the process of secretion), the washed cell samples (time points 0 and 60 min) were submitted for direct labeling with CyDyes as follows. The washed cell pellets were resuspended in 200 μl of a buffer containing 50 mm Tris (Sigma-Aldrich) and 1 m urea (Sigma-Aldrich), pH 8.5. The protein samples were labeled with CyDyes as described above with some modifications. According to the experimental design outlined in supplemental Table S1, each sample containing ∼109 cells was mixed with 200 pmol of Cy3 or Cy5 dye, and the internal standard sample containing aliquots from each sample was labeled with Cy2 dye. The reactions were incubated for 20 min on ice in the dark after which they were quenched by the addition of 20 μl of 10 mm lysine (Sigma-Aldrich) as described above. The labeled cells were washed twice with ice-cold 50 mm Tris-HCl, pH 8 and were disrupted with glass beads to extract proteins for two-dimensional gel electrophoresis as described above. To investigate whether possible cell lysis had occurred during the CyDye labeling process, colony-forming units, which measure viable bacterial numbers, were determined by plating GG cells incubated with CyDye labeling buffer (50 mm Tris, 1 m urea, pH 8.5) for 0, 10, and 20 min on MRS agar.
Two-dimensional Gel Electrophoresis and DeCyder Analyses
The labeled proteins were separated by IEF. IPG strips (24 cm, pH 3–10, nonlinear, Bio-Rad) were rehydrated in 500 μl of buffer, which contained 7 m urea, 2 m thiourea, 4% CHAPS, 50 mm DTT, 2 mm tributylphosphine, and 1% Bio-Lyte pH 3–10 (Bio-Rad), overnight at 20 °C using a Protean IEF Cell (Bio-Rad). Samples containing, in total, 96 μg (for total proteome analysis) or ∼105 μg (for surfome analysis) of protein in 50 mm DTT, 4 mm tributylphosphine, and 1% Bio-Lyte pH 3–10 were applied to the IPG strips via cup-loading near the acidic end of the strips according to the experimental design outlined in supplemental Table S1. IEF was performed using a Protean IEF Cell at 20 °C as follows: 15 min at 250 V, then linear ramping to 10,000 V for 40,000 V-h, and 40,000 V-h at 10,000 V (using a limit of 50 μA/strip). After IEF, the strips were equilibrated in a buffer containing 50 mm Tris-HCl, pH 6.8, 6 m urea, 2% SDS, 20% glycerol, and alternatively either 2% DTT (buffer A) or 2.5% iodoacetamide (buffer B), first in buffer A for 25 min and then in buffer B for 25 min.
The strips were loaded on 12% acrylamide gels that were subjected to electrophoresis in an Ettan DALTsix Electrophoresis Unit (GE Healthcare) at 80 V for 15 min and then 400 V for ∼3 h. The upper buffer was 2× TGS (50 mm Tris, 384 mm glycine, 0.2% (w/v) SDS; Bio-Rad), and the lower buffer was 1× TGS (25 mm Tris, 192 mm glycine, 0.1% (w/v) SDS). The gels were scanned between low fluorescence glass plates using an FLA-5100 laser scanner (Fujifilm) at wavelengths of 473 (for Cy2), 532 (Cy3), and 635 nm (Cy5) using voltages of 420, 410, and 400 V accordingly. All gels were scanned at 100-μm resolution. The gel images were cropped to identical size by removing areas extraneous to the protein spots with ImageQuant TL 7.0 software (GE Healthcare). After scanning, the gels were fixed in 30% ethanol and 0.5% acetic acid for a minimum of 60 min and then silver-stained (43).
Image and statistical analyses for the cropped DIGE gels were performed using DeCyder 2D 6.5 software (GE Healthcare). With the use of a batch processor function, the gels were first automatically analyzed in a differential in-gel analysis module, which normalized the Cy2, Cy3, and Cy5 image from each gel. Spot boundaries were detected, and spot volumes (protein abundances) were calculated. Then the spot volumes of Cy3 and Cy5 samples were compared with the spot volumes of the Cy2 sample (internal standard) to generate standard spot volumes, thereby correcting intergel variations. In the biological variation analysis module, the Cy2 images of four replicate gels were matched, and the standard spot volume ratios between all four gels were compared. Protein spots exhibiting a minimum of 1.5-fold (CyDye-labeled total proteome, t test p < 0.01) or 1.2-fold (CyDye-labeled intact cells, t test p < 0.05) difference in average spot volume ratios (average ratio ≥1.5/1.2 or ≤−1.5/−1.2) between the control and bile-challenged samples in at least three of four separate biological replicates were selected for identification.
To localize the selected protein spots of the surfome DIGE analysis on the silver-stained gels, an additional DIGE gel containing a Cy3-labeled surfome sample and a Cy5-labeled total proteome sample was included in the DeCyder analysis. The surfome two-dimensional map of this additional gel was matched with the surfome maps of the analytical surfome gels, and the total proteome map of the additional gel (containing about double the amount of protein spots on the surfome maps and mostly corresponding to the spot abundance on the silver-stained gels) was then used to localize the selected spots on the silver-stained gels.
Protein Identification
MS
MS-compatible silver staining (43) was performed to visualize the protein spots for identification. Protein spots of interest were in-gel digested with trypsin, and the peptides were recovered as described previously (36). The resulting peptides were analyzed by peptide mass fingerprinting (PMF) or by fragment ion analysis with LC-MS/MS. For the PMF, the mass spectra were acquired using an Ultraflex TOF/TOF instrument (Bruker Daltonics, Bremen, Germany) in positive ion reflector mode, and the instrument was externally calibrated using a standard peptide mixture from Bruker (P/N 206195, Bruker Daltonics). LC-MS/MS analysis for the tryptic peptides was performed using an Ultimate 3000 nano-LC system (Dionex, Sunnyvale, CA) and QSTAR Elite hybrid quadrupole TOF mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA) with nano-ESI ionization. The samples were first concentrated and desalted on a C18 trap column (10 mm × 150 μm, 3 μm, 120 Å; PROTECOLTM, SGE Analytical Science, Griesheim, Germany) followed by peptide separation on a PepMap100 C18 analytical column (15 cm × 75 μm, 5 μm, 100 Å; LC Packings, Sunnyvale, CA) at 200 nl/min. The separation gradient consisted of 0–50% B in 20 min, 50% B for 3 min, 50–100% B in 2 min, and 100% B for 3 min (buffer A, 0.1% formic acid; buffer B, 0.08% formic acid in 80% acetonitrile). MS data were acquired using Analyst QS 2.0 software. The information-dependent acquisition method consisted of a 0.5-s TOF-MS survey scan of m/z 400–1400. From every survey scan, the two most abundant ions with charge states 2+ to 4+ were selected for product ion scans. Once an ion was selected for MS/MS fragmentation, it was put on an exclusion list for 60 s.
Protein Identification
The PMF spectra were processed with FlexAnalysis version 3.0. Base-line subtraction for the raw spectra was performed with the algorithm Median, and peak detection was done using the following parameters: algorithm, Snap; signal to noise threshold, >7; relative intensity threshold, 3%; minimum intensity threshold, 50; maximal number of peaks, 100; quality factor threshold, 50; and Snap average composition averagine. The original MALDI-TOF spectra and peak lists are provided in supplemental Table S2 and supplemental Data S1. The PMF and LC-MS/MS data were searched with the local Mascot version 2.2 (Matrix Science, London, UK) against the in-house database of the published ORF set of GG, which contains 2944 protein entries (15), using the Biotools 3.0 (Bruker Daltonics) and ProteinPilot 2.0.1 (Applied Biosystems) interface, respectively. The search criteria for both Mascot searches were as follows: trypsin digestion with one missed cleavage allowed, carbamidomethyl modification of cysteine as a fixed modification, and oxidation of methionine as a variable modification. For the PMF spectra, the maximum peptide mass tolerance was ±80 ppm. For the LC-MS/MS spectra, both the maximum precursor ion mass tolerance and MS/MS fragment ion mass tolerance were 0.2 Da, and a peptide charge states of 1+, 2+, and 3+ were used. A successful identification was reported when a significant match (p < 0.05) was obtained. In addition, to consider the LC-MS/MS identification reliable, a minimum of two peptides with an ion score of at least 40 were required.
RESULTS AND DISCUSSION
The goal of the study described here was to obtain global insight into temporal alterations in mRNA expression and protein production in L. rhamnosus GG that occur in response to bile and thus to gain an understanding of the potential molecular mechanisms enabling survival of this microbe in the GIT. Stress response studies, including gene expression analyses, are commonly carried out from cell samples grown under not clearly defined conditions in flask cultures where the effect of different responses are hard to distinguish from each other. Therefore, to gain reliable data from such studies, targeted perturbation is essential. One typical feature of lactic acid bacteria, including the GG strain, is lactic acid production, resulting in a decrease in pH of the growth medium. Here the homogeneous bacterial samples before and after applying bile stress were withdrawn from pH-controlled bioreactor cultivations for the transcriptome and proteome analyses (Fig. 1 and supplemental Fig. S1). Strain GG was grown as four biological replicates in bioreactors where the pH of the cell culture media was maintained at 6.0, which approximately corresponds to the pH in duodenum (44). At logarithmic growth phase, all cultures were subjected to 0.2% ox gall, which is a commonly used model compound for bile stress conditions. The concentration of 0.2% was selected because it is estimated to correspond to the physiological bile concentration in the human small intestine (20). Growth experiments with 0.1–0.3% ox gall supplementation revealed that a 0.2% concentration reduced the growth rate of strain GG to ∼60% of non-stressed rate (data not shown). Transcriptomics and proteomics analyses were performed with cell samples collected simultaneously from the same bioreactors, giving a solid foundation for data comparison.
Fig. 1.
Work flow of proteome and transcriptome analyses of GG samples. 2-DE, two-dimensional gel electrophoresis.
The effect of bile shock on the transcriptome of GG was examined using a whole-genome DNA microarray. Alterations in the gene expression at the mRNA level were studied by comparing bile-challenged samples withdrawn at three time points (10, 30, and 120 min) after bile addition with a reference sample taken prior to the bile addition. Overall, differential transcription (≥2-fold up- or down-regulation, p ≤ 0.01) of 316 genes in response to bile was observed: 248 genes at 10 min (140 up- and 108 down-regulated genes), 133 genes at 30 min (73 up- and 60 down-regulated), and nine genes at 120 min (7 up- and 2 down-regulated) (Fig. 2A and supplemental Table S3). The transient nature of the bile stress response of GG strain was evident, and only six genes (5 up- and one down-regulated) were found to be differentially transcribed at all three time points examined.
Fig. 2.
Venn diagrams showing numbers of differentially expressed genes in response to bile stress. A, the number of differentially transcribed genes 10, 30, and 120 min after bile exposure. B, comparison of the number of differentially expressed genes at the transcriptome and proteome levels. Transcriptome results include the changes observed 10, 30, and 120 min after bile addition, and proteome results include changes in the total proteome and in the surfome 60 min after bile exposure.
The bile-specific changes at the total proteome and at the cell surface-exposed proteome (i.e. surfome) levels were monitored using DIGE. The DIGE methodology relies on the labeling of protein samples with three spectrally resolvable fluorescent CyDyes (Cy5/Cy3 and Cy2), which allow multiplexing of samples in the same two-dimensional gel (45). The proteome level bile response in GG were studied by comparing proteins from cell samples withdrawn immediately before and 60 min after the bile exposure. Approximately 800 separate protein spots were detected on the gels containing the labeled protein extracts (in the total proteome DIGE experiment), and ≥1.5-fold statistically significant (p < 0.01) changes in abundance of 35 protein spots were detected. Of these spots, 31 representing 23 different gene products could successfully be identified (Fig. 3A and Table I). The abundance of 14 different proteins was increased under bile stress, and the abundance of eight proteins was decreased. In addition, there was one protein for which the abundance was increased in one protein spot and decreased in another protein spot.
Fig. 3.
Proteome analysis of GG proteins before and after applying bile stress. A, representative overlay image of a two-dimensional DIGE gel containing proteins extracted from GG right before and 60 min after the addition of bile. The total amount of protein used for CyDye labeling was 96 μg. Protein spots appearing in red were more abundant 60 min after addition of bile, and the abundance of protein spots appearing in green was decreased after bile addition. Protein spots appearing in yellow showed no differences in abundance between the two time points. The numbered protein spots (1–21) cut from two-dimensional gels poststained with silver and identified by MS or MS/MS are listed in Table I. B, a representative overlay image of a two-dimensional DIGE gel containing protein samples of GG cells where the surface-exposed proteome was labeled with CyDyes before and 60 min after bile addition. The total amount of protein per gel was ∼105 μg. Protein spots appearing in red were more abundant in the cell surface-exposed proteome 60 min after bile addition, and proteins spots more abundant before bile addition appear in green. Protein spots appearing in yellow showed no differences in abundance between the two time points. The numbered protein spots (22–43) were identified as above and are listed in Table II.
Table I. Identified proteins from L. rhamnosus GG that were differentially abundant in response to bile stress in total proteome DIGE experiment and comparison of protein abundances with corresponding transcript levels.
| Spot no. | -Fold change (after/before bile) |
Theoretical molecular mass (kDa)/pI | Identif. typeb | Mowse score | Seq. cov.c | No. of peptides matchedd | Locus tag | Protein |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transcriptomic |
Proteomic,a 60 min | |||||||||||
| 10 min | 30 min | 120 min | Name | Function | ||||||||
| % | ||||||||||||
| 1 | −37.0a | −1.8 | 1.2 | −1.6 | 42.6/7.7 | MS/MS | 649 | 19 | 5 | LGG_00031 | P40 | Surface antigen |
| 2e | −1.2 | −1.2 | 1.1 | 2.0 | 23.5/5.3 | MS/MS | 724 | 27 | 3 | LGG_00238 | Pcp | Pyrrolidone-carboxylate peptidase |
| 64.9a | 6.8 | 2.8 | 2.0 | 26.1/5.3 | MS/MS | 419 | 35 | 2 | LGG_02913 | NagB | Glucosamine-6-phosphate deaminase/isomerase | |
| 3 | 2.5a | 1.5 | −1.1 | 1.7 | 27.7/6.0 | MS/MS | 223 | 7 | 3 | LGG_00534 | Glutamine amidotransferase | |
| 4e | 28.1a | 12.1 | 3.0 | 1.7 | 54.3/4.7 | MS | 73 | 21 | 12/25 | LGG_00914 | Conserved protein | |
| −1.0 | −1.0 | 1.0 | 1.7 | 63.3/4.8 | MS | 73 | 24 | 14/25 | LGG_01820 | Phosphoenolpyruvate-protein phosphotransferase | ||
| 5e | 1.2 | 1.7 | 1.1 | 1.5 | 67.3/4.8 | MS | 76 | 21 | 12/29 | LGG_00984 | PepF | Oligoendopeptidase F |
| 2.3 | 2.3 | 1.2 | 1.5 | 57.4/4.7 | MS | 114 | 41 | 16/29 | LGG_02239 | GroEL | 60-kDa chaperonin GroEL | |
| 6 | 11.3a | 3.7 | 1.6 | 1.8 | 44.1/5.0 | MS/MS | 2288 | 64 | 21 | LGG_01295 | TelA | Tellurite resistance protein |
| 7 | 3.5a | 2.7a | 1.1 | 1.5 | 96.3/5.2 | MS | 85 | 13 | 8/7 | LGG_01367 | ClpB | ATP-dependent chaperone ClpB |
| 8 | 1.2 | 1.8 | 1.6 | −1.9 | 71.7/5.3 | MS/MS | 112 | 7 | 2 | LGG_01478 | PepO | Endopeptidase O |
| 9 | −1.3 | 1.1 | 1.7 | −1.7 | 66.5/9.9 | MS/MS | 423 | 22 | 8 | LGG_01652 | OppA | ABC transporter, oligopeptide-binding protein |
| 10e | −1.0 | −1.0 | 1.0 | 1.6 | 63.3/4.8 | MS | 57 | 18 | 8/14 | LGG_01820 | Phosphoenolpyruvate-protein phosphotransferase | |
| 2.3 | 2.3 | 1.2 | 1.6 | 57.4/4.7 | MS | 48 | 19 | 6/14 | LGG_02239 | GroEL | 60-kDa chaperonin GroEL | |
| 11a | 1.1 | 1.1 | 1.2 | 1.8 | 9.3/4.6 | MS/MS | 106 | 13 | 2 | LGG_01821 | PtsH | Phosphocarrier protein HPr |
| 11b | 1.1 | 1.1 | 1.2 | −1.7 | 9.3/4.6 | MS/MS | 100 | 13 | 2 | LGG_01821 | PtsH | Phosphocarrier protein HPr |
| 12a | 7.0a | 4.8 | 1.2 | 2.7 | 81.6/5.8 | MS | 185 | 30 | 18/13 | LGG_01823 | ClpE | ATP-dependent Clp protease ATP-binding subunit |
| 12b | 7.0a | 4.8 | 1.2 | 2.4 | 81.6/5.8 | MS | 115 | 21 | 13/15 | LGG_01823 | ClpE | ATP-dependent Clp protease ATP-binding subunit |
| 12c | 7.0a | 4.8 | 1.2 | 2.4 | 81.6/5.8 | MS | 183 | 28 | 18/9 | LGG_01823 | ClpE | ATP-dependent Clp protease ATP-binding subunit |
| 12d | 7.0a | 4.8 | 1.2 | 2.2 | 81.6/5.8 | MS | 90 | 22 | 11/23 | LGG_01823 | ClpE | ATP-dependent Clp protease ATP-binding subunit |
| 12e | 7.0a | 4.8 | 1.2 | 2.0 | 81.6/5.8 | MS | 57 | 11 | 6/10 | LGG_01823 | ClpE | ATP-dependent Clp protease ATP-binding subunit |
| 12f | 7.0a | 4.8 | 1.2 | 1.5 | 81.6/5.8 | MS | 50 | 14 | 8/24 | LGG_01823 | ClpE | ATP-dependent Clp protease ATP-binding subunit |
| 13e | 19.7a | 10.0a | 9.1a | 2.2 | 33.9/6.4 | MS/MS | 363 | 18 | 3 | LGG_01930 | EcsA | ABC transporter, ATPase component |
| −2.5 | −1.4 | −1.6 | 2.2 | 30.4/11.0 | MS/MS | 295 | 24 | 5 | LGG_02484 | RplB | Large subunit/50 S ribosomal protein L2P | |
| 14 | −7.9a | −4.6a | −1.6 | −1.5 | 43.2/6.9 | MS | 268 | 59 | 22/14 | LGG_02050 | Glf | UDP-galactopyranose mutase |
| 15 | −6.2a | −2.7 | −1.3 | −1.5 | 27.4/9.5 | MS/MS | 1171 | 38 | 8 | LGG_02052 | Wze | Tyrosine-protein kinase |
| 16 | 1.2 | 1.4 | 1.3 | −1.6 | 26.0/5.2 | MS | 133 | 43 | 10/12 | LGG_02138 | GpmA | Phosphoglycerate mutase |
| 17 | 1.3 | 1.9 | −1.5 | 1.5 | 14.0/5.9 | MS | 47 | 25 | 3/14 | LGG_02218 | Transcriptional regulator, Xre family | |
| 18 | 2.3 | 2.3 | 1.2 | 1.8 | 57.4/4.7 | MS | 248 | 58 | 26/17 | LGG_02239 | GroEL | 60-kDa chaperonin GroEL |
| 19 | 2.3 | 2.2 | 1.2 | 1.6 | 10.0/4.7 | MS | 136 | 80 | 8/12 | LGG_02240 | GroES | 10-kDa chaperonin GroES |
| 20a | −1.3 | −2.0 | −1.0 | −2.4 | 82.5/6.5 | MS/MS | 157 | 9 | 4 | LGG_02296 | RtpR | Ribonucleoside-triphosphate reductase |
| 20b | −1.3 | −2.0 | −1.0 | −2.4 | 82.5/6.5 | MS/MS | 173 | 11 | 4 | LGG_02296 | RtpR | Ribonucleoside-triphosphate reductase |
| 20c | −1.3 | −2.0 | −1.0 | −1.9 | 82.5/6.5 | MS/MS | 157 | 7 | 4 | LGG_02296 | RtpR | Ribonucleoside-triphosphate reductase |
| 20d | −1.3 | −2.0 | −1.0 | −1.8 | 82.5/6.5 | MS/MS | 139 | 10 | 3 | LGG_02296 | RtpR | Ribonucleoside-triphosphate reductase |
| 21a | −5.0 | −4.1a | −3.0 | −1.9 | 59.9/6.6 | MS/MS | 522 | 18 | 8 | LGG_02546 | PyrG | CTP synthase |
| 21b | −5.0 | −4.1a | −3.0 | −1.8 | 59.9/6.6 | MS | 90 | 19 | 9/12 | LGG_02546 | PyrG | CTP synthase |
a t test p < 0.01.
b Identifation type: MS, MALDI-MS; MS/MS, LC-MS/MS.
c Sequence coverage.
d MALDI identifications: matched/unmatched peptides.
e Two proteins were identified from these spots.
Several previous studies indicate that bile especially affects the bacterial cell surface (24, 30, 46, 47). Thus, we studied the effect of bile stress on the surface-exposed proteome (i.e. surfome) of GG using DIGE labeling of intact GG cells (whole-cell DIGE labeling). This methodology has been used previously for labeling of bacterial surface-exposed proteins in bacterial species such as Porphyromonas gingivalis (48) and Legionella pneumophila (49). Possible cell lysis occurring during CyDye labeling was tested by plating GG cells incubated in the DIGE labeling buffer onto MRS agar. The plating assay revealed that the colony-forming unit counts of GG did not decrease during a 10-min treatment in DIGE buffer (data not shown). After a 20-min incubation in DIGE buffer, the colony-forming unit counts decreased about 20%, but because most of the labeling occurs during the first 10 min, labeling of intracellular proteins from lysed cells was probably not extensive. Also, the two-dimensional map of the surface-exposed proteome differed clearly from the total proteome map. On the DIGE gels representing the labeled surfome proteins, around 400 separate spots were detected. In the surfome analysis, at the minimum, 1.2-fold statistically significant (p < 0.05) changes in abundance were detected in 45 separate protein spots of which 29 could be identified. They represented 25 different gene products of which 17 different proteins were more abundant and eight proteins were less abundant after bile stress (Fig. 3B and Table II). Six of these proteins were differentially produced also at the total proteome level (five proteins with increased abundance and one with decreased abundance).
Table II. Identified proteins from L. rhamnosus GG that were differentially abundant in response to bile stress in surfome DIGE experiment and comparison of protein abundances with corresponding transcript levels.
| Spot no. | -Fold change (after/before bile) |
Theoretical molecular mass (kDa)/pI | Identif. typeb | Mowse score | Seq. cov.c | No. of peptides matchedd | Locus tag | Protein |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transcriptomic |
Proteomic,a 60 min | |||||||||||
| 10 min | 30 min | 120 min | Name | Function | ||||||||
| % | ||||||||||||
| 22 | 1.5 | 1.9 | −1.1 | 1.4 | 25.5/4.6 | MS | 167 | 52 | 14/24 | LGG_00252 | VanR | Two-component response regulator |
| 23 | 1.7 | 1.2 | 1.1 | 1.4 | 24.1/4.8 | MS | 94 | 45 | 8/25 | LGG_00740 | Gph | Hydrolase, haloacid dehalogenase-like family |
| 24a | 28.1e | 12.1 | 3.0 | 5.6 | 54.3/4.7 | MS | 62 | 14 | 7/14 | LGG_00914 | Conserved protein | |
| 24b | 28.1e | 12.1 | 3.0 | 5.5 | 54.3/4.7 | MS | 129 | 23 | 12/17 | LGG_00914 | Conserved protein | |
| 24cf | 28.1e | 12.1 | 3.0 | 5.0 | 54.3/4.7 | MS | 112 | 27 | 13/15 | LGG_00914 | Conserved protein | |
| −1.0 | −1.0 | 1.0 | 5 | 63.3/4.8 | MS | 65 | 20 | 11/15 | LGG_01820 | Phosphoenolpyruvate-protein phosphotransferase | ||
| 25 | −1.1 | −1.4 | 1.1 | 1.7 | 42.2/5.7 | MS | 126 | 46 | 12/21 | LGG_00934 | Pgk | Phosphoglycerate kinase |
| 26 | −1.1 | −1.4 | 1.0 | −1.4 | 47.1/4.4 | MS | 180 | 42 | 16/22 | LGG_00936 | Eno | Enolase |
| 27 | 1.4 | −1.2 | −1.2 | −1.3 | 34.0/6.2 | MS | 136 | 38 | 10/14 | LGG_01062 | GalU | UTP-glucose-1-phosphate uridylyltransferase |
| 28af | 1.1 | −1.1 | −1.1 | 1.6 | 55.2/5.0 | MS/MS | 215 | 16 | 4 | LGG_01181 | AtpA | F0F1-ATP synthase subunit α |
| 4.2e | 1.8 | 1.1 | 1.6 | 52.2/4.9 | MS/MS | 221 | 14 | 4 | LGG_01416 | HslU | Heat shock protein HslU | |
| 28bf | 1.1 | −1.1 | −1.1 | 1.5 | 55.2/5.0 | MS | 132 | 31 | 19/29 | LGG_01181 | AtpA | F0F1-ATP synthase subunit α |
| 4.2e | 1.8 | 1.1 | 1.5 | 52.2/4.9 | MS | 135 | 41 | 21/29 | LGG_01416 | HslU | Heat shock protein HslU | |
| 29 | 11.3e | 3.7 | 1.6 | 1.6 | 44.1/5.0 | MS | 98 | 25 | 8/10 | LGG_01295 | TelA | Tellurite resistance protein |
| 30 | −1.1 | −1.8 | −1.7 | −1.6 | 49.1/5.7 | MS | 51 | 10 | 5/8 | LGG_01323 | PdhD | Pyruvate dehydrogenase/dihydrolipoamide dehydrogenase |
| 31 | 3.5e | 2.7e | 1.1 | 1.3 | 96.3/5.2 | MS | 85 | 13 | 8/7 | LGG_01367 | ClpB | ATP-dependent chaperone ClpB |
| 32 | 1.3 | 1.2 | 1.1 | 1.4 | 85.2/5.1 | MS | 117 | 17 | 17/19 | LGG_01421 | PflB | Formate acetyltransferase |
| 33 | 1.1 | −1.2 | 1.1 | 2.2 | 23.1/6.0 | MS | 104 | 39 | 10/35 | LGG_01433 | Nitroreductase | |
| 34 | 2.0 | 1.8 | −1.2 | 3.9 | 67.2/4.6 | MS | 54 | 10 | 7/13 | LGG_01604 | DnaK | Chaperone protein DnaK |
| 35 | −1.2 | −1.1 | −1.0 | 1.4 | 22.7/4.7 | MS | 57 | 22 | 4/10 | LGG_01665 | Rpe | Ribulose-phosphate 3-epimerase |
| 36 | −5.3e | −2.5e | −2.1 | −1.4 | 62.5/6.1 | MS | 101 | 15 | 7/1 | LGG_01786 | ArgS | Arginyl-tRNA synthetase |
| 37a | 7.0e | 4.8 | 1.2 | 2.0 | 81.6/5.8 | MS | 66 | 10 | 8/12 | LGG_01823 | ClpE | ATP-dependent Clp protease ATP-binding subunit |
| 37b | 7.0e | 4.8 | 1.2 | 1.9 | 81.6/5.8 | MS/MS | 103 | 7 | 7 | LGG_01823 | ClpE | ATP-dependent Clp protease ATP-binding subunit |
| 37c | 7.0e | 4.8 | 1.2 | 1.5 | 81.6/5.8 | MS/MS | 274 | 10 | 4 | LGG_01823 | ClpE | ATP-dependent Clp protease ATP-binding subunit |
| 38a | 2.9 | 2.6 | −1.2 | 1.8 | 74.4/5.6 | MS | 113 | 17 | 12/13 | LGG_01864 | YuxL | Dipeptidyl aminopeptidase/acylaminoacyl-peptidase |
| 38bf | 2.9 | 2.6 | −1.2 | 1.6 | 74.4/5.6 | MS | 157 | 30 | 18/9 | LGG_01864 | YuxL | Dipeptidyl aminopeptidase/acylaminoacyl-peptidase |
| 1.1 | 1.1 | 1.0 | 1.6 | 71.6/5.5 | MS | 150 | 29 | 18/9 | LGG_02234 | MutL | DNA mismatch repair protein MutL | |
| 39 | −8.8e | −11.2e | −2.3 | −1.4 | 37.8/9.4 | MS | 92 | 21 | 9/12 | LGG_02045 | WelG | Glycosyltransferase, galactofuranosyltransferase |
| 40a | −1.3 | −2.0 | −1.0 | −2.1 | 82.5/6.5 | MS | 59 | 11 | 8/17 | LGG_02296 | RtpR | Ribonucleoside-triphosphate reductase |
| 40b | −1.3 | −2.0 | −1.0 | −2.0 | 82.5/6.5 | MS/MS | 167 | 8 | 3 | LGG_02296 | RtpR | Ribonucleoside-triphosphate reductase |
| 41 | −2.0 | −1.7 | −1.7 | 3.2 | 17.5/7.8 | MS | 178 | 49 | 10/2 | LGG_02470 | RpsE | Small subunit/30 S ribosomal protein S5P |
| 42 | −1.1 | −1.1 | 1.1 | −1.2 | 35.5/5.1 | MS | 120 | 28 | 11/13 | LGG_02523 | Ldh | l-Lactate dehydrogenase |
| 43 | −1.6 | −2.4 | 1.4 | −2.5 | 35.4/5.8 | MS | 127 | 56 | 12/45 | LGG_02838 | ManA | PTS, mannose-specific IIAB component |
a t test p < 0.05.
b Identification type: MS, MALDI-MS; MS/MS, LC-MS/MS.
c Sequence coverage.
d MALDI identifications: matched/unmatched peptides.
e t test p < 0.01.
f Two proteins were identified from these spots.
In conclusion, the microarray analyses coupled with proteomics revealed that the transcription of 316 genes and the production of 42 proteins in GG cells were altered when cells were challenged by 0.2% ox gall. Of these changes, a total of 14 could be confirmed by gene expression analysis both at the transcriptome and protein levels (Fig. 2B and Tables I and II). These identifications included pathways modulating cell envelope properties, regulatory systems, active removal of bile compounds from the cell, common stress responses, and central metabolic processes as detailed below. In the following sections, we discuss the bile-induced changes in the expression of 101 GG genes, including a total of 33 differentially produced proteins and 80 differentially transcribed genes.
GG Alters Cell Surface Properties and Expresses Multiple ABC-type Multidrug Transporters in Response to Bile
Exopolysaccharide (EPS)
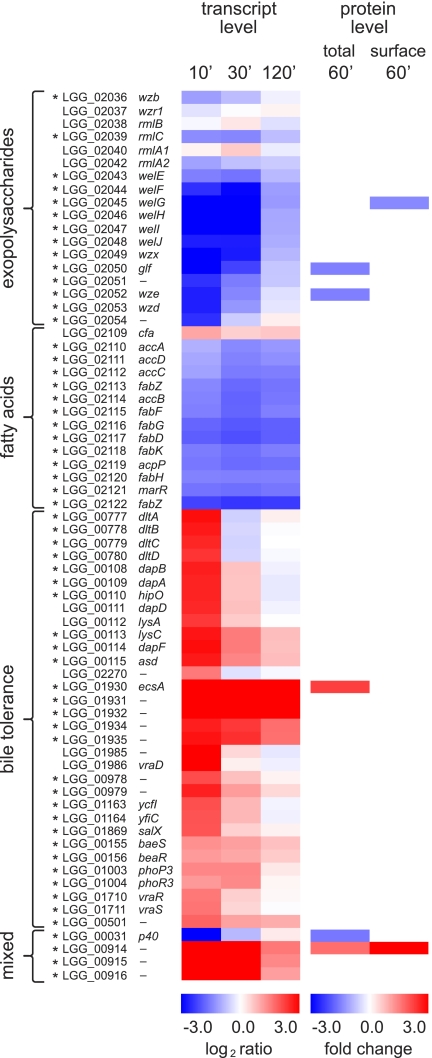
The bile shock was found to affect several cell envelope-related functions in GG (Fig. 4). A clear down-regulation was observed in EPS biosynthesis both at the transcriptome and proteome levels. At the transcriptome level, nearly the entire exopolysaccharide biosynthesis gene cluster LGG_02036–02053 was down-regulated, most remarkably at time points 10 and 30 min. A similar down-regulation was observed at the proteomic level: the relative abundances of Glf (LGG_02050) and Wze (LGG_02052) were found to be decreased 60 min after bile exposure in the total proteome analysis. In addition, surfome analysis revealed a decreased amount of WelG (LGG_02045) in response to bile.
Fig. 4.
Expression changes in selected genes coding for cell envelope- and bile tolerance-related functions. Changes at the transcript level are represented as log2 intensity ratio values 10, 30, and 120 min (′) after bile addition compared with the time point preceding the bile addition, and statistically significant changes (p < 0.01 at any time point) are marked with asterisks (*). Protein abundance changes in the total proteome and in the surface-exposed proteome are represented as -fold changes (standardized abundance 60 min after bile addition/right before bile addition). All the represented changes in protein abundances are statistically significant (p < 0.01 for total proteome; p < 0.05 for surfome).
Repression of EPS biosynthesis gene expression after bile exposure has been detected previously in microarray analyses in L. acidophilus (32). The observed down-regulation of EPS genes might thus be a common bile stress response in lactobacilli. Lebeer et al. (50) reported that although the inactivation of the EPS biosynthesis gene, welG, results in dramatically decreased exopolysaccharide production by GG strain, the mutant shows increased adhesion to Caco-2 cells and enhanced biofilm formation compared with wild type. Furthermore, it has been revealed that bile induces biofilm formation in GG (51). Weak adhesion in the presence of a thick EPS layer (especially long, galactose-rich EPS molecules) was speculated to result from shielding of adhesins on the cell surface (50). These previous results (50, 51) and the gene expression analyses presented here suggest a model where, in the absence of bile, GG cells are shielded by EPS, and this may provide protection under the harsh conditions of the stomach. The presence of bile could function as a signal of gut entrance, resulting in removal of EPS and concomitantly increased adherence of GG cells to gut.
Fatty Acids
Another cell envelope-related function that is likely to be affected by bile is fatty acid biosynthesis (Fig. 4). Long-chain saturated fatty acid biosynthetic genes were among the most highly repressed at the mRNA level in response to bile, referring to decreased production of saturated fatty acids. In L. reuteri, addition of bile in growth medium has been shown to decrease the saturated/unsaturated fatty acid ratio (52). In microarray analysis of Enterococcus faecalis, two gene clusters involved in fatty acid biosynthesis were repressed in bile-exposed cells (53). Decreased abundance of single proteins involved in fatty acid biosynthesis has been shown in proteomics studies with L. delbrueckii subsp. lactis (33) and L. casei (34). In contrast, there was an indication of increased transcription of the gene encoding cyclopropane-fatty-acyl-phospholipid synthase (cfa, LGG_02109), which was 1.8–2.3-fold up-regulated in biological replicate samples. However, the bile-induced up-regulation of cfa was statistically insignificant (p > 0.01). The increase in cyclopropane fatty acid content in the cell membrane has been shown to be a response to several different stress conditions in lactic acid bacteria: bile stress (52, 54), acid stress (55), heat shock (56), and osmotic stress (57). In Lactococcus lactis, a similar response has been revealed at the transcriptomic and proteomic levels in acid stress (58, 59). Our study, together with previous studies, suggests that membrane fatty acid composition plays an important role in bile resistance both in GG and in other lactic acid bacteria. These results suggest that increasing the synthesis of cyclic fatty acids and parallel strengthening of the cell membrane could comprise one survival strategy under stress conditions.
dlt Operon and Phospholipids
Bile was found to induce a strong and immediate up-regulation of transcription of all genes in the dlt operon (LGG_00777–00780) (Fig. 4). Lipoteichoic acids are secondary cell wall polymers of Gram-positive bacteria, and various substituents may be attached to them (60). The dlt operon up-regulated in response to bile stress in this study is involved in d-alanylation of lipoteichoic acids that, among others, is a means to add positive charges to otherwise negatively charged lipoteichoic acids. In lactobacilli, the dlt operon has been linked to better survival in gut conditions: in L. plantarum, the transcription of the dlt operon was increased under bile stress conditions (30), and the GG dltD mutant derivative exhibited lowered survival in gastric juice compared with the wild-type strain, whereas no major differences in adhesion, biofilm formation, or immunomodulation were detected (61). The dlt operon thus seems to affect the cell envelope integrity rather than cell signaling. Another gene affecting the charge of the cell surface that is possibly up-regulated after bile addition is lysylphosphatidylglycerol (LPG) synthetase (LGG_02270) catalyzing lysinylation of phosphatidylglycerol. A clear up-regulation (1.9–4.6-fold) of LPG synthetase was detected in biological replicate samples, but it was statistically insignificant because of the high variances of the data. Possibly related to consequent increased requirement of lysine residue, genes encoding the biosynthesis of lysine from aspartate via the diaminopimelic acid pathway (LGG_00108–00115 and LGG_00828) were strongly up-regulated (about 5–7-fold) directly after addition of bile. The function of LPG synthetase and of proteins encoded by the dlt operon is to increase the overall concentration of positive charges on the bacterial cell surface, and they may thereby play a role in bile resistance in GG by repulsion of cationic bile compounds. Lysinylation of membrane lipids and d-alanyl ester substitution of lipoteichoic acids have been described as important for the resistance of cationic antimicrobial peptides in many Gram-positive pathogenic bacteria (62–67). Our data suggest that bile compounds may induce similar bacterial resistance mechanisms based on the repulsion of positively charged molecules, and we propose that the alteration of surface charge may function in Gram-positive bacteria as a general defense mechanism against a wide variety of structurally and functionally dissimilar substrates.
Multidrug Transporters
Genes encoding an ABC-type multidrug transport system and a transcriptional regulator (LGG_01930–01932) were among the most highly induced genes in response to bile in GG (about 16-fold up-regulated immediately after addition of bile), and the up-regulation remained high (8–9-fold) 120 min after bile addition (Fig. 4). A similar increase in abundance was detected for an ATPase component of the ABC transporter (LGG_01930) at the proteome level. In addition, bile strongly increased transcription of two other multidrug/antimicrobial peptide transporter systems (LGG_00978–00979 and LGG_01163–01164; > 4-fold up-regulation), suggesting that ABC multidrug transporter systems may potentially be involved in the active removal of bile compounds from the GG cell. Multidrug transporters can be grouped into two main classes, ABC-type multidrug transporters and secondary multidrug transporters, and they can serve as a defense mechanism against inhibitory compounds such as antibiotics, host defense molecules, and bile by extruding a wide variety of structurally unrelated substrates from the cell (68, 69). Genome-wide transcriptional bile response studies of other Lactobacillus strains have shown that multidrug transporters may be important in bile tolerance in these species (26, 32). Furthermore, L. acidophilus NCFM derivatives containing deletion mutations in five selected transporter genes showed increased sensitivity to bile, confirming the role of multidrug transporters in bile resistance (70).
F0F1-ATP Synthase
The abundance of F0F1-ATP synthase subunit α (AtpA, LGG_01181) was observed to increase in the cell surface-exposed proteome of GG after the addition of bile. At the transcriptome or total proteome levels, no bile-induced effect in expression of AtpA was detected. F0F1-ATP synthase is known to be involved in the maintenance of the proton gradient across the cell membrane that is needed to maintain the proton motive force in the cell. A bile-induced increase in the mRNA level of the corresponding gene has been detected in L. plantarum (30) and E. faecalis (53). Bron et al. (30) speculated that bile stress might cause proton motive force dissipation, which is then compensated for by the increased expression of F0F1-ATPase. Kurdi et al. (71) reported that lactobacilli spontaneously accumulate bile acids (cholic acid), and this accumulation is driven by the transmembrane proton gradient. Thus, GG might require more F0F1-ATPases to be able to keep a sufficient proton gradient across the cell membrane during bile challenge, which could also prevent the intracellular pH from becoming too low (72).
Two-component Regulatory Systems and Bile Salt Hydrolase Modulate Cellular Response to Bile
Two-component Regulatory Systems
Through signal transduction and gene expression regulation, the abundance and activities of cellular proteins change, leading to establishment of a new homeostasis under stress conditions. Bacteria use two-component systems to sense various alterations in their environment (73). The mechanisms involved in sensing of bile compounds and regulating the gene expression accordingly are not well characterized but are likely to involve a two-component regulatory system. Some candidate components involved in signal transduction were identified here. Bile addition was found to increase the expression of two-component regulatory systems both at the transcriptome level (LGG_00155–00156, LGG_01003–01004, and LGG_01710–01711; >2-fold up-regulation) and at the surfome level (LGG_00252; 1.4-fold increase in abundance) (Fig. 4). Previous proteome studies have indicated increased production of proteins of two-component systems in response to bile (25, 74). Furthermore, the global transcriptional response of L. acidophilus NCFM to bile showed a strong and significant induction of a two-component regulatory system (32). A deletion mutation in the response regulator component of this two-component system caused an enhanced induction of the corresponding operon in response to bile, indicating a role of this two-component system in bile tolerance (32).
Bile Salt Hydrolase
The transcription of a gene encoding bile salt hydrolase (LGG_00501), which catalyzes deconjugation of glycine- or taurine-linked bile acids, was strongly up-regulated immediately upon bile exposure (Fig. 4). Bile salt hydrolases are highly specific for certain bile salts (75), and it has been proposed that deconjugation of bile salts may play a role in bile tolerance because of their detoxification properties (75, 76). Bile salt hydrolase activity may be a desirable feature of a probiotic because it helps to maximize its prospects of survival in gut conditions (76), and it has been shown that the better a probiotic survives in gut the better it can induce positive health effects in the host (77). In a study by Pfeiler et al. (32), two bile salt hydrolase (bsh) genes of L. acidophilus NCFM were not found to be differentially expressed in response to bile. Moreover, transcriptome studies of bile responses in L. plantarum WCFS1 have shown that the expression of bsh-1 was highly induced, whereas bsh-3 was highly reduced, and in two other bsh genes, no significant changes in expression levels were detected (30), supporting the idea that different bile salt hydrolases, even within one strain, are differentially regulated in response to bile. Overall, the exact function of different bile salt hydrolases has remained undefined. Our results suggest that under the conditions used a single bile salt hydrolase (LGG_00501) is induced in GG.
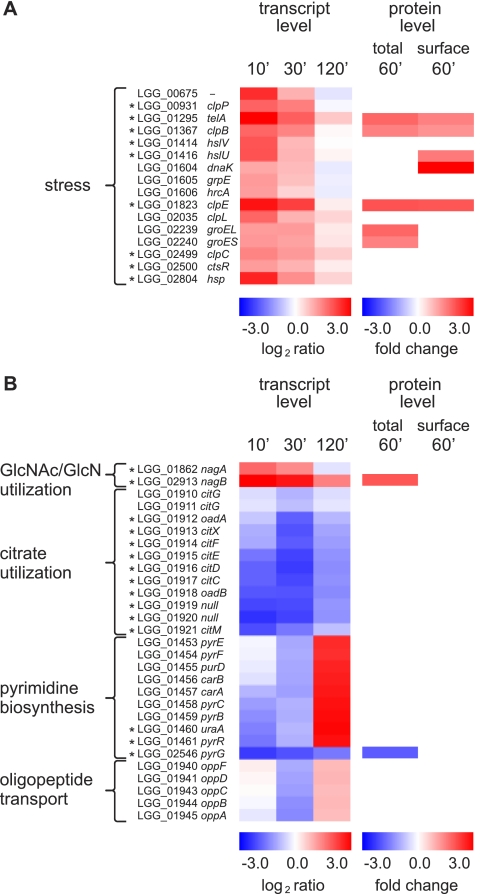
Bile Induces Common Stress Responses in GG
Chaperones and proteases related to several stress conditions were up-regulated in GG in response to bile (Fig. 5A). GroEL (LGG_02239) and GroES (LGG_2240) proteins were 1.8- and 1.6-fold more abundant in the total proteome 60 min after bile addition, respectively, and the abundance of DnaK (LGG_01604) in the surface-exposed proteome was increased 3.9-fold in response to bile stress. At the transcript level, the corresponding genes were more than 2-fold up-regulated 10 min after the bile shock, but the changes were not statistically significant (p > 0.01). In Bifidobacterium animalis, DnaK has been shown to be a surface-exposed human plasminogen receptor up-regulated in response to bile salts (78). The transcript levels of genes coding for the following Clp family proteins were 2.7–7-fold up-regulated: protease subunits ClpP (LGG_00931) and ClpQ (HslV, LGG_01414) and ATPase subunits ClpB (LGG_01367), ClpC (LGG_02499), ClpE (LGG_01823), and ClpY (HslU, LGG_01416). A similar increase in abundance was detected in proteins ClpB (1.5-fold increase in total proteome analysis and 1.3-fold increase in surfome analysis), ClpE (2.7-fold increase in total proteome analysis and 2.0-fold increase in surfome analysis), and HslU (1.5-fold increase in surfome analysis). ClpE protein was represented in our gels by six horizontally adjacent protein spots, suggesting charged post-translational modifications such as phosphorylation. In L. reuteri, the transcription of clpE and clpL was elevated in response to bile exposure, and gene-specific inactivations revealed an essential role for ClpL in survival of this bacterium under bile stress conditions (26). In L. acidophilus, up-regulation of groEL, dnaK, and clpP was detected in an in vitro gastrointestinal tract model, suggesting an important role for these proteins under GIT conditions (79). Although the bile-responsive Clp proteins in GG possibly include a wider range of members of this protein family than in L. reuteri or L. acidophilus, their specific functions in this bacterium remain to be studied. In the Gram-positive model bacterium Bacillus subtilis, dnaK and groESL are regulated by the HrcA repressor (80), and the genes encoding the Clp family of proteins are regulated by the CtsR repressor (81). In Lactobacillus gasseri, however, clpL was shown to belong to the HrcA regulon (82), but the regulation of stress protein expression in lactobacilli has not been clearly defined yet. In this study, statistically significant up-regulation of ctsR (LGG_02500) was observed, whereas the 2.0–2.8-fold up-regulation of hrcA (LGG_01606) in biological replicate samples was statistically insignificant (p > 0.01). The results suggest that the CtsR and HrcA regulons perform functions in response to bile stress conditions in strain GG.
Fig. 5.
Expression changes in selected genes coding for stress proteins (A) and central metabolic functions (B). Changes in transcript levels are represented as log2 intensity ratio values 10, 30, and 120 min (′) after bile addition compared with the time point preceding the bile addition, and statistically significant changes (p < 0.01 at any time point) are marked with asterisks (*). Protein abundance changes in the total proteome and in the surface-exposed proteome are represented as -fold changes (standardized abundance 60 min after bile addition/right before bile addition). All the represented changes in protein abundances are statistically significant (p < 0.01 for total proteome; p < 0.05 for surfome).
Other stress-related proteins up-regulated by bile were the tellurite resistance protein TelA (LGG_01295) and the DNA mismatch repair protein MutL (LGG_02234). TelA was up-regulated both at the mRNA level and in total proteome and surfome analyses. Kristoffersen et al. (83) observed a similar increase in the transcription of tellurite resistance gene in Bacillus cereus under bile stress conditions. Tellurite resistance protein has been suggested to be related to resistance against various toxic compounds (84), and it seems to be involved also in bile resistance. The abundance of MutL was increased in the surfome of GG after bile addition. Bile stress has been shown to induce DNA damage in bacteria (21). However, the location of TelA and MutL on the bacterial surface has not been reported previously, and the assessment of the biological significance of this finding requires further investigation.
Bile Affects Central Metabolic Processes
Carbohydrate Metabolism
Gene nagB (LGG_02913) encoding glucosamine-6-phosphate deaminase/isomerase, which is involved in glucosamine utilization, was strongly up-regulated after addition of bile (Fig. 5B). In fact, nagB was the most highly induced gene in the genome, showing a 65-fold increase at the mRNA level 10 min after bile shock. Also, a 2-fold increase in the amount of NagB protein was detected 60 min after the bile stress, suggesting an increased glucosamine utilization of GG in bile shock conditions. For nagA (LGG_01862), which encodes an enzyme degrading N-acetylglucosamine 6-phosphate to glucosamine 6-phosphate, the substrate of NagB (85), a 3.5-fold up-regulation was detected after bile addition. The MRS growth medium used in this study likely contains glucosamine because yeast extract is one component of the medium, and the fungal cell wall consists of N-acetylglucosamine-containing chitin. GG is able to grow on N-acetylglucosamine (15), and these results suggest that GG could utilize glucosamine as an energy source, particularly under bile stress conditions.
Transcript levels of genes central to citrate utilization (LGG_01910–01921), which entails converting citrate to acetate and oxaloacetate and further to pyruvate, were decreased immediately after bile addition (Fig. 5B), possibly indicating decreased citrate fermentation of GG under bile stress. Previously, citrate metabolism has been shown to be induced under acid and ethanol stress conditions in other lactic acid bacteria (86, 87), but similar induction was not observed in GG in response to bile stress under the conditions used here.
The abundance of ManA (PTS, mannose-specific IIAB component, LGG_02838) in the surface-exposed proteome of GG was found to decrease in response to bile stress. At the mRNA level, the detected 2.1–3.1-fold down-regulation of manA in biological replicate samples was statistically insignificant. The mannose phosphotransferase system is known to be involved in the transportation of glucose, in addition to mannose, into lactobacilli cells (88, 89). In L. plantarum, impaired expression of the mannose PTS operon was reported to result in peroxide sensitivity (90), but the oxidative stress typically caused by bile exposure (21) did not appear to result in increased expression of mannose PTS in GG. In L. lactis, components of mannose PTS have been shown to be targets of bacteriocins (91), and the reduction in abundance of mannose PTS in the surfome could be a means for GG to protect itself against bacteriocins in the gut conditions.
Previous studies have revealed that various carbohydrate metabolism enzymes are commonly found on the surfaces of different bacterial species (47, 92–97). Here we found that bile exposure caused several changes in the amounts of proteins related to carbohydrate metabolism in the surface-associated proteome of GG. The amounts of formate acetyltransferase (PflB, LGG_01421), phosphoglycerate kinase (Pgk, LGG_00934), and ribulose-phosphate 3-epimerase (Rpe, LGG_01665) were increased, and l-lactate dehydrogenase (Ldh, LGG_02523), enolase (Eno, LGG_00936), pyruvate dehydrogenase (PdhD, LGG_01323), and UTP-glucose-1-phosphate uridyltransferase (GalU, LGG_01062) were decreased in the surface-exposed proteome of GG in response to bile. No changes in the expression of the proteins were observed using the total proteome or transcriptome approaches, suggesting that the observed increases in the surface-exposed proteome is a result of relocalization of these proteins in response to bile. In Lactobacillus crispatus, enolase has been reported to be located on the cell surface (98), but to our knowledge, the differential abundance on the Lactobacillus cell surface in response to bile stress has not been shown previously. In a study by Ruiz et al. (47), the amount of enolase in the membrane protein fraction of Bifidobacterium longum after bile stress was increased. The amount of l-lactate dehydrogenase (Ldh) was decreased in the surface-exposed proteome of GG under bile stress, which is in accordance with results obtained for bile response in B. longum (47).
Nucleotide Metabolism
Expression of ribonucleoside-triphosphate reductase (RtpR, LGG_02296), an enzyme involved in deoxynucleotide biosynthesis, was down-regulated after bile addition at the total proteome and surfome levels. Also at the mRNA level, a noticeable (1.8–2.1-fold), although statistically insignificant, down-regulation was observed. A similar decrease in ribonucleotide reductase production under bile stress has been detected in a bile-resistant mutant of L. delbrueckii subsp. lactis (33) and in B. animalis (99). The activity of ribonucleotide reductase is an important regulation point in DNA replication, and control of DNA replication is an essential feature for a cell to survive in stress conditions. Irrespective of growth conditions, a general target of a cell is to maintain an approximately constant DNA/cell mass ratio (100). Thus, the observed decrease of ribonucleotide reductase expression under bile stress is probably a consequence of the reduced growth rate (see supplemental Fig. S1) and thereby also reduced DNA replication in GG after the bile addition.
Transcription of several pyrimidine biosynthesis genes, including members of the pyr gene cluster (pyrEFDAb1AaCBPR1), was transiently reduced in response to bile stress in GG (Fig. 5B). Also, the abundance of PyrG protein (LGG_02546) was reduced after bile addition. The expression pattern of pyrG, located in an operon separate from the pyr gene cluster pyrEFDAb1AaCBPR1 in the GG genome, differed from that of other pyr genes by showing a more permanent down-regulation after bile addition. Down-regulation of pyr gene transcription has been observed previously at high ethanol concentrations in L. lactis (101), and decreased production of Pyr proteins has been detected at high CO2 concentrations in L. plantarum (102). These results suggest that down-regulation of pyrimidine biosynthesis is a common response to several different stress conditions.
Protein Synthesis
The production of ribosomal components is a major metabolic expense, and the amount of translation machinery required by the cell varies with the growth rate. The optimal level of ribosomes and protein synthesis varies under different environmental conditions (103). Here we observed that in GG cells the mRNA levels for all ribosomal protein genes were >2-fold reduced in response to bile. However, with the exception of rpsN (LGG_02422), the reduction was statistically insignificant (p > 0.01). The ribosomal proteins were not present among the differentially expressed protein spots picked for identification from our total proteome gels, suggesting that their abundance in GG cells is not affected by bile stress. It is well established that in Escherichia coli and B. subtilis model organisms ribosomal protein synthesis is controlled primarily at the level of translation, and rRNA transcription is the rate-limiting step in ribosome synthesis (103).
In the surface-exposed proteome of GG, the abundance of one ribosomal protein (RpsE, LGG_02470) was found to be increased after bile stress. The localization of various ribosomal proteins on the cell surface has been reported for lactobacilli (92) and other Gram-positive bacteria (93, 94, 96, 104). Tjalsma et al. (94) have suggested that the cell wall is decorated with cytoplasmic proteins resulting from the lysis of a subpopulation of the cells during culturing. Ribosomal proteins appear to have high affinity for the bacterial cell wall, and they are suggested to be novel anchorless surface proteins. The function of surface-localized ribosomal proteins remains to be clarified, but currently, an immunomodulatory role has been suggested (105).
Proteolytic System
Production of several proteins involved in the proteolytic enzyme system was affected by bile stress; in the total proteome, PepO (LGG_01478) and OppA (LGG_01652) were less abundant, whereas Pcp (pyrrolidone-carboxylate peptidase, LGG_00238) and PepF (LGG_00984) were more abundant after the bile shock. Furthermore, in the surfome analysis, YuxL (LGG_01864) was found to be more abundant after bile addition. The results suggest a protective role for PcP, PepF, and YuxL under bile stress conditions. The transcription of several genes encoding oligopeptide transport system proteins (opp operon, LGG_01940–01945) may be reduced in response to bile. A clear (1.6–3.4-fold), although statistically insignificant, down-regulation in biological replicate samples was observed after bile addition (Fig. 5B). Of the genes corresponding to the bile-affected proteins, no statistically significant expression change was detected, pointing to a regulation mainly at the protein level rather than at the mRNA level.
Proteins Potentially Regulating Gut Epithelial Homeostasis
p40
Our results revealed strong down-regulation of a gene coding for a surface antigen protein, p40 (LGG_00031), after bile exposure at the transcriptome level (37-fold down-regulation). The down-regulation was detected also at the proteome level (1.6-fold down-regulation) (Fig. 4), although p40 is a secreted protein (106), which would be expected to be present inside the cell only to minor extent. Di Caro et al. (16) used transcriptome analysis to follow human responses to GG administration, revealing differential expression of over 400 genes in small bowel mucosa in response to GG. Although the exact mechanisms behind the effect of GG on specific human cellular pathways remain to be elucidated, in vitro studies have shown that GG promotes intestinal epithelial homeostasis through stimulating Akt activation, inhibiting mitogen-activated protein kinase activation, inhibiting TNF-induced cell apoptosis, and protecting the intestinal epithelial tight junctions (106–108). Secreted p40 and p75 proteins have especially been shown to regulate the gut epithelial cell responses without direct bacteria-human cell-cell contact (106, 108).
Conserved Proteins
In response to bile shock, there was a high (24–28-fold) increase in the transcript level of the operon LGG_00914–00916, encoding one conserved protein, one protein predicted to be secreted, and one conserved membrane protein (Fig. 4). The increased expression of the conserved protein LGG_00914 was also detected with the DIGE approaches; there was a 1.7-fold increase at the total proteome level and a 5.6-fold increase at the surfome level, which was the highest -fold change detected among surface-exposed proteins. Although the cellular function of this protein remains to be studied, the strong up-regulation of the entire operon suggests an important role under bile stress conditions in GG. Taken together, these results suggest a spectrum of diverse physiological responses of strain GG to bile stress (summarized in Fig. 6).
Fig. 6.
Model for physiological responses of GG to bile stress. The increased and decreased expression is represented by + and −, respectively.
Concluding Remarks
In this study, we explored the effects of bile stress on L. rhamnosus GG using complementary profiling of transcriptome and proteome level changes in response to 0.2% ox gall. The observed changes in gene expression seem to be associated with pathways contributing to adaptation of GG to bile stress at several levels. First, several two-component systems and multidrug transporters as well as a bile salt hydrolase were found to be up-regulated, suggesting their possible role in sensing and responding to bile challenge and in promoting active removal of bile compounds from the cells. Next, bile exposure induced changes in several cell envelope-related functions, which (possibly by strengthening the cell envelope structure) may enable cells to survive in the presence of this detergent. In addition, exopolysaccharide biosynthesis appears to be reduced under bile stress conditions, which is likely to alter the adhesion properties of GG in a way that is more advantageous for gut persistence. Bile stress can be a signal for gut entry for GG, which could result in a series of events resulting in improved adhesion ability in the new niche. The high number of cytoplasmic proteins identified by surfome analysis was interesting. It could be that the biological activity of the surface-exposed cytoplasmic proteins differs from their activity when expressed intracellularly; thus, a single protein might have different functions depending on its location as suggested previously by Jeffery (109). For example, intracellular enolase has a central role in carbohydrate metabolism, but when located on the surface, it is suggested to modulate adherence of bacteria to human cells and outcompete the pathogens (110, 111).
Acknowledgments
Elina Ahola-Iivarinen, Hanna Jefremoff, Saija Laakso, and Eeva-Marja Turkki are acknowledged for technical assistance.
Footnotes
* This work was supported by Academy of Finland Grants 210740 and 117746 and Finnish Funding Agency for Technology and Innovations Grant 201/08.
 This article contains supplemental Tables S1–S3, Data S1, and Fig. S1.
This article contains supplemental Tables S1–S3, Data S1, and Fig. S1.
1 The abbreviations used are:
- GIT
- gastrointestinal tract
- GG
- L. rhamnosus GG
- EPS
- exopolysaccharide
- PMF
- peptide mass fingerprinting
- ABC
- ATP-binding cassette
- LPG
- lysylphosphatidylglycerol
- PTS
- phosphotransferase system
- MRS
- de Man, Rogosa, and Sharpe.
REFERENCES
- 1. Qin J., Li R., Raes J., Arumugam M., Burgdorf K. S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., Mende D. R., Li J., Xu J., Li S., Li D., Cao J., Wang B., Liang H., Zheng H., Xie Y., Tap J., Lepage P., Bertalan M., Batto J. M., Hansen T., Le Paslier D., Linneberg A., Nielsen H. B., Pelletier E., Renault P., Sicheritz-Ponten T., Turner K., Zhu H., Yu C., Li S., Jian M., Zhou Y., Li Y., Zhang X., Li S., Qin N., Yang H., Wang J., Brunak S., Doré J., Guarner F., Kristiansen K., Pedersen O., Parkhill J., Weissenbach J., MetaHIT Consortium, Bork P., Ehrlich S. D., Wang J. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ley R. E., Peterson D. A., Gordon J. I. (2006) Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124, 837–848 [DOI] [PubMed] [Google Scholar]
- 3. Marco M. L., Pavan S., Kleerebezem M. (2006) Towards understanding molecular modes of probiotic action. Curr. Opin. Biotechnol. 17, 204–210 [DOI] [PubMed] [Google Scholar]
- 4. Resta S. C. (2009) Effects of probiotics and commensals on intestinal epithelial physiology: implications for nutrient handling. J. Physiol. 587, 4169–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Baarlen P., Troost F. J., van Hemert S., van der Meer C., de Vos W. M., de Groot P. J., Hooiveld G. J., Brummer R. J., Kleerebezem M. (2009) Differential NF-kappaB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc. Natl. Acad. Sci. U.S.A. 106, 2371–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doron S., Snydman D. R., Gorbach S. L. (2005) Lactobacillus GG: bacteriology and clinical applications. Gastroenterol. Clin. North Am. 34, 483–498, ix [DOI] [PubMed] [Google Scholar]
- 7. Szajewska H., Kotowska M., Mrukowicz J. Z., Armañska M., Mikołajczyk W. (2001) Efficacy of Lactobacillus GG in prevention of nosocomial diarrhea in infants. J. Pediatr. 138, 361–365 [DOI] [PubMed] [Google Scholar]
- 8. Kalliomäki M., Salminen S., Poussa T., Arvilommi H., Isolauri E. (2003) Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 361, 1869–1871 [DOI] [PubMed] [Google Scholar]
- 9. Isolauri E., Arvola T., Sütas Y., Moilanen E., Salminen S. (2000) Probiotics in the management of atopic eczema. Clin. Exp. Allergy 30, 1604–1610 [DOI] [PubMed] [Google Scholar]
- 10. Majamaa H., Isolauri E. (1997) Probiotics: a novel approach in the management of food allergy. J. Allergy Clin. Immunol. 99, 179–185 [DOI] [PubMed] [Google Scholar]
- 11. Viljanen M., Savilahti E., Haahtela T., Juntunen-Backman K., Korpela R., Poussa T., Tuure T., Kuitunen M. (2005) Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy 60, 494–500 [DOI] [PubMed] [Google Scholar]
- 12. Glück U., Gebbers J. O. (2003) Ingested probiotics reduce nasal colonization with pathogenic bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and beta-hemolytic streptococci). Am. J. Clin. Nutr. 77, 517–520 [DOI] [PubMed] [Google Scholar]
- 13. Hatakka K., Savilahti E., Pönkä A., Meurman J. H., Poussa T., Näse L., Saxelin M., Korpela R. (2001) Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ 322, 1327–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Näse L., Hatakka K., Savilahti E., Saxelin M., Pönkä A., Poussa T., Korpela R., Meurman J. H. (2001) Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. 35, 412–420 [DOI] [PubMed] [Google Scholar]
- 15. Kankainen M., Paulin L., Tynkkynen S., von Ossowski I., Reunanen J., Partanen P., Satokari R., Vesterlund S., Hendrickx A. P., Lebeer S., De Keersmaecker S. C., Vanderleyden J., Hämäläinen T., Laukkanen S., Salovuori N., Ritari J., Alatalo E., Korpela R., Mattila-Sandholm T., Lassig A., Hatakka K., Kinnunen K. T., Karjalainen H., Saxelin M., Laakso K., Surakka A., Palva A., Salusjärvi T., Auvinen P., de Vos W. M. (2009) Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human mucus binding protein. Proc. Natl. Acad. Sci. U.S.A. 106, 17193–17198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Caro S., Tao H., Grillo A., Elia C., Gasbarrini G., Sepulveda A. R., Gasbarrini A. (2005) Effects of Lactobacillus GG on genes expression pattern in small bowel mucosa. Dig. Liver Dis. 37, 320–329 [DOI] [PubMed] [Google Scholar]
- 17. Ventura M., O'Flaherty S., Claesson M. J., Turroni F., Klaenhammer T. R., van Sinderen D., O'Toole P. W. (2009) Genome-scale analyses of health-promoting bacteria: probiogenomics. Nat. Rev. Microbiol. 7, 61–71 [DOI] [PubMed] [Google Scholar]
- 18. De Angelis M., Gobbetti M. (2004) Environmental stress responses in Lactobacillus: a review. Proteomics 4, 106–122 [DOI] [PubMed] [Google Scholar]
- 19. Teusink B., Wiersma A., Molenaar D., Francke C., de Vos W. M., Siezen R. J., Smid E. J. (2006) Analysis of growth of Lactobacillus plantarum WCFS1 on a complex medium using a genome-scale metabolic model. J. Biol. Chem. 281, 40041–40048 [DOI] [PubMed] [Google Scholar]
- 20. Hofman A. F. (1998) Bile secretion and the enterohepatic circulation of bile acids, in Gastrointestinal and Liver Disease (Feldman M., Scharschmidt B. F., Sleisenger M. H. eds) 6th Ed., pp. 937–948, W. B. Saunders, Philadelphia [Google Scholar]
- 21. Begley M., Gahan C. G., Hill C. (2005) The interaction between bacteria and bile. FEMS Microbiol. Rev. 29, 625–651 [DOI] [PubMed] [Google Scholar]
- 22. Coleman R. (1987) Biochemistry of bile secretion. Biochem. J. 244, 249–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lebeer S., Vanderleyden J., De Keersmaecker S. C. J. (2008) Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 72, 728–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruiz L., Sánchez B., Ruas-Madiedo P., de Los Reyes-Gavilán C. G., Margolles A. (2007) Cell envelope changes in Bifidobacterium animalis ssp. lactis as a response to bile. FEMS Microbiol. Lett. 274, 316–322 [DOI] [PubMed] [Google Scholar]
- 25. Leverrier P., Dimova D., Pichereau V., Auffray Y., Boyaval P., Jan G. (2003) Susceptibility and adaptive response to bile salts in Propionibacterium freudenreichii: physiological and proteomic analysis. Appl. Environ. Microbiol. 69, 3809–3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whitehead K., Versalovic J., Roos S., Britton R. A. (2008) Genomic and genetic characterization of the bile stress response of probiotic Lactobacillus reuteri ATCC 55730. Appl. Environ. Microbiol. 74, 1812–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ding W. K., Shah N. P. (2007) Acid, bile, and heat tolerance of free and microencapsulated probiotic bacteria. J. Food Sci. 72, M446–450 [DOI] [PubMed] [Google Scholar]
- 28. Chateau N., Deschamps A. M., Hadj Sassi A. (1994) Heterogeneity of bile-salts resistance in the Lactobacillus isolates of a probiotic consortium. Lett. Appl. Microbiol. 18, 42–44 [Google Scholar]
- 29. Jacobsen C. N., Rosenfeldt Nielsen V., Hayford A. E., Møller P. L., Michaelsen K. F., Paerregaard A., Sandström B., Tvede M., Jakobsen M. (1999) Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 65, 4949–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bron P. A., Molenaar D., de Vos W. M., Kleerebezem M. (2006) DNA micro-array-based identification of bile-responsive genes in Lactobacillus plantarum. J. Appl. Microbiol. 100, 728–738 [DOI] [PubMed] [Google Scholar]
- 31. Lee K., Lee H. G., Choi Y. J. (2008) Proteomic analysis of the effect of bile salts on the intestinal and probiotic bacterium Lactobacillus reuteri. J. Biotechnol. 137, 14–19 [DOI] [PubMed] [Google Scholar]
- 32. Pfeiler E. A., Azcarate-Peril M. A., Klaenhammer T. R. (2007) Characterization of a novel bile-inducible operon encoding a two-component regulatory system in Lactobacillus acidophilus. J. Bacteriol. 189, 4624–4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burns P., Sánchez B., Vinderola G., Ruas-Madiedo P., Ruiz L., Margolles A., Reinheimer J., de los Reyes-Gavilán C. G. (2010) Inside the adaptation process of Lactobacillus delbrueckii subsp. lactis to bile. Int. J. Food Microbiol. 142, 132–141 [DOI] [PubMed] [Google Scholar]
- 34. Wu R., Sun Z., Wu J., Meng H., Zhang H. (2010) Effect of bile salts stress on protein synthesis of Lactobacillus casei Zhang revealed by 2-dimensional gel electrophoresis. J. Dairy Sci. 93, 3858–3868 [DOI] [PubMed] [Google Scholar]
- 35. Marco M. L., de Vries M. C., Wels M., Molenaar D., Mangell P., Ahrne S., de Vos W. M., Vaughan E. E., Kleerebezem M. (2010) Convergence in probiotic Lactobacillus gut-adaptive responses in humans and mice. ISME J. 4, 1481–1484 [DOI] [PubMed] [Google Scholar]
- 36. Koskenniemi K., Koponen J., Kankainen M., Savijoki K., Tynkkynen S., de Vos W. M., Kalkkinen N., Varmanen P. (2009) Proteome analysis of Lactobacillus rhamnosus GG using 2-D DIGE and mass spectrometry shows differential protein production in laboratory and industrial-type growth media. J. Proteome Res. 8, 4993–5007 [DOI] [PubMed] [Google Scholar]
- 37. Gentleman R. C., Carey V. J., Bates D. M., Bolstad B., Dettling M., Dudoit S., Ellis B., Gautier L., Ge Y., Gentry J., Hornik K., Hothorn T., Huber W., Iacus S., Irizarry R., Leisch F., Li C., Maechler M., Rossini A. J., Sawitzki G., Smith C., Smyth G., Tierney L., Yang J. Y., Zhang J. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smyth G. K., Speed T. (2003) Normalization of cDNA microarray data. Methods 31, 265–273 [DOI] [PubMed] [Google Scholar]
- 39. Baldi P., Long A. D. (2001) A bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 17, 509–519 [DOI] [PubMed] [Google Scholar]
- 40. Ritchie M. E., Silver J., Oshlack A., Holmes M., Diyagama D., Holloway A., Smyth G. K. (2007) A comparison of background correction methods for two-colour microarrays. Bioinformatics 23, 2700–2707 [DOI] [PubMed] [Google Scholar]
- 41. Yang Y. H., Thorne N. P. (2003) Normalization for two-color cDNA microarray data, in Science and Statistics: a Festschrift for Terry Speed (Goldstein D. R, ed) Vol. 40, pp. 403–418, Institute of Mathematical Statistics, Beachwood, OH [Google Scholar]
- 42. Edgar R., Domrachev M., Lash A. E. (2002) Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Connell K. L., Stults J. T. (1997) Identification of mouse liver proteins on two-dimensional electrophoresis gels by matrix-assisted laser desorption/ionization mass spectrometry of in situ enzymatic digests. Electrophoresis 18, 349–359 [DOI] [PubMed] [Google Scholar]
- 44. Fallingborg J. (1999) Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull. 46, 183–196 [PubMed] [Google Scholar]
- 45. Ünlü M., Morgan M. E., Minden J. S. (1997) Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis 18, 2071–2077 [DOI] [PubMed] [Google Scholar]
- 46. Gueimonde M., Noriega L., Margolles A., de los Reyes-Gavilan C. G., Salminen S. (2005) Ability of Bifidobacterium strains with acquired resistance to bile to adhere to human intestinal mucus. Int. J. Food Microbiol. 101, 341–346 [DOI] [PubMed] [Google Scholar]
- 47. Ruiz L., Couté Y., Sánchez B., de los Reyes-Gavilán C. G., Sanchez J. C., Margolles A. (2009) The cell-envelope proteome of Bifidobacterium longum in an in vitro bile environment. Microbiology 155, 957–967 [DOI] [PubMed] [Google Scholar]
- 48. Anaya C., Church N., Lewis J. P. (2007) Detection and identification of bacterial cell surface proteins by fluorescent labeling. Proteomics 7, 215–219 [DOI] [PubMed] [Google Scholar]
- 49. Khemiri A., Galland A., Vaudry D., Chan Tchi Song P., Vaudry H., Jouenne T., Cosette P. (2008) Outer-membrane proteomic maps and surface-exposed proteins of Legionella pneumophila using cellular fractionation and fluorescent labelling. Anal. Bioanal. Chem. 390, 1861–1871 [DOI] [PubMed] [Google Scholar]
- 50. Lebeer S., Verhoeven T. L., Francius G., Schoofs G., Lambrichts I., Dufrêne Y., Vanderleyden J., De Keersmaecker S. C. J. (2009) Identification of a gene cluster for the biosynthesis of a long, galactose-rich exopolysaccharide in Lactobacillus rhamnosus GG and functional analysis of the priming glycosyltransferase. Appl. Environ. Microbiol. 75, 3554–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lebeer S., Verhoeven T. L., Perea Vélez M., Vanderleyden J., De Keersmaecker S. C. J. (2007) Impact of environmental and genetic factors on biofilm formation by the probiotic strain Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 73, 6768–6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Taranto M. P., Fernandez Murga M. L., Lorca G., de Valdez G. F. (2003) Bile salts and cholesterol induce changes in the lipid cell membrane of Lactobacillus reuteri. J. Appl. Microbiol. 95, 86–91 [DOI] [PubMed] [Google Scholar]
- 53. Solheim M., Aakra A., Vebø H., Snipen L., Nes I. F. (2007) Transcriptional responses of Enterococcus faecalis V583 to bovine bile and sodium dodecyl sulfate. Appl. Environ. Microbiol. 73, 5767–5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Taranto M. P., Perez-Martinez G., Font de Valdez G. (2006) Effect of bile acid on the cell membrane functionality of lactic acid bacteria for oral administration. Res. Microbiol. 157, 720–725 [DOI] [PubMed] [Google Scholar]
- 55. Broadbent J. R., Larsen R. L., Deibel V., Steele J. L. (2010) Physiological and transcriptional response of Lactobacillus casei ATCC 334 to acid stress. J. Bacteriol. 192, 2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Broadbent J. R., Lin C. (1999) Effect of heat shock or cold shock treatment on the resistance of Lactococcus lactis to freezing and lyophilization. Cryobiology 39, 88–102 [DOI] [PubMed] [Google Scholar]
- 57. Guillot A., Obis D., Mistou M. Y. (2000) Fatty acid membrane composition and activation of glycine-betaine transport in Lactococcus lactis subjected to osmotic stress. Int. J. Food Microbiol. 55, 47–51 [DOI] [PubMed] [Google Scholar]
- 58. Budin-Verneuil A., Maguin E., Auffray Y., Ehrlich S. D., Pichereau V. (2005) Transcriptional analysis of the cyclopropane fatty acid synthase gene of Lactococcus lactis MG1363 at low pH. FEMS Microbiol. Lett. 250, 189–194 [DOI] [PubMed] [Google Scholar]
- 59. Budin-Verneuil A., Pichereau V., Auffray Y., Ehrlich D. S., Maguin E. (2005) Proteomic characterization of the acid tolerance response in Lactococcus lactis MG1363. Proteomics 5, 4794–4807 [DOI] [PubMed] [Google Scholar]
- 60. Neuhaus F. C., Baddiley J. (2003) A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67, 686–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Perea Vélez M., Verhoeven T. L., Draing C., Von Aulock S., Pfitzenmaier M., Geyer A., Lambrichts I., Grangette C., Pot B., Vanderleyden J., De Keersmaecker S. C. J. (2007) Functional analysis of D-alanylation of lipoteichoic acid in the probiotic strain Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 73, 3595–3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li M., Cha D. J., Lai Y., Villaruz A. E., Sturdevant D. E., Otto M. (2007) The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 66, 1136–1147 [DOI] [PubMed] [Google Scholar]
- 63. Li M., Lai Y., Villaruz A. E., Cha D. J., Sturdevant D. E., Otto M. (2007) Gram-positive three-component antimicrobial peptide-sensing system. Proc. Natl. Acad. Sci. U.S.A. 104, 9469–9474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Peschel A., Jack R. W., Otto M., Collins L. V., Staubitz P., Nicholson G., Kalbacher H., Nieuwenhuizen W. F., Jung G., Tarkowski A., van Kessel K. P., van Strijp J. A. (2001) Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with L-lysine. J. Exp. Med. 193, 1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thedieck K., Hain T., Mohamed W., Tindall B. J., Nimtz M., Chakraborty T., Wehland J., Jänsch L. (2006) The MprF protein is required for lysinylation of phospholipids in listerial membranes and confers resistance to cationic antimicrobial peptides (CAMPs) on Listeria monocytogenes. Mol. Microbiol. 62, 1325–1339 [DOI] [PubMed] [Google Scholar]
- 66. Peschel A., Otto M., Jack R. W., Kalbacher H., Jung G., Götz F. (1999) Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274, 8405–8410 [DOI] [PubMed] [Google Scholar]
- 67. Kovács M., Halfmann A., Fedtke I., Heintz M., Peschel A., Vollmer W., Hakenbeck R., Brückner R. (2006) A functional dlt operon, encoding proteins required for incorporation of D-alanine in teichoic acids in gram-positive bacteria, confers resistance to cationic antimicrobial peptides in Streptococcus pneumoniae. J. Bacteriol. 188, 5797–5805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Piddock L. J. (2006) Multidrug-resistance efflux pumps—not just for resistance. Nat. Rev. Microbiol. 4, 629–636 [DOI] [PubMed] [Google Scholar]
- 69. Putman M., van Veen H. W., Konings W. N. (2000) Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64, 672–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pfeiler E. A., Klaenhammer T. R. (2009) Role of transporter proteins in bile tolerance of Lactobacillus acidophilus. Appl. Environ. Microbiol. 75, 6013–6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kurdi P., van Veen H. W., Tanaka H., Mierau I., Konings W. N., Tannock G. W., Tomita F., Yokota A. (2000) Cholic acid is accumulated spontaneously, driven by membrane ΔpH, in many lactobacilli. J. Bacteriol. 182, 6525–6528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Senior A. E. (1990) The proton-translocating ATPase of Escherichia coli. Annu. Rev. Biophys. Biophys. Chem. 19, 7–41 [DOI] [PubMed] [Google Scholar]
- 73. Mascher T., Helmann J. D., Unden G. (2006) Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 70, 910–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sánchez B., Champomier-Vergès M. C., Anglade P., Baraige F., de Los Reyes-Gavilán C. G., Margolles A., Zagorec M. (2005) Proteomic analysis of global changes in protein expression during bile salt exposure of Bifidobacterium longum NCIMB 8809. J. Bacteriol. 187, 5799–5808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Patel A. K., Singhania R. R., Pandey A., Chincholkar S. B. (2010) Probiotic bile salt hydrolase: current developments and perspectives. Appl. Biochem. Biotechnol. 162, 166–180 [DOI] [PubMed] [Google Scholar]
- 76. Begley M., Hill C., Gahan C. G. (2006) Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 72, 1729–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Watson D., Sleator R. D., Hill C., Gahan C. G. M. (2008) Enhancing bile tolerance improves survival and persistence of Bifidobacterium and Lactococcus in the murine gastrointestinal tract. BMC Microbiol. 8, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Candela M., Centanni M., Fiori J., Biagi E., Turroni S., Orrico C., Bergmann S., Hammerschmidt S., Brigidi P. (2010) DnaK from Bifidobacterium animalis subsp. lactis is a surface-exposed human plasminogen receptor upregulated in response to bile salts. Microbiology 156, 1609–1618 [DOI] [PubMed] [Google Scholar]
- 79. Weiss G., Jespersen L. (2010) Transcriptional analysis of genes associated with stress and adhesion in Lactobacillus acidophilus NCFM during the passage through an in vitro gastrointestinal tract model. J. Mol. Microbiol. Biotechnol. 18, 206–214 [DOI] [PubMed] [Google Scholar]
- 80. Schulz A., Schumann W. (1996) hrcA, the first gene of the Bacillus subtilis dnaK operon encodes a negative regulator of class I heat shock genes. J. Bacteriol. 178, 1088–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Derré I., Rapoport G., Msadek T. (1999) CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria. Mol. Microbiol. 31, 117–131 [DOI] [PubMed] [Google Scholar]
- 82. Suokko A., Poutanen M., Savijoki K., Kalkkinen N., Varmanen P. (2008) ClpL is essential for induction of thermotolerance and is potentially part of the HrcA regulon in Lactobacillus gasseri. Proteomics 8, 1029–1041 [DOI] [PubMed] [Google Scholar]
- 83. Kristoffersen S. M., Ravnum S., Tourasse N. J., Økstad O. A., Kolstø A. B., Davies W. (2007) Low concentrations of bile salts induce stress responses and reduce motility in Bacillus cereus ATCC 14579. J. Bacteriol. 189, 5302–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Taylor D. E. (1999) Bacterial tellurite resistance. Trends Microbiol. 7, 111–115 [DOI] [PubMed] [Google Scholar]
- 85. Komatsuzawa H., Fujiwara T., Nishi H., Yamada S., Ohara M., McCallum N., Berger-Bächi B., Sugai M. (2004) The gate controlling cell wall synthesis in Staphylococcus aureus. Mol. Microbiol. 53, 1221–1231 [DOI] [PubMed] [Google Scholar]
- 86. Olguín N., Bordons A., Reguant C. (2009) Influence of ethanol and pH on the gene expression of the citrate pathway in Oenococcus oeni. Food Microbiol. 26, 197–203 [DOI] [PubMed] [Google Scholar]
- 87. Sánchez C., Neves A. R., Cavalheiro J., dos Santos M. M., García-Quintáns N., López P., Santos H. (2008) Contribution of citrate metabolism to the growth of Lactococcus lactis CRL264 at low pH. Appl. Environ. Microbiol. 74, 1136–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chaillou S., Postma P. W., Pouwels P. H. (2001) Contribution of the phosphoenolpyruvate:mannose phosphotransferase system to carbon catabolite repression in Lactobacillus pentosus. Microbiology 147, 671–679 [DOI] [PubMed] [Google Scholar]
- 89. Stevens M. J., Molenaar D., de Jong A., De Vos W. M., Kleerebezem M. (2010) σ54-mediated control of the mannose phosphotransferase system in Lactobacillus plantarum impacts on carbohydrate metabolism. Microbiology 156, 695–707 [DOI] [PubMed] [Google Scholar]
- 90. Stevens M. J., Molenaar D., de Jong A., de Vos W. M., Kleerebezem M. (2010) Involvement of the mannose phosphotransferase system of Lactobacillus plantarum WCFS1 in peroxide-stress tolerance. Appl. Environ. Microbiol. 76, 3748–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Diep D. B., Skaugen M., Salehian Z., Holo H., Nes I. F. (2007) Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc. Natl. Acad. Sci. U.S.A. 104, 2384–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Beck H. C., Madsen S. M., Glenting J., Petersen J., Israelsen H., Nørrelykke M. R., Antonsson M., Hansen A. M. (2009) Proteomic analysis of cell surface-associated proteins from probiotic Lactobacillus plantarum. FEMS Microbiol. Lett. 297, 61–66 [DOI] [PubMed] [Google Scholar]
- 93. Severin A., Nickbarg E., Wooters J., Quazi S. A., Matsuka Y. V., Murphy E., Moutsatsos I. K., Zagursky R. J., Olmsted S. B. (2007) Proteomic analysis and identification of Streptococcus pyogenes surface-associated proteins. J. Bacteriol. 189, 1514–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tjalsma H., Lambooy L., Hermans P. W., Swinkels D. W. (2008) Shedding & shaving: disclosure of proteomic expressions on a bacterial face. Proteomics 8, 1415–1428 [DOI] [PubMed] [Google Scholar]
- 95. Hansmeier N., Chao T. C., Kalinowski J., Pühler A., Tauch A. (2006) Mapping and comprehensive analysis of the extracellular and cell surface proteome of the human pathogen Corynebacterium diphtheriae. Proteomics 6, 2465–2476 [DOI] [PubMed] [Google Scholar]
- 96. Wilkins J. C., Beighton D., Homer K. A. (2003) Effect of acidic pH on expression of surface-associated proteins of Streptococcus oralis. Appl. Environ. Microbiol. 69, 5290–5296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang A., Xie C., Chen H., Jin M. (2008) Identification of immunogenic cell wall-associated proteins of Streptococcus suis serotype 2. Proteomics 8, 3506–3515 [DOI] [PubMed] [Google Scholar]
- 98. Antikainen J., Kuparinen V., Lähteenmäki K., Korhonen T. K. (2007) pH-dependent association of enolase and glyceraldehyde-3-phosphate dehydrogenase of Lactobacillus crispatus with the cell wall and lipoteichoic acids. J. Bacteriol. 189, 4539–4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sánchez B., Champomier-Vergès M. C., Stuer-Lauridsen B., Ruas-Madiedo P., Anglade P., Baraige F., de los Reyes-Gavilán C. G., Johansen E., Zagorec M., Margolles A. (2007) Adaptation and response of Bifidobacterium animalis subsp. lactis to bile: a proteomic and physiological approach. Appl. Environ. Microbiol. 73, 6757–6767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Herrick J., Sclavi B. (2007) Ribonucleotide reductase and the regulation of DNA replication: an old story and an ancient heritage. Mol. Microbiol. 63, 22–34 [DOI] [PubMed] [Google Scholar]
- 101. Maligoy M., Mercade M., Cocaign-Bousquet M., Loubiere P. (2008) Transcriptome analysis of Lactococcus lactis in coculture with Saccharomyces cerevisiae. Appl. Environ. Microbiol. 74, 485–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bringel F., Hammann P., Kugler V., Arsène-Ploetze F. (2008) Lactobacillus plantarum response to inorganic carbon concentrations: PyrR2-dependent and -independent transcription regulation of genes involved in arginine and nucleotide metabolism. Microbiology 154, 2629–2640 [DOI] [PubMed] [Google Scholar]
- 103. Paul B. J., Ross W., Gaal T., Gourse R. L. (2004) rRNA transcription in Escherichia coli. Annu. Rev. Genet. 38, 749–770 [DOI] [PubMed] [Google Scholar]
- 104. Doro F., Liberatori S., Rodríguez-Ortega M. J., Rinaudo C. D., Rosini R., Mora M., Scarselli M., Altindis E., D'Aurizio R., Stella M., Margarit I., Maione D., Telford J. L., Norais N., Grandi G. (2009) Surfome analysis as a fast track to vaccine discovery: identification of a novel protective antigen for group B Streptococcus hypervirulent strain COH1. Mol. Cell. Proteomics 8, 1728–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Spence J. M., Clark V. L. (2000) Role of ribosomal protein L12 in gonococcal invasion of Hec1B cells. Infect. Immun. 68, 5002–5010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yan F., Cao H., Cover T. L., Whitehead R., Washington M. K., Polk D. B. (2007) Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132, 562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yan F., Polk D. B. (2002) Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J. Biol. Chem. 277, 50959–50965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Seth A., Yan F., Polk D. B., Rao R. K. (2008) Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1060–G1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jeffery C. J. (2003) Moonlighting proteins: old proteins learning new tricks. Trends Genet. 19, 415–417 [DOI] [PubMed] [Google Scholar]
- 110. Pancholi V., Chhatwal G. S. (2003) Housekeeping enzymes as virulence factors for pathogens. Int. J. Med. Microbiol. 293, 391–401 [DOI] [PubMed] [Google Scholar]
- 111. Spurbeck R. R., Arvidson C. G. (2010) Lactobacillus jensenii surface-associated proteins inhibit Neisseria gonorrhoeae adherence to epithelial cells. Infect. Immun. 78, 3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]