Abstract
The seminal vesicles (SVs), like much of the male reproductive tract, depend on androgen-driven stromal-epithelial interactions for normal development, structure, and function. The primary function of the SVs is to synthesize proteins that contribute to the seminal plasma and this is androgen dependent. However, the cell-specific role for androgen action in adult SVs remains unclear. This study analyzed the SV in mice with targeted ablation of androgen receptors specifically in smooth muscle cells (PTM-ARKO) to determine in vivo whether it is androgen action in a subset of the SV stroma, the smooth muscle cells, that drives epithelial function and identity. These mice have significantly smaller SVs in adulthood with less smooth muscle and reduced epithelial cell height. Less epithelial cell proliferation was observed in adult PTM-ARKO SVs, compared with controls, and production of seminal proteins was reduced, indicating global impairment of epithelial cell function in PTM-ARKO SVs. None of these changes could be explained by altered serum testosterone or estradiol concentrations. We also demonstrate altered SV responsiveness to exogenous testosterone and estradiol in PTM-ARKO mice, indicating that smooth muscle androgen receptors may limit the SV epithelial proliferative response to exogenous estrogens. These results therefore demonstrate that the smooth muscle cells play a vital role in androgen-driven stromal-epithelial interactions in the SV, determining epithelial cell structure and function as well as limiting the SV epithelial proliferative response to exogenous estrogens.
Androgen signaling via the smooth muscle cells is vital for normal seminal vesicle structure and function.
The seminal vesicles (SVs), like much of the male reproductive tract, depend on androgen action for normal development and differentiation as well as for later structural and functional integrity (1,2,3). Testosterone (T) is synthesized by the testes (4) and, along with its more potent metabolite dihydrotestosterone (DHT), binds to the androgen receptor (AR) to modulate gene transcription in target cells (5). Blocking androgen action impairs male reproductive development such that XY males are born with a female phenotype with intraabdominal testes, no prostate, and no SVs (6,7,8,9,10). Androgen action in SVs is thought to be regulated by DHT rather than T because SV stromal cells express 5α-reductase type 2, which converts T to DHT, and 5α-reductase knockout mice have smaller SVs and prostates (11).
The primary function of the SVs is to synthesize proteins that contribute to the seminal plasma. This is important for the transport and nutrition of sperm as well as (in rodents) the formation of a copulatory plug after ejaculation; removal of the SVs from mice impairs fertility (12,13). SVs have a highly convoluted pseudostratified columnar epithelium with active protein secretory machinery. This epithelium is highly secretory producing fructose and prostaglandins as well as seminal fluid proteins such as SV secretion proteins (SVS), metallothionein-1 (Mt-1), and transglutiminase-4 (TGM4) (14,15,16,17,18,19,20,21,22). SV secretory function is androgen dependent (23), and castration after puberty results in involution of the SVs due to a gross reduction in secretions as well as cytological degeneration and apoptosis of the epithelium (24); these changes can be reversed by exogenous T (25), suggesting that androgen signaling is required for continued functional and structural homeostasis of SVs. Furthermore, it has been shown that altering the androgen-estrogen balance can affect adult male accessory sex organs because exogenous estrogens directly stimulate epithelial proliferation resulting in aberrant histological changes and even prostatic squamous metaplasia (26,27,28,29,30,31,32).
The SVs in adults are composed of epithelium surrounded by stromal cells, including an inner contractile layer of smooth muscle. The cell-specific role for androgen action in the SVs is poorly understood, with much of what we know being derived from prostate studies. Adult SVs express AR in all cell types (namely the stromal, smooth muscle, and epithelial cells) (33) and estrogen receptors (ERs)-α and -β (34,35). Normal male reproductive development and function is believed to depend on reciprocal interactions between the stroma and epithelium; tissue recombination studies, mostly on the prostate, have demonstrated that the stromal compartment is the key site for androgen action, determining the morphological and functional fate of the overlying epithelium, and regulating epithelial proliferation and apoptosis (36,37,38,39,40). Disruption of these hormone-driven interactions is implicated in several pathologies including prostate carcinogenesis (41). However, it is not known whether the epithelium relies on interactions with the entire stromal compartment or whether signaling from a subset of the SV stromal compartment, the smooth muscle cells, is sufficient to determine epithelial identity and function.
Advances in transgenic technology have enabled cell-specific gene ablation, providing new opportunities to investigate the cell-specific roles for androgen action. Testicular cell-specific AR knockout (ARKO) mice have been successfully generated by several investigators (42,43,44,45,46), including the generation of a testicular smooth muscle cell ARKO [termed PTM-ARKO (44)] using a smooth muscle promoter driven Cre recombinase mouse [namely myosin heavy chain (47)]. This promoter also resulted in Cre expression in the smooth muscle cells of the SVs, therefore providing a new opportunity to directly investigate whether it is androgen action in a subset of the SV stroma, the smooth muscle cells, that drives epithelial function and identity. As reported here, these mice have significantly smaller SVs in adulthood with abnormal histology and function, confirming similar observations in an independent study in which a probasin-driven Cre inexplicably resulted in Cre expression in the SV stroma cells (48). In addition to this, we demonstrate altered responsiveness to exogenous T and estradiol (E2) in our mice, indicating that smooth muscle ARs might limit the SV epithelial proliferative response to exogenous estrogens. Together these results demonstrate a vital role for AR signaling via the smooth muscle cells for normal SV structure and function and reinforce the evidence that stromal-epithelial interactions are important for adult sex accessory function.
Materials and Methods
Breeding of transgenic mice
Mice in which AR has been ablated from the smooth muscle cells of the testis have already been generated (44), using male mice heterozygous for Cre recombinase, and green fluorescent protein (GFP), both under the control of a smooth muscle myosin heavy-chain promoter (MH) (47). These mice also expressed Cre recombinase in the smooth muscle cells of the SV. The Cre-positive (ARflox positive) male offspring are termed PTM-ARKO, whereas the Cre-negative ARflox-positive littermates were used as controls. All mice were bred under standard conditions of care and use under licensed approval from the U.K. Home Office. Stud male MH mice and PTM-ARKO male offspring were genotyped for the presence of Cre using standard PCR as previously described (44).
Recovery of reproductive tissues
Male mice were culled at postnatal d 12–100 and SVs were recovered and weighed; these mice were also used to investigate the phenotype in the testis (44) and prostate (Welsh M, Moffat L, McNeilly A, Brownstein D, Saunders PTK, Sharpe RM, Smith LB, manuscript in preparation). In adults, seminal secretions were recovered, weighed, and snap frozen, and then the empty gland was reweighed. To collect fetal SVs, dams were culled at embryonic day (e)17.5, and reproductive tracts were recovered from male fetuses as previously described (44). Tissues were snap frozen for subsequent RNA analysis or fixed in Bouins for 6 h, processed, and embedded in paraffin wax as previously reported (10). Sections of SVs were stained with hematoxylin and eosin using standard protocols and examined for histological abnormalities.
Hormone implant study
Control (n = 5) and PTM-ARKO (n = 5) male mice (d 130–150) were implanted with a 1-cm SILASTIC brand implant (Dow Corning Corp., Midland, MI; inside diameter 1.47 mm, outside diameter 1.95 mm) containing T-E2 (Sigma-Aldrich, Poole, UK) at a concentration ratio 100 mg T to 10 mg E2, as used by Ricke et al. (49). Implants were left in situ for 13 wk, and then mice were culled, blood was collected by cardiac puncture, and SVs were recovered and weighed.
Hormone analysis
Immediately after culling, blood was collected from mice by cardiac puncture and sera were assayed for testosterone and estradiol as previously published (50,51). All samples from each mouse were run in a single assay, and the within-assay coefficients of variation were all less than 10%.
Immunohistochemical analysis
In addition to PCR genotyping, mice were examined by immunohistochemistry for the presence of Cre and absence of AR in SV smooth muscle cells, identified by immunoexpression of α-smooth muscle actin (SMA), using a previously published method (44).
Single immunohistochemistry was also performed using previously published methods (10,44). Adult SVs from PTM-ARKO and control males were stained for: 1) GFP as a marker for Cre expression (Abcam, Cambridge, UK; 1:100); 2) AR to confirm its deletion (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; 1:200); 3) SMA to identify the smooth muscle cell layer (Sigma-Aldrich; 1:4000); 4) pan-cytokeratin to identify the epithelial cells (Sigma-Aldrich; 1:1000); 4) ERα to confirm the SVs can respond to estradiol [Novocastra, Leica Microsystems (U.K.) Ltd., Milton Keynes, UK; 1:20]; and 5) phosphohistone H3 to identify mitotic cells (Upstate Biotechnology Inc., Lake Placid, NY; 1:1000). Cellular sites of expression were determined and slides photographed using a Provis AX70 microscope (Olympus Optical, London, UK) fitted with a Canon DS6031 camera (Canon Europe, Amsterdam, The Netherlands). To ensure reproducibility of results, representative SVs from at least three animals at each age were used, and sections from PTM-ARKO and control littermates were processed in parallel on the same slide on at least two occasions. Appropriate negative controls were included to ensure that any staining observed was specific. All antibodies used showed only minor nonspecific staining.
Quantification of SV smooth muscle thickness and epithelial cell height
Smooth muscle thickness and epithelial cell height were quantified in SV sections from adult PTM-ARKO and control (±T+E2) mice stained for SMA and cytokeratin, respectively; stereological analysis was undertaken on equivalent centrally located sections for each animal; therefore, the measurements should be at approximately equivalent points away from the SV distal tips. Sections were analyzed using Image-Pro Plus 6.2 software with a Stereology 5.0 plug-in (Media Cybernetics U.K., Berkshire, UK) with the ×20 objective on a Leitz DBRB microscope fitted with a Prior Pro-Scan automatic stage (Prior Scientific Instruments Ltd., Cambridge, UK). Epithelial cell height was measured in every 10th cell and smooth muscle thickness was measured in the adjacent smooth muscle area.
Quantification of cell mitosis in SVs
SV sections from control and PTM-ARKO adult mice (±T+E2) were immunostained for phosphohistone H3 (Upstate Biotechnology, Dundee, UK) as detailed above to identify mitotic cells. Positive and negative cells were counted separately in the epithelial compartment using Image-Pro Plus 6.2 software with a Stereology 5.0 plug-in (Media Cybernetics U.K.) with the ×20 objective on a Leitz DBRB microscope fitted with a Prior Pro-Scan automatic stage (Prior Scientific Instruments). The number of positive cells in the epithelial compartment was then divided by the total number of epithelial cells visible in section to calculate the epithelial proliferation index.
Analysis of apoptosis in SVs
Apoptotic cells were identified in d 12 and d 100 SVs from PTM-ARKO and control mice using terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling, as previously described (52). Very few apoptotic cells were identified in knockout or control SVs at either age; therefore, stereological quantification was deemed unnecessary.
Protein gels
SV secretions recovered from the glands at dissection were homogenized in PBS before freezing at −80 C. Protein concentration was quantified using a NanoDrop 1000 spectrophotometer (Thermo Scientific, Swedesboro, NJ), and 40 μg was heated to 70 C for 10 min and loaded onto a 4–12% (gradient) polyacrylamide gel. Gels were subjected to electrophoresis at 200 V under reducing conditions. Gels were stained with trypan blue to reveal the protein fingerprint. Samples from at least three PTM-ARKO and control males were run on at least three occasions.
RNA extraction and reverse transcription
RNA was isolated and quantified from frozen SVs from PTM-ARKO or control mice as previously described (44). Random hexamer primed cDNA was prepared using the Applied Biosystems TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA).
Determination of deletion of AR exon 2
RT-PCR was performed as previously described on cDNA synthesized from SVs from PTM-ARKO mice and control littermates using primers for AR exons 1 and 3 to reveal bands of 765 and 613 bp relating to mice with a wild type (WT), and/or excised exon 2 allele of AR, respectively. A weak central band was also identified; this was confirmed to be an artifact.
Quantitative analysis of gene expression
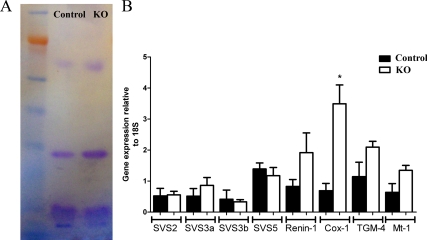
Quantitative PCR was performed for the following genes, using an ABI Prism 7500 sequence detection system (Applied Biosystems) and the Roche universal probe library (Roche, Welwyn, UK), according to the manufacturer’s instructions: SVS protein 2 (GACAGTCAGCTGTGGTTGGA, GCCTTTCTGACCAAGCATAAA), SVS3a (TCTCTGCTC CTCCTTCTGGA, AAGTATCCTTTTGTTCCACCA), SVS3b (GCCTTTCTCTGCTCCTCCTT, TTCCCAATAAACATGAGTGGTG), SVS4 (AGCTGAACATCTGGACCAA, TGAAGACATC ATGGGCTCTGT), SVS5 (GCAAGATGAGTCCCACCAG, CGCCGACTGAGAGAACCT), renin-1 (AAGAAAGTGTTCTCTGTCTACTACA, TCGCTACCTCCTAGCACCAC), transglutaminase-4 (TGM4) (CAGAGAGAGAGGTAGCAGGACCA, TCTCTCCCATTCACAG CGTA), Mt-1 (CACCAGATCTCGGAATGGAC, AGGAGCAGCAGCTCT TCTTG), cyclooxygenase (Cox)-1 (CTCTTCCAGGAGCTCACA, TCGATGTCACCGTACA GCTC). The expression of each gene was related to 18S, an internal positive control.
Statistical analysis
Data were analyzed using GraphPad Prism version 5 (GraphPad Software Inc., San Diego, CA) using a one-tailed unpaired t test, one-way ANOVA, or two-way ANOVA, as appropriate, followed by Bonferroni post hoc tests. Values are expressed as mean ± sem. Normality was confirmed using a Kolmogoron-Smirnov normality test.
Results
Characterization of SVs in mice with targeted deletion of AR from smooth muscle cells
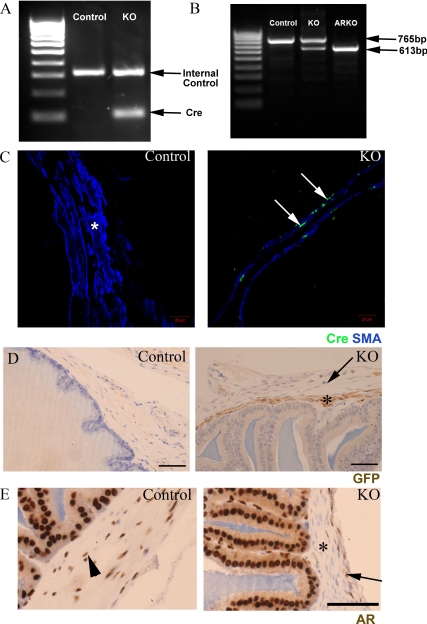
No reproductive phenotype was identified in adult MH Cre-positive, ARflox-negative male mice, demonstrating that Cre expression alone had no effect on SVs (data not shown). All PTM-ARKO males were hemizygous for X-linked ARflox, whereas approximately 50% of these males also carried the Cre transgene [Fig. 1A, knockout (KO)]; the ARflox-positive Cre-negative male littermates were used as controls. To confirm that Cre recombinase was functioning, we assayed for the deletion of exon 2 of the AR in PTM-ARKO SVs by RT-PCR. Control SVs expressed only the full-length (WT) band, whereas ARKO SVs expressed only the smaller KO band, confirming that exon 2 of the AR had been deleted from all cells (Fig. 1B). SVs from PTM-ARKO mice expressed both the WT and KO band, as expected, demonstrating that AR had been deleted from a proportion of SV cells (Fig. 1B). Immunohistochemistry demonstrated that Cre recombinase was expressed in SV smooth muscle cells in PTM-ARKO mice at (Fig. 1C), whereas SVs from control mice showed no specific Cre recombinase immunoexpression; Cre recombinase was never expressed in any other SV cell type in PTM-ARKO mice, including the outer stromal layer. As expected, GFP, which is coexpressed with Cre recombinase, was also expressed only in the smooth muscle layer of the PTM-ARKO SVs (Fig. 1D, arrow), whereas AR was absent in these cells (Fig. 1E, arrow; arrowhead indicates outer AR positive stromal layer).
Figure 1.
A, Characterization of Cre recombinase expression in adult PTM-ARKO seminal vesicles. Approximately 50% of male PTM-ARKOs were Cre positive, identified by the presence of a band at 100 bp. Control littermates were negative for Cre but were positive for the internal control gene. B, Deletion of AR in the adult SV was determined using RT-PCR spanning exon 2. Only the larger 765-bp WT band was seen in control SVs, whereas the smaller 613-bp KO band was seen in ARKO testes. Both bands were identified in PTM-ARKO SVs, showing deletion of AR in a proportion of cells. C, Immunohistochemistry for Cre recombinase (green) and SMA (blue) showed that Cre recombinase was expressed selectively (arrow) in the smooth muscle cells of the SVs but not in any other SV cell types or smooth muscle cells in control SVs (*). Note that the smooth muscle layer appears narrower in the PTM-ARKO than controls. D, GFP [expression of which is also driven by the MH promoter in this mouse (47)] was expressed in the smooth muscle cells in PTM-ARKO SVs at d 100 (*) but not in the outer stromal cell layer (arrow); GFP was not detected in any cells in control SVs. Scale bars, 50 μm. E, In contrast, AR was expressed in the smooth muscle cells of control SV (arrowhead) but not PTM-ARKO SV smooth muscle cells (*); AR continued to be expressed in the epithelial and outer stromal cells (arrow) in the PTM-ARKO SV. Scale bars, 25 μm.
Onset of Cre recombinase expression in PTM-ARKO SVs
PTM-ARKO SVs from e17.5 embryos were assayed for the deletion of exon 2 of the AR by RT-PCR, as used in Fig. 1B. SVs from control mice expressed only the full-length (WT) band, whereas SVs from PTM-ARKO mice expressed both the WT and smaller KO band (data not shown). Therefore, AR is deleted from smooth muscle cells around the age at which AR is normally first expressed (data not shown).
SV gross morphology in PTM-ARKO mice
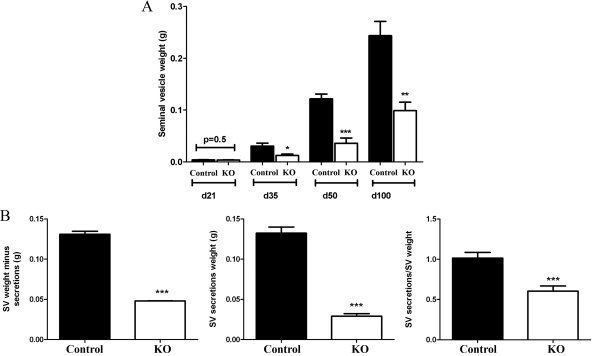
Serum T [control, 3.5 ± 0.94 ng/ml−1, n = 15; KO, 4.87 ± 1.2 ng/ml−1; (44)] and E2 (control, 5.2 ± 0.8 pg/ml−1, n = 3; KO, 5.7 ± 1.0 pg/ml−1, n = 5) concentrations were not significantly different in adult PTM-ARKO males, compared with age-matched control littermates. Body weight was not significantly different between PTM-ARKO and control males at any age (data not shown). The reproductive tract formed normally in PTM-ARKO males; however, SV weights were reduced at d 35, 50, and 100, compared with controls, but not at d 21 (Fig. 2A); prostate weight was also reduced in PTM-ARKO males (Welsh M, Moffat L, McNeilly A, Brownstein D, Saunders PTK, Sharpe RM, Smith LB, manuscript in preparation). These changes in SV weight could not be explained by correcting for body weight (data not shown). The weight of the emptied SVs was significantly reduced in PTM-ARKOs, compared with controls at d 100, as was the volume of SV secretions (Fig. 2B). The ratio of SV secretions was: emptied SV was also significantly reduced in PTM-ARKO mice, compared with controls (Fig. 2B), implying that the SVs from KO mice make disproportionately less secretions for their size.
Figure 2.
Gross morphology of PTM-ARKO seminal vesicles. A, Quantification of SV weight in PTM-ARKO mice at d 21–100 showing that there is a significant reduction at d 35 onward but not at d 21. B, Quantification of empty SV minus secretion and SV secretion weight as well as secretion weight relative to SV weight in PTM-ARKO mice at d 100. Values are means ± sem (n = 6–14 mice). *, P < 0.05, **, P < 0.01, ***, P < 0.001, compared with age-matched controls.
Postnatal SV histology
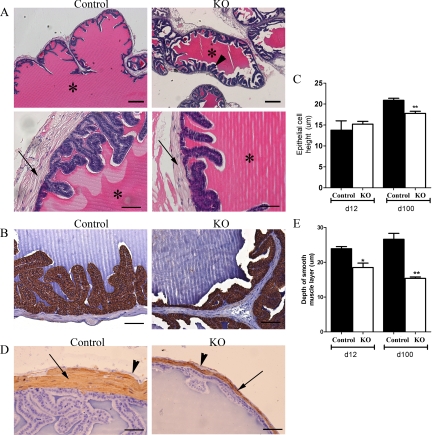
The SVs from both PTM-ARKO and control mice were composed of glands lined by epithelium surrounded by an inner smooth muscle layer and an outer stroma cell layer. The lumens of both the PTM-ARKO and control SVs were filled with a dense eosinophilic substance (Fig. 3A, asterisk). Tall secretory columnar epithelium was observed in the control SV, with typical folding of the mucosa (Fig. 3A), whereas the epithelium in the PTM-ARKO SVs was more cuboidal (Fig. 3B), as confirmed quantitatively (Fig. 3C). There was also an apparent increase in epithelial branching/folding in the PTM-ARKO, compared with controls (Fig. 3A, arrowhead). In addition to epithelial changes, there was a reduction in the depth of the smooth muscle layer in the PTM-ARKO SV, compared with the controls (Fig. 3D); this was confirmed quantitatively (Fig. 3E). Interestingly, the stromal compartment is mostly composed of smooth muscle cells in the mouse SVs, with only a thin SMA-negative outer layer (Fig. 3D, arrowhead).
Figure 3.
Histological analysis of PTM-ARKO seminal vesicles. A, Hematoxylin and eosin staining of d 100 PTM-ARKO and control SVs composed of epithelia surrounded by a stromal compartment (arrow); this epithelium appeared more folded in PTM-ARKO SVs than control (arrowhead). Note the dense eosinophilic seminal secretions in the lumen of both the PTM-ARKO and control SV (*). Scale bars, 400 and 50 μm, respectively. B, Immunohistochemistry for cytokeratin (brown), highlighting the epithelial cells in d 100 PTM-ARKO and control SVs. C, Note that epithelial cell height is significantly reduced in PTM-ARKOs at d 100 but not d 12, compared with age-matched controls. Scale bars, 50 μm. D, SMA immunostaining (brown) identifying the smooth muscle cell layer (arrow) directly surrounding the epithelium in d 100 PTM-ARKO and control SVs; note the presence of an outer SMA-negative layer (arrowhead). E, The smooth muscle layer is narrower in the PTM-ARKO than the control, as confirmed quantitatively. Values are means ± sem (n = 3 mice). *, P < 0.05, **, P < 0.01, compared with age-matched controls. Scale bars, 50 μm.
Proliferation and apoptosis in PTM-ARKO SVs
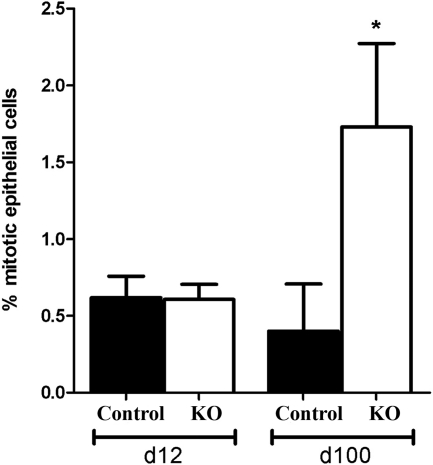
There was no significant difference in the number of mitotic cells in PTM-ARKO SVs at d 12, compared with controls; however, there was a significant increase in epithelial cell mitosis at d 100 in PTM-ARKOs compared with controls (Fig. 4). Very few apoptotic cells were identified in either KO or control SVs at d 12 or 100 (data not shown).
Figure 4.
Cell mitosis in PTM-ARKO seminal vesicles. Quantification of epithelial cell mitotic index in d 12 and 100 PTM-ARKO and control SVs. Values are means ± sem (n = 3 mice). *, P < 0.05, compared with age-matched control littermates.
Secretion of SV plasma proteins in PTM-ARKOs
Four main proteins were identified when SV secretions from PTM-ARKO and control mice were subjected to electrophoresis; there was no obvious difference in the protein fingerprint observed between PTM-ARKO and controls (Fig. 5A). Quantitative RT-PCR for genes known to be involved in SV function revealed no significant difference in expression of SVS2/3a/3b/5, renin-1, TGM-4, or Mt-1, but there was a significant increase in expression of Cox-1 mRNA, compared with controls (normalized to 18S expression; Fig. 5B).
Figure 5.
SV secretion production in PTM-ARKO mice. A, Seminal secretion protein expression in PTM-ARKO and control adult mice. There are four major proteins observed in the seminal secretions in both KO and control mice with no obvious differences in expression. B, Relative gene expression in SVs from d 100 PTM-ARKO and control mice. Note that the only gene that significantly changes is Cox-1, which is significantly increased in PTM-ARKO mice, compared with controls. Values are means ± sem (n = 3 mice). *, P < 0.05, compared with control littermates.
Expression of ERs in SVs from PTM-ARKO adult mice
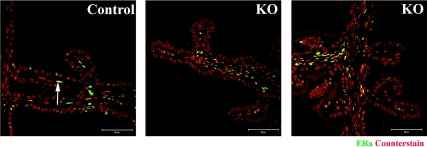
Immunohistochemistry demonstrated that ERα is expressed in stromal and occasional epithelial cells in both control and PTM-ARKO adult SVs (Fig. 6), therefore demonstrating that KO SVs are capable of responding to estrogens.
Figure 6.
ER expression in adult PTM-ARKO SVs. Immunoexpression of ERα (green) in adult PTM-ARKO and control SVs; nuclei are counterstained red.
Gross morphology of SVs from adult PTM-ARKO mice treated with exogenous T and E2
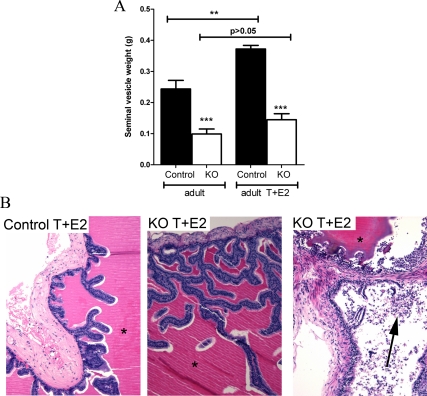
Treatment with estrogens is reported to stimulate epithelial proliferation in SVs and prostates (26,27,32) and induces prostate squamous metaplasia (31). Exogenous estrogens result in suppression of gonadotropins and so testicular T production. We therefore treated control and PTM-ARKO adult male mice with exogenous T and E2 (T+E2) for 13 wk to assess their response to estrogens without causing SV involution due to reduced serum T. This treatment resulted in normal serum T concentrations (3.5 ng/ml−1 ± 0.17 in controls and 4 ng/ml−1 ± 0.47 in PTM-ARKOs); this was not significantly different from untreated mice (44). Serum E2 concentrations were 2200 pg/ml−1 ± 57 in T+E2-treated controls and 2404 pg/ml−1 ± 57 in T+E2-treated KOs; this is an approximately 400-fold increase compared with serum estradiol concentrations in control and KO males not treated with T+E2. SVs recovered from adult control mice exposed to T+E2 were significantly larger than those from controls not treated with T+E2; conversely, there was no significant change in SV weight in PTM-ARKO mice after treatment with T+E2 (Fig. 7A).
Figure 7.
Gross morphology and histology of adult PTM-ARKO mice exposed to exogenous T and E2. A, Quantification of SV weight in adult PTM-ARKO mice exposed to T and E2 for 13 wk. Note the increase in SV weight in control but not KO mice after exposure to T+E2. B, Hematoxylin and eosin staining of adult PTM-ARKO and control SVs exposed to T+E2, with dense esinophilic seminal secretion in the lumen of both the PTM-ARKO and control SV (*). Note the densely packed cell nuclei in both the stroma and epithelia in control and PTM-ARKO SVs exposed to T+E2; this is more pronounced in KOs than controls, with desquamation of epithelial cells obvious in some KO SVs (arrow). Values are means ± sem (n = 3 mice). ***, P < 0.001, compared with control littermates; **, P < 0.01, compared with untreated control mice.
Histology of PTM-ARKO SVs exposed to T and E2
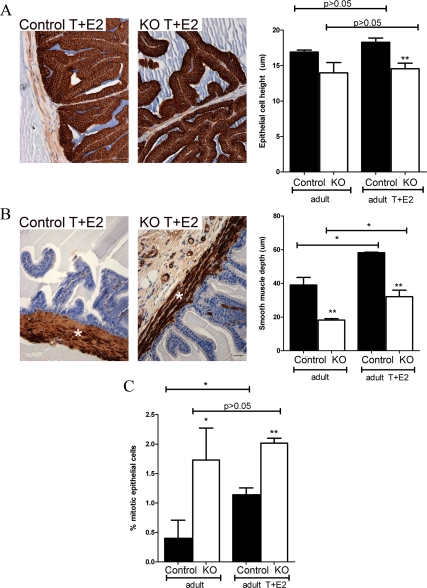
Upon gross histological inspection, the T+E2-treated control and PTM-ARKO SV contained eosinophilic secretions in the lumen and showed signs of hyperplasia and hypertrophy (Fig. 7B), as seen from the densely packed nuclei in the stroma and epithelium. This hyperplasia and hypertrophy was not obvious in untreated control or PTM-ARKO SVs (Fig. 3A) and was more severe in the T+E2-treated PTM-ARKO SV than in T+E2-treated control SVs (confirmed by an expert pathologist). This hyperplasia resulted in desquamation of epithelial cells in some KO SVs due to overcrowding of epithelial cells and sloughing off (Fig. 7B, right panel). There was also an apparent increase in epithelial branching in both the control and PTM-ARKO SVs exposed to T+E2 (Fig. 7B), compared with untreated control and PTM-ARKO SVs, respectively (Fig. 3A). Epithelial cell height did not significantly change in SVs from PTM-ARKO and control mice treated with T+E2, compared with untreated PTM-ARKO and control SVs, respectively (Fig. 8A). Epithelial cell height therefore remained significantly smaller in T+E2-treated PTM-ARKO SVs than controls (Fig. 8A). However, the depth of the SV smooth muscle cell layer significantly increased in T+E2-treated mice, compared with untreated mice (Fig. 8B); however, the smooth muscle layer remained significantly smaller in T+E2-treated PTM-ARKO SVs, compared with T+E2-treated controls (Fig. 8B). Quantification of the percentage of epithelial cells undergoing mitosis in adult PTM-ARKO and control SVs treated with T+E2 revealed significantly more epithelial cell mitosis in PTM-ARKO SVs than in controls, both with and without T+E2 exposure (Fig. 8C). There was a significant increase in epithelial mitosis in control SVs treated with T+E2, compared with untreated controls, but treatment with T+E2 did not significantly alter epithelial cell mitosis in PTM-ARKO SVs (Fig. 8C).
Figure 8.
Quantification of histological changes in adult PTM-ARKO seminal vesicles after exposure to T and E2. A, Epithelial cell height (immunostained for cytokeratin, brown) is significantly reduced in PTM-ARKO SVs treated with T+E2, compared with T+E2-treated controls (as reported in untreated mice). Exposure to T+E2 does not result in any change in epithelial cell height, compared with untreated PTM-ARKOs and controls, respectively. B, The depth of the smooth muscle cell layer (immunostained for SMA, brown, *) is significantly smaller in PTM-ARKO SVs treated with T+E2, compared with treated controls. Note that exposure to T+E2 results in a significant increase in this smooth muscle layer in PTM-ARKO and control SVs, compared with untreated PTM-ARKOs and controls, respectively. C, Quantification of epithelial cell mitosis revealed a significant increase in epithelial mitosis in control SVs treated with T+E2, compared with untreated controls. Note that there is significantly more epithelial cell mitosis in PTM-ARKO SVs than controls but that treatment with T+E2 does not significantly alter epithelial cell mitosis in PTM-ARKO mice. Values are means ± sem (n = 3 mice). *, P < 0.05, compared with control littermates or untreated mice; **, P < 0.01, compared with control littermates.
Discussion
Androgens and stromal-epithelial interactions are pivotal in the development and function of the male reproductive system (1,2); disruption of these can result in pathologies later in life. The cell-specific role for androgen action in adult SVs, however, remains unclear. The aim of this study was to investigate the impact of ablating androgen action specifically from SV smooth muscle cells on SV development and adult function. Our analysis revealed that the smooth muscle cells play an important role in androgen-driven stromal-epithelial interactions in the SV, determining epithelial cell structure and function as well as limiting the SV epithelial proliferative response to exogenous estrogens.
Cross-breeding ARflox female mice with smooth muscle myesin heavy chain-Cre male mice ablated AR from the smooth muscle cells in the SVs from as early as e17.5. In contrast to ARKO mice (43), PTM-ARKO males showed normal external sexual development and anogenital distance (44) and SVs formed normally, but there was a significant reduction in SV weight after puberty. Serum T and estrogen concentrations were normal in PTM-ARKO mice, confirming that the reduction in adult SV size must be due to ablation of AR from the SV smooth muscle. Furthermore, expression of Cre alone, without ARflox expression, did not result in any changes in SV weight or histology. The phenotype of the SVs in PTM-ARKOs is similar to that recently reported by Simanainen et al. (48), who deleted AR from the SV stromal smooth muscle using a probasin-driven Cre (named PEARKO mice); comparison of these two independent mouse models is summarized in Supplemental Table 1 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. The probasin-driven Cre is expressed from postnatal wk 5 in PEARKO mice, but it is unclear whether Cre expression is restricted solely to the smooth muscle layer or is expressed in all stromal cells in the SV; this uncertainty was not clarified by Jin et al. (53), who generated this probasin Cre mouse. Our PTM-ARKO mouse study therefore offers new evidence that AR signaling specifically via the smooth muscle cells alone is important for adult SV size and function.
SVs formed normally in the PTM-ARKO mice, and there was no obvious change in SV weight before d 35, even though AR ablation could be identified at e17.5. Because AR expression in the mesenchyme of the developing sex accessory organs is critical for normal development and epithelial identity (39,54), ablation of AR from the fetal smooth muscle might have been expected to affect SV formation. However, Cre expression, and hence AR deletion, will not be induced until the smooth muscle layer has actually differentiated; this happens during late fetal life, after the SV have been preprogrammed to form (9); therefore, onset of Cre expression in the PTM-ARKO is likely to be too late to affect SV formation. This PTM-ARKO model therefore offers a unique opportunity to interrogate androgen action specifically in the smooth muscle layer of the stroma without preventing SV formation in fetal life. However, we cannot rule out a prepubertal role for AR signaling via the smooth muscle cells of the SV because, even though there was no significant decrease in SV weight before d 35, a reduction in the depth of the smooth muscle layer could be identified at d 12.
Epithelial cells are normally pseudostratified and columnar in adult SVs, with epithelial cell height reported to be androgen dependent (55), possibly acting as a more sensitive marker of androgen action than gross changes in gland weight (55). Epithelial cell height was significantly reduced in adult PTM-ARKO SVs, demonstrating that the smooth muscle cells are important to specifying the identity of the overlying epithelium during development and in adulthood. This dedifferentiation of the SV epithelium in PTM-ARKOs may explain the reduction in epithelial cell function reflected in reduced SV secretion volume. Interestingly, there was a significant increase in SV epithelial cell height from d 12 to d 100 in controls, but this increase was less apparent in PTM-ARKOs, demonstrating that the SV epithelium differentiates as it matures and that this is dependent, at least in part, on AR signaling via the smooth muscle cells. The depth of the smooth muscle layer was also reduced in PTM-ARKO SVs, compared with controls. Unlike changes to the epithelium, the reduced depth of the smooth muscle layer was evident in PTM-ARKOs at both d 12 and 100. This agrees with the phenotype previously reported in PEARKO mice (Supplemental Table 1) (48) and suggests that AR signaling within the smooth muscle layer itself determines its depth and that this in turn may play a role in SV weight and epithelial function in adulthood.
The reduction in adult SV weight in PTM-ARKOs was due to a reduction in the weight of the SV tissue plus a reduction in SV secretions, a function of the epithelial cells. The reduction in SV secretions was disproportionate to the reduction in SV tissue weight, suggesting that ablation of AR specifically from the smooth muscle impairs, but does not completely abolish, normal adult SV secretory function. This is supported by the detection of a similar protein fingerprint for SV secretions from PTM-ARKO adult males to that in control males. PTM-ARKO SV epithelial cells are able to make the major proteins that contribute to seminal plasma, suggesting a global impact on epithelial cell function without selectively affecting production of specific proteins. This may result in smaller, more fragile plugs as reported in the PEARKO (48), thus explaining why PTM-ARKO mice rarely (one of 18 matings) produced copulatory plugs, unlike their control littermates (produced plugs in 17 of 24 matings). This impaired seminal plasma production may be a direct result of AR ablation from the SV smooth muscle or indirectly due to the thinner layer of smooth muscle in these mice. Our findings therefore confirm the important role for androgen-driven sex accessory organ function in fertility and demonstrate that the presence of a functioning androgen-responsive smooth muscle compartment is critical for normal SV epithelial function.
Interestingly, relative expression of some genes important in seminal fluid production (namely SVS2/3/5, Mt-1, and TGM-4) was not significantly altered in PTM-ARKO SVs, consistent with the view that ablating AR from the smooth muscle has a global impact on epithelial function, rather than impairing production of specific proteins. However, relative expression of Cox-1 mRNA was significantly increased in SVs from PTM-ARKO adults compared with controls. Cox-1 is constitutively expressed in mouse SV epithelium to produce prostaglandins (16), which have been suggested to be involved in sperm motility and capacitation (56) and relaxation of the smooth muscle of the uterus (57). It is not immediately clear why expression of Cox-1 increases in PTM-ARKO mice, but it is indicative of altered epithelial function in these mice; this may be a direct result of the absence of smooth muscle AR or an indirect consequence of the reduced smooth muscle layer in these mice.
Growth and development of an organ depends on not just differentiation of the tissue but also a balance of cell proliferation and apoptosis. Apoptosis was not obvious in the SV from either control or PTM-ARKO mice at d 12 or 100, but epithelial cell proliferation was significantly increased in adult PTM-ARKOs, resulting in obvious hyperplasia. Epithelial cell proliferation is dependent on androgen action in SVs and prostates (55), and it has been demonstrated using prostatic tissue recombination experiments that stromal, not epithelial, AR is important in regulating epithelial cell proliferation in the prostate (39,54,58). However, what controls epithelial cell proliferation in adult SVs is less clear. Our study demonstrates that ablating AR from smooth muscle cells increased epithelial cell proliferation, implying that androgen signaling via the smooth muscle normally suppresses epithelial cell proliferation in adulthood to maintain tissue homeostasis; this may be a direct effect on the epithelial cells themselves or an indirect effect due to changes in the proportion of smooth muscle in the SVs, i.e. as reported in the prostate (reviewed in Ref. 59). Interestingly, epithelial cell-specific ablation of AR in the prostate also results in increased proliferation (60,61). These results could imply that epithelial cell proliferation is differentially regulated in the prostate and SVs or that it is a balance of androgen action via either epithelial and/or smooth muscle cells that is important and impairing either is detrimental to normal tissue homeostasis.
Adult prostates and SVs are able to directly respond to both androgens and estrogens as they express both AR (33) and ERα and -β. Previous studies demonstrated that altering the androgen-estrogen balance can affect the adult male accessory sex organs (26,27,28,29,30,31,32). For example, exogenous estrogens directly stimulate epithelial cell proliferation in the SVs and prostate (26,27,32,62) as well as inducing prostate squamous metaplasia via ERα (31). We demonstrated that PTM-ARKO SVs continued to express ERα and therefore should still be capable of responding to estradiol treatment; therefore, we investigated whether a lack of smooth muscle AR affected PTM-ARKO SV response to exogenous E2. Because E2 exposure results in suppression of gonadotropins and thus of testicular T production, which would result in SV involution (63), we treated control and PTM-ARKO adult male mice with exogenous T and E2 (T+E2) for 13 wk to assess their response to estrogens without causing SV involution. This treatment regimen resulted in normal serum T levels in PTM-ARKOs and controls, but serum E2 concentrations were elevated 400-fold compared with nontreated PTM-ARKO and controls; therefore, any effects seen after this treatment are likely to be direct effects of estrogens. Exposure to T+E2 resulted in a significant increase in SV weight in controls but not PTM-ARKOs but had no effect on epithelial cell height, suggesting that estrogens do not directly affect epithelial cell differentiation. On the other hand, this treatment resulted in a significant increase in the depth of the smooth muscle layer in both control and PTM-ARKO SVs. As a similar increase was seen in both controls and PTM-ARKOs, which have no AR in the smooth muscle cells to respond to the T, this increase is likely to be mediated by the E2. Exposure to T+E2 also increased epithelial cell proliferation in both controls and PTM-ARKO SVs, resulting in hyperplasia and hypertrophy of the epithelium and stroma. This hyperplasia was more pronounced in the PTM-ARKO than in control and resulted in overcrowding of the epithelium and thus desquamation in PTM-ARKOs; the latter change was not seen in any of the SVs from controls examined. There were no obvious signs of inflammation in either controls or PTM-ARKO SVs treated with T+E2. Our results demonstrate that smooth muscle AR signaling suppresses epithelial cell proliferation in adult SVs and that, in its absence, estrogens can cause uncontrolled proliferation and hyperplasia in the SV.
In conclusion, we have successfully generated a PTM-ARKO mouse SV model that demonstrates the essential role for AR signaling via smooth muscle cells in normal SV function and male fertility. This mouse model provides a unique tool for identifying the underlying molecular mechanisms of androgen action in the SVs without preventing SV formation. Further investigations with these mice could provide new insight for the development of new male contraceptives and treatments for male infertility.
Acknowledgments
We are grateful to Karel De Gendt and Guido Verhoeven for providing the ARflox mice and Michael Kotlikoff for the smMHC-Cre mice. We thank Axel Thomson for advice in developing the testosterone/estradiol treatments. We thank Mark Fisken, Nancy Nelson, Chris McKinnell, and the members of the imaging facility and assay laboratory for technical assistance.
Footnotes
This work was supported by the United Kingdom Medical Research Council (WBS U.1276.00.002.0003.01).
Disclosure Summary: M.W., L.M., L.J., A.M., D.B., P.T.K.S., R.M.S., and L.B.S. have nothing to declare.
First Published Online May 5, 2010
Abbreviations: AR, Androgen receptor; Cox, cyclooxygenase; DHT, dihydrotestosterone; e, embryonic day; E2, estradiol; ER, estrogen receptor; GFP, green fluorescent protein; KO, knockout; MH, myosin heavy-chain promoter; Mt-1, metallothionein-1; PTM-ARKO, testicular smooth muscle cell ARKO; SMA, smooth muscle actin; SV, seminal vesicle; SVS, SV secretion protein; T, testosterone; TGM4, transglutiminase-4; WT, wild type.
References
- George FW, Wilson J 1994 Gonads and ducts in mammals. In: Knobil E, Neill JD, eds. The physiology of reproduction. 2nd ed. New York: Raven Press; 3–27 [Google Scholar]
- Mooradian AD, Morley JE, Korenman SG 1987 Biological actions of androgens. Endocr Rev 8:1–28 [DOI] [PubMed] [Google Scholar]
- Wilson JD, George FW, Griffin JE 1981 The hormonal control of sexual development. Science 211:1278–1284 [DOI] [PubMed] [Google Scholar]
- Pointis G, Latreille MT, Cedard L 1980 Gonado-pituitary relationships in the fetal mouse at various times during sexual differentiation. J Endocrinol 86:483–488 [DOI] [PubMed] [Google Scholar]
- Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS 1995 Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 16:271–321 [DOI] [PubMed] [Google Scholar]
- Lyon MF, Hawkes SG 1970 X-linked gene for testicular feminization in the mouse. Nature 227:1217–1219 [DOI] [PubMed] [Google Scholar]
- Quigley CA 2002 The postnatal gonadotropin and sex steroid surge-insights from the androgen insensitivity syndrome. J Clin Endocrinol Metab 87:24–28 (Editorial) [DOI] [PubMed] [Google Scholar]
- Brinkmann AO 2001 Molecular basis of androgen insensitivity. Mol Cell Endocrinol 179:105–109 [DOI] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM 2008 Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest 118:1479–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Marchetti NI, Sharpe RM 2006 Androgen-dependent mechanisms of Wolffian duct development and their perturbation by flutamide. Endocrinology 147:4820–4830 [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Cala KM, Hess DL, Russell DW 2001 Unexpected virilization in male mice lacking steroid 5α-reductase enzymes. Endocrinology 142:4652–4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitz B, Olds-Clarke P 1986 Effects of seminal vesicle removal on fertility and uterine sperm motility in the house mouse. Biol Reprod 35:608–617 [DOI] [PubMed] [Google Scholar]
- Pang SF, Chow PH, Wong TM 1979 The role of the seminal vesicles, coagulating glands and prostate glands on the fertility and fecundity of mice. J Reprod Fertil 56:129–132 [DOI] [PubMed] [Google Scholar]
- Gonzales GF 2001 Function of seminal vesicles and their role on male fertility. Asian J Androl 3:251–258 [PubMed] [Google Scholar]
- Luke MC, Coffey DS 1994 The male sex accessory tissue. In: Knobil E, Neill JD, eds. The physiology of reproduction. New York: Raven Press; 1435–1487 [Google Scholar]
- Balaji T, Ramanathan M, Sirinivasan M, Menon M 2008 Distribution of cyclooxygenase-1 and cycoloxygenase-2 in the mouse seminal vesicle. J Appl Biomed 6:97–107 [Google Scholar]
- Lundwall A, Malm J, Clauss A, Valtonen-Andre C, Olsson AY 2003 Molecular cloning of complementary DNA encoding mouse seminal vesicle-secreted protein SVS I and demonstration of homology with copper amine oxidases. Biol Reprod 69:1923–1930 [DOI] [PubMed] [Google Scholar]
- Kawano N, Yoshida M 2007 Semen-coagulating protein, SVS2, in mouse seminal plasma controls sperm fertility. Biol Reprod 76:353–361 [DOI] [PubMed] [Google Scholar]
- Lin TM, Ko K, Moore RW, Simanainen U, Oberley TD, Peterson RE 2002 Effects of aryl hydrocarbon receptor null mutation and in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on prostate and seminal vesicle development in C57BL/6 mice. Toxicol Sci 68:479–487 [DOI] [PubMed] [Google Scholar]
- Ialenti A, Santagada V, Caliendo G, Severino B, Fiorino F, Maffia P, Ianaro A, Morelli F, Di Micco B, Cartenì M, Stiuso P, Metafora V, Metafora S 2001 Synthesis of novel anti-inflammatory peptides derived from the amino-acid sequence of the bioactive protein SV-IV. Eur J Biochem 268:3399–3406 [DOI] [PubMed] [Google Scholar]
- Jonsson M, Lundwall A, Malm J 2006 The semenogelins: proteins with functions beyond reproduction? Cell Mol Life Sci 63:2886–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HJ, Luo CW, Chen YH 2002 Localization of the transglutaminase cross-linking site in SVS III, a novel glycoprotein secreted from mouse seminal vesicle. J Biol Chem 277:3632–3639 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Donjacour A 1987 Stromal-epithelial interactions in normal and abnormal prostatic development. Prog Clin Biol Res 239:251–272 [PubMed] [Google Scholar]
- Deanesly R, Parkes AS 1933 Size changes in the seminal vesicles of the mouse during development and after castration. J Physiol 78:442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deanesly R, Parkes AS 1936 Comparative activities of compounds of the androsterone-testosterone series. Biochem J 30:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco JJ, McPherson SJ, Wang H, Prins GS, Risbridger GP 2006 Transient neonatal estrogen exposure to estrogen-deficient mice (aromatase knockout) reduces prostate weight and induces inflammation in late life. Am J Pathol 168:1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco JJ, Handelsman DJ, Pedersen JS, Risbridger GP 2002 Direct response of the murine prostate gland and seminal vesicles to estradiol. Endocrinology 143:4922–4933 [DOI] [PubMed] [Google Scholar]
- Williams K, McKinnell C, Saunders PT, Walker M, Fisher JS, Turner KJ, Atanassova N, Sharpe M 2001 Neonatal exposure to potent and environmental oestrogens and abnormalities of the male reproductive system in the rat: evidence for importance of the androgen-oestrogen balance and assessment of the relevance to man. Hum Reprod Update 7:236–247 [DOI] [PubMed] [Google Scholar]
- McKinnell C, Atanassova N, Williams K, Fisher JS, Walker M, Turner KJ, Saunders TK, Sharpe RM 2001 Suppression of androgen action and the induction of gross abnormalities of the reproductive tract in male rats treated neonatally with diethylstilbestrol. J Androl 22:323–338 [PubMed] [Google Scholar]
- Rivas A, McKinnell C, Fisher JS, Atanassova N, Williams K, Sharpe RM 2003 Neonatal coadministration of testosterone with diethylstilbestrol prevents diethylstilbestrol induction of most reproductive tract abnormalities in male rats. J Androl 24:557–567 [DOI] [PubMed] [Google Scholar]
- Risbridger G, Wang H, Young P, Kurita T, Wang YZ, Lubahn D, Gustafsson JA, Cunha G, Wong YZ 2001 Evidence that epithelial and mesenchymal estrogen receptor-α mediates effects of estrogen on prostatic epithelium. Dev Biol 229:432–442 [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, Dhar MD, Ganjam VK, Parmigiani S, Welshons WV 1997 Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci USA 94:2056–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdic G, Millar MR, Saunders PT 1995 Immunolocalisation of androgen receptor to interstitial cells in fetal rat testes and to mesenchymal and epithelial cells of associated ducts. J Endocrinol 147:285–293 [DOI] [PubMed] [Google Scholar]
- Saunders PT, Maguire SM, Gaughan J, Millar MR 1997 Expression of oestrogen receptor β (ERβ) in multiple rat tissues visualised by immunohistochemistry. J Endocrinol 154:R13–R16 [DOI] [PubMed] [Google Scholar]
- Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS 1997 Tissue distribution and quantitative analysis of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology 138:4613–4621 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Alarid ET, Turner T, Donjacour AA, Boutin EL, Foster BA 1992 Normal and abnormal development of the male urogenital tract. Role of androgens, mesenchymal-epithelial interactions, and growth factors. J Androl 13:465–475 [PubMed] [Google Scholar]
- Donjacour AA, Cunha GR 1991 Stromal regulation of epithelial function. Cancer Treat Res 53:335–364 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y 1987 The endocrinology and developmental biology of the prostate. Endocr Rev 8:338–362 [DOI] [PubMed] [Google Scholar]
- Cunha GR 1976 Epithelial-stromal interactions in development of the urogenital tract. International Rev Cytol 47:137–194 [DOI] [PubMed] [Google Scholar]
- Higgins SJ, Young P, Cunha GR 1989 Induction of functional cytodifferentiation in the epithelium of tissue recombinants. II. Instructive induction of Wolffian duct epithelia by neonatal seminal vesicle mesenchyme. Development 106:235–250 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Wang YZ 2002 Role of stroma in carcinogenesis of the prostate. Differentiation 70:473–485 [DOI] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S 2004 Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA 101:6876–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lécureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G 2004 A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 101:1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Atanassova N, Sharpe RM, Smith LB 2009 Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J 23:4218–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Lin HY, Yeh SD, Yu IC, Wang RS, Chen YT, Zhang C, Altuwaijri S, Chen LM, Chuang KH, Chiang HS, Yeh S, Chang C 2007 Infertility with defective spermatogenesis and steroidogenesis in male mice lacking androgen receptor in Leydig cells. Endocrine 32:96–106 [DOI] [PubMed] [Google Scholar]
- Zhang C, Yeh S, Chen YT, Wu CC, Chuang KH, Lin HY, Wang RS, Chang YJ, Mendis-Handagama C, Hu L, Lardy H, Chang C 2006 Oligozoospermia with normal fertility in male mice lacking the androgen receptor in testis peritubular myoid cells. Proc Natl Acad Sci USA 103:17718–17723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin HB, Deng KY, Rishniw M, Ji G, Kotlikoff MI 2002 Smooth muscle expression of Cre recombinase and eGFP in transgenic mice. Physiol Genomics 10:211–215 [DOI] [PubMed] [Google Scholar]
- Simanainen U, McNamara K, Davey RA, Zajac JD, Handelsman DJ 2008 Severe subfertility in mice with androgen receptor inactivation in sex accessory organs but not in testis. Endocrinology 149:3330–3338 [DOI] [PubMed] [Google Scholar]
- Ricke WA, Ishii K, Ricke EA, Simko J, Wang Y, Hayward SW, Cunha GR 2006 Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer 118:2123–2131 [DOI] [PubMed] [Google Scholar]
- Corker CS, Davidson DW 1978 A radioimmunoassay for testosterone in various biological fluids without chromatography. J Steroid Biochem 9:373–374 [DOI] [PubMed] [Google Scholar]
- Mann GE, Lamming GE 1995 Effects of treatment with buserelin on plasma concentrations of oestradiol and progesterone and cycle length in the cow. Br Vet J 151:427–432 [DOI] [PubMed] [Google Scholar]
- Hooley RP, Paterson M, Brown P, Kerr K, Saunders PT 2009 Intra-testicular injection of adenoviral constructs results in Sertoli cell-specific gene expression and disruption of the seminiferous epithelium. Reproduction 137:361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, McKeehan K, Wang F 2003 Transgenic mouse with high Cre recombinase activity in all prostate lobes, seminal vesicle, and ductus deferens. Prostate 57:160–164 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Ricke W, Thomson A, Marker PC, Risbridger G, Hayward SW, Wang YZ, Donjacour AA, Kurita T 2004 Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol 92:221–236 [DOI] [PubMed] [Google Scholar]
- Nishino T, Wedel T, Schmitt O, Bühlmeyer K, Schönfelder M, Hirtreiter C, Schulz T, Kühnel W, Michna H 2004 Androgen-dependent morphology of prostates and seminal vesicles in the Hershberger assay: evaluation of immunohistochemical and morphometric parameters. Ann Anat 186:247–253 [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Yorimitsu A, Maruyama Y, Kubota T, Aso T, Bronson RA 1998 Prostaglandins induce calcium influx in human spermatozoa. Mol Hum Reprod 4:555–561 [DOI] [PubMed] [Google Scholar]
- Kelly RW 1981 Prostaglandin synthesis in the male and female reproductive tract. J Reprod Fertil 62:293–304 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Lung B 1978 The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool 205:181–193 [DOI] [PubMed] [Google Scholar]
- Lee C 1996 Role of androgen in prostate growth and regression: stromal-epithelial interaction. Prostate Suppl 6:52–56 [PubMed] [Google Scholar]
- Wu CT, Altuwaijri S, Ricke WA, Huang SP, Yeh S, Zhang C, Niu Y, Tsai MY, Chang C 2007 Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci USA 104:12679–12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanainen U, Allan CM, Lim P, McPherson S, Jimenez M, Zajac JD, Davey RA, Handelsman DJ 2007 Disruption of prostate epithelial androgen receptor impedes prostate lobe-specific growth and function. Endocrinology 148:2264–2272 [DOI] [PubMed] [Google Scholar]
- Pylkkänen L, Santti R, Newbold R, McLachlan JA 1991 Regional differences in the prostate of the neonatally estrogenized mouse. Prostate 18:117–129 [DOI] [PubMed] [Google Scholar]
- Evans GS, Chandler JA 1987 Cell proliferation studies in the rat prostate: II. The effects of castration and androgen-induced regeneration upon basal and secretory cell proliferation. Prostate 11:339–351 [DOI] [PubMed] [Google Scholar]