Abstract
Guided by evolutionary-developmental theories of biological sensitivity to context and reproductive development, the current research examined the interactive effects of early family environments and psychobiologic reactivity to stress on the subsequent timing and tempo of puberty. As predicted by the theory, among children displaying heightened biological sensitivity to context (i.e., higher stress reactivity), higher quality parent-child relationships forecast slower initial pubertal tempo and later pubertal timing while lower quality parent-child relationships forecast the opposite pattern. No such effects emerged among less context-sensitive children. Whereas sympathetic nervous system reactivity moderated the effects of parent-child relationships on both breast/genital and pubic hair development, adrenocortical activation only moderated the effect on pubic hair development. The current results build on previous research documenting what family contexts predict variation in pubertal timing by demonstrating for whom those contexts matter. In addition, the authors advance a new methodological approach for assessing pubertal tempo using piecewise growth curve analysis.
Pubertal maturation is neither unitary nor uniform. Indeed, there are multiple pubertal processes that occur in different people—and within the same person—at different ages and rates of development (Dorn, Dahl, Woodward, & Biro, 2006; Styne & Grumbach, 2002). This variation is not an isolated phenomenon, moreover, but part of a larger developmental continuum. At early points on the continuum are upstream developmental factors and processes (e.g., nutritional history, energetic expenditures, family conflict and cohesion) that influence the timing and tempo of puberty (reviewed in Ellis, 2004). At later points on the continuum are the downstream consequences of this variation—consequences that have substantial social and biological implications.
Epidemiologic evidence from ethnically diverse populations indicates that early-maturing girls, relative to their on-time or later developing peers, are at elevated risk for a variety of negative physical and mental health outcomes, including unhealthy weight gain, early initiation of substance use, early sexual initiation and pregnancy, emotional and behavioral problems, and mortality from cardiovascular disease and breast cancer (Bratberg, Nilsen, Holmen, & Vatten, 2007; Colditz & Frazier, 1999; Deardorff et al., 2005, 2007; Dick et al., 2000; Golub et al., 2008; Jacobsen, Oda, Knutsen & Fraser, 2009; Kelsey, Gammon, & John, 1993; Lakshman et al., 2009; Vo & Carney, 2007). This is particularly concerning given the ongoing trend toward younger age of breast development and menarche among girls in the United States (Euling et al, 2008; Biro et al., 2010).
Beyond the effects of pubertal timing, pubertal tempo—the rate at which adolescents progress through puberty—has also been implicated in development of psychopathology and physical health problems. Changing levels of exposure to steroid hormones underpin changes in pubertal tempo, with associated emotional and behavioral sequelae (Parry & Newton, 2001). A fast pubertal tempo not only causes maturational events to occur sooner, but may itself be psychologically challenging. Indeed, periods of faster pubertal maturation and rapid hormonal changes, including punctuated development of secondary sexual characteristics, negatively affect mood and adjustment in adolescents (Buchanan, Eccles, & Becker, 1992; Ge et al., 2003; Slap, Khalid, Paikoff, & Brooks-Gunn, 1994; Warren & Brooks-Gunn, 1989). In addition to the challenges of fast pubertal tempo, slow sexual maturation may also augment risk. Although research on physical health outcomes is limited, there is reason to believe that slowing of pubertal tempo may extend the window of vulnerability for breast carcinogenesis, given prolonged exposure to endogenous hormones during this period of high mammary cell proliferation and differentiation (Stoll, Vatten, & Kvinnsland, 1994).
These links between the timing and tempo of puberty and psychiatric and biomedical outcomes underscore the need to delineate the life experiences and pathways that influence pubertal maturation. This delineation would have great relevance to the long-term goal of informing early intervention and prevention strategies for high-risk youth. Toward this end, the current study—guided by evolutionary models of reproductive development and biological sensitivity to context—examined the interactive effects of early family environments and psychobiologic reactivity to stress on timing and tempo of puberty. In addition, this paper advances a new methodological approach to assessing pubertal tempo using growth curve analysis.
An Evolutionary-Developmental Model of Pubertal Maturation
An important framework for analyzing psychosocial influences on pubertal processes is Belsky, Steinberg, and Draper’s (1991) evolutionary theory of reproductive development, which focuses on the role of familial and ecological stressors in accelerating pubertal maturation. Drawing on life history theory in evolutionary biology, the theory posited that “a principal evolutionary function of early experience—the first 5–7 years of life—is to induce in the child an understanding of the availability and predictability of resources (broadly defined) in the environment, of the trustworthiness of others, and of the enduringness of close interpersonal relationships, all of which will affect how the developing person apportions reproductive effort” (p. 650). Belsky et al. theorized that humans have evolved to be sensitive to specific features of their early childhood environments, and that exposure to different environments biases children toward the development of different reproductive strategies. Ecological stressors in and around the family (e.g., scarcity or instability of resources) create conditions that undermine parental functioning and lower the quality of parental investment (such as by escalating marital conflict, increasing negativity and coercion in parent-child relationships, and reducing positivity and support in parent-child relationships). The theory posits that children respond to these familial and ecological contexts by developing in a manner that speeds pubertal maturation (i.e., provokes earlier onset of puberty or faster pubertal tempo leading to more advanced pubertal development at earlier ages), accelerates sexual activity, and orients the individual toward relatively unstable pairbonds. In contrast, children whose experiences in and around their families are characterized by relatively high levels of support and stability are hypothesized to develop in the opposite manner (Belsky et al., 1991). Either way, the theory postulates that children adaptively adjust pubertal development to match local conditions.
In a comprehensive review of the literature on Belsky et al.’s (1991) model, Ellis (2004, p. 935–36) concluded: “Empirical research to date has provided reasonable, though incomplete, support for the theory.” The most reliable empirical phenomenon, documented across a number of methodologically sound studies, is that greater parent-child warmth and cohesion forecasts later pubertal development in girls (prospective, longitudinal studies: Ellis, McFadyen-Ketchum, Dodge, Pettit, & Bates, 1999; Ellis & Essex, 2007; Graber, Brooks-Gunn, & Warren, 1995; Steinberg, 1988; cross-sectional or retrospective studies: Kim & Smith, 1998; Kim, Smith, & Palermiti, 1997; Miller & Pasta, 2000; Romans et al., 2003; Rowe, 2000). Although less consistently documented in the literature, an emerging body of research also suggests that harsh parenting, paternal social deviance, and child maltreatment predict earlier pubertal development in daughters (Belsky et al., 2007; Costello, Sung, Worthman, & Angold, 2007; Tither & Ellis, 2008). While these effects have proven replicable, effect sizes have been consistently small, with standardized regression coefficients typically around .20 or less.
Biological Sensitivity to Context
These small overall effects sizes (i.e., main effects) may reflect the fact the children differ in whether, how, and how much they are affected by rearing experiences (reviewed in Belsky, 2005; Belsky & Pleuss, 2009; Boyce & Ellis, 2005; Obradović & Boyce, 2009). Indeed, evolutionary-biological models posit that variation in susceptibility to environmental influence has been maintained by natural selection (Belsky, 2005; Boyce & Ellis, 2005; Ellis, Jackson, & Boyce, 2006; Wolf, van Doorn, & Weissing, 2008). An implication of this differential susceptibility, as articulated by Belsky (2000), is that the small main effects of parenting processes on pubertal timing may overestimate the impact of family environments in some children (low susceptibility, more fixed reproductive development) and underestimate it in others (high susceptibility, more plastic reproductive development). Boyce and Ellis (2005) have proposed that exaggerated autonomic and adrenocortical responses to psychosocial stressors may reflect a heightened biobehavioral susceptibility to environmental influences, or biological sensitivity to context (BSC). As measured in standardized laboratory assessments of stress reactivity, children displaying high BSC appear to have higher levels of mental and physical morbidities under harsh or stressful conditions, but unusually low levels within supportive and protective conditions (Boyce & Ellis, 2005; Obradović & Boyce, 2009). The current research tests the hypothesis that the effects of family environment on pubertal timing and tempo will depend on (be moderated by) BSC. Specifically, we predict that the link between more supportive parent-child relationships and later/slower pubertal maturation will be stronger in children who display greater BSC.
A variety of studies, employing diverse designs and methods of operationalizing individual susceptibility, have emerged in recent years offering empirical support for a theory of BSC. As reviewed by Ellis, Boyce, Belsky, Bakermans-Kranenburg, and van IJzendoorn (2011), the earlier work of Boyce, Ellis and colleagues formed a provisional empirical foundation for the biological sensitivity theory and provided explicit tests of its defining hypothesis. Further work has expanded on this evidentiary basis by testing the differential susceptibility hypothesis in longitudinal and experimental designs. Thus, for example, Quas, Bauer, and Boyce (2004) used a random assignment design to examine the effects of cortisol reactivity and the supportiveness of the interviewer on the capacity of 4–6 year olds to recall details of a fire alarm exposure two weeks prior. Relative to children with low hypothalamic-pituitary-adrenocortical (HPA) reactivity, children showing heightened cortisol responses to a set of standardized stressors had poorer memories for the exposure event when questioned by a non-supportive interviewer but better memories when interviewed supportively. In a longitudinal test of the biological sensitivity theory, Boyce and colleagues (2006) studied degree of father involvement and maternal depression in infancy, autonomic reactivity at age seven years, and presyndromal mental health outcomes at age nine. Among children with uninvolved fathers in infancy, the highest mental health symptom severity scores were found among nine-year-old children with depressed mothers and high autonomic reactivity, while the lowest scores were observed in those with low maternal depression and high reactivity. Finally, Obradovic and colleagues (Obradovic, Bush, Stamperdahl, Adler, & Boyce, in press) recently reported that parasympathetically reactive children showed either the lowest or highest rates of prosocial behavior in the context of kindergarten classrooms, depending upon the level of contextual adversity sustained at home. Taken together, these targeted extensions of evidence, using more advanced, non-cross sectional designs, offer deeper support for the BSC hypothesis. Extant research has been limited, however, by a focus on development of syndromal or presyndromal psychopathology rather than on normal developmental processes. The current research thus adds to the literature by examining the role of BSC in moderating a normative developmental outcome—puberty—with implications for health.
Assessment of Pubertal Tempo and Timing
Variation in timing of puberty is characterized by wide individual differences, such that the first signs of puberty may be evident anywhere between 7–13 and 9–13 years of age in girls and boys, respectively (Styne & Grumbach, 2002). Pubertal tempo, or maturational velocity, also varies widely across individuals. Although it takes 4 years on average to advance to adult-like breast development, this interval may range from 1.5 to over 6 years (Marshall & Tanner, 1969). Most boys will advance from child-like to adult-like genital development in 3 years, but again there is a wide range, from 1.5 to over 4.5 years depending on the child (Marshall & Tanner, 1970).
Although pubertal tempo has been measured in a variety of ways, common to the different methods of assessment is derivation of a score that represents how long it takes to complete pubertal development (or some portion thereof). One such method gauges the length of time between when an individual first exhibits signs of pubertal onset until full development (e.g., Tanner Stage 2-to-5; Biro et al., 2006). Alternatively, for girls, tempo has been assessed by calculating the time span from pubertal onset to peak height velocity, attainment of adult height, or menarche (e.g., Llop-Vinolas et al., 2004;Pantsiotou et al., 2008). Duration of puberty for boys has been assessed using the time period from attainment of testicular volume of 4 ml to final height (Bundak et al., 2007). Maturational velocity has also been measured by simply calculating the difference in an individual’s pubertal stage at two identified points in time (e.g., Laitinen-Krispijn, van der Ende, & Verhulst, 1999; Slap et al., 1994).
An inherent limitation of these time-based measures is that pubertal tempo is indexed as a single score; however, the velocity of pubertal maturation may vary substantially within individuals, with different—even opposite—tempos characterizing earlier vs. later stages of pubertal development (Marshall & Tanner, 1969, 1970). Such complexity can be modeled, however, through growth curve analysis. Our principal analytic goal was to examine timing and tempo of puberty, taking several issues into account: (1) individuals initiate puberty at different ages; (2) individuals proceed through puberty at different rates of development; (3) there may be intra-individual differences in rates of pubertal maturation, such that individuals who advance quickly through one stage may advance slowly through other stages; and (4) the components of puberty (i.e., breast/genital and pubic hair) may develop at different times/rates for different individuals.
Consider the central hypothesis in the current research: Children who experience high levels of psychosocial stress in and around their families and are biologically sensitive to context (designated here as our “first target group”) will have a faster initial tempo of puberty than their peers and attain more advanced levels of pubertal development at earlier ages; conversely, children who experience high levels of nurturance and support in and around their families and are biologically sensitive to context (designated here as our “second target group”) will display the opposite pattern of pubertal development. To test this hypothesis in a sophisticated manner that engaged the four issues enumerated above, we employed a piecewise (two-part) growth curve model of pubertal development (as explained in the Results section).
The growth model was applied to both overall development and breast/genital and pubic hair development separately. The first slope captured development prior to age 12.5 (initial pubertal tempo) and the second slope captured development after age 12.5 (subsequent pubertal tempo). Although our hypothesis only targeted initial pubertal tempo, we present results for both initial and subsequent tempo to demonstrate the utility of the piecewise approach. In terms of the first slope, the growth curve analysis enabled us to examine whether our first target group, relative to other participants, had earlier onset of puberty but similar initial pubertal tempo, thus maintaining a more advanced level of pubertal maturation through age 12.5; had earlier onset of puberty but slower initial tempo, thus decelerating to a comparable level of maturation by age 12.5; had the same onset of puberty and a similar initial tempo, thus maintaining comparable levels of maturation through age 12.5; or had the same onset of puberty but faster initial tempo, thus accelerating to a more advanced level of maturation by age 12.5. In terms of the second slope, this allowed us to address the analogous set of questions in relation to subsequent pubertal tempo and offset of puberty. Finally, the growth curve model enabled to us to simultaneously ask the matching, reciprocal set of questions in relation to our second target group. In total, compared with extant measures of pubertal tempo, the present application of growth curve modeling enables a much more detailed analysis of intra- and inter-individual differences in pubertal development—and thus more precise and fine-grained hypothesis-testing.
Goals of the Current Research
Of particular relevance to the current research are results from the Wisconsin Study of Families and Work indicating that higher quality parent-child relationships in preschool—more parental warmth and family positivity, less parent-child stress and conflict—forecast lower levels of pubertal maturation by daughters in the 5th grade (Ellis & Essex, 2007). Although this association proved robust against covariate adjustment, the unique effect of parent-child relationships on puberty was relatively small (β = −.22), after controlling for mother’s age at menarche, socioeconomic status, and body mass index. The current study (a) longitudinally extends Ellis and Essex (2007) by examining timing and tempo of pubertal development through the 9th grade, (b) increases the specificity of the analyses by examining breast/genital and pubic hair development separately, (c) expands the research by including both boys and girls and testing for sex differences, and (d) tests whether the documented effect of parent-child relationships on puberty is moderated by BSC. The prediction is that this effect will be stronger in more sensitive children. To our knowledge, this is the first study to apply growth curve modeling to puberty, to examine psychosocial antecedents of pubertal tempo, or to test for the moderating effects of stress physiology on relations between family environments and pubertal development.
Method
Participants
The children in this study represent a subset of those participating in an ongoing longitudinal study of child development, the Wisconsin Study of Families and Work (WSFW; see Hyde et al., 1995). The original sample comprised 570 women and their partners recruited during the second trimester of pregnancy from obstetric/gynecology clinics and a low income clinic in Milwaukee (80% of sample) and Madison (20%). We attempted to recruit as diverse a sample as possible in regard to ethnic heritage and social class, subject to the constraints that mothers and fathers had to be cohabiting when recruited, and obtaining prenatal care. Families were excluded if women were age 18 or under, unemployed, a student, or disabled. At initial recruitment (pregnancy), 95% of the 570 couples were married; 89% were Caucasian; mothers’ average age was 29.4 years (range=20–43) and fathers’ was 31.3 (range = 20–55). Mothers and fathers each had an average of 15 years of education (range = less than high school to professional degree); annual family income ranged from $7,500 to over $200,000 (Median = $45,000). Thirteen waves of data have been collected from pregnancy through adolescence. The present study relies primarily on the preschool data collections (T6 and T7; ages 3.5–5.2 years) for assessing the family environment, the first grade data collection (T9; ages 6.8–7.8 years) for assessing children’s biological reactivity to psychological stressors, and data collections in grades 3–9 (T10–T13; ages 8.6–16.5 years) for assessing pubertal development.
The present analyses are based on a subsample of 120 children that were selected from the first cohort of the WSFW in the summer following the 1st grade to participate in an intensive sub-study to develop the MacArthur Assessment Battery for Middle Childhood (Boyce et al., 2002). Because we wanted a balance of “high” and “low” symptom children for the substudy, we employed a broad definition to classify all WSFW kindergarteners as “high symptoms” if they were in the upper 20% of either internalizing or externalizing problems as reported by either mothers or teachers, and “low symptoms” otherwise. This resulted in an approximately equal number of children classified as “high symptoms” and “low symptoms”. The subset of 120 children was randomly selected from each of these two symptom groups to be representative of the larger sample. Thus, there were no statistically significant differences between the demographic profiles of these 120 families and the remainder (450) of the 570 original families. Of the subset of 120 families, 114 had puberty scores for at least one assessment period between 3rd and 9th grade. All subsequent analyses are based on N=114 families (67 girls).
Quality of Parent-Child Relationships: Preschool
A factor-analytically derived measure of parent-child relationship quality (called Parental Supportiveness; fully described in Ellis & Essex, 2007) was used as the main index of family environment. Based on preschool maternal interviews, the measure incorporated parental depression (Center for Epidemiological Studies-Depression scale; Radloff, 1977), family negativity and positivity (The Family Expressiveness Questionnaire; Halberstadt, 1986, 1991), marital compatibility and conflict (The Partner Role Quality Scale; Barnett and Marshall, 1989), parental security (The Parenting Stress Index [PSI]; Abidin, 1986), parental negativity-dissatisfaction and positivity-warmth (from the PSI and the Child-Rearing Practices Report [CRPR]; Block, 1965), and authoritarian and authoritative parenting styles (CRPR). These ten scales were combined using principle components analysis (PCA), and then component scores were saved. The first of the two extracted components (alpha reliability = .79) clearly indexed parent-child supportiveness and authoritative parenting, with high structure loadings on parental negativity-dissatisfaction (−.76), warm-positive parental behavior (.74), authoritative-democratic parenting style (.74), parental insecurity (−.72), and family positivity (.62). This first component was employed as the measure of Parental Supportiveness.
Biological Sensitivity to Context: First Grade
A four-hour home assessment was conducted to acquire physiological data, along with various other measures of child health and development. During a 20–30 minute component of the home visit, midway through the assessment procedures, children’s neurobiological reactivity to standardized stressors—BSC—was tested using a protocol for young children completed in a van stationed outside the family’s home (Alkon et al., 2003; Boyce et al., 2001). Children were monitored using thoracic and precordial electrodes and a Dinamap cardiac monitor (Model 1846 SX by Critikon, Inc.). Physiological response parameters employed in the protocol included a) respiratory sinus arrhythmia (RSA), a measure of parasympathetic influence on heart rate variability, b) pre-ejection period (PEP), an index of sympathetic activation of the heart, and c) salivary cortisol, the hormonal end product of the HPA axis. RSA, as described in the work of Porges (Porges, 1995) and Cacioppo (Cacioppo, Uchino et al., 1994), was derived from spectral analyses of interbeat interval data within the respiratory cycle-associated, high frequency (0.15–0.50 Hz) band of the heart rate power spectrum. The natural logarithm of the variance in high frequency heart period was calculated to estimate parasympathetic activity. PEP, the duration of isovolumetric ventricular contraction, was measured with impedance cardiography as the 70–100 millisecond interval between the onset of electromechanical systole (indicated by the ECG Q-wave) and left ventricular ejection (indicated by the B-point of the thoracic impedance signal). As described by Cacioppo and colleagues (Cacioppo, Berntson et al., 1994), thoracic impedance (Z0) and its first derivative (dZ/dt) were measured as resistance to a constant 4 mA, 100 kHz alternating current, using electrodes placed at the apex and base of the child’s thorax. Impedance data were ensemble averaged in 1-minute intervals, and each waveform was verified or edited prior to analysis. Autonomic reactivity was thus assessed as diminution in RSA (reflecting parasympathetic withdrawal) and shortening of PEP (reflecting a sympathetic effect on cardiac chronotropism).
Cortisol was assessed in saliva because it can be non-invasively collected and reflects the plasma concentration of the non-protein bound active fraction (Kirschbaum & Hellhammer, 1994). There were four collection times over the course of the four-hour, in-home assessment: one immediately following the arrival of the study team, a second and third prior to and following the reactivity protocol, and a fourth at the termination of the assessment procedures. Saliva samples were transported on ice from the families’ homes to the laboratory immediately after the assessment. Samples were temporarily stored at −20°C for pre-laboratory accounting and then transferred to the laboratory where they were stored at −70° C until assayed. Assays were conducted using the Pantex (Santa Monica, CA) 125I Cortisol RIA Kit modified for saliva. All samples were assayed in duplicate within the same assay. Results were considered acceptable only if they met the following criteria: For cortisol concentrations (ug/dl) < .055, required coefficient of variation (CV%) of the duplicate samples was <25%. For cortisol concentrations (ug/dl) > .055, required CV% of the duplicates was <15%. Repeat assays were performed on all samples not meeting these criteria until CVs of two scores were within an acceptable range. The detection limit of the assay (ED80) was .03 ug/dl. The mean inter-assay and intra-assay variations were 6.97% and 3.91%, respectively.
Because the neurobiological sensitivity protocol occurred in the middle of an intensive, four-hour home assessment, we anticipated that conventional change (Δ) scores might not adequately represent the dynamics of autonomic and adrenocortical responses within this study context. We have previously suggested (Boyce et al., 2006) that, in situations where achieving resting values is difficult, more predictive reactivity measures might be derived from a multidimensional response profile, encompassing measures such as maximal response intensity, response variability, or the up- or down-regulatory slope of measures over time. In the present study, change scores indeed showed minimal shifts from resting to task levels, and inspection of raw data suggested that neurobiological reactivity would be best operationalized as the slope of task measures over the course of the four challenge epochs (autonomic reactivity) or the four collection times during the four-hour visit (adrenocortical reactivity). Negative RSA slope scores denoted increasing parasympathetic withdrawal (i.e., higher RSA reactivity) and negative PEP slope scores denoted increasing sympathetic activation of the heart (i.e., higher PEP reactivity) over the 20–30 minute protocol and greater reactivity at measurement onset. Positive cortisol slope scores denoted increasing adrenocortical activation over the 4-hour visit.
Pubertal Development: Grades 3–9
The longitudinal design permits a rigorous assessment of timing and tempo of pubertal maturation. Following Ellis & Essex (2007), we used a multi-method, multi-informant approach to capture the components of puberty. Mothers and youth completed a self-administered Tanner stage measure based on description and visual inspection of line drawings (Morris & Udry, 1980) and the Pubertal Development Scale (PDS, Petersen et al., 1988). The development of secondary sex characteristics were characterized in a manner corresponding to specific hormonal signals (Dorn et al., 2006). Thus, within this framework, breast and genital development is mediated by gonadal hormones, while pubic hair development is mediated by adrenal hormones.
For Tanner staging, puberty at 3rd grade was based on the mother’s report, because youth were anticipated to be relatively poor informants of maturation at such a young age. In 5th grade, both youth- and mother-ratings of Tanner staging were collected. Given the high inter-rater reliability (girls: α=.85 for breast and α=.83 for pubic hair; boys: α=.84 for genital and α=.88 for pubic hair), the two data sources were averaged together. A similar strategy was employed in 7th grade. The reliability across informant for girls was excellent (α=.76 for breast and α=.75 for pubic hair). Reliability for boys was substantially less than that of girls, consistent with the idea that boys may hide development from their caregiver (α=.34 for genital and α=.44 for pubic hair). By 9th grade, we relied solely on youth-ratings of Tanner staging.
The mother-report PDS items were separated into gonadal and adrenal events using a scoring system that takes the normative timing of events and different endocrine correlates into account (Shirtcliff, Dahl, & Pollak, 2009). Growth of body hair and skin changes were combined (average α=.62 for boys and α=.50 for girls across grades 3–9) to form the adrenal score. For girls, growth spurt, breast growth and menstrual status were combined for the gonadal score (average α=.58). For boys, growth spurt, deepening of voice and facial hair growth were combined for the gonadal score (average α=.54) in a developmentally sensitive manner. Within each grade level, the PDS gonadal scores were then averaged with Tanner breast/genital and the PDS adrenal scores were averaged with Tanner pubic hair. For girls, PDS and Tanner stages were reliable (average α=.71 for gonadal/breast and α=.68 for adrenal/pubic across grades 3–9). Reliability was not as high for boys, possibly because there is little overlap of the Tanner stages with the PDS items (average α=.31 for gonadal/genital and α=.56 for adrenal/pubic). Although these two axes of puberty were considered separately at each of the four grade levels, they were also averaged into an integrative puberty composite at each grade level (average α=.64 for boys and α=.75 for girls).
Results
Graphical Presentation of Puberty Data
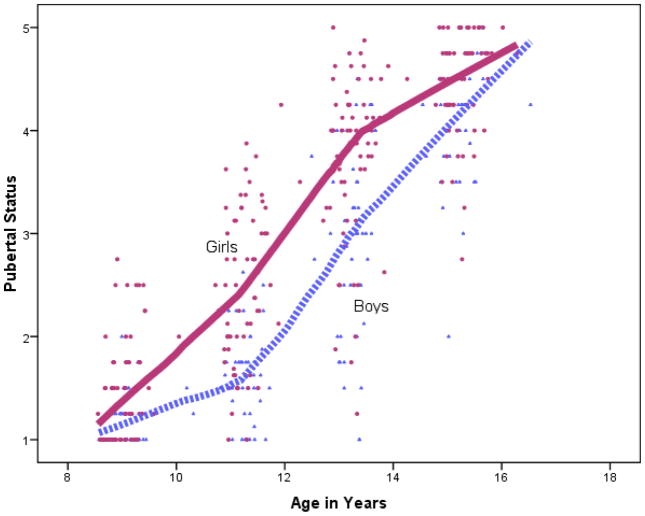
Our principal goal was to examine pubertal timing and tempo, taking into account that individuals initiate puberty at different ages, proceed through puberty at different developmental rates, and may display intra-individual differences in pubertal tempo such that individuals who advance quickly through one stage may advance slowly through other stages. These overarching analytic challenges are illustrated in the lowess curves and data in Figure 1 for the integrative puberty composite. There was substantial variability in pubertal stage. Most participants were stage 1 or 2 at around 9 years of age (3rd grade assessment) and then achieved stage 4 or 5 by around 15 years of age (9th grade assessment); there was substantial variability in stage within and across ages. Pubertal development did not appear to follow linear growth, which is consistent with Marshall and Tanner’s (1969, 1970) observations over four decades ago that growth may follow a sigmoid curve. Few subsequent studies have focused on intra-individual differences in puberty (Dorn et al., 2006). Although Figure 1 shows sex differences in pubertal maturation, the inflection points in the sigmoid curve (showing initial acceleration and later deceleration of pubertal growth) are at approximately the same ages in females and males.
Figure 1.
Scatterplot of relation between age and Tanner stage (as indexed by the integrative puberty composite) in girls and boys; pubertal maturation across the adolescent transition is highly variable and pubertal tempo appears nonlinear. Lines represent Lowess (locally weighted scatterplot smoothing) fraction curves fit to 50 data points. Lowess curves fit simple models to small subsets of the data, allowing different curves to be fit as the data builds up, point by point. The data is smoothed to fit 50 data points at a time until all 427 data points are modeled..
Modeling Between- and Within-Person Variability in Pubertal Development using Piecewise Growth Curves
Each participant provided up to 4 puberty assessments between 3rd and 9th grade. HLM is robust to missing data when individuals had fewer than 4 assessments; the program calculates coefficients using as much information as possible without necessitating pairwise or listwise deletion (Hox, 2002), A two-level hierarchical linear model separated within-individual variability (427 puberty scores across all assessments and participants) from between-individual sources of variability (N=114 participants with an average of 3.75 assessments each). The outcome was pubertal maturation. To capture pubertal growth over time, age was included as a Level 1, time-varying predictor. Thus, each individual’s trajectory was based on their specific age-in-months at the 4 assessment periods. This strategy does not assume that every child is the same age at each grade, but rather captures the age that each child achieves at each assessment while allowing the interval between assessments to vary from child to child and from wave to wave.
Age was centered at 12.5 years to capture pubertal timing, or the level of development achieved by 12.5 years old. Consequently, the intercept in the HLM model is the predicted level of development when that child is 12.5 years old. We selected 12.5 because (a) it captured a natural break in velocity between initial and subsequent tempo, (b) was at approximately the mid-point of our 4 pubertal assessments, and thus (c) allowed for 2 waves to be modeled within both the initial and subsequent pubertal tempo periods. Because it is age-standardized, this intercept captures timing more than status (Mustanski et al., 2004). In addition, sex differences in timing and tempo were controlled for at Level 2 of the analyses (as described below), capturing cross-level interactions. A potential limitation of this approach is that it would not work adequately if boys and girls did not have the same fundamental shape to their tempo and timing variables; however, as noted above, the lowess curves in Figure 1 do not provide evidence for this type of sex difference. Following Kreft and De Leeuw (1998), explained variance (R2) was calculated as [(unrestricted error-restricted error)/unrestricted error].
As expected, a linear growth model did not adequately capture pubertal tempo (based on the integrative puberty composite), χ2(113)=114, p=.40, likely because the growth parameter was mis-specified. We considered a sigmoid or polynomial curve, but opted to capture pubertal tempo with a piecewise (two-part) growth curve for several reasons. First, the piecewise components (two slopes) are linear, which is highly interpretable. Second, we expected the underlying maturational processes to be different (and perhaps opposing) between initial and subsequent pubertal development in accordance with Marshall and Tanner’s (1969, 1970) report that rate of early development was inversely correlated with later development. Third, for theoretical reasons, we were specifically interested in development prior to age 12.5 (early reproductive maturation); this component was simply captured by the first linear slope, while subsequent development was captured by the second linear slope. Initial tempo (early adolescent pubertal growth) was captured with age-before-12.5 as a linear predictor of puberty; subsequent tempo (later adolescent pubertal growth) was simultaneously captured with age-after-12.5 as a linear predictor of puberty.
First, we set up the Level 1 model to simultaneously capture the time periods of puberty. As notated in the equations above, these time periods included the intercept (at age 12.5), the age of the child prior to 12.5 years, and the age of the child after age 12.5 years. These predictors of puberty at Level 1, the intra-individual difference level, become outcomes of interest at Level 2, the inter-individual difference level. Both intra- and inter-individual variability in pubertal maturation were analyzed. Second, we examined the main effects of Parental Supportiveness and each of the three autonomic and adrenocortical reactivity slope scores (PEP, RSA, and cortisol), respectively, on initial tempo, subsequent tempo, and pubertal timing. Finally, we analyzed the interaction between Parental Supportiveness and each of the reactivity slope scores, controlling for gender and main effects. All models were computed first for the integrative puberty composite and then recomputed separately for breast/genital and pubic hair development as outcomes.
Analyzing the integrative puberty composite, the two-part growth model appeared to capture the intra- and inter-individual variability in pubertal maturation (see Figure 1). During the initial tempo period, participants advanced .45 stages/year until age 12.5, and this rate of advancement was significantly greater than zero, t(113)=17.4, p<.001. In addition, there was substantial intra-individual variability in initial pubertal tempo, SD=.22, and this level of variability was statistically significant, χ2(105)=207, p<.001. By age 12.5, participants had advanced to stage 2.8 on average, t(113)=29.3, p<.001, though there was still substantial variability in pubertal timing, SD=.936, χ2(105)=488, p<.001. Thereafter, participants advanced on average .55 stages/year through 9th grade, t(113)=20.3, p<.001. Substantial variability remained in later pubertal tempo as well, SD=.20, χ2(105)=132, p<.04.
Girls developed .24 stages/year faster on the integrative puberty composite than boys did during the initial tempo period, t(112)=5.2, p<.001, and had advanced by 1.1 stages more than boys by 12.5 years of age, t(112)=6.81, p<.001; thereafter, boys caught up by .27 stages/year during the subsequent tempo period, t(112)=5.43, p<.001. Gender explained 29.4% of the variability in initial tempo, 34.0% of the variability in pubertal timing, and 45.1% of the variability in subsequent tempo. Indeed, so much variability in subsequent tempo was explained by gender, Δχ2(1)=24.5, p<.001, that inter-individual differences were no longer statistically significant, SD=.148, χ2(104)=107.04, p=.40. We allowed subsequent tempo to vary, but results below emphasize initial tempo for both conceptual and statistical reasons. All models controlled for gender at the inter-individual difference level (Level 2). This allowed us to control for potential sex differences in family environment and BSC, as well as control for cross-level interactions between sex and pubertal timing and tempo, thereby capturing sex differences in rate or stage of maturation.
Main Effects of Parental Supportiveness
Building on analyses first presented in Ellis and Essex (2007), based on the integrative puberty composite, children from families with greater Parental Supportiveness showed slower initial pubertal tempo, β=−.06, t(111)=2.88, p<.005, were less developed by 12.5 years of age, β= −.24, t(111)=3.51, p<.001, and then showed faster subsequent pubertal tempo, β=.07, t(111)=3.46, p<.001. Parental supportiveness explained 8.8% of the variability in initial tempo, 8.8% of the variability in pubertal timing, and 17.4% of the variability in subsequent tempo. Results were similar when we separately examined breast/genital, ps<.04, or pubic hair development, ps<.004.
Interactions between Parental Supportiveness and Biological Sensitivity to Context
PEP
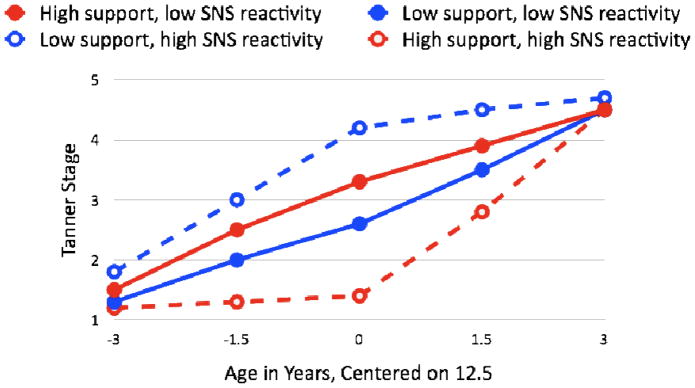
Analyzing the integrative puberty composite, there were no main effects of PEP slope scores on pubertal timing or tempo, ps>.28. However, Parental Supportiveness interacted consistently with PEP slope score to predict initial pubertal tempo, β=.08, t(109)=2.08, p<.05, pubertal timing, β=.35, t(109)=3.2, p<.002, and subsequent tempo after 12.5 years of age, β=−.10, t(109)=2.7, p<.008. The interaction effect explained 7.7% of the variability in initial tempo, 7.9% of the variability in pubertal timing, and 20.7% of the variability in subsequent tempo. Main effects of Parental Supportiveness also persisted, ps<.002. Figure 2 illustrates that children with more negative PEP slope scores (indicating greater sympathetic reactivity) experienced significantly faster initial maturation, displayed more advanced pubertal development by age 12.5, and then experienced less maturation after 12.5 years of age when they came from less supportive families (ps<.001). The opposite was true when children with more negative PEP slope scores came from more supportive families. Conversely, children with more positive PEP slope scores (indicating lower sympathetic reactivity) experienced similar trajectories of pubertal development regardless of whether they came from families with low or high levels of Parental supportiveness. The interactions remained statistically significant when breast/genital development and pubic hair development were examined separately (ps<.001).
Figure 2.
Variation in initial and subsequent pubertal tempo (based on integrative puberty composite) as a function of sympathetic nervous system (SNS) reactivity and Parental Supportiveness, controlling for sex. High versus low Parental Supportiveness only distinguishes pubertal tempo and timing in adolescents with high SNS reactivity.
RSA
Analyzing the integrative puberty composite, there was no evidence for main effects of RSA slope scores on pubertal timing or tempo, ps>.66. When we specifically modeled an interaction with Parental Supportiveness, there was a trend for an interaction on subsequent pubertal tempo, p=.07; otherwise, there was no evidence for an interaction between family environment and parasympathetic reactivity. The absence of a statistically significant interaction between Parental Supportiveness and RSA slope score in predicting either initial pubertal tempo or pubertal timing extended to the components of puberty as well; that is, there were no significant interactive effects on either the initial tempo or timing of breast/genital or pubic hair development. Therefore, no further RSA analyses were conducted.
Cortisol
Analyzing the integrative puberty composite, children with higher cortisol slope scores (increasing adrenocortical activation over the 4-hour visit) showed a trend toward slower initial tempo, β=−.43, t(111)=1.85, p=.06, and faster subsequent pubertal tempo, β=.17, t(111)=2.3, p=.05. This pattern of results became statistically significant when we specifically examined pubic hair development, ps<.05. Cortisol slope scores explained 1.0% of the variability in initial tempo of pubic hair development, 2.2% of the variability in pubic hair timing, and 9.4% of the variability in subsequent tempo. By contrast, cortisol slope scores did not significantly predict timing or tempo of breast/genital development, ps>.11. These results suggest that adrenocortical activation may be specifically associated with adrenal-hormone related development of secondary sex characteristics.
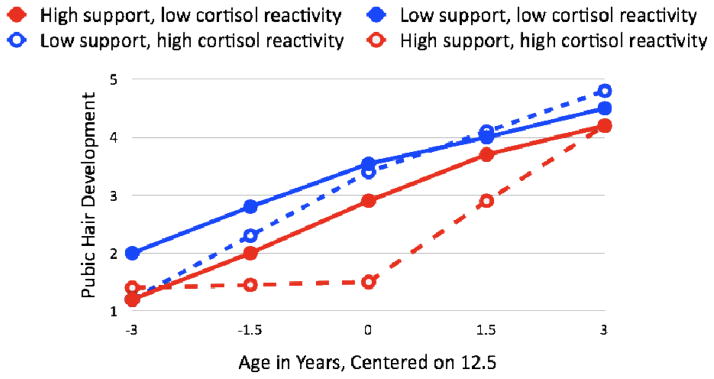
Analyzing the integrative puberty composite, there was no initial evidence that cortisol activation interacted with Parental Supportiveness to predict pubertal timing and tempo, ps>.75. When we examined breast/genital and pubic hair development separately, however, effects on pubic hair development emerged as above. As shown in Figure 3, there was a significant interaction between cortisol slope scores and Parental Supportiveness on initial tempo of pubic hair development, β= −.14, t(109)=2.35, p=.02. Children with higher adrenocortical activation and higher Parental Supportiveness experienced slower initial pubic hair development. The interaction explained 1.0% of the variability in initial tempo, 1.67% of the variability in pubertal timing and 10.4% of the variability in subsequent tempo. The main effects of Parental Supportiveness, ps<.001, and cortisol activation, ps<.03, on timing and tempo persisted, ps<.03 when pubic hair development was the outcome. There was no evidence for an interaction between cortisol activation and Parental Supportiveness in the model predicting breast/genital development, ps>.14.
Figure 3.
Variation in initial and subsequent tempo of pubic hair development as a function of cortisol reactivity and Parental Supportiveness, controlling for sex. High versus low Parental Supportiveness only distinguishes tempo and timing of pubic hair development in adolescents with high cortisol responsivity.
Discussion
In earlier analyses of the Wisconsin Study of Families and Work, Ellis and Essex (2007) documented a unique effect of Parental Supportiveness on the timing of pubertal maturation in girls. The current analyses extended this finding in two important ways. First and foremost, analyses tested for the moderating effect of BSC, highlighting that the link between Parental Supportiveness and puberty depended on two of the three indices of BSC: sympathetic nervous system reactivity (indexed by negative PEP slopes) and adrenocortical activation (indexed by positive cortisol slopes). Most critically, as predicted by the theory of BSC, higher levels of Parental Supportiveness predicted slower initial pubertal tempo and less advanced pubertal development by age 12.5 only among children who displayed higher BSC; conversely, lower levels of Parental Supportiveness predicted faster initial pubertal tempo and more advanced pubertal development by age 12.5 only among children who displayed higher BSC. Whereas sympathetic nervous system reactivity moderated the effects of Parental Supportiveness on both breast/genital and pubic hair development, adrenocortical activation only moderated the effect on pubic hair development. In sum, building on previous analyses documenting what family contexts predict variation in the timing of puberty, the present results established for whom those contexts matter. These results concur with Belsky’s (2000) conjecture that the small main effects of family processes on pubertal timing, as documented in past research, may overestimate the impact of family environments in some children (low susceptibility) and underestimate it in others (high susceptibility). Second, the current analyses further elucidated the outcome of interest, pubertal development, by examining adrenal and gonadal processes that occurred in different people—and within the same person—at different ages and rates of development over the whole course of puberty (from ages 8.6 to16.5 years) in girls and boys.
Dorn et al. (2006) remarked that attempting to understand children’s development without asking about the age of the child is tantamount to fallacy; within the developmental epoch of adolescence, it is arguably of similar importance to inquire about pubertal maturation. Although it is widely recognized that puberty is complex, recent theory and data have placed increasing emphasis on the multidimensional nature of puberty. Most fundamentally, puberty has distinct components and axes, including gonadal, adrenal, and growth hormone systems (Grumbach, 2002; Grumbach, Bin-Abbas, & Kaplan, 1998; Parker, 1991). Further, because pubertal processes unfold over a number of years, longitudinal research with repeated measurements of puberty are needed to capture maturation. The present study is one of the first investigations to longitudinally assess pubertal maturation and analytically examine how pubertal processes unfold within individuals over adolescence.
This approach proved fruitful: Compared with measures of pubertal timing, or pubertal status, or standard (time-based) indices of pubertal tempo, the current piecewise growth curve model yielded distinct and nuanced information about pubertal development. Specifically, we found that the previously documented effect of Parental Supportiveness on pubertal timing was underpinned by slower initial tempo and faster subsequent tempo of both breast/genital and pubic hair growth among children who experienced high Parental Supportiveness in preschool (and that these effects were moderated by BSC). These results not only underscore the dynamic and non-linear nature of pubertal development, but also demonstrate how analyzing the changing tempo of maturation across adolescence informs our understanding of intra-individual developmental processes. Because pubertal tempo has important developmental antecedents and sequelae, the benefit of such an analytic approach is that greater knowledge can be gained into the causes and effects of puberty in relation to a broad range of physical, psychological, biological, and social developmental processes.
Evolutionary Models of Adaptive Development under Stressful and Supportive Environmental Conditions
Central to an evolutionary-developmental perspective is the concept of conditional adaptation: “evolved mechanisms that detect and respond to specific features of childhood environments, features that have proven reliable over evolutionary time in predicting the nature of the social and physical world into which children will mature, and entrain developmental pathways that reliably matched those features during a species’ natural selective history” (Boyce & Ellis, 2005, p. 290; for a comprehensive treatment of conditional adaptation, see West-Eberhard, 2003). Because both stressful and supportive childhood environments have been a recurring part of the human experience throughout our evolutionary history, natural selection has shaped our developmental systems to respond adaptively to both contexts (Ellis et al., 2011). When people encounter stressful environments, therefore, it does not so much disturb their development as direct or regulate it toward strategies that are adaptive under stressful conditions. Conversely, when people encounter well-resourced and supportive environments, it directs or regulates development toward strategies that are adaptive in that context.
Within this theoretical framework, Boyce and Ellis (2005) proposed that children have conditional adaptations that detect and encode information about levels of support and adversity in early environments, as a basis for calibrating activation thresholds and response magnitudes within stress response systems to match those environments. Specifically, Boyce and Ellis (2005) postulated a curvilinear U-shaped relation between levels of early support versus adversity and the magnitude of biological response dispositions such that: (1) exposure to acutely stressful childhood environments up-regulates BSC, increasing the capacity and tendency of individuals to detect and respond to environmental dangers and threats; (2) exposure to especially supportive childhood environments also up-regulates BSC, increasing susceptibility to social resources and ambient support. By contrast, and typical of the large majority of children, (3) exposure to childhood environments that are not extreme in either direction down-regulates BSC, buffering individuals against the chronic stressors encountered in a world that is neither highly threatening nor consistently safe. Previous analyses of the Wisconsin Study of Families and Work provided support for this hypothesis specifically in relation to PEP: Growing up in either highly supportive or highly stressful home environments predicted development of high sympathetic nervous system reactivity (Ellis et al., 2005). In addition, growing up in highly stressful home environment predicted heightened adrenocortical activation.
Consider the u-shaped curve hypothesis and supporting PEP and cortisol data together with the current results and evolutionary-developmental model of pubertal maturation. Under conditions of early environmental stress and uncertainty, indexed by coercive and unsupportive family relationships, individuals developed heightened BSC (sympathetic and adrenocortical) and subsequently accelerated pubertal maturation in early adolescence, with family stress and context-sensitivity acting synergistically in the present study. Heightened BSC thus enabled a stronger pubertal response to adversity. According to evolutionary-developmental models, this response may represent a strategic—that is, functional—way of developing under stress. Over evolutionary history, responding to threatening or unpredictable childhood environments by growing up fast and hastening sexual maturity may have significantly increased the probability of reproducing at all. As Chisholm (1996) suggests, “When young mammals encounter conditions that are not favorable for survival—i.e., the conditions of environmental risk and uncertainty indexed by emotional stress during development—it will generally be adaptive for them to reproduce early” (p. 21).
The other side of the coin is that under conditions of early environmental protection and stability, indexed by positive and supportive family relationships, individuals also developed heightened BSC (Ellis et al., 2005), which in combination with high Parental Supportiveness forecast slower initial pubertal tempo and later pubertal timing in the current study. In this case, heightened BSC enhanced responsiveness to environmental resources and support. As suggested by evolutionary-developmental models, the resulting pattern of late sexual maturation may also constitute adaptive variation. Specifically, Ellis (2004) hypothesized that children have been selected to capitalize on the benefits of high quality parental investment and reduce the costs of low quality parental investment by contingently altering the length of childhood. Given high family resources and support and context-sensitivity, extending childhood by delaying onset of puberty or slowing pubertal tempo may function to enhance development of socio-competitive competencies that ultimately increase reproductive potential.
Variation in Results across Stress Response Systems
Notably, although the study hypothesis was generally supported, there were also important differences in results across the stress response systems. This variation may reflect the different levels at which environmental challenges are instantiated in the brain. Stress initially registers in the brain as limbic circuitry assesses the emotional content of incoming environmental information and activates hypothalamic nuclei and related neuroendocrine targets (Dedovic et al., 2009; Pruessner et al., 2010). A rapid, low threshold stress response is triggered first through the release of vagal, parasympathetic control over heart period, a chronotropic effect indirectly measured via changes in RSA and heart rate (Porges, 1995). The sympathetic adrenomedullary system, also quickly responsive through the activation of the locus coeruleus, is measurable in the periphery as increases in catecholamine levels (Goldstein & Kopin, 2008), or indirectly via autonomic changes in heart rate, PEP (Cacioppo et al., 1994; Cacioppo et al., 1998), or salivary alpha amylase (Granger et al., 2007; Nater & Rohleder, 2009). While release of the parasympathetic brake on heart rate occurs with relatively mild exposures to challenge, more stressful encounters are generally required to elicit sympathetic activation. The present investigation examined BSC by measuring physiological responses to moderate social, emotional, and cognitive challenges. In the context of such moderate challenges, parasympathetic reactivity (RSA) was likely provoked in the majority of participants, and the null findings in regard to RSA may have been observed because the threshold of reactivity was too low to distinguish between children with low and high BSC. By contrast, there were larger, and perhaps more differentiating and predictive individual differences in sympathetic nervous system reactivity (PEP), so that the clearest evidence in support of the hypothesized BSC effects derived from the measure of sympathetic reactivity (see also Ellis et al., 2005).
Although autonomic and HPA responses are highly integrated within brain circuitry, the hormonal cascade of the HPA axis occurs within a different and slower time frame than that of the autonomic nervous system (Sapolsky, Romero, & Munck, 2000), and HPA activation appears to be more specifically responsive to social evaluative challenges (Gunnar, Talge, & Herrera, 2009; Dickerson & Kemeny, 2004). Many laboratory and naturally occurring stressors may succeed in stimulating a stress response in other stress-responsive physiological systems (Gordis et al., 2006), but these challenges are often not sufficiently intense or specific to stimulate cortisol release (Cohen et al., 2000). Although cortisol reactivity was not consistently observed in response to the stress paradigm utilized in this study, changes in salivary cortisol over the course of the entire, four-hour home visit showed extensive individual differences, and the direction and slope of those changes were reflective of HPA activation. A subgroup of children demonstrated substantial HPA responsivity, and it was among these children that clear evidence for BSC effects also emerged.
It is notable that among children evincing HPA axis reactivity, differential susceptibility to Parental Supportiveness was observed specifically for pubic hair maturational timing and tempo. This finding concurs with the known developmental biology of pubic hair maturation, which is advanced by sex hormone release from the adrenal gland (Shirtcliff et al., 2009), often in conjunction with cortisol release in response to ACTH (Azziz et al, 2001; Shirtcliff et al, 2007). It is possible that HPA axis activity is more tightly coupled anatomically and functionally with pubic hair maturation, compared to breast/genital maturation (which is stimulated by gonadal hormones).
Limitations and Future Directions
Limitations of the present study should be noted because they provide important directions for future research. First, and foremost, the current research was based on a relatively small sample that was largely middle-class, white, and, at least at the outset, composed of intact families in a Midwestern state. Whereas the small sample limited our power to detect higher-order interactions in the data, the demographic homogeneity was likely to have underrepresented the high end of family stress and adversity. Undersampling high-risk families is concerning because dangerous or unpredictable family environments, which genuinely threaten children’s health and safety, may be most likely to accelerate pubertal maturation (see Ellis, Figueredo, Brumbach, & Schlomer, 2009). It will be especially important in future research, therefore, to study larger numbers of families that more fully capture the range of stress and adversity in the population.
Second, the current methods for assessing family environment constituted both a strength and weakness of this research. The strength was that family environment was measured prospectively, in preschool, prior to the onset of puberty; thus, pubertal maturation could not have influenced family dynamics. A potential weakness, however, was the unidimensional assessment of Parental Supportiveness. From an evolutionary-development perspective, both the harshness of childhood environments (stress-adversity versus nurturance-support, as estimated by the Parental Supportiveness measure), and the predictability versus unpredictability of childhood environments should influence sexual development (Brumbach, Figueredo, & Ellis, 2009; Ellis et al., 2009). Future research needs to more carefully assess harsh versus unpredictable environmental contexts, as well as differential susceptibility to these factors.
Third, the assessment of BSC also had advantages and disadvantages. The choice of psychophysiology measures for the purposes of this study had at least two important advantages: a) the standardized challenges within the reactivity protcol were developed as ecologically valid tasks, not unlike those encountered in the natural world among children of similar age, and b) the outcome variables (i.e., the timing and pace of pubertal development) are determined by neuroendocrine circuitry closely related to and influenced by the stress reactivity systems indexed within the reactivity protocol (see Ellis, 2004). Thus, in terms of both ecological validity and salience to outcomes, the current physiological measurement of BSC offered an optimal approach. Nonetheless, as reviewed in Ellis et al. (2011), an array of hierarchically organized neurogenomic and endophenotypic mechanisms may underlie variation in susceptibility to environmental influence. It will be important in future research to expand from single modalities of measurement (e.g., indexing BSC through psychophysiological responses) to multiple methods of assessment, ranging from behavioral indicators of environmental susceptibility to peripheral neuroendocrine pathways, brain circuitry, and both genetic and epigenetic variation.
Finally, our measures of puberty, though employing multiple informants and assessing multiple components of puberty, were imperfect. At the earliest and latest waves, different informants were used in order to be developmentally sensitive to the needs and knowledge of the family. Although Tanner staging by physical examination is the gold standard (Dorn et al., 2006), past research indicates that both self- and parent-ratings of pubertal development, using either the PDS or the Tanner drawings, are moderately to highly correlated with ratings by health-care professionals based on physical examinations (summarized in table 2 of Dorn et al., 2006). Further, self-reported pubertal maturation correlates well with sex hormones and is adequate for assessing development over time (Shirtcliff et al., 2009). Additional validation data for the current puberty measures, including correlations with height, weight, and adrenal androgens, are reported in Ellis and Essex (2007).
Toward an Integrated Understanding of Person X Environment Interactions in Pubertal Development
An expansive biomedical and behavioral literature has sought to characterize environmental influences on pubertal development (Ellis, 2004). Despite the indisputable contributions of this body of research, the current study—in the context of the differential susceptibility paradigm advanced in this special section—suggests that person x environment interactions may play an important role in regulating pubertal maturation. Specifically, individuals who display heightened BSC appear to be especially susceptible to both the development-enhancing and risk-promoting effects of family environments on pubertal growth. The current results using physiological indicators of BSC converge with other differential susceptibility research, which has shown that the effects of parent-child relationships on pubertal timing depend on genotypic indicators (i.e., variation in estrogen receptor-α [ESR1]; Manuck, Craig, Flory, Halder, & Ferrell, 2011) and behavioral indicators (i.e., variation in infant negative emotionality; Belsky et al., 2007) of susceptibility to environmental influence. Clearly, the path forward is integration across levels: elucidating the hierarchical and bidirectional relations between genotypes, endophenotypes and behavior that, together, constitute BSC and explain its role in moderating environmental effects on puberty.
The stakes are high. For example, a one year delay in age at menarche reduces risk for breast cancer by 10–20% (Bernstein, 2002). As policymakers and practitioners contemplate environmental manipulations to delay puberty, a clear implication of the differential susceptibility perspective is that the effects of such manipulations will not be homogeneous across individuals but vary according to BSC. Although in an ideal world interventions would be implemented to ameliorate adversity in all children, economic constraints dictate the need to identify particular groups for whom targeted programs will be most effective. In some circumstances, potential intervention targets could be selected using differential susceptibility screens. The current data suggest that prevention and intervention efforts aimed at altering pubertal timing or tempo will have a larger impact on biologically sensitive youth and, consequently, greater effects on puberty-related physical health (e.g., breast cancer) and mental health (e.g., anxiety) problems downstream.
Acknowledgments
Funding was provided by the John D. and Catherine T. MacArthur Foundation Research Network on Psychopathology and Development and National Institute of Mental Health grants R01-MH44340, P50-MH052354, P50-MH069315, and P50-MH084051. The authors thank the study participants who so generously committed their time to the project over the years and the dedicated staff of the Wisconsin Study of Families and Work.
Contributor Information
Bruce J. Ellis, John and Doris Norton School of Family and Consumer Sciences, University of Arizona
Elizabeth A. Shirtcliff, Department of Psychology, University of New Orleans
W. Thomas Boyce, Human Early Learning Partnership, University of British Columbia, Canada.
Julianna Deardorff, School of Public Health, University of California, Berkeley.
Marilyn J. Essex, Department of Psychiatry, University of Wisconsin School of Medicine and Public Health
References
- Abidin RR. Parenting Stress Index. Charlottesville, VA: Pediatric Psychology Press; 1986. [Google Scholar]
- Alkon A, Goldstein LH, Smider N, Essex M, Kupfer D, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Developmental Psychobiology. 2003;42:64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Azziz R, Fox LM, Zacur HA, Parker CR, Jr, Boots LR. Adrenocortical secretion of dehydroepiandrosterone in healthy women: highly variable response to adrenocorticotropin. J Clin Endocrinol Metab. 2001;86:2513–2517. doi: 10.1210/jcem.86.6.7587. [DOI] [PubMed] [Google Scholar]
- Barnett RC, Marshall NL. Preliminary manual for the Role-Quality Scales. Wellesley College: Center for Research on Women; 1989. [Google Scholar]
- Belsky J. Conditional and alternative reproductive strategies: Individual differences in susceptibility to rearing experience. In: Rodgers J, Rowe D, Miller W, editors. Genetic influences on human fertility and sexuality: Theoretical and empirical contributions from the biological and behavioral sciences. Boston: Kluwer; 2000. pp. 127–146. [Google Scholar]
- Belsky J. Differential susceptibility to rearing influences: An evolutionary hypothesis and some evidence. In: Ellis B, Bjorklund D, editors. Origins of the social mind: Evolutionary psychology and child development. New York: Guildford; 2005. pp. 139–163. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis-stress: Differential susceptibility to environmental influence. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development and reproductive strategy: An evolutionary theory of socialization. Child Development. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Houts RM, Friedman SL, DeHart G, Cauffman E, et al. Family rearing antecedents of pubertal timing. Child Development. 2007;78:1302–1321. doi: 10.1111/j.1467-8624.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. Journal of Mammary Gland Biology and Neoplasia. 2002;7:3–15. doi: 10.1023/a:1015714305420. [DOI] [PubMed] [Google Scholar]
- Biro FM, Huang G, Crawford PB, Lucky AW, Striegel-Moore R, Barton BA, et al. Pubertal correlates in black and white girls. Journal of Pediatrics. 2006;148:234–240. doi: 10.1016/j.jpeds.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Biro FM, Galvez MP, Greenspan LC, Succop PA, Vangeepuram N, Pinney SM, Teitelbaum S, Windham GC, Kushi LH, Wolff MS. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. 2010 doi: 10.1542/peds.2009-3079. Published online Aug 9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block JH. The Child-Rearing Practices Report (CRPR): A set of Q items for the description of parental socialization attitudes and values. Berkeley: Institute of Human Development; 1965. [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Alkon A, Goldsmith HH, Kraemer HC, Kupfer DJ. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:1510–1520. doi: 10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex M, Woodward HR, Measelle JR, Ablow JC, Kupfer DJ. The confluence of mental, physical, social and academic difficulties in middle childhood: I. Exploring the “headwaters” of early life morbidities. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:580–587. doi: 10.1097/00004583-200205000-00016. [DOI] [PubMed] [Google Scholar]
- Bratberg GH, Nilsen TI, Holmen TL, Vatten LJ. Early sexual maturation, central adiposity and subsequent overweight in late adolescence. A four-year follow-up of 1,605 adolescent Norwegian boys and girls: the Young HUNT study. BMC Public Health. 2007;12:54. doi: 10.1186/1471-2458-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbach BH, Figueredo AJ, Ellis BJ. Effects of harsh and unpredictable environments in adolescence on the development of life history strategies: A longitudinal test of an evolutionary model. Human Nature. 2009;20:25–51. doi: 10.1007/s12110-009-9059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychol Bull. 1992;111:62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- Bundak R, Darendeliler F, Gunoz H, Bas F, Saka N, Neyzi O. Analysis of puberty and pubertal growth in healthy boys. Eur J Pediatr. 2007;166:595–600. doi: 10.1007/s00431-006-0293-y. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology. 1994;31:586–598. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, Poehlmann KM, et al. Autonomic, neuroendocrine, and immune responses to psychological stress: the reactivity hypothesis. Ann N Y Acad Sci. 1998;840:664–673. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology. 1994;31:412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Chisholm JS. The evolutionary ecology of attachment organization. Human Nature. 1996;7:1–38. doi: 10.1007/BF02733488. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Frazier AL. Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev. 1995;4:567–571. [PubMed] [Google Scholar]
- Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. The stability of and intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Ann Behav Med. 2000;22:171–179. doi: 10.1007/BF02895111. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Sung M, Worthman C, Angold A. Pubertal maturation and the development of alcohol use and abuse. Drug and Alcohol Dependence. 2007;88(Suppl 1):S50–S59. doi: 10.1016/j.drugalcdep.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Deardorff J, Gonzales NA, Christopher FS, Roosa MW, Millsap R. Early puberty and adolescent pregnancy: the influence of alcohol use. Pediatrics. 2005;116:1451–1456. doi: 10.1542/peds.2005-0542. [DOI] [PubMed] [Google Scholar]
- Deardorff J, Hayward C, Wilson KA, Bryson S, Hammer LD, Agras S. Puberty and gender interact to predict social anxiety symptoms in early adolescence. J Adolesc Health. 2007;41:102–104. doi: 10.1016/j.jadohealth.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Dick DM, Rose RJ, Viken RJ, Kaprio J. Pubertal timing and substance use: Associations between and within families across late adolescence. Dev Psychol. 2000;36:180–189. [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10:30–56. [Google Scholar]
- Ellis BJ. Timing of pubertal maturation in girls: An integrated life history approach. Psychological Bulletin. 2004;130:920–958. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23 doi: 10.1017/S0954579410000611. this issue. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ. Family environments, adrenarche, and sexual maturation: A longitudinal test of a life history model. Child Development. 2007;78:1799–1817. doi: 10.1111/j.1467-8624.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Development and Psychopathology. 2005;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL. Fundamental dimensions of environmental risk: The impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Human Nature. 2009;20:204–268. doi: 10.1007/s12110-009-9063-7. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Jackson JJ, Boyce WT. The stress response systems: Universality and adaptive individual differences. Developmental Review. 2006;26:175–212. [Google Scholar]
- Ellis BJ, McFadyen-Ketchum S, Dodge KA, Pettit GS, Bates JE. Quality of early family relationships and individual differences in the timing of pubertal maturation in girls: a longitudinal test of an evolutionary model. Journal of Personality and Social Psychology. 1999;77:387–401. doi: 10.1037//0022-3514.77.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorenson T, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trands: panel findings. Pediatrics. 2008;121(Suppl):S172–191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: a meta-analysis. Endocr Regul. 2008;42:111–119. [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Collman GW, Foster PD, Kiimmel CA, Rajpert-De Meyts E, Reiter EO, et al. Public health implications of altered pubertal timing. Pediatrics. 2008;121(Suppl):S218–230. doi: 10.1542/peds.2007-1813G. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Graber JA, Brooks-Gunn J, Warren MP. The antecedents of menarcheal age: Heredity, family environment, and stressful life events. Child Development. 1995;66:346–359. doi: 10.1111/j.1467-8624.1995.tb00875.x. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, el-Sheikh M, Gordis EB, Stroud LR. Salivary alpha-amylase in biobehavioral research: recent developments and applications. Ann N Y Acad Sci. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Grumbach MM. The neuroendocrinology of human puberty revisited. Hormone Research. 2002;57(Suppl 2):2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- Grumbach MM, Bin-Abbas BS, Kaplan SL. The growth hormone cascade: progress and long-term results of growth hormone treatment in growth hormone deficiency. Horm Res. 1998;49(Suppl 2):41–57. [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AG. Family socialization of emotional expression and nonverabal communication styles and skills. Journal of Personality and Social Psychology. 1986;51:827–836. [Google Scholar]
- Halberstadt AG. Studies in emotion and social interaction. In: Feldman RS, Rime B, editors. Fundamentals of nonverbal behavior. New York, NY: Cambridge University Press; 1991. pp. 106–160. [Google Scholar]
- Hox J. Multilevel analysis techniques and applications. Lawrence Erlbaum Associates, Publishers; 2002. [Google Scholar]
- Hyde JS, Klein MH, Essex MJ, Clark R. Maternity leave and women’s mental health. Psychology of Women Quarterly. 1995;19:257–285. [Google Scholar]
- Jacobsen BK, Oda K, Knutsen SF, Fraser GE. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: the Adventist Health Study, 1976–88. International Journal of Epidemiology. 2009;38:245–252. doi: 10.1093/ije/dyn251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- Kim K, Smith PK. Retrospective survey of parental marital relations and child reproductive development. International Journal of Behavioral Development. 1998b;22:729–751. [Google Scholar]
- Kim K, Smith PK, Palermiti AL. Conflict in childhood and reproductive development. Evolution and Human Behavior. 1997;18:109–142. [Google Scholar]
- Kreft I, De Leeuw J. Introducing multilevel modeling. Thousand Oaks, CA: Sage; 1998. [Google Scholar]
- Laitinen-Krispijn S, Van der Ende J, Hazebroek-Kampschreur AA, Verhulst FC. Pubertal maturation and the development of behavioural and emotional problems in early adolescence. Acta Psychiatr Scand. 1999;99:16–25. doi: 10.1111/j.1600-0447.1999.tb05380.x. [DOI] [PubMed] [Google Scholar]
- Lakshman R, Forouhi NG, sharp SJ, Luben R, Bingham SA, Khaw KT, et al. Early age at menarche associated with cardiovascular disease and mortality. The Journal of clinical Endocrinology and Metabolism. 2009;94:4953–5960. doi: 10.1210/jc.2009-1789. [DOI] [PubMed] [Google Scholar]
- Llop-Viñolas D, Vizmanos B, Closa Monasterolo R, Escribano Subías J, Fernández-Ballart JD, Martí-Henneberg C. Onset of puberty at eight years of age in girls determines a specific tempo of puberty but does not affect adult height. Acta Paediatrica. 2004;93:874–879. doi: 10.1111/j.1651-2227.2004.tb02683.x. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Craig AE, Flory JD, Halder I, Ferrell RE. Reported Early Family Environment Covaries with Menarcheal Age as a Function of Polymorphic Variation in Estrogen Receptor-α (ESR1) Development and Psychopathology. 2011 doi: 10.1017/S0954579410000659. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WB, Pasta DJ. Early family environment, reproductive strategy, and contraceptive behavior: Testing a genetic hypothesis. In: Rodgers JL, Rowe DC, Miller WB, editors. Genetic influences on human fertility and sexuality. Boston: Kluwer Academic Publishers; 2000. pp. 183–230. [Google Scholar]
- Morris NM, Udry RJ. Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence. 1980;9(3):271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Mustanski BS, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental influences on pubertal development: longitudinal data from Finnish twins at ages 11 and 14. Developmental Psychology. 2004;40:1188–1198. doi: 10.1037/0012-1649.40.6.1188. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Obradović J, Boyce WT. Individual differences in behavioral, physiological, and genetic sensitivities to contexts: Implications for development and adaptation. Developmental Neuroscience. 2009;31:300–308. doi: 10.1159/000216541. [DOI] [PubMed] [Google Scholar]
- Obradović J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: Stress reactivity moderates the relation between contextual adversity and socio-emotional and cognitive development. Child Development (In press) [Google Scholar]
- Pantsiotou S, Papadimitriou A, Douros K, Priftis K, Nicolaidou P, Fretzayas A. Maturational tempo differences in relation to the timing of the onset of puberty in girls. Acta Pædiatrica. 2008;97:217–220. doi: 10.1111/j.1651-2227.2007.00598.x. [DOI] [PubMed] [Google Scholar]
- Parker LN. Adrenarche. Endocrinol Metab Clin North Am. 1991;20:71–83. [PubMed] [Google Scholar]
- Parry BL, Newton RP. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology. 2001;25(Suppl 5):S102–108. doi: 10.1016/S0893-133X(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, et al. Stress regulation in the central nervous system: Evidence from structural and functional neuroimaging studies in human populations. Psychoneuroendocrinology. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Quas JA, Bauer A, Boyce WT. Physiological reactivity, social support, and memory in early childhood. Child Development. 2004;75:797–814. doi: 10.1111/j.1467-8624.2004.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Romans SE, Martin M, Gendall K, Herbison GP. Age of menarche: The role of some psychosocial factors. Psychological Medicine. 2003;33:933–939. doi: 10.1017/s0033291703007530. [DOI] [PubMed] [Google Scholar]
- Rowe DC. Environmental and genetic influences on pubertal development: Evolutionary life history traits. In: Rodgers JL, Rowe DC, Miller WB, editors. Genetic influences on human fertility and sexuality: Theoretical and empirical contributions from the biological and behavioral sciences. Boston: Kluwer Academic Publishers; 2000. pp. 147–168. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Development. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff E, Zahn-Waxler C, Klimes-Dougan B, Slattery M. Salivary dehydroepiandrosterone responsiveness to social challenge in adolescents with internalizing problems. J Child Psychol Psychiatry. 2007;48:580–591. doi: 10.1111/j.1469-7610.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- Slap GB, Khalid N, Paikoff RL, Brooks-Gunn J. Evolving self-image, pubertal manifestations, and pubertal hormones: Preliminary findings in young adolescent girls. Journal of Adolescent Health. 1994;15:327–335. doi: 10.1016/1054-139x(94)90606-8. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Reciprocal relation between parent-child distance and pubertal maturation. Developmental Psychology. 1988;24:122–128. [Google Scholar]
- Stoll BA, Vatten LJ, Kvinnsland S. Does early physical maturity influence breast cancer risk? Acta Oncol. 1994;33:171–176. doi: 10.3109/02841869409098400. [DOI] [PubMed] [Google Scholar]
- Styne DM, Grumbach MM. Puberty in boys and girls. In: Pfaff D, Arnold A, Etgen AM, Fahrbach S, Rubin RT, editors. Hormones, brain and behavior. Vol. 4. San Diego: Elsevier Science; 2002. pp. 661–716. [Google Scholar]
- Tither JM, Ellis BJ. Impact of fathers on daughters’ age of menarche: A genetically and environmentally controlled sibling study. Developmental Psychology. 2008;44:1409–1420. doi: 10.1037/a0013065. [DOI] [PubMed] [Google Scholar]
- Vo C, Carney M. Ovarian cancer hormonal and environmental risk. Obstet Gynecol Clin N Am. 2007;34:687–700. doi: 10.1016/j.ogc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Warren MP, Brooks-Gunn J. Mood and behavior at adolescence: evidence for hormonal factors. Journal of Clinical Endocrinology and Metabolism. 1989;69:77–82. doi: 10.1210/jcem-69-1-77. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. New York: Oxford University Press; 2003. [Google Scholar]
- Wolf M, van Doorn GS, Weissing FJ. Evolutionary emergence of responsive and unresponsive personalities. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15825–15830. doi: 10.1073/pnas.0805473105. [DOI] [PMC free article] [PubMed] [Google Scholar]