Abstract
BACKGROUND
Hyponatremia is the most common electrolyte abnormality in hospitalized individuals.
METHODS
To investigate the association between serum sodium concentration and mortality, we conducted a prospective cohort study of 98,411 adults hospitalized between 2000 and 2003 at 2 teaching hospitals in Boston, Massachusetts. The main outcome measures were in-hospital, 1-year, and 5-year mortality. Multivariable logistic regression and Cox proportional hazards models were used to compare outcomes in patients with varying degrees of hyponatremia against those with normal serum sodium concentration.
RESULTS
Hyponatremia (serum sodium concentration <135 mEq/L) was observed in 14.5% of patients on initial measurement. Compared with patients with normonatremia (135–144 mEq/L), those with hyponatremia were older (67.0 vs 63.1 years, P <.001) and had more comorbid conditions (mean Deyo-Charlson Index 1.9 vs 1.4, P <.001). In multivariable-adjusted models, patients with hyponatremia had an increased risk of death in hospital (odds ratio 1.47, 95% confidence interval [CI], 1.33–1.62), at 1 year (hazard ratio 1.38, 95% CI, 1.32–1.46), and at 5 years (hazard ratio 1.25, 95% CI, 1.21–1.30). The increased risk of death was evident even in those with mild hyponatremia (130–134 mEq/L; odds ratio 1.37, 95% CI, 1.23–1.52). The relationship between hyponatremia and mortality was pronounced in patients admitted with cardiovascular disease, metastatic cancer, and those admitted for procedures related to the musculoskeletal system. Resolution of hyponatremia during hospitalization attenuated the increased mortality risk conferred by hyponatremia.
CONCLUSION
Hyponatremia, even when mild, is associated with increased mortality.
Keywords: Epidemiology, Hospitalization, Hyponatremia, Mortality, Outcomes
The serum sodium concentration in humans is normally between 135 and 144 mEq/L. Hyponatremia (serum sodium concentration <135 mEq/L) implies a relative excess of total body water to sodium and is seen in a variety of medical conditions including congestive heart failure, liver disease, the syndrome of inappropriate antidiuretic hormone, and as a result of medications (eg, thiazide diuretics, psychotropic agents, and chemotherapeutic agents).1 Hyponatremia is the most common electrolyte abnormality in hospitalized individuals, affecting up to 30% of patients in some series.2 The risk of death during hospitalization is increased by more than 50% in patients admitted with hyponatremia compared with normonatremia,2–5 but the magnitude of the risk according to the actual degree of hyponatremia has not been well studied. In addition, previous studies have not examined differences in the risk of death with hyponatremia in different patient subpopulations, among which the rates and causes of hyponatremia may vary. Further, long-term mortality after hospitalization with hyponatremia has not previously been investigated. We therefore studied the short- and long-term mortality of more than 95,000 patients admitted with and without hyponatremia, from mild to severe cases.
METHODS
Source Population
We extracted administrative and laboratory data from individuals admitted to 2 academic teaching hospitals in Boston, Massachusetts. Brigham and Women’s Hospital (BWH) is a 777-bed teaching hospital. Massachusetts General Hospital (MGH) is a 902-bed teaching hospital. The 2 hospitals provide primary as well as tertiary care to an ethnically and socioeconomically diverse population within eastern Massachusetts and the surrounding region.
Data Sources
Data on all patients admitted for at least 48 hours to BWH or MGH between January 1, 2000 and December 31, 2002 were obtained through the Research Patient Data Registry, a registry maintained by Partners Healthcare System, which is the administrative body that oversees operations at BWH and MGH. The Research Patient Data Registry serves as a central clinical data warehouse for over 1.8 million inpatients and outpatients; the database contains information on patient demographics, diagnoses and procedures, medications, inpatient and outpatient encounters, health care providers, and laboratory results. The database was designed for research and quality improvement purposes and has been accessed previously for clinical studies.6,7
The following data were retrieved: demographics, length of stay, vital status at hospital discharge and up to 5 years following discharge, International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes (up to 12 per patient), Center for Medicare and Medicaid Services diagnosis-related group, and inpatient plasma sodium and glucose measurements. The primary analyses examined the first inpatient sodium measurement as the exposure of interest. We used the Deyo modification of the Charlson index (D-CI)8 to estimate comorbidity. The D-CI is the sum of the weighted number of comorbid conditions based on 17 diagnostic categories identified from ICD-9-CM diagnosis codes. The D-CI has been shown to perform adequately as a comorbidity adjustment tool using administrative data.9 We used ICD-9-CM codes and diagnosis-related groups to identify medical conditions present during hospitalization.
Study Population
During the 3-year enrollment period there were 201,143 admissions (140,816 unique patients, age ≥18 years) lasting at least 48 hours. We selected for analysis the initial admission during the study period. Of the 140,816 index admissions, 98,411 had at least one sodium measurement and constituted the study cohort; the number of sodium measurements ranged from 1 (44% of cohort) to 229. Of the 42,405 patients with no sodium measurements, 69% were admitted to the Obstetrics service for labor and delivery.
Assessment of Mortality
Information on vital status at discharge was obtained from hospital administrative records. Information on vital status through April 23, 2007 among survivors of hospitalization was obtained from the Social Security Death Index (SSDI).
Statistical Analysis
All analyses were performed with SAS version 9.1 (SAS Institute, Cary, NC). Continuous variables were expressed as means ± standard deviations or medians with interquartile ranges and tested by the Student’s t test or Wilcoxon rank sum test, as appropriate. Categorical variables were described as proportions and compared using the chi-squared test.
In-hospital mortality across categories of hyponatremia and normonatremia was compared by fitting logistic regression models. Longer-term mortality (ie, following discharge) was compared by fitting Cox proportional hazards models. Survival times were censored at the latest available date of death in the database, April 23, 2007. To explore the extent of confounding, we fitted increasingly adjusted models, first adjusting for age alone, then for age, sex, and D-CI, and then additionally for the following a priori selected indicator variables based on the presence or absence of ICD-9-CM codes (see Appendix): acute myocardial infarction, congestive heart failure, sepsis, metastatic cancer, pneumonia, chronic kidney disease, liver disease, gastrointestinal bleeding, syndrome of inappropriate antidiuretic hormone, and volume depletion.
Appendix.
Administrative Codes for Diagnoses and Major Diagnostic Categories
| Diagnosis | ICD-9 code |
| Acute MI | 410.x |
| Congestive heart failure | 428.x |
| Sepsis | 038.x, 112.5, 112.81, 020.2, 790.7 |
| Pneumonia | 480.x, 481, 482.x, 483.x, 484.x, 485, 486 |
| Chronic kidney disease | 582.x, 583.x, 585.x, 586, 588.x |
| Liver disease | 570, 571 |
| Gastrointestinal hemorrhage | 578.x |
| Syndrome of inappropriate antidiuretic hormone | 253.6 |
| Hypothyroidism | 244.x |
| Adrenal insufficiency | 255.4x |
| Polydipsia | 783.5 |
| Volume depletion | 276.5 |
| Metastatic cancer | 196.x, 197.x, 198.x, 199.x |
| Major diagnostic category* | Diagnosis-related group codes |
| Circulatory system (surgical) | 104–120 |
| Circulatory system (medical) | 121–145 |
| Musculoskeletal system (surgical) | 209–234 |
| Nervous system (surgical) | 1–8 |
| Nervous system (medical) | 9–35 |
| Respiratory system (medical) | 78–102 |
The most common admission diagnoses (based on ICD-9 codes) for each of the Major Diagnostic Categories were as follows:
○ Circulatory system (medical) – unspecified chest pain (12%); congestive heart failure (12%); acute myocardial infarction (8%); syncope and collapse (8%); and intermediate coronary syndrome (7%).
○ Circulatory system (surgical) – chronic ischemic heart disease (14%); coronary atherosclerosis of native coronary artery (10%); intermediate coronary syndrome (9%); aortic valve disorders (7%); and acute myocardial infarction, unspecified (6%).
○ Musculoskeletal system (surgical) – osteoarthrosis (28%); mechanical complication of internal orthopedic device (4%); lumbar spinal stenosis (4%); fracture of femur (3%); and other complication of internal prosthetic device (2%).
○ Nervous system (surgical) – neoplasm of brain (unspecified) (12%); nonruptured cerebral aneurysm (7%); subarachnoid hemorrhage (6%); carotid artery stenosis or occlusion (5%); and benign neoplasm of cranial nerves (5%).
○ Nervous system (medical) – acute cerebrovascular disease (20%); convulsions (10%); intracerebral hemorrhage (5%); transient cerebral ischemia (4%); and cerebral artery occlusion with infarction (3%).
○ Respiratory system (medical) – pneumonia (24%); chronic airway obstruction (6%); asthma (6%); shortness of breath (5%); and pulmonary embolism (4%).
We investigated whether the association between hyponatremia and mortality differed in clinical subgroups by including interaction terms in multivariable logistic regression models of in-hospital mortality. For multivariable models involving subgroups, we excluded the corresponding variable from the list of covariates. The assumption of proportional hazards was confirmed by inspecting log-log survival curves. Two-tailed P values <.05 were considered statistically significant.
RESULTS
Clinical Characteristics
Among 98,411 individuals admitted during the study period, hyponatremia (serum sodium concentration <135 mEq/L) was present on the first sodium determination in 14,290 (14.5%) and at some point during hospitalization in an additional 5093 (total, 19.7%). Hyponatremia on admission was mild (130–134 mEq/L) in the majority of patients (83.0%) and severe (<120) in only 0.2% (Table 1).
Table 1.
Prevalence of Hyponatremia, Uncorrected and Corrected for Glucose Concentration
| Sodium Concentration (mEq/L) |
% Misclassified* | ||||||
|---|---|---|---|---|---|---|---|
| 135–144 | <135 | 130–134 | 125–129 | 120–124 | <120 | ||
| Initial value during admission, n (%) | 81,031 (82.3%) | 14,290 (14.5%) | 11,853 (12.0%) | 1856 (1.9%) | 415 (0.4%) | 166 (0.2%) | — |
| Hillier10 (2-piece linear regression), n (%)† | 82,377 (83.7%) | 12,562 (12.8%) | 10,469 (10.6%) | 1591 (1.6%) | 353 (0.4%) | 149 (0.2%) | 12.1% |
| Hillier10 (linear regression), n (%)‡ | 83,423 (84.8%) | 11,615 (11.8%) | 9671 (9.8%) | 1472 (1.5%) | 328 (0.3%) | 144 (0.1%) | 18.7% |
| Katz,11 n (%)§ | 82,574 (83.9%) | 12,617 (12.8%) | 10,513 (10.7%) | 1601 (1.6%) | 353 (0.4%) | 150 (0.2%) | 11.7% |
Percentages were calculated based on the number of index admissions between January 1, 2000 and December 31, 2002, lasting ≥2 days and having at least 1 sodium measurement (n = 98,656).
The percentage of patients initially diagnosed as having hyponatremia (Na <135, n = 81,234) who were re-classified as having normonatremia (Na 135–144) or hypernatremia (Na >144) after correction for hyperglycemia. Admission glucose was measured in 94.6% of patients with hyponatremia on admission.
Nacorrected = Naadmission = [4.0 * (glucoseadmission −100 mg/dL)] if glucose >440; if glucose ≤440 Nacorrected = Naadmission + [1.6 * (glucoseadmission − 100 mg/dL)].
Nacorrected = Naadmission + [2.4 * (glucoseadmission − 100 mg/dL)].
Nacorrected = Naadmission + [1.6 * (glucoseadmission − 100 mg/dL)].
Hyperglycemia (glucose ≥200 mg/dL) was present in 15.8% of patients admitted with hyponatremia (glucose was measured in 94.7%). When the initial serum sodium concentration was corrected for hyperglycemia using 3 different formulas, 11.7% to 18.7% of the 14,290 initially hyponatremic patients were recategorized as having normo- or hypernatremia (Table 1). All subsequent analyses were performed using initial sodium values corrected for initial glucose concentration above 100 mg/dL according to the 2-piece linear regression model described by Hillier et al.10 After excluding 3472 individuals with hypernatremia (Na >144 mEq/L), the final study cohort consisted of 94,939 patients with normonatremia or hyponatremia.
Demographic and clinical characteristics of patients with and without hyponatremia are shown in Table 2. Women accounted for approximately half of all admissions with and without hyponatremia overall, but two thirds of admissions with severe hyponatremia. Notable differences in the clinical characteristics of those with hyponatremia compared with normonatremia included a higher frequency of congestive heart failure, sepsis, pneumonia, metastatic disease, and volume depletion. Compared with those with normonatremia, patients admitted with hyponatremia had more comorbidity (mean D-CI 1.9 vs 1.4, P <.001).
Table 2.
Characteristics of Hospitalized Individuals with and without Hyponatremia
| Sodium Concentration (mEq/L) |
P Value* | ||||||
|---|---|---|---|---|---|---|---|
| 135–144 (n = 82,377) | <135 (n = 12,562) | 130–134 (n = 10,469) | 125–129 (n = 1591) | 120–124 (n = 353) | <120 (n = 149) | ||
| Mean age ± SD, years | 63.1 | 67.0 | 66.0 | 71.5 | 72.8 | 73.1 | <.001 |
| Female (%) | 51.1 | 51.2 | 50.8 | 51.4 | 55.5 | 66.4 | .83 |
| Race and ethnicity (%) | |||||||
| White | 81.2 | 82.7 | 82.3 | 84.8 | 84.1 | 87.3 | <.001 |
| Black | 7.0 | 5.3 | 5.6 | 3.7 | 4.3 | 2.0 | <.001 |
| Hispanic | 5.0 | 4.1 | 4.3 | 3.0 | 4.3 | 2.7 | <.001 |
| Asian | 1.4 | 1.8 | 1.7 | 2.1 | 2.3 | 1.3 | .0009 |
| Medical diagnoses (%) | |||||||
| Acute MI | 6.0 | 6.0 | 5.9 | 6.3 | 6.2 | 5.4 | .91 |
| Congestive heart failure | 9.5 | 12.9 | 12.0 | 17.4 | 16.7 | 16.8 | <.001 |
| Sepsis | 2.4 | 5.2 | 4.9 | 6.9 | 5.7 | 4.7 | <.001 |
| Pneumonia | 4.5 | 8.5 | 7.9 | 11.6 | 9.6 | 11.4 | <.001 |
| CKD | 2.0 | 3.0 | 3.0 | 3.4 | 3.7 | 2.0 | <.001 |
| Liver disease | 1.3 | 3.4 | 2.9 | 5.2 | 6.8 | 8.1 | <.001 |
| GI bleed | 2.1 | 2.4 | 2.3 | 3.1 | 3.7 | 3.4 | .05 |
| SIADH | 0.1 | 1.8 | 1.0 | 4.5 | 11.1 | 11.4 | <.001 |
| Hypothyroidism | 6.4 | 7.5 | 7.4 | 7.4 | 8.2 | 15.4 | <.001 |
| Adrenal insufficiency | 0.2 | 0.3 | 0.2 | 0.5 | 0.3 | 3.4 | .002 |
| Polydispia | 0.0 | 0.1 | 0.0 | 0.1 | 0.9 | 2.7 | <.001 |
| Volume depletion | 3.6 | 8.6 | 7.3 | 13.0 | 21.0 | 24.8 | <.001 |
| Metastatic disease | 6.4 | 10.8 | 10.4 | 12.8 | 15.0 | 9.4 | <.001 |
| Major diagnostic category (%) | |||||||
| Circulatory system: surgical | 16.7 | 17.8 | 18.7 | 15.0 | 7.9 | 4.0 | .002 |
| Circulatory system: medical | 9.0 | 8.2 | 7.9 | 10.1 | 9.9 | 10.1 | .006 |
| Musculoskeletal system: surgical | 12.2 | 8.3 | 8.9 | 5.8 | 3.7 | 2.0 | <.001 |
| Nervous system: surgical | 5.0 | 3.3 | 3.5 | 2.3 | 1.7 | 0.7 | <.001 |
| Nervous system: medical | 5.7 | 4.2 | 3.9 | 6.1 | 5.7 | 1.3 | <.001 |
| Respiratory system: medical | 5.1 | 7.5 | 6.8 | 10.7 | 13.0 | 10.7 | <.001 |
| Mean D-CI | 1.4 | 1.9 | 1.9 | 2.2 | 2.3 | 1.7 | <.001 |
MI = myocardial infarction; CKD = chronic kidney disease; GI = gastrointestinal; SIADH = syndrome of inappropriate antidiuretic hormone; D-CI = Deyo-Charlson Index.
Sodium values corrected for admission glucose.
P values compare those with and without hyponatremia (<135 vs 135–144).
Mortality
Patients with hyponatremia had higher in-hospital, 1-year, and 5-year mortality rates than patients without hyponatremia (Table 3). Differences in mortality persisted after multivariable adjustment in all categories of hyponatremia except for serum sodium concentration <120 mEq/L. Compared with patients with normonatremia, the multivariable-adjusted risk of death after 5 years following admission was increased by 24% in patients with admission serum sodium concentration 130–134 mEq/L (P <.001); 33% in those with serum sodium concentration 125–129 mEq/L (P <.001); 29% in those with serum sodium concentration 120–124 mEq/L (P =.003); and 9% in those with admission serum sodium concentration <120 mEq/L (P =.52) (Table 3). Repeating mortality and survival analyses using sodium values unadjusted for admission glucose concentration or using nadir rather than initial sodium values resulted in no substantial differences. Further adjustment for the 6 most common major diagnostic categories or race did not materially affect the results.
Table 3.
Mortality in Patients with and without Hyponatremia
| Sodium Concentration (mEq/L) |
||||||
|---|---|---|---|---|---|---|
| 135–144 (n = 82,377) | <135 (n = 12,562) | 130–134 (n = 10,469) | 125–129 (n = 1591) | 120–124 (n = 353) | <120 (n = 149) | |
| Crude in-hospital mortality (%) | 2.4 | 5.4 | 4.8 | 8.9 | 8.5 | 6.7 |
| Age-adjusted | 1 (ref) | 2.08 (1.90–2.28) | 1.87 (1.69–2.07) | 3.20 (2.67–3.83) | 2.93 (2.00–4.28) | 2.24 (1.17–4.28) |
| Age, sex, D-CI-adjusted | 1 (ref) | 1.88 (1.72–2.06) | 1.69 (1.53–1.87) | 2.88 (2.40–3.45) | 2.56 (1.74–3.77) | 2.29 (1.19–4.42) |
| Multivariable-adjusted | 1 (ref) | 1.47 (1.33–1.62) | 1.37 (1.23–1.52) | 2.01 (1.64–2.45) | 1.67 (1.09–2.56) | 1.46 (0.73–2.91) |
| Crude 1-year mortality (%) | 11.7 | 21.4 | 19.8 | 28.5 | 33.1 | 22.2 |
| Age-adjusted | 1 (ref) | 1.65 (1.57–1.73) | 1.58 (1.50–1.68) | 1.88 (1.68–2.11) | 2.31 (1.87–2.86) | 1.29 (0.86–1.95) |
| Age, sex, D-CI-adjusted | 1 (ref) | 1.51 (1.44–1.59) | 1.45 (1.37–1.53) | 1.76 (1.57–1.97) | 2.13 (1.72–2.63) | 1.42 (0.94–2.14) |
| Multivariable-adjusted | 1 (ref) | 1.38 (1.32–1.46) | 1.35 (1.28–1.43) | 1.53 (1.36–1.71) | 1.78 (1.44–2.21) | 1.03 (0.68–1.56) |
| Crude 5-year mortality (%) | 42.3 | 54.8 | 53.6 | 61.0 | 60.6 | 59.7 |
| Age-adjusted | 1 (ref) | 1.42 (1.37–1.46) | 1.39 (1.34–1.44) | 1.55 (1.43–1.68) | 1.59 (1.35–1.87) | 1.34 (1.04–1.72) |
| Age, sex, D-CI adjusted | 1 (ref) | 1.34 (1.30–1.39) | 1.31 (1.26–1.36) | 1.50 (1.38–1.62) | 1.53 (1.30–1.80) | 1.31 (1.12–1.86) |
| Multivariable-adjusted | 1 (ref) | 1.25 (1.21–1.30) | 1.24 (1.19–1.29) | 1.33 (1.23–1.44) | 1.29 (1.09–1.53) | 1.09 (0.84–1.41) |
Sodium values corrected for admission glucose. Multivariable models adjusted for age, sex, Deyo-Charlson Index (D-CI), and individual diagnoses (acute myocardial infarction, congestive heart failure, sepsis, metastatic cancer, pneumonia, chronic kidney disease, liver disease, gastrointestinal bleeding, syndrome of inappropriate antidiuretic hormone, volume depletion).
Subgroup Analyses
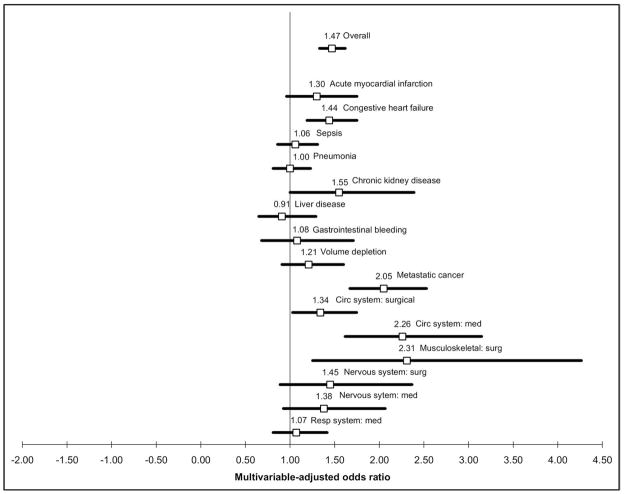
The multivariable-adjusted odds of in-hospital mortality associated with hyponatremia (serum sodium concentration <135 mEq/L) differed across clinical subgroups (Figure). For example, hyponatremia was not associated with an increased risk of death compared with normonatremia in patients admitted with pneumonia, sepsis, liver disease, or medical admissions related to the respiratory system. The risk of in-hospital mortality with hyponatremia was pronounced in patients admitted with metastatic cancer (odds ratio [OR] 2.05, 95% confidence interval [CI], 1.67–2.53), for medical admissions related to the cardiovascular system (OR 2.26, 95% CI, 1.62–3.15), and for surgical admissions related to the musculoskeletal system (OR 2.31, 95% CI, 1.25–4.27). The associations between degree of hyponatremia and in-hospital mortality are shown in Table 4 for those subgroups in which a statistically significant interaction was detected. In the setting of pneumonia, sepsis, and medical admissions related to the respiratory system, there was no increased risk of death at any degree of hyponatremia. For liver disease, a statistically significantly increased risk of death was found only for those with serum sodium concentration 120–124 mEq/L.
Figure.
Odds ratios for death in patients with versus without hyponatremia, according to clinical subgroups. Values above one represent a higher risk of death in those with hyponatremia. Odds ratio point estimates are listed above each box, and 95% confidence intervals are represented by solid lines. P values for interaction terms between hyponatremia (Na <135) and the subgroup of interest were as follows: acute myocardial infarction .30, congestive heart failure .57, sepsis .0002, pneumonia <.0001, chronic kidney disease .67, liver disease .004, gastrointestinal bleeding .18, volume depletion .07, metastatic cancer .005, circulatory system (surgical) .69, circulatory system (medical) .02, musculoskeletal system (surgical) .01, nervous system (surgical) .73, nervous system (medical) .26, respiratory system (medical) .002.
Table 4.
In-hospital Mortality in Selected Subgroups of Patients with and without Hyponatremia
| Condition | Sodium Concentration (mEq/L) |
|||||
|---|---|---|---|---|---|---|
| Total n (% Mortality) | 135–144 | 130–134 | 125–129 | 120–124 | <120 | |
| Sepsis | 2632 (25%) | 1 (ref) | 1.00 (0.79–1.26) | 1.36 (0.88–2.11) | 1.65 (0.64–4.24) | 0.38 (0.04–3.21) |
| Pneumonia | 4761 (16%) | 1 (ref) | 0.98 (0.78–1.23) | 1.09 (0.71–1.69) | 1.70 (0.71–4.08) | — |
| Liver disease | 1462 (16%) | 1 (ref) | 0.73 (0.48–1.10) | 1.32 (0.71–2.45) | 2.63 (1.00–6.94) | 0.56 (0.07–4.83) |
| Metastatic cancer | 6612 (7%) | 1 (ref) | 1.78 (1.41–2.24) | 3.25 (2.20–4.81) | 3.43 (1.61–7.33) | 4.80 (1.27–18.17) |
| Circulatory system (medical) | 8421 (3%) | 1 (ref) | 2.19 (1.52–3.17) | 2.73 (1.41–5.28) | 1.35 (0.26–6.90) | 2.97 (0.35–25.16) |
| Musculoskeletal system (surgical) | 11,079 (0.6%) | 1 (ref) | 2.10 (1.08–4.07) | 4.60 (1.35–15.73) | — | — |
| Respiratory system (medical) | 5153 (7%) | 1 (ref) | 1.03 (0.75–1.40) | 1.38 (0.81–2.35) | 0.82 (0.25–2.66) | 0.60 (0.07–5.35) |
Sodium values corrected for admission glucose. Values are shown for subgroups of patients in whom statistically significant interaction was detected for the association between hyponatremia (Na <135) and in-hospital mortality. P values for interaction terms were: sepsis .0002, pneumonia <.0001, liver disease .004, metastatic cancer .005, circulatory system (medical) .02, musculoskeletal system (surgical) .01, respiratory system (medical) .002.
Change in Serum Sodium Concentration
A total of 52,468 patients with hypo- or normonatremia had 2 or more sodium determinations, enabling analyses of the relation with change in serum sodium concentration during hospitalization. Patients were categorized into 4 groups, according to the change in serum sodium concentration between the first and final sodium measurement (unadjusted for glucose concentration). The majority were normonatremic on both measurements. Hyponatremia resolved in 3794 (7.2%), persisted in 4524 (8.6%), and was acquired during hospitalization in 1974 (3.8%). Mortality (in hospital, 1-year, and 5-year) was highest and comparable in those with persistent or acquired hyponatremia, lower in those with hyponatremia that resolved, and lowest in those with normonatremia at both first and last serum sodium measurements (Table 5).
Table 5.
Mortality in Patients with At Least 2 Serum Sodium Determinations According to Initial and Final Sodium Values
| Persistent Normonatremia (n = 42,176) | Resolution of Hyponatremia (n = 3794) | Persistent Hyponatremia (n = 4524) | Acquired Hyponatremia (n = 1974) | |
|---|---|---|---|---|
| Crude in-hospital mortality (%) | 1.8% | 3.9% | 6.2% | 5.9% |
| Multivariable-adjusted odds ratio for death | 1 (ref) | 1.26 (1.03–1.52) | 2.37 (2.03–2.77) | 2.44 (1.97–3.03) |
| Crude 1-year mortality (%) | 11.1% | 18.5% | 23.5% | 22.7% |
| Multivariable-adjusted hazard ratio for death at 1 year after discharge | 1 (ref) | 1.19 (1.09–1.31) | 1.55 (1.43–1.67) | 1.54 (1.37–1.72) |
| Crude 5-year mortality (%) | 25.4% | 38.5% | 40.8% | 41.1% |
| Multivariable-adjusted hazard ratio for death at 5 years after discharge | 1 (ref) | 1.18 (1.11–1.25) | 1.32 (1.25–1.39) | 1.40 (1.30–1.51) |
Categorization is according to normonatremia (135–144 mEq/L) and hyponatremia (<135 mEq/L) on initial versus final serum sodium determination (unadjusted for glucose concentration). Multivariable models adjusted for age, sex, Deyo-Charlson Index, and individual diagnoses (acute myocardial infarction, congestive heart failure, sepsis, metastatic cancer, pneumonia, chronic kidney disease, liver disease, gastrointestinal bleeding, syndrome of inappropriate antidiuretic hormone, volume depletion).
DISCUSSION
The prevalence of hyponatremia (first measured serum sodium concentration <135 mEq/L) in individuals hospitalized for at least 2 days is approximately 13%. Our estimate is higher than the 5.5% prevalence reported in the 39-hospital study by Zilberberg et al,5 which included all admissions irrespective of length of stay. Anderson et al12 found a prevalence of 2.4% when defining hyponatremia as serum sodium concentration <130 mEq/L; our results using this definition are similar. Our study confirms an association between hyponatremia and mortality, and extends previous reports in several ways. We found that the risk of mortality in individuals with hyponatremia is evident even in mild cases (serum sodium concentration 130–134 mEq/L), which constitute the majority of cases of hyponatremia. Typically, previous studies have examined hyponatremia as a binary variable (present or absent), but our large sample size allowed us to examine several different categories of serum sodium concentrations as well as the relation with change in serum sodium concentration during hospitalization. We were able to study the long-term impact on survival in addition to in-hospital mortality by extending the observation period following hospitalization, and demonstrated that the increased risk of death persists 5 years beyond discharge. We also found that 12.1% of patients initially identified with hyponatremia were hyperglycemic, such that they would otherwise have been normonatremic or hypernatremic in the absence of the associated shifts in free water from the intracellular space. The effect of hyperglycemia on serum sodium was accounted for by using a formula experimentally derived in human studies.10
Anderson et al12 reported that 16% of cases of hyponatremia in medical or surgical patients were due to hyperglycemia (glucose >300 mg/dL), but most subsequent epidemiologic studies of hyponatremia have not accounted for hyperglycemia. We speculated that there may be substantial differential misclassification of hyponatremia due to hyperglycemia given the strong association between hyperglycemia and in-hospital mortality even among those with undiagnosed diabetes.13 Importantly, we found only a small difference in the absolute prevalence of hyponatremia when corrected for hyperglycemia, and no effect on the association between hyponatremia and mortality.
The significance of hyponatremia varies according to the clinical context (Figure). While the risk of death in hyponatremic patients was higher for most diagnoses, we found no increased risk of in-hospital mortality with hyponatremia (serum sodium concentration <135 mEq/L) in the setting of sepsis, liver disease, or respiratory diseases including pneumonia. Using a large administrative database of hospitalized patients with pneumonia, Zilberberg14 also found no increased risk of death with hyponatremia (serum sodium concentration <135 mEq/L) compared with normonatremia, whereas Nair et al15 reported a 7% increased risk of death in a single-center study. We found no increased risk of death in any category of hyponatremia in sepsis, pneumonia, or medical admissions for respiratory diseases, but an increased risk of death in liver disease with more severe hyponatremia (serum sodium concentration 120–124 mEq/L). Previous studies in cirrhosis have yielded conflicting reports, with several reports16–20 of an increased risk of death with hyponatremia and at least one21 showing no association after multivariable adjustment for disease severity.
We documented a heightened association between hyponatremia and in-hospital mortality in the setting of metastatic cancer, heart disease, and patients admitted for orthopedic surgical procedures. The prognostic value of low serum sodium concentrations for mortality has been previously described for acute myocardial infarction,22–24 congestive heart failure,25–28 and cancer,29–31 but not to our knowledge in orthopedics.
The association between hyponatremia and outcomes following orthopedic surgical procedures deserves further attention. McPherson and Dunsmuir32 found hyponatremia (Na <130 mEq/L) pre- or postoperatively in 2.8% of all patients undergoing surgery for fracture of the femoral neck. No published study has examined outcomes associated with hyponatremia in this large patient population. We found in patients admitted for orthopedic surgical procedures a 2.10-fold increased risk of death with mild hyponatremia (serum sodium concentration 130–134 mEq/L) and a 4.60-fold increased risk of death with more severe hyponatremia (serum sodium concentration 125–129 mEq/L). We could not differentiate between preoperative hyponatremia (ie, as a potential cause for falls leading to admission for an orthopedic procedure33) and postoperative (ie, potentially iatrogenic) hyponatremia. Further studies should elucidate the reasons for the associations we observed between hyponatremia and mortality and whether they are due to severity of comorbid disease or whether hyponatremia itself represents a potentially treatable perioperative risk factor.
Our study cannot determine whether a causal link exists between hyponatremia and mortality. When severe or acute in onset, hyponatremia can lead to life-threatening cerebral edema. The reasons underlying our finding of increased mortality in less severe hyponatremia are less clear. Hyponatremia can be a marker of severity of illness, which may be predictive of but not causally related to mortality. For example, hyponatremia may signal the presence of important physiologic derangements in the case of cardiovascular disease (eg, low effective circulating volume with consequent impairments of glomerular filtration rate and distal sodium delivery, elevated levels of arginine vasopressin, or neurohormonal activation). Alternatively, the association between mild hyponatremia and mortality may have a physiologic basis. Maintenance of serum osmolarity and sodium concentrations within tight boundaries is a hallmark of all terrestrial mammals.34 Sodium concentrations affect the 3-dimensional conformations of proteins and enzymes, and play a critical role in maintaining electrical gradients across cell membranes, in nerve-impulse transmission, and in muscle excitation.35 The effects of abnormal serum sodium concentrations on cerebral function are well known,33,36 but the effects on other organ systems deserve further study. Notably, patients with chronic hyponatremia may have subtle but significant decrements in neurological function, in the absence of overt cerebral edema; the mechanism(s) of these effects are not as yet clear.33
Hyponatremia is common in the hospitalized population but extremely heterogenous in terms of causes; our results from subgroup analyses confirm that the heterogeneity extends to its prognostic significance as well. Whether hyponatremia is a rational target for interventional studies (eg, water restriction, loop diuretics, and the newly introduced V2-receptor antagonists37) likely depends on the clinical context. Because hyponatremia may not be associated with mortality in sepsis and pneumonia, the benefit of correcting sodium concentration in those populations may be limited. In contrast, hyponatremia appears to be strongly associated with mortality in heart disease, cancer, and in patients undergoing orthopedic procedures; these groups could be targeted to examine the potential benefit of strategies to normalize serum sodium concentrations. Indeed, we found that improvements in serum sodium concentration attenuated the increased risk of death conferred by hyponatremia.
Several limitations of our study deserve mention. We relied on ICD-9-CM and diagnosis-related group codes to classify patients in subgroups and for use in multivariable models. The accuracy of ICD-9-CM codes has been studied extensively both for identifying specific medical conditions and for use in the D-CI for risk-adjustment. ICD-9-CM codes have reasonable positive predictive values (PPV) for identifying common medical conditions such as heart failure (PPV 94.3%),38 acute myocardial infarction (PPV 94.1%),39 pneumonia (PPV 72.6%–80.8%),40 sepsis (PPV 88.9%–97.7%),41 and chronic kidney disease (PPV 85.7%–97.5%).42 The accuracy of ICD-9-CM codes for diagnoses such as syndrome of inappropriate antidiuretic hormone and volume depletion have not been studied. The extent of misclassification in our study due to the inaccuracy of ICD-9-CM codes is not known and may have affected our estimates of mortality within clinical subgroups. The D-CI has been shown to be comparable with other comorbidity measures for risk adjustment and mortality prediction,9,43 but to be inferior to more complex models incorporating additional numerical laboratory values.44,45 The 2 hospitals included in these analyses are teaching hospitals and tertiary care centers in an urban setting; the prevalence of hyponatremia may differ in other hospital settings.
We were not able to define causes or chronicity for hyponatremia in our cohort. The distinction between euvolemic, hypervolemic, and hypovolemic hyponatremia is a critical first step in differential diagnosis, but impractical when performing a large-scale epidemiologic study such as ours. In addition to a detailed clinical evaluation, this distinction requires diagnostic testing that is not available for all of the patients. For example, an accurate distinction between euvolemic and hypovolemic hyponatremia requires measurement of urine electrolytes or urea, given that “subclinical” hypovolemia can lead to an exaggerated release of vasopressin and subsequent hyponatremia.46–48
We used the SSDI to obtain vital status following discharge. The SSDI has a reported accuracy of 93.2%.49 Misclassification of vital status was likely nondifferential with respect to the presence or absence of hyponatremia, and therefore would bias our estimates towards the null hypothesis.
To conclude, hyponatremia is common in the hospitalized population, and even mild cases are independently associated with a higher risk of in-hospital and long-term mortality. Resolution of hyponatremia during hospitalization attenuated the increased mortality risk conferred by hyponatremia. There are important differences in the prognostic significance of hyponatremia across clinical settings, several of which should be the focus for interventional investigations.
Acknowledgments
Funding: Investigator-initiated grant from Astellas Pharma US, Inc.
Footnotes
Conflict of Interest: Dr. Mount reported receiving lecture fees from Astellas Pharma, US, Inc. Astellas had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Authorship: Dr. Waikar had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Brenner BM, Rector FC. Brenner & Rector’s the Kidney. 8. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 2.Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119(7 Suppl 1):S30–S35. doi: 10.1016/j.amjmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Clayton JA, Le Jeune IR, Hall IP. Severe hyponatraemia in medical in-patients: aetiology, assessment and outcome. QJM. 2006;99:505–511. doi: 10.1093/qjmed/hcl071. [DOI] [PubMed] [Google Scholar]
- 4.Gill G, Huda B, Boyd A, et al. Characteristics and mortality of severe hyponatraemia—a hospital-based study. Clin Endocrinol (Oxf) 2006;65:246–249. doi: 10.1111/j.1365-2265.2006.02583.x. [DOI] [PubMed] [Google Scholar]
- 5.Zilberberg MD, Exuzides A, Spalding J, et al. Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients. Curr Med Res Opin. 2008;24:1601–1608. doi: 10.1185/03007990802081675. [DOI] [PubMed] [Google Scholar]
- 6.Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med. 2004;141:745–752. doi: 10.7326/0003-4819-141-10-200411160-00005. [DOI] [PubMed] [Google Scholar]
- 7.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes for Acute Renal Failure. J Am Soc Nephrol. 2006;17:1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 9.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42:355–360. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- 10.Hillier TA, Abbott RD, Barrett EJ. Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med. 1999;106:399–403. doi: 10.1016/s0002-9343(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 11.Katz MA. Hyperglycemia-induced hyponatremia—calculation of expected serum sodium depression. N Engl J Med. 1973;289:843–844. doi: 10.1056/NEJM197310182891607. [DOI] [PubMed] [Google Scholar]
- 12.Anderson RJ, Chung HM, Kluge R, Schrier RW. Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Ann Intern Med. 1985;102:164–168. doi: 10.7326/0003-4819-102-2-164. [DOI] [PubMed] [Google Scholar]
- 13.Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 14.Zilberberg MD, Exuzides A, Spalding J, et al. Hyponatremia and hospital outcomes among patients with pneumonia: a retrospective cohort study. BMC Pulm Med. 2008;8:16. doi: 10.1186/1471-2466-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair V, Niederman MS, Masani N, Fishbane S. Hyponatremia in community-acquired pneumonia. Am J Nephrol. 2007;27:184–190. doi: 10.1159/000100866. [DOI] [PubMed] [Google Scholar]
- 16.Ruf AE, Kremers WK, Chavez LL, et al. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336–343. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]
- 17.Biggins SW, Rodriguez HJ, Bacchetti P, et al. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32–39. doi: 10.1002/hep.20517. [DOI] [PubMed] [Google Scholar]
- 18.Heuman DM, Abou-Assi SG, Habib A, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802–810. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 19.Londono MC, Cardenas A, Guevara M, et al. MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut. 2007;56:1283–1290. doi: 10.1136/gut.2006.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porcel A, Diaz F, Rendon P, et al. Dilutional hyponatremia in patients with cirrhosis and ascites. Arch Intern Med. 2002;162:323–328. doi: 10.1001/archinte.162.3.323. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg A, Hammerman H, Petcherski S, et al. Hyponatremia and long-term mortality in survivors of acute ST-elevation myocardial infarction. Arch Intern Med. 2006;166:781–786. doi: 10.1001/archinte.166.7.781. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg A, Hammerman H, Petcherski S, et al. Prognostic importance of hyponatremia in acute ST-elevation myocardial infarction. Am J Med. 2004;117:242–248. doi: 10.1016/j.amjmed.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Singla I, Zahid M, Good CB, et al. Effect of hyponatremia (<135 mEq/L) on outcome in patients with non-ST-elevation acute coronary syndrome. Am J Cardiol. 2007;100:406–408. doi: 10.1016/j.amjcard.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 25.Milo-Cotter O, Cotter G, Weatherley BD, et al. Hyponatraemia in acute heart failure is a marker of increased mortality but not when associated with hyperglycaemia. Eur J Heart Fail. 2008;10:196–200. doi: 10.1016/j.ejheart.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) J Am Coll Cardiol. 2008;52:347–356. doi: 10.1016/j.jacc.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Lee DS, Austin PC, Rouleau JL, et al. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 28.Tribouilloy C, Rusinaru D, Mahjoub H, et al. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. 2008;29:339–347. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 29.Berghmans T, Paesmans M, Body JJ. A prospective study on hyponatraemia in medical cancer patients: epidemiology, aetiology and differential diagnosis. Support Care Cancer. 2000;8:192–197. doi: 10.1007/s005200050284. [DOI] [PubMed] [Google Scholar]
- 30.Hsu HH, Chen YC, Tian YC, et al. Role of serum sodium in assessing hospital mortality in cancer patients with spontaneous tumour lysis syndrome inducing acute uric acid nephropathy. Int J Clin Pract. 2007 [Epub ahead of print] [PubMed] [Google Scholar]
- 31.Kimura T, Kudoh S, Hirata K, et al. Prognostic factors in elderly patients with unresectable non-small cell lung cancer. Anticancer Res. 2001;21(2B):1379–1383. [PubMed] [Google Scholar]
- 32.McPherson E, Dunsmuir RA. Hyponatraemia in hip fracture patients. Scott Med J. 2002;47:115–116. doi: 10.1177/003693300204700506. [DOI] [PubMed] [Google Scholar]
- 33.Renneboog B, Musch W, Vandemergel X, et al. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119:71.e1–71.e78. doi: 10.1016/j.amjmed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Hill RW, Wyse GA, Anderson M. Animal Physiology. 2. Sunderland, MA: Sinauer Associates; 2008. [Google Scholar]
- 35.Guyton AC, Hall JE. Textbook of Medical Physiology. 11. Philadelphia: Elsevier Saunders; 2006. [Google Scholar]
- 36.Rose BD, Post TW. Clinical Physiology of Acid-base and Electrolyte Disorders. 5. New York: McGraw-Hill, Medical Pub. Division; 2001. [Google Scholar]
- 37.Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet. 2008;371(9624):1624–1632. doi: 10.1016/S0140-6736(08)60695-9. [DOI] [PubMed] [Google Scholar]
- 38.Lee DS, Donovan L, Austin PC, et al. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43:182–188. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Kiyota Y, Schneeweiss S, Glynn RJ, et al. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Aronsky D, Haug PJ, Lagor C, Dean NC. Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20:319–328. doi: 10.1177/1062860605280358. [DOI] [PubMed] [Google Scholar]
- 41.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 42.Winkelmayer WC, Schneeweiss S, Mogun H, et al. Identification of individuals with CKD from Medicare claims data: a validation study. Am J Kidney Dis. 2005;46:225–232. doi: 10.1053/j.ajkd.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 43.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42:801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 44.Tabak YP, Johannes RS, Silber JH. Using automated clinical data for risk adjustment: development and validation of six disease-specific mortality predictive models for pay-for-performance. Med Care. 2007;45:789–805. doi: 10.1097/MLR.0b013e31803d3b41. [DOI] [PubMed] [Google Scholar]
- 45.Pine M, Jordan HS, Elixhauser A, et al. Enhancement of claims data to improve risk adjustment of hospital mortality. JAMA. 2007;297:71–76. doi: 10.1001/jama.297.1.71. [DOI] [PubMed] [Google Scholar]
- 46.Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83:905–908. doi: 10.1016/0002-9343(87)90649-8. [DOI] [PubMed] [Google Scholar]
- 47.Decaux G, Musch W. Clinical laboratory evaluation of the syndrome of inappropriate secretion of antidiuretic hormone. Clin J Am Soc Nephrol. 2008;3:1175–1184. doi: 10.2215/CJN.04431007. [DOI] [PubMed] [Google Scholar]
- 48.Musch W, Thimpont J, Vandervelde D, et al. Combined fractional excretion of sodium and urea better predicts response to saline in hyponatremia than do usual clinical and biochemical parameters. Am J Med. 1995;99:348–355. doi: 10.1016/s0002-9343(99)80180-6. [DOI] [PubMed] [Google Scholar]
- 49.Wentworth DN, Neaton JD, Rasmussen WL. An evaluation of the Social Security Administration master beneficiary record file and the National Death Index in the ascertainment of vital status. Am J Public Health. 1983;73:1270–1274. doi: 10.2105/ajph.73.11.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]