Abstract
Cocaine is a widely abused drug without a U.S. Food and Drug Administration-approved medication. There is a recognized, promising anticocaine medication to accelerate cocaine metabolism, producing biologically inactive metabolites via a route similar to the primary cocaine-metabolizing pathway [i.e., cocaine hydrolysis catalyzed by butyrylcholinesterase (BChE) in plasma]. An ideal, therapeutically valuable mutant of human BChE should have not only a significantly improved catalytic activity against (−)-cocaine but also certain selectivity for (−)-cocaine over neurotransmitter acetylcholine (ACh), such that one would not expect systemic administration of the BChE mutant to interrupt cholinergic transmission. The present study accounting for the mutation-caused changes of the catalytic activities of BChE against both (−)-cocaine and ACh by means of molecular modeling and site-directed mutagenesis has led to identification of three BChE mutants that have not only a considerably improved catalytic efficiency against (−)-cocaine but also the desirable selectivity for (−)-cocaine over ACh. Two representative BChE mutants have been confirmed to be potent in actual protection of mice from acute toxicity (convulsion and lethality) of a lethal dose of cocaine (180 mg/kg). Pretreatment with the BChE mutant (i.e., 1 min before cocaine administration) dose-dependently protected mice against cocaine-induced convulsions and lethality. In particular, all mice pretreated with the mutant (e.g., 0.02 mg or more of A199S/F227A/S287G/A328W/E441D BChE) survived. The in vivo data reveal the primary factor (i.e., the relative catalytic efficiency), determining the efficacy in practical protection of mice from the acute cocaine toxicity and future direction for further improving the efficacy of the enzyme in the cocaine overdose treatment.
Introduction
Cocaine is a well known, widely abused drug (United Nations Office on Drugs and Crime, 2010). There is no U.S. Food and Drug Administration-approved medication specific for cocaine abuse treatment. The disastrous medical and social consequences of cocaine abuse have made a high priority the development of an anticocaine medication (Karila et al., 2008; Xi and Gardner, 2008). It would be an ideal anticocaine medication to accelerate cocaine metabolism producing biologically inactive metabolites via a route similar to the primary cocaine-metabolizing pathway [i.e., cocaine hydrolysis catalyzed by butyrylcholinesterase (BChE) in plasma] (Landry et al., 1993; Kamendulis et al., 1996; Carrera et al., 2004; Meijler et al., 2005; Zhan et al., 2005; Gorelick, 2008). Unfortunately, wild-type BChE has a low catalytic activity against naturally occurring (−)-cocaine (kcat = 4.1 min−1 and KM = 4.5 μM) (Gatley, 1991; Sun et al., 2002a; Darvesh et al., 2003; Giacobini, 2003; Hamza et al., 2005). It is interesting to design human BChE mutants that may be regarded as a cocaine hydrolase, with a significantly improved catalytic activity against (−)-cocaine.
It has been well known that computational design of high-activity mutants of an enzyme is extremely challenging, particularly when the chemical reaction process is rate-determining for the enzymatic reaction (Gao and Zhan, 2005, 2006; Gao et al., 2006). Generally speaking, for computational design of a mutant enzyme with an improved catalytic activity for a given substrate, one needs to design possible amino acid mutations that can accelerate the rate-determining step of the catalytic reaction process (Zhan et al., 2003; Hamza et al., 2005; Zhan and Gao, 2005) whereas other steps of the reaction are not slowed down by the mutations. The detailed reaction pathway for BChE-catalyzed hydrolysis of (−)-cocaine was uncovered by extensive molecular dynamics simulations (Zhan et al., 2003; Hamza et al., 2005) and reaction coordinate calculations (Zhan et al., 2003; Zhan and Gao, 2005) using quantum mechanics and hybrid quantum mechanics/molecular mechanics. It has been known (Zhan et al., 2003; Hamza et al., 2005; Pan et al., 2005; Gao and Zhan, 2006) that the rate-determining step of (−)-cocaine hydrolysis catalyzed by the A328W/Y332A and A328W/Y332G mutants of BChE is the first step of the chemical reaction process. Therefore, starting from the A328W/Y332A or A328W/Y332G mutant, rational design of BChE mutants against (−)-cocaine has been focused on decreasing the energy barrier for the first reaction step without significantly affecting the other reaction steps. We have developed unique computational strategies and protocols based on the virtual screening of rate-determining transition states of the enzymatic reaction to design enzyme mutants with improved catalytic activity (Pan et al., 2005, 2007, 2008; Zheng et al., 2008, 2010; Yang et al., 2009). The computational design was followed by in vitro experiments, including site-directed mutagenesis, protein expression, and fast enzyme activity screening using the culture medium. The integrated computational-experimental studies have led to the discovery of some BChE mutants with a significantly improved catalytic efficiency against (−)-cocaine (Pan et al., 2005, 2007, 2008; Zheng et al., 2008; Yang et al., 2009; Yang et al., 2010). One of our designed and discovered high-activity mutants of human BChE (i.e., the A199S/S287G/A328W/Y332G mutant) (Pan et al., 2005) has been validated by an independent group of scientists (Brimijoin et al., 2008) who concluded that this mutant is “a true cocaine hydrolase with a catalytic efficiency that is 1000-fold greater than wild-type BChE.”
Despite the success of our previous integrated computational-experimental efforts, we did not account for substrate-selectivity of the enzyme in the design of BChE mutants. In particular, it is well known that as a cousin of acetylcholinesterase, BChE also catalyzes hydrolysis of neurotransmitter acetylcholine (ACh) (Gorelick, 1997). So far, ACh is the only known natural substrate of BChE in the body. An ideal, therapeutically valuable mutant of human BChE should have not only a significantly improved catalytic activity against (−)-cocaine but also certain selectivity for (−)-cocaine over ACh. It is interesting to design mutants of human BChE with a significantly improved catalytic activity against (−)-cocaine without significantly increasing the catalytic activity against ACh compared with the wild-type enzyme. Our further integrated computational-experimental effort reported here has accounted for the mutation-caused changes of the catalytic activities of BChE against both (−)-cocaine and ACh, leading to the identification of three BChE mutants that are satisfactory for both the catalytic activity and selectivity. Furthermore, we have developed stable cell lines to express two representative mutants, one with a higher catalytic efficiency (kcat/KM) and the other with a larger catalytic rate constant (kcat), in a relatively larger scale and used the purified BChE mutants to test their in vivo potency in actual protection of mice from the acute cocaine toxicity. The in vivo data clearly demonstrate the relative importance of the kcat and kcat/KM values of the enzyme in the cocaine detoxification.
Materials and Methods
In Vitro Activity Tests
Materials.
Cloned Pfu DNA polymerase and DpnI endonuclease were obtained from Stratagene (La Jolla, CA). [3H](−)-Cocaine (50 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). All oligonucleotides were synthesized by the Integrated DNA Technologies, Inc (Coralville, IA). The QIAprep Spin Plasmid Miniprep Kit and QIAGEN plasmid purification kit and QIAquick PCR purification kit were obtained from QIAGEN (Valencia, CA). Human embryonic kidney 293T/17 cells were from the American Type Culture Collection (Manassas, VA). Dulbecco's modified Eagle's medium (DMEM) was purchased from Thermo Fisher Scientific (Waltham, MA). 3,3′,5,5′-Tetramethylbenzidine was obtained from Sigma-Aldrich (St. Louis, MO). Anti-BChE (mouse monoclonal antibody) was purchased from AntibodyShop (Gentofte, Denmark), and goat anti-mouse IgG HRP conjugate was from Zymed Laboratories (South San Francisco, CA).
Site-Directed Mutagenesis.
Site-directed mutagenesis of human BChE cDNA was performed by using the QuikChange method (Braman et al., 2000). Further mutation(s) required to produce a new BChE mutant cDNA was/were generated from the cDNA corresponding to the A199S/S287G/A328W/Y332G mutant of human BChE in a pRc/CMV expression plasmid (Masson et al., 1999). Using plasmid DNA as template and primers with specific base-pair alterations, mutations were made by polymerase chain reaction with Pfu DNA polymerase for replication fidelity. The PCR product was treated with DpnI endonuclease to digest the parental DNA template. Modified plasmid DNA was transformed into Escherichia coli, amplified, and purified. The DNA sequences of the mutants were confirmed by DNA sequencing.
Protein Expression.
Both the wild-type and mutants of human BChE were expressed and their enzyme activity against (−)-cocaine were assayed at the same time under the same experimental conditions; the wild type was used a standard reference. The proteins (wild-type and mutants of BChE) were expressed in human embryonic kidney cell line 293T/17. Cells were grown to 80 to 90% confluence in six-well dishes and then transfected by Lipofectamine 2000 complexes of 4 μg of plasmid DNA per each well. Cells were incubated at 37°C in a CO2 incubator for 24 h and cells were moved to 60-mm culture vessel and cultured for 4 more days. The culture medium [10% fetal bovine serum in Dulbecco's modified Eagle's medium (DMEM)] was harvested for the BChE activity assays.
Enzyme Activity Assays.
To measure (−)-cocaine and benzoic acid, the product of (−)-cocaine hydrolysis catalyzed by BChE, we used sensitive radiometric assays based on toluene extraction of [3H](−)-cocaine labeled on its benzene ring (Sun et al., 2002b). In brief, to initiate the enzymatic reaction, 100 nCi of [3H](−)-cocaine was mixed with 100 μl of culture medium. The enzymatic reactions proceeded at room temperature (25°C) with varying concentrations of (−)-cocaine. The reactions were stopped by adding 200 μl of 0.05 M HCl, which neutralized the liberated benzoic acid whereas ensuring a positive charge on the residual (−)-cocaine. [3H]Benzoic acid was extracted by 1 ml of toluene and measured by scintillation counting. Finally, the measured (−)-cocaine concentration-dependent radiometric data were analyzed by using the standard Michaelis-Menten kinetics so that the catalytic parameters were determined along with the use of a well established standard enzyme-linked immunosorbent assay protocol (Pan et al., 2008). The BChE activity assays with [3H]ACh are similar to the assays with [3H](−)-cocaine. The primary difference is that the enzymatic reaction was stopped by addition of 200 μl of 0.2 M HCl containing 2 M NaCl and that the product was [3H]acetic acid for the ACh hydrolysis.
Preparation of BChE Mutants in Large Scale
Materials.
Lentivirus related plasmids were kindly provided by Dr. Louis Hersh at College of Medicine, University of Kentucky (Lexington, KY). Plasmid purification kit was purchased from QIAGEN. Q fast flow (QFF) anion exchanger was supplied by GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK). Hypatite C was supplied by Clarkson Chem. Co. (Williamsport, PA). Fibrous cellulose powder CF11 was from Whatman Co. (Sanford, ME). Nondenatured 4 to 20% gradient polyacrylamide gel, free-style 293F cells, free-style 293 expression medium, 5% penicillin and streptomycin, and 0.05% trypsin-EDTA were purchased from Invitrogen (Carlsbad, CA). Poly(l-proline) was obtained from Sigma-Aldrich.
Generation of Recombinant Lentivirus-Expressing Mutants of Human BChE.
The BChE mutant cDNAs in lentivirus plasmids were constructed in pCSC-SP-PW vector at ApaI and XhoI sites by PCR with BChE mutants in pRC/CMV plasmids as templates. Forward primer is gagggcccaaggtgcacggcccacgt (ApaI), and backward primer is ccgctcgagttagagacccacacaactttct (Xho1). The sequences of constructs were confirmed by DNA sequencing. To prepare lentiviruses encoding BChE mutants, 293 FT cells were cultured in DMEM/10% fetal calf serum medium with 250 ng/ml G418 (Invitrogen). Cells were transfected at approximately 70% confluence by lentivirus plasmid encoding BChE (BChE/pCSC-SP-PW) or its mutants together with three other packaging plasmids, pMDL-gp.RRE, pRSV.Rev, and pVSVG, at a mass ratio of 10.0:6.5:2.5:3.5. Transfection was achieved by calcium phosphate-mediated procedure. In brief, for a 10-cm dish cells, total DNAs (approximately 22.5 μg) were mixed first, and then 500 μl of 0.25 M freshly diluted CaCl2 was added and mixed before another 500 μl of 2× BES-buffered solution was gently added and gently mixed. The mixture was incubated at room temperature for 15 min and then added drop-wise onto a 10-cm dish. The cells were cultured in 3% CO2 at 37°C. Culture medium was changed 12 to 16 h after transfection and medium was collected three times at a 24-h interval beginning 24 h after the post-transfection change of medium. The medium was filtered through a 0.45-μm cellulose acetate filter and spun in Beckman SW28 rotor at 19,400 rpm for 2 h at the room temperature to collect the virus particles. Lentivirus was suspended in Hank's balanced salt solution and separated into small-volume aliquots to store at −80°C.
Expression of the BChE Mutants.
Large-scale preparation of enzyme (BChE mutant) was first achieved by infecting 293F cells with lentivirus followed by resuspending attached 293F cells in suspension culture in free-style 293 expression medium. 293F cells were routinely suspension cultured in serum-free medium according to manufacturer's instruction at 8% CO2, 37°C on orbit shaker at 125 rpm. The day before infection, cells were loaded at 1 × 105/ml and cultured steadily in free-style 293 expression medium with 1% FBS. Cells began to attach to the plate soon after the change of culture condition. Lentivirus was then added to infect cells for 1 day with two intermittent additions of the virus. Infected cells were suspended by 0.05% trypsin-EDTA and seeded at 2 to 10 cells/well in 96-well plate in 1% FBS free-style medium again to culture for another 14 to 21 days without changing medium and shaking until single clones clearly appeared. Single-clone cell lines from 96-well plates were chosen to culture in 48-well plates, then 12-well plates and 6-well plates in 1% FBS free-style 293 expression medium. High-expression single-clone cell lines were screened and selected by determining BChE activity in medium. Selected cell-line cells were then changed back to suspension culture, and the culture volume increased from six-well plate to a 125-ml flask and a series of larger flasks, and finally to a 2-L flask. Poly(l-proline) was added at 1 μM to the free-style 293 expression medium. Culture medium was changed every 2 to 3 days and collected to store at 4°C in sterilized bottles for protein purification. The enzyme, mostly in tetramers, was secreted in medium. The cultured medium contained 4 to 10 mg/l enzyme.
Enzyme Purification.
Large-scale purification of each enzyme in medium was achieved by using an ion exchanger and adsorption chromatographies. In brief, 8 liters of medium with a BChE mutant was diluted with the same volume of 20 mM Tris-HCl, pH 7.4. Equilibrated QFF anion exchanger was added to diluted medium in 1% of its volume and incubated at 4°C with occasional stirring for 1 h. More than 95% enzyme activity was found to bind to the resin after this incubation. The suspension was then packed in a column (5 × 50 cm), and the medium was allowed to flow through rapidly with the aid of suction (50–100 ml/min). The QFF resin was repacked again in a washing buffer after all of the medium was excluded. After washing the column with 20 mM Tris-HCl, pH 7.0, overnight at 4°C, the enzyme was eluted by 20 mM phosphate buffer, pH 7.0, plus 0.3 M NaCl. The eluate was desalted to 20 mM Tris-HCl, pH 7.0, by Millipore centrifugal filter device. The desalted eluate was applied to a Hypatite C column (2.5 × 22 cm), which was packed with fibrous cellulose powder CF11 at a ratio of 1:1. The column was washed by 20 mM Tris-HCl, pH 7.0, and the enzyme was eluted by 10 mM sodium phosphate buffer, pH 7.0, containing 0.3 M NaCl. The purified enzyme was dialyzed against phosphate-buffered saline and stored at 4°C or −80°C. The entire purification process was carried out in cold room at 4°C. The purified enzymes were provided for in vivo activity tests described below.
In Vivo Activity Tests
Subjects.
Male NIH-Swiss mice (27–30 g) were obtained from Harlan (Indianapolis, IN) and were housed in groups of four to six mice per cage. All mice were allowed ad libitum access to food and water and were maintained on a 12-h light/dark cycle with lights on at 6:30 AM in a room kept at a temperature of 21 to 22°C. Each mouse was used only once, and each dosing condition contained eight mice. Experiments were performed in the same colony room in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The experimental protocols were approved by the University Committee on the Use and Care of Animals at the University of Michigan.
Drugs.
Naturally occurring cocaine [i.e., (−)-cocaine HCl (Tyco-Mallinckrodt, Hazelwood, MO)], was dissolved in sterile water and was administered at a volume of 0.01 ml/g i.p. The BChE mutant was diluted to different concentrations in phosphate-buffered saline and administered intravenously at a volume of 0.2 ml/mouse.
Behavioral Assay.
Cocaine-induced toxicity was characterized by the occurrence of convulsions and lethality. Cocaine-induced convulsions were defined as loss of righting posture for at least 5 s with the simultaneous presence of clonic limb movements (Ko et al., 2007). Lethality was defined as cessation of observed movement and respiration. After intraperitoneal administration of cocaine, mice were immediately placed individually in Plexiglas containers (16 × 28 × 20 cm high) for observation. The presence or absence of convulsions and lethality were recorded for 60 min after cocaine administration.
Drug Administration.
The mouse was placed in a small restraint chamber (outer tube diameter, 30 mm; inner tube diameter, 24 mm; Harvard Apparatus, Inc., Holliston, MA) that left the tail exposed. The tail was cleansed with an alcohol wipe, then a 30-gauge, 0.5-ml precision glide needle (Thermo Fisher Scientific) was inserted into one of the side veins for infusion. The intravenous injection volume of BChE mutant for different doses was 0.2 ml of per mouse, and it was given 1 min before administration of cocaine 180 mg/kg i.p. (LD100). Sterile gauze and pressure were applied to the injection site to staunch the bleeding.
Data Analysis.
Data from the behavioral toxicity studies (i.e., percentage of mice showing affected responses) were analyzed with Fisher's exact probability test. Protective effects of the BChE mutant were compared with those of phosphate-buffered saline. The criterion for significance was set at p < 0.05. The value of ED100conv was determined when 100% of mice showed convulsions. Such value was used to compare the degree of shifts of cocaine's dose-response curve in subjects pretreated with different BChE mutants.
Results
BChE Mutant Design: Insights from Molecular Modeling.
Our goal of the present study was to identify BChE mutants that have significantly improved catalytic activity against (−)-cocaine without a significant change in the catalytic activity against ACh, compared with wild-type BChE to make sure that the cocaine detoxification with BChE mutants will not affect the cholinergic transmission. Based on the catalytic mechanisms for BChE-catalyzed hydrolyzes of (−)-cocaine and ACh shown in Fig. 1, A and B (Zhan et al., 2003; Gao and Zhan, 2005, 2006; Zhan and Gao, 2005; Gao et al., 2006), our rational design of BChE mutants in this study was focused on the hydrogen bonding interactions between the carbonyl oxygen of the substrate and the oxyanion hole. Our previous computational studies (Zhan et al., 2003; Gao and Zhan, 2005, 2006; Zhan and Gao, 2005; Gao et al., 2006) have revealed that the fundamental reaction pathway for BChE-catalyzed hydrolysis of (−)-cocaine is similar to that for BChE-catalyzed hydrolysis of ACh in terms of the formation and breaking of covalent bonds during the reaction processes. As shown in Fig. 1, A and B, for both (−)-cocaine and ACh, the BChE-catalyzed hydrolysis consists of acylation and deacylation. The acylation is initiated by the attack of the hydroxyl oxygen of Ser198 side chain at the carbonyl carbon of the substrate. Although the hydroxyl oxygen of Ser198 side chain gradually approaches the carbonyl carbon of the substrate, the carbonyl oxygen of the substrate gradually becomes negatively charged, and the hydroxyl hydrogen of the Ser198 side chain gradually transfers to a nitrogen atom of His438 side chain. Thus, the carbonyl oxygen of the substrate forms stronger and stronger hydrogen bonds with the oxyanion hole (consisting of Gly116, Gly117, and Ala199) from the Michaelis-Menten complex to the transition state and to the intermediate during the acylation process. In this way, the hydrogen bonds between the carbonyl oxygen of the substrate and the oxyanion hole of BChE help to stabilize the transition state and, thus, to decrease the activation free energy of the BChE-catalyzed hydrolysis. The primary difference between the (−)-cocaine and ACh hydrolyzes catalyzed by wild-type BChE exists in the number of potential hydrogen bonds between the carbonyl oxygen of the substrate and the oxyanion hole of the enzyme. The carbonyl oxygen of ACh can potentially form three hydrogen bonds with the oxyanion hole (two in the Michaelis-Menten complex and three in the transition state), whereas the carbonyl oxygen of (−)-cocaine can only potentially form two hydrogen bonds with the oxyanion hole of wild-type BChE (Gao and Zhan, 2005, 2006). The mechanistic insights suggest that certain amino acid mutations that can increase a potential hydrogen bond between the carbonyl oxygen of the substrate and the oxyanion hole could significantly increase the catalytic activity of BChE.
Fig. 1.
Schematic representation of hydrolyzes of (−)-cocaine (A) and ACh (B) catalyzed by BChE mutant including the A199S mutation; (−)-cocaine binding with wild- type BChE (C) and the A199S/F227A/S287G/A328W/E441D mutant (D); and ACh binding with wild-type BChE (E) and the A199S/F227A/S287G/A328W/E441D mutant (F).
In light of the above analysis, the desirable amino acid mutations on BChE should be those that can increase a potential hydrogen bond of the oxyanion hole with the carbonyl oxygen of (−)-cocaine but not with the carbonyl oxygen of ACh. The BChE mutants corresponding to such type of desirable mutations may be expected to have a significantly improved catalytic activity against (−)-cocaine without a significantly increased catalytic activity against ACh. Hence, the same computational modeling methods (including molecular dynamics simulations and energy minimizations using Amber program) (Case et al., 2004) used in our previous studies (Gao and Zhan, 2005, 2006; Pan et al., 2005) were used, in the present study, to identify the desirable amino acid mutations on BChE. The computational modeling suggested that A199S/S287G/A328W/Y332G, A199S/F227A/S287G/A328W/E441D, and A199S/F227A/S287G/A328W/Y332G/E441D might be the desirable sets of mutations. Depicted in Fig. 1, C to F, are the modeled structures of representative Michaelis-Menten complexes for the (−)-cocaine and ACh hydrolyzes.
As seen in Fig. 1C, the carbonyl oxygen of (−)-cocaine can only have two potential hydrogen bonds with the NH groups of Gly117 and Ala199 backbones in the oxyanion hole of wild-type BChE; the hydrogen bond with Ala199 backbone is insignificant in the Michaelis-Menten complex, but it is expected to be significantly stronger in the transition state (Gao and Zhan, 2005). For the (−)-cocaine hydrolysis catalyzed by each of these BChE mutants, the carbonyl oxygen of (−)-cocaine can have one more potential hydrogen bond with the hydroxyl group (OH) of Ser199 side chain, in addition to the two potential hydrogen bonds with the Gly117 and Ser199 backbones. Depicted in Fig. 1D is the modeled structure of (−)-cocaine binding with the A199S/F227A/S287G/A328W/E441D mutant as an example; the modeled structures of (−)-cocaine binding with the other two mutants are similar to this one.
Compared with (−)-cocaine interacting with wild-type BChE, the carbonyl oxygen of ACh can have one more hydrogen bond with the NH group of Gly116 backbone, in addition to the two potential hydrogen bonds with the Gly117 and Ala199 backbones, as seen in Fig. 1E. One might expect the same mutations that increase a potential hydrogen bond of the oxyanion hole with the carbonyl oxygen of (−)-cocaine also to increase a potential hydrogen bond with the carbonyl oxygen of ACh. However, the modeled structures reveal that the expected additional hydrogen bond between the carbonyl oxygen of ACh and the hydroxyl group of Ser199 side chain does not exist in any of the modeled structures for ACh binding with the BChE mutants. Depicted in Fig. 1F is the modeled structure of ACh binding with the A199S/F227A/S287G/A328W/E441D mutant as an example; the modeled structures of ACh binding with the other two mutants are similar to this one. These modeling results suggest that the A199S/S287G/A328W/Y332G, A199S/F227A/S287G/A328W/E441D, and A199S/F227A/S287G/A328W/Y332G/E441D mutations should not significantly increase the catalytic activity of BChE against ACh while significantly improving the catalytic activity against (−)-cocaine.
In Vitro Activity of the BChE Mutants against (−)-Cocaine.
Based on the computational insights, we carried out in vitro experimental tests, including site-directed mutagenesis, protein expression, and in vitro enzyme activity assays, on the A199S/S287G/A328W/Y332G, A199S/F227A/S287G/A328W/E441D, and A199S/F227A/S287G/A328W/Y332G/E441D mutants of human BChE. To minimize the possible systematic experimental errors of in vitro kinetic data, we expressed the enzymes and performed kinetic studies with wild-type BChE and the mutants under the same conditions and compared the catalytic efficiencies of the mutants to the corresponding catalytic efficiencies of wild-type BChE against (−)-cocaine and ACh. Michaelis-Menten kinetics of the enzymatic hydrolysis of (−)-cocaine or ACh was determined by performing the sensitive radiometric assays using [3H](−)-cocaine (labeled on its benzene ring) or [3H]ACh (labeled on its acetyl group) with varying concentrations of substrate. Depicted in Fig. 2 are the measured kinetic data. Summarized in Table 1 are the determined kinetic parameters.
Fig. 2.
Plots of measured reaction rates (with error bars) versus the substrate concentration for the (−)-cocaine and ACh hydrolyzes catalyzed by wild-type BChE and its mutants. The reaction rates were determined by using a sensitive radiometric assays based on toluene extraction of [3H](−)-cocaine labeled on its benzene ring. See Materials and Methods for details.
TABLE 1.
Kinetic parameters determined for (−)-cocaine and ACh hydrolyses catalyzed by wild-type BChE and its mutants
Unless indicated otherwise, all kinetic parameters listed in this table were determined in the present study. Relative catalytic efficiency (kcat/KM) is the ratio of the kcat/KM value of the mutant to that of wild-type BChE against the same substrate.
| Substrate and Enzyme | KM | kcat | kcat/KM | Relative Catalytic Efficiency |
|---|---|---|---|---|
| μM | min−1 | M−1min−1 | ||
| (−)-Cocaine | ||||
| Wild-type BChEa | 4.5 | 4.1 | 9.1 × 105 | 1 |
| A199S/S287G/A328W/Y332Gb | 3.1 | 3060 | 9.9 × 108 | 1080 |
| A199S/F227A/S287G/A328W/E441D | 1.1 | 1730 | 1.6 × 109 | 1730 |
| A199S/F227A/S287G/A328W/Y332G/E441D | 3.5 | 4430 | 1.3 × 109 | 1390 |
| ACh | ||||
| Wild-type BChE | 148 | 86,000 | 5.8 × 108 | 1 |
| A199S/S287G/A328W/Y332G | 36 | 7500 | 2.1 × 108 | 0.36 |
| A199S/F227A/S287G/A328W/E441D | 27 | 14,600 | 5.4 × 108 | 0.93 |
| A199S/F227A/S287G/A328W/Y332G/E441D | 75 | 13,800 | 1.8 × 108 | 0.32 |
Data from Sun et al. (2002a).
The kinetic parameters reported in Yang et al. (2010). The experimental measurement in the present study was able to reproduce the kinetic parameters.
The three mutants of human BChE collected in Table 1 all have a considerably improved catalytic efficiency against (−)-cocaine. The A199S/S287G/A328W/Y332G mutant has a 1080-fold improved catalytic efficiency against (−)-cocaine (kcat/KM = 9.9 × 108 M−1 · min−1) compared with the wild-type enzyme (kcat/KM = 9.1 × 105 M−1 · min−1). Both kcat and KM values of the A199S/F227A/S287G/A328W/E441D mutant against (−)-cocaine are significantly lower than the corresponding values of the A199S/S287G/A328W/Y332G mutant. Overall, the A199S/F227A/S287G/A328W/E441D mutant has a 1730-fold improved catalytic efficiency against (−)-cocaine (kcat/KM = 1.6 × 109 M−1 · min−1) compared with the wild-type enzyme. Starting from the A199S/F227A/S287G/A328W/E441D mutant, the additional Y332G mutation produces the A199S/F227A/S287G/A328W/Y332G/E441D mutant. The additional Y332G mutation significantly increases both the kcat and KM values of the A199S/F227A/S287G/A328W/E441D mutant against (−)-cocaine. Overall, the catalytic efficiency (kcat/KM = 1.3 × 109 M−1 · min−1) of the A199S/F227A/S287G/A328W/Y332G/E441D mutant is slightly lower than that of the A199S/F227A/S287G/A328W/E441D mutant.
In Vitro Activity of the BChE Mutants against Acetylcholine.
Regarding the catalytic activity of wild-type BChE against ACh, we obtained kcat = 86,000 min−1 and KM = 148 μM. The KM value of 148 μM is nearly identical with the KM value of 150 μM reported by Gao et al. (2008). Our determined kcat value of 86,000 min−1 is slightly larger than, but reasonably close to, the kcat value of 61,200 min−1 reported by Gao et al. (2008). It is remarkably notable in Table 1 that the same mutations that considerably improve the catalytic efficiency of BChE against (−)-cocaine do not increase the catalytic efficiency of BChE against ACh at all. Compared with the wild-type enzyme (kcat/KM = 5.8 × 108 M−1 · min−1), all of the mutants listed in Table 1 have significantly lower kcat and KM values against ACh. Overall, the catalytic efficiency (kcat/KM = 5.4 × 108 M−1 · min−1) of the A199S/F227A/S287G/A328W/E441D mutant against ACh is slightly lower than that of the wild-type enzyme (∼0.93-fold), whereas the other two mutants even have a significantly lower catalytic efficiency against ACh (approximately one third).
Furthermore, as seen in Table 1, the catalytic efficiency of wild-type BChE against ACh (kcat/KM = 5.8 × 108 M−1 · min−1) is ∼640-fold higher than that of the same enzyme against (−)-cocaine (kcat/KM = 9.1 × 105 M−1 · min−1). However, it is interesting to note that for each of the three BChE mutants, the catalytic efficiency against (−)-cocaine (kcat/KM = 9.9 × 108 to 1.6 × 109 M−1 · min−1) becomes significantly higher than that of any enzyme (wild-type BChE or mutant) in Table 1 against ACh (kcat/KM = 1.8–5.8 × 108 M−1 · min−1). These BChE mutants not only have a considerably improved catalytic efficiency against (−)-cocaine but also have the desirable selectivity for (−)-cocaine over ACh.
In Vivo Activity of the BChE Mutants in Protection of Mice from Cocaine Toxicity.
The in vitro activity data discussed above suggest that all of the three BChE mutants have the desirable high catalytic efficiency and substrate selectivity for (−)-cocaine. Within the three promising BChE mutants, we expressed and purified the A199S/S287G/A328W/Y332G and A199S/F227A/S287G/A328W/E441D mutants in large scale for the purpose of in vivo studies. We are interested in testing these two representative mutants in vivo because these two mutants both have their own unique mutation(s): Y332G mutation in the A199S/S287G/A328W/Y332G mutant; and F227A and E441D mutations in the A199S/F227A/S287G/A328W/E441D mutant. It is interesting to know which mutant is actually more potent in the practical detoxification of cocaine.
For the large-scale expression and purification of these two BChE mutants, we developed the stable cell lines in 293F by using lentivirus plasmids encoding the cDNAs of the BChE mutants. Each mutant enzyme was purified by using an ion exchanger and adsorption chromatographies. The catalytic activities of the purified enzymes were confirmed by using the same in vitro assays mentioned above.
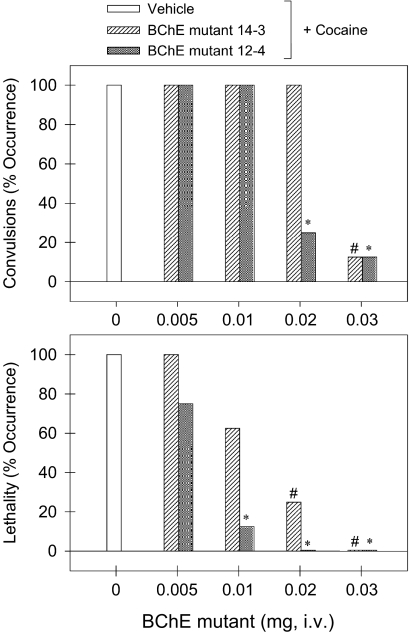
The purified mutant enzymes were used to study their in vivo activity in protection of male NIH-Swiss mice (27–30 g) from acute toxicity of a lethal dose of cocaine (180 mg/kg). Figure 3 compares the protective effects of the BChE mutants. Intraperitoneal administration of cocaine 180 mg/kg produced convulsions and lethality in all tested mice (n = 8). Pretreatment with the BChE mutant (i.e., 1 min before cocaine administration) dose-dependently protected mice against cocaine-induced convulsions and lethality. In particular, the A199S/S287G/A328W/Y332G mutant of human BChE at the dose of 0.03 mg of produced full protection in mice after receiving a lethal dose of cocaine 180 mg/kg (p < 0.05). The A199S/F227A/S287G/A328W/E441D mutant of human BChE was more potent than the A199S/S287G/A328W/Y332G mutant. As seen in Fig. 3, the A199S/F227A/S287G/A328W/E441D mutant at 0.02 mg also produced full protection in mice after receiving a lethal dose of cocaine 180 mg/kg (p < 0.05). Therefore, the minimum dose of the enzyme required to produce full protection of the mice from the acute toxicity of 180 mg/kg cocaine was determined to be 0.03 mg for the A199S/S287G/A328W/Y332G mutant and 0.02 mg for the A199S/F227A/S287G/A328W/E441D mutant.
Fig. 3.
The potency of protective effects of the BChE mutants against cocaine-induced toxicity. “BChE mutant 12-4” represents the A199S/F227A/S287G/A328W/E441D mutant of human BChE, whereas “BChE mutant 14-3” refers to the A199S/S287G/A328W/Y332G mutant. The BChE mutant (in milligrams per mouse) was administered intravenously 1 min before administration of cocaine 180 mg/kg i.p. Each data point represents the percentage of mice (n = 8 for each dosing condition) exhibiting cocaine-induced convulsions or lethality. The asterisks represent a significant difference from the condition of mice pretreated with phosphate-buffered saline (*, p < 0.05). See Materials and Methods for details.
Discussion
The present study accounting for the mutation-caused changes of the catalytic activities of BChE against both (−)-cocaine and ACh has led to identification of three BChE mutants that not only have a considerably improved catalytic efficiency against (−)-cocaine but also have the desirable selectivity for (−)-cocaine over ACh. Development of the stable cell lines for large-scale expression of the BChE mutants has allowed us to confirm the in vivo potency of two representative BChE mutants in the actual protection of mice from the acute cocaine toxicity. The minimum doses of enzyme required to protect all of the mice from the acute toxicity of 180 mg/kg cocaine have been determined to be 0.02 and 0.03 mg for the A199S/F227A/S287G/A328W/E441D and A199S/S287G/A328W/Y332G mutants, respectively. These mutants of human BChE provided significantly better in vivo protection of mice from the acute cocaine toxicity than the well known cocaine esterase and its mutants reported to date (Cooper et al., 2006; Ko et al., 2007; Collins et al., 2009; Gao et al., 2009; Brim et al., 2010; Narasimhan et al., 2010). The in vivo potency of these two mutants is roughly equivalent to that of the most efficient cocaine hydrolase reported previously (Zheng et al., 2008), which also provided the full protection of the mice at a similar dose (0.01 and 0.03 mg). But the present study accounted for the substrate selectivity for the first time.
We are not aware of any study determining whether administration of BChE produces significant decreases in ACh levels. Published case reports on clinical use of partially purified human BChE did not mention any adverse effects related to altered ACh activity (Gorelick, 2008). On the other hand, it has been known that BChE is overexpressed in patients with Alzheimer's disease (Guillozet et al., 1997) and the presence of BChE with β-amyloid plaques dramatically amplifies the toxicity of β-amyloid (Barber et al., 1996). In addition, increased BChE gene expression correlates with neurodegenerative disorders (Mack and Robitzki, 2000). In light of these published reports, without further studies, we do not know for sure that the administration of a BChE mutant with significantly increased activity against ACh will not cause any unexpected adverse effects for the patients, particularly those who also suffer from Alzheimer's disease or other neurodegenerative disorders. In any case, it is reasonable to assume that the administration of a BChE mutant with substrate selectivity for (−)-cocaine over ACh will less likely cause unexpected adverse effects.
Based on the in vitro activity data, the A199S/S287G/A328W/Y332G mutant has a significantly higher kcat value (3060 min−1) against (−)-cocaine than the A199S/F227A/S287G/A328W/E441D mutant (kcat = 1730 min−1). On the other hand, the A199S/F227A/S287G/A328W/E441D mutant has a significantly lower KM value (1.1 μM) than the A199S/S287G/A328W/Y332G mutant (KM = 3.1 μM). Overall, the catalytic efficiency (kcat/KM) of the A199S/F227A/S287G/A328W/E441D mutant is higher than that of the A199S/S287G/A328W/Y332G mutant. According to the relative magnitudes of the kcat values, when the (−)-cocaine concentration is much higher than the KM value such that the enzyme is nearly saturated with (−)-cocaine, the A199S/S287G/A328W/Y332G mutant is expected to be more effective than the A199S/F227A/S287G/A328W/E441D mutant. However, according to the relative magnitudes of the kcat/KM values, when the (−)-cocaine concentration is much lower than the KM value, the A199S/F227A/S287G/A328W/E441D mutant is expected to be more effective than the A199S/S287G/A328W/Y332G mutant.
The determined relative magnitudes of the minimum doses of the BChE mutants producing the full protection of mice from the acute cocaine toxicity clearly demonstrate that the catalytic efficiency (i.e., the kcat/KM value) of the BChE mutant is the primary factor determining the in vivo potency in the practical protection of the mice from the acute cocaine toxicity, which contrasts with the usual perception that the catalytic rate constant (kcat) is the primary factor. Furthermore, because of the fact that the A199S/F227A/S287G/A328W/E441D mutant with a larger kcat/KM value and a smaller kcat value (compared with the A199S/S287G/A328W/Y332G mutant) is more potent in the practical protection of the mice from the acute cocaine toxicity, the cocaine concentration in the blood of mice overdosed with 180 mg/kg cocaine was not higher than KM value (3.1 μM) of the A199S/S287G/A328W/Y332G mutant in the presence of the BChE mutant. In light of this finding, for further improvement of the efficacy in cocaine detoxification, future rational design of new BChE mutants should focus on the kcat/KM value rather than the kcat value alone.
Acknowledgments
We acknowledge the Center for Computational Sciences at the University of Kentucky for supercomputing time on an IBM X-series Cluster with 340 nodes or 1360 processors.
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA013930, R01-DA025100, R01-DA021416, R01-DA023213].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.068494.
- BChE
- butyrylcholinesterase
- ACh
- acetylcholine
- DMEM
- Dulbecco's modified Eagle's medium
- QFF
- Q fast flow
- PCR
- polymerase chain reaction
- FBS
- fetal bovine serum
- BES
- N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid.
Authorship Contributions
Participated in research design: Zhan, Tai, Woods, and Ko.
Conducted experiments: Xue, Ko, Tong, Yang, Hou, Fang, Liu, and Zheng.
Contributed new reagents or analytic tools: Tai.
Performed data analysis: Zhan, Xue, Ko, and Zheng.
Wrote or contributed to the writing of the manuscript: Zhan, Xue, Tong, Tai, Ko, and Woods.
Other: Zhan, Woods, and Ko acquired funding for the research.
References
- Barber KL, Mesulam MM, Kraft GA, Klein WL. (1996) Butyrylcholinesterase alters the aggregation of β amyloid. Soc Neurosci Abstr 22:1172 [Google Scholar]
- Braman J, Papworth C, Greener A. (2000) Site-Directed Mutagenesis Using Double-Stranded Plasmid DNA Templates, in The Nucleic Acid Protocols Handbook (Rapley R. ed) pp 835–844, Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- Brim RL, Nance MR, Youngstrom DW, Narasimhan D, Zhan CG, Tesmer JJ, Sunahara RK, Woods JH. (2010) A thermally stable form of bacterial cocaine esterase: a potential therapeutic agent for treatment of cocaine abuse. Mol Pharmacol 77:593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimijoin S, Gao Y, Anker JJ, Gliddon LA, Lafleur D, Shah R, Zhao Q, Singh M, Carroll ME. (2008) A cocaine hydrolase engineered from human butyrylcholinesterase selectively blocks cocaine toxicity and reinstatement of drug seeking in rats. Neuropsychopharmacology 33:2715–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera MR, Kaufmann GF, Mee JM, Meijler MM, Koob GF, Janda KD. (2004) Treating cocaine addiction with viruses. Proc Natl Acad Sci USA 101:10416–10421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case DA, Darden TA, Cheatham TE I, Simmerling CL, Wang J. RED, R Luo, Merz KM, Wang B, Pearlman DA, Crowle M, Brozell S, Tsui V, Gohlke H, J Mongan, Hornak V, Cui G, Beroza P, Schafmeister C, Caldwell JW, Ross WS, Kollman PA. (2004) AMBER 8, University of California, San Francisco: [Google Scholar]
- Collins GT, Brim RL, Narasimhan D, Ko MC, Sunahara RK, Zhan CG, Woods JH. (2009) Cocaine esterase prevents cocaine-induced toxicity and the ongoing intravenous self-administration of cocaine in rats. J Pharmacol Exp Ther 331:445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Narasimhan D, Sunahara RK, Mierzejewski P, Jutkiewicz EM, Larsen NA, Wilson IA, Landry DW, Woods JH. (2006) Rapid and robust protection against cocaine-induced lethality in rats by the bacterial cocaine esterase. Mol Pharmacol 70:1885–1891 [DOI] [PubMed] [Google Scholar]
- Darvesh S, Hopkins DA, Geula C. (2003) Neurobiology of butyrylcholinesterase. Nat Rev Neurosci 4:131–138 [DOI] [PubMed] [Google Scholar]
- Gao D, Cho H, Yang W, Pan Y, Yang G, Tai HH, Zhan CG. (2006) Computational design of a human butyrylcholinesterase mutant for accelerating cocaine hydrolysis based on the transition-state simulation. Angew Chem Int Ed Engl 45:653–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Narasimhan DL, Macdonald J, Brim R, Ko MC, Landry DW, Woods JH, Sunahara RK, Zhan CG. (2009) Thermostable variants of cocaine esterase for long-time protection against cocaine toxicity. Mol Pharmacol 75:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Zhan CG. (2005) Modeling effects of oxyanion hole on the ester hydrolysis catalyzed by human cholinesterases. J Phys Chem B 109:23070–23076 [DOI] [PubMed] [Google Scholar]
- Gao D, Zhan CG. (2006) Modeling evolution of hydrogen bonding and stabilization of transition states in the process of cocaine hydrolysis catalyzed by human butyrylcholinesterase. Proteins 62:99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, LaFleur D, Shah R, Zhao Q, Singh M, Brimijoin S. (2008) An albumin-butyrylcholinesterase for cocaine toxicity and addiction: catalytic and pharmacokinetic properties. Chem Biol Interact 175:83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ. (1991) Activities of the enantiomers of cocaine and some related compounds as substrates and inhibitors of plasma butyrylcholinesterase. Biochem Pharmacol 41:1249–1254 [DOI] [PubMed] [Google Scholar]
- Giacobini E. (2003) Butyrylcholinesterase: Its Function and Inhibitors, Martin Dunitz, London: [Google Scholar]
- Gorelick DA. (1997) Enhancing cocaine metabolism with butyrylcholinesterase as a treatment strategy. Drug Alcohol Depend 48:159–165 [DOI] [PubMed] [Google Scholar]
- Gorelick DA. (2008) Pharmacokinetic approaches to treatment of drug addiction. Expert Rev Clin Pharmacol 1:277–290 [DOI] [PubMed] [Google Scholar]
- Guillozet AL, Smiley JF, Mash DC, Mesulam MM. (1997) Butyrylcholinesterase in the life cycle of amyloid plaques. Ann Neurol 42:909–918 [DOI] [PubMed] [Google Scholar]
- Hamza A, Cho H, Tai HH, Zhan CG. (2005) Molecular dynamics simulation of cocaine binding with human butyrylcholinesterase and its mutants. J Phys Chem B 109:4776–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamendulis LM, Brzezinski MR, Pindel EV, Bosron WF, Dean RA. (1996) Metabolism of cocaine and heroin is catalyzed by the same human liver carboxylesterases. J Pharmacol Exp Ther 279:713–717 [PubMed] [Google Scholar]
- Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, et al. (2008) New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol 11:425–438 [DOI] [PubMed] [Google Scholar]
- Ko MC, Bowen LD, Narasimhan D, Berlin AA, Lukacs NW, Sunahara RK, Cooper ZD, Woods JH. (2007) Cocaine esterase: interactions with cocaine and immune responses in mice. J Pharmacol Exp Ther 320:926–933 [DOI] [PubMed] [Google Scholar]
- Landry DW, Zhao K, Yang GX, Glickman M, Georgiadis TM. (1993) Antibody-catalyzed degradation of cocaine. Science 259:1899–1901 [DOI] [PubMed] [Google Scholar]
- Mack A, Robitzki A. (2000) The key role of butyrylcholinesterase during neurogenesis and neural disorders: an antisense-5′butyrylcholinesterase-DNA study. Prog Neurobiol 60:607–628 [DOI] [PubMed] [Google Scholar]
- Masson P, Xie W, Froment MT, Levitsky V, Fortier PL, Albaret C, Lockridge O. (1999) Interaction between the peripheral site residues of human butyrylcholinesterase, D70 and Y332, in binding and hydrolysis of substrates. Biochim Biophys Acta 1433:281–293 [DOI] [PubMed] [Google Scholar]
- Meijler MM, Kaufmann GF, Qi L, Mee JM, Coyle AR, Moss JA, Wirsching P, Matsushita M, Janda KD. (2005) Fluorescent cocaine probes: a tool for the selection and engineering of therapeutic antibodies. J Am Chem Soc 127:2477–2484 [DOI] [PubMed] [Google Scholar]
- Narasimhan D, Nance MR, Gao D, Ko MC, Macdonald J, Tamburi P, Yoon D, Landry DM, Woods JH, Zhan CG, et al. (2010) Structural analysis of thermostabilizing mutations of cocaine esterase. Protein Eng Des Sel 23:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Gao D, Yang W, Cho H, Yang G, Tai HH, Zhan CG. (2005) Computational redesign of human butyrylcholinesterase for anticocaine medication. Proc Natl Acad Sci USA 102:16656–16661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Gao D, Yang W, Cho H, Zhan CG. (2007) Free energy perturbation (FEP) Simulation on the transition states of cocaine hydrolysis catalyzed by human butyrylcholinesterase and its mutants. J Am Chem Soc 129:13537–13543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Gao D, Zhan CG. (2008) Modeling the catalysis of anti-cocaine catalytic antibody: competing reaction pathways and free energy barriers. J Am Chem Soc 130:5140–5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Pang YP, Lockridge O, Brimijoin S. (2002a) Re-engineering butyrylcholinesterase as a cocaine hydrolase. Mol Pharmacol 62:220–224 [DOI] [PubMed] [Google Scholar]
- Sun H, Shen ML, Pang YP, Lockridge O, Brimijoin S. (2002b) Cocaine metabolism accelerated by a re-engineered human butyrylcholinesterase. J Pharmacol Exp Ther 302:710–716 [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (2010) World Drug Report 2010, United Nations Publication, Sales no. E.10.XI.13, New York, NY: [Google Scholar]
- Xi ZX, Gardner EL. (2008) Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Curr Drug Abuse Rev 1:303–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Pan Y, Zheng F, Cho H, Tai HH, Zhan CG. (2009) Free-energy perturbation simulation on transition states and redesign of butyrylcholinesterase. Biophys J 96:1931–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Xue L, Fang L, Chen X, Zhan CG. (2010) Characterization of a high-activity mutant of human butyrylcholinesterase against (−)-cocaine. Chem Biol Interact 187:148–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan CG, Deng SX, Skiba JG, Hayes BA, Tschampel SM, Shields GC, Landry DW. (2005) First-principle studies of intermolecular and intramolecular catalysis of protonated cocaine. J Comput Chem 26:980–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan CG, Gao D. (2005) Catalytic mechanism and energy barriers for butyrylcholinesterase-catalyzed hydrolysis of cocaine. Biophys J 89:3863–3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan CG, Zheng F, Landry DW. (2003) Fundamental reaction mechanism for cocaine hydrolysis in human butyrylcholinesterase. J Am Chem Soc 125:2462–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Yang W, Ko MC, Liu J, Cho H, Gao D, Tong M, Tai HH, Woods JH, Zhan CG. (2008) Most efficient cocaine hydrolase designed by virtual screening of transition states. J Am Chem Soc 130:12148–12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Yang W, Xue L, Hou S, Liu J, Zhan CG. (2010) Design of high-activity mutants of human butyrylcholinesterase against (−)-cocaine: structural and energetic factors affecting the catalytic efficiency. Biochemistry 49:9113–9119 [DOI] [PMC free article] [PubMed] [Google Scholar]