Abstract
We have reported previously novel drug-induced inactivation and reactivation of human 5-hydroxytryptamine7 (5-HT7) receptors in a recombinant cell line. To explain these novel observations, a homodimer structure displaying protomer-protomer cross-talk was proposed. To determine whether these novel observations and interpretations are due to an artifactual G protein-coupled receptor (GPCR) mechanism unique to the recombinant cell line, we explored the properties of r5-HT7 receptors expressed by cortical astrocytes in primary culture. As in the recombinant cell line, risperidone, 9-OH-risperidone, methiothepin, and bromocriptine were found to potently inactivate r5-HT7 receptors. As in the recombinant cell line, exposure of risperidone-inactivated astrocyte r5-HT7 receptors to competitive antagonists resulted in the reactivation of r5-HT7 receptors. The potencies of the reactivating drugs closely correlated with their affinities for h5-HT7 receptors. These results indicate the novel inactivating and reactivating property of drugs is not due to an artifact of the recombinant cell line expressing h5-HT7 receptors but is an intrinsic property of 5-HT7 receptors in vitro and ex vivo. This evidence suggests that a native (nonmutated) GPCR, in its native membrane environment (cortical astrocyte primary culture), can function as a homodimer with protomer-protomer cross-talk. Homodimers may be a common GPCR structure. The experimental design used in our studies can be used to explore the properties of other GPCRs in their native forms in recombinant cells, primary cultures expressing the endogenous GPCRs, and possibly in vivo. The homodimer structure and protomer-protomer cross-talk offer new avenues of research into receptor dysfunction in disease states and the development of novel drugs.
Introduction
The 5-HT7 receptor is one of 13 serotonin GPCRs (Bard et al., 1993; Lovenberg et al., 1993; Shen et al., 1993; Teitler and Herrick-Davis, 1994; Hoyer et al., 2002). In a series of studies, we have reported unusual properties of drugs interacting with the h5-HT7 receptor expressed in HEK293 cells (Smith et al., 2006; Knight et al., 2009; Toohey et al., 2009). It was first noted that the exposure of the recombinant cells expressing the h5-HT7 receptor to low levels of risperidone, 9-OH-risperidone, methiothepin, and bromocriptine resulted in an inactivation of the receptor. This was demonstrated by exposing the cells to the drugs for 30 min, washing out the drugs, then stimulating with 10 μM 5-HT. The potencies of the four “inactivating antagonists” closely matched their affinities for the h5-HT7 receptor (Knight et al., 2009). Radioligand binding studies using [3H]5-HT revealed that risperidone and 9-OH-risperidone, which fully inactivate the receptor, bind only 50% of the receptors in a “wash-resistant” (pseudoirreversible) manner. Radioligand binding studies using [3H]risperidone also revealed that 50% of the receptor binding is wash-resistant. Exposing the cells to drugs that bind but do not inactivate the receptor (competitive antagonists), after inactivation by risperidone or 9-OH-risperidone, resulted in a reactivation of the receptor (Teitler et al., 2010). These drugs also were found to “release” the wash-resistant [3H]risperidone bound to the h5-HT7 receptor. The potencies of the “reactivating antagonists” in reactivating the h5-HT7 receptor strongly correlated with their affinities for the orthosteric binding site, despite the presence of the pseudoirreversibly bound risperidone at the orthosteric binding site. This indicated that an orthosteric binding site was available to the reactivating drugs despite the presence of risperidone occluding the orthosteric site. A homodimeric quaternary structure for the h5-HT7 receptor capable of protomer-protomer cross-talk was proposed. This implies that drugs interacting with the orthosteric binding site of one protomer allosterically modulate the binding properties and function of the other protomer. Consistent with this hypothesis, bioluminescence resonance energy transfer analysis indicated that h5-HT7 receptors have a high propensity to form homodimers in HEK293 cells.
A major challenge in the field of GPCR oligomer structure and function is to demonstrate the presence of oligomers in primary cell culture preparations or in vivo (Pin et al., 2007). This is because the strategies being used to detect dimers and higher-order structures involve mutating the GPCRs and transfecting them into cells and/or solubilizing receptors from recombinant cell lines and performing Western blot analysis. Thus, although the literature suggests the possibility of oligomer formation for GPCRs in vivo, there has been no report of an experimental design that can be used to detect the structure and function of endogenously expressed GPCR oligomers in primary culture. Biochemical and biophysical techniques inevitably involve conditions that leave doubt as to the relevance of the findings to the in vivo situation.
Therefore, a major question concerning our observations of the 5-HT7 receptor's properties in HEK293 cells was whether we could observe similar effects in a primary cell culture expressing the 5-HT7 receptor. We decided to explore the possibility of using rat cortical astrocytes in primary culture to investigate whether these properties would be observed in the native 5-HT7 receptor endogenously expressed in a brain cell. Rat cortical astrocytes have been shown to produce a robust 5-HT-induced stimulation of cAMP production (Hirst et al., 1997; Shimizu et al., 1998). This response is mediated through the r5-HT7 receptor, as determined by pharmacological analysis and detection of r5-HT7 receptor mRNA in rat cortical astrocytes (Hirst et al., 1997, 1998). Therefore, we endeavored to establish the 5-HT7 receptor nature of the cAMP stimulation in rat cortical primary culture using (2R)-1-[(3-hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine hydrochloride (SB269970), a selective 5-HT7 receptor antagonist that was not available at the time of the original reports on the rat cortical astrocytes, and to determine whether the r5-HT7 receptor expressed in rat cortical primary astrocytes displays the same or similar novel regulatory properties observed in the recombinant cell lines. Given that the radioligand binding and signal transduction studies in the recombinant cell lines strongly indicate a homodimeric structure with protomer-protomer cross-talk, similar results in the astrocyte primary culture would be the first demonstration of a native GPCR endogenously expressed in its native cellular environment displaying homodimeric protomer-protomer cross-talk.
Materials and Methods
Reagents
Risperidone and 9-OH-risperidone were purchased from Research Diagnostics (Flanders, NJ); bromocriptine, clozapine, and SB269970 were from Sigma-Aldrich (St. Louis, MO). Mianserin and cyproheptadine were from Sigma/RBI (Natick, MA). 5-Chloro-N-[4-methoxy-3-(1-piperazinyl)phenyl]-3-methyl-benzo[b]thiophen-2-sulfonamide hydrochloride (SB270146) was from Tocris Bioscience (Ellisville, MO); mesulergine was generously provided by Novartis Pharmaceuticals (East Hanover, NJ), and 2-(2-dimethylamino ethylthio)-3-phenyl quinoline (ICI169369) was provided by Dr. Bryan Roth (University of North Carolina, Chapel Hill, NC). All other reagents were of analytical grade and were obtained from various suppliers.
Cell Culture
Astrocytes were cultured as described previously (Kaech and Banker, 2006). The protocol used to prepare and maintain rat primary cortical astrocyte cultures yields nearly pure astrocytes as demonstrated by glial fibrillary acidic protein immunostaining. Brains from postnatal day 1 rat pups were placed in a dish containing calcium- and magnesium-free Hanks' balanced salt solution (HBSS), cerebral hemispheres were removed, and meninges were stripped away. Hemispheres were chopped, and cells were dissociated and finally plated in complete glial MEM (MEM supplemented with 0.6% glucose, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% horse serum) at a density of 7.5 × 106 cells per 75-cm2 flask and maintained at 37°C (5% CO2/95% air), with media changes every 3 to 4 days, until confluence. At confluence, astrocytes were frozen in cell-freezing medium at 2 × 106 cells/ml. Cells used in these experiments were thawed from a frozen state and plated at a density of 1.8 × 105 cells per 100-mm tissue culture dish and maintained as above until confluence, generally 2 to 3 weeks. The day before the assay, media were changed to a serum-free medium (MEM supplemented with 0.6% glucose).
cAMP Accumulation
Concentration-Response Curves.
Astrocytes were lifted in 1 ml of diluted trypsin/EDTA (1:3 in PBS) followed by the addition of 5 ml/dish HBSS. Cells were centrifuged at 330g for 3 min. The pellet was resuspended in LANCE stimulation buffer (PerkinElmer Life and Analytical Sciences, Waltham, MA), containing 500 μM 3-isobutyl-1-methylxanthine, and cells were counted, added to tubes containing stimulation buffer with or without antagonists, and incubated for 30 min. Pargyline (10 μM) was tested and found to have no effect on the potency of 5-HT-stimulated activity and therefore was not included. Cells were added to a 96-well opaque plate containing 5-HT and incubated for 30 min. The reaction was terminated, and cells were lysed with the addition of LANCE cAMP Detection Mix (PerkinElmer Life and Analytical Sciences), and time-resolved fluorescence resonance energy transfer (TR-FRET) was measured by the Victor3 1420 plate-reader as reported previously (Knight et al., 2009). Data were analyzed using Prism (version 5.0; GraphPad Software Inc., San Diego, CA).
Wash-Resistant Drug Effects.
Astrocytes were lifted in 1 ml of diluted trypsin/EDTA (1:3 in PBS) followed by the addition of 5 ml per dish of HBSS buffer. Astrocytes were centrifuged at 330g for 3 min, and the pellet was resuspended in fresh HBSS buffer. Cells were counted and added to tubes containing 1 μM drug. Cell suspensions were incubated for 30 min at 37°C and thoroughly washed three times: tubes were centrifuged at 330g for 3 min, and the pellet was resuspended in fresh HBSS buffer and incubated for 10 min. After the final wash, cells were resuspended in stimulation buffer. Cells were counted and added to a 96-well opaque plate containing stimulation buffer in the absence or presence of 10 μM 5-HT, incubated for 30 min, followed by the addition of Detection Mix. TR-FRET was measured, and data were analyzed as above.
Reactivation Concentration-Response Curves.
Astrocytes were lifted in 1 ml of diluted trypsin/EDTA (1:3 in PBS) followed by the addition of 5 ml/dish HBSS buffer. Astrocytes were centrifuged at 330g for 3 min, and the pellet was resuspended in fresh HBSS buffer. Cells were added to tubes containing HBSS or 7 nM risperidone. Cell suspensions were incubated for 30 min at 37°C followed by the addition of varying concentrations of a noninactivating antagonist. Cell suspensions were incubated for 90 min at 37°C and thoroughly washed three times: tubes were centrifuged at 330g for 3 min, and the pellet was resuspended in fresh HBSS buffer and incubated for 10 min. After the final wash, cells were resuspended in stimulation buffer. Cells were counted and added to a 96-well opaque plate containing stimulation buffer in the absence or presence of 10 μM 5-HT, incubated for 30 min, followed by the addition of detection mix. TR-FRET was measured, and data were analyzed as above.
Results
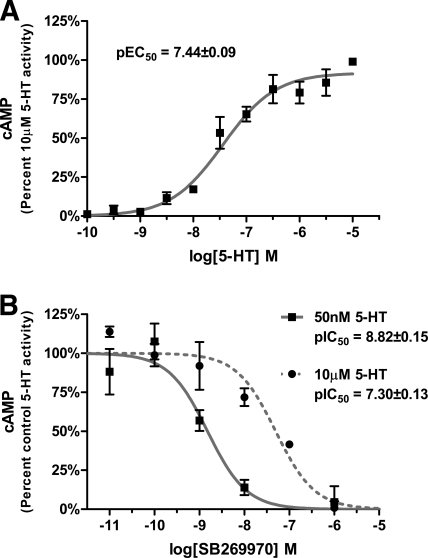
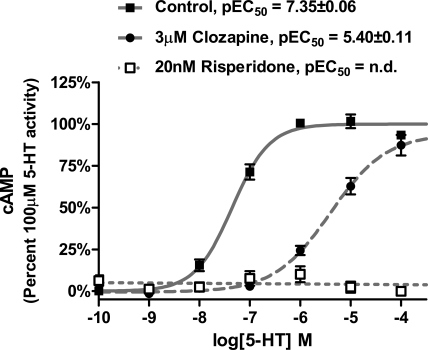
To characterize the role of the r5-HT7 receptor in the 5-HT stimulation of cAMP in rat cortical astrocytes, basic pharmacological studies were performed (Figs. 1 and 2). 5-HT stimulates cAMP production with an EC50 of 36 nM (Fig. 1A), which was consistent with previous reports (Hirst et al., 1997; Shimizu et al., 1998). The concentration-response curve of SB269970, a potent and selective 5-HT7 receptor antagonist (Lovell et al., 2000; Thomas et al., 2000), was right-shifted by increasing the concentration of 5-HT from 50 nM to 10 μM, indicating that the 5-HT-induced stimulation of cAMP is mediated through the r5-HT7 receptor (Fig. 1B). Clozapine, a potent, nonselective 5-HT7 receptor antagonist (Smith et al., 2006; Knight et al., 2009), also displayed competitive antagonist properties, shifting the 5-HT concentration-response curve to the right (Fig. 2). In contrast, risperidone, an “inactivating antagonist” at h5-HT7 receptors in HEK293 cells (Smith et al., 2006; Knight et al., 2009), displayed noncompetitive antagonist properties, eliminating any 5-HT-stimulated cAMP production (Fig. 2). Taken together, the results in Figs. 1 and 2 indicate the 5-HT-stimulated cAMP production in rat cortical astrocytes is mediated by the r5-HT7 receptor, SB269970 and clozapine are competitive antagonists at the r5-HT7 receptor, and risperidone is a noncompetitive antagonist at the r5-HT7 receptor. Hirst et al. (1998) reported the detection of mRNA for the 5-HT6 receptor, which has been shown to couple to Gs, and might play a role in the stimulation of cAMP in the astrocytes. The results in Fig. 1 indicate that this is unlikely because the selective 5-HT7 receptor antagonist SB269970 completely antagonizes the 5-HT-induced stimulation of cAMP. In addition, the 5-HT6-selective antagonist SB270146 (Routledge et al., 2000) produced no inhibition of 50 nM 5-HT at concentrations ranging from 10−9 to 10−5M (data not shown). Therefore, it seems that the r5-HT6 receptor is not involved in the 5-HT-induced stimulation of cAMP observed in these studies.
Fig. 1.
5-HT stimulation of cAMP in rat cortical astrocytes is mediated by r5-HT7 receptors. A, 5-HT potently stimulates cAMP production in rat cortical astrocytes (EC50 = 36 nM). B, potent competitive antagonism of 5-HT-mediated stimulation of cAMP by SB269970, a selective 5-HT7 antagonist. Treatment with SB269970 results in potent and complete inhibition of 50 nM or 10 μM 5-HT. Increasing the 5-HT concentration from 50 nM to 10 μM results in a parallel, rightward shift in the SB269970 inhibition curve, consistent with competitive antagonism. Results are the means ± S.E.M. of three independent experiments performed in triplicate.
Fig. 2.
Competitive effects of clozapine and noncompetitive effects of risperidone on 5-HT stimulation of cAMP production in rat cortical astrocytes. 5-HT stimulates cAMP production potently, with an EC50 value of 45 nM, similar to previous results (Fig. 1A). Coincubation with 3 μM clozapine produced a surmountable rightward shift in the 5-HT concentration-response curve with an EC50 value of 4 μM, consistent with competitive antagonism by clozapine. Coincubation of astrocytes with 20 nM risperidone produced a complete, insurmountable (noncompetitive) inhibition of 5-HT stimulation of cAMP production. Results are the means ± S.E.M. of three independent experiments performed in triplicate; n.d., not determinable.
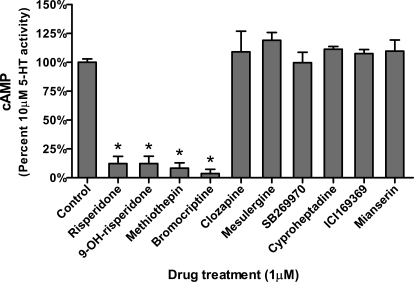
Ten 5-HT7 antagonists were screened for their ability to inactivate the r5-HT7 receptor using 1 μM concentrations (Fig. 3). Risperidone, 9-OH-risperidone, methiothepin, and bromocriptine produced a wash-resistant inhibition of 10 μM 5-HT-stimulated cAMP production. Clozapine, mesulergine, mianserin, ICI169369, SB269970, and cyproheptadine did not produce a wash-resistant effect on the r5-HT7 receptor, displaying no significant inhibition of 5-HT-stimulated cAMP production after washout. These results are consistent with similar results observed in h5-HT7 receptor-expressing HEK293 cells (Knight et al., 2009).
Fig. 3.
Effects of pretreatment, followed by washout, of four “inactivating antagonists” and six noninactivating antagonists (Knight et al., 2009). Risperidone, 9-OH-risperidone, bromocriptine, and methiothepin inactivate 5-HT7-mediated cAMP production in astrocytes (one-way analysis of variance with Dunnett's post test, p < 0.0001). Clozapine, cyproheptadine, ICI169369, mesulergine, mianserin, and SB269970 did not inactivate the receptor. Results are the means ± S.E.M. of three independent experiments performed in triplicate.
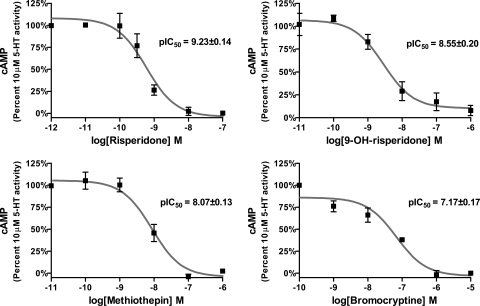
Risperidone, 9-OH-risperidone, methiothepin, and bromocriptine were tested for their potencies in producing the wash-resistant inactivation of the rat cortical astrocyte 5-HT7 receptor (Fig. 4). Varying concentrations of these four inactivating antagonists were incubated with the rat cortical astrocytes, washed out, and the cells stimulated with 10 μM 5-HT. The four inactivating antagonists displayed potencies indistinguishable from those determined using the recombinant cell line (Table 1) (Smith et al., 2006; Knight et al., 2009).
Fig. 4.
Concentration-dependent inactivation of r5-HT7 receptors expressed by rat cortical astrocytes. Thirty-minute exposure of risperidone, 9-OH-risperidone, methiothepin, or bromocriptine to rat cortical astrocytes, followed by washout of the drugs, resulted in the concentration-dependent loss of r5-HT7 receptor activity as defined by the ability of 10 μM 5-HT to stimulate cAMP production. The potencies of the drugs closely match their potencies in inactivating h5-HT7 receptors expressed in HEK293 cells (Table 1). Results are the means ± S.E.M. of three independent experiments performed in triplicate.
TABLE 1.
Inactivation potencies of “inactivating antagonists” at r5-HT7 receptors in rat cortical astrocytes and at h5-HT7 receptors in HEK293 cells
Two-way analysis of variance with Bonferroni post test determined no significant potency differences between cell types (p = 0.816).
| Drug | Potency of Inactivation (pIC50) |
|
|---|---|---|
| Rat Cortical Astrocytes | HEK293 Cells | |
| M | ||
| Risperidone | 9.23 ± 0.14 | 8.88 ± 0.14a |
| 9-OH-risperidone | 8.55 ± 0.20 | 8.41 ± 0.19a |
| Methiothepin | 8.07 ± 0.13 | 8.52 ± 0.19a |
| Bromocryptine | 7.17 ± 0.17 | 7.10 ± 0.14a |
pIC50 values are from Knight et al. (2009).
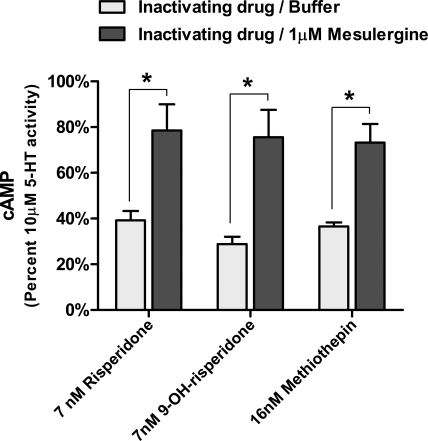
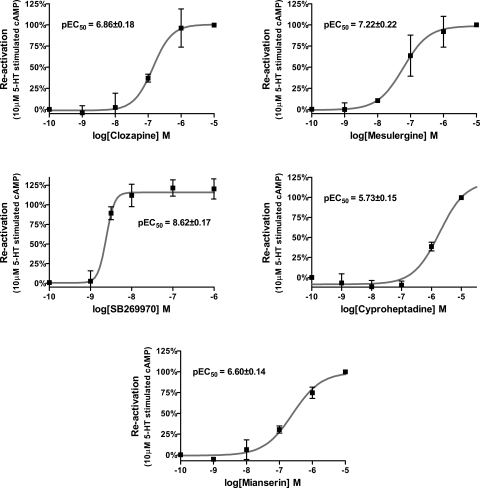
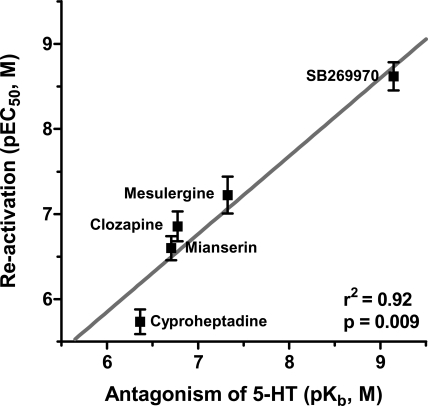
To determine whether the inactivating effects of risperidone, 9-OH-risperidone, and methiothepin could be reversed by a 5-HT7 competitive antagonist, the cells were exposed to 1 μM mesulergine after exposure to the inactivating antagonists (Fig. 5). Mesulergine was capable of reactivating the partially inactivated r5-HT7 receptor expressed on rat cortical astrocytes. The potencies of other 5-HT7 competitive antagonists in reactivating the inactivated r5-HT7 receptor were then examined (Fig. 6). Clozapine, mesulergine, SB269970, cyproheptadine, and mianserin, in a concentration-dependent manner, reactivated the risperidone-inactivated r5-HT7 receptors expressed in rat cortical astrocytes. The potencies of these drugs in reactivating the inactivated r5-HT7 receptor correlated closely with the pKb values for these drugs in antagonizing 50 nM 5-HT stimulation of cAMP in the rat cortical astrocytes (Fig. 7 and Table 2).
Fig. 5.
Reversal of risperidone, 9-OH-risperidone, or methiothepin-induced partial inactivation of r5-HT7 receptors in rat cortical astrocytes by mesulergine. Mesulergine (1 μM) was applied to cells that had been partially inactivated by previous exposure to 7 nM risperidone, 7 nM 9-OH-risperidone, or 16 nM methiothepin (drug concentrations are double their Ki values for the receptor). After 90-min mesulergine exposure, 10 μM 5-HT was applied for 30 min, and cAMP production was measured. These results indicate that exposure of competitive antagonists to inactivated r5-HT7 receptors in astrocytes reverses the inactivation. Results are the means ± S.E.M. of four independent experiments performed in triplicate.
Fig. 6.
Concentration-dependent reversal of risperidone-induced partial inactivation of r5-HT7 receptors in rat cortical astrocytes by competitive antagonists. Rat cortical astrocytes were exposed to 7 nM risperidone and then exposed to increasing concentrations of the competitive antagonists clozapine, mesulergine, SB269970, cyproheptadine, and mianserin for 90 min. Cells were washed thoroughly, followed by the addition of 10 μM 5-HT. The potencies of the competitive antagonists to reverse r5-HT7 inactivation closely match their potencies (pKb) as antagonists of 5-HT-stimulated cAMP production in rat cortical astrocytes (Table 2). Results are the means ± S.E.M. of three independent experiments performed in triplicate.
Fig. 7.
Correlation of competitive antagonists' potencies to reactivate risperidone-inactivated r5-HT7 receptors (ordinate) and potencies to inhibit 5-HT-stimulated cAMP production in rat cortical astrocytes (abscissa). The correlation is highly significant (r2 = 0.92, p < 0.01), and the points define a line with slope and intercept not significantly different from 1 and 0, respectively. This indicates that the reversal of risperidone's inactivating effect is occurring through the r5-HT7 receptor orthosteric binding site. Results are the means ± S.E.M. of three independent experiments performed in triplicate.
TABLE 2.
Potencies of drugs as r5-HT7 receptor antagonists versus potencies as reactivators of risperidone-inactivated r5-HT7 receptors
Concentration-response curves for the inhibition of 50 nM 5-HT-stimulated cAMP in rat cortical astrocytes were generated for each drug (data not shown). The IC50 values of these curves were used to calculate pKb values. The potencies are highly correlated (r2 = 0.92; Fig. 7).
| Drug | Rat Cortical Astrocytes |
|
|---|---|---|
| Antagonism of 5-HT (pKb) | Potency of Reactivation (pEC50) | |
| Clozapine | 6.77 ± 0.170 | 6.86 ± 0.18 |
| Mesulergine | 7.32 ± 0.16 | 7.22 ± 0.22 |
| SB269970 | 9.14 ± 0.25 | 8.62 ± 0.17 |
| Cyproheptadine | 6.36 ± 0.12 | 5.73 ± 0.15 |
| Mianserin | 6.70 ± 0.14 | 6.60 ± 0.14 |
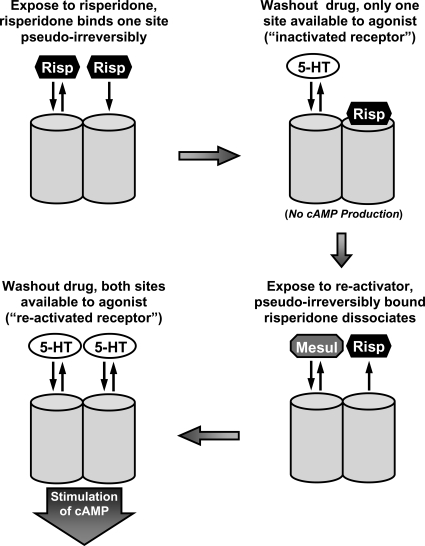
Figure 8 is a schematic (originally presented by Teitler et al., 2010) depicting 5-HT7 receptor function that seems to explain the anomalous results reported herein using primary cultures of rat cortical astrocytes and previous studies using HEK293 cells expressing h5-HT7 receptors. The similarity of the data in the two systems (i.e., the inactivation produced by risperidone, 9-OH-risperidone, methiothepin, and bromocriptine) and the reversal of this inactivation by competitive antagonists through the 5-HT7 receptor seems to indicate that the receptor is operating as a homodimer in both cell types. In the recombinant cells, we were able to use radioligand binding studies to demonstrate that a maximum of 50% of the h5-HT7 receptors were occupied by risperidone or 9-OH-risperidone in a pseudoirreversible manner, yet these drugs can completely inactivate h5-HT7-dependent cAMP production in these cells. This is a key observation, indicating that the binding of these drugs to one receptor orthosteric binding site is producing an allosteric effect on another receptor's orthosteric binding site and function. This, in turn, strongly indicates a homodimeric structure. The ability of competitive antagonists to release the bound risperidone, returning the receptor to an activated state, is consistent and supportive of the homodimer model. The competitive antagonists seem to be binding to the second protomer, producing an allosteric effect on the first risperidone-bound protomer, resulting in the decreased affinity of that protomer for risperidone. The release of the pseudoirreversibly bound risperidone restores the receptor to its initial state that can be stimulated by 5-HT (Teitler et al., 2010).
Fig. 8.
Schematic presentation of a homodimeric receptor model consistent with the anomalous effects of drugs on the 5-HT7 receptor (Teitler et al., 2010). An inactivating antagonist, risperidone (Risp), binds to the orthosteric binding sites of a 5-HT7 receptor homodimer (depicted as two adjacent cylinders). This results in a pseudoirreversible complex between risperidone and one protomer, the inactivation of the 5-HT7 receptor, and the inability of the second protomer to bind risperidone pseudoirreversibly. The second protomer retains reversible drug binding properties, indicated by equilibrium double arrows. The binding of a noninactivating antagonist, such as mesulergine (Mesul), to the second protomer releases the pseudoirreversibly bound risperidone from the first protomer, leaving a reactivated receptor capable of binding agonists, such as serotonin (5-HT), at both protomers. [Reproduced from Teitler M, Toohey N, Knight JA, Klein MT, and Smith C (2010) Clozapine and other competitive antagonists re-activate risperidone-inactivated h5-HT7 receptors: radioligand binding and functional evidence for GPCR homodimer protomer interactions. Psychopharmacology (Berl) 212:687–697. Fig. 10. Copyright © Springer-Verlag Berlin Heidelberg. Used with permission.]
Discussion
In previous studies, we have reported apparently anomalous effects of antagonists on the activity of the h5-HT7 receptor expressed in HEK293 cells (Smith et al., 2006; Knight et al., 2009; Toohey et al., 2009). The key finding of those studies was that risperidone binds to 50% of the h5-HT7 receptors in a pseudoirreversible manner, causing complete inactivation of the receptor after washing out risperidone. 9-OH-risperidone displayed similar properties. Methiothepin and bromocriptine also inactivated the h5-HT7 receptor; however, they were shown to occupy all receptors pseudoirreversibly after exposure to the drug and the washout procedure. Exposure of risperidone-inactivated h5-HT7 receptor-expressing cells to competitive antagonists resulted in the release of the pseudoirreversibly bound risperidone and the reactivation of the receptor (Teitler et al., 2010). Competitive antagonists could not reverse the effects of saturating concentrations of methiothepin or bromocriptine after washout of these two drugs.
Two fundamental principles were necessary to interpret those results. First, drugs bind to unoccupied binding sites (i.e., the presence of pseudoirreversibly bound risperidone should prevent the subsequent binding of another drug to the same orthosteric binding site of the h5-HT7 receptor). Second, orthosteric binding sites and allosteric binding sites display distinctly different pharmacological profiles (i.e., drugs that bind to the orthosteric binding site with high affinities do not bind to allosteric sites on a GPCR monomer with high affinities, and vice versa). Therefore, the ability of h5-HT7 receptor antagonists to potently reactivate the receptor, despite the presence of the pseudoirreversibly bound risperidone occluding the orthosteric binding site, presented an anomaly.
A monomeric model of h5-HT7 receptor function could not reasonably account for these observations. However, a homodimeric structure, in which the binding of risperidone to one protomer allosterically changes the properties of the other protomer, can account for these observations. The homodimeric structure can explain risperidone's ability to bind only 50% of 5-HT7 receptors in a pseudoirreversible manner: the risperidone-occupied protomer allosterically modulates the affinity of risperidone for the second protomer, allowing risperidone to bind reversibly at the second protomer's orthosteric site. With this second orthosteric site available to bind other ligands, competitive antagonists are able to bind to the risperidone-inactivated h5-HT7 receptor dimers and cause the release of risperidone and the concomitant reactivation of the receptor (Teitler et al., 2010). This model includes the concept that the inactivation of one protomer fully inactivates the homodimer. These results are similar to results observed with the 5-HT2C receptor (Herrick-Davis et al., 2005). These data demonstrate a native (nonmutated), homodimeric GPCR displaying protomer-protomer cross-talk in live cells. Using the ability of competitive antagonists to reverse the effects of a pseudoirreversible antagonist represents a novel, noninvasive experimental design to investigate the presence and properties of endogenous GPCR homodimers.
The observations reported previously involved experiments using a recombinant cell line expressing the h5-HT7 receptor (Teitler et al., 2010). The presence of a native GPCR homodimer in primary culture displaying protomer-protomer cross-talk has not been reported (Pin et al., 2007). The presence of homodimers in recombinant cell lines can be detected using various techniques, but evidence for the presence of GPCR homodimers in an in vivo situation has remained elusive. Protomer-protomer cross-talk has also not been convincingly demonstrated for a GPCR homodimer in any cell culture system.
To determine whether the properties of the h5-HT7 receptor expressed in HEK293 cells are relevant to the in vivo situation, we used rat cortical astrocytes in primary culture. Astrocytes express a functional 5-HT7 receptor that produces a robust cAMP stimulation upon exposure to 5-HT (Hirst et al., 1997; Shimizu et al., 1998). We first demonstrated that the 5-HT response is due to the r5-HT7 receptor by using the 5-HT7-selective antagonist SB269970. This selective antagonist completely inhibits the 5-HT-induced stimulation of cAMP in a competitive manner, with a potency consistent with its affinity for the 5-HT7 receptor (Fig. 1). The nonselective antagonist clozapine also produced a potent competitive antagonism of the 5-HT-induced stimulation of cAMP, indicating that this response does not involve another 5-HT receptor (Fig. 2).
Risperidone, 9-OH-risperidone, methiothepin, and bromocriptine were found to potently inactivate the r5-HT7 receptor (Figs. 3 and 4; Table 1). Clozapine, mesulergine, cyproheptadine, SB269970, and mianserin did not inactivate the receptor, although they are antagonists at the 5-HT7 receptor with moderate to high affinity (Fig. 3). These results are indistinguishable from those observed in the HEK293 cells expressing the h5-HT7 receptor (Table 1) (Knight et al., 2009).
Clozapine, mesulergine, cyproheptadine, SB269970, and mianserin were found to reactivate the risperidone-inactivated receptor (Figs. 5 and 6; Table 2). The potencies of these drugs in reactivating the receptor closely correlate with their potencies as antagonists of 50 nM 5-HT-induced stimulation of cAMP in rat cortical astrocytes (Fig. 7 and Table 2). Again, these results are very similar to the results observed in the HEK293 cells expressing the h5-HT7 receptor (Teitler et al., 2010).
Given the fundamental principles that drugs bind to unoccupied binding sites and that orthosteric and allosteric binding sites display distinctly different pharmacological profiles, the same conclusions drawn from the work on the HEK293 cells can be drawn from the work presented herein using rat cortical astrocytes. As shown in Fig. 8, we propose a model in which the binding of one risperidone molecule to a r5-HT7 homodimer results in pseudoirreversible binding to that protomer, inactivation of the receptor dimer, and the inability of the second protomer to bind risperidone pseudoirreversibly, although other binding properties of that protomer are preserved. The orthosteric binding site on the second protomer remains available to bind 5-HT7 receptor antagonists, thus explaining the ability of the apparently occupied 5-HT7 receptor orthosteric site to interact with other competitive antagonists. The binding of a competitive antagonist to the second (non–risperidone-bound) protomer induces an allosteric effect on the first protomer that results in the release of risperidone, allowing the reactivation of the receptor. This model and its schematic representation in Fig. 8 have been presented previously (Teitler et al., 2010). Methiothepin and the other inactivating antagonists that bind both protomers pseudoirreversibly do not provide competitive antagonists with access to unoccupied protomers, and thus the receptors cannot be reactivated. However, when nonsaturating levels of methiothepin are used to partially inactivate receptors, competitive antagonists can bind to unoccupied protomers and reactivate the receptors. This is demonstrated in Figs. 4 and 5, in which 16 nM methiothepin, which partially inactivates the receptor (Fig. 4), was used to inactivate r5-HT7 receptors and mesulergine treatment recovered 5-HT activity (Fig. 5).
This model is strongly based on the work in the recombinant cell line (Smith et al., 2006; Knight et al., 2009; Teitler et al., 2010). Although being able to demonstrate the unusual regulation of the 5-HT7 receptor by drugs in a primary culture is critical, there are drawbacks to using this system, relative to the recombinant cell line. The low expression levels of the r5-HT7 receptor have not allowed the detection of a radioligand binding signal in our laboratory or the laboratories of groups previously reporting 5-HT7 receptor function in cultured astrocytes (Hirst et al., 1997). The homodimer model presented in Fig. 8 does depend on data from the recombinant cell line (Teitler et al., 2010); however, the reversal of the inactivating effect of risperidone by 5-HT7 competitive antagonists is difficult to interpret without a dimer model.
The biochemical mechanism responsible for the pseudoirreversible binding of the “inactivating antagonists” observed in the astrocyte studies, as well as the studies on the h5-HT7 receptor, is beyond the scope of this investigation. There is no scaffolding protein known to bind the inactivating (or noninactivating antagonists) with the high affinities and the order of affinities observed in these studies.
In summary, the observations that provide evidence for the h5-HT7 receptor existing as a homodimer and displaying protomer-protomer cross-talk in a recombinant cell line are also evident in rat cortical astrocytes. This is an important step in developing evidence for the existence of GPCR homodimers in vivo and identifying the function of protomer-protomer interactions. The strategy of finding pseudoirreversible drugs that inactivate GPCRs and reactivating the receptors with competitive antagonists is a novel one and can lead to the demonstration of other receptors displaying homodimeric behavior (i.e., protomer-protomer cross-talk). If many GPCRs do exist as homodimers, there is a great deal of information to be learned regarding the purpose of the homodimeric structure, the possible role of homodimer dysfunction in disease states, and the possible development of novel drugs that produce different protomer-protomer effects at the receptor.
Acknowledgments
We thank Dr. Tara Lindsley for help with the astrocyte culture procedures and Dr. Katharine Herrick-Davis for advice and general discussions.
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant MH56650].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.069278.
- 5-HT
- 5-hydroxytryptamine
- GPCR
- G protein-coupled receptor
- HEK
- human embryonic kidney
- HBSS
- Hanks' balanced salt solution
- TR-FRET
- time-resolved fluorescence energy transfer
- MEM
- minimum essential medium
- PBS
- phosphate-buffered saline
- SB269970
- (2R)-1-[(3-hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine hydrochloride
- SB270146
- 5-chloro-N-[4-methoxy-3-(1-piperazinyl)phenyl]-3-methyl-benzo[b]thiophen-2-sulfonamide hydrochloride
- ICI169369
- 2-(2-dimethylamino ethylthio)-3-phenyl quinoline.
Authorship Contributions
Participated in research design: Smith, Toohey, Knight, Klein, and Teitler.
Conducted experiments: Smith, Toohey, and Knight.
Performed data analysis: Smith, Toohey, Knight, Klein, and Teitler.
Wrote or contributed to the writing of the manuscript: Smith, Toohey, Knight, Klein, and Teitler.
References
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. (1993) Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem 268:23422–23426 [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Harrigan TJ, Mazurkiewicz JE. (2005) Inhibition of serotonin 5-hydroxytryptamine2c receptor function through heterodimerization: receptor dimers bind two molecules of ligand and one G-protein. J Biol Chem 280: 40144–40151 [DOI] [PubMed] [Google Scholar]
- Hirst WD, Cheung NY, Rattray M, Price GW, Wilkin GP. (1998) Cultured astrocytes express messenger RNA for multiple serotonin receptor subtypes, without functional coupling of 5-HT1 receptor subtypes to adenylyl cyclase. Brain Res Mol Brain Res 61:90–99 [DOI] [PubMed] [Google Scholar]
- Hirst WD, Price GW, Rattray M, Wilkin GP. (1997) Identification of 5-hydroxytryptamine receptors positively coupled to adenylyl cyclase in rat cultured astrocytes. Br J Pharmacol 120:509–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71:533–554 [DOI] [PubMed] [Google Scholar]
- Kaech S, Banker G. (2006) Culturing hippocampal neurons. Nat Protoc 1:2406–2415 [DOI] [PubMed] [Google Scholar]
- Knight JA, Smith C, Toohey N, Klein MT, Teitler M. (2009) Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5-hydroxytryptamine7 receptor by risperidone, 9-OH-risperidone, and other inactivating antagonists. Mol Pharmacol 75:374–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell PJ, Bromidge SM, Dabbs S, Duckworth DM, Forbes IT, Jennings AJ, King FD, Middlemiss DN, Rahman SK, Saunders DV, et al. (2000) A novel, potent, and selective 5-HT(7) antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl) phenol (SB-269970). J Med Chem 43:342–345 [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW. (1993) A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11:449–458 [DOI] [PubMed] [Google Scholar]
- Pin JP, Neubig R, Bouvier M, Devi L, Filizola M, Javitch JA, Lohse MJ, Milligan G, Palczewski K, Parmentier M, et al. (2007) International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol Rev 59:5–13 [DOI] [PubMed] [Google Scholar]
- Routledge C, Bromidge SM, Moss SF, Price GW, Hirst W, Newman H, Riley G, Gager T, Stean T, Upton N, et al. (2000) Characterization of SB-271046: a potent, selective and orally active 5-HT(6) receptor antagonist. Br J Pharmacol 130:1606–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Monsma FJ, Jr., Metcalf MA, Jose PA, Hamblin MW, Sibley DR. (1993) Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem 268:18200–18204 [PubMed] [Google Scholar]
- Shimizu M, Nishida A, Zensho H, Miyata M, Yamawaki S. (1998) Agonist-induced desensitization of adenylyl cyclase activity mediated by 5-hydroxytryptamine7 receptors in rat frontocortical astrocytes. Brain Res 784:57–62 [DOI] [PubMed] [Google Scholar]
- Smith C, Rahman T, Toohey N, Mazurkiewicz J, Herrick-Davis K, Teitler M. (2006) Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor. Mol Pharmacol 70:1264–1270 [DOI] [PubMed] [Google Scholar]
- Teitler M, Herrick-Davis K. (1994) Multiple serotonin receptor subtypes: molecular cloning and functional expression. Crit Rev Neurobiol 8:175–188 [PubMed] [Google Scholar]
- Teitler M, Toohey N, Knight JA, Klein MT, Smith C. (2010) Clozapine and other competitive antagonists re-activate risperidone-inactivated h5-HT7 receptors: radioligand binding and functional evidence for GPCR homodimer protomer interactions. Psychopharmacology (Berl) 212:687–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR, Atkinson PJ, Ho M, Bromidge SM, Lovell PJ, Villani AJ, Hagan JJ, Middlemiss DN, Price GW. (2000) [3H]-SB-269970—A selective antagonist radioligand for 5-HT(7) receptors. Br J Pharmacol 130:409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toohey N, Klein MT, Knight J, Smith C, Teitler M. (2009) Human 5-HT7 receptor-induced inactivation of forskolin-stimulated adenylate cyclase by risperidone, 9-OH-risperidone and other “inactivating antagonists.” Mol Pharmacol 76:552–559 [DOI] [PMC free article] [PubMed] [Google Scholar]