Abstract
Cytochrome P450 2J2 (CYP2J2) epoxygenase converts arachidonic acid to four regioisomeric epoxyeicosatrienoic acids (EETs) that exert multiple biological effects in the cardiovascular system and in various human solid cancers. However, it is unknown whether this enzyme is expressed or plays any role in malignant hematological diseases. In this study, we found strong and highly selective CYP2J2 expression in five human-derived malignant hematological cell lines and in leukemia cells from peripheral blood and bone marrow in 36 of 42 patients (86%) with malignant hematologic diseases. Furthermore, increased levels of EETs were detected in urine and blood samples from these patients. Addition of exogenous EET or CYP2J2 overexpression in cultured human-derived malignant hematologic cell lines markedly accelerated proliferation and attenuated apoptosis. Addition of the selective CYP2J2 inhibitor compound 26 (C26; 1-[4-(vinyl) phenyl]-4-[4-(diphenyl-hydroxymethyl)-piperidinyl]-butanone hydrochloride) inhibited cell proliferation and increased apoptosis, an effect that was significantly reversed by EET. CYP2J2 overexpression and exogenous EET activated AMP-activated protein kinase, c-Jun NH2-terminal kinase, and phosphatidylinositol 3-kinase/Akt signaling pathways, and increased epidermal growth factor receptor phosphorylation levels. CYP2J2 overexpression also enhanced malignant xenograft growth, which was efficiently inhibited by oral administration of C26 in Tie2-CYP2J2 transgenic mice and in severe combined immunodeficiency (SCID) xenograft mice. Together, these results suggest that CYP2J2 plays a key role in the pathogenesis of human hematologic malignant diseases. Selective inhibition of CYP2J2 may be a promising therapeutic strategy for these conditions.
Introduction
Studies using purified and/or recombinant cytochrome P450 (P450) epoxygenases have demonstrated that multiple P450 enzymes can metabolize arachidonic acid to four regioisomeric epoxyeicosatrienoic acids (5,6-, 8,9-, 11,12-, and 14,15-EETs), albeit with different catalytic efficiencies (Capdevila et al., 1992; Zeldin, 2001; Kroetz and Zeldin, 2002). One of the predominant epoxygenase isoforms involved in EET formation belongs to the CYP2 gene family (Spiecker and Liao, 2005). Although expressed primarily in the liver, many P450 enzymes are expressed in extrahepatic organs, including lung, kidney, and gastrointestinal tissues (Zeldin et al., 1997; Enayetallah et al., 2004). CYP2J2, a unique CYP2J gene found in humans, is a major enzyme located in extrahepatic tissues, with predominant expression in the cardiovascular system, including endothelial cells (Node et al., 1999) and cardiomyocytes (Wu et al., 1996), and is active in the biosynthesis of EETs (Wu et al., 1997).
EETs are known to have diverse biological functions. To maintain cardiovascular homeostasis they vasodilate vascular beds by activating large-conductance Ca2+-activated K+ channels (BKCa) in smooth muscle cells (Harder et al., 1995; Campbell and Harder, 1999; Roman, 2002), protect endothelial cells from inflammatory injury and apoptosis, up-regulate endothelial nitric-oxide synthase (eNOS) and increase its activity and enhance angiogenesis (Wang et al., 2005; Fleming, 2007). Other studies have shown that EETs reduce blood pressure and attenuate ischemia/reperfusion injury in the heart (Harder et al., 1995; Kroetz and Zeldin, 2002; Roman, 2002). In addition to their vasodilatory effects, EETs possess potent anti-inflammatory properties (Node et al., 1999). Similar to their roles in the cardiovascular system (Elbekai and El-Kadi, 2006; Larsen et al., 2006), CYP2J2 and EETs also play an important role in human malignant diseases. In recent publications, we demonstrated that CYP2J2 was overexpressed in various human solid cancers and human-derived cancer cell lines. In addition, the addition of exogenous EETs or recombinant adenoassociated viral vector-mediated overexpression of CYP2J2 dramatically enhanced the proliferation of cancer cells in vitro and malignant growth of xenograft tumors in vivo (Jiang et al., 2005), and promoted metastasis of MDA-MB-231 cell xenograft tumors in lungs. In contrast, antisense oligonucleotides to CYP2J2 or nonspecific P450 inhibitors significantly attenuated these neoplastic and malignant phenotypes (Jiang et al., 2005, 2007). These findings suggest that inhibition of CYP2J2-mediated EET biosynthesis may represent a novel approach for the treatment of human cancers (Jiang et al., 2007). It is noteworthy that we recently identified a novel class of selective inhibitors of CYP2J2 with marked antitumor properties both in vitro and in vivo (Chen et al., 2009).
It remains unclear whether CYP2J2 and EETs play a role in human hematologic malignant tumors as they do in human solid tumors. Herein, we demonstrate specific overexpression of CYP2J2 in human hematologic tumor cells and increased EET production in patients with leukemia or lymphoma, as well as in various hematological tumor cell lines. Furthermore, we show that EET treatment significantly enhances the malignant leukemia phenotypes in vitro and in vivo and, in contrast, administration of the selective CYP2J2 inhibitor C26 attenuates EET production and dramatically inhibits these phenotypes in hematologic malignant diseases.
Materials and Methods
Materials.
TRIzol, cell culture medium, and fetal bovine serum were purchased from Invitrogen (Carlsbad, CA). Reverse transcriptase-polymerase chain reaction (RT-PCR) kit was from Takara Bio Co. Ltd. (Dalian, China). Anthra[1–9-cd]pyrazol-6(2H)-one (SP600125), 11,12-EET, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO). Antibody against CYP2J2 was purchased from Abcam Inc. (Cambridge, MA). Antibodies against epidermal growth factor receptor (EGFR), p-EGFR, phosphatidylinositol 3-kinase (PI3K), Bcl-2, Bcl-xL, Bax, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β-actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against Akt/p-Akt, AMPK/p-AMPK, and JNK/p-JNK were purchased from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase-conjugated secondary antibodies (goat anti-mouse IgG and goat anti-rabbit IgG) were purchased from KPL (Gaithersburg, MD). Enhanced chemiluminescence reagents were purchased from Pierce, Inc. (Rockford, IL); polyvinylidene difluoride membranes, prestained protein markers, and SDS-polyacrylamide gel electrophoresis gels were from Bio-Rad, Inc. (Hercules, CA). 2′-Amino-3′-methoxyflavone (PD98059), apigenin, l-NG-nitroarginine methyl ester, and 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) were purchased from Cayman Chemical Co. (Ann Arbor, MI). 4-(3′-chloroanilino)-6,7-dimethoxy-quinazoline (AG1478), 2-chloro-5-nitrobenzanilide (GW9662), Akt inhibitor, protein kinase C inhibitor peptide 19–36 (RFARKGALRQKNVHEVKN), and Compound C were purchased from Calbiochem/EMD (Darmstadt, Germany). siRNAs were purchase from Ribobio Co. (Guangzhou, China). All other reagents were purchased from standard commercial suppliers unless otherwise indicated.
Patient Samples.
After informed consent was obtained, 42 patients at Tongji Hospital with hematologic malignant diseases, including acute leukemia, chronic leukemia, and lymphoma, were recruited. Five milliliters of peripheral blood and 5 ml of morning urine were collected from each patient. Bone marrow samples were also collected from 20 of these patients. In addition, surgically resectioned pathological tissue samples from 20 patients with lymphoma were obtained and cut into 4-μm thick sections for immunohistochemistry. Thirty healthy subjects or patients with nonhematologic malignant diseases were recruited as control subjects. Their blood and morning urine were collected, and bone marrow smears were obtained from two of the control subjects. All human research protocols were approved by the Clinical Research Committees of Tongji Medical College and were carried out according to the guidelines of the National Institutes of Health. Plasma and white blood cells (WBC) were isolated from peripheral blood by centrifugation, and plasma was frozen at −80°C for measurements of the stable EET metabolite [14,15-dihydroxyeicosatrienoic acid (14,15-DHET)] and WBCs were used for CYP2J2 expression analysis by Western blotting, immunohistochemistry, or confocal microscopy. Bone marrow and peripheral blood smears were obtained for further CYP2J2 expression analysis.
Cell Lines.
K562, HL-60, Raji, MOLT-4, SP2/0, Jurkat, and EL4 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained as recommended by the source. Cells were cultured in RPMI 1640 medium, adjusted to contain 4 mM l-glutamine, 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 10% fetal bovine serum, 100 units/ml penicillin, and 65 units/ml streptomycin. All cell cultures were maintained at 37°C in constant humidified incubator containing 95% air/5% CO2 atmosphere.
Synthesis of C26.
The design and synthesis of high-affinity and selective CYP2J2 inhibitors derived from terfenadone, a derivative of the drug terfenadine, has been described in detail by Lafite et al. (2006). We synthesized an additional novel hydrochloride salt compound, 1-[4-(vinyl) phenyl]-4-[4-(diphenyl-hydroxymethyl)-piperidinyl]-butanone hydrochloride, labeled as compound 26 (C26) (Chen et al., 2009).
Analysis of CYP2J2 Expression by RT-PCR.
Total RNA was isolated from cells using TRIzol reagent. Semiquantitative analysis of the expression of CYP2J2 mRNA was done using RT-PCR. Expression of GAPDH mRNA was used as an internal standard. RNA was reverse-transcribed using the Takara Bio RT-PCR kit, according to the manufacturer's protocol. The PCR mixture contained 5 μl of cDNA, 1× PCR buffer, 1.5 mM MgCl2, 0.8 mM deoxynucleotide triphosphates, 1 unit of Taq DNA polymerase, and 100 nM concentrations of each primer for CYP2J2 (sense primer, 5′-CTCCTACTGGGCACTGTCGC-3′; antisense primer, 5′-TGGGCCTCCTCCTGAAT-3′) or for GAPDH (sense primer, 5′-ACCACAGTCCATGCCATCAC-3′; antisense primer, 5′-TCCACCACCCTGTTGCTGTA-3′). PCR products were resolved in 1% agarose gels stained with ethidium bromide. The relative intensity of CYP2J2 compared with GAPDH was calculated for each sample by densitometry.
Western Blotting.
Proteins from cell lysates of cultures or peripheral white blood cells (20 μg) were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. After blocking in 5% nonfat milk, protein blots were incubated with specific antibodies followed by incubation with a peroxidase-conjugated secondary antibody in blocking buffer. The bands were visualized with the enhanced chemiluminescence method.
Analysis of CYP2J2 Expression by Confocal Laser-Scanning Microscopy.
To confirm CYP2J2 expression and its localization, confocal laser-scanning microscopy was performed using peripheral blood and bone marrow smears. After fixation with acetone and ethanol (1:1) at room temperature for 10 min, slides were washed three times with phosphate-buffered saline. After blocking, slides were incubated with CYP2J2 antibodies for 16 h at 4°C, followed by incubation with rhodamine B isothiocyanate-conjugated goat anti-mouse IgG antibodies for 1 h at room temperature. Nuclear staining was performed with Hoechst 33258 at room temperature for 10 min after washing. Once free staining materials were removed with three more washes, slides were available for scanning under a confocal laser-scanning microscope (FV500; Olympus, Tokyo, Japan) equipped with digital imaging. Ten high-power field images were captured for each slide.
Determination of 14,15-DHET Levels.
CYP2J2 metabolizes arachidonic acid to four EETs that are further metabolized to DHETs. To further investigate CYP2J2 function, we measured plasma and urinary concentrations of the major CYP2J2-dependent epoxidation products from arachidonic acid. Given the instability of EETs, the concentration of the stable 14,15-EET metabolite 14,15-DHET (which was converted from 14,15-EET, the highest expressed EET), was determined in urine and plasma of patients. For this purpose, the urine and plasma were preserved with triphenylphosphine and stored at −80°C until analyzed. Eicosanoids were extracted from urine and plasma thrice with ethyl acetate after acidification with acetic acid (Jiang et al., 2005; Yang et al., 2007). After evaporation, the samples were dissolved in N,N-dimethylformamide (AMRESCO, Solon, OH) and the concentration of 14,15-DHET was determined by an ELISA kit (Detroit R&D, Detroit, MI), according to the manufacturer's instructions.
Transfection.
Transfection was performed with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Cells were plated in 96-well or 6-well plates at a density of approximately 104 or 106 cells/well. The cells were harvested 48 h after transfection with plasmid or siRNAs. Plasmid DNA for transient transfection was prepared with the TaKaRa MiniBEST Plasmid Purification Kit (Takara Bio) and siRNAs were chemically synthesized.
Cell Growth Assays.
The effects of CYP2J2, EETs, and C26 on the growth of leukemia cells was determined using the MTT assay as described previously (Jiang et al., 2005). This assay measures the conversion of MTT to formazan crystals by enzymes in the mitochondria of metabolically active cells. The cells were seeded at 104 cells per well (five replicates each in 96-well plates) in serum-free medium for 24 h for synchronization. Cells transfected with pcDNA3.1-CYP2J2 or CYP2J2-specific siRNAs were treated with EET and/or C26 as indicated in the figure legends. MTT was added to cells for 3 h, and then the formed crystals were dissolved in DMSO and the intensity of the color in each well was measured at a wavelength of 490 nm using a microplate reader.
BrdU Cell Proliferation Assay.
To examine the effects of CYP2J2-derived EET and C26 on the growth of leukemia cells, we carried out BrdU cell proliferation assays. As described previously (Wegiel et al., 2008), the BrdU immunolabeling assay was performed using a nonisotopic immunoassay kit for the quantitation of bromodeoxyuridine incorporation into newly synthesized DNA of actively proliferating cells according to manufacturer's instructions (Calbiochem/Merck). In brief, transfected cells were incubated in a 96-well plastic plate using serum-free medium. BrdU was then added, and cells were cultured for another 8 h. BrdU incorporated into the DNA was determined by measuring the absorbance at 450 nm on an ELISA plate reader.
Determination of Cell Apoptosis by Flow Cytometry.
After treatments with EET and/or C26 as described above, the cells were harvested and resuspended in binding buffer and incubated with FITC-conjugated Annexin V and propidium iodide (Annexin V-FITC kit; Bender MedSystems, San Bruno, CA) according to the manufacturer's protocol and then analyzed with a FACStar-Plus flow cytometer (BD Biosciences, Franklin Lakes, NJ). To exclude necrotic cells, only the cells with Annexin V-positive and propidium iodide-negative staining were counted for early stages of apoptosis.
Colorimetric Assay for the Measurement of Caspase-3 Activity.
Caspase-3 activity was measured using a colorimetric assay kit according to manufacturer's instructions (R&D Systems, Minneapolis, MN). In brief, the treated cells were collected and lysed by the addition of lysis buffer. After centrifugation, a caspase-specific peptide that was conjugated to a color reporter molecule and reaction buffer were added to the supernatant and incubated at 37°C for 2 h in the dark. Release of the chromophore by the caspase enzymatic activity was quantified spectrophotometrically at a wavelength of 405 nm.
Mice and Leukemia Models.
Tie2-CYP2J2 transgenic mice that express CYP2J2 in an endothelial-specific manner were generated in D.Z.'s laboratory at the National Institute of Environmental Health Sciences, National Institutes of Health (Lee et al., 2010). Male C57BL/6 and Tie2-CYP2J2 transgenic mice on a C57BL/6 genetics background, aged 6 to 8 weeks, were used for tumor-bearing recipients. The animals were housed in microisolator cages and fed ad libitum access to food and water. All animal studies were approved by the Animal Research Committee of Tongji Medical College and done according to guidelines set forth by the National Institutes of Health. Lymphoma was induced by intraperitoneal injection of the appropriate dose of EL4 cells (2 × 106) in a volume of 0.1 ml of serum-free RPMI 1640 medium as described previously (Boyer et al., 1997).
Ten SCID mice per group were injected with cyclophosphamide (150 mg/kg i.p.) on days 0 and 1, respectively, to inactivate the immune system and then inoculated intravenously with 107 K562 cells (human-derived leukemia cells) on day 2 as described previously (Shiotsu et al., 2000). C26 in 1% DMSO or vehicle, respectively, was administrated orally at a dose of 0.25 mg/kg/day.
Histology.
After the animals were sacrificed, organs were removed, fixed in formalin, and embedded in paraffin. Four-micrometer thick sections were prepared and stained with hematoxylin and eosin for histological analysis. Proliferation status was assessed by immunostaining with PCNA antibodies. Apoptosis was determined using terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining according to manufacturer's instructions (R&D Systems). Images were acquired on an inverted microscope (TE 2000; Nikon, Tokyo, Japan) equipped with digital imaging. For each slide, 10 high-power field images were captured.
Statistics.
Data are presented as mean ± S.E. The Wilcoxon test, the Student's t test, and analysis of variance were performed, respectively, to determine statistical significance among treatment groups, as appropriate. In all cases, statistical significance was defined as p < 0.05.
Results
Expression of CYP2J2 in Leukemia Cells from Patients with Hematologic Malignant Disease and Human-Derived Leukemia Cell Lines.
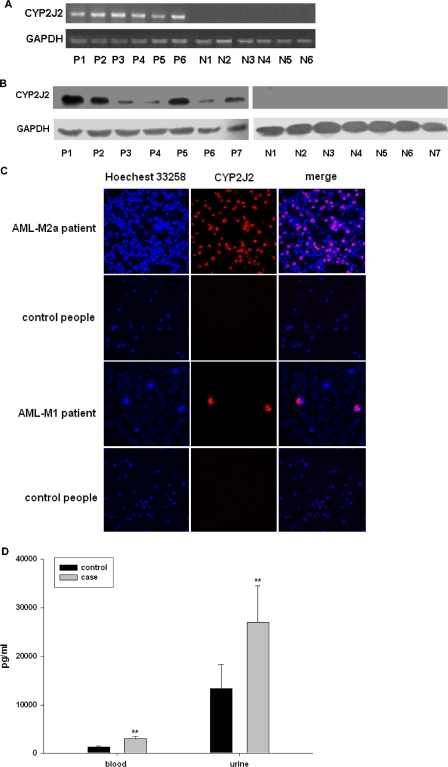
We found that CYP2J2 mRNA and protein was abundantly expressed in malignant leukemia and lymphoma cells in peripheral blood but not in normal WBCs of healthy volunteers (Fig. 1, A and B, the detail clinical data of the patients are in Supplemental Table 1). CYP2J2 expression was observed in virtually all leukemia and lymphoma cells but not in normal cells. We further investigated the expression of CYP2J2 in bone marrow and peripheral blood smears using a confocal laser-scanning microscopy. As predicted, CYP2J2 was abundantly expressed in the cytoplasm of nucleated cells from patients but not in cells from healthy volunteers (Fig. 1C), suggesting that CYP2J2 is expressed exclusively in cells from patients with hematologic malignancy.
Fig. 1.
Selective expression of CYP2J2 in white blood cells in patients with hematologic malignant diseases. A, CYP2J2 mRNA levels. Total RNA was isolated from WBC in healthy volunteers (V) and in patients (P) with leukemia or lymphoma. Semiquantitative analysis of the expression of CYP2J2 mRNA was done using a multiplex RT-PCR technique as described under Materials and Methods. B, CYP2J2 protein levels. Protein from WBC lysates in patients with leukemia or lymphoma and healthy volunteers were subjected to Western blot analysis as described under Materials and Methods. C, CYP2J2 subcellular localization. Peripheral blood smears and bone marrow smears from patients with leukemia or lymphoma and from healthy volunteers were subject to confocal microscopy as described under Materials and Methods. D, 14,15-DHET levels from healthy volunteers and patients with leukemia or lymphoma. 14,15-DHET levels were determined by ELISA as described under Materials and Methods. *, p < 0.01 versus control.
To evaluate the activity of CYP2J2, we measured the level of the stable 14,15-EET metabolite 14,15-DHET in plasma and urine from patients with leukemia/lymphoma and healthy volunteers. Results show that the concentrations of 14,15-DHET were significantly higher in urine and plasma from patients than from healthy volunteers (Fig. 1D), suggesting that expression of CYP2J2 in hematologic malignant disease may result in increased production of CYP epoxygenase metabolites. To exclude effects of other epoxygenases on increase in EETs production, we detected expression of other two important human epoxygenases CYP2C8 and CYP2C9 in white blood cells from six acute leukemia patients. Results showed that no CYP2C8 and CYP2C9 mRNA was detectable in white blood cells of the patients, which suggest that the overexpression of CYP2J2 in leukemia cells is the major contributor of elevation in EETs level in the plasma and urine in patients with hematologic malignant disease.
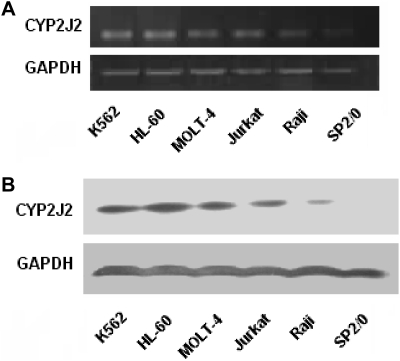
The expression of CYP2J2 in leukemia cell lines was also examined. Results show that abundant CYP2J2 mRNA and protein were present in five human malignant cell lines (i.e., K562, HL-60, MOLT-4, Jurkat, and Raji) but not in the nonmalignant cell line SP2/0 (Fig. 2). Thus, all of the human leukemia and lymphoma cells examined selectively and highly expressed CYP2J2, and significantly increased levels of P450 epoxygenase products were observed in urine and blood from patients with hematologic malignant diseases.
Fig. 2.
Selective expression of CYP2J2 in human-derived leukemia cell lines. A, CYP2J2 mRNA levels. Total RNAs were isolated from different cell lines. Semiquantitative analysis of the expression of CYP2J2 mRNA was done using a multiplex RT-PCR technique as described under Materials and Methods. B, CYP2J2 protein levels. Cells from different cell lines were collected and proteins from cell lysates were then subject to Western blot analysis as described under Materials and Methods.
CYP2J2 and EETs Promote In Vitro Human Leukemia Cell Proliferation.
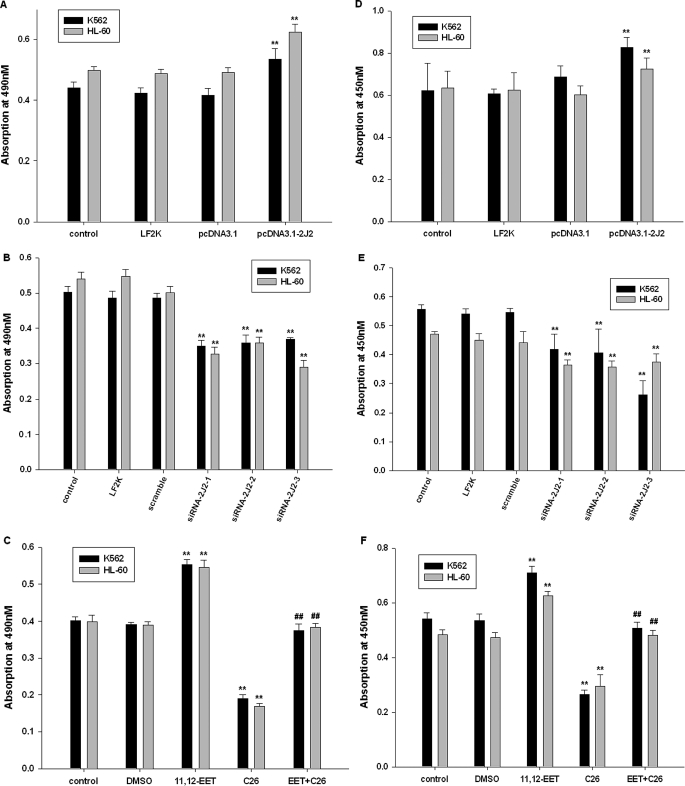
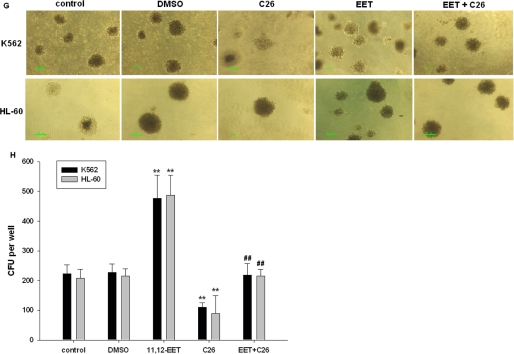
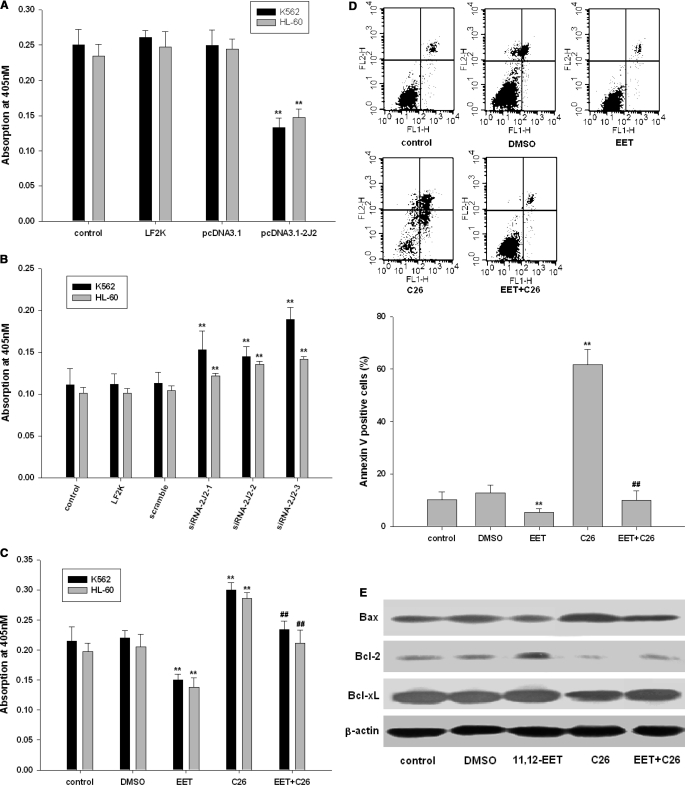
On the basis of these results and our previous observations (Jiang et al., 2005, 2007), we hypothesized that CYP2J2 and its epoxygenase metabolites (EETs) may contribute to the neoplastic phenotype of leukemia cells. First, we determined the effect of pcDNA3.1-CYP2J2, CYP2J2-specific siRNAs, exogenous EETs, and the CYP2J2 inhibitor C26 on proliferation of K562 and HL-60 cells in vitro over a 48-h period. As predicted, pcDNA3.1-CYP2J2 (1 μg/ml) transfection caused a significant increase in the number of viable K562 and HL-60 cells as determined by MTT assay (Fig. 3A). In contrast, addition of CYP2J2-specific siRNAs (100 nM) decreased the number of viable cells (Fig. 3B). 11,12-EET (1 μM) treatment increased the viable cell number, but C26 decreased viable cell numbers (Fig. 3C). It is noteworthy that 11,12-EET treatment reversed the effects of C26 on viable cell numbers. BrdU cell proliferation assays also showed that CYP2J2 promotes cancer cell proliferation (Fig. 3, D and E), whereas C26 treatment has significant antiproliferative effects in cancer cells (Fig. 3F). 11,12-EET treatment revised the effects of C26 on cell proliferation. In colony formation assay, which reflect the tumorigenic property of the treated cells, we observed the same results (Fig. 3, G and H). Together, these data demonstrate that CYP2J2-derived EETs promote proliferation in human leukemia cells and suggests a role for P450 epoxygenases in the neoplastic leukemia phenotype.
Fig. 3.
CYP2J2 and EETs promote human leukemia cell proliferation in vitro. A, effect of pcDNA3.1-CYP2J2 (1 μg/ml) on number of K562 and HL-60 cells. B, effect of CYP2J2 specific siRNAs (100 nM) on number of K562 and HL-60 cells. C, effect of 11,12-EET (1 μM) and/or C26 (10 μM) on number of K562 and HL-60 cells. D, effect of pcDNA3.1-CYP2J2 (1 μg/ml) on proliferation of K562 and HL-60 cells. E, effect of CYP2J2-specific siRNAs (100 nM) on proliferation of K562 and HL-60 cells. F, effect of 11,12-EET (1 μM) and/or C26 (10 μM) on proliferation of K562 and HL-60 cells. G and H, effect of 11,12-EET (1 μM) and/or C26 (10 μM) on colony formation assay of K562 and HL-60 cells. Data are expressed as absorptions, which are reported as mean ± S.E. (n = 5); **, p < 0.01 versus control; ##, p < 0.01 versus EET treatment.
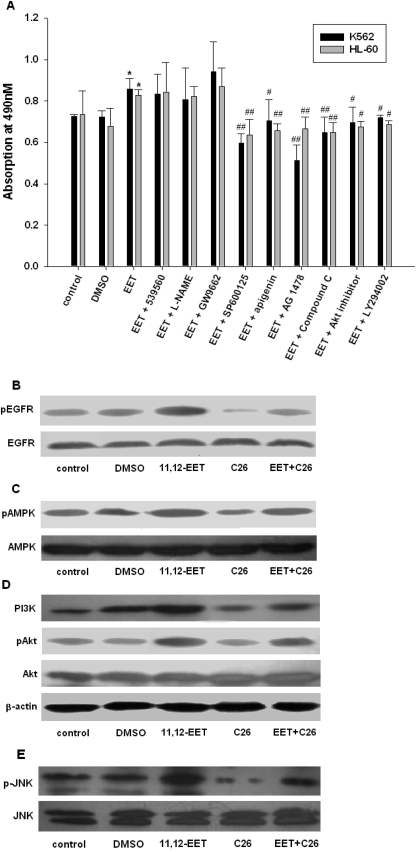
Using K562 and HL-60 cells as representative cell lines, we investigated the influence of CYP2J2 and EETs on the expression of a variety of growth factors reported to play important roles in EET-mediated cell proliferation. In addition, to examine the importance of activating these growth factor signaling pathways on EET-induced enhancement of cell survival and proliferation, MTT proliferation assays were performed on cells incubated with individual EETs in the presence or absence of inhibitors of epidermal growth factor, PKC, eNOS, PPARγ, JNK, AMPK, Akt, and PI3K. With the exception of PKC, eNOS, and PPARγ inhibitors, all other tested inhibitors decreased EET-mediated proliferation in K562 and HL-60 cells (Fig. 4A), indicating the important roles for these intracellular signaling pathways in the EET-mediated regulation of cell proliferation. We then used K562 cells to examine the effect of 11,12-EET and C26 on expression and phosphorylation status of these important signaling molecules. Protein expression levels of PKC, eNOS, and PPARγ were not altered by EET treatment (data not shown). However, phosphorylated EGFR and AMPK were increased by EET (Fig. 4, B and C). Conversely, treatment with C26 decreased the phosphorylation level of EGFR and AMPK and this effect could be reversed by adding EET. We also examined the expression and phosphorylation status of the downstream PI3K/Akt and JNK signaling pathways. Similar to EGFR and AMPK, EET treatment increased levels of PI3K, p-Akt, and JNK, whereas C26 attenuated these effects (Fig. 4, D and E). Treatment with EET reversed the effects of C26. Thus, consistent with the effects on cell proliferation, EETs significantly activate intracellular growth factor signaling molecules in human leukemia cells.
Fig. 4.
Effects of EET administration on the epidermal growth factor signaling pathway in human leukemia cells. A, cellular proliferation was stimulated by 11,12-EETs (1 μM) and decreased by inhibitors of JNK (SP600125), mitogen-activated protein kinase (apigenin), EGFR (AG1478), AMPK (Compound C), Akt (Akt inhibitor), and PI3K (LY294002), but not by inhibitors of PKC (539560), eNOS (l-NG-nitroarginine methyl ester; l-NAME), and PPARγ (GW9662). B, increased phosphorylation of EGFR in K562 cells after 11,12-EET (1 μM) treatment is reversed by C26 (10 μM). C, increased phosphorylation of AMPK in K562 cells after 11,12-EET (1 μM) treatment is reversed by C26 (10 μM). D, increased expression of PI3K and p-Akt in K562 cells after 11,12-EET (1 μM) treatment is reversed by C26 (10 μM). E, increased phosphorylation of JNK in K562 cells after 11,12-EET (1 μM) treatment is reversed by C26 (10 μM). Results shown are mean ± S.E. (n = 5); *, p < 0.05 versus control; #, p < 0.05 versus EET.
Collectively, these results suggest that P450 epoxygenases and their metabolites (EETs) promote viability, stimulate proliferation of human leukemia cells, and activate growth factor signaling pathways. Conversely, C26 and siRNA to CYP2J2 blocks proliferation of these cells.
CYP2J2 Inhibitor Activates Caspase-3 and Enhances Human Leukemia Cell Apoptosis.
We next examined the effect of EET and C26 on the activity of caspase-3, an intracellular cysteine protease activated during the cascade of events leading to apoptosis (Roy et al., 2001) and on apoptosis in the leukemia cell lines K562 and HL-60. Using a colorimetric assay, we found that CYP2J2 significantly decreased the activity of caspase-3 (Fig. 5A). In contrast, CYP2J2-specific siRNAs or addition of C26 to inhibit CYP2J2 significantly increased caspase-3 activity (Fig. 5, B and C). Addition of 11,12-EET attenuated the effect of C26 on caspase-3 activity. To verify the apoptotic effects of C26, C26-treated K562 cells were stained with Annexin V/propidium iodide, which allows for the identification of apoptotic cells by flow cytometry. Treatment with C26 (10 μM, 24 h) significantly increased the percentage of apoptotic cells compared with control and DMSO-treated cells. Furthermore, addition of 11,12-EET significantly attenuated C26-induced apoptosis (Fig. 5D). Western blot analysis for apoptosis-related proteins revealed that addition of 11,12-EET up-regulated the antiapoptotic proteins Bcl-2 and Bcl-xL but down-regulated the proapoptotic protein Bax and thus increased the ratio of Bcl-2/Bax. In contrast, C26 treatment prevented these antiapoptotic changes (Fig. 5E). Together, these data suggest that P450 epoxygenases and EETs protect human leukemia cells from apoptosis through regulatory effects on proapoptotic and antiapoptotic protein expression. On the other hand, application of C26 increases the activity of caspase-3 and induces apoptosis in human leukemia cells.
Fig. 5.
CYP2J2 inhibitor activates caspase-3 and enhances human leukemia cell apoptosis. A, effect of pcDNA3.1-CYP2J2 (1 μg/ml) on caspase-3 activity in K562 and HL-60 cells. B, effect of CYP2J2 specific siRNAs (100 nM) on caspase-3 activity in K562 and HL-60 cells. C, effect of 11,12-EET (1 μM) and/or C26 (10 μM) on caspase-3 activity of K562 and HL-60 cells. Data are reported as mean ± S.E. (n = 5). D, analysis by flow cytometry of K562 cells treated with 11,12-EET (1 μM) and/or C26 (10 μM) using Annexin V-FITC and propidium iodide. The lower left quadrant represents nonapoptotic cells, the lower right quadrant represents early apoptotic cells (Annexin positive, propidium iodide negative), and the upper right quadrant represents Annexin V- and propidium iodide-positive late apoptotic or necrotic cells. The graph represents the mean number of Annexin V-positive K562 cells expressed as percentage of control untreated cells ± S.E. (n = 3). E, the effects of 11,12-EET (1 μM) and/or C26 (10 μM) on apoptosis-related proteins in K562 cells. *, p < 0.01 versus control; #, p < 0.05 versus EET.
CYP2J2 Promotes Murine Xenograft Tumor Growth in Leukemia Models.
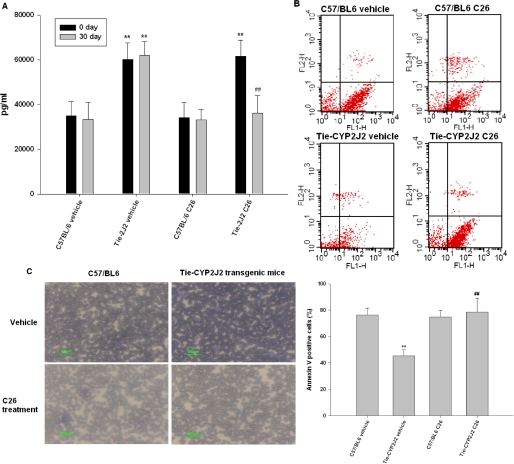
We next examined the in vivo role of CYP2J2 on tumor growth. Wild-type C57BL/6 and Tie2-CYP2J2 transgenic mice, which overexpress human CYP2J2 in an endothelial-specific manner, were intraperitoneally injected with 2 × 106 EL4 lymphoma cells per mouse and then orally administered C26 or vehicle. Urine samples were collected at 4 weeks and urinary 14,15-DHET levels were measured. As expected, Tie2-CYP2J2 mice secreted significantly more 14,15-DHET than wild-type C57BL/6 mice. Furthermore, C26 significantly decreased 14,15-DHET levels in Tie2-CYP2J2 transgenic mice but not in wild-type C57BL/6 mice (Fig. 6A).
Fig. 6.
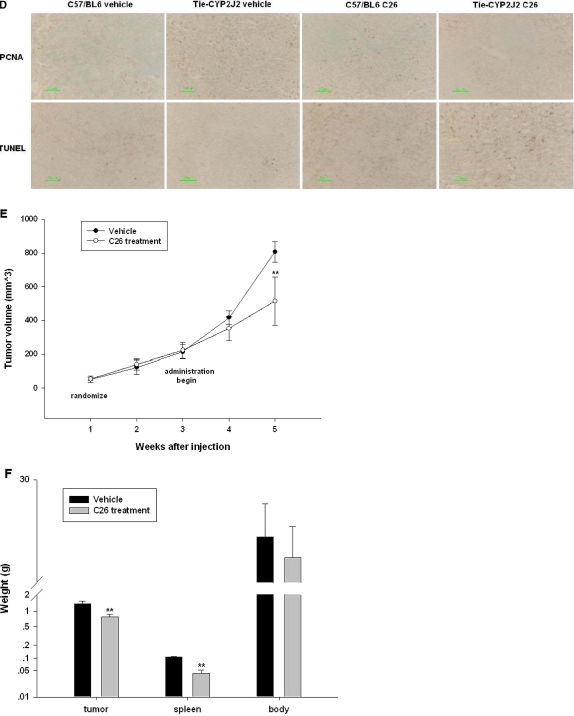
CYP2J2 promotes murine xenograft tumor growth in leukemia models. C57BL/6 and Tie2-CYP2J2 transgenic mice were injected with 2 × 106 EL4 cells per mouse. The mice were then randomized to vehicle control versus C26 treatment. A, 14,15-DHET levels in the urine during treatment with C26 or vehicle control. B, analysis by flow cytometry of peripheral blood nucleated cells from C57BL/6 and Tie2-CYP2J2 transgenic mice treated with C26 or vehicle control using Annexin V-FITC and propidium iodide. Graph represents the mean number of Annexin-positive cells expressed as percentage of control untreated cells ± S.E. (n = 6). C, Wright-Giemsa staining of peripheral blood smear. D, PCNA and TUNEL staining of spleen section slices. **, p < 0.01 versus C57BL/6 vehicle; ##, p < 0.01 versus Tie2-CYP2J2 vehicle. SCID mice were injected with 107 K562 cells per mouse. The mice were then randomized to vehicle control versus C26 treatment. E, tumor volume as measured weekly in vehicle control and C26-treated mice. Tumor volume was monitored by digital caliper on a weekly basis and calculated as length × width2 × π/6. F, tumor, spleen, and body weight of mice 4 weeks after randomization into vehicle control and C26 treatment groups. **, p < 0.01 versus vehicle.
Because dysregulated apoptosis plays a central role in defective hematopoiesis in human myelodysplastic syndrome, we next examined apoptosis in Tie2-CYP2J2 transgenic mice. At the end of the study, peripheral blood was aspirated and analyzed for viable tumor cells and for apoptosis. Decreased apoptosis was observed in Tie2-CYP2J2 transgenic mice [Annexin V-positive population in transgenic mice, 45.23 ± 4.85% (n = 6), versus C57BL/6, 76.25 ± 5.45% (n = 6); P ≤ 0.01] and C26 treatment increased apoptosis [Annexin V-positive population in transgenic mice without treatment, 45.23 ± 4.85% (n = 6) versus transgenic mice with C26 treatment, 78.65 ± 10.41% (n = 6); P ≤ 0.01] (Fig. 6B). Monitoring of hematologic abnormalities in transgenic mice revealed that increased numbers of immature blasts and atypical dysplastic white cells were seen on peripheral blood smears (Fig. 6C). We also labeled liver and spleen sections with a PCNA antibody to evaluate proliferation and used TUNEL staining to measure apoptosis. Histological evaluation of spleen sections revealed a large number of mitotic figures indicative of active proliferation and fewer apoptotic bodies in transgenic mice (Fig. 6D). Immunohistochemical staining for PCNA showed a higher proliferative index consistent with leukemia cell invasion, compared with wild-type C57BL/6 mice, which had much less mitotic activity. TUNEL staining of spleen sections from Tie2-CYP2J2 transgenic mice indicated significantly fewer apoptotic bodies compared with wild-type C57BL/6 mice. Inhibition of CYP2J2 activity by C26 treatment in Tie2-CYP2J2 transgenic mice reverses these effects (Fig. 6D). Liver section slices showed similar patterns (data not shown).
We also examined the effects of CYP2J2 and C26 on tumor growth in an in vivo leukemia model. SCID mice were injected subcutaneously with 107 K562 leukemia cells per mouse. The tumor volumes were measured using a digital caliper once a week. When tumors reached an average of 200 mm3 in volume, C26 was orally administrated on a daily basis. The difference in tumor volume between the vehicle and treatment groups was apparent within 1 week after treatment of the animals and became statistically significant after 2 weeks (Fig. 6E). At the end of the treatment period, C26 was associated with decreased tumor and spleen weight without changes in body weight (Fig. 6F). Together, these data are consistent with our in vitro findings and indicate that the selective CYP2J2 inhibitor C26 inhibits leukemia cell tumor growth in vivo.
Discussion
CYP2J2 is a major enzyme found in extrahepatic tissue, with predominant expression in the cardiovascular system, including endothelial cells (Node et al., 1999) and cardiomyocytes (Wu et al., 1996). Our previous studies demonstrated a role for CYP2J2-derived EETs in promoting the neoplastic phenotype of cancer cells. Increased CYP2J2 expression and activity was associated with increased tumor growth and lung metastasis (Jiang et al., 2007). However, the expression of CYP2J2 in the hematopoietic system in normal and pathological conditions has not yet been reported, and the effects of CYP2J2 inhibition on leukemia cell growth have not been examined. In the present study, we found abundant expression of CYP2J2 in leukemia cell lines and cells from the bone marrow and peripheral blood of patients with leukemia and lymphoma. It is noteworthy that CYP2J2 expression was absent in peripheral white blood cells and bone marrow smears from healthy volunteers. EET levels in urine and blood in patients with leukemia and lymphoma also were much higher than that in urine and blood from healthy volunteers. We excluded possible effect on EET level of other two important human epoxygenases, CYP2C8 and CYP2C9, in white blood cells from six patients with acute leukemia because they are not expressed in leukemia cells. Although there still are many other P450 enzymes able to produce EETs, their yield is extremely low. Thus, the overexpression of CYP2J2 in leukemia cells is the major contributor of elevation in EET levels in the plasma and urine of patients with hematologic malignant disease. These data suggest that CYP2J2 may play an important role in human leukemia and lymphoma.
We also found that CYP2J2 overexpression and EETs significantly promote proliferation and inhibit apoptosis in leukemia cells in vitro. It is noteworthy that the specific CYP2J2 inhibitor C26 significantly inhibited leukemia cell proliferation in vitro and tumor growth in vivo, suggesting that CYP2J2 may be a key therapeutic target for these conditions.
Acute myeloid leukemia is an aggressive, heterogeneous disease with numerous cytogenetic abnormalities and mutations within key signaling pathways involved in cell differentiation, proliferation, and survival (Van Etten, 2007). These key signaling molecules include the PI3K/Akt signaling network (Martelli et al., 2007), RAS signaling pathways (Flotho et al., 2007), Wnt signaling (Mikesch et al., 2007), transforming growth factor-β (Lin et al., 2005), FLT-3 (Markovic et al., 2005), Cotylenin A (Honma, 2002), cAMP response element-binding protein (Kinjo et al., 2005), EGFR (Bacchiocchi et al., 2005; Nishioka et al., 2010), JNK (Lagadinou et al., 2008), and AMPK (Campàs et al., 2003; Drakos et al., 2009). Considering these complex signaling events, we hypothesized that CYP2J2-derived EETs may participate in hematological malignant diseases through one or more of these pathways. Our investigation of the potential mechanisms of CYP2J2 involvement in leukemogenesis demonstrated that activation of the EGFR, PI3K/Akt, AMPK, and JNK pathways, which are known to be activated by CYP2J2 and EETs, are associated with leukemia cell proliferation as well as inhibition of leukemia cell apoptosis by elevating the Bcl-2/Bax ratio.
In summary, we have identified a novel molecule participating in hematologic malignancy and found a selective inhibitor of CYP2J2 with marked antihematologic malignant properties in vitro and in vivo. CYP2J2 inhibitors hold promise for use in targeted combination therapy for the control of malignant diseases of the hematopoietic system.
Supplementary Material
Acknowledgments
We thank the following people for their efforts in CYP2J2 inhibitor synthesis: Drs. Guiling Li, Jin Kui Du, and Ruo Fei Guan.
This work was supported by the China Natural Science Foundation Committee [Grants 30540087, 30430320]; the 973 Program [Grants 2007CB512004, 2002CB513107]; and the International Project. This work was also supported, in part, by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences [Grant Z01-ES025034].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.174805.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- P450
- cytochrome P450
- EET
- epoxyeicosatrienoic acid
- eNOS
- endothelial nitric-oxide synthase
- RT-PCR
- reverse transcriptase-polymerase chain reaction
- SP600125
- anthra[1–9-cd]pyrazol-6(2H)-one
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- EGFR
- epidermal growth factor receptor
- PI3K
- phosphatidylinositol 3-kinase
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- AMPK
- AMP-activated kinase
- JNK
- c-Jun NH2-terminal kinase
- PD98059
- 2′-amino-3′-methoxyflavone
- LY294002
- 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one
- AG-1478
- 4-(3′-chloroanilino)-6,7-dimethoxy-quinazoline
- GW9662
- 2-chloro-5-nitrobenzanilide
- WBC
- white blood cell
- 14,15-DHET
- 14,15-dihydroxyeicosatrienoic acid
- C26
- compound 26 [1-[4-(vinyl) phenyl]-4-[4-(diphenyl-hydroxymethyl)-piperidinyl]-butanone hydrochloride]
- DHET
- dihydroxyeicosatrienoic acid
- ELISA
- enzyme-linked immunosorbent assay
- siRNA
- small interfering RNA
- DMSO
- dimethyl sulfoxide
- FITC
- fluorescein isothiocyanate
- PCNA
- proliferating cell nuclear antigen
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick-end labeling
- PKC
- protein kinase C
- PPAR
- peroxisome proliferator-activated receptor
- SCID
- severe combined immunodeficiency
- BrdU
- 5-bromo-2′-deoxyuridine.
Authorship Contributions
Participated in research design: C. Chen, Wei, Ma, and Zhou.
Conducted experiments: C. Chen, Wei, Rao, Wu, Yang, and F. Chen.
Performed data analysis: Wang.
Wrote or contributed to the writing of the manuscript: C. Chen, Dackor, Zeldin, and Wang.
References
- Bacchiocchi R, Baldanzi G, Carbonari D, Capomagi C, Colombo E, van Blitterswijk WJ, Graziani A, Fazioli F. (2005) Activation of alpha-diacylglycerol kinase is critical for the mitogenic properties of anaplastic lymphoma kinase. Blood 106:2175–2182 [DOI] [PubMed] [Google Scholar]
- Boyer MW, Vallera DA, Taylor PA, Gray GS, Katsanis E, Gorden K, Orchard PJ, Blazar BR. (1997) The role of B7 costimulation by murine acute myeloid leukemia in the generation and function of a CD8+ T-cell line with potent in vivo graft-versus-leukemia properties. Blood 89:3477–3485 [PubMed] [Google Scholar]
- Campàs C, Lopez JM, Santidrián AF, Barragán M, Bellosillo B, Colomer D, Gil J. (2003) Acadesine activates AMPK and induces apoptosis in B-cell chronic lymphocytic leukemia cells but not in T lymphocytes. Blood 101:3674–3680 [DOI] [PubMed] [Google Scholar]
- Campbell WB, Harder DR. (1999) Endothelium-derived hyperpolarizing factors and vascular cytochrome P450 metabolites of arachidonic acid in the regulation of tone. Circ Res 84:484–488 [DOI] [PubMed] [Google Scholar]
- Capdevila JH, Falck JR, Estabrook RW. (1992) Cytochrome P450 and the arachidonate cascade. FASEB J 6:731–736 [DOI] [PubMed] [Google Scholar]
- Chen C, Li G, Liao W, Wu J, Liu L, Ma D, Zhou J, Elbekai RH, Edin ML, Zeldin DC, et al. (2009) Selective inhibitors of CYP2J2 related to terfenadine exhibit strong activity against human cancers in vitro and in vivo. J Pharmacol Exp Ther 329:908–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakos E, Atsaves V, Li J, Leventaki V, Andreeff M, Medeiros LJ, Rassidakis GZ. (2009) Stabilization and activation of p53 downregulates mTOR signaling through AMPK in mantle cell lymphoma. Leukemia 23:784–790 [DOI] [PubMed] [Google Scholar]
- Elbekai RH, El-Kadi AO. (2006) Cytochrome P450 enzymes: central players in cardiovascular health and disease. Pharmacol Ther 112:564–587 [DOI] [PubMed] [Google Scholar]
- Enayetallah AE, French RA, Thibodeau MS, Grant DF. (2004) Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J Histochem Cytochem 52:447–454 [DOI] [PubMed] [Google Scholar]
- Fleming I. (2007) Epoxyeicosatrienoic acids, cell signaling and angiogenesis. Prostaglandins Other Lipid Mediat 82:60–67 [DOI] [PubMed] [Google Scholar]
- Flotho C, Kratz C, Niemeyer CM. (2007) Targeting RAS signaling pathways in juvenile myelomonocytic leukemia. Curr Drug Targets 8:715–725 [DOI] [PubMed] [Google Scholar]
- Harder DR, Campbell WB, Roman RJ. (1995) Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res 32:79–92 [DOI] [PubMed] [Google Scholar]
- Honma Y. (2002) Cotylenin A—a plant growth regulator as a differentiation-inducing agent against myeloid leukemia. Leuk Lymphoma 43:1169–1178 [DOI] [PubMed] [Google Scholar]
- Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, Ning YG, Xiao X, Zeldin DC, Wang DW. (2005) Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res 65:4707–4715 [DOI] [PubMed] [Google Scholar]
- Jiang JG, Ning YG, Chen C, Ma D, Liu ZJ, Yang S, Zhou J, Xiao X, Zhang XA, Edin ML, et al. (2007) Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res 67:6665–6674 [DOI] [PubMed] [Google Scholar]
- Kinjo K, Sandoval S, Sakamoto KM, Shankar DB. (2005) The role of CREB as a proto-oncogene in hematopoiesis. Cell Cycle 4:1134–1135 [DOI] [PubMed] [Google Scholar]
- Kroetz DL, Zeldin DC. (2002) Cytochrome P450 pathways of arachidonic acid metabolism. Curr Opin Lipidol 13:273–283 [DOI] [PubMed] [Google Scholar]
- Lafite P, Dijols S, Buisson D, Macherey AC, Zeldin DC, Dansette PM, Mansuy D. (2006) Design and synthesis of selective, high-affinity inhibitors of human cytochrome P450 2J2. Bioorg Med Chem Lett 16:2777–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagadinou ED, Ziros PG, Tsopra OA, Dimas K, Kokkinou D, Thanopoulou E, Karakantza M, Pantazis P, Spyridonidis A, Zoumbos NC. (2008) c-Jun N-terminal kinase activation failure is a new mechanism of anthracycline resistance in acute myeloid leukemia. Leukemia 22:1899–1908 [DOI] [PubMed] [Google Scholar]
- Larsen BT, Gutterman DD, Hatoum OA. (2006) Emerging role of epoxyeicosatrienoic acids in coronary vascular function. Eur J Clin Invest 36:293–300 [DOI] [PubMed] [Google Scholar]
- Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, et al. (2010) Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J 24:3770–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HK, Bergmann S, Pandolfi PP. (2005) Deregulated TGF-beta signaling in leukemogenesis. Oncogene 24:5693–5700 [DOI] [PubMed] [Google Scholar]
- Markovic A, MacKenzie KL, Lock RB. (2005) FLT-3: a new focus in the understanding of acute leukemia. Int J Biochem Cell Biol 37:1168–1172 [DOI] [PubMed] [Google Scholar]
- Martelli AM, Tazzari PL, Evangelisti C, Chiarini F, Blalock WL, Billi AM, Manzoli L, McCubrey JA, Cocco L. (2007) Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside. Curr Med Chem 14:2009–2023 [DOI] [PubMed] [Google Scholar]
- Mikesch JH, Steffen B, Berdel WE, Serve H, Müller-Tidow C. (2007) The emerging role of Wnt signaling in the pathogenesis of acute myeloid leukemia. Leukemia 21:1638–1647 [DOI] [PubMed] [Google Scholar]
- Nishioka C, Ikezoe T, Yang J, Yokoyama A. (2010) Long-term exposure of leukemia cells to multi-targeted tyrosine kinase inhibitor induces activations of AKT, ERK and STAT5 signaling via epigenetic silencing of the PTEN gene. Leukemia 24:1631–1640 [DOI] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. (1999) Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285:1276–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman RJ. (2002) P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82:131–185 [DOI] [PubMed] [Google Scholar]
- Roy S, Bayly CI, Gareau Y, Houtzager VM, Kargman S, Keen SL, Rowland K, Seiden IM, Thornberry NA, Nicholson DW. (2001) Maintenance of caspase-3 proenzyme dormancy by an intrinsic “safety catch” regulatory tripeptide. Proc Natl Acad Sci USA 98:6132–6137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiotsu Y, Neckers LM, Wortman I, An WG, Schulte TW, Soga S, Murakata C, Tamaoki T, Akinaga S. (2000) Novel oxime derivatives of radicicol induce erythroid differentiation associated with preferential G(1) phase accumulation against chronic myelogenous leukemia cells through destabilization of Bcr-Abl with Hsp90 complex. Blood 96:2284–2291 [PubMed] [Google Scholar]
- Spiecker M, Liao JK. (2005) Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch Biochem Biophys 433:413–420 [DOI] [PubMed] [Google Scholar]
- Van Etten RA. (2007) Aberrant cytokine signaling in leukemia. Oncogene 26:6738–6749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wei X, Xiao X, Hui R, Card JW, Carey MA, Wang DW, Zeldin DC. (2005) Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J Pharmacol Exp Ther 314:522–532 [DOI] [PubMed] [Google Scholar]
- Wegiel B, Bjartell A, Tuomela J, Dizeyi N, Tinzl M, Helczynski L, Nilsson E, Otterbein LE, Härkönen P, Persson JL. (2008) Multiple cellular mechanisms related to cyclin A1 in prostate cancer invasion and metastasis. J Natl Cancer Inst 100:1022–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Chen W, Murphy E, Gabel S, Tomer KB, Foley J, Steenbergen C, Falck JR, Moomaw CR, Zeldin DC. (1997) Molecular cloning, expression, and functional significance of a cytochrome P450 highly expressed in rat heart myocytes. J Biol Chem 272:12551–12559 [DOI] [PubMed] [Google Scholar]
- Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. (1996) Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem 271:3460–3468 [DOI] [PubMed] [Google Scholar]
- Yang S, Lin L, Chen JX, Lee CR, Seubert JM, Wang Y, Wang H, Chao ZR, Tao DD, Gong JP, et al. (2007) Cytochrome P-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-alpha via MAPK and PI3K/Akt signaling pathways Am J Physiol Heart Circ Physiol 293:H142–H151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldin DC. (2001) Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem 276:36059–36062 [DOI] [PubMed] [Google Scholar]
- Zeldin DC, Foley J, Goldsworthy SM, Cook ME, Boyle JE, Ma J, Moomaw CR, Tomer KB, Steenbergen C, Wu S. (1997) CYP2J subfamily cytochrome P450s in the gastrointestinal tract: expression, localization, and potential functional significance. Mol Pharmacol 51:931–943 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.