Abstract
β2-Agonists are the most effective bronchodilators for the rapid relief of asthma symptoms, but for unclear reasons, their effectiveness may be decreased during severe exacerbations. Because peroxidase activity and nitrogen oxides are increased in the asthmatic airway, we examined whether salbutamol, a clinically important β2-agonist, is subject to potentially inactivating nitration. When salbutamol was exposed to myeloperoxidase, eosinophil peroxidase or lactoperoxidase in the presence of hydrogen peroxide (H2O2) and nitrite (NO2−), both absorption spectroscopy and mass spectrometry indicated formation of a new metabolite with features expected for the nitrated drug. The new metabolites showed an absorption maximum at 410 nm and pKa of 6.6 of the phenolic hydroxyl group. In addition to nitrosalbutamol (m/z 285.14), a salbutamol-derived nitrophenol, formed by elimination of the formaldehyde group, was detected (m/z 255.13) by mass spectrometry. It is noteworthy that the latter metabolite was detected in exhaled breath condensates of asthma patients receiving salbutamol but not in unexposed control subjects, indicating the potential for β2-agonist nitration to occur in the inflamed airway in vivo. Salbutamol nitration was inhibited in vitro by ascorbate, thiocyanate, and the pharmacological agents methimazole and dapsone. The efficacy of inhibition depended on the nitrating system, with the lactoperoxidase/H2O2/NO2− being the most affected. Functionally, nitrated salbutamol showed decreased affinity for β2-adrenergic receptors and impaired cAMP synthesis in airway smooth muscle cells compared with the native drug. These results suggest that under inflammatory conditions associated with asthma, phenolic β2-agonists may be subject to peroxidase-catalyzed nitration that could potentially diminish their therapeutic efficacy.
Introduction
Asthma is a chronic inflammatory disease characterized by bronchial smooth muscle contraction and episodic narrowing of the airway. This, along with edema of the bronchial wall and accumulation of airway mucus, limits airflow and gas exchange. Treatment of acute asthma exacerbations typically includes inhalation of β2-adrenergic agonists, which activate β2-adrenergic receptors (β2ARs) on bronchial smooth muscle cells, triggering an increase in cAMP that leads to smooth muscle relaxation. β2-Agonists are currently the most potent and effective bronchodilators in clinical use. However, for unknown reasons, their ability to relieve bronchoconstriction during severe asthma exacerbations (i.e., status asthmaticus) may be reduced and even harmful (Suissa et al., 1994; Abramson et al., 2003).
Elevated levels of inflammatory cells, particularly neutrophils (PMN) and eosinophils (EOS), as well as their secretory products, have been detected in asthmatic airways, and their amounts are further increased during clinical exacerbations of the disease (Bousquet et al., 2000; Wu et al., 2000; Aldridge et al., 2002; Andreadis et al., 2003). Upon activation, EOS and PMN generate superoxide (O2•−) and hydrogen peroxide (H2O2) and secrete unique peroxidases, eosinophil peroxidase (EPO) by EOS and myeloperoxidase (MPO) by PMN. These enzymes functionally resemble lactoperoxidase (LPO) that is normally present in lung lining fluid (Conner et al., 2002; El-Chemaly et al., 2003).
Nitric oxide (NO•) is generated by constitutive and inducible forms of nitric-oxide synthases that are present in many cell types within the respiratory tract, including airway and alveolar epithelial cells, macrophages, neutrophils, mast cells, and vascular endothelial and smooth muscle cells. In asthmatic airways there is increased synthesis of NO• as evidenced by increased levels of NO• in exhaled breath, accumulation of nitrite (NO2−) and nitrate (NO3−), and the presence of nitrated tyrosine residues in proteins (Gaston et al., 1994; MacPherson et al., 2001; Ramesh et al., 2001; Andreadis et al., 2003). Previous work indicated that nitrogen oxides may reduce the bronchodilatory effect of salbutamol in asthmatics (Kanazawa et al., 2002), presumably by inactivation of adenylate cyclase. Impairment of β2AR function has also been attributed to peroxynitrite oxidation of cysteine residues and/or nitration of tyrosine residues present in the extracellular domains in the β1- and β2-receptors (Lewis et al., 2005).
Because of their phenolic character, β-agonists such as salbutamol (also referred to as albuterol) (Fig. 1) are potentially subject to peroxidative metabolism. Consistent with this notion, we have shown that the airway peroxidases LPO and MPO can catalyze oxidation of β2-agonists in vitro (Reszka et al., 2009). Given that inflamed airways are also the source of NO2−, we were interested to examine the effect of NO2− on the peroxidative metabolism of the agonist. Nitrite is an excellent substrate for peroxidases, which oxidize it to the nitrogen dioxide radical (NO2•), a species involved in oxidation and nitration of biological targets (Reszka et al., 1997; van der Vliet et al., 1997; Burner et al., 2000; Brück et al., 2001; Brennan et al., 2002; Van Dalen et al., 2006). Results of our study show that in the presence of NO2− airway peroxidases can convert salbutamol to nitrated species with reduced capacity to bind β2-ARs and stimulate cAMP synthesis. Putative metabolites of salbutamol nitration were also detected in exhaled breath condensates (EBCs) from asthmatics, thereby suggesting that metabolism of salbutamol by peroxidases and NO2− in the inflamed airway may be a mechanism that underlies impaired β2-agonist efficacy in certain clinical settings.
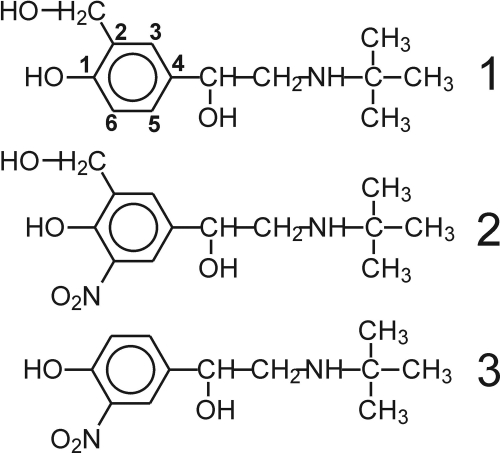
Fig. 1.
Structures of salbutamol (1), 6-nitrosalbutamol (2), and salbutamol-derived nitrophenol (3).
Materials and Methods
Materials.
Salbutamol hemisulfate (1) [4-[2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxymethyl)phenol sulfate (2:1), also known as albuterol] was from MP Biochemicals, Inc. (Solon, OH). LPO (from bovine milk; EC 1.11.1.7) and all other chemicals (hydrogen peroxide, 30%), ascorbic acid (AA), methimazole (1-methyl-3H-imidazole-2-thione), dapsone (diamino-diphenyl sulfone), and diethylenetriamine pentaacetic acid (DTPA) were obtained from Sigma-Aldrich (St. Louis, MO). LPO concentration was determined using ε412 of 1.12 × 105 M−1 · cm−1 (Jenzer et al., 1986). MPO from human leukocytes [lyophilized powder, 25 U, RZ (reinheit zahl purity value) (A429/A280) of 0.61] was obtained from Axxora, LLC (San Diego, CA) and reconstituted with 0.25 ml of distilled water before use. Human EPO (460 U/mg protein) (Calbiochem, San Diego, CA) was reconstituted with 0.240 ml of water. The concentration of H2O2 was determined using ε240 of 39.4 M−1 · cm−1 (Nelson and Kiesow, 1972). Intact salbutamol shows absorption maximum at 277 nm, and its molar absorptivity (ε277) has been determined to be 1.8 × 103 M−1 · cm−1 in pH 7.0 phosphate buffer. All chemicals were used as received, and stock solutions of reagents were prepared in glass-distilled water.
Exhaled Breath Condensate Samples.
EBC samples were collected from childhood asthmatics hospitalized after an asthma exacerbation during 15 min of quiet breathing through a single-use disposable RTube collector (Respiratory Research, Inc., Charlottesville, VA) while subjects were wearing nose clips. Two aluminum sleeves were used to cool the device at an initial temperature of −80°C before collection. All EBC samples were taken within 3 h of the last albuterol dose. After collection, the plunger was used to pool the condensed material within the tube into a single sample (approximately 1.0 ml). Samples were stored in the reaction tube at a temperature of −80°C until thawed and processed for analysis.
Spectrophotometric Measurements.
Spectra were measured by using an Agilent diode array spectrophotometer model 8453 (Agilent Technologies, Santa Clara, CA). Nitration of salbutamol was studied by measuring absorption spectra at designated time points after the start of the reaction. Samples were prepared in 100 mM phosphate buffers, pH 7.0. All buffers contained DTPA (200 μM) or were pretreated with Chelex-100 before use, and measurements were performed at an ambient temperature of 20°C. Typically, the reaction was started by the addition of a small aliquot of H2O2 or the desired peroxidase. Time course measurements were carried out after changes in absorbance at 410 nm in 30-s intervals versus absorbance at 800 nm, where none of the compounds absorb.
To determine molar absorptivity of the mixture of salbutamol-derived metabolites, the absorbance at 410 nm was measured after multiple additions of peroxidases, H2O2, and NO2− until A410 stabilized, indicating that all salbutamol was consumed. These experiments were conducted at various initial concentrations of the drug. Based on these measurements ε410 for the mixture of metabolites was estimated to be 4550 ± 227 M−1 · cm−1 (n = 14) at pH 7.0. The effect of pH on ionization of the compound's phenolic moiety was determined by measuring absorption spectra of samples prepared by adding equal volumes of nitrated salbutamol (25 μl) to buffers (0.1 M, 475 μl) of pH ranging from 4 to 9.
Mass Spectrometry.
Experiments were performed on a Thermo Fisher Scientific (Waltham, MA) LTQ-FT, a hybrid instrument consisting of a linear ion trap and a Fourier transform ion cyclotron resonance (ICR) mass spectrometer (Micromass, Manchester, UK). Liquid chromatography separation of the sample components used a Waters (Milford, MA) XBridge C18 3.5-μm, 2.1 × 100-mm column, Finnigan Surveyor MS pump (Thermo Fisher Scientific), and Finnigan Micro AS autosampler (Thermo Fisher Scientific). A 200 μl/min gradient elution of water and acetonitrile, both containing 0.1% formic acid, took place as follows: 5 to 32% acetonitrile in the first 8 min followed by a rapid rise to 95% acetonitrile within the next minute and held at 95% for an additional 7 min. The entire eluant was introduced into the LTQ-FT using the standard electrospray ionization source for the instrument with a spray voltage of 5 kV and a capillary temperature of 275°C. Autogain control was used and set at 500,000 with a maximum injection time of 1250 ms for FT-ICR full scans. Collision-induced dissociation, MS/MS, was executed in the linear trap with an autogain control setting of 10,000 and a maximum injection time of 500 ms. FT-ICR full scans were acquired in the positive ion mode at 100,000 resolving power at m/z 400. Mass accuracy errors for salbutamol and its nitrated analogs were below 250 ppb. The positive-ion MS/MS experiments were performed simultaneously in the linear trap portion of the instrument using helium as a collision gas, isolation widths of 2 atomic mass units, normalized collision energies of 35 to 40, a q value of 0.250, and an excitation time of 30 ms.
Samples for MS experiments were prepared using ∼50 μM salbutamol, 5.9 mM H2O2, 5.8 mM NaNO2, and 0.25 μM LPO (or 0.19 U/ml MPO). The reaction was allowed to proceed until A410 ceased to increase. Before the MS analysis, proteins were removed by using a Centricon centrifugal filter device with a 10-kDa cut-off filter (Millipore Corporation, Billerica, MA). Experiments were also performed on samples prepared using lower concentrations of reactants, 20 μM salbutamol, 200 μM NaNO2, and 200 μM H2O2, MPO, or EPO (0.1 U/ml). All of these systems produced similar results. The newly detected salbutamol nitration products are 6-nitrosalbutamol (2) 4-[2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxymethyl)-6-nitrophenol and salbutamol-derived nitrophenol (3) 4-[2-(tert-butylamino)-1-hydroxyethyl]-2-nitrophenol.
Efficacy of Salbutamol Conversion.
Salbutamol (10 μM) was exposed to MPO (0.1 U/ml), NaNO2 (100 μM), and H2O2 (100 μM) in phosphate buffer (0.1 M, pH 7.0). After 3-h reaction, when no further changes in the absorbance at 410 nm were observed, the sample was analyzed by a Q-TOF2 mass spectrometer (Micromass, Manchester, UK). Before the analysis, equal volumes of the sample were mixed with either 50/50 acetonitrile/H2O, 0.1% formic acid, or methanol, then each sample was directly infused into the mass spectrometer. The presence and relative amount of unreacted salbutamol was monitored by measuring its mass at m/z 240.16 (M+H+), 260.14 (M+Na+), and their relative amounts based on their ion counts. Five runs were performed for each solvent. Similar analysis was performed on intact salbutamol (10 μM) without performing nitration. A comparison of the salbutamol ion counts in the sample before nitration to the salbutamol ion counts in the same sample after nitration indicated that approximately 98% of the salbutamol was converted in the process.
Extraction of Products from EBC.
Salbutamol and derived products were extracted from the exhaled breath condensate using an Oasis MCX 1cc extraction cartridge (Waters). The cartridge was first washed with 1 ml of methanol followed by 1 ml of water. The sample was acidified with formic acid to pH ∼ 3, loaded, and washed with 1 ml of formic acid (2% in water) and vacuum-dried. Neutral substances were eluted with 0.25 ml of methanol and then bases (salbutamol and related products) were extracted with 0.25 ml of ammonia (5% in methanol) then acidified and diluted with 2% formic acid.
β2-AR Receptor Binding and cAMP Generation.
Receptor affinity was assessed by measuring the ability of native and nitrated salbutamol to displace binding of the β2AR antagonist 125I-CYP as described previously (McGraw and Liggett, 1997). Binding assays were carried out with crude membrane preparations from airway smooth muscle cells that transgenically overexpress the human β2-AR (McGraw et al., 1999).
The ability of native and nitrated salbutamol to stimulate cAMP production in airway smooth muscle cells was measured by a fluorescent detection assay using a commercially available kit (Mediomics, St. Louis, MO). For these studies, cells were grown to near-confluence in 96-well plates. After washing with phosphate-buffered saline, cells were treated for 15 min with either phosphate-buffered saline vehicle or the indicated concentration of salbutamol. The media were then removed, and the reaction was halted by using the supplied stop buffer, after which the resultant sample was processed for fluorescent detection per the manufacturer's instructions. The cAMP content for each sample was determined by extrapolating values to a standard curve prepared with known concentrations of cAMP.
Results
Reaction of Salbutamol with Peroxidase/H2O2/NO2−.
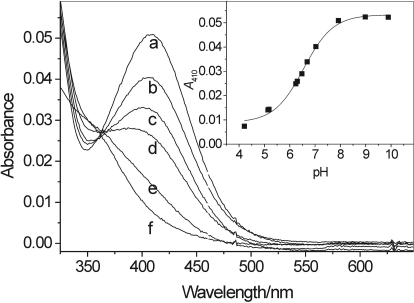
We have reported that in the presence of LPO(MPO)/H2O2 salbutamol undergoes oxidation to a new species, most likely a o,o′-dimer, absorbing in the UV range, approximately 315 nm (Reszka et al., 2009). The reaction was, however, slow, presumably because of the inability of the drug to react efficiently with compound II of the peroxidases. In preliminary studies, we found that NO2− substantially facilitates the peroxidative reaction and affords a different metabolite, clearly distinguishable by its absorption spectrum. Figure 2A shows spectra observed during interaction of salbutamol with LPO and H2O2 in the presence of NaNO2. The concentration of LPO used is in the range found in the lavage fluid of human subjects, 3.2 nM (0.25 ng/μl) (El-Chemaly et al., 2003). During the reaction, the color of the sample changed to yellow, and the new species formed exhibited absorption maxima at 410 and 314 nm. The inset in Fig. 2A shows the time course of the reaction measured at these two wavelengths. The yellow color and the absorption maximum at 410 nm suggest formation of a nitrated phenol with the nitro group in position ortho with respect to the phenolic –OH group. These features are similar to those described for 3-nitrotyrosine, in which the phenolic -OH and -NO2 groups are ortho to each other (Sokolovsky et al., 1966; Monzani et al., 2004). Because there is only one unoccupied ortho position in salbutamol, the new product absorbing at 410 nm was tentatively attributed to 6-nitrosalbutamol (Fig. 1, compound 2), although related salbutamol-derived nitrated phenol and other oxidation products may also be formed. No characteristic absorption at 410 nm was observed when either LPO or NO2− or H2O2 was omitted, confirming that nitration of salbutamol is an enzymatic process totally dependent on the simultaneous presence of the drug, NO2−, LPO, and H2O2.
Fig. 2.
Interaction of salbutamol with LPO/H2O2/NaNO2 (A and B) and MPO/H2O2/NaNO2 (C and D) systems. A and C, absorption spectra recorded during interaction of salbutamol (20 μM), with H2O2 (310 μM), NaNO2 (300 μM), and LPO (4 nM) (A) or MPO (0.2 U/ml) (C). Spectra were recorded every 30 s. Arrows indicate direction of changes. Absorption peaks at 350 and 276 nm in spectrum a (before H2O2 addition) in A and C are from NO2− and salbutamol, respectively. Insets show time course of absorbance changes at 410 and 314 nm after bolus addition of H2O2. B, nitration of salbutamol (100 μM) measured at 410 nm versus [H2O2] (●) in the presence of NaNO2 (250 μM) and LPO (6.5 nM), and versus [NaNO2] (■) in the presence of H2O2 (104 μM) and LPO (10 nM). D, nitration of salbutamol (10 μM) measured at 410 nm versus [H2O2] (●) in the presence of NaNO2 (1000 μM) and versus [NaNO2] (■) in the presence of H2O2 (220 μM) and MPO (0.1 U/ml). All experiments were carried out in phosphate buffer (0.1 M, pH 7.0) containing DTPA (0.2 mM).
The molar absorptivity of the reaction mixture at 410 nm derived from salbutamol was estimated to be 4550 ± 227 M−1 · cm−1 (n = 14) at pH 7.0. This value is not far from the molar absorptivity reported for 3-nitrotyrosine of 4000 M−1 · cm−1 at 422 nm at pH 7.5 (Monzani et al., 2004) and 4100 M−1 · cm−1 at 428 nm at pH 8 (Sokolovsky et al., 1966; Riordan et al., 1967). It is also close to molar absorptivities determined for a number of ortho-nitrophenols in alkaline solutions (Rapoport et al., 1961).
The dependence of salbutamol nitration on [H2O2] was investigated measuring ΔA410 at constant [NaNO2] of 250 μM. When the concentration of H2O2 was varied from 0 to 1000 μM, ΔA410 initially increased reaching maximum at near 250 μM H2O2, and then decreased (Fig. 2B). This decrease in efficacy at higher concentrations of the peroxide was probably caused by inactivation of the enzyme by excess H2O2 because A410 increased to near the maximal value when additional doses of LPO were applied (data not shown). When the concentration of NaNO2 was varied from 0 to 250 μM at constant H2O2 concentration (250 μM), ΔA410 monotonously increased, reaching a maximum value approximately 250 μM NaNO2 (Fig. 2B). At higher concentrations of NaNO2, ΔA410 started to plateau, suggesting that nearly all of the H2O2 was consumed under these conditions.
Because either MPO or EPO may be present in the airways of patients with asthma (Andreadis et al., 2003), we next examined whether these peroxidases also support nitration of salbutamol. Figure 2C shows spectra observed during interaction of salbutamol with MPO/H2O2/NO2−. It may be appreciated that these spectra are similar to those generated by the LPO/H2O2/NO2− system (Fig. 2A), suggesting formation of the same products. The inset in Fig. 2C shows kinetic traces recorded at 410 and 314 nm. As with LPO, the efficacy of salbutamol nitration by MPO depends on [H2O2] and [NaNO2] as shown in Fig. 2D. Similar observations were made when MPO was replaced with EPO (not shown), confirming that all three peroxidases can support nitration and oxidation of salbutamol.
We further found that the absorption spectrum of nitrated products derived from salbutamol depended on pH (Fig. 3), most likely caused by changes in protonation of the compounds' phenolic –OH group. The intensity of the peak at 410 nm increased when the pH was raised from 4 to 8 and remained essentially unchanged above pH 8. Based on the dependence of A410 on pH (Fig. 3, inset), the apparent pKa of the phenolic –OH in the nitrated compound was determined to be 6.6. This value is in agreement with pKa of 6.8 reported for 3-nitrotyrosine (Riordan et al., 1967) and is close to pKa values of other ortho-nitrophenols (Rapoport et al., 1961). All of these observations further support the conclusion that the product absorbing at 410 nm can be linked to 6-nitrosalbutamol (2) and/or a related ortho-nitrophenol.
Fig. 3.
Absorption spectra of nitrosalbutamol measured at pH 7.9, 7.0, 6.7, 6.3, 5.2, and 4.2 (spectra a-f, respectively). Inset, A410 plotted versus pH. The apparent pKa of 6.6 was determined for the phenolic –OH group in nitrated salbutamol-derived products.
Efficacy of Salbutamol Conversion.
The efficacy of the enzymatic conversion of salbutamol was determined by analysis of the sample by Q-TOF2 mass spectrometer. Salbutamol (10 μM) was exposed to MPO (0.1 U/ml), NaNO2 (100 μM), and H2O2 (100 μM) in phosphate buffer (0.1 M, pH 7.0), and the reaction was continued until A410 reached maximum. A comparison of the salbutamol ion counts in the sample before nitration to the salbutamol ion counts in the same sample after nitration indicated that approximately 98% of the salbutamol was converted in the process.
To relate these observations to more physiological conditions we compared the efficacy of the reaction (measured as a change in ΔA410) carried out at 20°C with that performed at 37°C using MPO of low (0.2 U/ml) and high (1 U/ml) activity. The higher MPO activity is to mimic the severe inflammation during acute asthma attack. Concentrations of other reactants were kept low, salbutamol 5 μM, H2O2 45 μM, NaNO2 50 μM, in pH 7.0 phosphate buffer. When the experiment was performed at 37°C and with MPO of 1 U/ml the maximum level of nitro-salbutamol was attained in 5 min (ΔA410 = 0.026; n = 2). In contrast, at 20°C and with MPO of 0.2 U/ml even 60 min was not enough to reach the same level (n = 2). Thus, under more physiologic conditions the reaction is rapid, which suggests that it may play a role in rapid inactivation of the agonist during a severe asthma attack.
Mass Spectrometry.
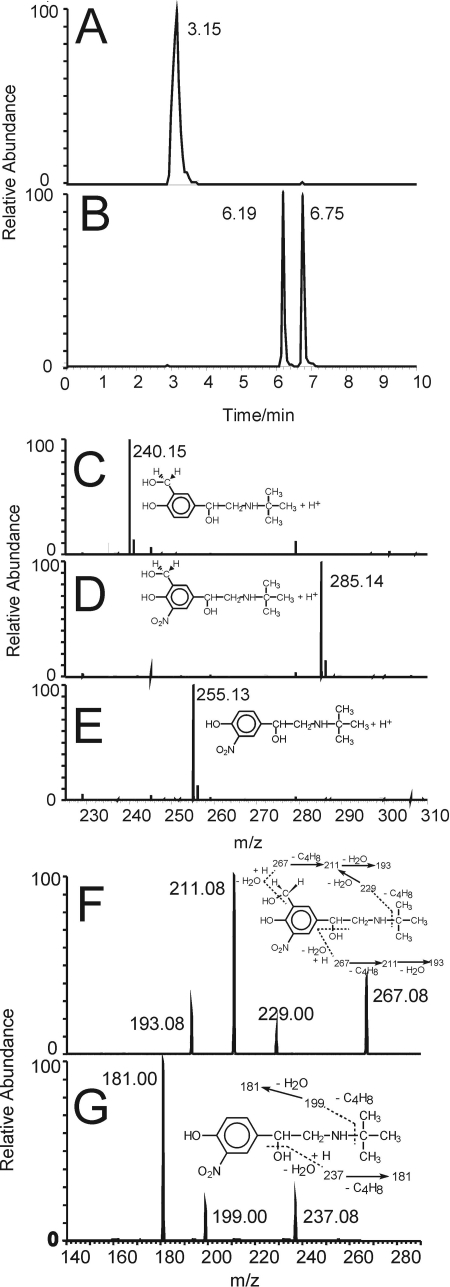
Samples of native salbutamol and salbutamol treated with LPO(MPO, EPO)/H2O2/NO2− in pH 7.0 phosphate buffer containing DTPA were analyzed by HPLC/mass spectrometry. Figure 4A shows HPLC elution profile of intact salbutamol (retention time 3.15 min), and Fig. 4C shows its mass spectrum (m/z 240.15947; error 207 ppb). Two mono-nitrated products were detected in samples of salbutamol treated with LPO(MPO)/H2O2/NO2− with retention times of 6.19 and 6.75 min (Fig. 4B). These species were identified as nitrated salbutamol, 2 (m/z 285.14456, error: 215 ppb; Fig. 4D) and a salbutamol-derived nitrophenol 3 (m/z 255.13397, error: 142 ppb; Fig. 4E), consistent with the elemental composition of their protonated ions C13H21N2O5+ and C12H19N2O4+, respectively. Structures of these metabolites are shown in Fig. 1. To further confirm identity of these ions, the MS/MS spectra of ions 2 and 3 were acquired (Fig. 4, F and G). The fragmentation pattern of the parent ion 2 indicates elimination of a water molecule (m/z 267.08), the isobutene fragment (C4H8) (m/z 229.00), loss of both C4H8 and H2O (m/z 211.08), and another water molecule (m/z 193.08) (Fig. 4F). The fragmentation pattern of the parent ion 3 indicates elimination of water molecule (m/z 237.08), the isobutene fragment (C4H8) m/z 199.00, and the loss of both C4H8 and H2O (m/z 181.00) (Fig. 4G). These data further support the assignment of the parent ions to nitrated phenolics 2 and 3, respectively. MS spectra of salbutamol treated with MPO and EPO nitrating systems showed formation of the same nitrated species (not shown).
Fig. 4.
A and B, HPLC elution profiles of salbutamol (1, retention time 3.15 min) (A) and two mono-nitrated metabolites of salbutamol (2, retention time 6.19 and 3, retention time 6.75 min) (B). Nitration of salbutamol was carried out using a LPO/H2O2/NO2− nitration system in phosphate buffer, pH 7.0, containing DTPA (0.1 mM). C-E, positive ion electrospray ionization mass spectra of salbutamol 1 (m/z 240.15947, error: 207 ppb, eluted at 3.15 min) (C), nitrosalbutamol 2 (m/z 285.14456, error: 215 ppb, eluted at 6.19 min) (D), and salbutamol-derived nitrophenol 3 (m/z 255.13397, error: 142 ppb, eluted at 6.75 min) (E). F and G, MS/MS spectra and fragmentation patterns of the molecular ions 2 (m/z 285.14) (F) and 3 (m/z 255.13) (G).
We also considered formation of salbutamol dimers during enzymatic nitration of the drug. However, mass spectrometry investigations did not indicate the presence of these products in our samples, possibly because in the presence of high concentrations of nitrite nitration of phenolics is preferred over their dimerization.
Mass Spectrometry of Exhaled Breath Condensate.
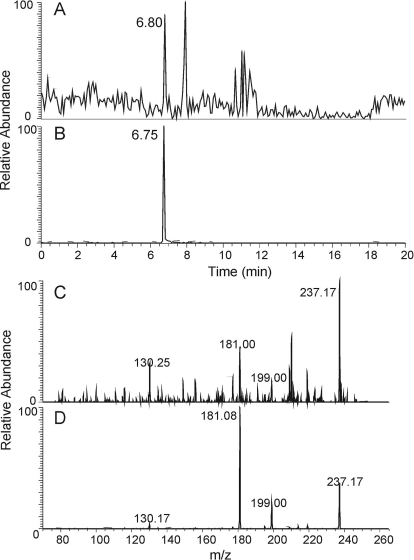
The preceding in vitro experiments offered strong evidence that salbutamol and other β2-agonists are subject to peroxidase-catalyzed nitration. Because both peroxidase activity and nitrite content are elevated in the inflamed airway, we next wanted to determine whether drug nitration could occur in vivo. To examine this possibility, we performed mass spectrometric analysis of exhaled breath condensates from asthmatic patients who were being treated with salbutamol for an asthma exacerbation. Preliminary mass spectroscopy analyses were suggestive but not definitive for the presence of nitrated species similar to those observed in the in vitro reactions caused by high signal-to-noise ratio. Therefore to increase the concentration of potential nitrated drug metabolites, we pooled condensates from three different subjects and analyzed it as a single concentrated sample. Figure 5A shows the HPLC elution profile of the EBC sample with a peak at 6.80 min, which coincides with the HPLC peak (6.75 min) from salbutamol-derived nitrophenol 3, m/z 255.13 (Fig. 5B). The MS/MS spectrum of the EBC fraction eluting at 6.8 min (Fig. 5C) shows product ions of m/z of 237.17, 199.00, 181.00, and 130.25, which are the same as those from nitrophenol 3 eluting at 6.75 min prepared in vitro (Figs. 4G and 5D), which indicates a similar pattern of fragmentation and the same parent ion. The m/z 181 product ion arising from the loss of isobutene and water was isolated in the linear trap and further fragmented, resulting in a MS3 experiment. MS3 product ions from the parent matched the standard; both gave m/z 136 as the most abundant ion along with strong abundances for m/z 117, 133, 135, and 136 (not shown). Together, these preliminary data (the same retention times, the same parent ion of m/z 255.13, and the same fragments observed in MSn from both samples) strongly suggest that oxidation and nitration of salbutamol can occur in the airways of asthmatics.
Fig. 5.
A and B, HPLC elution profile of molecular ions of m/z 255.13 in breath condensate of asthma patients treated with salbutamol (A; retention time 6.80 min) and from a salbutamol nitrated in vitro (with MPO(LPO)/H2O2/NO2−) (B; retention time 6.75 min). C and D, MS/MS spectra of the parent ion of m/z 255.13 in breath condensate (C) and from compound 3 produced by nitration of salbutamol by LPO/H2O2/NO2− in vitro (D). Peaks at m/z 237.17, 199.00, 181.08, and 130.25, originating from the parent ion of m/z 255.13 in D, coincide with peaks in C.
Effect of Ascorbate and Thiocyanate on Nitration of Salbutamol.
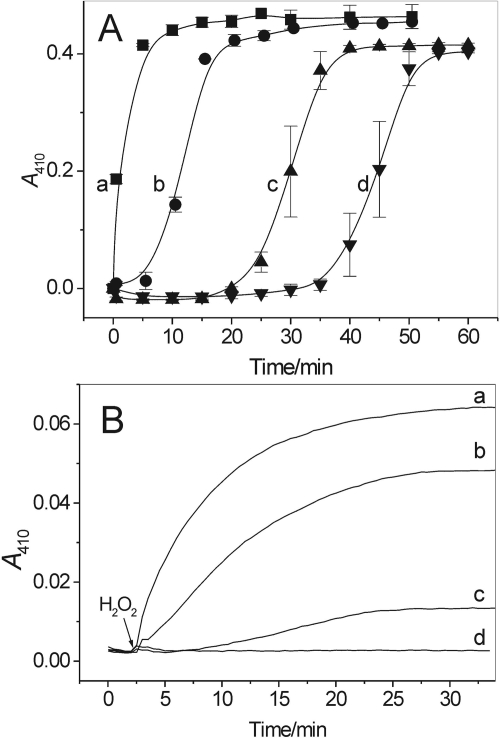
AA and sodium thiocyanate (NaSCN) are natural constituents of airway lining fluids with concentrations near 100 μM and 20 to 120 μM, respectively (Tenovuo and Mäkinen, 1976; Cross et al., 1994). Figure 6A shows that AA in a concentration-dependent manner delayed the appearance of the peak at 410 nm, suggesting that nitration of salbutamol by LPO/H2O2/NO2− is also delayed. However, the resumption of the reaction after a lag period suggests that the inhibitory action of AA is not caused by inhibition/inactivation of the enzyme, but rather by its interaction with the drug-derived phenoxyl radicals (Reszka et al., 2009) and/or with NO2• radicals (Reszka et al., 1999). Thus, although AA at physiological concentrations only transiently inhibits nitration of the drug, supplementation of the system with AA at higher than physiological concentrations, e.g., ∼ 1 mM, completely prevented nitration of salbutamol (not shown).
Fig. 6.
Nitration of salbutamol by LPO/H2O2/NO2−: effect of ascorbate (A) and thiocyanate (B). A, ascorbate in a concentration-dependent manner causes a lag in net nitration of the drug. Shown are A410 versus time traces recorded during interaction of salbutamol (100 μM), with H2O2 (4 mM), NaNO2 (3.5 mM), and LPO (67 nM) in the absence and presence of 55, 88, and 109 μM ascorbate (traces a-d, respectively). B, thiocyanate inhibits nitration of salbutamol (20 μM) by LPO (10 nM), H2O2 (312 μM), and NaNO2 (300 μM). Shown are A410 versus time traces recorded in the absence (trace a) and presence of 10, 25, and 50 μM NaSCN (traces b-d, respectively). n = 2.
Figure 6B shows the effects of NaSCN on nitration of the agonist by LPO/H2O2/NaNO2. On increasing the concentration of NaSCN from 0 to 50 μM there is a gradual inhibition of the reaction. However, NaSCN at 50 μM completely prevented nitration of the drug (Fig. 6, trace d). This inhibitory action is most likely caused by preferential oxidation of SCN− by the LPO/H2O2 system and rapid depletion of H2O2.
Effect of Methimazole and Dapsone on Nitration of Salbutamol.
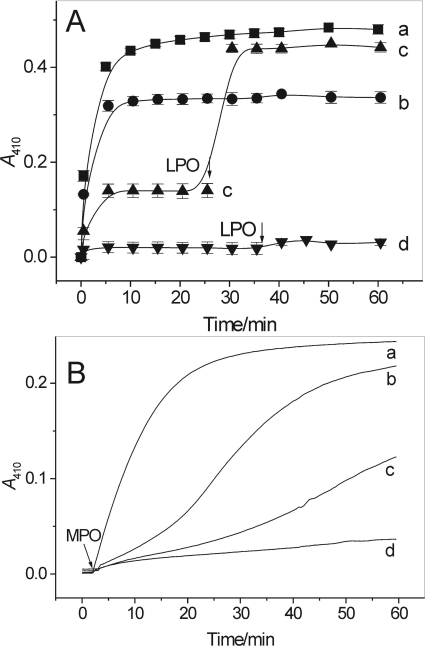
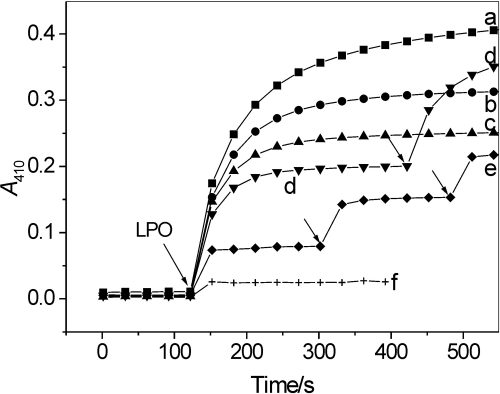
Methimazole, an antithyroid drug, and dapsone, an anti-inflammatory and antileprotic agent, are potent inhibitors of LPO and MPO (McGirr et al., 1990; Kettle and Winterbourn, 1991; Bozeman et al., 1992). In agreement with this, both compounds in a concentration-dependent manner inhibited nitration of salbutamol by LPO/H2O2/NO2− (Figs. 7A and 8). Methimazole at a concentration close to 100 μM completely stopped the reaction (Fig. 7A, trace d). Dapsone at the concentration of 50 μM inhibited LPO/H2O2/NO2− nitration of salbutamol by approximately 86% (Fig. 8). However, in contrast to AA or NaSCN, the methimazole- and dapsone-dependent inhibition was permanent, presumably caused by inactivation of the enzyme, because the reaction resumed when LPO was readded (Fig. 7A, trace c, and Fig. 8, traces d and e).
Fig. 7.
Inhibition by methimazole of salbutamol nitration by LPO/H2O2/NO2− (A) and MPO/H2O2/NO2− (B) systems measured as a change in absorbance at 410 nm. A, salbutamol (100 μM) reacted with LPO (73 nM), NaNO2 (3.5 mM), and H2O2 (4 mM) in pH 7.0 buffer in the absence (trace a) and presence of 27, 55, and 109 μM methimazole (traces b-d, respectively). In traces c and d additional doses of LPO were added at the time indicated by arrows. n = 2. B, salbutamol (50 μM) reacted with MPO (0.2 U/ml), NaNO2 (5 mM), and H2O2 (2.1 mM) in pH 7.0 buffer in the absence (trace a) and presence of 25, 50, and 100 μM methimazole (traces b-d,). Shown are typical kinetic traces.
Fig. 8.
Inhibition by dapsone of salbutamol nitration by LPO/H2O2/NO2− measured as a change in absorbance at 410 nm. Salbutamol (97 μM) reacted with LPO (65 nM), NaNO2 (3.4 mM), and H2O2 (4 mM) in phosphate buffer, pH 7.0, in the absence (trace a) and presence of 2.5, 5, 10, 50, and 100 μM dapsone (traces b-f, respectively). In traces d and e additional doses of LPO were added at the time indicated by arrows. n = 2.
The effects of methimazole on nitration catalyzed by MPO and EPO were somewhat different. In contrast to its apparent irreversible inhibition of LPO, the inhibitory action of methimazole on MPO was transitory. The A410 versus time traces recorded at various concentrations of methimazole are shown in Fig. 7B. The S shape of these traces indicates that methimazole affects the process only at its initial stage, presumably because it is a good MPO substrate and competes with other reactants for the active site of the enzyme and/or the nitrite-derived oxidants. At low concentrations, however, methimazole is rapidly consumed, after which the nitration process is resumed as suggested by the increased rate of the reaction. A similar effect of methimazole was observed when MPO was replaced by EPO (not shown).
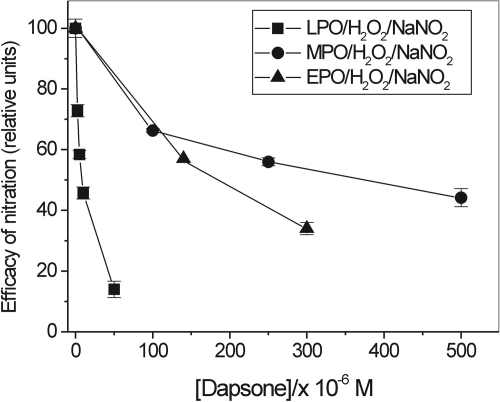
Figure 9 illustrates the effects of dapsone on nitration carried out by LPO, MPO, and EPO systems. Dapsone seems to be a potent blocker of nitration catalyzed by LPO even at high concentrations of NaNO2 and H2O2 used in these experiments. Its inhibitory effect on nitration catalyzed by EPO was stronger than on nitration supported by MPO (Fig. 9), in agreement with a prior report that EPO is more sensitive to dapsone than is MPO (Bozeman et al., 1992; Thomas et al., 1994).
Fig. 9.
Inhibition by dapsone of salbutamol nitration by peroxidase/H2O2/NO2− systems. The extent of nitration is expressed as a change in ΔA410 versus control (dapsone omitted) taken as 100% after 10, 30, and 60 min reaction for LPO, MPO, and EPO nitrating systems, respectively. LPO system: [Salbutamol] = 100 μM, [NaNO2] = 3.4 mM, [H2O2] = 4 mM, [LPO] = 65 nM. MPO system: [Salbutamol] = 50 μM, [NaNO2] = 5 mM, [H2O2] = 5 mM, [MPO] = 0.2 U/ml. EPO system: [Salbutamol] = 50 μM, [NaNO2] = 5 mM, [H2O2] = 2 mM, [EPO] = 0.075 U/ml. Reactions were carried out in phosphate buffer, pH 7.0, containing dimethyl sulfoxide (5% v/v) (n = 2).
Effect of Salbutamol Transformation on β2AR Binding and cAMP Generation.
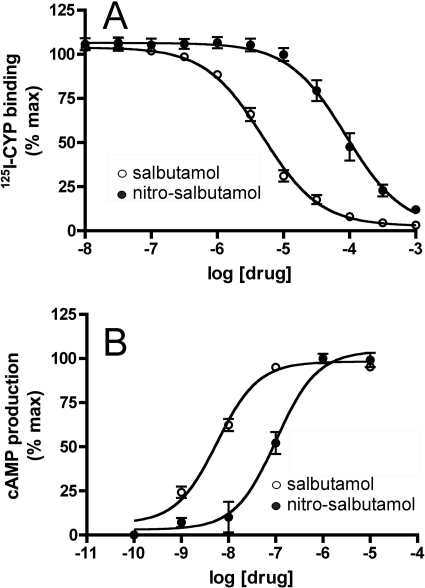
The ability of β2-agonists such as salbutamol to stimulate receptor signaling depends on their ability to bind to the receptor and induce conformational changes in β2AR structure that facilitate activation of its associated G proteins. Addition of a nitrite moiety to a β2-agonist such as salbutamol could potentially impair agonist-mediated signal transduction by altering the agonist's ability to bind to the β2AR, or alternatively, the nitrated agonist might bind the receptor but fail to induce the conformational changes in receptor structure necessary for signal transduction. Therefore, to determine whether the affinity of nitrated salbutamol for the β2AR was different from that of the native drug, we carried out competition binding assays using primary airway smooth muscle cells that were derived from transgenic mice overexpressing the human β2AR (McGraw et al., 1999). As shown in Fig. 10A, salbutamol transformed by LPO/H2O2/NO2− displaced binding of the nonselective β2AR antagonist 125I-CYP to airway smooth muscle cell membranes by nearly 1.7 orders of magnitude less than that of native salbutamol, indicating that nitration of the parent compound markedly reduced its capacity to bind the β2AR.
Fig. 10.
A, 125I-CYP displacement from β2-receptors by intact (○) and nitrated (●) salbutamol. B, cAMP production by smooth muscle cells stimulated by intact (○) and nitrated (●) salbutamol.
To establish whether the reduction in receptor affinity was associated with decreased signal transduction, we compared the ability of native salbutamol and the nitrated reaction products to stimulate cAMP synthesis in murine airway smooth muscle cells. Consistent with the reduction in binding affinity, we found that the ability of the nitrated salbutamol product to stimulate cAMP was more than two orders of magnitude less than that of the parent compound (Fig. 10B), with EC50 1.02 ± 0.05 × 10−7 M [confidence interval (0.588–1.77) × 10−7 M] versus 5.74 ± 1.20 × 10−9 M [confidence interval (3.86–8.52) × 10−9 M], respectively (p < 0.01, n = 3).
Discussion
Salbutamol is a short-acting β2-adrenergic agonist frequently used to relieve acute airway bronchospasm. However, its therapeutic effectiveness may be decreased in severe exacerbations of the disease. The molecular mechanisms responsible for this decreased effectiveness are poorly understood. One feature of severe asthma exacerbations is marked airway inflammation characterized by an influx of cells with significant peroxidase content and activity (i.e., EOS and PMN). The in vitro studies presented here show that, in the presence of NO2−, salbutamol can undergo oxidation and nitration in reactions catalyzed by airway peroxidases. These findings raise the possibility that a similar process may occur in severe asthma exacerbations, which are characterized by increased airway peroxidase activity and elevated content of nitrogen oxides. Preliminary results indicating the presence of the oxidatively modified drug (compound 3) in breath condensate samples from asthmatic patients undergoing salbutamol therapy strongly support this hypothesis.
In vitro-generated nitrated salbutamol shows both substantially diminished affinity for the β2-AR and decreased ability to stimulate cAMP production in airway smooth muscle cells. Because the therapeutic activity of β2-agonists depends on molecular interactions between the agonist and amino acid residues that induce or stabilize an “active” receptor conformation, the resultant change in agonist structure caused by the introduction of the highly electrophilic nitro group to the aromatic ring could play a role in the agonist's pharmacologic activity. Given the resultant reduction in β2-AR activation observed in airway smooth muscle cells, the occurrence of salbutamol nitration in vivo could have important clinical implications for therapeutic efficacy of β2-agonists in severe asthma exacerbations. However, our study also demonstrates that it may be possible to prevent the peroxidase- and nitrite-dependent inactivation of the drug by using antioxidants or peroxidase inhibitors. Should nitration of the β2-agonist be responsible for its diminished physiological responsiveness, these approaches might be useful in maintaining the therapeutic activity of the drug in some clinical settings.
Mechanistic Considerations.
Salbutamol is a phenolic compound (Fig. 1) and, therefore, similar to tyrosine and other phenolics, may be subject to the oxidative metabolism and nitration when exposed to peroxidases and NO2−. The process probably involves formation of free radical intermediates, the corresponding phenoxyl radical (PhO•) from salbutamol and the NO2• radical from NO2−. Both species can be formed enzymatically by LPO, MPO, and EPO systems (Burner et al., 2000; Brück et al., 2001; Brennan et al., 2002; Monzani et al., 2004; Van Dalen et al., 2006; Reszka et al., 2009). At concentrations of NO2− markedly higher than the concentration of salbutamol, oxidation of NO2− to NO2• may prevail, and oxidation of salbutamol may be accomplished by its reaction with NO2•. In either case, formation of a nitrated phenol could then be accomplished via radical-radical recombination: PhO• + NO2• → PhOH-NO2.
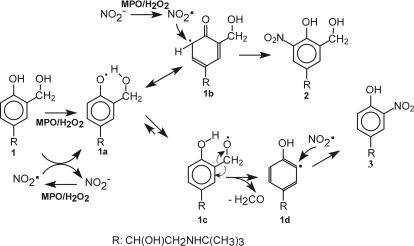
The scheme in Fig. 11 illustrates the proposed pathway for the oxidation and nitration of salbutamol using MPO as a typical peroxidase. It is suggested that MPO/H2O2 concomitantly oxidizes 1 to the corresponding phenoxyl radical (1a) and oxidizes NO2− to NO2•. 1a rearranges to 1b (only one of several resonance forms is shown), which reacts with NO2• according to eq. 1, yielding 2.
Fig. 11.
Proposed mechanisms of the enzymatic nitration of the aromatic moiety of salbutamol. Salbutamol (1) is oxidized to the phenoxyl-type radical (1a), which rearranges to a resonance structure 1b (only one of several resonance forms is shown), in which the unpaired electron is localized in the aromatic ring. The concomitantly generated NO2• radicals add to 1b to form nitrated salbutamol, which rearranges to 2 (m/z 285.14). In an alternative pathway, the radical 1a undergoes intramolecular hydrogen atom transfer from the –CH2OH moiety at C3 to form a methoxyl-type radical 1c, which after elimination formaldehyde (H2CO), produces the aromatic radical 1d, which reacts with NO2• to form the nitrophenol 3 (m/z = 255.13). In general, the mechanisms of nitration catalyzed by EPO and LPO systems may be similar to that shown in the scheme for MPO. R = CH(OH)CH2NHC(CH3)3.
The species, which eluted at 6.75 min and produced a molecular ion of m/z 255.13 (Fig. 4E), was assigned to a mono-nitrated salbutamol derivative 3 (Fig. 1). This assignment is consistent with elemental analysis (C12H19N2O4+, exact mass 255.13397) and further supported by MS/MS spectrum obtained from this molecular ion (Fig. 4G). Formation of this nitrophenol was unexpected as it required the side chain at C3 of the aromatic ring, –CH2OH, to be eliminated. We verified that the putative phenolic precursor of 3, a salbutamol analog, in which the –CH2OH moiety was replaced with –H, (m/z 210), was not present as an impurity in salbutamol samples. This indicates that the corresponding radical (1d in Fig. 11) had to be produced in situ, during enzymatic oxidation of salbutamol itself. In the proposed mechanism (Fig. 11), the initially generated phenoxyl radical, 1a, undergoes intramolecular hydrogen atom transfer from the adjacent –CH2OH moiety to generate the aryl-methoxyl-type radical 1c. After formaldehyde elimination, the remaining aromatic radical 1d adds NO2• to form 3. It is noteworthy that results of our analyses of a sample prepared from pooled asthmatic EBCs suggests that 3 could be generated in the asthmatic airway (Fig. 5). It is noteworthy that if this transformation does indeed occur, it should be a matter of concern because of the cytotoxic and carcinogenic nature of formaldehyde. Further studies are needed to confirm unequivocally the oxidative degradation of salbutamol and release of formaldehyde in vivo.
Nitration supported by EPO most likely proceeds via a similar mechanism. We note that nitration catalyzed by LPO may differ from that driven by MPO or EPO, because, in contrast to these two peroxidases, the compound I of LPO probably does not oxidize NO2− to NO2• but converts NO2− directly to nitrate (NO3−) in one step (Brück et al., 2001). The NO2• radical presumably is generated by oxidation of NO2− by LPO compound II (Brück et al., 2001), the latter could be produced by the reduction of LPO compound I by salbutamol. We have previously reported that salbutamol undergoes oxidation by LPO/H2O2 alone, but the process is not very efficient, because only a small portion of the drug was oxidized (Reszka et al., 2009). It was concluded that this was caused by the slow interaction of the drug with LPO compound II, which accumulates in the system. In this situation, addition of NO2−, which readily reacts with LPO compound II and reduces it to native LPO, accelerates the enzyme turnover and generates NO2•. Thus, all enzymes studied generate species required for nitration of the drug.
The absorption spectrum of nitrated salbutamol depends on pH with the pKa (phenol) of 6.6. This is significantly less than the pKa (phenol) of 9.4 reported for salbutamol itself (Croes et al., 1995). For comparison the corresponding pKa values for nitrotyrosine and tyrosine are 6.8 and 10.2, respectively (Riordan et al., 1967; Sokolovsky et al., 1967). Thus, the presence of the electron-withdrawing nitro group renders the phenolic –OH group in 2 and 3 almost 1000-fold more acidic than in salbutamol. Thus, at physiological pH, nitrosalbutamol will be present mostly with its phenolic –OH group ionized. Therefore, if interaction of salbutamol with its β2-AR involves hydrogen bonding, the nitrated agonist will be unable to participate in this type of interaction. This may be another possible reason the affinity of the nitrated agonist for the β2-ARs decreased (Fig. 10A) in addition to its structural incompatibility to the receptor.
We found that AA and NaSCN at physiological concentrations inhibit nitration of salbutamol. However, the effect of AA was transient because of its rapid consumption. It is expected that in inflamed airways concentrations of these compounds will be significantly decreased, even in the absence of the drug, so their interference with nitration reactions should be minimal. However, one can speculate that administration of ascorbate could be a potential mean to control or even prevent the oxidative inactivation of the drug in vivo.
Pharmacological agents methimazole and dapsone were also effective in inhibiting the reaction. Methimazole, a suicidal inhibitor of thyroid peroxidase and LPO (McGirr et al., 1990), is used in the treatment of hyperthyroid conditions. Salbutamol nitration was also inhibited by dapsone, an anti-inflammatory and antileprotic drug that has been reported to have corticosteroid-sparing activity in severe asthma (Dewey et al., 2002). Although both drugs displayed inhibitory activity, there were differences between methimazole- and dapsone-dependent inhibition of nitration that depended on the enzymatic systems used. Both of these agents efficiently and permanently inhibited nitration catalyzed by LPO, presumably by poisoning the enzyme. However, in contrast to LPO, the effect of dapsone on the reaction catalyzed by MPO and EPO was less pronounced, even at high concentrations of the inhibitor (Fig. 9). Nitration by EPO systems seemed to be more sensitive to dapsone than nitration catalyzed by MPO. This is in agreement with prior reports showing that dapsone affected EPO to a larger extent than MPO (Bozeman et al., 1992).
We emphasize that there are multiple targets in the airways, which may undergo enzymatic modification during severe exacerbation of the disease. This includes tyrosine residues in proteins (Gaston et al., 1994; MacPherson et al., 2001; Ramesh et al., 2001; Andreadis et al., 2003) and components of β2-AR itself, such as cysteine and tyrosine present in the extracellular domains of the receptor (Lewis et al., 2005). In addition, ascorbic acid and glutathione, the natural antioxidants present in the airway lining fluid undergo rapid depletion, which may facilitate oxidative injury. Although the present study focused on the enzymatic transformation of salbutamol, the clinically most important bronchodilator, as an additional factor that potentially may play a role in the therapeutic control of asthma, the presented data do not preclude the possibility that the diminished bronchorelaxation activity of the agonist may also be caused by oxidative modification of these other targets.
In summary, we have demonstrated that airway peroxidases catalyze nitration of the β2-agonist salbutamol. The transformed salbutamol binds to β2-AR on smooth muscle cells with diminished affinity and reduced capacity to stimulate cAMP synthesis. If nitration of the agonist is responsible for its diminished bronchorelaxation effects during a severe asthma attack, results of this study suggest that antioxidants or peroxidase inhibitors may be useful in maintaining the drug's therapeutic activity. Results of our preliminary studies show that related phenolic β2-agonists (fenoterol, formoterol, salmeterol, and terbutaline) undergo similar oxidation and nitration reactions. Further studies are needed to verify formation of nitrated salbutamol and related β2-agonists in the airways of asthmatic patients and their role in bronchodilator resistance and treatment failure.
This study was partially supported by a Veteran Administration Merit Review Grant and a Research Initiative Project, the Cystic Fibrosis Foundation, and the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL092121].
The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
Portions of this work were presented previously: Reszka KJ, McGraw D, and Britigan B (2009) Airway peroxidases can catalyze inactivating oxidation/nitration of β-agonists, at the American Thoracic Society International Conference; 2009 May 15–20; San Diego, CA. American Thoracic Society, New York, NY.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.170027.
- β2-AR
- β2-adrenergic receptor
- AA
- ascorbic acid
- EBC
- exhaled breath condensate
- EPO
- eosinophil peroxidase
- LPO
- lactoperoxidase
- MPO
- myeloperoxidase
- NaSCN
- sodium thiocyanate
- EOS
- eosinophil
- PMN
- neutrophil
- DTPA
- diethylenetriamine pentaacetic acid
- MS/MS
- tandem mass spectrometry
- FT
- Fourier transform
- ICR
- ion cyclotron resonance
- CYP
- cyanopindolol
- HPLC
- high-performance liquid chromatography
- ppb
- parts per billion.
Authorship Contributions
Participated in research design: Reszka, Sallans, Macha, McGraw, Butsch Kovacic, and Britigan.
Conducted experiments: Reszka, Sallans, Macha, and Brown.
Contributed new reagents or analytic tools: Butsch Kovacic.
Performed data analysis: Reszka, Sallans, Macha, McGraw, Butsch Kovacic, and Britigan.
Wrote or contributed to the writing of the manuscript: Reszka, Sallans, Macha, McGraw, Butsch Kovacic, and Britigan.
Other: Reszka, McGraw, and Britigan acquired funding for the research.
References
- Abramson MJ, Walters J, Walters EH. (2003) Adverse effects of β-agonists: are they clinically relevant? Am J Respir Med 2:287–297 [DOI] [PubMed] [Google Scholar]
- Aldridge RE, Chan T, van Dalen CJ, Senthilmohan R, Winn M, Venge P, Town GI, Kettle AJ. (2002) Eosinophil peroxidase produces hypobromous acid in the airways of stable asthmatics. Free Radic Biol Med 33:847–856 [DOI] [PubMed] [Google Scholar]
- Andreadis AA, Hazen SL, Comhair SA, Erzurum SC. (2003) Oxidative and nitrosative events in asthma. Free Radic Biol Med 35:213–225 [DOI] [PubMed] [Google Scholar]
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. (2000) Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 161:1720–1745 [DOI] [PubMed] [Google Scholar]
- Bozeman PM, Learn DB, Thomas EL. (1992) Inhibition of the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase by dapsone. Biochem Pharmacol 44:553–563 [DOI] [PubMed] [Google Scholar]
- Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, et al. (2002) A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem 277:17415–17427 [DOI] [PubMed] [Google Scholar]
- Brück TB, Fielding RJ, Symons MC, Harvey PJ. (2001) Mechanism of nitrite-stimulated catalysis by lactoperoxidase. Eur J Biochem 268:3214–3222 [DOI] [PubMed] [Google Scholar]
- Burner U, Furtmuller PG, Kettle AJ, Koppenol WH, Obinger C. (2000) Mechanism of reaction of myeloperoxidase with nitrite. J Biol Chem 275:20597–20601 [DOI] [PubMed] [Google Scholar]
- Conner GE, Salathe M, Forteza R. (2002) Lactoperoxidase and hydrogen peroxide metabolism in the airway. Am J Respir Crit Care Med 166:S57–S61 [DOI] [PubMed] [Google Scholar]
- Croes K, McCarthy PT, Flanagan RJ. (1995) HPLC of basic drugs and quaternary ammonium compounds on microparticulate strong cation-exchange materials using methanolic or aqueous methanol eluents containing an ionic modifier. J Chromatogr A 693:289–306 [Google Scholar]
- Cross CE, van der Vliet A, O'Neill CA, Louie S, Halliwell B. (1994) Oxidants, antioxidants, and respiratory tract lining fluids. Environ Health Perspect 102 (Suppl 10):185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey A, Bara A, Dean TP, Walters EH. (2002) Dapsone as an oral corticosteroid sparing agent for asthma. Cochrane Database Syst Rev, doi: 10.1002/14651858.CD003268 [DOI] [PubMed] [Google Scholar]
- El-Chemaly S, Salathe M, Baier S, Conner GE, Forteza R. (2003) Hydrogen peroxide-scavenging properties of normal human airway secretions. Am J Respir Crit Care Med 167:425–430 [DOI] [PubMed] [Google Scholar]
- Gaston B, Drazen JM, Loscalzo J, Stamler JS. (1994) The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med 149:538–551 [DOI] [PubMed] [Google Scholar]
- Jenzer H, Jones W, Kohler H. (1986) On the molecular mechanism of lactoperoxidase-catalyzed H2O2 metabolism and irreversible enzyme inactivation. J Biol Chem 261:15550–15556 [PubMed] [Google Scholar]
- Kanazawa H, Hirata K, Yoshikawa J. (2002) Nitrogen oxides reduce albuterol-induced bronchodilation in patients with bronchial asthma. Respiration 69:490–495 [DOI] [PubMed] [Google Scholar]
- Kettle AJ, Winterbourn CC. (1991) Mechanism of inhibition of myeloperoxidase by anti-inflammatory drugs. Biochem Pharmacol 41:1485–1492 [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Hoque A, Walton TM, Kooy NW. (2005) Potential role of nitration and oxidation reactions in the effects of peroxynitrite on the function of β-adrenoceptor sub-types in the rat. Eur J Pharmacol 518:187–194 [DOI] [PubMed] [Google Scholar]
- MacPherson JC, Comhair SA, Erzurum SC, Klein DF, Lipscomb MF, Kavuru MS, Samoszuk MK, Hazen SL. (2001) Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generation of reactive nitrogen species. J Immunol 166:5763–5772 [DOI] [PubMed] [Google Scholar]
- McGirr LG, Jatoe SD, O'Brien PJ. (1990) Myeloperoxidase catalysed cooxidative metabolism of methimazole: oxidation of glutathione and NADH by free radical intermediates. Chem Biol Interact 73:279–295 [DOI] [PubMed] [Google Scholar]
- McGraw DW, Liggett SB. (1997) Heterogeneity in β-adrenergic receptor kinase expression in the lung accounts for cell-specific desensitization of the β2-adrenergic receptor. J Biol Chem 272:7338–7344 [DOI] [PubMed] [Google Scholar]
- McGraw DW, Forbes SL, Kramer LA, Witte DP, Fortner CN, Paul RJ, Liggett SB. (1999) Transgenic overexpression of β2-adrenergic receptors in airway smooth muscle alters myocyte function and ablates bronchial hyperreactivity. J Biol Chem 274:32241–32247 [DOI] [PubMed] [Google Scholar]
- Monzani E, Roncone R, Galliano M, Koppenol WH, Casella L. (2004) Mechanistic insight into the peroxidase catalyzed nitration of tyrosine derivatives by nitrite and hydrogen peroxide. Eur J Biochem 271:895–906 [DOI] [PubMed] [Google Scholar]
- Nelson DP, Kiesow LA. (1972) Enthalpy of decomposition of hydrogen peroxide by catalase at 25°C (with molar extinction coefficients of H2O2 solutions in the UV). Anal Biochem 49:474–478 [DOI] [PubMed] [Google Scholar]
- Ramesh G, Jindal SK, Ganguly NK, Dhawan V. (2001) Increased nitric oxide production by neutrophils in bronchial asthma. Eur Respir J 17:868–871 [DOI] [PubMed] [Google Scholar]
- Rapoport M, Hancock CK, Meyers EA. (1961) The correlation of the electronic spectra and acidity of 4-substituted 2-nitrophenols with substituent constants. J Am Chem Soc 83:3489–3494 [Google Scholar]
- Reszka KJ, Matuszak Z, Chignell CF. (1997) Lactoperoxidase-catalyzed oxidation of the anticancer agent mitoxantrone by nitrogen dioxide (NO2•) radicals. Chem Res Toxicol 10:1325–1330 [DOI] [PubMed] [Google Scholar]
- Reszka KJ, Matuszak Z, Chignell CF, Dillon J. (1999) Oxidation of biological electron donors and antioxidants by a reactive lactoperoxidase metabolite from nitrite (NO2−): an EPR and spin trapping study. Free Radic Biol Med 26:669–678 [DOI] [PubMed] [Google Scholar]
- Reszka KJ, McGraw DW, Britigan BE. (2009) Peroxidative metabolism of β2-agonists salbutamol and fenoterol and their analogues. Chem Res Toxicol 22:1137–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JF, Sokolovsky M, Vallee BL. (1967) Environmentally sensitive tyrosyl residues. Nitration with tetranitromethane. Biochemistry 6:358–361 [DOI] [PubMed] [Google Scholar]
- Sokolovsky M, Riordan JF, Vallee BL. (1966) Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry 5:3582–3589 [DOI] [PubMed] [Google Scholar]
- Sokolovsky M, Riordan JF, Vallee BL. (1967) Conversion of 3-nitrotyrosine to 3-aminotyrosine in peptides and proteins. Biochem Biophys Res Commun 27:20–25 [DOI] [PubMed] [Google Scholar]
- Suissa S, Ernst P, Boivin JF, Horwitz RI, Habbick B, Cockroft D, Blais L, McNutt M, Buist AS, Spitzer WO. (1994) A cohort analysis of excess mortality in asthma and the use of inhaled β-agonists. Am J Respir Crit Care Med 149:604–610 [DOI] [PubMed] [Google Scholar]
- Tenovuo J, Mäkinen KK. (1976) Concentration of thiocyanate and ionizable iodine in saliva of smokers and nonsmokers. J Dent Res 55:661–663 [DOI] [PubMed] [Google Scholar]
- Thomas EL, Jefferson MM, Joyner RE, Cook GS, King CC. (1994) Leukocyte myeloperoxidase and salivary lactoperoxidase: identification and quantitation in human mixed saliva. J Dent Res 73:544–555 [DOI] [PubMed] [Google Scholar]
- van Dalen CJ, Winterbourn CC, Kettle AJ. (2006) Mechanism of nitrite oxidation by eosinophil peroxidase: implications for oxidant production and nitration by eosinophils. Biochem J 394:707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet A, Eiserich JP, Halliwell B, Cross CE. (1997) Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. J Biol Chem 272:7617–7625 [DOI] [PubMed] [Google Scholar]
- Wu W, Samoszuk MK, Comhair SA, Thomassen MJ, Farver CF, Dweik RA, Kavuru MS, Erzurum SC, Hazen SL. (2000) Eosinophils generate brominating oxidants in allergen-induced asthma. J Clin Invest 105:1455–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]