Abstract
Previous studies suggested that differences between the behavioral effects of cocaine and analogs of benztropine were related to the relatively slow onset of action of the latter compounds. Several N-substituted benztropine analogs with a relatively fast onset of effects were studied to assess whether a fast onset of effects would render the effects more similar to those of cocaine. Only one of the compounds increased locomotor activity, and the increases were modest compared with those of 10 to 20 mg/kg cocaine. In rats trained to discriminate 10 mg/kg cocaine from saline none of the compounds produced more than 40% cocaine-like responds up to 2 h after injection. None of the compounds produced place-conditioning when examined up to 90 min after injection, indicating minimal abuse liability. The compounds had 5.6 to 30 nM affinities at the dopamine transporter (DAT), with uniformly lower affinities at norepinephrine and serotonin transporters (from 490-4600 and 1420–7350 nM, respectively). Affinities at muscarinic M1 receptors were from 100- to 300-fold lower than DAT affinities, suggesting minimal contribution of those sites to the behavioral effects of the compounds. Affinities at histaminic H1 sites were from 11- to 43-fold lower than those for the DAT. The compounds also had affinity for sigma, 5-hydroxytryptamine1 (5-HT1), and 5-HT2 receptors that may have contributed to their behavioral effects. Together, the results indicate that a slow onset of action is not a necessary condition for reduced cocaine-like effects of atypical DAT ligands and suggest several mechanisms that may contribute to the reduced cocaine-like efficacy of these compounds.
Introduction
Previous studies indicated that dopamine (DA) transport inhibitors with varying potency produce behavioral effects that differ little from those of cocaine (e.g., Kuhar et al., 1991). More recently, several “atypical” DA uptake inhibitors were identified that do not completely reproduce cocaine's behavioral effects, despite inhibiting DA uptake with high DA transporter (DAT) affinity (Tanda et al., 2009). For example, benztropine (BZT) analogs have been reported that did not stimulate locomotor activity as effectively as cocaine, did not fully substitute for cocaine in rats trained to discriminate cocaine from saline injections (Newman et al., 1995; Katz et al., 1999, 2004), were not self-administered to the same degree as cocaine or other DA uptake inhibitors (Woolverton et al., 2000; Ferragud et al., 2009; Hiranita et al., 2009b), did not produce place conditioning comparable with cocaine (Li et al., 2005; Velázquez-Sánchez et al., 2009), and were less effective than cocaine in stimulating nucleus accumbens shell DA levels (Tanda et al., 2005, 2009). Although BZT analogs may be the most thoroughly studied atypical DAT inhibitors, examples from other structural classes exist, including analogs of the σ receptor ligand, rimcazole (Katz et al., 2003), and some analogs of cocaine (Navarro et al., 2009).
Structure-activity studies have indicated that BZT analogs bind to the DAT in a manner that differs from that for standard DAT inhibitors (Newman et al., 1995). Beuming et al. (2008) used a DAT model based on the crystallized structure of the bacterial leucine transporter (Yamashita et al., 2005) to model DAT binding. BZT analogs, as opposed to cocaine analogs, preserved a distance between Tyr156 and Asp79 that allowed hydrogen bonding between the two residues. That bond was suggested to close the binding pocket, shielding the binding site from extracellular space (Beuming et al., 2008). Furthermore, studies of the binding of the radiolabeled BZT analog, N-(n-butyl)-3α-[bis(4′-fluorophenyl)methoxy]-tropane (JHW 007), indicate that, as opposed to other DAT inhibitors, its binding is insensitive to sodium (Kopajtic et al., 2010), suggesting again that DAT binding of BZT analogs differs from that for other DAT inhibitors.
Pharmacological studies have suggested conformational changes in the DAT induced by uptake inhibitors, consistent with the modeling studies. Loland et al. (2008) found that cocaine analogs were less potent as DA uptake inhibitors in cells transfected with a DAT mutant (Y335A) that assumes an inward-facing conformation than in cells with WT DAT. In contrast, BZT analogs had more similar potencies for the two forms of DAT. Furthermore, there was a relationship between the behavioral effects of the compounds and the change in DA-uptake inhibition potency in Y335A- and WT-transfected cells. The behavioral effects of compounds for which there was a large ratio were comparable with those of cocaine. In contrast, a small ratio predicted atypical behavioral effects.
One BZT analog, JHW 007, is of interest because it blocks some of the effects of cocaine. When administered before cocaine, JHW 007 antagonized the stimulation of locomotor activity normally produced by cocaine (Desai et al., 2005a). Furthermore, combinations of cocaine with JHW 007, in contrast to typical DA uptake inhibitors, are less than additive, and at some concentrations JHW 007 antagonized the effects of cocaine on extracellular DA, consistent with the antagonism of cocaine's behavioral effects (Tanda et al., 2009). Finally, JHW 007 antagonizes the self-administration of cocaine (Hiranita et al., 2009b) as well as the place conditioning induced by cocaine (Velázquez-Sánchez et al., 2010).
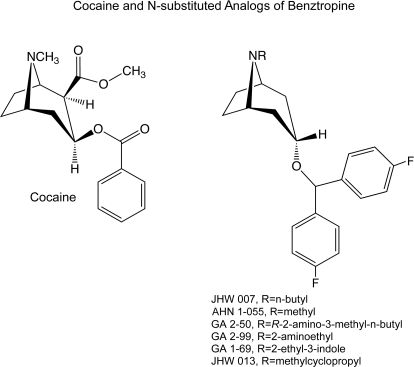
One feature of JHW 007 that may contribute to its atypical actions, as well as its cocaine-antagonist effects, is its slow onset of action. Desai et al. (2005a) reported that the rate at which JHW 007 displaced [125I]2β-carboisopropoxy-3β-(4-iodophenyl)tropane ([125I]RTI-121), a ligand used to label the DAT in vivo, was approximately 10-fold lower than that of cocaine. Furthermore, the locomotor-stimulant effects of cocaine early after its injection were disproportionately greater than that predicted from its DAT occupancy. Those findings together suggested that a slow onset of action may contribute to the atypical actions of JHW 007. Preliminary behavioral studies with a series of N-substituted BZT analogs shown in Fig. 1 indicated that the onsets of effects of N-(indole-3″-ethyl)-3α-(4′,4″-difluoro-diphenylmethoxy)tropane HCl (GA 1-69), (R)-2″-amino-3″-methyl-n-butyl-3α-(4′,4″-difluoro-diphenylmethoxy)tropane HBr (GA 2-50), N-2″aminoethyl-3α-(4′,4″-difluoro-diphenylmethoxy)tropane HBr (GA 2-99), and N-(cyclopropylmethyl)-3α-(4′,4″-difluoro-diphenylmethoxy)tropane HCl (JHW 013) were substantially faster than that for JHW 007. Thus, to examine the hypothesis that a slow onset was critical for atypical effects of DAT inhibitors, we studied the acute behavioral effects of this group of N-substituted BZT analogs.
Fig. 1.
Chemical structures of cocaine, the BZT analogs studied, and reference compounds.
Materials and Methods
Subjects.
The subjects were Swiss-Webster mice and Sprague-Dawley rats that, respectively, weighed 20 to 25 and 180 to 200 g when received (both from Taconic Farms, Germantown, NY). They were housed in a temperature- and humidity-controlled animal facility with a 12-h light/dark cycle (lights on at 7:00 AM), with all experiments conducted during the light phase. Subjects were allowed to habituate to the animal facility for at least 1 week before experiments. Food and water were available at all times except during experimental sessions. Experiments were conducted in accordance with National Institutes of Health Guidelines under Institutional Animal Care and Use Committee-approved protocols.
Dopamine Transporter Binding Assay.
Brains from male Sprague-Dawley rats weighing 200 to 225 g (Taconic Farms) were removed, and the striatum was dissected and quickly frozen. Membranes were prepared by homogenizing tissues in 20 volumes (w/v) of ice-cold modified sucrose-phosphate buffer (0.32 M sucrose, 7.74 mM Na2HPO4, 2.26 mM NaH2PO4, pH adjusted to 7.4) using a Brinkmann Instruments (Westbury, NY) Polytron (setting 6 for 20 s) and centrifuged at 50,000g for 10 min at 4°C. The resulting pellet was resuspended in buffer, recentrifuged, and suspended in buffer again to a concentration of 10 mg/ml original wet weight (OWW).
Ligand binding experiments were conducted in assay tubes containing 0.5 ml of sucrose-phosphate buffer. Each tube contained 0.5 nM [3H](−)-2-β-carbomethoxy-3-β-(4-fluorophenyl)tropane-1,5-napthalenedisulfonate (WIN 35,428) (specific activity 84 Ci/mmol; PerkinElmer Life and Analytical Sciences, Waltham, MA) and 1.0 mg of striatal tissue (OWW). The reaction was started with the addition of tissue, and the tubes were incubated for 120 min on ice. Nonspecific binding was determined using 0.1 mM cocaine HCl (Sigma-Aldrich, St. Louis, MO). The affinities of these compounds were reported previously (Agoston et al., 1997; Robarge et al., 2000) but were assayed again because previous results were obtained with a HEPES buffer, which typically renders 3-fold lower Ki values than the presently used buffer (unpublished data).
Norepinephrine Transporter Binding Assay.
Frontal cortex was dissected from brains of male Sprague-Dawley rats (Taconic Farms) and frozen for later use. Tissue was thawed and homogenized in 20 volumes (w/v) of 50 mM Tris containing 120 mM NaCl and 5 mM KCl (pH 7.4 at 25°C), using a Brinkmann Instruments Polytron (at setting 6 for 20 s). The tissue was centrifuged at 50,000g for 10 min at 4°C. The resulting pellet was resuspended in buffer and centrifuged again. The final pellet was resuspended in cold buffer to a concentration of 80 mg/ml OWW.
Ligand binding experiments were conducted in assay tubes containing 0.5 ml of buffer, 0.5 nM [3H]nisoxetine (PerkinElmer Life and Analytical Sciences), and 8 mg of frontal cortex tissue OWW. The reaction was started with the addition of the tissue, and the tubes were incubated for 60 min at 0 to 4°C. Nonspecific binding was determined using 1 μM desipramine (Sigma-Aldrich).
Serotonin Transporter Binding Assay.
Midbrain was dissected from brains of male Sprague-Dawley rats (Taconic Farms) and frozen for later use. Tissue was thawed and homogenized in 20 volumes (w/v) of 50 mM Tris containing 120 mM NaCl and 5 mM KCl (pH 7.4 at 25°C), using a Brinkmann Instruments Polytron (at setting 6 for 20 s). The tissue was centrifuged at 50,000g for 10 min at 4°C. The resulting pellet was resuspended in buffer and centrifuged again. The final pellet was resuspended in cold buffer to a concentration of 15 mg/ml (OWW).
Ligand binding experiments were conducted in assay tubes containing 0.5 ml of buffer, 1.4 nM [3H]citalopram (PerkinElmer Life and Analytical Sciences), and 1.5 mg of midbrain tissue. The reaction was started with the addition of the tissue, and the tubes were incubated for 60 min at 25°C (room temperature). Nonspecific binding was determined using 10 μM fluoxetine (Sigma-Aldrich).
M1 Receptor Binding Assay.
Frozen rat brains excluding cerebellum were thawed in ice-cold buffer (10 mM Tris-HCl, 320 mM sucrose, pH 7.4) and homogenized with a Brinkmann Instruments Polytron in a volume of 10 ml/g of tissue. The homogenate was centrifuged at 1,000g for 10 min at 4°C. The resulting supernatant was then centrifuged at 10,000g for 20 min at 4°C. The resulting pellet was resuspended in a volume of 200 mg/ml OWW in 10 mM Tris buffer, pH 7.4.
Ligand binding assays were conducted in tubes containing 0.5 ml of buffer (10 mM Tris-HCl, 5 mM MgCl2), 3 nM [3H]pirenzepine (Perkin-Elmer Life and Analytical Sciences), and 20 mg of brain tissue OWW. The reaction was started with the addition of the tissue, and the tubes were incubated for 60 min in a 37°C water bath. Quinuclidinyl benzilate (Sigma-Aldrich), 100 μM final concentration, was used to determine nonspecific binding.
σ1 Receptor Binding.
Frozen whole guinea pig brains (minus cerebellum) were thawed on ice, weighed, and homogenized (with a glass and Teflon homogenizer) in 10 mM Tris-HCl with 0.32 M sucrose, pH 7.4 (10 ml/g tissue). The homogenate was centrifuged at 1000g for 10 min at 4°C. The supernatant was collected into a clean centrifuge tube, and the remaining pellet was resuspended by vortex in 10 ml of buffer (tissue) and centrifuged again at 50,000g for 15 min at 4°C. The resulting pellet was resuspended at 3 ml/g (OWW) in 10 mM Tris-HCl with 0.32 M sucrose, pH 7.4, and mixed by vortexing. The pellet was gently resuspended in experimental buffer to 80 mg/ml (OWW).
Ligand binding experiments were conducted in polypropylene assay tubes containing 0.5 ml of 50 mM Tris-HCl buffer, pH 8.0. Each tube contained 3 nM [3H](+)-pentazocine (Perkin Elmer Life and Analytical Sciences) and 8.0 mg of tissue (OWW). Nonspecific binding was determined using 10 μM haloperidol. The reaction was started with the addition of tissue, and the tubes were incubated for 120 min at room temperature.
σ2 Receptor Binding.
Tissue was prepared as described above for σ1 receptor binding. Ligand binding experiments were conducted in polypropylene assay tubes containing 0.5 ml of 50 mM Tris-HCl buffer, pH 8.0. Each tube contained 3 nM [3H]-1,3-di-ortho-tolylguanidine (DTG) (Perkin Elmer Life and Analytical Sciences), 200 nM (+)-pentazocine, and 8.0 mg of tissue (OWW). Nonspecific binding was determined using 100 μM haloperidol.
Incubations for all binding assays were terminated by rapid filtration through Whatman (Clifton, NJ) GF/B filters, presoaked in polyethylenimine, using a Brandel R48 filtering manifold (Brandel Inc., Gaithersburg, MD). The filters were washed twice with 5 ml of ice-cold buffer and transferred to scintillation vials. Beckman Ready Safe (3.0 ml) was added, and the vials were counted the next day using a Beckman 6000 liquid scintillation counter (Beckman Coulter, Fullerton, CA) at 50% efficiency. Assays were typically conducted in at least three independent experiments, each performed in triplicate.
The IC50 values from the displacement data were computed using a nonlinear, least-squares regression analysis (Prism; GraphPad Software, Inc., San Diego, CA). Inhibition constants (Ki values) were calculated using the equation of Cheng and Prusoff (1973), the concentration of radioligand used in the assay, and the historical value for the Kd value of the radioligand determined in this laboratory.
Receptor Screen.
The compounds were also screened for their activity at various receptor sites by examining their competition with the appropriate radioligands (ProfilingScreen procured from MDS Panlabs Pharmacology Services, Bothell, WA). The screen consisted of assays designed to assess the activity of the compounds at various mammalian receptors listed in Table 1. Each compound was tested in duplicate in each assay at a concentration of 10 μM. Concurrent vehicle and reference standards were conducted with each assay. If displacement at 10 μM was more than 50% the result was considered positive, otherwise a Ki value was considered to be more than 100 μM. For JHW 013, all positive results were repeated at concentrations from 0.1 to 100 μM in log-unit increments, to obtain an estimate of the affinity for the site. For the other compounds, selected positive results were repeated to obtain affinity estimates. For sites at which activity was identified, IC50 values were computed as described using the obtained IC50 value, the concentration of radioligand used in the assay, and the MDS Panlabs historical value for the Kd of the radioligand. Because IC50 values were determined from four concentrations of cold compound, the derived binding constants should be interpreted as estimates. Significant details of the assay procedures are also provided in Table 1.
TABLE 1.
Summary of assay conditions used for assessing activity at various receptor sites in competition for the specified radioligand
Further details can be found in the MDS Panlabs catalogue (MDS Panlabs Pharmacology Services).
| Assay Target | Ligand | Nonspecific Binding | Tissue | Incubation |
|---|---|---|---|---|
| Adenosine A1 | 1 nM [3H]DPCPX | 100 μM R(−)-PIA | Human recombinant CHO cells | 90 min at 25°C |
| Adenosine A2A | 0.05 μM [3H]CGS-21680 | 50 μM NECA | Human recombinant HEK-293 cells | 90 min at 25°C |
| Adrenergic α1, nonselective | 0.25 nM [3H]prazosin | 0.1 μM Prazosin | Rat brain | 30 min at 25°C |
| Adrenergic α1A | 0.25 nM [3H]prazosin | 10 μM Phentolamine | Rat submaxillary gland | 60 min at 25°C |
| Adrenergic α1B | 0.25 nM [3H]prazosin | 10 μM Phentolamine | Rat liver | 60 min at 25°C |
| Adrenergic α2, nonselective | 0.7 nM [3H]rauwolscine | 1 μM Yohimbine | Rat cortex | 30 min at 25°C |
| Adrenergic α2A | 1 nM [3H]MK-912 | 10 μM WB-4101 | Human recombinant insect Sf9 cells | 60 min at 25°C |
| Adrenergic β, nonselective | 0.25 nM [3H]dihydroalprenolol | 1 μM S(−)-propranolol | Rat brain | 20 min at 25°C |
| Adrenergic β1 | 0.03 nM [125I]-cyanopindolol | 100 μM (S)- propranolol | Human recombinant Rex 16 cells | 120 min at 25°C |
| Adrenergic β2 | 0.2 nM [3H]CGP-12177 | 10 μM ICI-118551 | Human recombinant CHO-NBR1 cells | 60 min at 25°C |
| Ca++ channel-L, dihydropyridine | 0.1 nM [3H]nitrendipine | 1 μM Nifedipine | Rat cortex | 90 min at 25°C |
| Dopamine D1 | 1.4 nM [3H]SCH 23390 | 10 μM (+)-Butaclamol | Human recombinant CHO cells | 120 min at 37°C |
| Dopamine D2 | 0.16 nM [3H]spiperone | 10 μM Haloperidol | Human recombinant CHO cells | 120 min at 25°C |
| GABAA, agonist site (muscimol) | 1 nM [3H]muscimol | 100 nM Muscimol | Rat brain (minus cerebellum) | 10 min at 4°C |
| GABAA, benzodiazepine, central | 1 nM [3H]flunitrazepam | 10 μM Diazepam | Rat brain (minus cerebellum) | 60 min at 25°C |
| GABAA, chloride channel | 3 nM [3H]TBOB | 200 μM Picrotoxin | Rat cortex | 20 min at 25°C |
| GABAB, nonselective | 0.6 nM [3H]CGP-54626 | 100 nM CGP-54626 | Rat brain | 20 min at 25°C |
| Glutamate, NMDA, phencyclidine | 4 nM [3H]TCP | 1 μM MK-801 | Rat cortex | 45 min at 25°C |
| Glutamate, nonselective | 3.75 nM [3H]l-glutamic acid | 50 μM L-Glutamic acid | Rat brain | 30 min at 37°C |
| Glycine, strychnine-sensitive | 10 nM [3H]strychnine | 1 mM Glycine | Rat spinal cord | 10 min at 4°C |
| Insulin | 0.03 nM [125I]insulin | 1 μM Insulin | Rat liver | l6 h at 4°C |
| Muscarinic M2 | 0.29 nM [3H]N-methylscopolamine (0.8 nM for JHW 013) | 1 μM Atropine | Human recombinant insect sf9 cells (human recombinant CHO cells) | 60 min at 25°C (120 min at 25°C) |
| Muscarinic M3 | 0.29 nM [3H]N-methylscopolamine (0.8 nM for JHW 013) | 1 μM Atropine | Human recombinant insect sf9 cells (human recombinant CHO cells) | 60 min at 25°C (120 min at 25°C) |
| Nicotinic acetylcholine | 0.1 nM [125I]epibatidine | 300 μM (−)-Nicotine | Human IMR-32 cells | 60 min at 25°C |
| Opiate, nonselective | 1.0 nM [3H]naloxone | 1 μM Naloxone | Rat brain | 40 min at 25°C |
| Opiate-δ | 0.9 nm [3H]naltrindole | 10 μM Naloxone | Human recombinant CHO cells | 120 min at 25°C |
| Opiate-κ | 0.6 nM [3H]diprenorphine | 1 μM Naloxone | Human recombinant HEK-293 cells | 60 min at 25°C |
| Opiate-μ | 0.6 nM [3H]diprenorphine | 10 μM Naloxone | Human recombinant CHO cells | 60 min at 25°C |
| Phorbol ester | 3 nM [3H]PDBu | 1 μM PDBu | Mouse brain | 60 min at 25°C |
| K+ channel [KATP] | 5 nM [3H]glyburide | 1 μM Glyburide | Hamster pancreatic β cells HIT-T15 | 120 min at 25°C |
| K+ channel hERG | 1.5 nM [3H]astemizole | 10 μM Astemizole | Human recombinant HEK-293 cells | 60 min at 25°C |
| Progesterone | 2 nM [3H]R-5020 | 0.41 μM Progesterone | Bovine uterus | 16 h at 4°C |
| 5-HT1, nonselective | 2 nM [3H]5-HT | 10 μM 5-HT | Rat cortex | 10 min at 37°C |
| 5-HT2, nonselective | 0.5 nM [3H]ketanserin | 1 μM Ketanserin | Rat brain | 40 min at 25°C |
| Na+ Channel, site 2 | 5 nM [3H]batrachotoxin A 20-α-benzoate | 100 μM Veratridine | Rat brain | 60 min at 37°C |
NMDA, N-methyl-d-aspartate; CGP-12177, 4-[3-[(1,1-dimethylethyl)amino]-2-hydroxypropoxy]-1,3-dihydro-2H-benzimidazol-2-one; CGP-54626, [S-(R*,R*)]-[3-[[1-(3,4-dichlorophenyl)ethyl]amino]-2-hydroxypropyl](cyclohexylmethyl) phosphinic acid; CGS 21680, 2-[p-(2-carboxyethyl)phenethylamino]-5′-N-ethylcarboxamidoadenosine; DPCPX, 8-cyclopentyl-1,3-dipropylxanthine; ICI 118551, (±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol; MK801, (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen; MK-912, l-657,743, (2S-trans)-1,3,4,5′,6,6′,7,12b-octahydro-1′,3′-dimethyl-spiro[2H-benzofuro[2,3-a]quinolizine-2,4′(1′H)-pyrimidin]-2′(3′H)-one hydrochloride hydrate; NECA, 5′-N-ethylcarboxamidoadenosine; PDBu, phorbol-12,13-dibutyrate; PIA, N6-phenylisopropyladenosine; R-5020, 17α,21-dimethyl-19-nor-4,9-pregnadiene-3,20-dione; SCH 23390, R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine; TBOB, t-butylbicycloorthobenzoate; TCP, thienyl cyclohexylpiperidine; WB-4101, 2-(2,6-dimethoxyphenoxyethyl)aminomethyl-1,4-benzodioxane hydrochloride.
Locomotor Activity.
Experimentally naive mice were placed singly in clear acrylic chambers (40 cm3) contained within monitors (Accuscan Instruments, Inc., Columbus, OH) that were equipped with light-sensitive detectors. The detectors were spaced 2.5 cm apart along two perpendicular walls with infrared light sources mounted on the opposing walls and directed at the detectors. Activity counts were registered for each interruption of a light beam. Mice were injected (intraperitoneally in volumes of 1 ml/100 g), and then immediately placed in the apparatus for 60 min or for 8 h in other experiments. Activity counts were collected every 10 min. Mice were used only once, and each dose was studied in six to eight mice. The doses of the N-substituted BZT analogs tested in half log-unit steps were as follows: GA 1-69, 0.03 to 1.0 mg/kg; GA 2-50, 3.0 to 30.0 mg/kg; GA 2-99, 3.0 to 30.0 mg/kg; and JHW 013, 0.1 to 10.0 mg/kg. The doses of cocaine tested were 5.0 to 40.0 mg/kg in one-third log-unit steps.
Cocaine Discrimination.
Details have been described previously (Katz et al., 2004). In brief, experimentally naive male rats were individually housed and maintained at 325 to 350 g by providing 10 to 15 g of food daily at least 1 h after testing. Experiments were conducted at the same time daily, with subjects placed in 29.2 × 24.2 × 21-cm operant-conditioning chambers (modified ENV-001; MED Associates, St. Albans, VT) containing two response keys (levers requiring a downward force of 0.4 N) with pairs of green and yellow light-emitting diodes above each. A dispenser delivered 45-mg food pellets (BioServ, Frenchtown, NJ) to a tray located between the response keys, and a light was mounted near the ceiling to provide overall illumination. The chamber was contained within a ventilated enclosure that provided sound attenuation and was supplied with white noise to further mask extraneous noise.
Rats were initially trained with food reinforcement to press both levers, and they were subsequently trained to press one after cocaine (10 mg/kg i.p.) and the other after saline (intraperitoneal) injection. Each response produced an audible click. The ratio of responses to food pellets [fixed ratio (FR)] was gradually increased until, under the final conditions, the completion of 20 consecutive responses on the cocaine- or saline-appropriate lever produced food. Incorrect responses reset the FR response requirement. The right versus left assignments of cocaine and saline keys were counterbalanced among subjects. Subjects were injected and placed in chambers. Sessions started after a 5-min timeout period during which lights were off and responses had no scheduled consequences, other than producing a click. After the timeout, the house light was turned on until the completion of the FR 20-response requirement and the presentation of food. Sessions ended after 20 food presentations or 15 min, whichever occurred first, and they were conducted 5 days per week, with cocaine or saline sessions scheduled in a double-alternation sequence.
Testing sessions were initiated after subjects met the criteria on four consecutive sessions of at least 85% cocaine- or saline-appropriate responding (two sessions of each) over the entire session and the first FR. Test sessions were conducted with the presession administration of different doses of cocaine or the N-substituted BZT analogs. Test sessions were identical to training sessions with the exception that 20 consecutive responses on either lever were reinforced.
Place Conditioning.
Details have been described previously (Li et al., 2002). In brief, subjects were first habituated to handling followed by the experiment proper, which was conducted with subjects placed daily in acrylic test chambers (Accuscan Instruments, Inc.). The chambers were divided into two 20 × 40-cm compartments separated by a clear acrylic 10 × 6-cm guillotine door. One compartment was black with a floor constructed of stainless-steel mesh, under which there was β-chip bedding. The other compartment was black with a floor constructed of stainless-steel rods, under which there was no bedding. Infrared light sources directed at detectors on the opposing walls were spaced 2.5 cm apart and allowed recording of time spent in each compartment and locomotor activity (total number of beams interrupted).
During the first preconditioning phase (four consecutive 15-min daily sessions) of the experiments, subjects were placed in the chamber close to the middle, with both compartments accessible. The time spent in each compartment was recorded on the fourth day. If a subject spent more than 67% of its time on one side, it was removed from the study. Approximately 25% of the subjects were removed based on this criterion.
During the conditioning phase (eight consecutive daily 30-min sessions) injections of saline or the test compound were administered before alternate sessions, with subjects placed in one of the two compartments. The door between compartments was in place, restricting the subject to the one compartment. The treatments that alternated with vehicle injections in different groups of subjects were: saline, cocaine (10 mg/kg), GA 1-69 (1–17 mg/kg), GA 2-50 (3–17 mg/kg), GA 2-99 (3–17 mg/kg), and JHW 013 (1–10 mg/kg). Each of the N-substituted BZT analogs was first studied when injected immediately before subjects were placed in the chamber. In separate studies, subjects were injected and placed back in their home cages until 45 or 90 min later when they were placed into the test chamber. The effect on locomotor activity of the first administration of each treatment was assessed by tabulating the number of photocell beams broken during the 30-min exposure period.
The postconditioning phase was one 15-min session that was conducted 1 day after the last conditioning session. This session was conducted exactly as in the preconditioning phase. Subjects were placed in the chamber close to the middle with both compartments accessible. The time spent in each compartment was recorded.
Compounds.
The compounds studied were cocaine hydrochloride (Sigma-Aldrich), and several N-substituted analogs of BZT (Fig. 1): GA 1-69, GA 2-50, GA 2-99, and JHW 013. The synthesis of these analogs was conducted in the Medicinal Chemistry Section of the National Institute on Drug Abuse Intramural Research Program and has been described previously (Agoston et al., 1997). All compounds were dissolved in distilled water with heat and sonication, as necessary except cocaine, which was dissolved in saline. All solutions were administered at 1 ml/kg i.p. except GA 2-50, which was given at 2 and 3.4 ml/kg at the higher doses because of solubility limitations.
Statistical Analysis.
Radioligand binding data were analyzed by using Prism software (GraphPad Software Inc.). The Ki values from individual experiments were determined and averaged to provide a single value with S.E.M. To obtain a more accurate resolution of potential multiple-site binding, the data from the σ-receptor binding assays were globally fit to one- or two-site binding models. The two models were compared by F test to determine which model better fit the data, and those Ki values and their 95% confidence limits are reported. Locomotor activity in mice was assessed with counts collected during each successive 10-min epoch for 8 h after injection. Because effects were most prominent in the first hour after injection, these data were further analyzed as a function of dose using standard analysis of variance (ANOVA) and linear regression techniques.
For cocaine discrimination, overall rate of responding on both keys and the percentage of responses emitted on the cocaine-appropriate key were calculated. The mean values for the group of subjects were calculated for each measure at each drug dose tested. Because the percentage of responses emitted on the cocaine-appropriate key is a relative measure, it is largely independent of the rate of response. However, when response rates are extremely low, the small sample size may render the measure an unreliable indication of the discriminative effect of the drug. Therefore, a data exclusion criterion was implemented by which the percentage of cocaine-appropriate responding was not calculated if fewer than half of the subjects responded at a particular dose. This data exclusion criterion was met once in the course of these studies (for GA 2-99 at 17.0 mg/kg administered 60 min before testing).
The data were analyzed using standard ANOVA and linear regression techniques to calculate the dose producing a half-maximal effect (50% cocaine-appropriate responding). For these analyses, points on the linear part of the ascending portions of the dose-effect curves were used. Pairs of ED50 values were considered to be significantly different if their 95% confidence limits did not overlap. Differences in the effectiveness of selected pairs of compounds were assessed by comparing maximal effects with Student's t test.
For place conditioning, the time spent in the compound-paired compartment during the postconditioning session was expressed as a difference from that during the last preconditioning session [conditioned place preference (CPP) score]. Because the time spent in the compound-paired compartment is an allocation of total time, it is largely independent of the subjects' activity level. The exception to this is when activity is virtually eliminated, which did not occur in the present study. The locomotor activity was expressed as counts/minute during the first compound conditioning sessions. The differences in group means were analyzed by one-way ANOVA followed by Dunnett's multiple comparisons of treatments versus saline controls detecting change from control in either direction. The effects of single doses of cocaine were compared with vehicle controls by unpaired t tests for independent samples. Effects with a calculated p value < 0.05 were considered statistically significant.
Results
Receptor Binding.
The affinities (Ki values) of the BZT analogs at the various primary sites studied are shown in Table 2. Affinity at the dopamine transporter varied from 5.59 nM for GA 2-99 to 29.2 nM for GA 1-69. Affinities at monoamine transporters were uniformly lower, varying from 490 to 4600 and 1420 to 7350 nM, respectively, for the serotonin and norepinephrine transporters (Table 2). In general, the N-substituted compounds retained selectivity for the dopamine transporter over M1 muscarinic and H1 histaminic sites. GA 1-69, GA 2-50, and GA 2-99 had from 100- to 300-fold lower affinity for M1 and 11- to 43-fold lower affinity for H1 sites. JHW 013 was less selective than the other compounds (Table 2). The affinities of GA 2-50 and JHW 013 for σ1 receptors were similar to or greater than the affinities of the compounds for the DAT. In contrast, selectivity for the DAT over σ receptors varied more substantially across the compounds studied. Affinities of GA 1-69 and GA 2-99 for σ1 receptors were approximately 15- to 20-fold lower than their affinities for the DAT, whereas those for GA 2-50 and JHW 013 were, respectively, similar to or greater than their DAT affinities. Only GA 2-99 had an affinity for σ2 receptors that was appreciably lower than its affinity for the DAT.
TABLE 2.
Affinities of N-substituted BZT analogs in binding to the transporters for dopamine, norepinephrine, and serotonin, and M1 muscarinic, histamine H1, σ1, and σ2 receptors
Numbers are Ki values in nM with S.E.M. or 95% confidence limits.
| Target | GA 1-69 | GA 2-50 | GA 2-99 | JHW 013 |
|---|---|---|---|---|
| DAT | 29.2 ± 3.24 | 13.2 ± 1.50 | 5.59 ± 0.619 | 24.6 ± 1.70 |
| SERT | 490 ± 56.4 | 3870 ± 135 | 4600 ± 680 | 1420 ± 116 |
| NET | 7350 ± 934 | 2130 ± 160 | 1420a ± 125 | 1640 ± 153 |
| M1 | 3280 ± 221b | 4020 ± 592b | 1250 ± 138b | 257 ± 28.9b |
| H1 | 333 ± 22.6c | 218 ± 15.5c | 240 ± 32.6c | 48.3 ± 5.54 |
| σ1 | 430 (385–479) | 12.1 (10.9–13.5) | 129 (110–150) | 6.90 (6.31–7.57) |
| σ2 | 65.8 (50.8–85.1)d | 18.1 (15.0–21.8)d | 156 (111–219)d | 25.9 (20.0–33.8)d |
SERT, serotonin transporter; NET, norepinephrine transporter.
Data were published in Kulkarni et al., 2004.
Data were published in Robarge et al., 2000.
Data were published in Campbell et al., 2005.
The Ki values reported are from a [3H]DTG binding assay in which the data uniformly modeled better for two than one binding site. The Ki value for the higher affinity site is displayed. The obtained high-affinity site is the site recognized as the σ2 receptor, whereas the low-affinity site is currently not identified. Values for the low-affinity DTG site and their 95% confidence limits were as follows: GA 1-69, 14,600 (3860–55,000); GA 2-50, 6500 (2830–14,900); GA 2-99, 8490 (424–171,000); JHW 013, 12,100 (4280–34,000).
The results of the binding screen show activities at sites that varied across the different compounds (Table 3). Sites at which all of the compounds tested uniformly had activity that was less than 10 μM, or within 1000-fold of the compound's affinity at the DAT, included: α-adrenergic receptors, DA D2 receptors, muscarinic M2 receptors, muscarinic M3 receptors, μ-opioid receptors, κ-opioid receptors, and 5-HT2 receptors.
TABLE 3.
Estimated Ki values of N-substituted BZT analogues at binding sites examined in the receptor screen
Values are in nanomolars unless otherwise specified.
| Assay | GA 1-69 | GA 2-50 | GA 2-99 | JHW 013 |
|---|---|---|---|---|
| Adenosine A1 | 36.8 | >100 μM | >100 μM | >100 μM |
| Adenosine A2A | >100 μM | >100 μM | >100 μM | >100 μM |
| Adrenergic α1, nonselective | N.T. | N.T. | N.T. | 113 |
| Adrenergic α1A | <10 μM | <10 μM | <10 μM | N.T. |
| Adrenergic α1B | <10 μM | <10 μM | <10 μM | N.T. |
| Adrenergic α2A | <10 μM | <10 μM | <10 μM | 1440 |
| Adrenergic β, nonselective | N.T. | N.T. | N.T. | >100 μM |
| Adrenergic β1 | >100 μM | 18.3 | >100 μM | N.T. |
| Adrenergic β2 | >100 μM | >100 μM | >100 μM | N.T. |
| Ca++ channel L-type, dihydropyridine | <10 μM | 511,000 | 256 | 478 |
| Dopamine D1 | <10 μM | 315,000 | <10 μM | 813 |
| Dopamine D2S | <10 μM | <10 μM | <10 μM | N.T. |
| Dopamine D2L | N.T. | N.T. | N.T. | 586 |
| Dopamine D3 | N.T. | N.T. | N.T. | 28 |
| GABAA, agonist site | >100 μM | >100 μM | >100 μM | >100 μM |
| GABAA, benzodiazepine, central | >100 μM | >100 μM | >100 μM | N.T. |
| GABAA, chloride channel | N.T. | N.T. | N.T. | >100 μM |
| GABAB, nonselective | N.T. | N.T. | N.T. | >100 μM |
| Glutamate, NMDA, phencyclidine | >100 μM | >100 μM | >100 μM | N.T. |
| Glutamate, nonselective | N.T. | N.T. | N.T. | >100 μM |
| Glycine, strychnine-sensitive | N.T. | N.T. | N.T. | 36,000 |
| Insulin | N.T. | N.T. | N.T. | >100 μM |
| Muscarinic M2 | 449 ± 31 | 618 ± 2 | 1420 ± 230 | 1360 |
| Muscarinic M3 | 245 ± 41 | 913 ± 189 | 376 ± 79 | 5390 |
| Na+ channel, site 2 | 9670 ± 260 | 55.1 ± 5 | 321 ± 6 | 168 |
| Nicotinic acetylcholine | 43,400 | >100 μM | >100 μM | 5190 |
| Opiate, nonselective | N.T. | N.T. | N.T. | 3260 |
| Opiate-δ | 3530 | >100 μM | 88,500 | N.T. |
| Opiate-κ | <10 μM | <10 μM | 1590 | N.T. |
| Opiate-μ | <10 μM | <10 μM | 643 | N.T. |
| Phorbol ester | N.T. | N.T. | N.T. | >100 μM |
| K+ channel [KATP] | >100 μM | >100 μM | >100 μM | >100 μM |
| K+ channel hERG | 99.1 ± 4 | 854 ± 163 | 871 ± 28 | N.T. |
| Progesterone | N.T. | N.T. | N.T. | 9100 |
| 5-HT1 (nonselective) | 73.9 | 58.7 | 93.0 | 8100 |
| 5-HT2 (nonselective) | <10 μM | <10 μM | <10 μM | 100 |
N.T., not tested.
Locomotor Activity.
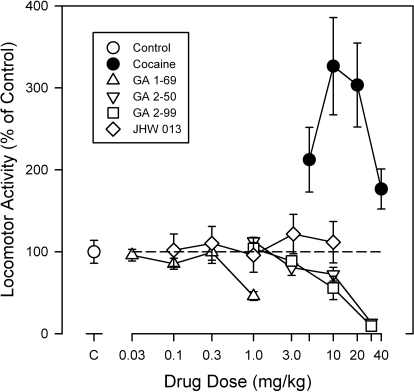
Cocaine produced dose-related increases in locomotor activity during the first 30 min after injection (Fig. 2, ●). Levels of activity were increased at all of the doses examined, and the dose-effect curve had a characteristic bell shape. The ANOVA indicated a significant overall effect of dose (F4,20 = 4.26; p = 0.012), and post hoc tests indicated that doses of 10 and 20 mg/kg significantly increased activity. In contrast to the effects of cocaine, none of the N-substituted BZT analogs significantly increased activity (Fig. 2, open symbols). At the higher doses of GA 1-69, GA 2-50, and GA 2-99, post hoc tests indicated significant decreases in locomotor activity. With JHW 013 there was a nonsignificant ANOVA, with small nonsignificant increases in activity at the highest two doses. Because of this small effect, and because in previous studies several BZT analogs had a long duration of action, we examined the effects of the N-substituted BZT analogs over an 8-h period.
Fig. 2.
Dose-dependent effects of N-substituted BZT analogs on locomotor activity in mice. Ordinates: horizontal locomotor activity counts after drug administration as a percentage of control values. Abscissae: dose of drug in milligrams per kilogram log scale. Each point represents the average effect determined in six to eight mice. Vertical bars represent the standard error of the mean. The data are from the 30-min period immediately after drug administration. Note that none of the BZT analogs produced a stimulation of activity that was equivalent to that of cocaine, and that only with JHW 013 was there a trend toward stimulated locomotor activity.
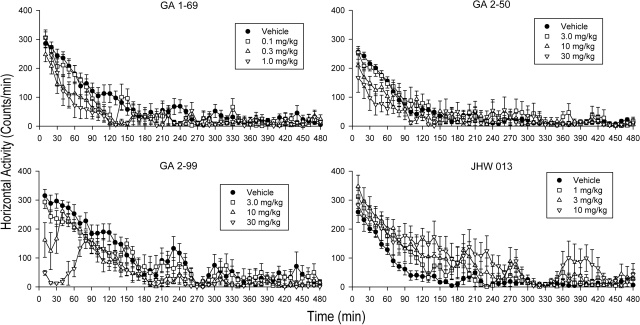
GA 1-69 (0.01–1.0 mg/kg) produced dose- and time-related decreases in locomotor activity (Fig. 3, top left). Decreases in activity were significant between 20 and 40 min after injection depending on dose and returned to approximate control levels at approximately 150 min after injection. Two-way ANOVA of the effects of GA 1-69 indicated significant effects of time (F47,1410 = 85.3; p < 0.001), dose (F5,1410 = 9.43; p < 0.001), and their interaction (F235,1410 = 2.05; p < 0.0011). Post hoc comparisons with control indicated that all of the significant effects were decreases in locomotor activity (P < 0.05), and that those decreases were obtained predominantly within the first 2½ h after injection. Likewise, both GA 2-50 and GA 2-99 produced dose- and time-related decreases in locomotor activity (Fig. 3, top right and bottom left, respectively). Effects of GA 2-50 at 30 mg/kg, and GA 2-99 at 10 and 30 mg/kg were significant at the first time point examined (10 min after injection). The two-way ANOVAs indicated significant effects of time (F values = 61.9 and 37.0, respectively; p < 0.001), dose (F values = 18.2 and 28.6, respectively; p < 0.001), and their interactions (F values = 1.55 and 2.26; p < 0.001). Post hoc comparisons with control indicated that all of the significant effects were decreases in locomotor activity (p < 0.05), and that those decreases were obtained predominantly within the first hour after injection for each compound.
Fig. 3.
Time course of effects of benztropine analogs on locomotor activity in mice. Ordinates: horizontal locomotor activity counts after drug administration. Abscissae: time since injection and placement of subject in experimental chamber. Effects of several doses are shown (see symbol keys) as well as the effects of vehicle injection (●). Each point represents the average effect determined in six mice. Vertical bars represent the standard error of the mean. Points represent average total counts for successive 10-min time periods up to 480 min (8 h) after injection. Note that GA 1-69 (top left), GA 2-50 (top right), and GA 2-99 (bottom left) did not increase locomotor activity at any time after injection, and the predominant effect was a decrease in activity that lasted no more than 2 h after injection. Only JHW 013 (bottom right) increased locomotor activity, and that effect was modest.
In contrast to the effects of the other compounds, JHW 013 (at doses from 0.1 to 10.0 mg/kg) significantly increased locomotor activity at some time points after injection (Fig. 3, bottom right). Two-way ANOVA of the effects of JHW 013 indicated significant effects of time (F47,1656 = 74.4; p < 0.001), dose (F5,1656 = 2.95; p = 0.026), and the interaction of the two (F235,1656 = 1.32; p = 0.002). Post hoc comparisons indicated that the significant increases in locomotor activity (P < 0.05) were obtained predominantly at 70 to 200 min after injection, although at 3.0 mg/kg increases in activity were obtained immediately after injection.
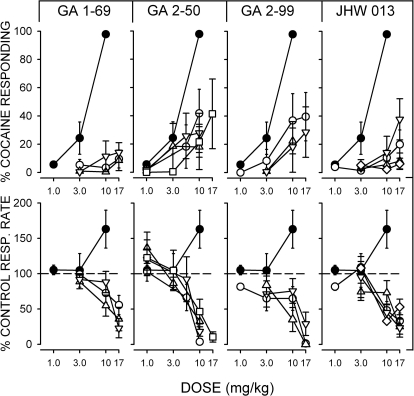
Cocaine Discrimination.
As has been shown previously, cocaine produced a dose-related increase in the percentage of drug-appropriate responses in subjects trained to discriminate cocaine (10 mg/kg) from saline (Fig. 4, filled symbols). ANOVA indicated a significant effect of dose (F2,16 = 55.6; p < 0.001) and an ED50 value of 3.73 mg/kg (95% confidence limits 3.06 to 4.63). In contrast to the effects of cocaine, none of the N-substituted BZT analogs fully substituted for cocaine at any of the doses studied (Fig. 4, top) across the range of doses from those that had no effects to those that virtually eliminated responses (Fig. 4, bottom). Each of the BZT analogs was studied up to 120 min after injection, and JHW 013 was studied 180 min after injection. If increasing the time between injection and testing appreciably increased the maximum substitution, a still longer time was studied until there were no substantial increases in maximum substitution or there was an apparent decrease in potency. The maximum substitution that was obtained was no more than 40% with any of the studied doses and times after injection. The ANOVAs of the effects indicated that only GA 2-99 had effects that were significantly related to dose (F2,41 = 3.55; p = 0.038, with F values for the effect of dose for the other compounds < 2.74 and all p > 0.05). None of the compounds had effects that depended on time (F values < 0.938; p > 0.475). In contrast to the negative outcomes in substituting for cocaine, all of the compounds had effects of dose on response rates (Fig. 4, bottom) that were significant (F values > 9.82; p < 0.015), with only the effects of JHW 013 significant with respect to time (F3,5 = 6.46; p = 0.036).
Fig. 4.
Effects of various doses of cocaine and N-substituted BZT analogs in rats trained to discriminate injections of cocaine (10 mg/kg) from saline at various times after injection. Ordinates at the top indicate percentage of responses on the cocaine-appropriate key, and ordinates at the bottom indicate the rates at which responses were emitted (as a percentage of response rates after saline administration). Abscissae: drug dose in milligram per kilogram (log scale). Each point represents the effect in six rats. ●, the effects of cocaine, reproduced in each for reference. ○, the effects obtained with the drug injected 5 min before testing. △, the effects obtained with the drug injected 60 min before testing. □, the effects obtained with the drug injected 90 min before testing. ▽, the effects obtained with the drug injected 2 h before testing. ♢, the effects obtained with the drug injected 3 h before testing.
Place Conditioning and Locomotor Activity.
The increase from preconditioning to postconditioning in time spent in the drug-paired compartment (CPP score) with cocaine (10 mg/kg) was significantly greater (t test, t14 = 2.32; p < 0.05) than that produced by saline (Fig. 5, compare filled bars). In addition, the first exposure to this dose of cocaine significantly increased locomotor activity over that obtained with saline (Fig. 5, bottom, compare filled and open circles; t14 = 4.54; p = 0.0005).
Fig. 5.
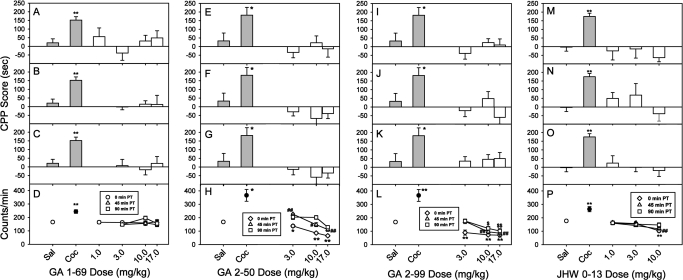
Place conditioning (A-C, E-G, I-K, and M-O) and locomotor effects (D, H, L, and P) for N-substituted BZT analogs administered to rats at different times before placement in the conditioning chamber. A, E, I, and M, results from the place conditioning effects of the drugs given immediately before conditioning sessions. B, F, J, and N, results from the 45-min pretreatment before conditioning sessions. C, G, K, and O, results for the 90-min pretreatment condition. D, H, L, and P, the effects of the compounds on locomotor activity (measured in counts of beam breaks per minute) during the first exposure to the substance. Data are presented as means ± S.E.M. Statistical significance is indicated as follows: * for the 0-min pretreatment, # for the 45-min pretreatment, and $ for the 90-min pretreatment. One symbol represents p < 0.05 and two symbols represent p < 0.01, as determined by t test versus saline group for the cocaine groups and by ANOVA followed by the Dunnett's test versus saline group for each of the novel compounds.
Doses of GA 1-69 from 1.0 to 17.0 mg/kg failed to produce a significant place conditioning when administered immediately before conditioning sessions (Fig. 5A; F4,43 = 0.925; p = 0.458). Because increased time between injection and conditioning sessions can increase effectiveness in place conditioning (e.g., De Beun et al., 1992), conditioning sessions were also conducted with injections administered at 45 and 90 min before conditioning sessions. GA 1-69 was ineffective in conditioning when administered 45 min before (Fig. 5B; F3,36 = 0.0988; p = 0.960) or 90 min before (Fig. 5C; F3,36 = 0.297; p = 0.827) conditioning sessions. There were no significant effects of GA I-69 on locomotor activity at any of the times tested (Fig. 5D; 0 min: F4,43 = 0.775, p = 0.548; 45 min: F3,36 = 0.755, p = 0.527; 90 min: F3,36 = 2.28, p = 0.0955).
GA 2-50 from 3.0 to 17.0 mg/kg immediately before sessions was ineffective in producing a significant change in time spent in the compound-paired compartment (Fig. 5E; F3,28 = 0.551; p = 0.651). The absence of significant place conditioning with GA 2-50 was obtained across this range of doses despite dose-dependent and significant decreases in locomotor activity (Fig. 5H, diamonds; F3,28 = 33.4; p < 0.0001), with 17 mg/kg producing the maximum decrease (Dunnett's test q = 8.90, q < 0.01). Neither the 45-min (Fig. 5F; F3,28 = 1.53; p = 0.228) nor the 90-min (Fig. 5G; F3,28 = 1.11, p = 0.362) pretreatment time significantly increased CPP scores across the range of doses examined. At 45 min after the first administration of GA 2-50 locomotor activity was significantly altered (F3,28 = 19.3; p < 0.0001), with 3.0 mg/kg producing a significant increase (Dunnett's test q = 3.79; p < 0.01) and 17 mg/kg producing a significant decrease (Dunnett's test q = 3.64; p < 0.01). At 90 min after injection, one-way ANOVA showed an overall significant difference in locomotor activity counts (F3,28 = 7.76; p < 0.001), although the effects were noticeably less than those obtained at earlier time points.
As with the other compounds, neither GA 2-99 (3–17 mg/kg) nor JHW 013 (1–10 mg/kg) produced significant place conditioning when given immediately before conditioning sessions (respectively, Fig. 5I, F3,28 = 0.829, p = 0.489 and Fig. 5M, F3,36 = 0.517, p = 0.673). Neither a 45- nor a 90-min increase in the time between injection and placement in the chambers for conditioning increased the efficacy of either compound (45 min GA 2-99: Fig. 5J, F3,28 = 1.53, p = 0.229; 45 min JHW 013: Fig. 5N, F3,36 = 1.32, p = 0.284; 90 min GA 2-99: Fig. 5K, F3,28 = 0.0560, p = 0.982; 90 min JHW 013: Fig. 5O, F3,36 = 0.269, p = 0.847). GA 2-99 also significantly decreased locomotor activity in a dose-dependent manner with maximal decreases in locomotor activity obtained immediately after injection and lesser decreases at 45 and 90 min after injection (Fig. 5L). The effects were significant at each pretreatment time (0 min: F3,28 = 14.1, p < 0.0001; 45 min: F3,28 = 8.83, p < 0.001; 90 min: F3,28 = 8.56, p < 0.001; Fig. 2D). When tested immediately and 45 min after injection, JHW 013 also produced significant decreases in locomotor activity (0 min: F3,36 = 10.4, p < 0.0001; 45 min: F3,36 = 8.58, p = 0.0002). However, no significant effects on locomotor activity were observed when JHW 013 was injected 90 min before conditioning sessions (F3,36 = 1.45, p = 0.244) (Fig. 5P).
Discussion
The presently studied N-substituted BZT analogs were less effective than cocaine in producing stimulant-like behavioral effects that indicate abuse liability. These compounds showed affinity for the DAT that ranged from approximately 5 to 30 nM and were previously shown to inhibit the uptake of DA (Agoston et al., 1997). Actions at the DAT suggest cocaine-like in vivo effects (Kuhar et al., 1991) that were not obtained in the present study, results similar to those obtained with several other BZT analogs (Newman et al., 2009; Tanda et al., 2009).
The reduced cocaine-like effects of the present BZT analogs could be caused by their pharmacokinetics. Syed et al. (2008) reported brain-to-plasma partition coefficients for GA1-69 (1.32) and JHW 013 (1.51) that were lower than several previously reported BZT analogs that ranged from 2.0 to 5.6 (Raje et al., 2003). In contrast, the brain-to-plasma ratio for GA 2-50 was reported to be approximately 10, depending on the dose administered (Othman et al., 2008). Comparable studies have not been conducted with GA 2-99. Because the brain-to-plasma ratios were calculated over large time periods, probably more important are concentrations in brain shortly after injection, which were 900, 800, and 610 ng/g for GA 1-69, GA 2-50, and JHW 013, respectively, each at 5.0 mg/kg i.v. (Othman et al., 2008; Syed et al., 2008). These values reflect estimated molar concentrations from 65 to 150 times their Ki values. Because the pharmacokinetic studies were designed to examine blood-brain barrier transport, values are based on total brain concentrations, including those that presumably bound nonspecifically. Thus, although definitive information is not presently available, it seems that unless more than 98% of available drug is otherwise occupied more than adequate concentrations of the drugs are available in brain for DAT binding.
In addition to their activity at the DAT, the compounds were relatively selective for the DAT among the monoamine transporters, having affinities at the norepinephrine transporter and serotonin transporter, respectively, that ranged from 67- to 250-fold and 17- to 800-fold lower than their DAT affinities. Moreover, the compounds had affinities at the DAT that were from 10- to 300-fold higher than at M1 receptors and 2- to 43-fold higher than at H1 receptors. Previous studies have suggested that actions at M1 and H1 sites are unlikely contributors to the reduced effectiveness of the BZT analogs compared with cocaine (see Tanda et al., 2009 for a review).
Whether actions at a site other than the DAT are responsible for the reduced cocaine-like effects of these and other BZT analogs has been of considerable interest. The present results of the receptor screen suggest other sites that may be involved, and parsimony suggests that the sites first considered are those at which all of the BZT analogs had affinity that approached that for the DAT. For example, whereas GA 2-50 had ∼18 nM affinity for adrenergic β1 receptors, the other compounds studied had undetectable affinity at that site up to concentrations of 10 μM, suggesting that the diminished cocaine-like effects, which were exhibited by all of the compounds, were not caused by actions at adrenergic β1 receptors.
The sites at which all four compounds had discernable affinity included α-adrenergic, dopaminergic, opioid, serotonin, and σ receptors. A previous study of other BZT analogs (Katz et al., 2004) reported low affinity of those compounds at adrenergic α1 and α2 receptors, opioid-δ, and opioid-κ receptors, suggesting that high affinity at those sites is not necessary for the diminished cocaine-like effects. In unpublished studies (R. I. Desai, A. H. Newman, D. K. Grandy, and J. L. Katz), JHW 007 antagonized the locomotor stimulant effects of cocaine in DA D2R knockout and wild-type mice. In that study, the locomotor-stimulant effects of cocaine were diminished in the DA D2R knockout compared with WT mice as in previous studies (Kelly et al., 1998; Chausmer et al., 2002). However, the cocaine antagonist effects of JHW 007 were evident, suggesting that at least the antagonism of cocaine's locomotor-stimulant effects by BZT analogs, and probably their diminished cocaine-like effects as well, do not depend on actions at DA D2Rs. Some studies suggest that actions at 5-HT2A and 5-HT2C receptors can block the effects of cocaine (Bubar and Cunningham, 2006). The actions of the BZT analogs at those sites, beyond the nonselective 5-HT2 assay conducted in the present study, will be further addressed in future studies.
Several previous studies have indicated that σ-receptor antagonists can block several of the effects of cocaine, including locomotor stimulation, sensitization, and place conditioning (see review by Matsumoto 2009). It is noteworthy that the σ antagonists that block some of the above-mentioned effects of cocaine were not effective in blocking the self-administration of cocaine (Hiranita et al., 2010). However, we have found that combined treatment with σ-receptor antagonists and dopamine uptake inhibitors blocks the self-administration of cocaine, whereas the drugs administered alone do not (Hiranita et al., 2009a). Those findings suggest the actions of BZT analogs at σ receptors may be interacting with their activity at the dopamine transporter to limit their cocaine-like efficacy and possibly contribute to their cocaine-antagonist effects. GA 2-50 and JHW 013 had affinities at σ1 or σ2 receptors that were similar to their affinities at the DAT. Ongoing studies are directed at the hypothesis that actions at σ receptors combined with their affinities at the DAT are critical for the decreased cocaine-like behavioral effects of the present compounds.
Previous studies with several N-substituted BZT analogs showed that cocaine-like effects became more prominent with time after injection. For example, the N-methyl analog of the present compounds [3α-[bis-(4-fluorophenyl)methoxy]tropane (AHN 1-055)] (Fig. 1) produced complete substitution for the discriminative-stimulus effects of cocaine at 90 min after injection, but not at 5 min after injection. Other BZT analogs became more effective, although they did not fully substitute for cocaine (Katz et al., 1999). Compared with cocaine, several N-substituted BZT analogs had a slow time course for DAT binding in vivo (Desai et al., 2005a, b) and had a relatively slow onset of increases in extracellular DA levels detected by in vivo microdialysis (Tanda et al., 2005, 2009). Pharmacokinetic studies indicated that these compounds reached sufficient concentrations in the brain to bind to the dopamine transporter shortly after systemic injection (Raje et al., 2003), suggesting a relatively slow association with the DAT, which was confirmed in vitro for the N-butyl BZT analog, JHW 007 (Kopajtic et al., 2010). These observations suggest that some aspect of the slow onset of action was important for the diminished cocaine-like effects of these DAT ligands (Tanda et al., 2009).
In contrast with those results, the present compounds were less effective than cocaine and those effects did not become greater with time. Only JHW 013 increased locomotor activity, although still less than cocaine. The other compounds only decreased locomotor activity, and those decreases diminished within 2 h after injection. As described above, pharmacokinetic studies indicated that brain-to-plasma partition coefficients varied for the present compounds, but likely are in brain at sufficient concentrations to bind at the DAT. Thus the present N-substituted compounds provide a distinctive set of pharmacodynamic and pharmacokinetic effects that suggest that a slow in vivo association may not be necessary for the diminished cocaine-like effects of BZT analogs.
A previous study suggested that the Tyr335 residue in the DAT is critical for regulating the equilibrium between open and closed states of the DAT (Loland et al., 2002). In addition, the potencies of cocaine and its analogs for inhibiting DA transport in cells transfected with a Y335A DAT mutant were decreased by approximately 100-fold compared with potencies with WT DAT. In contrast, potencies of BZT analogs were only 7- to 58-fold less in cells with the Y335A mutant (Loland et al., 2008). Furthermore, there was a significant relationship between the decrease in potencies of the BZT analogs caused by this DAT mutation and their behavioral effectiveness. The only one of the present BZT analogs studied, GA 2-99, like other BZT analogs had a ratio of potencies in the Y335A mutant that was lower than those obtained with cocaine or WIN 35,428 (Loland et al., 2008). Together, these results suggest that the BZT structure promotes conformational changes of the DAT that may lead to effects unlike those of cocaine, and that those conformational changes in vivo may occur faster than previously anticipated.
In summary, the present compounds, like other BZT analogs, have high affinity for the DAT, but are less effective than cocaine in producing various effects related to drug abuse. Subsequent studies will examine the potential of these compounds to antagonize the effects of cocaine. The present compounds depart from those previously reported in that their effectiveness is especially low, and their onsets of action are relatively fast. Sites other than the DAT have been identified, and an alternate mode of action at the DAT has been suggested as mechanisms that may be responsible for the reduced cocaine-like efficacy of these compounds. Subsequent studies will examine those mechanisms and the interaction of these drugs with cocaine to better assess their potential as treatments for cocaine abuse and further investigate mechanistic hypotheses.
Acknowledgments
We thank Ahmed Othman, Gianluigi Tanda, and Takato Hiranita for advice on the manuscript and Patty Ballerstadt for administrative assistance.
This work was supported by the National Institutes of Health, National Institute on Drug Abuse Intramural Research Program.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.173260.
- DA
- dopamine
- DAT
- DA transporter
- 5-HT
- 5-hydroxytryptamine
- ANOVA
- analysis of variance
- BZT
- benztropine
- OWW
- original wet weight
- CPP
- conditioned place preference
- WT
- wild type
- FR
- fixed ratio
- DTG
- 1,3-di-ortho-tolylguanidine
- CHO
- Chinese hamster ovary
- HEK
- human embryonic kidney
- GA 1-69
- N-(indole-3″-ethyl)-3α-(4′,4″-difluoro-diphenylmethoxy)tropane HCl
- GA 2-50
- (R)-2″-amino-3″-methyl-n-butyl-3α-(4′,4″-difluoro-diphenylmethoxy)tropane HBr
- GA 2-99
- N-2″aminoethyl-3α-(4′,4″-difluoro-diphenylmethoxy)tropane HBr
- JHW 013
- N-(cyclopropylmethyl)-3α-(4′,4″-difluoro-diphenylmethoxy)tropane HCl
- JHW 007
- N-(n-butyl)-3α-[bis(4′-fluorophenyl)methoxy]-tropane
- WIN 35,428
- (−)-2-β-carbomethoxy-3-β-(4-fluorophenyl)tropane-1,5-napthalenedisulfonate
- AHN 1-055
- 3α-[bis-(4-fluorophenyl)methoxy]tropane
- [125I]RTI-121
- [125I]2β-carboisopropoxy-3β-(4-iodophenyl)tropane.
Authorship Contributions
Participated in research design: Li, Kopajtic, O'Callaghan, and Katz.
Conducted experiments: Li, Kopajtic, and O'Callaghan.
Contributed new reagents or analytic tools: Agoston, Cao, and Newman.
Performed data analysis: Li, Kopajtic, O'Callaghan, and Katz.
Wrote or contributed to the writing of the manuscript: Li, Kopajtic, Cao, Newman, and Katz.
References
- Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, Newman AH. (1997) Novel N-substituted-3α-[bis (4′fluorophenyl methoxy)] tropane analogues: selective ligands for the dopamine transporter. J Med Chem 40:4329–4339 [DOI] [PubMed] [Google Scholar]
- Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, et al. (2008) The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci 11:780–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. (2006) Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem 6:1971–1985 [DOI] [PubMed] [Google Scholar]
- Campbell VC, Kopajtic TA, Newman AH, Katz JL. (2005) Assessment of the influence of histaminergic actions on cocaine-like effects of 3α-diphenylmethoxytropane analogues. J Pharmacol Exp Ther 315:631–640 [DOI] [PubMed] [Google Scholar]
- Chausmer AL, Elmer GI, Rubinstein M, Low MJ, Grandy DK, Katz JL. (2002) Cocaine-induced locomotor activity and cocaine discrimination in dopamine D2 receptor mutant mice. Psychopharmacology 163:54–61 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- De Beun R, Jansen E, Geerts NE, Slangen JL, Van de Poll NE. (1992) Temporal characteristics of appetitive stimulus effects of luteinizing hormone-releasing hormone in male rats. Pharmacol Biochem Behav 42:445–450 [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, French D, Newman AH, Katz JL. (2005a) Relationship between in vivo occupancy at the dopamine transporter and behavioral effects of cocaine, GBR 12909 [1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl) piperazine]and benztropine analogs. J Pharmacol Exp Ther 315:397–404 [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. (2005b) Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci 25:1889–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferragud A, Velázquez-Sánchez C, Hernández-Rabaza V, Nácher A, Merino V, Cardá M, Murga J, Canales JJ. (2009) A dopamine transport inhibitor with markedly low abuse liability suppresses cocaine self-administration in the rat. Psychopharmacology 207:281–289 [DOI] [PubMed] [Google Scholar]
- Hiranita T, Kopajtic T, Newman AH, Katz JL. (2009a) Regulation of cocaine self-administration in rats by sigma (σ) receptors, in Neuroscience Meeting Planner; 2009 Oct 17–21; Chicago, IL Program 496.1 Society for Neuroscience, Washington, DC [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. (2009b) Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther 329:677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL. (2010) Reinforcing effects of σ-receptor agonists in rats trained to self-administer cocaine. J Pharmacol Exp Ther 332:515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Kline RH, Allen AC, Newman AH. (1999) Novel 3α-diphenylmethoxytropane analogs: selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine. J Pharmacol Exp Ther 288:302–315 [PubMed] [Google Scholar]
- Katz JL, Kopajtic TA, Agoston GE, Newman AH. (2004) Effects of N-substituted analogs of benztropine: diminished cocaine-like effects in dopamine transporter ligands. J Pharmacol Exp Ther 309:650–660 [DOI] [PubMed] [Google Scholar]
- Katz JL, Libby TA, Kopajtic T, Husbands SM, Newman AH. (2003) Behavioral effects of rimcazole analogues alone and in combination with cocaine. Eur J Pharmacol 468:109–119 [DOI] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow JR, Fang Y, Gerhardt GA, Grandy DK, et al. (1998) Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci 18:3470–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopajtic TA, Liu Y, Surratt CK, Donovan DM, Newman AH, Katz JL. (2010) Dopamine transporter-dependent and -independent striatal binding of the benztropine analog JHW 007, a cocaine antagonist with low abuse liability. J Pharmacol Exp Ther 335:703–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. (1991) The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 14:299–302 [DOI] [PubMed] [Google Scholar]
- Kulkarni SS, Grundt P, Kopajtic T, Katz JL, Newman AH. (2004) Structure-activity relationships at monoamine transporters for a series of N-substituted 3α-(bis[4-fluorophenyl]methoxy)tropanes: comparative molecular field analysis, synthesis, and pharmacological evaluation. J Med Chem 47:3388–3398 [DOI] [PubMed] [Google Scholar]
- Li SM, Newman AH, Katz JL. (2005) Place conditioning and locomotor effects of N-substituted, 4′,4"-difluorobenztropine analogs in rats. J Pharmacol Exp Ther 313:1223–1230 [DOI] [PubMed] [Google Scholar]
- Li SM, Yin LL, Shi J, Lin ZB, Zheng JW. (2002) The effect of 7-nitroindazole on the acquisition and expression of d-methamphetamine-induced place preference in rats. Eur J Pharmacol 435:217–223 [DOI] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. (2008) Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol 73:813–823 [DOI] [PubMed] [Google Scholar]
- Loland CJ, Norregaard L, Litman T, Gether U. (2002) Generation of an activating Zn(2+) switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proc Natl Acad Sci USA 99:1683–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR. (2009) Targeting sigma receptors: novel medication development for drug abuse and addiction. Exp Rev Clin Pharmacol 2:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro HA, Howard JL, Pollard GT, Carroll FI. (2009) Positive allosteric modulation of the human cannabinoid (CB) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br J Pharmacol 156:1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Kline RH, Allen AC, Izenwasser S, George C, Katz JL. (1995) Novel 4′ substituted and 4′,4" disubstituted 3α-(diphenylmethoxy)tropane analogs as potent and selective dopamine uptake inhibitors. J Med Chem 38:3933–3940 [DOI] [PubMed] [Google Scholar]
- Othman AA, Newman AH, Eddington ND. (2008) The novel N-substituted benztropine analog GA2-50 possesses pharmacokinetic and pharmacodynamic profiles favorable for a candidate substitute medication for cocaine abuse. J Pharm Sci 97:5453–5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raje S, Cao J, Newman AH, Gao H, Eddington ND. (2003) Evaluation of the blood-brain barrier transport, population pharmacokinetics, and brain distribution of benztropine analogs and cocaine using in vitro and in vivo techniques. J Pharmacol Exp Ther 307:801–808 [DOI] [PubMed] [Google Scholar]
- Robarge MJ, Agoston GE, Izenwasser S, Kopajtic T, George C, Katz JL, Newman AH. (2000) Highly selective chiral N-substituted 3α-[bis(4′-fluorophenyl)methoxyl] tropane analogues for the dopamine transporter: synthesis and comparative molecular field analysis. J Med Chem 43:1085–1093 [DOI] [PubMed] [Google Scholar]
- Syed SA, Newman AH, Othman AA, Eddington ND. (2008) Population pharmacokinetics, brain distribution, and pharmacodynamics of 2nd generation dopamine transporter selective benztropine analogs developed as potential substitute therapeutics for treatment of cocaine abuse. J Pharm Sci 97:1993–2007 [DOI] [PubMed] [Google Scholar]
- Tanda G, Ebbs A, Newman AH, Katz JL. (2005) Effects of 4′-chloro-3α-(diphenylmethoxy)-tropane on mesostriatal, mesocortical, and mesolimbic dopamine transmission: comparison with effects of cocaine. J Pharmacol Exp Ther 313:613–620 [DOI] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Ebbs AL, Tronci V, Green JL, Tallarida RJ, Katz JL. (2009) Combinations of cocaine with other dopamine uptake inhibitors: assessment of additivity. J Pharmacol Exp Ther 330:802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Katz JL. (2009) Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Adv Pharmacol 57:253–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velázquez-Sánchez C, Ferragud A, Hernández-Rabaza V, Nácher A, Merino V, Cardá M, Murga J, Canales JJ. (2009) The dopamine uptake inhibitor 3α-[bis(4′-fluorophenyl)metoxy]-tropane reduces cocaine-induced early-gene expression, locomotor activity, and conditioned reward. Neuropsychopharmacology 34:2497–2507 [DOI] [PubMed] [Google Scholar]
- Velázquez-Sánchez C, Ferragud A, Murga J, Cardá M, Canales JJ. (2010) The high affinity dopamine uptake inhibitor, JHW 007, blocks cocaine-induced reward, locomotor stimulation and sensitization. Eur Neuropsychopharmacol 20:501–508 [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Rowlett JK, Wilcox KM, Paul IA, Kline RH, Newman AH, Katz JL. (2000) 3′- and 4′-Chloro-substituted analogs of benztropine: intravenous self-administration and in vitro radioligand binding studies in rhesus monkeys. Psychopharmacology 147:426–435 [DOI] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. (2005) Crystal structure of a bacterial homologue of Na+/Cl-dependent neurotransmitter transporters. Nature 437:215–223 [DOI] [PubMed] [Google Scholar]