Abstract
The coupled interplay between activation and inactivation gating is a functional hallmark of K+ channels1,2. This coupling has been experimentally demonstrated from ion interaction effects3,4, cysteine accessibility1 and is associated with a well-defined boundary of energetically coupled residues2. The structure of KcsA in its fully open conformation, as well as four other partial openings, richly illustrates the structural basis of activation-inactivation gating5. Here, we have identified the mechanistic principles by which movements on the inner bundle gate trigger conformational changes at the selectivity filter, leading to the non-conductive C-type inactivated state. Analysis of a series of KcsA open structures suggests that as a consequence of the hinge bending and rotation of TM2, the aromatic ring of Phe103 tilts towards residues Thr74 and Thr75 in the pore helix as well as Ile100 in the neighboring subunit. This allows the network of hydrogen bonds among residues W67, E71, and D80 to destabilize the selectivity filter6,7, facilitating entry to its non-conductive conformation. Mutations at position 103, affect gating kinetics in a size-dependent way: small side chain substitutions F103A and F103C severely impair inactivation kinetics, while larger side chains (F103W) have more subtle effects. This suggests that the allosteric coupling between the inner helical bundle and the selectivity filter might rely on straightforward mechanical deformation propagated through a network of steric contacts. Average interactions calculated from molecular dynamics simulations show favourable open state interaction-energies between Phe103 and surrounding residues. Similar interactions were probed in the Shaker K-channel where inactivation was impaired in the mutant I470A. We propose that side chain rearrangements at position 103 mechanically couple activation and inactivation in KcsA and a variety of other K channels.
The K-channel pore domain contains all the molecular elements necessary for ion conduction, activation and inactivation gating8,9. Activation is classically associated with conformational rearrangements at the inner helix bundle5,10,11,12 while C-type inactivation involves structural changes at the selectivity filter and its relationship with permeant ions4,5,13,14,15. Coupling between these two molecular “gates” has been experimentally demonstrated based on the expectation that the conformation of one gate must bias the conformational equilibrium of the other. High affinity permeant ions like Rb+ and Cs+ (which interact intimately with the selectivity filter) slow down the closure of the internal gate of many K+ channels4,16,17. Mutations at or near the selectivity filter lead to a variety of gating effects in K+ channels11,18,19,20, while block by the N-terminal inactivating particle, which tends to lock open the inner gate, greatly accelerates entry into the C-type inactivated state3. Experiments on Kir 1.1 have shown that gating by either intracellular protons or extracellular K+ ions appear to be coupled through conformational changes near the selectivity filter21.
Taken together, these studies suggest that most K+ channels open through coupled gates at both ends of the permeation path. Accordingly, a minimal kinetic cycle coupling these two dynamical elements1,5,22 involves transitions between four states, Closed inner gate with Open-conductive filter (C/O), Open inner gate with Open-conductive filter (O/O), Open inner gate with Inactivated filter (O/I) and Closed inner gate with Inactivated filter (C/I). Observed kinetic behaviors can be described in terms of equilibrium population shifts among four pre-existing states (C/O, O/O, O/I, and C/I). As in the concerted MWC model23, Opening of the inner gate (O/O ↔ O/I) greatly stabilizes the inactivated conformation of the filter, whereas transitioning into a C-type inactivated filter (O/I ↔ C/I) biases the conformation of the inner gate towards the closed state (Supplementary figure S1).
In KcsA, cross-talk between the activation gate and the selectivity filter was first documented by EPR spectroscopy on a limited set of residues in the pore helix12, where opening of the inner gate leads to subtle conformational changes in the pore helix and outer vestibule (Supplementary materials, Figure S2). These conformational rearrangements, associated with C-type inactivation at the selectivity filter6,7, are also coupled to the opening of the activation gate24. In an accompanying paper5, a molecular mechanism for C-type inactivation is suggested from the crystal structure of the Open-Inactivated (O/I) state and a series of partially open-inactivated structures. Using these structures as baseline, we have sought to elucidate the mechanistic principles underlying the conformational coupling between activation and inactivation gates in KcsA.
We first estimated the degree of allosteric coupling between proton-dependent activation and C-type inactivation through bi-directional perturbation in each of the gates. The conformation of the inner bundle gate was monitored by EPR spectroscopy while influencing the stability of the conductive conformation of the selectivity filter in two different ways: by inhibiting C-type inactivation through the E71A mutant or, by slowing down entry into C-type inactivation in the presence of Rb+ ions. In both cases, the pH-dependence of the inner bundle gate conformation shifts towards lower proton concentrations (Figure 1a). This effect was also found when the channels were bathed in Cs+, but is absent in the presence of impermeant ions (Supplementary materials, Figure S3). Since the selectivity filter mostly inactivates from the open-state24, we interpret these shifts as the energetic cost to be paid by the activation gate to favor the collapse of the selectivity filter. Therefore, stabilizing the conductive conformation of the filter reduces the amount of energy required to open the inner gate. Consistently, the structure of a fully open KcsA (32 Å diameter opening) in the presence of Rb+ ions at 3.3 Å resolution shows that the filter is unable to fully enter the collapsed inactivated state, when compared to the K+ occupied pore (Supplementary materials, Figure S4).
Figure 1.
Conformational coupling between activation and inactivation gates in K+ channels. a. Defining coupling from the selectivity filter to the activation gate by modulating the rate and extent of inactivation. Left panel, KcsA macroscopic currents obtained at 100 mV from liposome-reconstituted KcsA in 100 mM symmetric KCl (red), 100 mM symmetric RbCl (navy blue) or the mutant E71A in 100 mM symmetric KCl (light blue). The right panel shows the extent of inner gate opening for these three conditions in KcsA spin labeled at G116C, and monitored from the amplitude of the central resonance line normalized by the total number of spins. Asolectin-reconstituted spin-labeled channels in 100 mM symmetric KCl (red circles), 100 mM symmetric RbCl (navy blue circles) or the mutant E71A in 100 mM symmetric KCl (light blue circles). b. Defining coupling from the activation gate to the selectivity filter by modulating the extent of inner gate opening. Left, macroscopic currents at 100 mV and 100 mM symmetric KCl for wild-type KcsA (red) or Chymotrypsin C-terminal truncated channel KcsA Δ-125. Right panel, EPR spectra from the spin labeled Y82C mutant in the closed, conductive state (pH 7, black trace); open, inactivated state (pH 3, blue trace); and C-terminal truncated open, inactivated state (pH 3, red trace). EPR spectra were normalized by the total number of spins in the sample.
If gate coupling is indeed bi-directional, a perturbation at the activation gate should also modify the energetic stability of the selectivity filter. Figure 1b shows that deletion of the channel C-terminal helix bundle (KcsA-Δ125) sharply increases the rate and extent of inactivation without major effects on activation kinetics, leading to very low open probabilities at steady state. Accordingly, truncation of the channel C-terminal domain leads to an increase in the extent of gate opening (Supplementary materials, Figure S5), and a subtle increase in dipolar coupling (increased proximity) between spin labels at position Tyr8212 (Figure 1b, right panel) consistent with the proposed “collapsing” or “pinching” of the selectivity filter25.
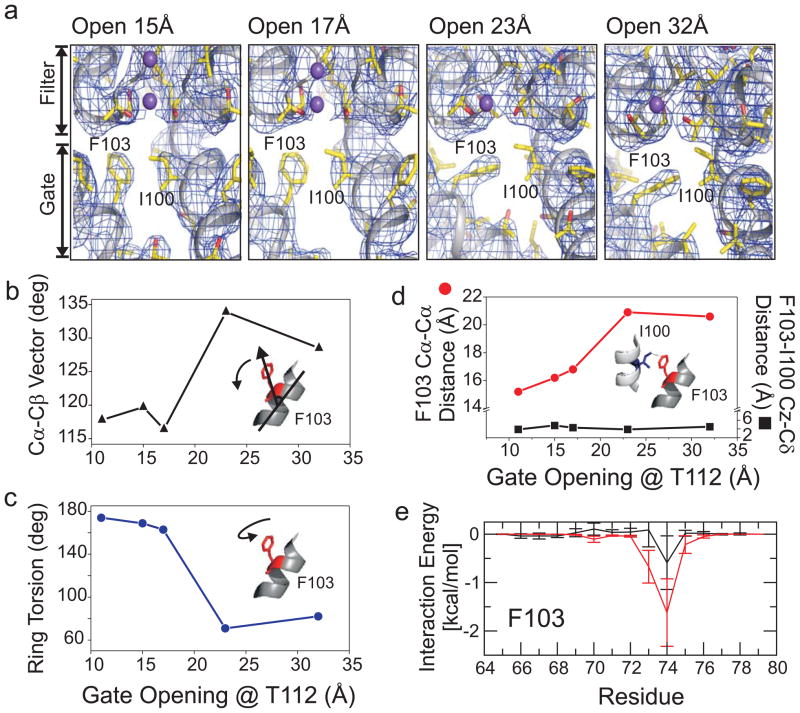
Evaluation of a series of KcsA structures with various degrees of gate opening5 suggests a likely mechanism underlying the transition from the C/O state to the O/I state. A sequential analysis of these crystal structures (Figure 2a, Supplementary material, Figure S6) revealed step-wise conformational changes at Phe103, a residue in close proximity to the C-terminal end of the KcsA pore helix. Phe103 changes its rotameric conformation as a function of channel opening (Figure 2b–d) so that given its spatial proximity to the selectivity filter, a steric clash could in principle promote the collapse of the filter. In fact, the side chain of Phe103 is absolutely obligated to change its rotameric state in response to the conformational change of the inner helix associated with the opening of the intracellular activation gate (Supplementary material, figure S7). Additional pieces of information provide some clues to the mode of action of Phe103 and how it affects the stability of the conductive state of the selectivity filter.
Figure 2.
Structural basis for allosteric coupling in KcsA. a. Conformational rearrangements in the KcsA aqueous cavity in a series of partially opened structures5. In each panel, the composite omit map (contoured at 2σ) of the channel corresponding to two neighbouring subunits is shown as a blue mesh. Transmembrane helices are represented as ribbons, and residues Ile100 and Phe103 are shown in stick representation, while ions are shown as space filling spheres. Dependence of the Phe103 side chain tilting (b.), Torsional angle (c.) Cα-Cα inter-subunit distance, and distance between F103 and Ile100 from adjacent subunits (d.) with the degree of opening of the inner bundle gate at Thr112. e. Interaction energies of residue F103 in TM2 with individual side chains from residues in the pore helix.
EPR spectroscopy indicates that when KcsA undergoes activation gating, a spin label attached to the mutant F103C undergoes an increase in its steric contacts (Supplementary materials, Figure S8). Furthermore, we have shown that when maximally open, the activation gate appears to compress the first helical turn (C-terminal end) of the pore helix, leading to a slight shortening the helical pitch5. These observations are suggestive of the steric nature of the allosteric coupling with the selectivity filter. To probe the molecular origin of the coupling between the activation gate and the selectivity filter, we examined the average interactions between the aromatic ring of Phe103 with residues along the pore helix during a molecular dynamics simulation. Significant non-polar van der Waals interactions (−2.4 kcal/mol) were found between the Phe103 side chain with Ile100 in the neighboring subunit and Thr74 and Thr75 in the same subunit, with additional minor interactions with Met96 (Figure 2e). These observations are consistent with ssNMR chemical-shift mapping suggesting a role for the interaction between Ile100 and residues Thr72-Thr75 in activation-inactivation coupling26 and help explain the intrinsic cooperativity of the process27.
The implicit prediction of these calculations is that severing the integrity of this coupling element should stop the communication between the two gates, generating a non-inactivating channel. Figure 3 shows that this is indeed the case. Mutating Phe103 to Ala inhibits entry into the inactivated conformation, as shown by steady-state single channel (Figure 3a, left) and macroscopic (Fig 3a, right, inset) currents at +100 mV. Further, interaction energies are sharply decreased in the F103A (Supplementary figure S9). Mutations at position 103 seemed to influence C-type inactivation in a size-dependent way: small side chain substitutions F103A and F103C limited entry into the inactivated state, while the larger F103W allowed the channel to inactivate (Figure 3a).
Figure 3.
Role of Phe103 in allosteric coupling with the selectivity filter. a. The mutation F103A sharply reduces the rate and extent of C-type inactivation. Left, representative single channel recordings from asolectin reconstituted channels in symmetric 100 mM KCl. Right, the extent of steady-state inactivation from the ratio between peak (Ipeak) and steady state (I∞) current plotted as a function of side chain volume for four substitutions at position 103. Multiple side-chain substitutions at position 103 show a volume dependence of the extent of inactivation, as derived from macroscopic currents during pH pulse experiments (inset). b. Mutation of Phe103 disrupts C-type inactivation in the deeply inactivated mutant E71H. Top, representative single channel recordings in symmetric 100 mM KCl from the E71H mutant background and the E71H-F103A double mutant. Bottom, crystal structure of E71H-F103A KcsA, determined at 3 Å resolution. Shown are the 2Fo−Fc electron density map (magenta mesh contoured at 2σ) of the filter corresponding to residues 70–80 from two diagonally symmetric subunits and the Fo−Fc omit map (cyan mesh contoured at 3σ) corresponding to ions in the filter.
Further support for the role of Phe103 in the allosteric communication between activation and inactivation gates comes from introducing the mutation F103A on the background of the extremely inactivating mutant E71H7. E71H readily enters the inactivated state by strengthening the interaction with Asp80 at the top of the selectivity filter, even in the closed or partially open states, leading to very short mean open times and a low steady state open probability7. In single channel traces, substitution of the Phe103 by an alanine on the E71H frame greatly destabilizes the inactivated conformation, increasing both mean open times and steady state open probability (Fig 3b, top panel). In agreement with this, the crystal structure of E71H-F103A KcsA, determined at 3 Å resolution (Fig 3b, bottom), shows the conductive form of the selectivity filter with its full complement of K+ ions, similar to that observed in closed KcsA.
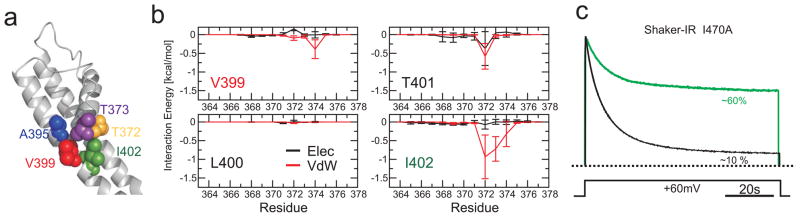
Given the high degree of functional and structural conservation among K+ channel pore domains, it is tempting to suggest that a common structural motif mediates the communication between gates in the K+ channel superfamily. To assess if the information flowing between the two gates in Kv-channels follows the same pathway as in KcsA, we evaluated the role of the Phe103-equivalent residues, a position which is often occupied by a bulky isoleucine (Supplementary Figure S10). Interaction energy calculations for Ile402 in Kv1.2 suggest strong van der Waals coupling with Thr372 and Thr373 (equivalent to Thr74 and Thr75 in KcsA) in the pore helix of the same subunit as in KcsA (Figure 4b). Mutating the same position in the pore domain of Shaker Kv-channel (Ile470) to Alanine effectively destabilizes the C-type inactivated state as determined from Cut-open oocyte voltage clamp experiments (Figure 4c). This is in agreement with the effect of mutant I470C in Shaker28, which severely reduced the rate of C-type inactivation, and with the role of the analogous position on hERG (Phe656) channels in inactivation gating29. Interestingly, when residue Ile470 is mutated to a tyrosine in Shaker, an inverted inactivating phenotype was observed, reminiscent of the fast inactivation of hERG channels30.
Figure 4.
A common gate coupling mechanism in K+ channels. a. Structural representation of the interface between the inner bundle gate (S6) and the pore helix in the Kv 1.2 pore domain (2R9R) with key amino acid side chains shown as van der Waals spheres (Equivalent KcsA/Kv 1.2 positions: Met 96/Ala391, blue; Ile100/Val399, Red; and Phe103/Ile401, green; T74/T373, yellow; T75/T374, purple). b. Interaction free energies of selected Kv1.2 residues in TM2 (equivalent to those in KcsA) with individual side chains from residues in the pore helix. c. Equivalent role of Ile470 in gate coupling in Shaker. Representative Cut-open oocyte voltage clamp traces obtained from N-type inactivation-removed Shaker (Shaker-IR), black trace and the mutation I470A (green trace) expressed in Xenopus oocytes (n=5 for I470A). Currents were normalized to the peak current.
The basic features of the network of residues forming this allosteric communication pathway appear to be conserved from prokaryotes to eukaryotes. Key among these residues is Phe103 (in KcsA) and its equivalent position in Kv channels. Although the nature of the interactions between Phe103 and the pore helix remains to be elucidated, the apparent volume dependence in the C-type inactivation coupling suggest that a steric component must play a significant role. More importantly, the present mechanism can lend a structural basis to the complex interplay between C-type inactivation and pore block in hERG channels, with obvious clinical relevance29.
Methods Summary
Single channel and macroscopic current measurements were carried out by liposome patch clamping as described in the accompanying manuscript7. The conformational state of the inner bundle gate was evaluated by CW-EPR and fluorescence spectroscopy. Both site-directed spin and fluorophore labeling were carried out at position G116 before channel reconstitution onto pre-formed asolectin liposomes. Crystals of the E71H-F103A mutant and of the open KcsA mutant in Rb+ ions were obtained in complex with a Fab fragment by the sitting drop method in 20–25 % PEG400 (v/v), 50 mM magnesium acetate, 50 mM sodium acetate (pH 5.4–5.6) at 20 °C. The structures were determined by molecular replacement the Fab fragment and the extracellular part of WT KcsA (PDB 1K4C) without the selectivity filter as search model. MD simulations were performed with an all-atom system that included the open inactive KcsA channel7 embedded in dipalmitoylphosphatidylcholine (DPPC) lipids and surrounded by an aqueous solution of 200 mM KCl. Average interaction energies were calculated from MD simulations performed using the programs CHARMM and NAMD with the all-atom PARAM27 force field.
Methods
Molecular biology and protein expression
For generating KcsA-OM construct, PCR overlapping method was used to introduce all mutations at once. KcsA-OM was expressed and purified as described elsewhere31,32 with some modifications. Protein expression was carried out in E. coli at RT and 20 mM BaCl2 for 18 h. Protein purification was carried out by solubilizing KcsA containing E. coli membranes with 10 mM DodecylMaltoside (ANATRACE) in a buffer with 150 mM KCl and 50 mM Tris-Cl pH 7.0. Solubilization mixture is spun down @ 100,000 g and the supernatant was loaded on to a cobalt column (TALON) and washed extensively with 10 mM imidazole followed by elution with 500 mM imidazole. After purification, protein was loaded on to a Superdex HR 200 (Amersham) size exclusion chromatography to assess proper folding and stability of KcsA.
Spin labeling of cysteine mutants for EPR and reconstitution in liposomes
KcsA cysteine mutants while still bound to the cobalt column were washed extensively with 0.5 mM TCEP/TCYP and eluted with buffer that has been previously degassed. Once the protein was eluted, it was concentrated to 3–4 mg/ml and labeled with methane-thiosulfonate spin-label (MTS-SL; Toronto Research Chemical) in at 10:1 SL to channel molar ratio. The excess of spin label was removed before reconstitution by passing the mixture through a desalting column (PD-10 column, AMERSHAM). KcsA spin-labeled mutant were reconstituted in preformed asolectin liposome in 1:400 channel to lipid molar ratio. After diluting the protein-liposome mixture with a buffer containing 150 mM KCl and 50 mM tris-Cl pH 7.0, the sample was incubated with ~0.5 g of Bio-Beads (Bio-Rad) overnight. The samples were spun down at 100,000 g for 2 hours. For eliciting pH-dependent conformational changes, the samples were re-suspended with a high capacity buffer at the desired pH and/or ionic strength and then spun down to a small pellet, which was resuspended in a small volume for EPR measurements12,33,34.
Fluorescence Spectroscopy
Purified KcsA-OM 116C were labeled with a 10 fold molar excess of a mixture of Alexa-Fluor350 (Donor) and Alexa-Fluor 488 (Acceptor), where the proportion of acceptor varies from 0 to 1. After 2 hours the labeling reactions were stopped by the addition of large excess of L-Cysteine, and the excess of probe was removed by a PD10 desalting colomn (Amersham), followed by a gel exclusion chromatography on a Amersham Sephadex 200 FPLC. Each of the labeled samples were split into 2 fractions, one of them being submitted to Chymotrypsin digestion as previously described. The samples (truncated and non truncated) were then normalized to the same donor concentration and the fluorescence spectra (λex = 350) were recorded on a Steady State - photon counting fluorimeter (QM-4 PTI) from 400 to 650 nm. The normalized fluorescence intensity (FDA/FD) is plotted as a function of the acceptor molar fraction (a), and the experimental plot were fitted with the equation IDA/ID =1 − E +E(1 − a)n−1, where E is the transfer efficiency, and n the oligomeric state. Our experimental results were best fitted when n=4 (tetramer).
Liposome patch clamp
Liposome-reconstituted KcsA was patch clamped following the method of Delcour et al.35, with some modifications6,36. KcsA was reconstituted as above at protein-to-lipid rations varying from 1:100 to 1:5000. The proteoliposome suspension was centrifuged for 1 h at 100,000 g, and the pellet, corresponding to 10 mg of lipids, was resuspended in 60 μl of rehydration buffer. Typically, three drops of the suspension were dried overnight in a desiccation chamber under vacuum for 24 h, at which time 20 μl of rehydration buffer were applied to each dried drop. Rehydration was allowed proceed for 5 h, yielding liposomes suitable for patch clamp. All patch-clamp measurements were done in symmetrical conditions: 200 mM KCl and MOPS buffer, pH 4.0, at room temperature. Single-channel currents were recorded with an Axopatch 200B patch clamp amplifier, and currents were sampled at 40 kHz with analogue filter set to 2 kHz (−3 dB). Pipette resistances were 2 MΩ.
Crystallization of KcsA-OM
Mutant E71H/F103A in the KcsA_OM background was expressed and purified to homogeneity as described above. is the channel was crystallized in the presence of antibody Fab fragment by sitting drop method as described previously37. For crystals of KcsA-OM with Rb+, after complex formation, K+ was exchanged for 150mM RbCl by gel filtration. Cubic shaped crystal of KcsA-Fab complex appeared after a week in a sitting drop with the following composition 20–25 % PEG400 (v/v), 50 mM magnesium acetate, 50 mM sodium acetate (pH 5.4–6.0) at RT. Based on our previous experience, PEG concentration in the reservoir was increased regularly to decrease the water content in the drop to achieve reduced B-factors. Crystals diffracted to Bragg spacing of 3.3 Å. Data was collected on beamlines 23ID (GMCA) 24IDC (NECAT) at the Advanced Photon Source and processed with HKL200038.
Crystallographic analysis
Structures were solved by molecular replacement using only Fab fragment and extracellular part of WT KcsA (PDB 1K4C) without the selectivity filter as search model to reduce the biasing of model prediction, as the expected conformation is supposed to be different from the closed state. In the first cycle of refinement using CNS39, electron density corresponding to the intracellular part with TM2 splayed open appeared, which was filled by manual rebuilding using O program40. Selectivity filter was built with side-chain density corresponding to V76, Y78 and D80 as markers. Multiple cycles of manual rebuilding and refinement was carried out till the complete model is built into the electron density while minimizing Rfactor values. Electron density could be seen only up to residue 117 in open-inactivated and 112 in open-activated structures, and unresolved side-chains were assigned as alanines. Data collection and refinement statistics are given in Supplementary Table 1. One-dimensional electron density profiles along the symmetry axis of the selectivity filter for structures in Rb+ were obtained as described elsewhere. Briefly, CNS37,41 is used to calculate the difference Fourier maps of open-activated structure at 3.3 Å and the closed state structure (PDB 1R3I) at 2.4 Å in the presence of Rb+. We did not scale down the resolution of the closed state as we are interested in qualitative comparison of the electron density peaks corresponding to the ions in the filter binding sites.
Molecular dynamics simulations
The simulation system was represented by an atomic model of the open inactive KcsA channel5 embedded in dipalmitoylphosphatidylcholine (DPPC) lipids and surrounded by an aqueous solution of 150 mM KCl. The model contained the KcsA tetramer (404 amino acid residues), 112 DPPC molecules, 7,325 water molecules and 2 K+ ions in the pore at positions S1 and S4. To ensure electrical neutrality and mimic a 150 mM KCl concentration, 18 K+ and 32 Cl− ions were added to the bulk solution. The system was set up using CHARMM42 following a previously described methodology 43. Constant pressure molecular dynamics simulations were done using the NAMD program 44. The F103A mutation system was generated in silico on all four KcsA subunits and carefully equilibrated before the production run. Interaction energies were calculated between the side-chain of residue 103 with residues 60 to 80 on the same subunit averaged over all four subunits during an 11.5 ns molecular dynamics trajectory. The Kv1.2 simulations were described previously in45. Error bars are standard deviations over interaction energy averages sampled once every ps. Adiabatic energy maps were obtained by varying the \chi1 and \chi2 dihedral angles of Phe103 in one subunit of KcsA and calculating the energy of those conformations after performing a minimization. The system consisted of the KcsA tetramer in vacuum. Atoms far from the Phe103 were fixed in space (residues 22 to 28, 46 to 65 and 115 to 124) and heavy atoms near Phe103 (all others except for the Phe103 sidechain) were harmonically restrained with a force constant of 100 kcal/(mol*A2).
Supplementary Material
Acknowledgments
We thank Drs. F. Bezanilla, H Mchaourab and R. Nakamoto for helpful discussions and comments on the manuscript. Dr. K. Locher for comments on the manuscripts. Dr. R Mackinnon kindly provided the Fab-expressing hybridoma cells. The members of the Perozo lab for comments on the manuscript. We are thankful to Dr. Kanagalaghatta R. Rajashankar, Dr. Ruslan Sanishvili and the staff at the NE-CAT 24ID and GM-CA 23ID beamlines at the Advanced Photon Source, Argonne National Laboratory. This work was supported in part by NIH grant R01-GM57846 and by a gift from the Palmer family to E.P., R01-GM62342 to B.R. and a NRSA postdoctoral fellowship to A.C.P.
Footnotes
Author Contributions
E.P. and L.G.C. conceived the project. L.G.C and D.M.C generated constructs, performed biochemical analysis, expressed, purified and crystallized the proteins. L.G.C. performed EPR experiments. L.G.C, V.J. and E.P collected X-ray diffraction data. L.G.C and V.J. determined and analyzed the structures. A.C.P and B.R. performed computation analysis. O.D and L.G.C conducted FRET measurements. S.C. measured inactivation in truncated KcsA and in rubidium ions. J.F.C.M measured E71H single channel activity and made the F103A mutation. L.G.C measured F103X mutant series electrophysiology. D.G.C measured electrophysiology in Shaker channel. E.P., L.G.C and V.J. analyzed the data and wrote the paper.
Author information
The atomic coordinates of the mutant E71H-F103A in the OM-KcsA background and of OM-KcsA in Rb+ ions have been deposited in the Protein data Bank under accession codes 3HPL and 3FB7, respectively. Reprints and permissions information is available at www.nature.com/reprints.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Panyi G, Deutsch C. Cross talk between activation and slow inactivation gates of Shaker potassium channels. J Gen Physiol. 2006;128:547–559. doi: 10.1085/jgp.200609644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadovsky E, Yifrach O. Principles underlying energetic coupling along an allosteric communication trajectory of a voltage-activated K+ channel. Proc Natl Acad Sci U S A. 2007;104:19813–19818. doi: 10.1073/pnas.0708120104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baukrowitz T, Yellen G. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+ channel. Science. 1996;271:653–656. doi: 10.1126/science.271.5249.653. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Barneo J, Hoshi T, Heinemann SH, Aldrich RW. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1:61–71. [PubMed] [Google Scholar]
- 5.Cuello L, Jogini V, Perozo E. Structural mechanism of C-type inactivation in K+ channnels. Nature. 2010 doi: 10.1038/nature09153. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordero-Morales JF, et al. Molecular determinants of gating at the potassium-channel selectivity filter. Nat Struct Mol Biol. 2006;13:311–318. doi: 10.1038/nsmb1069. [DOI] [PubMed] [Google Scholar]
- 7.Cordero-Morales JF, et al. Molecular driving forces determining potassium channel slow inactivation. Nat Struct Mol Biol. 2007;14:1062–1069. doi: 10.1038/nsmb1309. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong CM. Voltage-gated K channels. Sci STKE. 2003;2003:re10. doi: 10.1126/stke.2003.188.re10. [DOI] [PubMed] [Google Scholar]
- 9.Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2002;419:35–42. doi: 10.1038/nature00978. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y, et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Holmgren M, Jurman ME, Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19:175–184. doi: 10.1016/s0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 12.Perozo E, Cortes DM, Cuello LG. Structural rearrangements underlying K+-channel activation gating. Science. 1999;285:73–78. doi: 10.1126/science.285.5424.73. [DOI] [PubMed] [Google Scholar]
- 13.Choi KL, Aldrich RW, Yellen G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc Natl Acad Sci U S A. 1991;88:5092–5095. doi: 10.1073/pnas.88.12.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- 15.Kiss L, LoTurco J, Korn SJ. Contribution of the selectivity filter to inactivation in potassium channels. Biophys J. 1999;76:253–263. doi: 10.1016/S0006-3495(99)77194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demo SD, Yellen G. Ion effects on gating of the Ca(2+)-activated K+ channel correlate with occupancy of the pore. Biophys J. 1992;61:639–648. doi: 10.1016/S0006-3495(92)81869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swenson RP, Jr, Armstrong CM. K+ channels close more slowly in the presence of external K+ and Rb+ Nature. 1981;291:427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]
- 18.Alagem N, Yesylevskyy S, Reuveny E. The pore helix is involved in stabilizing the open state of inwardly rectifying K+ channels. Biophys J. 2003;85:300–312. doi: 10.1016/S0006-3495(03)74475-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman ML, Blanke ML, Krovetz HS, Vandongen AM. Allosteric effects of external K(+) ions mediated by the aspartate of the GYGD signature sequence in the Kv2.1 K(+) channel. Pflugers Arch. 2006;451:776–792. doi: 10.1007/s00424-005-1515-2. [DOI] [PubMed] [Google Scholar]
- 20.Proks P, Capener CE, Jones P, Ashcroft FM. Mutations within the P-loop of Kir6.2 modulate the intraburst kinetics of the ATP-sensitive potassium channel. J Gen Physiol. 2001;118:341–353. doi: 10.1085/jgp.118.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulte U, Weidemann S, Ludwig J, Ruppersberg J, Fakler B. K(+)-dependent gating of K(ir)1.1 channels is linked to pH gating through a conformational change in the pore. Journal of Physiology. 2001;534:49–58. doi: 10.1111/j.1469-7793.2001.t01-1-00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben-Abu Y, Zhou Y, Zilberberg N, Yifrach O. Inverse coupling in leak and voltage-activated K+ channel gates underlies distinct roles in electrical signaling. Nat Struct Mol Biol. 2009;16:71–79. doi: 10.1038/nsmb.1525. [DOI] [PubMed] [Google Scholar]
- 23.Monod J, Wyman J, Changeux JP. On the Nature of Allosteric Transitions: A Plausible Model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 24.Chakrapani S, Cordero-Morales JF, Perozo E. A quantitative description of KcsA gating I: macroscopic currents. J Gen Physiol. 2007;130:465–478. doi: 10.1085/jgp.200709843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Jurman ME, Yellen G. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 1996;16:859–867. doi: 10.1016/s0896-6273(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 26.Ader C, et al. Coupling of activation and inactivation gate in a K+-channel: potassium and ligand sensitivity. EMBO J. 2009;28:2825–2834. doi: 10.1038/emboj.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotem D, Mason A, Bayley H. Inactivation of the KcsA potassium channel explored with heterotetramers. J Gen Physiol. 2010;135:29–42. doi: 10.1085/jgp.200910305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olcese R, Sigg D, Latorre R, Bezanilla F, Stefani E. A conducting state with properties of a slow inactivated state in a shaker K(+) channel mutant. J Gen Physiol. 2001;117:149–163. doi: 10.1085/jgp.117.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Seebohm G, Sanguinetti MC. Position of aromatic residues in the S6 domain, not inactivation, dictates cisapride sensitivity of HERG and eag potassium channels. Proc Natl Acad Sci U S A. 2002;99:12461–12466. doi: 10.1073/pnas.192367299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klement G, Nilsson J, Arhem P, Elinder F. A tyrosine substitution in the cavity wall of a k channel induces an inverted inactivation. Biophys J. 2008;94:3014–3022. doi: 10.1529/biophysj.107.119842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuello LG, Romero JG, Cortes DM, Perozo E. pH-dependent gating in the Streptomyces lividans K+ channel. Biochemistry. 1998;37:3229–3236. doi: 10.1021/bi972997x. [DOI] [PubMed] [Google Scholar]
- 32.Cortes DM, Perozo E. Structural dynamics of the Streptomyces lividans K+ channel (SKC1): oligomeric stoichiometry and stability. Biochemistry. 1997;36:10343–10352. doi: 10.1021/bi971018y. [DOI] [PubMed] [Google Scholar]
- 33.Liu YS, Sompornpisut P, Perozo E. Structure of the KcsA channel intracellular gate in the open state. Nat Struct Biol. 2001;8:883–887. doi: 10.1038/nsb1001-883. [DOI] [PubMed] [Google Scholar]
- 34.Cuello LG, Cortes DM, Perozo E. Molecular architecture of the KvAP voltage-dependent K+ channel in a lipid bilayer. Science. 2004;306:491–495. doi: 10.1126/science.1101373. [DOI] [PubMed] [Google Scholar]
- 35.Delcour AH, Martinac B, Adler J, Kung C. Modified reconstitution method used in patch-clamp studies of Escherichia coli ion channels. Biophys J. 1989;56:631–636. doi: 10.1016/S0006-3495(89)82710-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortes DM, Cuello LG, Perozo E. Molecular architecture of full-length KcsA: role of cytoplasmic domains in ion permeation and activation gating. J Gen Physiol. 2001;117:165–180. doi: 10.1085/jgp.117.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 38.Otwinowski ZMW. Processing of X-ray diffraction data collected in oscillation mode. Methods in Enzymology. 1997;276:307–332. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 39.Brunger AT, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 40.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47 (Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 41.Morais-Cabral JH, Zhou Y, MacKinnon R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 2001;414:37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- 42.Brooks BR, et al. CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. Journal of Computational Chemistry. 1983;4:187–217. [Google Scholar]
- 43.Berneche S, Roux B. Molecular dynamics of the KcsA K(+) channel in a bilayer membrane. Biophys J. 2000;78:2900–2917. doi: 10.1016/S0006-3495(00)76831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips JC, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26 :1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jogini V, Roux B. Dynamics of the Kv1.2 voltage-gated K+ channel in a membrane environment. Biophys J. 2007;93:3070–3082. doi: 10.1529/biophysj.107.112540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.