Abstract
Globally, developed nations spend a significant amount of their resources on healthcare initiatives that poorly translate into increased population life expectancy. As an example, the United States devotes sixteen percent of its gross domestic product to healthcare, the highest level in the world, but falls behind other nations that enjoy greater individual life expectancy. These observations point to the need for pioneering avenues of drug discovery to increase lifespan with controlled costs. In particular, innovative drug development for metabolic disorders such as diabetes mellitus (DM) becomes increasingly critical given that the number of diabetic individuals will increase exponentially over the next twenty years. Here we discuss the elucidation and targeting of novel cellular pathways that are intimately tied to oxidative stress in DM for new treatment strategies. Pathways that involve wingless, NAD+ precursors, and cytokines govern complex biological pathways that determine both cell survival and longevity during DM and its complications. Furthermore, the role of these entities as biomarkers for disease can further enhance their utility irrespective of their treatment potential. Greater understanding of the intricacies of these unique cellular mechanisms will shape future drug discovery for DM to provide focused clinical care with limited or absent long-term complications.
Keywords: biomarkers, diabetes, oxidative stress
Healthcare and metabolic disease
Compared with other nations throughout the world, the United States devotes 16% of the gross domestic product to healthcare and spending for each individual equal to $7,290, the highest levels in the world 1. Total spending on pharmaceuticals is also the highest in the world with $878 per individual. Yet, life expectancy in years in the United States equals 78.1 years and trails behind other countries such as Japan that allots 8% of the gross domestic product on healthcare, spends $2,514 for each individual, and has a life expectancy of 82.6 years. Furthermore, the United States is ranked as having the highest level of obesity in the population at 34.3% while countries such as Japan have a 3.4% level of obesity 1. These statistics are the results of multiple factors, but also can support the arguments for not only improved preventive health measures, but also new directions to treat multiple disorders that can lead to improved lifespan while minimizing economic burden.
In particular, one can consider diabetes mellitus (DM), a metabolic disorder closely associated with increased weight gain 2, 3. DM reaches approximately 20 million individuals in the United States and more than 165 million individuals worldwide 4. By 2030, DM may affect more than 360 million individuals. Additionally, a significant portion of the population has undiagnosed diabetes, illustrating the need for improved early diagnosis 5. The incidence of impaired glucose tolerance in the young also raises further concerns 6. Individuals with impaired glucose tolerance have a greater than twice the risk for the development of diabetic complications than individuals with normal glucose tolerance 7.
Type 1 insulin-dependent DM is present in 5-10 percent of all diabetics, but is increasing in adolescent minority groups 2, 8. Furthermore, Type 1 DM leads to long-term complications throughout the body involving cardiovascular, renal, and nervous system disease 9. Type 1 DM is associated with the presence of alleles of the Human leukocyte antigen (HLA) class II genes within the major histocompatibility complex (MHC). The disorder is considered to have autoimmune origins resulting from inflammatory infiltration of the islets of Langerhans and the selective destruction of β-cells in the pancreas that leads to insulin loss 10. In Type 1 DM, activation of T-cell clones that are capable of recognizing and destroying β-cells lead eventually to severe insulin deficiency. These T-cell clones are able to escape from thymus control that yield high affinity for major histocompatibility complex (MHC) molecules with T-cell receptors but incorrect low affinity for self-peptides. Once released into the bloodstream, these T-cell clones can become activated to destroy self-antigens. In many cases, the insulin gene (INS) and the human MHC or HLA complex are believed to contain the loci with IDDM1 and IDDM2 to account for the susceptibility to Type 1 DM with defective antigen presentation 11, 12. Interestingly, a HLA class II molecule has been linked to Type 1 DM inheritance. HLA-DQ that lacks a charged aspartic acid (Asp-57) in the β-chain is believed to lead to the ineffective presentation of autoantigen peptides during thymus selection of T-cells 13. Animal models that involve the nonobese diabetic (NOD) mice further support these findings, since these mice spontaneously develop diabetes with the human predisposing HLA-DQ corresponding molecule of H2 I-Ag. Yet, NOD mice without H2 I-Ag do not develop diabetes 14.
Upon initial diagnosis, approximately ninety percent of individuals with Type 1 DM have elevated titers of autoantibodies (Type 1A DM). The remaining ten percent of Type 1 DM individuals do not have serum autoantibodies and are described as having maturity-onset diabetes of the young (MODY) that can be a result of β-cell dysfunction with autosomal-dominant inheritance (Type 1B DM) 15. Other variables reported in patients with Type 1 DM include the presence of insulin resistance that is usually characteristic of Type 2 DM and can lead to neurological and vascular disease 16, 17. Interestingly, there is a converse overlap with Type 1 and Type 2 DM, since almost ten percent of Type 2 DM patients may have elevated serum autoantibodies 18.
Monogenic inheritance does not appear to lead to Type 1 DM. Prior work demonstrates that multiple loci with possible epistatic interactions among other loci may be responsible for genetic transmission 19, 20. In addition, several environmental factors may have a role with Type 1 DM such that investigations have suggested that Type 1 DM in monozygotic twins can occur with a cumulative risk of seventy percent from birth to 35 years of age 21, 22. Other studies indicate a concordance between monozygotic twins to be approximately fifty percent 23, suggesting that environmental factors also may lead to a predisposition for Type 1 DM. Loss of autoimmunity in Type 1 DM can be precipitated also by the exposure to infectious agents 24.
Type 2 noninsulin-dependent DM represents at least 80 percent of all diabetics, usually in individuals over 40 years of age, and is dramatically increasing in incidence as a result of changes in human behavior and increased body mass index 2, 8. Type 2 DM is characterized by a progressive deterioration of glucose tolerance with early β-cell compensation for insulin resistance (achieved by β-cell hyperplasia). This is subsequently followed by progressive decrease in β-cells mass. In contrast, gestational diabetes mellitus that represents glucose intolerance during some cases of pregnancy usually subsides after delivery.
Insulin resistance or defective insulin action occurs when physiological levels of insulin produce a subnormal physiologic response. Skeletal muscle and liver are two of the primary insulin-responsive organs responsible for maintaining normal glucose homeostasis. Insulin lowers the level of blood glucose through suppression of hepatic glucose production and stimulation of peripheral glucose uptake, but metabolic disorders can result in insulin resistance and elevated serum glucose levels. Although insulin resistance forms the basis for the development of Type 2 DM, elevated serum glucose levels also are a result of the concurrent impairment in insulin secretion. This abnormal insulin secretion may be a result of defective β-cell function, chronic exposure to free fatty acids and hyperglycemia, and the loss of inhibitory feedback through plasma glucagon levels 25.
Patients with DM can develop multiple complications that include immune dysfunction 26, sarcopenia 27, depression 28, hepatic dysfunction 29, renal disease 30, anemia and hematological disease 31-33, neurodegenerative disorders 8, 34, 35, and cardiovascular disease 8, 36. Interestingly, patients with DM are at risk for the development of cognitive disorders 26, 37. In a prospective population based study of 6,370 elderly individuals, patients with DM had almost twice the risk for the development of dementia 38. DM also has been found to increase the risk for vascular dementia in elderly subjects 39, 40. Although some studies have found that diabetic patients may have significantly less neuritic plaques and neurofibrillary tangles than non-diabetic patients 41, other investigators report a modest adjusted relative risk of Alzheimer's disease in patients with diabetes as compared with those without diabetes to be 1.3 42, 43. Additional studies have described the reduced expression of genes encoding insulin in Alzheimer's patients that suggests a potential link between DM and the development of Alzheimer's disease 43. Alzheimer's disease can be the result of a number of etiologies 44, 45, such as changes in cerebral blood flow and metabolism with aging 46, sialylation and glycosylation of amyloid plaques 47, 48, aberrant cell cycle induction 49-51, amyloid toxicity 51-55, chemokine induction 56, exogenous toxins 57, alteration in muscarinic and nicotinic pathways 46, 58, and intracellular calcium changes 59. Yet, other studies point to metabolic dysfunction 60-62. For example, in animal models with brain/neuronal insulin receptor knockouts, loss of insulin signaling appears to be linked to increased phosphorylation of the microtubule-associated protein tau that occurs during Alzheimer's disease 63.
Oxidative stress, apoptotic injury, mitochondria, and diabetes
Many of the cellular pathways that lead to diabetic complications and insulin resistance have been linked to the generation of free radicals and oxidative stress 2, 3, 34, 35, 62, 64, 65. In animal studies with Type 1 diabetic animals, oxidative stress leads to DNA damage in renal cortical cells 66. Although early effects of elevated glucose may increase the presence of potentially protective pathways 67, more prolonged exposure of elevated glucose can lead to reactive oxygen species (ROS) 32, 68 and can be detrimental even if glucose levels are controlled 69. In addition, elevated levels of ceruloplasmin during hyperglycemia are suggestive of increased ROS 70. A number of treatment entities seek to ameliorate the effects of oxidative stress during DM 55, 71-75.
Oxidative stress also may promote the onset of DM by decreasing insulin sensitivity and destroying the insulin-producing cells within the pancreas. For example, ROS can penetrate through cell membranes and cause damage to β-cells of pancreas 76, 77. A high fat diet 78 or free fatty acids also have been shown to release ROS and contribute to mitochondrial DNA damage and impaired pancreatic β-cell function 79. Interestingly in non-diabetic rats, hyperglycemia has been shown to increase muscle protein carbonyl content and elevated levels of malondialdehyde and 4-hydroxynonenal, indicators of oxidative stress and lipid peroxidation 80. These biomarkers of oxidative stress and insulin resistance suggest that ROS contribute to the pathogenesis of hyperglycemia-induced insulin resistance 81, 82 as well as insulin induced ROS 73. Hyperglycemia can lead to increased production of ROS in several cell types 82, 83. For example, with increased age in a rat model of nonobese Type 2 DM, increased levels of 8-OHdG and HNE-modified proteins in pancreatic beta-cells have been reported 84. Elevated glucose also has been shown to increase antioxidant enzyme levels in human endothelial cells, suggesting that elevated glucose levels may lead to a reparative process to protect cells from oxidative stress injury 85. Chronic hyperglycemia is not necessary to lead to oxidative stress injury, since even short periods of hyperglycemia, generate ROS, such as in vascular cells 86. Recent clinical correlates support these experimental studies to show that acute glucose swings in addition to chronic hyperglycemia can trigger oxidative stress mechanisms during Type 2 DM, demonstrating the importance for therapeutic interventions during acute and sustained hyperglycemic episodes 87.
At the cellular level, ROS are oxygen free radicals and other chemical entities that can lead to cell injury if left unchecked 49, 88, 89. Oxidative stress can result in hepatic injury 90, 91, pancreatitis 92, impaired cognition 93, 94, neuronal injury 44, 49, 95-99, Parkinson's disease 100-103, complications of epilepsy 104, cardiovascular disease 105-107, ocular disease 108, age-related disorders 109, metal ion injury 110, uncontrolled pain sensation 111, and promote xenobiotic toxicity 112, 113. ROS consist of superoxide free radicals, hydrogen peroxide, singlet oxygen, nitric oxide (NO), and peroxynitrite 34, 44, 83. ROS usually occur at low levels during normal physiological conditions and are scavenged by endogenous antioxidant systems that include superoxide dismutase (SOD), glutathione peroxidase, catalase, and vitamin D3 114, 115. Additional pathways include vitamins C, E, and K 104, 116-119.
Oxidative stress results in cell injury through apoptotic and non-apoptotic pathways. In regards to programmed cell death (apoptosis), apoptosis can occur during DM 2, 3, 120, 121, anesthetic exposure 122, tissue ischemia 123-126, bone fatigue 127, neurodegenerative disorders 34, 128-130 and Alzheimer's disease 51-54, 59, 131-136, plasticity associated with ischemic preconditioning 137, aging-related diseases 35, 138, 139, and toxic conditions during development 122, 140.
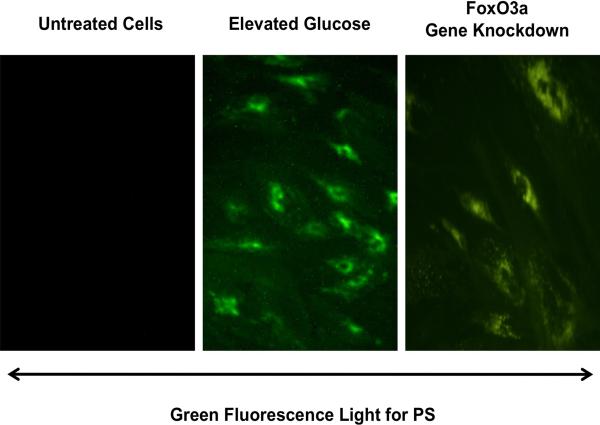
During apoptosis, the cleavage of genomic DNA into fragments occurs 129, 141, 142 as a later event during apoptotic injury 142-145 after the exposure of membrane phosphatidylserine (PS) residues 146, 147. Membrane PS exposure occurs in neurons, vascular cells, and inflammatory microglia during reduced oxygen exposure 50, 89, 141, 148, 149, β-amyloid (Aβ) exposure 53, 55, nitric oxide exposure 150-154, and during the administration of agents that induce the production of reactive oxygen species (ROS), such as 6-hydroxydopamine 103 (Figure 1). Membrane PS externalization also occurs on platelets and has been associated with clot formation in the vascular system 155.
Figure 1. Transfection of FoxO3a siRNA in endothelial cells prevents apoptotic phosphatidylserine (PS) exposure during elevated D-glucose.
Representative images illustrate that gene knockdown of FoxO3a with FoxO3a siRNA (siRNA) significantly blocks endothelial cell membrane PS externalization assessed by annexin V phycoerythrin (green fluorescence). FoxO3a siRNA alone was not toxic and non-specific scrambled siRNA did not reduce PS exposure during elevated D-glucose.
Membrane PS exposure also is involved in the activation of inflammatory cells such as microglia of the central nervous system that can dispose of injured cells 156, 157. For their own survival, microglia and non-neuronal cells of the brain are dependent upon several intracellular pathways, such as mTOR 158, 159 and zinc regulation 160. Non-neuronal cells of the brain can be beneficial to modulate neurogenesis 161, to function as immune surveillance for toxic products 162, such as for β-amyloid 48, to block foreign organisms and viral agents from proliferating in the brain 163, to modulate vascular growth 164, and to allow for the repair of tissues composed of neuronal and vascular cells 158, 165. Yet, microglia have another side that may be detrimental to an organism. They can generate ROS 166, 167, may worsen events with oxidative stress injury 168, and activate cytokines that in some circumstances may initially lead to cell proliferation 169, but later can result in the demise of cells 56, 163, 164. For these reasons, it is important to understand the mechanisms that can activate microglia. Membrane PS exposure can become a signal for microglia to dispose of injured cells 61, 145, 170-172. This process can be controlled by caspase 1 and caspase 3 89, 173, 174. Increased expression of the phosphatidylserine receptor (PSR) on microglia also occurs to facilitate activation of microglia 116, 175.
Oxidative stress and apoptotic cell death during disorders such as DM are also strongly associated to cellular energy maintenance and intact mitochondrial function 8, 26, 64, 176-178. ROS exposure can result in the opening of the mitochondrial membrane permeability transition pore 145, 179-181, reduce mitochondrial NAD+ stores, and result in apoptotic cell injury 44. Free fatty acids also can lead to ROS release, mitochondrial DNA damage, and impaired pancreatic β-cell function 79. In patients with Type 2 DM, skeletal muscle mitochondria have been described to be smaller than those in control subjects 182. A decrease in the levels of mitochondrial proteins and mitochondrial DNA in adipocytes also has been correlated with the development of type 2 DM 183.
Innovative drug discovery for DM
Multiple pathways may lead to a loss in cell survival and longevity during DM that are broad in nature and consist of several precipitating factors. Yet, oxidant-induced injury and the cellular pathways responsible for this signaling are thought to be primary mediators of the disability that ensues during DM. As a result, novel and pioneering directions for drug discovery are required for safe and effective treatments for DM. In addition, it is the understanding of the complex nature of cellular pathways and their intimate relationship that is critical for the development of successful drug platforms. Here we present novel cellular pathways that are strongly bound to oxidant pathways in DM. These pathways involve wingless genes with Wnt, NAD+ precursors with nicotinamide, forkhead transcription factors of the “O” class, and the cytokine and growth factor erythropoietin (EPO), each of which determine cellular development, survival and injury mechanisms, and longevity.
Novel cellular pathways and diabetes
Wnt
Proteins derived from the Drosophila Wingless (Wg) and the mouse Int-1 genes are secreted cysteine-rich glycosylated proteins that play a role in a variety of cellular functions 53, 72, 175, 184. Wnt proteins determine multiple cellular functions that involve embryonic cell proliferation, cell differentiation, and cell survival that involve neurons, cardiomyocytes, endothelial cells, red blood cells, tumors, adipose tissue as well as several other cell types 185-198. Recent work in clinical disease indicates that abnormalities in Wnt pathways, such as with transcription factor 7-like 2 gene, may impart increased risk for type 2 DM in some populations 199-201 and have a strong association with the development of obesity 202 (Table 1). The family member Wnt5b has been shown to have an elevated expression in adipose tissue, the pancreas, and the liver in patients with DM, suggesting control of metabolic pathways by Wnt 203. In addition, clinical studies in patients with coronary artery disease and the combined metabolic syndrome with hypertension, hyperlipidemia, and DM have shown impaired Wnt signaling through a missense mutation in LRP-6 204. Experimental studies in mice with hyperglycemia through a high fat diet also show increased expression of Wnt3a and Wnt7a 205.
Table 1.
Summary of Clinical Outcomes and Signaling Pathways with Wnt, Nicotinamide, and Erythropoietin
| Therapeutic Presentation and Potential During Diabetes Mellitus (DM) | Clinical Outcomes | Signaling Pathways |
|---|---|---|
| Wnt | Wnt pathways, such as with transcription factor 7-like 2 gene, may impart increased risk for type 2 DM Wnt may have an association with the development of obesity Wnt has an elevated expression in adipose tissue, the pancreas, and the liver in patients with DM Impaired Wnt signaling through a missense mutation in LRP-6 during metabolic syndrome |
Vascular/renal cell early and late apoptotic programs decreased by Wnt Wnt utilizes EPO for protection against elevated glucose Protection by Wnt through Akt and Secreted Frizzled-related protein pathways |
| Nicotinamide | Nicotinamide can maintain normal fasting blood glucose and improve glucose utilization in animal models of DM Nicotinamide can limit peripheral nerve injury during elevated glucose Nicotinamide protects β-cell function in islet-cell antibody-positive first-degree relatives of Type 1 DM Nicotinamide combined with intensive insulin therapy reduces HbA1c levels Nicotinamide can reduce intestinal absorption of phosphate and prevent the development of hyperphosphatemia |
Nicotinamide functions through transcription factors of the forkhead family and caspases Nicotinamide has an inverse relationship with sirtuins that can alter cell survival and cell longevity |
| Erythropoietin (EPO) | EPO is often low in DM, suggesting an impaired EPO response EPO in diabetic patients with severe, resistant congestive heart failure can decrease fatigue, increase left ventricular ejection fraction, and significantly decrease hospitalization stay EPO can serve to reverse the complications of anemia during DM EPO can protect vascular cells during DM |
EPO protection in the hematological and vascular systems relies upon modulation of FoxO and Wnt EPO fosters eythroid progenitor cell development through FoxO activity EPO during elevated glucose and similar to other models of oxidative stress can block cell degeneration through Wnt Protection by EPO is governed by the maintenance of mitochondrial membrane potential |
It also has been suggested that intact Wnt family members may offer glucose tolerance and increased insulin sensitivity 206 as well as protect glomerular mesangial cells from elevated glucose induced apoptosis 207. Animals that over express Wnt10b with a high-fat diet experienced a reduction in bodyweight, hyperinsulinemia, triglyceride plasma levels, and improved glucose homeostasis 208. Cell culture studies demonstrate that the Wnt1 protein that can be controlled by the growth factor EPO is necessary and sufficient to impart cellular protection during elevated glucose exposure 72, 209, 210. EPO maintains the expression of Wnt1 during elevated glucose exposure and prevents loss of Wnt1 expression that would occur in the absence of EPO during elevated glucose. In addition, blockade of Wnt1 with a Wnt1 antibody can neutralize the protective capacity of EPO, illustrating that Wnt1 is a critical component in the cytoprotection of EPO during elevated glucose exposure 72.
Wnt may foster cellular protection during DM through the novel regulation of protein kinase B (Akt) (Table 1). Activation of Akt can promote cell survival, such as during cell proliferation 211, progenitor cell development 169, blood-brain barrier permeability 212, inflammation 163, 213. Ischemic-preconditioning 214, neurodegeneration 103, hyperglycemia 67, 215, hypoxia 216, amyloid toxicity 52, 53, 131, 132, 217, excitotoxicity 95, amyloid production 218, cardiomyopathy 219, cellular aging 220, and oxidative stress 145, 171, 221. Akt activation also can modulate microglial cell activation 145, 171, 180, regulate transcription factors 222, maintain mitochondrial membrane potential (ΔΨm ), prevent cytochrome c release 150, 180, 223, and block caspase activity 180, 216, 223. However, as a “pro-survival pathway”, it should be recognized that Akt activation may be deleterious, such as during cancer resistance to chemotherapy 224.
A number of observations support the dependence of Wnt on Akt activation and support the premise that Wnt can modulate DM complications through Akt 192, 225, 226. For example, neuronal cell differentiation requires Wnt signaling and trophic factor induction through Akt activity 227 and differentiation of cardiomyocytes proceeds only with Akt activation 228. Wnt also has been shown in preadipocytes to increase Akt phosphorylation 229 and the Wnt-induced secreted protein in a fibroblast cell line uses Akt to block apoptotic death 230. Secreted Frizzled-related proteins (sFRPs), which can modulate Wnt signaling, also employ Akt for cardiac tissue repair 231, reduction in tissue injury during pressure overload cardiac hypertrophy is tied to Akt activation 232, and cardiac ischemic preconditioning appear to rely upon Akt 233. In the neuronal system, Wnt over-expression can independently increase the phosphorylation and the activation of Akt to promote neuronal protection. Inhibition of the phosphatidylinositol 3-kinase (PI 3-K) pathway or gene silencing of Akt expression prevents Wnt from blocking apoptotic injury and microglial activation 53.
Nicotinamide
As the amide form of vitamin B3 (niacin), nicotinamide or nicotinic acid which is the water soluble form vitamin B3, is obtained through synthesis or as a dietary source and supplement 234. The principal form of niacin in dietary plant sources is nicotinic acid that is rapidly absorbed through the gastrointestinal epithelium 235. Nicotinamide is subsequently generated through the conversion of nicotinic acid in the liver or through the hydrolysis of NAD+ 62. After nicotinamide is obtained in the body, it functions as the precursor for the coenzyme ß-nicotinamide adenine dinucleotide (NAD+) 61, 236 and also is essential for the synthesis of nicotinamide adenine dinucleotide phosphate (NADP+) 237. Initially, nicotinamide is changed to its mononucleotide form (NMN) with the enzyme nicotinic acid/nicotinamide adenylyltransferase yielding the dinucleotides NAAD+ and NAD+. NAAD+ also yields NAD+ through NAD+ synthase 238 or NAD+ can be synthesized through nicotinamide riboside kinase that phosphorylates nicotinamide riboside to NMN 239, 240.
The cellular pathways of nicotinamide are essential for energy metabolism and may directly impact normal physiology as well as disease progression 241-244. Nicotinamide through NAD+ has a critical physiological role in cellular metabolism and can be directly utilized by cells to synthesize NAD+ 34, 61, 116. Nicotinamide also participates in energy metabolism through the tricarboxylic acid cycle by utilizing NAD+ in the mitochondrial respiratory electron transport chain for the production of ATP, DNA synthesis, and DNA repair 245-247. In addition, nicotinamide can significantly increase NAD+ levels in vulnerable regions of the ischemic brain, suggesting that nicotinamide may prevent cell injury through the maintenance of NAD+ levels 248. During axonal degeneration, nicotinamide also may promote protection through NAD+-dependent mechanisms 249. Nicotinamide also appears to function directly at the level of mitochondrial membrane pore formation 61, 250, 251 to prevent the release of cytochrome c 252. Nicotinamide can prevent mitochondrial membrane depolarization during exposure to either tert-butylhydroperoxide or atractyloside 253. There are other mechanisms that nicotinamide may use to maintain cellular metabolic homeostasis through the maintenance of mitochondrial membrane potential 181, 250. Nicotinamide can phosphorylate Bad 252 to prevent mitochondrial membrane depolarization and subsequent cytochrome c release. Nicotinamide also may inhibit the assembly of the mitochondrial permeability transition pore complex similar to the action of cyclosporin A 254 as well as stabilize cellular energy metabolism through ATP pathways 255.
In addition to its role in metabolism, nicotinamide can be essential for cellular differentiation, such as for human embryonic stem cells 256. Nicotinamide has protean endocrine effects 257, 258, can scavenge ROS, and offers cellular protection for both neuronal 253, 259, 260 and vascular cells 34, 61, 116, 236. In neuronal cell populations, nicotinamide protects against free radical injury 181, anoxia 261, excitotoxicity 262, homocysteine toxicity 263, ethanol-induced neuronal injury 264, and oxygen-glucose deprivation 253, 265. In cortical neurons, nicotinamide blocks cell injury during ROS generating toxins such as tertiary butylhydroperoxide 266. Nicotinamide also can protect both rod and cone photoreceptor cells against N-methyl-N-nitrosourea toxicity 267, 268 as well as against glycation end products in all layers of the retina 269. In animal studies, nicotinamide improves cognitive function, cell survival, and reduces edema following cortical trauma 270-275, limits axonal degeneration 249, reduces cerebral ischemia 276-278 sometimes more effectively in models that were absent of comorbidities 279, prevents spinal cord injury 280, 281, and lessens disability in models of Parkinson's disease 100, 282, 283.
In regards to the vascular system 253, 259, 260, nicotinamide promotes vascular integrity 61, 116, 236 which may be crucial for tissue growth and repair 284. Nicotinamide can protect the function of the blood brain barrier 270, 271, influence arteriolar dilatation and blood flow 285, increase skin vascular permeability 286, inhibit atherosclerotic plaque formation through inhibition of poly(ADP-ribose) polymerase 287, and foster platelet production through megakaryocyte maturation 288. Nicotinamide can enhance endothelial cell viability during ROS exposure 181, 250, 261, 289. Nicotinamide also may reverse a previously sustained early apoptotic injury 61, 181, 250, 252, 253, 261, suggesting that apoptosis prior to reaching genomic DNA degradation is dynamic and reversible in nature 61, 181, 251, 261. Yet, some studies in mice suggest that nicotinamide may either prevent or contribute to atherosclerotic plaques over a three to six month progression 290. Although the mechanisms are not clear, it is conceivable that these events may occur during oxidative stress and the production of acidosis-induced cellular toxicity 291-293. Nicotinamide cannot prevent cellular injury during intracellular acidification paradigms 181.
Since nicotinamide is closely aligned with cellular energy management, it may play a significant role during DM and the complications of this disorder (Table 1). For example, nicotinamide appears to have a close relationship with metabolic pathways that may lead to clinical cognitive changes 294. Nicotinamide also has been shown to maintain normal fasting blood glucose with streptozotocin-induced DM in animal models 295, 296. Nicotinamide can limit peripheral nerve injury during elevated glucose 297, reverse Type 1 DM in mice with acetyl-lcarnitine 298, and block oxidant stress 242, 250, 252, 264, 299. Nicotinamide also affects levels of O-N-acetylglucosamin(O-GlcNAc)ylated proteins 300 and can significantly improve glucose utilization, prevent excessive lactate production in ischemic animal models 301. In clinical conditions, oral nicotinamide administration, nicotinamide (1200mg/m2/day) protects β-cell function and prevents clinical disease in islet-cell antibody-positive first-degree relatives of Type 1 DM 302. Nicotinamide administration (25mg/kg) has been shown in patients with recent onset Type 1 DM combined with intensive insulin therapy for up to two years after diagnosis to reduce HbA1c levels 303. Also relevant to patients with DM and renal insufficiency, nicotinamide can reduce intestinal absorption of phosphate and prevent the development of hyperphosphatemia 304. As a caveat for caution, some studies have reported that prolonged exposure to nicotinamide may lead to impaired β-cell function and reduction in cell growth 305, 306 as well as elevated nicotinamide levels may foster DM 307. Furthermore, nicotinamide also may inhibit P450 and hepatic metabolism 308 and play a role in the progression of other disorders such as Parkinson's disease 283.
One novel pathway that may control some of the beneficial effects of nicotinamide during DM involves the forkhead transcription factors of the “O” class (FoxOs) 309, 310 (Table 1). These transcription factors either inhibit or activate target gene expression by binding bind to DNA through the forkhead domain that relies upon fourteen protein-DNA contacts 309, 311-314. The term for these transcription factors is derived in part from imaging studies. On X-ray crystallography 315 or nuclear magnetic resonance imaging 316, the forkhead domain is described as a “winged helix” as a result of a butterfly-like appearance. The original nomenclature for these proteins, such as forkhead in rhabdomyosarcoma (FKHR), the Drosophila gene fork head (fkh), and Forkhead RElated ACtivator (FREAC)-1 and -2, has been replaced 317. The current nomenclature for human Fox proteins places all letters in uppercase, otherwise only the initial letter is listed as uppercase for the mouse, and for all other chordates the initial and subclass letters are in uppercase 318. Members of this family that include FoxO1, FoxO3, FoxO4, and FoxO6 are found throughout the body 82, 191, 317. These proteins are expressed in tissues of the reproductive system of males and females, skeletal muscle, the cardiovascular system, lung, liver, pancreas, spleen, thymus, and the nervous system 157, 313, 314, 319. Modulation of FoxOs is a viable therapeutic target for systems that involve metabotropic glutamate receptors 96, neurotrophins 320, cancer 157, 313, 321, and cytokines 222 to foster intended cell survival.
Interestingly, FoxO proteins can modulate cell cycle progression to prevent tumor growth 157, 313, 322. For example, administration of the Bcr-Abl tyrosine kinase inhibitor imatinib in chronic myelogenous leukemia cell lines blocks cell proliferation and promotes apoptotic cell death through FoxO3a and increased TRAIL production 323. The transcription factor E2F-1 that that oversees cell cycle progression increases expression of FoxO1 and FoxO3a to lead to cell cycle arrest 324. Other work indicates that FoxO proteins utilize the p53 upstream regulator p19(Arf) through Myc to block cell cycle induction and lymphoma progression 325.
Since attempted initiation of the cell cycle such as in neurons may be detrimental and can lead to cell death 49, 50, 326, 327, one may consider the ability of FoxO proteins to block cell cycle progression to be beneficial in these circumstances. In regards to cell metabolism and DM, FoxO proteins may be cytoprotective. Interferon-gamma driven expression of tryptophan catabolism by cytotoxic T lymphocyte antigen 4 may activate Foxo3a to protect dendritic cells from injury in nonobese diabetic mice 328. In addition, adipose tissue-specific expression of Foxo1 in mice improves glucose tolerance and sensitivity to insulin during an elevated fat diet 329. FoxO proteins also may protect against diminished mitochondrial energy levels known to occur during insulin resistance such as in the elderly populations 2, 3, 8. In caloric restricted mice that have decreased energy reserves, Foxo1, Foxo3a, and Foxo4 mRNA levels were noted to progressively increase over two years 330. These observations complement studies in Drosophila and mammalian cells that demonstrate an increase in insulin signaling to regulate cellular metabolism during the up-regulation of FoxO1 expression 331.
However, the role of FoxO proteins in different cell systems can be variable and do not consistently point to a beneficial effect of FoxO proteins. FoxO3a controls early activation and subsequent apoptotic injury in microglia through caspase action of caspase 3, 8, and 9 55, 75, illustrating that targeting FoxO3a activity may limit apoptotic caspase activity and promote cell survival (Figure 1). In clinical conditions, analysis of the genetic variance in FOXO1a and FOXO3a on metabolic profiles, age-related diseases, fertility, fecundity, and mortality in patients have observed higher HbA1c levels and increased mortality risk associated with specific haplotypes of FOXO1a 332. These clinical observations may indicate that elevated glucose levels can reduce post-translational phosphorylation of FOXO1, FOXO3a, and FOXO4 and initiate cellular apoptosis 333. In addition, mice with a constitutively active Foxo1 transgene have increased microsomal triglyceride transfer protein and high plasma triglyceride levels 334. Increased transcriptional activity of FoxO1, such as by the Sirt1 activator resveratrol, also can decrease insulin mediated glucose uptake and result in insulin resistance 335. Overexpression of Foxo1 in skeletal muscles of mice can lead to reduced skeletal muscle mass and poor glycemic control 336. Other studies that block the expression of Foxo1 in normal and cachectic mice 337 or reduce FoxO3 expression 338 demonstrate positive effects with an increase in skeletal muscle mass or resistance to muscle atrophy.
As the pathways with cellular metabolism and FoxOs begin to unravel, nicotinamide becomes an attractive agent to consider for DM 61, 62, 116, 236. Nicotinamide inhibits FoxO protein activity through phosphorylation 253 and may be protective through two separate mechanisms of post-translational modification of FoxO3a 35, 157, 191, 314, 317. Nicotinamide not only can maintain phosphorylation of FoxO3a and inhibit its activity to potentially block caspase 3 activity 253, but also can preserve the integrity of the FoxO3a protein to block FoxO3a proteolysis that can yield pro-apoptotic amino-terminal fragments 253. During oxidative stress, an initial inhibitory phosphorylation of FoxO3a at the regulatory phosphorylation sites (Thr32 and Ser253) occurs 253, 339. Yet, loss of phosphorylated FoxO3a expression appears to subsequently result over twelve hours, possibly by caspase degradation, which can raise the vulnerability of neurons to apoptotic injury 253. The loss of both FoxO3a phosphorylation and the integrity of this transcription factor may then lead to apoptosis. FoxO3a proteolysis occurs during cell injury yielding an amino-terminal (Nt) fragment that can become biologically active and lead to cellular injury 340. Nicotinamide, through the phosphorylation of FoxO3a blocks apoptotic cell injury and prevents caspase 3 activity 253.
Nicotinamide is closely linked to cell longevity pathways that involve not only FoxOs, but also sirtuins 116, 341, 342. FoxO proteins are deacetylated by histone deacetylases. These include the sirtuin Sirt1, a NAD+-dependent deacetylase and the mammalian ortholog of the silent information regulator 2 (Sir2) protein 310, that can control multiple processes such as cell injury, lifespan, and metabolism 343, 344. FoxO proteins and sirtuins have been associated with cell longevity and aging as shown by early studies linking DAF-16 in Caenorhabditis elegans 157, 310, 344-346. Furthermore, sirtuins are tied to cellular metabolism 343, 347 and increased cell survival 344, 345, 348-350. Yet, the relationship among nicotinamide, FoxO transcription factors, and sirtuins is not entirely clear (Table 1). For example, some studies suggest that stimulation of Sirt1 during starvation is dependent upon FoxO3a activity as well as p53 351. During exercise, an up-regulation of FoxO3a and Sirt1 activity is observed in the heart of rats 352, suggesting that physical activity may be beneficial for the cardiovascular system through FoxO proteins. Other work has shown that Sirt1 may repress the activity of FoxO1, FoxO3a, and FoxO4, illustrating that cellular longevity may benefit from reduction in FoxO protein generated apoptosis 353.
However, nicotinamide prevents oxidant-induced apoptotic injury usually in a specific concentration range. Administration of nicotinamide in a range of 5.0 - 25.0 mmol/L significantly protects cells during oxidative stress injuries. This concentration range is similar to other injury paradigms in both animal models 268 and in cell culture models 61, 181, 250. In contrast to these cytoprotective concentrations of nicotinamide that also can modulate offers gene regulation 354, a reduction in nicotinamide levels during nicotinamidase expression supports increased cellular survival and longevity 348, 350. Nicotinamide can block cellular Sir2 by intercepting an ADP-ribosyl-enzyme-acetyl peptide intermediate with the regeneration of NAD+ (transglycosidation) 355. Physiological concentrations of nicotinamide noncompetitively inhibit Sir2, suggesting that nicotinamide is a physiologically relevant regulator of Sir2 enzymes 356. Interestingly, nicotinamidase expression which reduces nicotinamide concentrations prevents both apoptotic late DNA degradation and early PS exposure that appears to depend upon increased Sirt1 activity and may serve to modulate inflammatory cell activation 348, 350. In addition, inhibition of sirtuin (Sirt1) activity either by pharmacological methods or siRNA gene silencing is detrimental to cell survival during oxidative stress and blocks nicotinamidase protection, further supporting that Sirt1 activity may be necessary for nicotinamidase protection during oxidative stress. As a result, in relation to cell longevity, it is the lower concentrations of nicotinamide that can function as an inhibitor of sirtuins that are necessary for the promotion of increased lifespan and cellular survival 250, 252, 253, 261, 348, 350, 357, at least in yeast and metazoans 116, 341, 342. Sirtuins also may prevent nicotinamide from assisting with DNA repair by altering the accessibility of DNA damaged sites for repair enzymes 358. Furthermore, sirtuin activators, at least at the experimental animal level, may promote glucose homeostasis and insulin sensitivity 62, 343, 344, 350, 359 while also reducing the risk of obesity 360.
Erythropoietin
The growth factor and cytokine EPO is approved by the Food and Drug Administration for the treatment of anemia, but continued new work has identified this agent for the potential treatment of multiple disorders 209, 361. Clinical considerations include treatment for depression 362, Alzheimer's disease 52, 363, 364, Parkinson's disease 365, immune system dysfunction 150, 222, 223, 366, 367, neurodegeneration 52, 71, 150, 223, 368-371, cardiovascular disorders 180, 216, 222, 372-379, spinal cord injury 380, 381, brain edema 382, fertility 383, trauma 384-386, shock 387-389, infection 390-392, pulmonary disease 393-395, renal disease 68, 396-398, gastrointestinal disorders 399-401, ocular disease 402-404, and metabolic disorders 2, 33, 34, 71, 72, 405. New studies further support the use of intravitreal EPO injections in patients 406.
EPO is required for erythropoiesis 407-409, but also functions in other organs and tissues, such as the brain, heart, and vascular system 216, 222, 223, 410-412. EPO production is believed to occur throughout the body 83, 361, 413 and can be detected in the breath of healthy individuals 414. The principal organs of EPO production and secretion are the kidney, liver, brain, and uterus 210, 339, 415.
In regards to EPO during DM, plasma EPO is often low in diabetic patients with anemia 416 or without anemia 417. The inability of these individuals to produce EPO in response to a declining hemoglobin levels suggests an impaired EPO response during DM 418 (Table 1). Yet, increased EPO secretion during diabetic pregnancies may represent the body's attempt at endogenous protection against the complications of DM 419, 420. This potential cytoprotective capacity of EPO may be important during complications of DM, such as those that involve cognitive impairment. For example, EPO may improve cognitive ability. EPO may reduce in animal models apoptotic pathways during periods of hyperoxia in the developing brain 421, 422. Furthermore, clinical disorders may have periods of hyperoxia followed by cerebral hypoperfusion and hypoxia that can lead to cerebral injury with associated oxidative stress 423. EPO under these conditions also may be protective since it can promote neurite outgrowth 424 and also may regulate hemoglobin levels that have recently been associated with cognitive decline 425. Elevated EPO concentrations during infant maturation have been correlated with increased Mental Development Index scores 426 and EPO may prevent toxic effects of agents used to control cognitive function such as haloperidol 427.
In relation to clinical relevance, EPO in diabetic as well as non-diabetic patients with severe, resistant congestive heart failure can decrease fatigue, increase left ventricular ejection fraction, and significantly decrease hospitalization stay 428 (Table 1). In addition, EPO can serve to reverse the complications of anemia during DM 33. Experimental work during elevated glucose also has demonstrated that EPO can significantly improve vascular cell survival in a 1.0 ng/ml range 72. EPO administration in patients also can significantly increase plasma levels of EPO well above this range of 1.0 ng/ml that has been associated with potential EPO cellular protection in patients with cardiac or renal disease 429, 430, illustrating that the effects of EPO observed during in vitro studies may parallel the cellular processes altered by EPO in patients with DM 426.
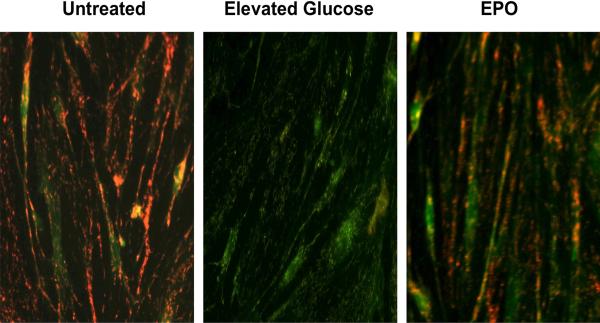
Protection in the hematological and vascular system by EPO may rely upon modulation of FoxOs and Wnt signaling 209, 361 (Table 1). EPO fosters eythroid progenitor cell development through the regulation of FoxO activity 361, 413, 431 and may require regulation of specific gene expression through an EPO-FoxO3a association to promote erythropoiesis in cultured cells 432. In addition, EPO exerts cellular protection through Wnt signaling. Cell culture studies demonstrate that the Wnt1 protein is necessary and sufficient to impart cellular protection during elevated glucose exposure 72. EPO maintains the expression of Wnt1 during elevated glucose exposure and prevents loss of Wnt1 expression that would occur in the absence of EPO during elevated glucose. In addition, blockade of Wnt1 with a Wnt1 antibody can neutralize the protective capacity of EPO, illustrating that Wnt1 is a critical component in the cytoprotection of EPO during elevated glucose exposure 72. Furthermore, EPO during elevated glucose and similar to other models of oxidative stress can block neuronal degeneration 71, prevents renal cell apoptosis 405, apoptotic DNA degradation, and degeneration in cardiac and vascular cell models 180, 216, 222, 411, 433. Protection by EPO also is related to the maintenance of mitochondrial membrane potential, since loss of mitochondrial membrane potential is known to lead to apoptotic cell injury 166, 434. EPO has the capacity to prevent the depolarization of the mitochondrial membrane that also affects the release of cytochrome c 150, 216, 435 (Figure 2).
Figure 2. Erythropoietin (EPO) blocks mitochondrial depolarization during elevated D-glucose.
Elevated D-glucose (20 mM) resulted in a significant decrease in the red/green fluorescence intensity ratio of mitochondria using a cationic membrane potential indicator JC-1 within 48 hours when compared with untreated endothelial cells, illustrating that elevated D-glucose leads to mitochondrial membrane depolarization. In contrast, pre-treatment with EPO (10 ng/ml) during elevated D-glucose significantly increased the red/green fluorescence intensity of mitochondria in endothelial cells, indicating that mitochondrial membrane potential was restored by EPO.
Biomarkers and future considerations
Primary cellular pathways that may be critical for the development of new drug therapies also may possess great utility to function as biomarkers that can predict the diagnosis, onset, or progression of disease. Biomarkers can be used for the determination of specific genes, proteins, or products of cellular and biological processes. In addition, biomarkers can represent the response of cells or tissues to therapeutic strategies 81. Many of the biological outcomes described for Wnt, nicotinamide, and EPO encompass necessary cellular pathways for development, growth, and survival. Yet, these entities also hold the potential to further disease progression or be set in motion during disease onset as an endogenous protective response. In regards to Wnt, signaling for this glycoprotein may indicate the onset of early tissue injury during conditions such as elevated glucose 72, amyloid toxicity 53, or cardiac ischemia 233, 436 and alert the need for rapid responsive treatments to limit cell injury. Yet, increased Wnt expression also may suggest disease progression as well as a poor prognostic response to prior therapies 34, 177, 192. Aberrant Wnt signaling has been associated with advanced prostate cancer and bone metastases 437, the genesis of cancer stem cells 185, the reliance of FoxO pathways to promote unregulated cell proliferation in cancer 438, and the vulnerability of patients with Type 2 DM to develop colorectal tumors 439.
Independent from Wnt, FoxOs and EPO may serve as essential biomarkers. For example, the absence of FoxO proteins can suggest tumor progression 440. On the converse side, the up-regulation of FoxO proteins can indicate a positive response to chemotherapy 321, 441, 442 and also be an indicator and responsive agent to ischemic tissue 443. In some scenarios, expression of FoxO proteins may suggest tissue protection and recovery, since FoxO3a activity may enhance vascular smooth muscle antioxidant properties in aged animals and be beneficial to the cardiovascular system during physical exertion 352. In a similar manner, EPO also may be a positive indicator of cytoprotective responses 209, 210, 339. Although EPO has not been shown to correlate with Psychomotor Development Index or an overall incidence of neurodevelopmental impairment, in clinical studies infants with elevated EPO possessed higher Mental Development Index scores than infants with lower EPO concentrations, suggesting that the presence of EPO may correlate with a positive developmental course. Advanced cognitive function also may rely upon appropriate levels of EPO that can be followed since both low and high levels of EPO in the elderly can be associated with diminished cognitive function 425. Yet, the presence of EPO may affect a number of other systems in addition to the brain 444. Recent work has shown that the presence of a truncated form of EPO receptor can function as a dominant negative regulator of EPO signaling and lead to hypertension, suggesting that monitoring of this biomarker may identify individuals susceptible to hypertension 445. Although EPO has recently been reported to prevent drug-induced fibrosis and possible endothelial damage during chemotherapy 446, it is important to note that EPO also may foster tumor progression 447, 448. EPO and its receptor are present in tumor specimens, may block tumor cell apoptosis through Akt 449, enhance metastatic disease, 450, and complicate radiotherapy by assisting with tumor angiogenesis 451.
Drug development for any disorder becomes a complex enterprise, especially for disorders such as DM with the multiple complications of this disease that can ensue through oxidant stress pathways. Nevertheless, elucidation of novel pathways and their biological role that have an intimate relationship with agents such as Wnt, nicotinamide, and EPO are vital for the successful treatment of clinical disease. In addition, defining the ability of these agents to function as potential biomarkers for disease can further enhance the utility of new entities irrespective of their treatment potential. Given that the present percentage of the gross domestic product for U.S. healthcare spending is the highest in the world, life expectancy in the U.S. trails behind other countries, and that the number of individuals affected by DM globally is expected to climb exponentially over the years, it becomes critical to understand both the clinical efficacy of novel treatments as well as the potential complications of these agents especially in a variety of circumstances that may not only involve essential cellular repair but also undesirable cellular proliferation.
Acknowledgments
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639), NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
Disclosure: No conflict of interest exists
References
- 1.Development OfEC-oa OECD Health Data 2009. 2009 www.ecosante.org/oecd.htm.
- 2.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14(16):1729–1738. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007 Feb;4(1):63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. Jama. 2009 May 27;301(20):2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 5.Rebecchi KR, Wenke JL, Go EP, Desaire H. Label-free quantitation: a new glycoproteomics approach. J Am Soc Mass Spectrom. 2009 Jun;20(6):1048–1059. doi: 10.1016/j.jasms.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Dabelea D, Bell RA, D'Agostino RB, Jr., et al. Incidence of diabetes in youth in the United States. JAMA. 2007 Jun 27;297(24):2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 7.Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev. 2000 Jul-Aug;16(4):230–236. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr122>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Maiese K. Diabetic stress: new triumphs and challenges to maintain vascular longevity. Expert Rev Cardiovasc Ther. 2008 Mar;6(3):281–284. doi: 10.1586/14779072.6.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daneman D. Type 1 diabetes. Lancet. 2006 Mar 11;367(9513):847–858. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 10.Bonner-Weir S. Life and death of the pancreatic beta cells. Trends Endocrinol Metab. 2000 Nov;11(9):375–378. doi: 10.1016/s1043-2760(00)00305-2. [DOI] [PubMed] [Google Scholar]
- 11.Awata T, Kurihara S, Kikuchi C, et al. Evidence for association between the class I subset of the insulin gene minisatellite (IDDM2 locus) and IDDM in the Japanese population. Diabetes. 1997 Oct;46(10):1637–1642. doi: 10.2337/diacare.46.10.1637. [DOI] [PubMed] [Google Scholar]
- 12.Baisch JM, Weeks T, Giles R, Hoover M, Stastny P, Capra JD. Analysis of HLA-DQ genotypes and susceptibility in insulin-dependent diabetes mellitus. N Engl J Med. 1990 Jun 28;322(26):1836–1841. doi: 10.1056/NEJM199006283222602. [DOI] [PubMed] [Google Scholar]
- 13.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15-21;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 14.Lund T, O'Reilly L, Hutchings P, et al. Prevention of insulin-dependent diabetes mellitus in non-obese diabetic mice by transgenes encoding modified I-A beta-chain or normal I-E alpha-chain. Nature. 1990 Jun 21;345(6277):727–729. doi: 10.1038/345727a0. [DOI] [PubMed] [Google Scholar]
- 15.Permutt MA, Wasson J, Cox N. Genetic epidemiology of diabetes. J Clin Invest. 2005 Jun;115(6):1431–1439. doi: 10.1172/JCI24758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kernan WN, Inzucchi SE, Viscoli CM, Brass LM, Bravata DM, Horwitz RI. Insulin resistance and risk for stroke. Neurology. 2002 Sep 24;59(6):809–815. doi: 10.1212/wnl.59.6.809. [DOI] [PubMed] [Google Scholar]
- 17.Orchard TJ, Olson JC, Erbey JR, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003 May;26(5):1374–1379. doi: 10.2337/diacare.26.5.1374. [DOI] [PubMed] [Google Scholar]
- 18.Pietropaolo M, Barinas-Mitchell E, Pietropaolo SL, Kuller LH, Trucco M. Evidence of islet cell autoimmunity in elderly patients with type 2 diabetes. Diabetes. 2000 Jan;49(1):32–38. doi: 10.2337/diabetes.49.1.32. [DOI] [PubMed] [Google Scholar]
- 19.Bottino R, Trucco M. Multifaceted therapeutic approaches for a multigenic disease. Diabetes. 2005 Dec;54(Suppl 2):S79–86. doi: 10.2337/diabetes.54.suppl_2.s79. [DOI] [PubMed] [Google Scholar]
- 20.Davies JL, Kawaguchi Y, Bennett ST, et al. A genome-wide search for human type 1 diabetes susceptibility genes. Nature. 1994 Sep 8;371(6493):130–136. doi: 10.1038/371130a0. [DOI] [PubMed] [Google Scholar]
- 21.Kyvik KO, Green A, Beck-Nielsen H. Concordance rates of insulin dependent diabetes mellitus: a population based study of young Danish twins. Bmj. 1995 Oct 7;311(7010):913–917. doi: 10.1136/bmj.311.7010.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melanitou E. The autoimmune contrivance: genetics in the mouse model. Clin Immunol. 2005 Dec;117(3):195–206. doi: 10.1016/j.clim.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Redondo MJ, Yu L, Hawa M, et al. Heterogeneity of type I diabetes: analysis of monozygotic twins in Great Britain and the United States. Diabetologia. 2001 Mar;44(3):354–362. doi: 10.1007/s001250051626. [DOI] [PubMed] [Google Scholar]
- 24.Luppi P, Rossiello MR, Faas S, Trucco M. Genetic background and environment contribute synergistically to the onset of autoimmune diseases. J Mol Med. 1995 Aug;73(8):381–393. doi: 10.1007/BF00240137. [DOI] [PubMed] [Google Scholar]
- 25.Del Prato S, Marchetti P. Beta- and alpha-cell dysfunction in type 2 diabetes. Horm Metab Res. 2004 Nov-Dec;36(11-12):775–781. doi: 10.1055/s-2004-826163. [DOI] [PubMed] [Google Scholar]
- 26.Hao J, Shen W, Tian C, et al. Mitochondrial nutrients improve immune dysfunction in the type 2 diabetic Goto-Kakizaki rats. J Cell Mol Med. 2009 Apr;13(4):701–711. doi: 10.1111/j.1582-4934.2008.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008 Aug;24(3):455–469. vi. doi: 10.1016/j.cger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 28.McIntyre RS, Rasgon NL, Kemp DE, et al. Metabolic syndrome and major depressive disorder: co-occurrence and pathophysiologic overlap. Curr Diab Rep. 2009 Feb;9(1):51–59. doi: 10.1007/s11892-009-0010-0. [DOI] [PubMed] [Google Scholar]
- 29.Wu SY, Wang GF, Liu ZQ, et al. Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta Pharmacol Sin. 2009 Feb;30(2):202–208. doi: 10.1038/aps.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guarnieri G, Zanetti M, Vinci P, Cattin MR, Barazzoni R. Insulin resistance in chronic uremia. J Ren Nutr. 2009 Jan;19(1):20–24. doi: 10.1053/j.jrn.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Aso Y, Suganuma R, Wakabayashi S, et al. Anemia is associated with an elevated serum level of high-molecular-weight adiponectin in patients with type 2 diabetes independently of renal dysfunction. Transl Res. 2009 Oct;154(4):175–182. doi: 10.1016/j.trsl.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Gossai D, Lau-Cam CA. The effects of taurine, taurine homologs and hypotaurine on cell and membrane antioxidative system alterations caused by type 2 diabetes in rat erythrocytes. Adv Exp Med Biol. 2009;643:359–368. doi: 10.1007/978-0-387-75681-3_37. [DOI] [PubMed] [Google Scholar]
- 33.Singh DK, Winocour P, Farrington K. Erythropoietic stress and anemia in diabetes mellitus. Nat Rev Endocrinol. 2009 Apr;5(4):204–210. doi: 10.1038/nrendo.2009.17. [DOI] [PubMed] [Google Scholar]
- 34.Maiese K. Triple play: Promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008 April - May;62(4):218–232. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maiese K, Chong Z, Li F. Reducing oxidative stress and enhancing neurovascular longevity during diabetes mellitus. In: Maiese K, editor. Neurovascular Medicine: Pursuing Cellular Longevity for Healthy Aging. Oxford University Press; New York, NY: 2009. pp. 540–564. ISBN13: 978-0-19-532669-7, ISBN10: 0-19-532669-5. [Google Scholar]
- 36.Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007 Aug 15;298(7):765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 37.Kuhad A, Bishnoi M, Tiwari V, Chopra K. Suppression of NF-kappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav. 2009 Apr;92(2):251–259. doi: 10.1016/j.pbb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999 Dec 10;53(9):1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 39.Schnaider Beeri M, Goldbourt U, Silverman JM, et al. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology. 2004 Nov 23;63(10):1902–1907. doi: 10.1212/01.wnl.0000144278.79488.dd. [DOI] [PubMed] [Google Scholar]
- 40.Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004 Oct 12;63(7):1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- 41.Beeri MS, Silverman JM, Davis KL, et al. Type 2 diabetes is negatively associated with Alzheimer's disease neuropathology. J Gerontol A Biol Sci Med Sci. 2005 Apr;60(4):471–475. doi: 10.1093/gerona/60.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maiese K, Chong ZZ, Hou J, Shang YC. New strategies for Alzheimer's disease and cognitive impairment. Oxid Med Cell Longev. 2009;2(5):279–290. doi: 10.4161/oxim.2.5.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J Alzheimers Dis. 2005 Feb;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 44.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005 Feb;75(3):207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer's disease. Brain Res Brain Res Rev. 2005 Jul;49(1):1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maiese K, Holloway HH, Larson DM, Soncrant TT. Effect of acute and chronic arecoline treatment on cerebral metabolism and blood flow in the conscious rat. Brain Res. 1994;641(1):65–75. doi: 10.1016/0006-8993(94)91816-3. [DOI] [PubMed] [Google Scholar]
- 47.Kim HS, Lim JY, Sul D, et al. Neuroprotective effects of the new diterpene, CBNU06 against beta-amyloid-induced toxicity through the inhibition of NF-kappaB signaling pathway in PC12 cells. Eur J Pharmacol. 2009 Nov 10;622(1-3):25–31. doi: 10.1016/j.ejphar.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Salminen A, Kaarniranta K. Siglec receptors and hiding plaques in Alzheimer's disease. J Mol Med. 2009 Jul;87(7):697–701. doi: 10.1007/s00109-009-0472-1. [DOI] [PubMed] [Google Scholar]
- 49.Chong ZZ, Li F, Maiese K. Attempted Cell Cycle Induction in Post-Mitotic Neurons Occurs in Early and Late Apoptotic Programs Through Rb, E2F1, and Caspase 3. Curr Neurovasc Res. 2006 Feb;3(1):25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin SH, Chong ZZ, Maiese K. Cell cycle induction in post-mitotic neurons proceeds in concert with the initial phase of programmed cell death in rat. Neurosci Lett. 2001 Sep 14;310(2-3):173–177. doi: 10.1016/s0304-3940(01)02118-8. [DOI] [PubMed] [Google Scholar]
- 51.Majd S, Zarifkar A, Rastegar K, Takhshid MA. Different fibrillar Abeta 1-42 concentrations induce adult hippocampal neurons to reenter various phases of the cell cycle. Brain Res. 2008 Jul 7;1218:224–229. doi: 10.1016/j.brainres.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 52.Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005 Dec;2(5):387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007 Jun;19(6):1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Majd S, Rastegar K, Zarifkar A, Takhshid MA. Fibrillar beta-amyloid (Abeta) (1-42) elevates extracellular Abeta in cultured hippocampal neurons of adult rats. Brain Res. 2007 Dec 14;1185:321–327. doi: 10.1016/j.brainres.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 55.Shang YC, Chong ZZ, Hou J, Maiese K. The forkhead transcription factor FoxO3a controls microglial inflammatory activation and eventual apoptotic injury through caspase 3. Curr Neurovasc Res. 2009 Feb;6(1):20–31. doi: 10.2174/156720209787466064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bakshi P, Margenthaler E, Laporte V, Crawford F, Mullan M. Novel role of CXCR2 in regulation of gamma-secretase activity. ACS Chem Biol. 2008 Dec 19;3(12):777–789. doi: 10.1021/cb800167a. [DOI] [PubMed] [Google Scholar]
- 57.Lu J, Wu DM, Zheng YL, et al. Trace amounts of copper exacerbate beta amyloid-induced neurotoxicity in the cholesterol-fed mice through TNF-mediated inflammatory pathway. Brain Behav Immun. 2009 Feb;23(2):193–203. doi: 10.1016/j.bbi.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Bitner RS, Nikkel AL, Markosyan S, Otte S, Puttfarcken P, Gopalakrishnan M. Selective alpha7 nicotinic acetylcholine receptor activation regulates glycogen synthase kinase3beta and decreases tau phosphorylation in vivo. Brain Res. 2009 Apr 10;1265:65–74. doi: 10.1016/j.brainres.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 59.Vaisid T, Barnoy S, Kosower NS. Calpastatin overexpression attenuates amyloid-beta-peptide toxicity in differentiated PC12 cells. Neuroscience. 2008 Oct 28;156(4):921–931. doi: 10.1016/j.neuroscience.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 60.Erol A. Unraveling the Molecular Mechanisms Behind the Metabolic Basis of Sporadic Alzheimer's Disease. J Alzheimers Dis. 2009 Feb 16; doi: 10.3233/JAD-2009-1047. [DOI] [PubMed] [Google Scholar]
- 61.Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003 May;24(5):228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 62.Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14(9):3446–3485. doi: 10.3390/molecules14093446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schubert M, Gautam D, Surjo D, et al. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Newsholme P, Haber EP, Hirabara SM, et al. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007 Aug 15;583(Pt 1):9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szabo C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br J Pharmacol. 2009 Mar;156(5):713–727. doi: 10.1111/j.1476-5381.2008.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simone S, Gorin Y, Velagapudi C, Abboud HE, Habib SL. Mechanism of oxidative DNA damage in diabetes: tuberin inactivation and downregulation of DNA repair enzyme 8-oxo-7,8-dihydro-2'-deoxyguanosine-DNA glycosylase. Diabetes. 2008 Oct;57(10):2626–2636. doi: 10.2337/db07-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaddini L, Villa M, Matteucci A, et al. Early effects of high glucose in retinal tissue cultures Renin-Angiotensin system-dependent and -independent signaling. Neurobiol Dis. 2009 Aug;35(2):278–285. doi: 10.1016/j.nbd.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 68.Singh DK, Winocour P, Farrington K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol. 2008 Apr;4(4):216–226. doi: 10.1038/ncpneph0757. [DOI] [PubMed] [Google Scholar]
- 69.Barbosa NB, Oliveira C, Araldi D, Folmer V, Rocha JB, Nogueira CW. Acute diphenyl diselenide treatment reduces hyperglycemia but does not change delta-aminolevulinate dehydratase activity in alloxan-induced diabetes in rats. Biol Pharm Bull. 2008 Dec;31(12):2200–2204. doi: 10.1248/bpb.31.2200. [DOI] [PubMed] [Google Scholar]
- 70.Memisogullari R, Bakan E. Levels of ceruloplasmin, transferrin, and lipid peroxidation in the serum of patients with Type 2 diabetes mellitus. J Diabetes Complications. 2004 Jul-Aug;18(4):193–197. doi: 10.1016/S1056-8727(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 71.Chattopadhyay M, Walter C, Mata M, Fink DJ. Neuroprotective effect of herpes simplex virus-mediated gene transfer of erythropoietin in hyperglycemic dorsal root ganglion neurons. Brain. 2009 Apr;132(Pt 4):879–888. doi: 10.1093/brain/awp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007 Aug;4(3):194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin J, Zheng S, Chen A. Curcumin attenuates the effects of insulin on stimulating hepatic stellate cell activation by interrupting insulin signaling and attenuating oxidative stress. Lab Invest. 2009 Dec;89(12):1397–1409. doi: 10.1038/labinvest.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu W, Liu P, Tao S, et al. Berberine inhibits aldose reductase and oxidative stress in rat mesangial cells cultured under high glucose. Arch Biochem Biophys. 2008 Jul 15;475(2):128–134. doi: 10.1016/j.abb.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 75.Shang YC, Chong ZZ, Hou J, Maiese K. FoxO3a Governs Early Microglial Proliferation and Employs Mitochondrial Depolarization with Caspase 3, 8, and 9 Cleavage During Oxidant Induced Apoptosis. Curr Neurovasc Res. 2009 Nov 1;6(4):223–238. doi: 10.2174/156720209789630302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen H, Li X, Epstein PN. MnSOD and catalase transgenes demonstrate that protection of islets from oxidative stress does not alter cytokine toxicity. Diabetes. 2005 May;54(5):1437–1446. doi: 10.2337/diabetes.54.5.1437. [DOI] [PubMed] [Google Scholar]
- 77.Lepore DA, Shinkel TA, Fisicaro N, et al. Enhanced expression of glutathione peroxidase protects islet beta cells from hypoxia-reoxygenation. Xenotransplantation. 2004 Jan;11(1):53–59. doi: 10.1111/j.1399-3089.2004.00082.x. [DOI] [PubMed] [Google Scholar]
- 78.Ribeiro MC, Barbosa NB, de Almeida TM, et al. High-fat diet and hydrochlorothiazide increase oxidative stress in brain of rats. Cell Biochem Funct. 2009 Oct;27(7):473–478. doi: 10.1002/cbf.1599. [DOI] [PubMed] [Google Scholar]
- 79.Rachek LI, Thornley NP, Grishko VI, LeDoux SP, Wilson GL. Protection of INS-1 cells from free fatty acid-induced apoptosis by targeting hOGG1 to mitochondria. Diabetes. 2006 Apr;55(4):1022–1028. doi: 10.2337/diabetes.55.04.06.db05-0865. [DOI] [PubMed] [Google Scholar]
- 80.Haber CA, Lam TK, Yu Z, et al. N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: possible role of oxidative stress. Am J Physiol Endocrinol Metab. 2003 Oct;285(4):E744–753. doi: 10.1152/ajpendo.00355.2002. [DOI] [PubMed] [Google Scholar]
- 81.Maiese K. Marking the onset of oxidative stress: Biomarkers and novel strategies. Oxid Med Cell Longev. 2009;2(1):1. doi: 10.4161/oxim.2.1.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maiese K, Hou J, Chong ZZ, Shang YC. Erythropoietin, forkhead proteins, and oxidative injury: biomarkers and biology. ScientificWorldJournal. 2009;9:1072–1104. doi: 10.1100/tsw.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008;5(2):125–142. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ihara Y, Toyokuni S, Uchida K, et al. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999 Apr;48(4):927–932. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- 85.Ceriello A, dello Russo P, Amstad P, Cerutti P. High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes. 1996 Apr;45(4):471–477. doi: 10.2337/diab.45.4.471. [DOI] [PubMed] [Google Scholar]
- 86.Yano M, Hasegawa G, Ishii M, et al. Short-term exposure of high glucose concentration induces generation of reactive oxygen species in endothelial cells: implication for the oxidative stress associated with postprandial hyperglycemia. Redox Rep. 2004;9(2):111–116. doi: 10.1179/135100004225004779. [DOI] [PubMed] [Google Scholar]
- 87.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006 Apr 12;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 88.De Felice FG, Velasco PT, Lambert MP, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007 Apr 13;282(15):11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 89.Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21(3):262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 90.Nomoto M, Miyata M, Yin S, et al. Bile acid-induced elevated oxidative stress in the absence of farnesoid X receptor. Biol Pharm Bull. 2009 Feb;32(2):172–178. doi: 10.1248/bpb.32.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walsh KB, Toledo AH, Rivera-Chavez FA, Lopez-Neblina F, Toledo-Pereyra LH. Inflammatory mediators of liver ischemia-reperfusion injury. Exp Clin Transplant. 2009 Jun;7(2):78–93. [PubMed] [Google Scholar]
- 92.Escobar J, Pereda J, Arduini A, et al. Cross-talk between oxidative stress and pro-inflammatory cytokines in acute pancreatitis: a key role for protein phosphatases. Curr Pharm Des. 2009;15(26):3027–3042. doi: 10.2174/138161209789058075. [DOI] [PubMed] [Google Scholar]
- 93.Hammoud DA, Hoffman JM, Pomper MG. Molecular neuroimaging: from conventional to emerging techniques. Radiology. 2007 Oct;245(1):21–42. doi: 10.1148/radiol.2451060731. [DOI] [PubMed] [Google Scholar]
- 94.Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev. 2007 Sep;17(3):259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- 95.Campos-Esparza MR, Sanchez-Gomez MV, Matute C. Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Calcium. 2009 Apr;45(4):358–368. doi: 10.1016/j.ceca.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 96.Chong ZZ, Li F, Maiese K. Group I Metabotropic Receptor Neuroprotection Requires Akt and Its Substrates that Govern FOXO3a, Bim, and beta-Catenin During Oxidative Stress. Curr Neurovasc Res. 2006 May;3(2):107–117. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.He Z, Lu Q, Xu X, Huang L, Chen J, Guo L. DDPH ameliorated oxygen and glucose deprivation-induced injury in rat hippocampal neurons via interrupting Ca2+ overload and glutamate release. Eur J Pharmacol. 2009 Jan 28;603(1-3):50–55. doi: 10.1016/j.ejphar.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 98.Lehtinen MK, Tegelberg S, Schipper H, et al. Cystatin B deficiency sensitizes neurons to oxidative stress in progressive myoclonus epilepsy, EPM1. J Neurosci. 2009 May 6;29(18):5910–5915. doi: 10.1523/JNEUROSCI.0682-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ye J, Han Y, Wang C, Yu W. Cytoprotective effect of polypeptide from Chlamys farreri on neuroblastoma (SH-SY5Y) cells following HO exposure involves scavenging ROS and inhibition JNK phosphorylation. J Neurochem. 2009 Oct;111(2):441–451. doi: 10.1111/j.1471-4159.2009.06328.x. [DOI] [PubMed] [Google Scholar]
- 100.Anderson DW, Bradbury KA, Schneider JS. Broad neuroprotective profile of nicotinamide in different mouse models of MPTP-induced parkinsonism. Eur J Neurosci. 2008 Aug;28(3):610–617. doi: 10.1111/j.1460-9568.2008.06356.x. [DOI] [PubMed] [Google Scholar]
- 101.Morissette M, Al Sweidi S, Callier S, Di Paolo T. Estrogen and SERM neuroprotection in animal models of Parkinson's disease. Mol Cell Endocrinol. 2008 Aug 13;290(1-2):60–69. doi: 10.1016/j.mce.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 102.Morissette M, Le Saux M, D'Astous M, et al. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008 Feb;108(3-5):327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 103.Rodriguez-Blanco J, Martin V, Herrera F, Garcia-Santos G, Antolin I, Rodriguez C. Intracellular signaling pathways involved in post-mitotic dopaminergic PC12 cell death induced by 6-hydroxydopamine. J Neurochem. 2008 Oct;107(1):127–140. doi: 10.1111/j.1471-4159.2008.05588.x. [DOI] [PubMed] [Google Scholar]
- 104.Sales Santos I, da Rocha Tomé A, Saldanha G, Ferreira P, Militão G, de Freitas R. Oxidative stress in the hippocampus during experimental seizures can be ameliorated with the antioxidant ascorbic acid. Oxid Med Cell Longev. 2009;2(4):23–30. doi: 10.4161/oxim.2.4.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maiese K, Chong ZZ, Shang YC, Hou J. Therapeutic promise and principles: Metabotropic glutamate receptors. Oxid Med Cell Longev. 2008 Jul 1;1(1):1–14. doi: 10.4161/oxim.1.1.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Probst-Hensch NM, Imboden M, Felber Dietrich D, et al. Glutathione S-transferase polymorphisms, passive smoking, obesity, and heart rate variability in nonsmokers. Environ Health Perspect. 2008 Nov;116(11):1494–1499. doi: 10.1289/ehp.11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009 Sep;15(9):1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 108.Fatma N, Kubo E, Toris CB, Stamer WD, Camras CB, Singh DP. PRDX6 attenuates oxidative stress- and TGFbeta-induced abnormalities of human trabecular meshwork cells. Free Radic Res. 2009 Sep;43(9):783–795. doi: 10.1080/10715760903062887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gomes P, Simão S, Silva E, et al. Aging increases oxidative stress and renal expression of oxidant and antioxidant enzymes that are associated with an increased trend in systolic blood pressure. Oxid Med Cell Longev. 2009;2(3):19–26. doi: 10.4161/oxim.2.3.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pedersen MO, Jensen R, Pedersen DS, et al. Metallothionein-I+II in neuroprotection. Biofactors. 2009 Jul-Aug;35(4):315–325. doi: 10.1002/biof.44. [DOI] [PubMed] [Google Scholar]
- 111.Chuang HH, Lin S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):20097–20102. doi: 10.1073/pnas.0902675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Astiz M, de Alaniz MJ, Marra CA. Effect of pesticides on cell survival in liver and brain rat tissues. Ecotoxicol Environ Saf. 2009 Oct;72(7):2025–2032. doi: 10.1016/j.ecoenv.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 113.Slotkin TA, Seidler FJ. Protein kinase C is a target for diverse developmental neurotoxicants: transcriptional responses to chlorpyrifos, diazinon, dieldrin and divalent nickel in PC12 cells. Brain Res. 2009 Mar 31;1263:23–32. doi: 10.1016/j.brainres.2009.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hamden K, Carreau S, Jamoussi K, et al. 1Alpha,25 dihydroxyvitamin D3: therapeutic and preventive effects against oxidative stress, hepatic, pancreatic and renal injury in alloxan-induced diabetes in rats. J Nutr Sci Vitaminol (Tokyo) 2009 Jun;55(3):215–222. doi: 10.3177/jnsv.55.215. [DOI] [PubMed] [Google Scholar]
- 115.Regulska M, Leskiewicz M, Budziszewska B, et al. Inhibitory effects of 1,25-dihydroxyvitamin D(3) and its low-calcemic analogues on staurosporine-induced apoptosis. Pharmacol Rep. 2007 Jul-Aug;59(4):393–401. [PubMed] [Google Scholar]
- 116.Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem. 2006;13(8):883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li J, Wang H, Rosenberg PA. Vitamin K prevents oxidative cell death by inhibiting activation of 12-lipoxygenase in developing oligodendrocytes. J Neurosci Res. 2009 Jul;87(9):1997–2005. doi: 10.1002/jnr.22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ozsoy N, Candoken E, Akev N. Implications for degenerative disorders: Antioxidative activity, total phenols, flavonoids, ascorbic acid, β-carotene, α-tocopherol in Aloe vera. Oxid Med Cell Longev. 2009;2(2):99–106. doi: 10.4161/oxim.2.2.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Then SM, Mazlan M, Mat Top G, Wan Ngah WZ. Is vitamin E toxic to neuron cells? Cell Mol Neurobiol. 2009 Jun;29(4):485–496. doi: 10.1007/s10571-008-9340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.El-Mir MY, Detaille D, G RV, et al. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J Mol Neurosci. 2008;34(1):77–87. doi: 10.1007/s12031-007-9002-1. [DOI] [PubMed] [Google Scholar]
- 121.Kui L, Weiwei Z, Ling L, et al. Ghrelin inhibits apoptosis induced by high glucose and sodium palmitate in adult rat cardiomyocytes through the PI3K-Akt signaling pathway. Regul Pept. 2009 Jun 5;155(1-3):62–69. doi: 10.1016/j.regpep.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 122.Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009 Apr;110(4):813–825. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Han Z, Xiao MJ, Shao B, Zheng RY, Yang GY, Jin K. Attenuation of ischemia-induced rat brain injury by 2-(-2-benzofuranyl)-2-imidazoline, a high selectivity ligand for imidazoline I(2) receptors. Neurol Res. 2009 May;31(4):390–395. doi: 10.1179/174313209X444116. [DOI] [PubMed] [Google Scholar]
- 124.Maiese K. From the Bench to the Bedside: The Molecular Management of Cerebral Ischemia. Clinical Neuropharm. 1998;21(1):1–7. [PubMed] [Google Scholar]
- 125.Maiese K, Pek L, Berger SB, Reis DJ. Reduction in focal cerebral ischemia by agents acting at imidazole receptors. J Cereb Blood Flow Metab. 1992;12(1):53–63. doi: 10.1038/jcbfm.1992.7. [DOI] [PubMed] [Google Scholar]
- 126.Nakka VP, Gusain A, Mehta SL, Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol. 2008 Feb;37(1):7–38. doi: 10.1007/s12035-007-8013-9. [DOI] [PubMed] [Google Scholar]
- 127.Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res. 2009 Apr;24(4):597–605. doi: 10.1359/JBMR.081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arboleda G, Morales LC, Benitez B, Arboleda H. Regulation of ceramide-induced neuronal death: cell metabolism meets neurodegeneration. Brain Res Rev. 2009 Mar;59(2):333–346. doi: 10.1016/j.brainresrev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 129.Maiese K, Vincent AM. Critical temporal modulation of neuronal programmed cell injury. Cell Mol Neurobiol. 2000;20(3):383–400. doi: 10.1023/A:1007070311203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhong J, Zheng W, Huang L, et al. PrP106-126 amide causes the semi-penetrated poration in the supported lipid bilayers. Biochim Biophys Acta. 2007 Jun;1768(6):1420–1429. doi: 10.1016/j.bbamem.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 131.Burgos-Ramos E, Puebla-Jimenez L, Arilla-Ferreiro E. Minocycline provides protection against beta-amyloid(25-35)-induced alterations of the somatostatin signaling pathway in the rat temporal cortex. Neuroscience. 2008 Jul 17;154(4):1458–1466. doi: 10.1016/j.neuroscience.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 132.Burgos-Ramos E, Puebla-Jimenez L, Arilla-Ferreiro E. Minocycline prevents Abeta(25-35)-induced reduction of somatostatin and neprilysin content in rat temporal cortex. Life Sci. 2009 Feb 13;84(7-8):205–210. doi: 10.1016/j.lfs.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 133.Casoli T, Di Stefano G, Giorgetti B, et al. Release of beta-amyloid from high-density platelets: implications for Alzheimer's disease pathology. Ann N Y Acad Sci. 2007 Jan;1096:170–178. doi: 10.1196/annals.1397.082. [DOI] [PubMed] [Google Scholar]
- 134.Kelley BJ, Knopman DS. Alternative medicine and Alzheimer disease. Neurologist. 2008 Sep;14(5):299–306. doi: 10.1097/NRL.0b013e318172cf4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liang WS, Dunckley T, Beach TG, et al. Altered neuronal gene expression in brain regions differentially affected by Alzheimer's disease: a reference data set. Physiol Genomics. 2008 Apr 22;33(2):240–256. doi: 10.1152/physiolgenomics.00242.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Verdaguer E, Susana Gde A, Clemens A, Pallas M, Camins A. Implication of the transcription factor E2F-1 in the modulation of neuronal apoptosis. Biomed Pharmacother. 2007 Aug;61(7):390–399. doi: 10.1016/j.biopha.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 137.Sommer C. Neuronal plasticity after ischemic preconditioning and TIA-like preconditioning ischemic periods. Acta Neuropathol. 2009 May;117(5):511–523. doi: 10.1007/s00401-008-0473-0. [DOI] [PubMed] [Google Scholar]
- 138.Braga M, Sinha Hikim AP, Datta S, et al. Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis. 2008 Jun;13(6):822–832. doi: 10.1007/s10495-008-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maiese K, Chong ZZ, Kang J. Transformation into treatment: Novel therapeutics that begin within the cell. In: Maiese K, editor. Neuronal and Vascular Plasticity: Elucidating Basic Cellular Mechanisms for Future Therapeutic Discovery. Kluwer Academic Publishers; Norwell, MA: 2003. pp. 1–26. [Google Scholar]
- 140.Gross J, Machulik A, Amarjargal N, et al. Expression of apoptosis-related genes in the organ of Corti, modiolus and stria vascularis of newborn rats. Brain Res. 2007 Aug 8;1162:56–68. doi: 10.1016/j.brainres.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 141.Maiese K, Ahmad I, TenBroeke M, Gallant J. Metabotropic glutamate receptor subtypes independently modulate neuronal intracellular calcium. J Neurosci Res. 1999;55:472–485. doi: 10.1002/(SICI)1097-4547(19990215)55:4<472::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 142.Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J Neurosci Res. 2000;59(4):568–580. doi: 10.1002/(SICI)1097-4547(20000215)59:4<568::AID-JNR13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 143.Dombroski D, Balasubramanian K, Schroit AJ. Phosphatidylserine expression on cell surfaces promotes antibody- dependent aggregation and thrombosis in beta2-glycoprotein I-immune mice. J Autoimmun. 2000;14(3):221–229. doi: 10.1006/jaut.2000.0365. [DOI] [PubMed] [Google Scholar]
- 144.Jessel R, Haertel S, Socaciu C, Tykhonova S, Diehl HA. Kinetics of apoptotic markers in exogeneously induced apoptosis of EL4 cells. J Cell Mol Med. 2002;6(1):82–92. doi: 10.1111/j.1582-4934.2002.tb00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003 Sep;64(3):557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- 146.Maiese K, Vincent A, Lin SH, Shaw T. Group I and Group III metabotropic glutamate receptor subtypes provide enhanced neuroprotection. J Neurosci Res. 2000;62(2):257–272. doi: 10.1002/1097-4547(20001015)62:2<257::AID-JNR10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 147.Mari C, Karabiyikoglu M, Goris ML, Tait JF, Yenari MA, Blankenberg FG. Detection of focal hypoxic-ischemic injury and neuronal stress in a rodent model of unilateral MCA occlusion/reperfusion using radiolabeled annexin V. Eur J Nucl Med Mol Imaging. 2004 May;31(5):733–739. doi: 10.1007/s00259-004-1473-5. [DOI] [PubMed] [Google Scholar]
- 148.Maiese K. The dynamics of cellular injury: transformation into neuronal and vascular protection. Histol Histopathol. 2001;16(2):633–644. doi: 10.14670/HH-16.633. [DOI] [PubMed] [Google Scholar]
- 149.Vincent AM, Maiese K. Direct temporal analysis of apoptosis induction in living adherent neurons. J Histochem Cytochem. 1999;47(5):661–672. doi: 10.1177/002215549904700508. [DOI] [PubMed] [Google Scholar]
- 150.Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003 Mar 1;71(5):659–669. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- 151.Chong ZZ, Lin SH, Kang JQ, Maiese K. The tyrosine phosphatase SHP2 modulates MAP kinase p38 and caspase 1 and 3 to foster neuronal survival. Cell Mol Neurobiol. 2003 Oct;23(4-5):561–578. doi: 10.1023/A:1025158314016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maiese K, Boccone L. Neuroprotection by peptide growth factors against anoxia and nitric oxide toxicity requires modulation of protein kinase C. J Cereb Blood Flow Metab. 1995;15(3):440–449. doi: 10.1038/jcbfm.1995.55. [DOI] [PubMed] [Google Scholar]
- 153.Maiese K, Boniece IR, Skurat K, Wagner JA. Protein kinases modulate the sensitivity of hippocampal neurons to nitric oxide toxicity and anoxia. J Neurosci Res. 1993;36(1):77–87. doi: 10.1002/jnr.490360109. [DOI] [PubMed] [Google Scholar]
- 154.Maiese K, TenBroeke M, Kue I. Neuroprotection of lubeluzole is mediated through the signal transduction pathways of nitric oxide. J Neurochem. 1997;68(2):710–714. doi: 10.1046/j.1471-4159.1997.68020710.x. [DOI] [PubMed] [Google Scholar]
- 155.Leytin V, Allen DJ, Mykhaylov S, Lyubimov E, Freedman J. Thrombin-triggered platelet apoptosis. J Thromb Haemost. 2006 Dec;4(12):2656–2663. doi: 10.1111/j.1538-7836.2006.02200.x. [DOI] [PubMed] [Google Scholar]
- 156.Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009 Mar;228(1):149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Maiese K, Chong ZZ, Shang YC, Hou J. A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Med Res Rev. 2009 May;29(3):395–418. doi: 10.1002/med.20139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007 Feb;19(2):263–272. [PMC free article] [PubMed] [Google Scholar]
- 159.Dello Russo C, Lisi L, Tringali G, Navarra P. Involvement of mTOR kinase in cytokine-dependent microglial activation and cell proliferation. Biochem Pharmacol. 2009 Nov 1;78(9):1242–1251. doi: 10.1016/j.bcp.2009.06.097. [DOI] [PubMed] [Google Scholar]
- 160.Lee SJ, Cho KS, Koh JY. Oxidative injury triggers autophagy in astrocytes: the role of endogenous zinc. Glia. 2009 Sep;57(12):1351–1361. doi: 10.1002/glia.20854. [DOI] [PubMed] [Google Scholar]
- 161.Martin I, Andres CR, Vedrine S, et al. Effect of the oligodendrocyte myelin glycoprotein (OMgp) on the expansion and neuronal differentiation of rat neural stem cells. Brain Res. 2009 Aug 11;1284:22–30. doi: 10.1016/j.brainres.2009.05.070. [DOI] [PubMed] [Google Scholar]
- 162.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009 Jul;9(7):465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Williams R, Dhillon NK, Hegde ST, et al. Proinflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia. 2009 May;57(7):734–743. doi: 10.1002/glia.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao L, Ma W, Fariss RN, Wong WT. Retinal vascular repair and neovascularization are not dependent on CX3CR1 signaling in a model of ischemic retinopathy. Exp Eye Res. 2009 Jun;88(6):1004–1013. doi: 10.1016/j.exer.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal. 2005 Sep-Oct;7(9-10):1223–1233. doi: 10.1089/ars.2005.7.1223. [DOI] [PubMed] [Google Scholar]
- 166.Maiese K, Chong ZZ. Insights into oxidative stress and potential novel therapeutic targets for Alzheimer disease. Restor Neurol Neurosci. 2004;22(2):87–104. [PubMed] [Google Scholar]
- 167.Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch Biochem Biophys. 1998;353(2):312–321. doi: 10.1006/abbi.1998.0658. [DOI] [PubMed] [Google Scholar]
- 168.Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008 Oct;28(10):1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- 169.Wu Y, Peng H, Cui M, Whitney NP, Huang Y, Zheng JC. CXCL12 increases human neural progenitor cell proliferation through Akt-1/FOXO3a signaling pathway. J Neurochem. 2009 May;109(4):1157–1167. doi: 10.1111/j.1471-4159.2009.06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chong ZZ, Kang JQ, Maiese K. Metabotropic glutamate receptors promote neuronal and vascular plasticity through novel intracellular pathways. Histol Histopathol. 2003 Jan;18(1):173–189. doi: 10.14670/HH-18.173. [DOI] [PubMed] [Google Scholar]
- 171.Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003 Oct 1;74(1):37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- 172.Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol. 2005 Feb;15(1):101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 173.Chong ZZ, Kang J, Li F, Maiese K. mGluRI Targets Microglial Activation and Selectively Prevents Neuronal Cell Engulfment Through Akt and Caspase Dependent Pathways. Curr Neurovasc Res. 2005 Jul;2(3):197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006 Aug;3(3):187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006 Jan;21(1):103–124. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.He XL, Wang YH, Gao M, Li XX, Zhang TT, Du GH. Baicalein protects rat brain mitochondria against chronic cerebral hypoperfusion-induced oxidative damage. Brain Res. 2009 Jan 16;1249:212–221. doi: 10.1016/j.brainres.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 177.Maiese K, Chong ZZ, Shang YC, Hou J. Rogue proliferation versus restorative protection: where do we draw the line for Wnt and forkhead signaling? Expert Opin Ther Targets. 2008 Jul;12(7):905–916. doi: 10.1517/14728222.12.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Plecita-Hlavata L, Lessard M, Santorova J, Bewersdorf J, Jezek P. Mitochondrial oxidative phosphorylation and energetic status are reflected by morphology of mitochondrial network in INS-1E and HEP-G2 cells viewed by 4Pi microscopy. Biochim Biophys Acta. 2008 Jul-Aug;1777(7-8):834–846. doi: 10.1016/j.bbabio.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 179.Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001 Jan 26;276(4):2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 180.Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c, and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003 Mar;23(3):320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- 181.Lin SH, Vincent A, Shaw T, Maynard KI, Maiese K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J Cereb Blood Flow Metab. 2000;20(9):1380–1391. doi: 10.1097/00004647-200009000-00013. [DOI] [PubMed] [Google Scholar]
- 182.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002 Oct;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 183.Choo HJ, Kim JH, Kwon OB, et al. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006 Apr;49(4):784–791. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- 184.Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007 Jun;30(6):268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Espada J, Calvo MB, Diaz-Prado S, Medina V. Wnt signalling and cancer stem cells. Clin Transl Oncol. 2009 Jul;11(7):411–427. doi: 10.1007/s12094-009-0380-4. [DOI] [PubMed] [Google Scholar]
- 186.Jozwiak J, Kotulska K, Grajkowska W, et al. Upregulation of the WNT pathway in tuberous sclerosis-associated subependymal giant cell astrocytomas. Brain Dev. 2007 Jun;29(5):273–280. doi: 10.1016/j.braindev.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 187.Jozwiak J, Wlodarski P. Hamartin and tuberin modulate gene transcription via beta-catenin. J Neurooncol. 2006 Sep;79(3):229–234. doi: 10.1007/s11060-006-9134-0. [DOI] [PubMed] [Google Scholar]
- 188.Kikuchi A, Yamamoto H, Sato A. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol. 2009 Mar;19(3):119–129. doi: 10.1016/j.tcb.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 189.Lee JH, Lee EO, Kang JL, Chong YH. Concomitant degradation of beta-catenin and GSK-3 beta potently contributes to glutamate-induced neurotoxicity in rat hippocampal slice cultures. J Neurochem. 2008 Aug;106(3):1066–1077. doi: 10.1111/j.1471-4159.2008.05444.x. [DOI] [PubMed] [Google Scholar]
- 190.Luo JM, Dai CF, Lin SY, Huang PQ. Asymmetric syntheses and Wnt signal inhibitory activity of melleumin A and four analogues of melleumins A and B. Chem Asian J. 2009 Feb 2;4(2):328–335. doi: 10.1002/asia.200800355. [DOI] [PubMed] [Google Scholar]
- 191.Maiese K, Hou J, Chong ZZ, Shang YC. A fork in the path: Developing therapeutic inroads with FoxO proteins. Oxid Med Cell Longev. 2009;2(3):119–126. doi: 10.4161/oxim.2.3.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008 Apr;118(1):58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mercado-Gomez O, Hernandez-Fonseca K, Villavicencio-Queijeiro A, Massieu L, Chimal-Monroy J, Arias C. Inhibition of Wnt and PI3K signaling modulates GSK-3beta activity and induces morphological changes in cortical neurons: role of tau phosphorylation. Neurochem Res. 2008 Aug;33(8):1599–1609. doi: 10.1007/s11064-008-9714-9. [DOI] [PubMed] [Google Scholar]
- 194.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009 Jan;66(2):236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sutton LP, Honardoust D, Mouyal J, Rajakumar N, Rushlow WJ. Activation of the canonical Wnt pathway by the antipsychotics haloperidol and clozapine involves dishevelled-3. J Neurochem. 2007 Jul;102(1):153–169. doi: 10.1111/j.1471-4159.2007.04527.x. [DOI] [PubMed] [Google Scholar]
- 196.Vettor R, Milan G, Franzin C, et al. THE ORIGIN OF INTERMUSCULAR ADIPOSE TISSUE AND ITS PATHOPHYSIOLOGICAL IMPLICATIONS. Am J Physiol Endocrinol Metab. 2009 Sep 8; doi: 10.1152/ajpendo.00229.2009. [DOI] [PubMed] [Google Scholar]
- 197.Wang Z, Havasi A, Gall JM, Mao H, Schwartz JH, Borkan SC. Beta-catenin promotes survival of renal epithelial cells by inhibiting Bax. J Am Soc Nephrol. 2009 Sep;20(9):1919–1928. doi: 10.1681/ASN.2009030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wilusz M, Majka M. Role of the Wnt/beta-catenin network in regulating hematopoiesis. Arch Immunol Ther Exp (Warsz) 2008 Jul-Aug;56(4):257–266. doi: 10.1007/s00005-008-0029-y. [DOI] [PubMed] [Google Scholar]
- 199.Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006 Mar;38(3):320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 200.Lehman DM, Hunt KJ, Leach RJ, et al. Haplotypes of Transcription Factor 7-Like 2 (TCF7L2) Gene and Its Upstream Region Are Associated With Type 2 Diabetes and Age of Onset in Mexican Americans. Diabetes. 2007 Feb;56(2):389–393. doi: 10.2337/db06-0860. [DOI] [PubMed] [Google Scholar]
- 201.Scott LJ, Bonnycastle LL, Willer CJ, et al. Association of transcription factor 7-like 2 (TCF7L2) variants with type 2 diabetes in a Finnish sample. Diabetes. 2006 Sep;55(9):2649–2653. doi: 10.2337/db06-0341. [DOI] [PubMed] [Google Scholar]
- 202.Guo YF, Xiong DH, Shen H, et al. Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J Med Genet. 2006 Oct;43(10):798–803. doi: 10.1136/jmg.2006.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kanazawa A, Tsukada S, Sekine A, et al. Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. Am J Hum Genet. 2004 Nov;75(5):832–843. doi: 10.1086/425340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mani A, Radhakrishnan J, Wang H, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007 Mar 2;315(5816):1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Al-Aly Z, Shao JS, Lai CF, et al. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2007 Dec;27(12):2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 206.Wright WS, Longo KA, Dolinsky VW, et al. Wnt10b Inhibits Obesity in ob/ob and Agouti Mice. Diabetes. 2007 Feb;56(2):295–303. doi: 10.2337/db06-1339. [DOI] [PubMed] [Google Scholar]
- 207.Lin CL, Wang JY, Huang YT, Kuo YH, Surendran K, Wang FS. Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol. 2006 Oct;17(10):2812–2820. doi: 10.1681/ASN.2005121355. [DOI] [PubMed] [Google Scholar]
- 208.Aslanidi G, Kroutov V, Philipsberg G, et al. Ectopic expression of Wnt10b decreases adiposity and improves glucose homeostasis in obese rats. Am J Physiol Endocrinol Metab. 2007 Sep;293(3):E726–736. doi: 10.1152/ajpendo.00248.2007. [DOI] [PubMed] [Google Scholar]
- 209.Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: Elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008;85:194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008 Apr;19(2):145–155. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gayer CP, Chaturvedi LS, Wang S, Craig DH, Flanigan T, Basson MD. Strain-induced proliferation requires the phosphatidylinositol 3-kinase/AKT/glycogen synthase kinase pathway. J Biol Chem. 2009 Jan 23;284(4):2001–2011. doi: 10.1074/jbc.M804576200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.An J, Zhang C, Polavarapu R, Zhang X, Yepes M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein induce Akt phosphorylation in the ischemic brain. Blood. 2008 Oct 1;112(7):2787–2794. doi: 10.1182/blood-2008-02-141630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Slaets H, Dumont D, Vanderlocht J, et al. Leukemia inhibitory factor induces an antiapoptotic response in oligodendrocytes through Akt-phosphorylation and up-regulation of 14-3-3. Proteomics. 2008 Mar;8(6):1237–1247. doi: 10.1002/pmic.200700641. [DOI] [PubMed] [Google Scholar]
- 214.Dreixler JC, Hemmert JW, Shenoy SK, et al. The role of Akt/protein kinase B subtypes in retinal ischemic preconditioning. Exp Eye Res. 2009 Mar;88(3):512–521. doi: 10.1016/j.exer.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006 Feb;116(2):344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002 Dec 3;106(23):2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 217.Burgos-Ramos E, Martos-Moreno GA, Lopez MG, et al. The N-terminal tripeptide of insulin-like growth factor-I protects against beta-amyloid-induced somatostatin depletion by calcium and glycogen synthase kinase 3 beta modulation. J Neurochem. 2009 Apr;109(2):360–370. doi: 10.1111/j.1471-4159.2009.05980.x. [DOI] [PubMed] [Google Scholar]
- 218.Tamagno E, Guglielmotto M, Giliberto L, et al. JNK and ERK1/2 pathways have a dual opposite effect on the expression of BACE1. Neurobiol Aging. 2009 Oct;30(10):1563–1573. doi: 10.1016/j.neurobiolaging.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 219.Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther. 2008 Jan;324(1):160–169. doi: 10.1124/jpet.107.125773. [DOI] [PubMed] [Google Scholar]
- 220.Tajes M, Yeste-Velasco M, Zhu X, et al. Activation of Akt by lithium: Pro-survival pathways in aging. Mech Ageing Dev. 2009 Jan 3;130(4):253–261. doi: 10.1016/j.mad.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 221.Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-x(L) and caspase 1, 3, and 9. Exp Cell Res. 2004 Jun 10;296(2):196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 222.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007 Apr;150(7):839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003 Mar;138(6):1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fujiwara M, Izuishi K, Sano T, et al. Modulating effect of the PI3-kinase inhibitor LY294002 on cisplatin in human pancreatic cancer cells. J Exp Clin Cancer Res. 2008;27:76. doi: 10.1186/1756-9966-27-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chong ZZ, Li F, Maiese K. Activating Akt and the brain's resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005 Jan;20(1):299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chong ZZ, Maiese K. Targeting WNT, protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system. Histol Histopathol. 2004 Apr;19(2):495–504. doi: 10.14670/hh-19.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fukumoto S, Hsieh CM, Maemura K, et al. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276(20):17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- 228.Naito AT, Akazawa H, Takano H, et al. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res. 2005 Jul 22;97(2):144–151. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- 229.Longo KA, Kennell JA, Ochocinska MJ, Ross SE, Wright WS, MacDougald OA. Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J Biol Chem. 2002;277(41):38239–38244. doi: 10.1074/jbc.M206402200. [DOI] [PubMed] [Google Scholar]
- 230.Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002;16(1):46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mirotsou M, Zhang Z, Deb A, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007 Jan 30;104(5):1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.van de Schans VA, van den Borne SW, Strzelecka AE, et al. Interruption of Wnt signaling attenuates the onset of pressure overload-induced cardiac hypertrophy. Hypertension. 2007 Mar;49(3):473–480. doi: 10.1161/01.HYP.0000255946.55091.24. [DOI] [PubMed] [Google Scholar]
- 233.Barandon L, Dufourcq P, Costet P, et al. Involvement of FrzA/sFRP-1 and the Wnt/frizzled pathway in ischemic preconditioning. Circ Res. 2005 Jun 24;96(12):1299–1306. doi: 10.1161/01.RES.0000171895.06914.2c. [DOI] [PubMed] [Google Scholar]
- 234.DiPalma JR, Thayer WS. Use of niacin as a drug. Annu Rev Nutr. 1991;11:169–187. doi: 10.1146/annurev.nu.11.070191.001125. [DOI] [PubMed] [Google Scholar]
- 235.Rex A, Fink H. Pharmacokinetic aspects of reduced nicotinamide adenine dinucleotide (NADH) in rats. Front Biosci. 2008;13:3735–3741. doi: 10.2741/2962. [DOI] [PubMed] [Google Scholar]
- 236.Li F, Chong ZZ, Maiese K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front Biosci. 2004;9:2500–2520. doi: 10.2741/1412. [DOI] [PubMed] [Google Scholar]
- 237.Jackson TM, Rawling JM, Roebuck BD, Kirkland JB. Large supplements of nicotinic acid and nicotinamide increase tissue NAD+ and poly(ADP-ribose) levels but do not affect diethylnitrosamine-induced altered hepatic foci in Fischer-344 rats. J Nutr. 1995 Jun;125(6):1455–1461. doi: 10.1093/jn/125.6.1455. [DOI] [PubMed] [Google Scholar]
- 238.Wojcik M, Seidle HF, Bieganowski P, Brenner C. Glutamine-dependent NAD+ synthetase. How a two-domain, three-substrate enzyme avoids waste. J Biol Chem. 2006 Nov 3;281(44):33395–33402. doi: 10.1074/jbc.M607111200. [DOI] [PubMed] [Google Scholar]
- 239.Khan JA, Forouhar F, Tao X, Tong L. Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert Opin Ther Targets. 2007 May;11(5):695–705. doi: 10.1517/14728222.11.5.695. [DOI] [PubMed] [Google Scholar]
- 240.Khan JA, Xiang S, Tong L. Crystal structure of human nicotinamide riboside kinase. Structure. 2007 Aug;15(8):1005–1013. doi: 10.1016/j.str.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 241.Depeint F, Bruce WR, Shangari N, Mehta R, O'Brien PJ. Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact. 2006 Oct 27;163(1-2):94–112. doi: 10.1016/j.cbi.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 242.Hara N, Yamada K, Shibata T, Osago H, Hashimoto T, Tsuchiya M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem. 2007 Aug 24;282(34):24574–24582. doi: 10.1074/jbc.M610357200. [DOI] [PubMed] [Google Scholar]
- 243.Williams AC, Ramsden DB. Pellagra: A clue as to why energy failure causes diseases? Med Hypotheses. 2007;69(3):618–628. doi: 10.1016/j.mehy.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 244.Williams AC, Ramsden DB. Hydrogen symbioses in evolution and disease. Qjm. 2007 Jul;100(7):451–459. doi: 10.1093/qjmed/hcm045. [DOI] [PubMed] [Google Scholar]
- 245.Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. Enzymology of NAD+ homeostasis in man. Cell Mol Life Sci. 2004 Jan;61(1):19–34. doi: 10.1007/s00018-003-3161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lin SJ, Guarente L. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr Opin Cell Biol. 2003 Apr;15(2):241–246. doi: 10.1016/s0955-0674(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 247.Hageman GJ, Stierum RH. Niacin, poly(ADP-ribose) polymerase-1 and genomic stability. Mutat Res. 2001 Apr 18;475(1-2):45–56. doi: 10.1016/s0027-5107(01)00078-1. [DOI] [PubMed] [Google Scholar]
- 248.Sadanaga-Akiyoshi F, Yao H, Tanuma S, et al. Nicotinamide attenuates focal ischemic brain injury in rats: with special reference to changes in nicotinamide and NAD+ levels in ischemic core and penumbra. Neurochem Res. 2003 Aug;28(8):1227–1234. doi: 10.1023/a:1024236614015. [DOI] [PubMed] [Google Scholar]
- 249.Wang J, Zhai Q, Chen Y, et al. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol. 2005 Aug 1;170(3):349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chong ZZ, Lin SH, Maiese K. Nicotinamide Modulates Mitochondrial Membrane Potential and Cysteine Protease Activity during Cerebral Vascular Endothelial Cell Injury. J Vasc Res. 2002;39(2):131–147. doi: 10.1159/000057762. [DOI] [PubMed] [Google Scholar]
- 251.Maiese K, Lin S, Chong ZZ. Elucidating neuronal and vascular injury through the cytoprotective agent nicotinamide. Curr Med Chem-Imm, Endoc. & Metab. Agents. 2001;1(3):257–267. [Google Scholar]
- 252.Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res. 2005 Oct;2(4):271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004 Jul;24(7):728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- 254.Halestrap AP, Woodfield KY, Connern CP. Oxidative stress, thiol reagents, and membrane potential modulate the mitochondrial permeability transition by affecting nucleotide binding to the adenine nucleotide translocase. J Biol Chem. 1997 Feb 7;272(6):3346–3354. doi: 10.1074/jbc.272.6.3346. [DOI] [PubMed] [Google Scholar]
- 255.La Piana G, Marzulli D, Consalvo MI, Lofrumento NE. Cytochrome c-induced cytosolic nicotinamide adenine dinucleotide oxidation, mitochondrial permeability transition, and apoptosis. Arch Biochem Biophys. 2003 Feb 15;410(2):201–211. doi: 10.1016/s0003-9861(02)00687-2. [DOI] [PubMed] [Google Scholar]
- 256.Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009 Oct 2;5(4):396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 257.Aoyagi S, Archer TK. Nicotinamide uncouples hormone-dependent chromatin remodeling from transcription complex assembly. Mol Cell Biol. 2008 Jan;28(1):30–39. doi: 10.1128/MCB.01158-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bruno V, Battaglia G, Copani A, et al. Activation of class II or III metabotropic glutamate receptors protects cultured cortical neurons against excitotoxic degeneration. Eur J Neurosci. 1995;7(9):1906–1913. doi: 10.1111/j.1460-9568.1995.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 259.Anderson DW, Bradbury KA, Schneider JS. Neuroprotection in Parkinson models varies with toxin administration protocol. Eur J Neurosci. 2006 Dec;24(11):3174–3182. doi: 10.1111/j.1460-9568.2006.05192.x. [DOI] [PubMed] [Google Scholar]
- 260.Feng Y, Paul IA, LeBlanc MH. Nicotinamide reduces hypoxic ischemic brain injury in the newborn rat. Brain Res Bull. 2006 Mar 31;69(2):117–122. doi: 10.1016/j.brainresbull.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lin SH, Chong ZZ, Maiese K. Nicotinamide: A Nutritional Supplement that Provides Protection Against Neuronal and Vascular Injury. J Med Food. 2001;4(1):27–38. doi: 10.1089/10966200152053686. Spring. [DOI] [PubMed] [Google Scholar]
- 262.Slomka M, Zieminska E, Salinska E, Lazarewicz JW. Neuroprotective effects of nicotinamide and 1-methylnicotinamide in acute excitotoxicity in vitro. Folia Neuropathol. 2008;46(1):69–80. [PubMed] [Google Scholar]
- 263.Slomka M, Zieminska E, Lazarewicz J. Nicotinamide and 1-methylnicotinamide reduce homocysteine neurotoxicity in primary cultures of rat cerebellar granule cells. Acta Neurobiol Exp (Wars) 2008;68(1):1–9. doi: 10.55782/ane-2008-1666. [DOI] [PubMed] [Google Scholar]
- 264.Ieraci A, Herrera DG. Nicotinamide Protects against Ethanol-Induced Apoptotic Neurodegeneration in the Developing Mouse Brain. PLoS Med. 2006 Feb 21;3(4):e101. doi: 10.1371/journal.pmed.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Shen CC, Huang HM, Ou HC, Chen HL, Chen WC, Jeng KC. Protective effect of nicotinamide on neuronal cells under oxygen and glucose deprivation and hypoxia/reoxygenation. J Biomed Sci. 2004 Jul-Aug;11(4):472–481. doi: 10.1007/BF02256096. [DOI] [PubMed] [Google Scholar]
- 266.Sonee M, Martens JR, Evers MR, Mukherjee SK. The effect of tertiary butylhydroperoxide and nicotinamide on human cortical neurons. Neurotoxicology. 2003 Jun;24(3):443–448. doi: 10.1016/S0161-813X(03)00019-6. [DOI] [PubMed] [Google Scholar]
- 267.Kiuchi K, Kondo M, Ueno S, et al. Functional rescue of N-methyl-N-nitrosourea-induced retinopathy by nicotinamide in Sprague-Dawley rats. Curr Eye Res. 2003 Jun;26(6):355–362. doi: 10.1076/ceyr.26.5.355.15435. [DOI] [PubMed] [Google Scholar]
- 268.Kiuchi K, Yoshizawa K, Shikata N, Matsumura M, Tsubura A. Nicotinamide prevents N-methyl-N-nitrosourea-induced photoreceptor cell apoptosis in Sprague-Dawley rats and C57BL mice. Exp Eye Res. 2002 Mar;74(3):383–392. doi: 10.1006/exer.2001.1127. [DOI] [PubMed] [Google Scholar]
- 269.Reber F, Geffarth R, Kasper M, et al. Graded sensitiveness of the various retinal neuron populations on the glyoxal-mediated formation of advanced glycation end products and ways of protection. Graefes Arch Clin Exp Ophthalmol. 2003 Mar;241(3):213–225. doi: 10.1007/s00417-002-0528-1. [DOI] [PubMed] [Google Scholar]
- 270.Hoane MR, Gilbert DR, Holland MA, Pierce JL. Nicotinamide reduces acute cortical neuronal death and edema in the traumatically injured brain. Neurosci Lett. 2006 Nov 6;408(1):35–39. doi: 10.1016/j.neulet.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 271.Hoane MR, Kaplan SA, Ellis AL. The effects of nicotinamide on apoptosis and blood-brain barrier breakdown following traumatic brain injury. Brain Res. 2006 Dec 13;1125(1):185–193. doi: 10.1016/j.brainres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 272.Hoane MR, Pierce JL, Holland MA, Anderson GD. Nicotinamide treatment induces behavioral recovery when administered up to 4 hours following cortical contusion injury in the rat. Neuroscience. 2008 Jun 26;154(3):861–868. doi: 10.1016/j.neuroscience.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hoane MR, Pierce JL, Kaufman NA, Beare JE. Variation in chronic nicotinamide treatment after traumatic brain injury can alter components of functional recovery independent of histological damage. Oxid Med Cell Longev. 2008;1(1):45–52. doi: 10.4161/oxim.1.1.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Holland MA, Tan AA, Smith DC, Hoane MR. Nicotinamide treatment provides acute neuroprotection and GFAP regulation following fluid percussion injury. J Neurotrauma. 2008 Feb;25(2):140–152. doi: 10.1089/neu.2007.0312. [DOI] [PubMed] [Google Scholar]
- 275.Wallis RA, Panizzon KL, Girard JM. Traumatic neuroprotection with inhibitors of nitric oxide and ADP- ribosylation. Brain Res. 1996;710(1-2):169–177. doi: 10.1016/0006-8993(95)01278-8. [DOI] [PubMed] [Google Scholar]
- 276.Yang J, Klaidman L, Chang M, et al. Nicotinamide therapy protects against both necrosis and apoptosis in a stroke model. Pharmacol Biochem Behav. 2002;73(4):901–910. doi: 10.1016/s0091-3057(02)00939-5. [DOI] [PubMed] [Google Scholar]
- 277.Gupta S, Kaul CL, Sharma SS. Neuroprotective effect of combination of poly (ADP-ribose) polymerase inhibitor and antioxidant in middle cerebral artery occlusion induced focal ischemia in rats. Neurol Res. 2004 Jan;26(1):103–107. doi: 10.1179/016164104773026624. [DOI] [PubMed] [Google Scholar]
- 278.Sakakibara Y, Mitha AP, Ayoub IA, Ogilvy CS, Maynard KI. Delayed treatment with nicotinamide (vitamin B3) reduces the infarct volume following focal cerebral ischemia in spontaneously hypertensive rats, diabetic and non-diabetic Fischer 344 rats. Brain Res. 2002 Mar 22;931(1):68–73. doi: 10.1016/s0006-8993(02)02263-1. [DOI] [PubMed] [Google Scholar]
- 279.Macleod MR, O'Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004 May;35(5):1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- 280.Brewer KL, Hardin JS. Neuroprotective effects of nicotinamide after experimental spinal cord injury. Acad Emerg Med. 2004 Feb;11(2):125–130. doi: 10.1111/j.1553-2712.2004.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 281.Isbir CS, Ak K, Kurtkaya O, et al. Ischemic preconditioning and nicotinamide in spinal cord protection in an experimental model of transient aortic occlusion. Eur J Cardiothorac Surg. 2003 Jun;23(6):1028–1033. doi: 10.1016/s1010-7940(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 282.Williams A, Ramsden D. Nicotinamide: a double edged sword. Parkinsonism Relat Disord. 2005 Nov;11(7):413–420. doi: 10.1016/j.parkreldis.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 283.Williams AC, Cartwright LS, Ramsden DB. Parkinson's disease: the first common neurological disease due to auto-intoxication? Qjm. 2005 Mar;98(3):215–226. doi: 10.1093/qjmed/hci027. [DOI] [PubMed] [Google Scholar]
- 284.Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009 May;117(5):481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 285.Giulumian AD, Meszaros LG, Fuchs LC. Endothelin-1-induced contraction of mesenteric small arteries is mediated by ryanodine receptor Ca2+ channels and cyclic ADP-ribose. J Cardiovasc Pharmacol. 2000 Dec;36(6):758–763. doi: 10.1097/00005344-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 286.Pietrzak L, Mogielnicki A, Buczko W. Nicotinamide and its metabolite N-methylnicotinamide increase skin vascular permeability in rats. Clin Exp Dermatol. 2009 Apr;34(3):380–384. doi: 10.1111/j.1365-2230.2008.02922.x. [DOI] [PubMed] [Google Scholar]
- 287.Oumouna-Benachour K, Hans CP, Suzuki Y, et al. Poly(ADP-ribose) polymerase inhibition reduces atherosclerotic plaque size and promotes factors of plaque stability in apolipoprotein E-deficient mice: effects on macrophage recruitment, nuclear factor-kappaB nuclear translocation, and foam cell death. Circulation. 2007 May 8;115(18):2442–2450. doi: 10.1161/CIRCULATIONAHA.106.668756. [DOI] [PubMed] [Google Scholar]
- 288.Giammona LM, Fuhrken PG, Papoutsakis ET, Miller WM. Nicotinamide (vitamin B3) increases the polyploidisation and proplatelet formation of cultured primary human megakaryocytes. Br J Haematol. 2006 Nov;135(4):554–566. doi: 10.1111/j.1365-2141.2006.06341.x. [DOI] [PubMed] [Google Scholar]
- 289.Slominska EM, Yuen A, Osman L, Gebicki J, Yacoub MH, Smolenski RT. Cytoprotective effects of nicotinamide derivatives in endothelial cells. Nucleosides Nucleotides Nucleic Acids. 2008 Jun;27(6):863–866. doi: 10.1080/15257770802146528. [DOI] [PubMed] [Google Scholar]
- 290.Mateuszuk L, Khomich TI, Slominska E, et al. Activation of nicotinamide N-methyltrasferase and increased formation of 1-methylnicotinamide (MNA) in atherosclerosis. Pharmacol Rep. 2009 Jan-Feb;61(1):76–85. doi: 10.1016/s1734-1140(09)70009-x. [DOI] [PubMed] [Google Scholar]
- 291.Vincent AM, Maiese K. Nitric oxide induction of neuronal endonuclease activity in programmed cell death. Exp Cell Res. 1999;246(2):290–300. doi: 10.1006/excr.1998.4282. [DOI] [PubMed] [Google Scholar]
- 292.Vincent AM, TenBroeke M, Maiese K. Metabotropic glutamate receptors prevent programmed cell death through the modulation of neuronal endonuclease activity and intracellular pH. Exp Neurol. 1999;155(1):79–94. doi: 10.1006/exnr.1998.6966. [DOI] [PubMed] [Google Scholar]
- 293.Vincent AM, TenBroeke M, Maiese K. Neuronal intracellular pH directly mediates nitric oxide-induced programmed cell death. J Neurobiol. 1999;40(2):171–184. doi: 10.1002/(sici)1097-4695(199908)40:2<171::aid-neu4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 294.Young GS, Jacobson EL, Kirkland JB. Water maze performance in young male Long-Evans rats is inversely affected by dietary intakes of niacin and may be linked to levels of the NAD+ metabolite cADPR. J Nutr. 2007 Apr;137(4):1050–1057. doi: 10.1093/jn/137.4.1050. [DOI] [PubMed] [Google Scholar]
- 295.Reddy S, Bibby NJ, Wu D, Swinney C, Barrow G, Elliott RB. A combined casein-free-nicotinamide diet prevents diabetes in the NOD mouse with minimum insulitis. Diabetes Res Clin Pract. 1995 Aug;29(2):83–92. doi: 10.1016/0168-8227(95)01109-9. [DOI] [PubMed] [Google Scholar]
- 296.Hu Y, Wang Y, Wang L, et al. Effects of nicotinamide on prevention and treatment of streptozotocin-induced diabetes mellitus in rats. Chin Med J (Engl) 1996 Nov;109(11):819–822. [PubMed] [Google Scholar]
- 297.Stevens MJ, Li F, Drel VR, et al. Nicotinamide reverses neurological and neurovascular deficits in streptozotocin diabetic rats. J Pharmacol Exp Ther. 2007 Jan;320(1):458–464. doi: 10.1124/jpet.106.109702. [DOI] [PubMed] [Google Scholar]
- 298.Cresto JC, Fabiano de Bruno LE, Cao GF, et al. The association of acetyl-l-carnitine and nicotinamide remits the experimental diabetes in mice by multiple low-dose streptozotocin. Pancreas. 2006 Nov;33(4):403–411. doi: 10.1097/01.mpa.0000236740.07854.b1. [DOI] [PubMed] [Google Scholar]
- 299.Chlopicki S, Swies J, Mogielnicki A, et al. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br J Pharmacol. 2007 Sep;152(2):230–239. 300. doi: 10.1038/sj.bjp.0707383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lee HI, Cho HJ, Han JA, et al. Transient downregulation of protein O-N-acetylglucosaminylation by treatment of high-dose nicotinamide in human cells. Exp Mol Med. 2008 Apr 30;40(2):246–253. doi: 10.3858/emm.2008.40.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Tam D, Tam M, Maynard KI. Nicotinamide modulates energy utilization and improves functional recovery from ischemia in the in vitro rabbit retina. Ann N Y Acad Sci. 2005 Aug;1053:258–268. doi: 10.1196/annals.1344.023. [DOI] [PubMed] [Google Scholar]
- 302.Olmos PR, Hodgson MI, Maiz A, et al. Nicotinamide protected first-phase insulin response (FPIR) and prevented clinical disease in first-degree relatives of type-1 diabetics. Diabetes Res Clin Pract. 2006 Mar;71(3):320–333. doi: 10.1016/j.diabres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 303.Crino A, Schiaffini R, Ciampalini P, et al. A two year observational study of nicotinamide and intensive insulin therapy in patients with recent onset type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2005 Aug;18(8):749–754. doi: 10.1515/jpem.2005.18.8.749. [DOI] [PubMed] [Google Scholar]
- 304.Eto N, Miyata Y, Ohno H, Yamashita T. Nicotinamide prevents the development of hyperphosphataemia by suppressing intestinal sodium-dependent phosphate transporter in rats with adenine-induced renal failure. Nephrol Dial Transplant. 2005 Jul;20(7):1378–1384. doi: 10.1093/ndt/gfh781. [DOI] [PubMed] [Google Scholar]
- 305.Liu HK, Green BD, Flatt PR, McClenaghan NH, McCluskey JT. Effects of long-term exposure to nicotinamide and sodium butyrate on growth, viability, and the function of clonal insulin secreting cells. Endocr Res. 2004 Feb;30(1):61–68. doi: 10.1081/erc-120028485. [DOI] [PubMed] [Google Scholar]
- 306.Reddy S, Salari-Lak N, Sandler S. Long-term effects of nicotinamide-induced inhibition of poly(adenosine diphosphate-ribose) polymerase activity in rat pancreatic islets exposed to interleukin-1 beta. Endocrinology. 1995;136(5):1907–1912. doi: 10.1210/endo.136.5.7720637. [DOI] [PubMed] [Google Scholar]
- 307.Zhou SS, Li D, Sun WP, et al. Nicotinamide overload may play a role in the development of type 2 diabetes. World J Gastroenterol. 2009 Dec 7;15(45):5674–5684. doi: 10.3748/wjg.15.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Gaudineau C, Auclair K. Inhibition of human P450 enzymes by nicotinic acid and nicotinamide. Biochem Biophys Res Commun. 2004 May 7;317(3):950–956. doi: 10.1016/j.bbrc.2004.03.137. [DOI] [PubMed] [Google Scholar]
- 309.Maiese K, Chong ZZ, Shang YC. “Sly as a FOXO”: New paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007 Nov;4(4):295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008 Apr 8;14(5):219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Coffer PJ. When less is more: the PI3K pathway as a determinant of tumor response to dietary restriction. Cell Res. 2009 Jul;19(7):797–799. doi: 10.1038/cr.2009.81. [DOI] [PubMed] [Google Scholar]
- 312.Jacobsen EA, Ananieva O, Brown ML, Chang Y. Growth, differentiation, and malignant transformation of pre-B cells mediated by inducible activation of v-Abl oncogene. J Immunol. 2006 Jun 1;176(11):6831–6838. doi: 10.4049/jimmunol.176.11.6831. [DOI] [PubMed] [Google Scholar]
- 313.Maiese K, Chong ZZ, Shang YC, Hou J. Clever cancer strategies with FoxO transcription factors. Cell Cycle. 2008 Dec 15;7(24):3829–3839. doi: 10.4161/cc.7.24.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond) 2009 Feb;116(3):191–203. doi: 10.1042/CS20080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993 Jul 29;364(6436):412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 316.Jin C, Marsden I, Chen X, Liao X. Sequence specific collective motions in a winged helix DNA binding domain detected by 15N relaxation NMR. Biochemistry. 1998 Apr 28;37(17):6179–6187. doi: 10.1021/bi980031v. [DOI] [PubMed] [Google Scholar]
- 317.Maiese K, Chong Z, Hou J, Shang Y. The “O” Class: Crafting clinical care with FoxO transcription factors. Adv Exp Med Biol. 2009;665:242–260. doi: 10.1007/978-1-4419-1599-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000 Jan 15;14(2):142–146. [PubMed] [Google Scholar]
- 319.Lappas M, Lim R, Riley C, Rice GE, Permezel M. Localisation and expression of FoxO1 proteins in human gestational tissues. Placenta. 2009 Mar;30(3):256–262. doi: 10.1016/j.placenta.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 320.Zheng WH, Kar S, Quirion R. FKHRL1 and its homologs are new targets of nerve growth factor Trk receptor signaling. J Neurochem. 2002 Mar;80(6):1049–1061. doi: 10.1046/j.0022-3042.2002.00783.x. [DOI] [PubMed] [Google Scholar]
- 321.Fei M, Lu M, Wang Y, et al. Arsenic trioxide-induced growth arrest of human hepatocellular carcinoma cells involving FOXO3a expression and localization. Med Oncol. 2009;26(2):178–185. doi: 10.1007/s12032-008-9105-8. [DOI] [PubMed] [Google Scholar]
- 322.Lei H, Quelle FW. FOXO transcription factors enforce cell cycle checkpoints and promote survival of hematopoietic cells after DNA damage. Mol Cancer Res. 2009 Aug;7(8):1294–1303. doi: 10.1158/1541-7786.MCR-08-0531. [DOI] [PubMed] [Google Scholar]
- 323.Kikuchi S, Nagai T, Kunitama M, Kirito K, Ozawa K, Komatsu N. Active FKHRL1 overcomes imatinib resistance in chronic myelogenous leukemia-derived cell lines via the production of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Sci. 2007 Dec;98(12):1949–1958. doi: 10.1111/j.1349-7006.2007.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Nowak K, Killmer K, Gessner C, Lutz W. E2F-1 regulates expression of FOXO1 and FOXO3a. Biochim Biophys Acta. 2007 Apr;1769(4):244–252. doi: 10.1016/j.bbaexp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 325.Bouchard C, Lee S, Paulus-Hock V, Loddenkemper C, Eilers M, Schmitt CA. FoxO transcription factors suppress Myc-driven lymphomagenesis via direct activation of Arf. Genes Dev. 2007 Nov 1;21(21):2775–2787. doi: 10.1101/gad.453107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Bethea CL, Reddy AP, Tokuyama Y, Henderson JA, Lima FB. Protective actions of ovarian hormones in the serotonin system of macaques. Front Neuroendocrinol. 2009 Jul;30(2):212–238. doi: 10.1016/j.yfrne.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Chong ZZ, Kang JQ, Maiese K. Essential cellular regulatory elements of oxidative stress in early and late phases of apoptosis in the central nervous system. Antioxid Redox Signal. 2004 Apr;6(2):277–287. doi: 10.1089/152308604322899341. [DOI] [PubMed] [Google Scholar]
- 328.Fallarino F, Bianchi R, Orabona C, et al. CTLA-4-Ig activates forkhead transcription factors and protects dendritic cells from oxidative stress in nonobese diabetic mice. J Exp Med. 2004 Oct 18;200(8):1051–1062. doi: 10.1084/jem.20040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Nakae J, Cao Y, Oki M, et al. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes. 2008 Mar;57(3):563–576. doi: 10.2337/db07-0698. [DOI] [PubMed] [Google Scholar]
- 330.Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, Mori N. Effects of aging and caloric restriction on the gene expression of Foxo1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microsc Res Tech. 2002 Nov 15;59(4):331–334. doi: 10.1002/jemt.10213. [DOI] [PubMed] [Google Scholar]
- 331.Puig O, Tjian R. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 2005 Oct 15;19(20):2435–2446. doi: 10.1101/gad.1340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Kim JR, Jung HS, Bae SW, et al. Polymorphisms in FOXO gene family and association analysis with BMI. Obesity (Silver Spring) 2006 Feb;14(2):188–193. doi: 10.1038/oby.2006.24. [DOI] [PubMed] [Google Scholar]
- 333.Marchetti V, Menghini R, Rizza S, et al. Benfotiamine counteracts glucose toxicity effects on endothelial progenitor cell differentiation via Akt/FoxO signaling. Diabetes. 2006 Aug;55(8):2231–2237. doi: 10.2337/db06-0369. [DOI] [PubMed] [Google Scholar]
- 334.Kamagate A, Dong HH. Foxo1 integrates insulin signaling to VLDL production. Cell Cycle. 2008;7(20):3162–3170. doi: 10.4161/cc.7.20.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ni YG, Wang N, Cao DJ, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci U S A. 2007 Dec 18;104(51):20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Kamei Y, Miura S, Suzuki M, et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem. 2004 Sep 24;279(39):41114–41123. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- 337.Liu CM, Yang Z, Liu CW, et al. Effect of RNA oligonucleotide targeting Foxo-1 on muscle growth in normal and cancer cachexia mice. Cancer Gene Ther. 2007 Dec;14(12):945–952. doi: 10.1038/sj.cgt.7701091. [DOI] [PubMed] [Google Scholar]
- 338.Sandri M, Lin J, Handschin C, et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006 Oct 31;103(44):16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25(11):577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 340.Charvet C, Alberti I, Luciano F, et al. Proteolytic regulation of Forkhead transcription factor FOXO3a by caspase-3-like proteases. Oncogene. 2003 Jul 17;22(29):4557–4568. doi: 10.1038/sj.onc.1206778. [DOI] [PubMed] [Google Scholar]
- 341.Porcu M, Chiarugi A. The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol Sci. 2005 Feb;26(2):94–103. doi: 10.1016/j.tips.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 342.Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007 Aug 13;26(37):5489–5504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- 343.Taylor DM, Maxwell MM, Luthi-Carter R, Kazantsev AG. Biological and potential therapeutic roles of sirtuin deacetylases. Cell Mol Life Sci. 2008 Dec;65(24):4000–4018. doi: 10.1007/s00018-008-8357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Zschoernig B, Mahlknecht U. SIRTUIN 1: regulating the regulator. Biochem Biophys Res Commun. 2008 Nov 14;376(2):251–255. doi: 10.1016/j.bbrc.2008.08.137. [DOI] [PubMed] [Google Scholar]
- 345.Tang BL, Chua CE. SIRT1 and neuronal diseases. Mol Aspects Med. 2008 Jun;29(3):187–200. doi: 10.1016/j.mam.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 346.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89(4):1121–1132. [PubMed] [Google Scholar]
- 347.Canto C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009 Sep;20(7):325–331. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Balan V, Miller GS, Kaplun L, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008 Oct 10;283(41):27810–27819. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Bureau G, Longpre F, Martinoli MG. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J Neurosci Res. 2008 Feb 1;86(2):403–410. doi: 10.1002/jnr.21503. [DOI] [PubMed] [Google Scholar]
- 350.Chong ZZ, Maiese K. Enhanced Tolerance against Early and Late Apoptotic Oxidative Stress in Mammalian Neurons through Nicotinamidase and Sirtuin Mediated Pathways. Curr Neurovasc Res. 2008 Aug;5(3):159–170. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004 Dec 17;306(5704):2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 352.Ferrara N, Rinaldi B, Corbi G, et al. Exercise Training Promotes SIRT1 Activity in Aged Rats. Rejuvenation Res. 2008 Dec 10;11(1):139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 353.Motta MC, Divecha N, Lemieux M, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004 Feb 20;116(4):551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 354.Lee HI, Jang SY, Kang HT, Hwang ES. p53-, SIRT1-, and PARP-1-independent downregulation of p21WAF1 expression in nicotinamide-treated cells. Biochem Biophys Res Commun. 2008 Apr 4;368(2):298–304. doi: 10.1016/j.bbrc.2008.01.082. [DOI] [PubMed] [Google Scholar]
- 355.Jackson MD, Schmidt MT, Oppenheimer NJ, Denu JM. Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases. J Biol Chem. 2003 Dec 19;278(51):50985–50998. doi: 10.1074/jbc.M306552200. [DOI] [PubMed] [Google Scholar]
- 356.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002 Nov 22;277(47):45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- 357.Cai AL, Zipfel GJ, Sheline CT. Zinc neurotoxicity is dependent on intracellular NAD levels and the sirtuin pathway. Eur J Neurosci. 2006 Oct;24(8):2169–2176. doi: 10.1111/j.1460-9568.2006.05110.x. [DOI] [PubMed] [Google Scholar]
- 358.Kruszewski M, Szumiel I. Sirtuins (histone deacetylases III) in the cellular response to DNA damage--facts and hypotheses. DNA Repair (Amst) 2005 Nov 21;4(11):1306–1313. doi: 10.1016/j.dnarep.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 359.Yoshizaki T, Milne JC, Imamura T, et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009 Mar;29(5):1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Zillikens MC, van Meurs JB, Rivadeneira F, et al. SIRT1 genetic variation is related to BMI and risk of obesity. Diabetes. 2009 Dec;58(12):2828–2834. doi: 10.2337/db09-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. Jama. 2005 Jan 5;293(1):90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Girgenti MJ, Hunsberger J, Duman CH, Sathyanesan M, Terwilliger R, Newton SS. Erythropoietin induction by electroconvulsive seizure, gene regulation, and antidepressant-like behavioral effects. Biol Psychiatry. 2009 Aug 1;66(3):267–274. doi: 10.1016/j.biopsych.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 363.Ma R, Xiong N, Huang C, et al. Erythropoietin protects PC12 cells from beta-amyloid(25-35)-induced apoptosis via PI3K/Akt signaling pathway. Neuropharmacology. 2009 May-Jun;56(6-7):1027–1034. doi: 10.1016/j.neuropharm.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 364.Sun ZK, Yang HQ, Pan J, et al. Protective effects of erythropoietin on tau phosphorylation induced by beta-amyloid. J Neurosci Res. 2008 Oct;86(13):3018–3027. doi: 10.1002/jnr.21745. [DOI] [PubMed] [Google Scholar]
- 365.McLeod M, Hong M, Mukhida K, Sadi D, Ulalia R, Mendez I. Erythropoietin and GDNF enhance ventral mesencephalic fiber outgrowth and capillary proliferation following neural transplantation in a rodent model of Parkinson's disease. Eur J Neurosci. 2006 Jul;24(2):361–370. doi: 10.1111/j.1460-9568.2006.04919.x. [DOI] [PubMed] [Google Scholar]
- 366.Cuzzocrea S, Mazzon E, di Paola R, et al. Erythropoietin reduces the degree of arthritis caused by type II collagen in the mouse. Arthritis Rheum. 2005 Mar;52(3):940–950. doi: 10.1002/art.20875. [DOI] [PubMed] [Google Scholar]
- 367.Thorne M, Moore CS, Robertson GS. Lack of TIMP-1 increases severity of experimental autoimmune encephalomyelitis: Effects of darbepoetin alfa on TIMP-1 null and wild-type mice. J Neuroimmunol. 2009 Jun 25;211(1-2):92–100. doi: 10.1016/j.jneuroim.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 368.Sanchez PE, Fares RP, Risso JJ, et al. Optimal neuroprotection by erythropoietin requires elevated expression of its receptor in neurons. Proc Natl Acad Sci U S A. 2009 Jun 16;106(24):9848–9853. doi: 10.1073/pnas.0901840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Sanchez PE, Navarro FP, Fares RP, et al. Erythropoietin receptor expression is concordant with erythropoietin but not with common beta chain expression in the rat brain throughout the life span. J Comp Neurol. 2009 Jun 1;514(4):403–414. doi: 10.1002/cne.22020. [DOI] [PubMed] [Google Scholar]
- 370.Slotkin TA, Seidler FJ, Fumagalli F. Targeting of neurotrophic factors, their receptors, and signaling pathways in the developmental neurotoxicity of organophosphates in vivo and in vitro. Brain Res Bull. 2008 Jul 1;76(4):424–438. doi: 10.1016/j.brainresbull.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.van der Kooij MA, Groenendaal F, Kavelaars A, Heijnen CJ, van Bel F. Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res Rev. 2008 Nov;59(1):22–33. doi: 10.1016/j.brainresrev.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 372.Andreotti F, Agati L, Conti E, et al. Update on phase II studies of erythropoietin in acute myocardial infarction. Rationale and design of Exogenous erythroPoietin in Acute Myocardial Infarction: New Outlook aNd Dose Association Study (EPAMINONDAS). J Thromb Thrombolysis. 2009 Nov;28(4):489–495. doi: 10.1007/s11239-009-0363-x. [DOI] [PubMed] [Google Scholar]
- 373.Brunner S, Winogradow J, Huber BC, et al. Erythropoietin administration after myocardial infarction in mice attenuates ischemic cardiomyopathy associated with enhanced homing of bone marrow-derived progenitor cells via the CXCR-4/SDF-1 axis. Faseb J. 2009 Feb;23(2):351–361. doi: 10.1096/fj.08-109462. [DOI] [PubMed] [Google Scholar]
- 374.Incagnoli P, Ramond A, Joyeux-Faure M, Pepin JL, Levy P, Ribuot C. Erythropoietin improved initial resuscitation and increased survival after cardiac arrest in rats. Resuscitation. 2009 Jun;80(6):696–700. doi: 10.1016/j.resuscitation.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 375.Li CL, Jiang J, Fan YQ, Fu GS, Wang JA, Fan WM. Knockout of the tumor necrosis factor a receptor 1 gene can up-regulate erythropoietin receptor during myocardial ischemia-reperfusion injury in mice. Chin Med J (Engl) 2009 Mar 5;122(5):566–570. [PubMed] [Google Scholar]
- 376.Schlecht-Bauer D, Antier D, Machet MC, Hyvelin JM. Short- and Long-Term Cardioprotective Effect of Darbepoetin-alpha: Role of Bcl-2 Family Proteins. J Cardiovasc Pharmacol. 2009 Jul 10; doi: 10.1097/FJC.0b013e3181b04d01. [DOI] [PubMed] [Google Scholar]
- 377.Smith K, Semple D, Bhandari S, Seymour AM. Cellular basis of uraemic cardiomyopathy: a role for erythropoietin? Eur J Heart Fail. 2009 Aug;11(8):732–738. doi: 10.1093/eurjhf/hfp093. [DOI] [PubMed] [Google Scholar]
- 378.Timmer SA, De Boer K, Knaapen P, Gotte MJ, Van Rossum AC. The potential role of erythropoietin in chronic heart failure: from the correction of anemia to improved perfusion and reduced apoptosis? J Card Fail. 2009 May;15(4):353–361. doi: 10.1016/j.cardfail.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 379.Uitterdijk A, Groenendijk BC, van Der Giessen WJ. Stem cell therapy for chronic heart failure. Hellenic J Cardiol. 2009 Mar-Apr;50(2):127–132. [PubMed] [Google Scholar]
- 380.Matis GK, Birbilis TA. Erythropoietin in spinal cord injury. Eur Spine J. 2009 Mar;18(3):314–323. doi: 10.1007/s00586-008-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Yoo JY, Won YJ, Lee JH, et al. Neuroprotective effects of erythropoietin posttreatment against kainate-induced excitotoxicity in mixed spinal cultures. J Neurosci Res. 2009 Jan;87(1):150–163. doi: 10.1002/jnr.21832. [DOI] [PubMed] [Google Scholar]
- 382.Okutan O, Turkoglu OF, Gok HB, Beskonakli E. Neuroprotective effect of erythropoietin after experimental cold injury-induced vasogenic brain edema in rats. Surg Neurol. 2008 Nov;70(5):498–502. doi: 10.1016/j.surneu.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 383.Karaca M, Odabasoglu F, Kumtepe Y, Albayrak A, Cadirci E, Keles ON. Protective effects of erythropoietin on ischemia/reperfusion injury of rat ovary. Eur J Obstet Gynecol Reprod Biol. 2009 Jun;144(2):157–162. doi: 10.1016/j.ejogrb.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 384.Harder Y, Amon M, Schramm R, et al. Erythropoietin reduces necrosis in critically ischemic myocutaneous tissue by protecting nutritive perfusion in a dose-dependent manner. Surgery. 2009 Apr;145(4):372–383. doi: 10.1016/j.surg.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 385.Rotter R, Menshykova M, Winkler T, et al. Erythropoietin improves functional and histological recovery of traumatized skeletal muscle tissue. J Orthop Res. 2008 Dec;26(12):1618–1626. doi: 10.1002/jor.20692. [DOI] [PubMed] [Google Scholar]
- 386.Zhu L, Wang HD, Yu XG, et al. Erythropoietin prevents zinc accumulation and neuronal death after traumatic brain injury in rat hippocampus: in vitro and in vivo studies. Brain Res. 2009 Sep 15;1289:96–105. doi: 10.1016/j.brainres.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 387.Aoshiba K, Onizawa S, Tsuji T, Nagai A. Therapeutic effects of erythropoietin in murine models of endotoxin shock. Crit Care Med. 2009 Mar;37(3):889–898. doi: 10.1097/CCM.0b013e31819b8371. [DOI] [PubMed] [Google Scholar]
- 388.Contaldo C, Elsherbiny A, Lindenblatt N, et al. Erythropoietin enhances oxygenation in critically perfused tissue through modulation of nitric oxide synthase. Shock. 2009 Jun;31(6):599–606. doi: 10.1097/SHK.0b013e31818b9cc4. [DOI] [PubMed] [Google Scholar]
- 389.Simon F, Calzia E, Radermacher P, Schelzig H. Beneficial effects of erythropoietin in models of shock and organ failure-nothing is simple and easy. Shock. 2009 Feb;31(2):220–221. doi: 10.1097/SHK.0b013e3181890733. [DOI] [PubMed] [Google Scholar]
- 390.Casals-Pascual C, Idro R, Picot S, Roberts DJ, Newton CR. Can erythropoietin be used to prevent brain damage in cerebral malaria? Trends Parasitol. 2009 Jan;25(1):30–36. doi: 10.1016/j.pt.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 391.Mihaila RG, Rezi EC, Boitan M, Zaharie AV, Olteanu A, Deac M. Erythropoietin and the pro-inflammatory cytokines in chronic C hepatitis. Hepatogastroenterology. 2009 May-Jun;56(91-92):751–755. [PubMed] [Google Scholar]
- 392.Picot S, Bienvenu AL, Konate S, et al. Safety of epoietin beta-quinine drug combination in children with cerebral malaria in Mali. Malar J. 2009;8:169. doi: 10.1186/1475-2875-8-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.MacRedmond R, Singhera GK, Dorscheid DR. Erythropoietin inhibits respiratory epithelial cell apoptosis in a model of acute lung injury. Eur Respir J. 2009 Jun;33(6):1403–1414. doi: 10.1183/09031936.00084608. [DOI] [PubMed] [Google Scholar]
- 394.Tascilar O, Cakmak GK, Tekin IO, et al. Protective effects of erythropoietin against acute lung injury in a rat model of acute necrotizing pancreatitis. World J Gastroenterol. 2007 Dec 14;13(46):6172–6182. doi: 10.3748/wjg.v13.i46.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Wu H, Dong G, Liu H, Xu B, Li D, Jing H. Erythropoietin attenuates ischemiareperfusion induced lung injury by inhibiting tumor necrosis factor-alpha and matrix metalloproteinase-9 expression. Eur J Pharmacol. 2009 Jan 14;602(2-3):406–412. doi: 10.1016/j.ejphar.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 396.Chang YK, Choi DE, Na KR, et al. Erythropoietin attenuates renal injury in an experimental model of rat unilateral ureteral obstruction via anti-inflammatory and anti-apoptotic effects. J Urol. 2009 Mar;181(3):1434–1443. doi: 10.1016/j.juro.2008.10.105. [DOI] [PubMed] [Google Scholar]
- 397.Chen HH, Tarng DC, Lee KF, Wu CY, Chen YC. Epoetin alfa and darbepoetin alfa: effects on ventricular hypertrophy in patients with chronic kidney disease. J Nephrol. 2008 Jul-Aug;21(4):543–549. [PubMed] [Google Scholar]
- 398.Song YR, Lee T, You SJ, et al. Prevention of acute kidney injury by erythropoietin in patients undergoing coronary artery bypass grafting: a pilot study. Am J Nephrol. 2009;30(3):253–260. doi: 10.1159/000223229. [DOI] [PubMed] [Google Scholar]
- 399.Luo YH, Li ZD, Liu LX, Dong GH. Pretreatment with erythropoietin reduces hepatic ischemia-reperfusion injury. Hepatobiliary Pancreat Dis Int. 2009 Jun;8(3):294–299. [PubMed] [Google Scholar]
- 400.Schmeding M, Hunold G, Ariyakhagorn V, et al. Erythropoietin reduces ischemiareperfusion injury after liver transplantation in rats. Transpl Int. 2009 Mar 20; doi: 10.1111/j.1432-2277.2009.00861.x. [DOI] [PubMed] [Google Scholar]
- 401.Ucan BH, Irkorucu O, Cakmak GK, et al. Erythropoietin: a possible cytoprotective cytokine in acute necrotizing pancreatitis. J Hepatobiliary Pancreat Surg. 2009;16(4):530–537. doi: 10.1007/s00534-009-0082-x. [DOI] [PubMed] [Google Scholar]
- 402.Tsai JC, Song BJ, Wu L, Forbes M. Erythropoietin: a candidate neuroprotective agent in the treatment of glaucoma. J Glaucoma. 2007 Sep;16(6):567–571. doi: 10.1097/IJG.0b013e318156a556. [DOI] [PubMed] [Google Scholar]
- 403.Wang ZY, Shen LJ, Tu L, et al. Erythropoietin protects retinal pigment epithelial cells from oxidative damage. Free Radic Biol Med. 2009 Apr 15;46(8):1032–1041. doi: 10.1016/j.freeradbiomed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 404.Zhong YS, Liu XH, Cheng Y, Min YJ. Erythropoietin with retrobulbar administration protects retinal ganglion cells from acute elevated intraocular pressure in rats. J Ocul Pharmacol Ther. 2008 Oct;24(5):453–459. doi: 10.1089/jop.2008.0021. [DOI] [PubMed] [Google Scholar]
- 405.Toba H, Sawai N, Morishita M, et al. Chronic treatment with recombinant human erythropoietin exerts renoprotective effects beyond hematopoiesis in streptozotocin-induced diabetic rat. Eur J Pharmacol. 2009 Jun 10;612(1-3):106–114. doi: 10.1016/j.ejphar.2009.03.065. [DOI] [PubMed] [Google Scholar]
- 406.Lagreze WA, Feltgen N, Bach M, Jehle T. Feasibility of intravitreal erythropoietin injections in humans. Br J Ophthalmol. 2009 Dec;93(12):1667–1671. doi: 10.1136/bjo.2008.156794. [DOI] [PubMed] [Google Scholar]
- 407.Di Giacomo V, Sancilio S, Caravatta L, Rana RA, Di Pietro R, Cataldi A. Regulation of CREB activation by P38 mitogen activated protein kinase during human primary erythroblast differentiation. Int J Immunopathol Pharmacol. 2009 Jul-Sep;22(3):679–688. doi: 10.1177/039463200902200313. [DOI] [PubMed] [Google Scholar]
- 408.Koh SH, Noh MY, Cho GW, Kim KS, Kim SH. Erythropoietin increases the motility of human bone marrow-multipotent stromal cells (hBM-MSCs) and enhances the production of neurotrophic factors from hBM-MSCs. Stem Cells Dev. 2009 Apr;18(3):411–421. doi: 10.1089/scd.2008.0040. [DOI] [PubMed] [Google Scholar]
- 409.Tilling L, Chowienczyk P, Clapp B. Progenitors in motion: mechanisms of mobilization of endothelial progenitor cells. Br J Clin Pharmacol. 2009 Oct;68(4):484–492. doi: 10.1111/j.1365-2125.2009.03486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Mikati MA, Hokayem JA, Sabban ME. Effects of a single dose of erythropoietin on subsequent seizure susceptibility in rats exposed to acute hypoxia at p10. Epilepsia. 2007 Jan;48(1):175–181. doi: 10.1111/j.1528-1167.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- 411.Moon C, Krawczyk M, Paik D, et al. Erythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective properties. J Pharmacol Exp Ther. 2006 Mar;316(3):999–1005. doi: 10.1124/jpet.105.094854. [DOI] [PubMed] [Google Scholar]
- 412.Um M, Gross AW, Lodish HF. A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007 Mar;19(3):634–645. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 413.Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008 Apr;141(1):14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 414.Schumann C, Triantafilou K, Krueger S, et al. Detection of erythropoietin in exhaled breath condensate of nonhypoxic subjects using a multiplex bead array. Mediators Inflamm. 2006;2006(5):18061. doi: 10.1155/MI/2006/18061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Li F, Chong ZZ, Maiese K. Erythropoietin on a Tightrope: Balancing Neuronal and Vascular Protection between Intrinsic and Extrinsic Pathways. Neurosignals. 2004 Nov-Dec;13(6):265–289. doi: 10.1159/000081963. [DOI] [PubMed] [Google Scholar]
- 416.Mojiminiyi OA, Abdella NA, Zaki MY, El Gebely SA, Mohamedi HM, Aldhahi WA. Prevalence and associations of low plasma erythropoietin in patients with Type 2 diabetes mellitus. Diabet Med. 2006 Aug;23(8):839–844. doi: 10.1111/j.1464-5491.2006.01893.x. [DOI] [PubMed] [Google Scholar]
- 417.Symeonidis A, Kouraklis-Symeonidis A, Psiroyiannis A, et al. Inappropriately low erythropoietin response for the degree of anemia in patients with noninsulin-dependent diabetes mellitus. Ann Hematol. 2006 Feb;85(2):79–85. doi: 10.1007/s00277-005-1102-9. [DOI] [PubMed] [Google Scholar]
- 418.Thomas MC, Cooper ME, Tsalamandris C, MacIsaac R, Jerums G. Anemia with impaired erythropoietin response in diabetic patients. Arch Intern Med. 2005 Feb 28;165(4):466–469. doi: 10.1001/archinte.165.4.466. [DOI] [PubMed] [Google Scholar]
- 419.Teramo K, Kari MA, Eronen M, Markkanen H, Hiilesmaa V. High amniotic fluid erythropoietin levels are associated with an increased frequency of fetal and neonatal morbidity in type 1 diabetic pregnancies. Diabetologia. 2004 Oct;47(10):1695–1703. doi: 10.1007/s00125-004-1515-3. [DOI] [PubMed] [Google Scholar]
- 420.Teramo KA, Widness JA. Increased fetal plasma and amniotic fluid erythropoietin concentrations: markers of intrauterine hypoxia. Neonatology. 2009;95(2):105–116. doi: 10.1159/000153094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Kaindl AM, Sifringer M, Koppelstaetter A, et al. Erythropoietin protects the developing brain from hyperoxia-induced cell death and proteome changes. Ann Neurol. 2008 Nov;64(5):523–534. doi: 10.1002/ana.21471. [DOI] [PubMed] [Google Scholar]
- 422.Yis U, Kurul SH, Kumral A, et al. Effect of erythropoietin on oxygen-induced brain injury in the newborn rat. Neurosci Lett. 2008 Dec 31;448(3):245–249. doi: 10.1016/j.neulet.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 423.He Z, Huang L, Wu Y, Wang J, Wang H, Guo L. DDPH: improving cognitive deficits beyond its alpha 1-adrenoceptor antagonism in chronic cerebral hypoperfused rats. Eur J Pharmacol. 2008 Jul 7;588(2-3):178–188. doi: 10.1016/j.ejphar.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 424.Berkingali N, Warnecke A, Gomes P, et al. Neurite outgrowth on cultured spiral ganglion neurons induced by erythropoietin. Hear Res. 2008 Sep;243(1-2):121–126. doi: 10.1016/j.heares.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 425.Shah RC, Wilson RS, Tang Y, Dong X, Murray A, Bennett DA. Relation of hemoglobin to level of cognitive function in older persons. Neuroepidemiology. 2009;32(1):40–46. doi: 10.1159/000170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Bierer R, Peceny MC, Hartenberger CH, Ohls RK. Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics. 2006 Sep;118(3):e635–640. doi: 10.1542/peds.2005-3186. [DOI] [PubMed] [Google Scholar]
- 427.Pillai A, Dhandapani KM, Pillai BA, Terry AV, Jr., Mahadik SP. Erythropoietin prevents haloperidol treatment-induced neuronal apoptosis through regulation of BDNF. Neuropsychopharmacology. 2008 Jul;33(8):1942–1951. doi: 10.1038/sj.npp.1301566. [DOI] [PubMed] [Google Scholar]
- 428.Silverberg DS, Wexler D, Iaina A, Schwartz D. The interaction between heart failure and other heart diseases, renal failure, and anemia. Semin Nephrol. 2006 Jul;26(4):296–306. doi: 10.1016/j.semnephrol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 429.Mason-Garcia M, Beckman BS, Brookins JW, et al. Development of a new radioimmunoassay for erythropoietin using recombinant erythropoietin. Kidney Int. 1990 Nov;38(5):969–975. doi: 10.1038/ki.1990.299. [DOI] [PubMed] [Google Scholar]
- 430.Namiuchi S, Kagaya Y, Ohta J, et al. High serum erythropoietin level is associated with smaller infarct size in patients with acute myocardial infarction who undergo successful primary percutaneous coronary intervention. J Am Coll Cardiol. 2005 May 3;45(9):1406–1412. doi: 10.1016/j.jacc.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 431.Cariou A, Claessens YE, Pene F, et al. Early high-dose erythropoietin therapy and hypothermia after out-of-hospital cardiac arrest: a matched control study. Resuscitation. 2008 Mar;76(3):397–404. doi: 10.1016/j.resuscitation.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 432.Bakker WJ, van Dijk TB, Parren-van Amelsvoort M, et al. Differential regulation of Foxo3a target genes in erythropoiesis. Mol Cell Biol. 2007 May;27(10):3839–3854. doi: 10.1128/MCB.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Avasarala JR, Konduru SS. Recombinant erythropoietin down-regulates IL-6 and CXCR4 genes in TNF-alpha-treated primary cultures of human microvascular endothelial cells: implications for multiple sclerosis. J Mol Neurosci. 2005;25(2):183–189. doi: 10.1385/JMN:25:2:183. [DOI] [PubMed] [Google Scholar]
- 434.Leuner K, Hauptmann S, Abdel-Kader R, et al. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer's disease? Antioxid Redox Signal. 2007 Oct;9(10):1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- 435.Miki T, Miura T, Yano T, et al. Alteration in erythropoietin-induced cardioprotective signaling by postinfarct ventricular remodeling. J Pharmacol Exp Ther. 2006 Apr;317(1):68–75. doi: 10.1124/jpet.105.095745. [DOI] [PubMed] [Google Scholar]
- 436.Barandon L, Couffinhal T, Ezan J, et al. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation. 2003 Nov 4;108(18):2282–2289. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- 437.Emami KH, Corey E. When prostate cancer meets bone: control by wnts. Cancer Lett. 2007 Aug 18;253(2):170–179. doi: 10.1016/j.canlet.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 438.Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with {beta}-Catenin Inhibits {beta}-Catenin/T Cell Factor Activity. J Biol Chem. 2008 Apr 4;283(14):9224–9230. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- 439.Sun J, Jin T. Both Wnt and mTOR signaling pathways are involved in insulin-stimulated proto-oncogene expression in intestinal cells. Cell Signal. 2008 Jan;20(1):219–229. doi: 10.1016/j.cellsig.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 440.Lynch RL, Konicek BW, McNulty AM, et al. The progression of LNCaP human prostate cancer cells to androgen independence involves decreased FOXO3a expression and reduced p27KIP1 promoter transactivation. Mol Cancer Res. 2005 Mar;3(3):163–169. doi: 10.1158/1541-7786.MCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 441.Hegde PS, Rusnak D, Bertiaux M, et al. Delineation of molecular mechanisms of sensitivity to lapatinib in breast cancer cell lines using global gene expression profiles. Mol Cancer Ther. 2007 May;6(5):1629–1640. doi: 10.1158/1535-7163.MCT-05-0399. [DOI] [PubMed] [Google Scholar]
- 442.Hoekstra AV, Ward EC, Hardt JL, et al. Chemosensitization of endometrial cancer cells through AKT inhibition involves FOXO1. Gynecol Oncol. 2008 Mar;108(3):609–618. doi: 10.1016/j.ygyno.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 443.Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007 Dec 28;28(6):941–953. doi: 10.1016/j.molcel.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 444.Dharmarajan TS, Widjaja D. Erythropoiesis-stimulating agents in anemia: use and misuse. J Am Med Dir Assoc. 2009 Nov;10(9):607–616. doi: 10.1016/j.jamda.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 445.Ioka T, Tsuruoka S, Ito C, et al. Hypertension induced by erythropoietin has a correlation with truncated erythropoietin receptor mRNA in endothelial progenitor cells of hemodialysis patients. Clin Pharmacol Ther. 2009 Aug;86(2):154–159. doi: 10.1038/clpt.2009.74. [DOI] [PubMed] [Google Scholar]
- 446.Sigounas G, Salleng KJ, Mehlhop PD, Sigounas DG. Erythropoietin ameliorates chemotherapy-induced fibrosis of the lungs in a preclinical murine model. Int J Cancer. 2008 Jun 15;122(12):2851–2857. doi: 10.1002/ijc.23426. [DOI] [PubMed] [Google Scholar]
- 447.Kokhaei P, Abdalla AO, Hansson L, et al. Expression of erythropoietin receptor and in vitro functional effects of epoetins in B-cell malignancies. Clin Cancer Res. 2007 Jun 15;13(12):3536–3544. doi: 10.1158/1078-0432.CCR-06-2828. [DOI] [PubMed] [Google Scholar]
- 448.Maiese K, Li F, Chong ZZ. Erythropoietin and cancer. JAMA. 2005;293(15):1858–1859. doi: 10.1001/jama.293.15.1858-a. [DOI] [PubMed] [Google Scholar]
- 449.Hardee ME, Rabbani ZN, Arcasoy MO, et al. Erythropoietin inhibits apoptosis in breast cancer cells via an Akt-dependent pathway without modulating in vivo chemosensitivity. Mol Cancer Ther. 2006 Feb;5(2):356–361. doi: 10.1158/1535-7163.MCT-05-0196. [DOI] [PubMed] [Google Scholar]
- 450.Lai SY, Grandis JR. Understanding the presence and function of erythropoietin receptors on cancer cells. J Clin Oncol. 2006 Oct 10;24(29):4675–4676. doi: 10.1200/JCO.2006.08.1190. [DOI] [PubMed] [Google Scholar]
- 451.Ceelen W, Boterberg T, Smeets P, et al. Recombinant human erythropoietin alpha modulates the effects of radiotherapy on colorectal cancer microvessels. Br J Cancer. 2007 Mar 12;96(5):692–700. doi: 10.1038/sj.bjc.6603568. [DOI] [PMC free article] [PubMed] [Google Scholar]