Abstract
Francisella tularensis, the causative agent of tularemia, is currently considered a category A bioterrorism agent due to its high virulence. Infection with F. tularensis results in an inflammatory response that plays an important role in the pathogenesis of the disease; however, the cellular mechanisms regulating this response are poorly understood. Glycogen synthase kinase-3β (GSK3β) is a serine/threonine protein kinase that has recently emerged as a key regulatory switch in the modulation of the inflammatory response. In this study, we investigated the effect of GSK3β inhibition in regulating F. tularensis LVS-induced inflammatory responses. F. tularensis LVS infection of murine peritoneal macrophages induced a TLR2 dependent phosphorylation of GSK3β. Inhibition of GSK3β resulted in a significant decrease in the production of pro-inflammatory cytokine IL-6, IL-12p40 and TNF-α, as well as a significant increase in the production of the anti-inflammatory cytokine IL-10. GSK3β regulated the F. tularensis LVS-induced cytokine response by differentially affecting the activation of transcription factors NF-κB and CREB. Inhibition of GSK3β by lithium in vivo suppressed the inflammatory response in mice infected with F. tularensis LVS and conferred a survival advantage. In addition, we show that the production of IFN-γ contributed to the development of tularemia and to the fatal outcome of the infected animals, depending on the timing and the relative level of the IFN-γ produced. IFN-γ potentiated F. tularensis LVS-induced cytokine production by increasing GSK3β activity and the nuclear translocation of NF-κB. Taken together, these results demonstrate a regulatory function of GSK3β in modulating inflammatory responses that can be detrimental to the host during an F. tularensis LVS infection, and suggest that inhibition of GSK3β may represent a novel therapeutic approach in the treatment of tularemia.
Keywords: F. tularensis, GSK3β, inflammation, IFN-γ
1. Introduction
Francisella tularensis, a gram-negative, facultative, intracellular coccobacillus, is the causative agent of the disease tularemia in humans and other mammals. There are four closely related subspecies of F. tularensis: tularensis (type A), holartica (type B), mediasiatica, and novicida, with type A being the most virulent in humans (Forsman et al., 1990). Due to its ability to infect via multiple routes, its ease of dissemination, and its high infectivity, morbidity and rates of mortality, the Center for Disease Control and Prevention has classified this pathogen as a category A bioterrorism agent (Santic et al., 2006). This has lead to intensive investigations on the pathogenesis of this microorganism and on the development of a vaccine or immunotherapeutic means for the prevention/treatment of this infectious disease. Since the fully virulent strains of F. tularensis are highly infectious, most research on the pathogenesis of F. tularensis has used an attenuated live vaccine strain (LVS) derived from the type B strain of F. tularenis. Although attenuated for humans, F. tularensis LVS is virulent in mice and results in a disease that closely resembles human tularemia (Elkins et al., 2003).
F. tularensis is believed to replicate intracellularly, mainly within macrophages, during infection of the mammalian host. Following invasion of macrophages, F. tularensis disrupt the normal process of phagosome-lysosome fusion that leads to pathogen destruction and escape into the host cell cytosol, where bacterial replication occurs (Clements et al., 2005; Cole et al., 2006; Sjostedt, 2006). The response of macrophages to Francisella infection involves the release of multiple inflammatory cytokines including IL-6, IL-12, and TNF-α (Cole et al., 2008; Parsa et al., 2006). These cytokines, which may be produced within minutes of infection, are critical immunoregulatory determinants of disease pathogenesis and progression. They regulate the antimicrobial activity of macrophages and influence the interactions between macrophages and lymphocytes that are relevant for effective anti-pathogen activity. However, an inability to regulate the inflammatory response can result in tissue damage and toxicity that is detrimental to the host. In this regard, it has been shown that the pronounced inflammatory response induced by F. tularensis infection is responsible for most of the tissue damage that occurs in tularemia (Cole et al., 2006).
The ability of the innate immune system to recognize and respond to microbial components has been largely attributed to the family of type I transmembrane receptors called Toll-like receptors (TLRs) (Akira et al., 2001; Kaisho and Akira, 2000). Recognition of microbial products by TLRs leads to the activation of a variety of signal transduction pathways that regulate the nature, magnitude, and duration of the inflammatory response. Recently, we (Katz et al., 2006) and others (Cole et al., 2006; Cole et al., 2007; Li et al., 2006; Malik et al., 2006) have demonstrated that TLR2 is required for the inflammatory cytokine response to F. tularensis LVS. However, the underlying cellular mechanisms that directly regulate the inflammatory cytokine response after TLR stimulation are currently unknown.
Glycogen synthase kinase-3 (GSK3) is a serine/threonine protein capable of phosphorylating and inactivating glycogen synthase, a key enzyme in glycogen metabolism (Cohen and Frame, 2001). It also participates in the regulation of a multitude of cellular processes, ranging from cell membrane-to-nucleus signaling, gene transcription, translation, and cytoskeletal organization to cell cycle progression and survival (Dugo et al., 2006; Jope et al., 2007). This multi-tasking is achieved by the many substrates phosphorylated by GSK3 and the convergence on GSK3 of many regulatory intracellular signaling pathways. In mammals, there exist two isoforms of GSK3, GSK3α (Ser21) and GSK3β (Ser9), which are encoded by different genes and are highly homologous (Jope and Johnson, 2004). Unique to GSK3β is its reported involvement in NF-κB-mediated cell survival. Homozygous deletion of the GSK3β gene in mice leads to the defect in NF-κB activity and embryonic lethality characterized by extensive liver degeneration (Hoeflich et al., 2000). Several mechanisms play a part in controlling the actions of GSK3, including phosphorylation, protein complex formation, and subcellular distribution (Jope and Johnson, 2004). Unlike most kinases, GSK3 is active in resting cells and its activity can be inhibited through N-terminal serine phosphorylation by a wide range of extracellular stimuli, including insulin, epidermal growth factor, and fibroblast growth factor (Dugo et al., 2006). Dysregulation of GSK3 has been implicated in the pathogenesis of several prevalent pathological conditions, such as diabetes and/or insulin resistant, Alzheimer’s disease, and cancer (Jope and Johnson, 2004; Jope et al., 2007). Recently, it was indicated that the link between these pathological conditions and GSK3 can be attribute to the association between GSK3 and inflammation (Jope et al., 2007), since GSK3β has been shown to be a vital factor in the inflammatory process (Martin et al., 2005).
In the present study, we investigated in a murine model the function of GSK3 in regulating the inflammatory response to F. tularensis LVS infection. We show that GSK3β differentially regulates the production of pro- and anti-inflammatory cytokine production following infection with F. tularensis LVS, and that inhibition of GSK3β suppresses systemic inflammation and confers protection against F. tularensis LVS infection in vivo. Our results also provide insight into the cellular mechanisms by which GSK3β regulates the TLR2-mediated inflammatory response to F. tularensis LVS and identify IFN-γ as a key player in the fatal outcome of the host following F. tularensis LVS infection.
2. Materials and Methods
2.1. Bacteria
Frozen stocks and fresh cultures of F. tularensis LVS (ATCC 29684; American Type Culture Collection, Rockville, MD), kindly provided by Dr. Karen Elkins (Division of Bacterial and Parasitic Products, CBER/FDA, Bethesda, MD), were prepared as described previously (Katz et al., 2006). Briefly, bacterial cultures were grown to mid-log phase in Mueller-Hinton (MH) broth (BD Biosciences, Sparks, MD) supplemented with IsoVitaleX Enrichment (BD Biosciences) and ferric pyrophosphate. The number of bacteria in the suspension was determined at the optical density (OD) 600 nm by extrapolating from a standard curve, and confirmed by plating serial dilutions on supplemented MH agar plates.
2.2. Mice
C57BL/6 wild type (wt) and TLR2 knockout (TLR2−/−) mice were bred and maintained within an environmentally controlled, pathogen-free animal facility at the University of Alabama at Birmingham (UAB). The original TLR2−/− breeding pairs were obtained under a material transfer agreement from Dr. Shikuo Akira (Osaka University, Osaka, Japan). Female mice were 8–10 weeks of age when used in the studies. All studies were performed according to the National Institutes of Health guidelines, and protocols were approved by the UAB Institutional Animal Care and Use Committee.
2.3. Murine peritoneal macrophage cell cultures
Peritoneal macrophages from female C57BL/6 wt or TLR2−/− mice were induced by the intraperitoneal (i.p.) injection of 1 ml of 3% BBL Brewer modified thioglycollate medium (BD Biosciences) and were isolated as previously described (Zhang et al., 2005). Macrophages were cultured in RPMI 1640 medium supplemented with 5% fetal calf serum, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 1.5 mg/ml of sodium bicarbonate, and 25 mM HEPES (complete medium). For some studies, macrophages were pretreated with the PI3K inhibitor LY294002 (Calbiochem, La Jolla, CA), the Akt inhibitor Akt-i (Calbiochem), or with the GSK3 inhibitor LiCl (Sigma) or BIO (Calbiochem) for 1 h prior to stimulation with F. tularensis LVS at different multiplicities of infection (MOIs) for various times. IFN-γ-treated macrophages were obtained by pre-culturing macrophages with 200 U/ml of mouse recombinant IFN-γ (eBiocience, San Diego, CA) overnight, or by culturing macrophages with both IFN-γ and F. tularensis LVS.
2.4. GSK3β siRNA transfection assay
Murine macrophage-like cell line Raw 264.7 cells were cultured in 6-well plates in complete medium until 60–80% confluent. The cells were transfected with either control siRNA or siRNA targeted for the GSK3β gene (Santa Cruz Biotechnology, Santa Cruz, CA), according to the manufacture’s protocol.
2.5. Western blot
Macrophage whole cell lysates were prepared from cell cultures grown in 24-well plates, as previously described (Zhang et al., 2005). Equivalent amounts of protein from cell lysates were separated by SDS-PAGE on a 10% Tris-HCl gel (Bio-Rad Laboratories, Hercules, CA). Protein was electrotransferred to immobilon-P transfer membranes (Millipore, Bedford, MA), and probed with specific antibodies against total p38, GSK3β, Akt or the phosphorylated form of Akt (Ser473), GSK3α/β (Ser21/9), NF-κB p65 (Ser536) and CREB (Ser133). Detection was carried out using HRP-linked rabbit IgG antibody, followed by ECL Western blotting detection reagents (Amersham Biosciences UK limited, Little Chalfont Buckinghamshire, United Kingdom). All antibodies were purchased from Cell Signaling Technology (Beverly, MA). Densitometer scans of the blots were performed using the AlphaImager 2000 documentation and analysis system (Alpha Innotech, San Leandro, CA).
2.6. Nuclear NF-κB and CREB DNA-binding activity
Nuclear extracts of macrophages were prepared from cell cultures grown in 10 mm tissue culture dishes by using a nuclear extract kit (Active Motif, Carlsbad, CA), according to the manufacture’s protocol. Levels of NF-κB p65 and CREB activation in the nuclear extracts were quantified by TransAM NF-κB p65 and TransAM pCREB assay kits (Active Motif) and expressed as the OD at 450 nm, according to the manufacturer’s instructions.
2.7. Mice infection model
Female C57BL/6 mice (8–10 weeks of age, 17–21 g body weight) were injected i.p. with different doses of F. tularensis LVS in 500 μl of PBS. For GSK3β inhibition, LiCl was given i.p. at 100 μg/g body weight 1 h before or after F. tularensis LVS infection. Serum samples were collected at 6, 24, 48 or 72 h after infection. Mice were monitored over a 14-day period for weight loss and survival.
2.8. Cytokine analysis
The levels of IL-10, IL-12p40, IL-6, and TNF-α in the supernatants of macrophage cultures and the levels of IL-10, IL-12p40, IL-6, TNF-α and IFN-γ in mice serum samples were determined by an enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions (IL-10, IL-12p40 and IL-6 ELISA kits, BD Biosciences; IFN-γ and TNF-α ELISA kits, eBioscience).
2.9. FACS analysis
Peritoneal macrophages from C57BL/6 wt mice were cultured with F. tularensis LVS at an MOI of 10 with or without IFN-γ (200 U/ml) for 24 h. Cells were then harvested, washed and suspended in fluorescence-activated cell sorting (FACS) buffer and stained with phycoerythrin (PE)-labeled anti-mouse TLR2 antibody or mouse IgG1 isotype control antibody (eBioscience) for 30 min on ice. The cells were washed, suspended in FACS buffer, and immediately analyzed using a FACSCalibur apparatus (BD Bioscience), as described previously (Katz et al., 2006).
2.10. Statistical analysis
Statistical significance between cytokine levels was evaluated by ANOVA and the Tukey multiple-comparisons test using the InStat program (Graphpad Software, San Diego, CA). The Kaplan-Meier survival analyses were performed using a log rank test. Differences between groups were considered significant at a P value of < 0.05.
3. Results
3.1. F. tularensis LVS induces TLR2-mediated phosphorylation of GSK3β in murine macrophages
We have previously reported that the induction of cytokine production and the up-regulation of CD80, CD86, CD40, and MHCII molecules by murine bone marrow-derived dendritic cells following F. tularensis LVS stimulation was dependent on the presence of TLR2 (Katz et al., 2006). Cole et al. (Cole et al., 2006) also reported that F. tularensis LVS specifically activates NF-κB activity in murine macrophages through TLR2. To address whether F. tularensis LVS mediates GSK3 phosphorylation, as well as the involvement of TLR2 signaling in this event, peritoneal macrophages from wt and TLR2−/− mice were stimulated with F. tularensis LVS at an MOI of 10 for 0 – 120 min. Phosphorylation of the two GSK3 isoforms GSK3α (ser9) and GSK3β (ser21) was assessed by Western blot. F. tularensis LVS induced Ser9 phosphorylation of GSK3β in macrophages from wt, but not TLR2−/− mice (Fig. 1A). Phophorylation was observed as early as 10 min and persisted through the 120 min of stimulation. No Ser21 phosphorylation of GSK3α was observed in wt and TLR2−/− macrophages after F. tularensis LVS stimulation.
Figure 1.

F. tularensis LVS induces TLR2-mediated phosphorylation of GSK3β in murine macrophages via a PI3K-Akt dependent pathway. (A) Peritoneal macrophages from wt and TLR2−/− mice were stimulated with F. tularensis LVS at an MOI of 10 for 0 – 120 min. The phosphorylation of Akt (Ser473) and GSK3α/β (Ser9/21) were analyzed by Western Blotting. Total GSK3β in each sample was analyzed to ensure equal protein loading. (B) Macrophages from wt mice were pretreated with 20 μM LY294002 (LY) or 100 nM Akt-i or medium only for 1 h before stimulation with F. tularensis LVS (MOI = 10) for 30 min. Untreated cultures served as negative controls. The phosphorylation of GSK3β (Ser9) was analyzed by Western Blotting. Total GSK3β in each sample was analyzed to ensure equal protein loading. Blots are representative of three independent experiments.
We next determined if F. tularnesis LVS mediated GSK3β phosphorylation via a PI3K-Akt-dependent pathway. PI3K is a heterodimeric enzyme that consists of a regulatory subunit and a catalytic subunit, which mediates the recruitment and subsequent activation of signaling proteins with pleckstrin-homology domains, including the serine-threonine kinase Akt (Cantley, 2002). The PI3K-Akt signaling pathway is a major regulator of GSK3 in response to insulin and many other growth factors (Jope et al., 2007). Our results indicate that Akt is phosphorylated in wt macrophages infected with F. tularensis LVS (Fig. 1A). Pre-incubation of macrophages with the PI3K inhibitor LY294002 or the Akt inhibitor Akt-i significantly reduced the phosphorylation of GSK3β following F. tularensis LVS stimulation (Fig. 1B). These results indicate that the signaling of F. tularensis LVS through TLR2 results in the Ser9 phosphorylation of GSK3β via a PI3K-Akt-dependent pathway.
3.2 GSK3β differentially regulates pro- and anti-inflammatory cytokine responses in F. tularensis LVS-stimulated macrophages
To address the role of GSK3β in the regulation of the F. tularensis LVS-induced inflammatory response, we first examined the dose-dependent cytokine production in our system. Peritoneal macrophages from wt mice were stimulated with live F. tularensis LVS at different MOIs (0, 0.1, 1, 10 and 100), culture supernatants were harvested 24 h post infection and assayed by ELISA for IL-6, IL-10, IL-12p40 and TNF-α. Our results indicate that F. tularensis LVS induced a robust production of the pro-inflammatory cytokines TNF-α, IL-6 and IL-12p40 and a limited production of the anti-inflammatory cytokine IL-10 (data not shown). The optimal induction of cytokines was observed at an MOI of 10. We next investigated the effect of lithium chloride (LiCl), a potent and selective GSK3 inhibitor, on F. tularensis LVS-induced cytokine production. Macrophages were pre-incubated with different concentrations of LiCl for 1 h before stimulation with F. tularensis LVS at an MOI of 10 for 24 h. The production of the pro-inflammatory cytokines IL-6, IL-12p40 and TNF-α was significantly reduced in a dose-dependent manner in cells pretreated with LiCl (Fig. 2A). In contrast, inhibition of GSK3β by LiCl resulted in a significant increase in the production of the anti-inflammatory cytokine IL-10. A similar cytokine profile was observed with the GSK3 inhibitor 2′Z,3′E-6-bromoindirubin-3′-oxime (BIO) (Fig. 2B). We also assessed the effect of LiCl and BIO on F. tularensis LVS-induced GSK3β phosphorylation. As expected, LiCl and BIO increased the phosphorylation of GSK3β, thus inhibiting GSK3β activity (Fig. 2C). Since previous studies have indicated that LiCl can alter the bacterial cell wall, and thus its viability (Pavlovich and Tkacheva, 1990; Scolari et al., 2006), we next assessed the effect of this GSK3 inhibitor on F. tularensis LVS by incubating the bacteria with LiCl at a therapeutic concentration (~ 1 mM) or at a concentration that results in maximum GSK3 inhibition (20 mM). Treatment of the bacteria with LiCl for 24 h had no effect on growth or viability of F. tularensis (data not shown). Furthermore, macrophage viability was not affected by either LiCl or BIO (data not shown).
Figure 2.
Inhibition of GSK3β differentially regulates F. tularensis LVS-mediated pro- and anti-inflammatory cytokine production by murine macrophages. (A-B) Peritoneal macrophages from wt mice were preincubated with different concentrations of LiCl or BIO for 1 h, and then stimulated with F. tularensis LVS (MOI = 10) for 24 h. The levels of TNF-α, IL-6, IL-12p40, IL-10 in the culture supernatant were determined by ELISA. Data represent the mean ± SEM of five separate experiments. Significant differences were seen at P < 0.05 (*), P < 0.01 (**) and P < 0.001 (***), respectively, compared with the cells treated with LVS only. (C) Peritoneal macrophages from wt mice were preincubated with BIO (10 μM) or LiCl (20 mM) for 1 h, and then stimulated with F. tularensis LVS (MOI = 10) for 30 min. Untreated cultures served as negative controls. The phosphorylation of GSK3β (Ser9) was analyzed by Western Blotting. Total GSK3β in each sample was analyzed to ensure equal protein loading. Blots are representative of three independent experiments.
To further demonstrate the definitive involvement of GSK3β in regulating F. tularensis LVS-mediated cytokine production, we next used siRNA specific for GSK3β in Raw 264.7 cells. Silencing GSK3β by RNA interference for 96 h strongly reduced GSK3β activity as compared with that of untransfected cells or cells transfected with control siRNA (Fig. 3A). In the absence of stimulation, cells pretreated with control siRNA or siRNA targeting GSK3β showed no substantial difference in cytokine production compared with that of untransfected cells (data not shown). In contrast, macrophages pretreated with GSK3β siRNA and subsequently stimulated with F. tularensis LVS showed a decreased level of TNF-α production (Fig. 3B) compared with that of untransfected cells or cells transfected with control siRNA. Moreover, an increase in the level of IL-10 production was observed in F. tularensis LVS-stimulated macrophages pretreated with GSK3β siRNA. These results demonstrate that GSK3β plays a regulatory role in TLR2-mediated cytokine production in F. tularensis LVS-infected macrophages by promoting the production of pro-inflammatory cytokines and suppressing anti-inflammatory cytokine production.
Figure 3.
Inhibition of GSK3β with specific siRNA suppresses TNF-α and enhances IL-10 by F. tularensis-stimulated macrophages. (A) Raw 264.7 cells were pretreated with medium, control siRNA or siRNA specific for GSK3β for 96 h and the level of total GSK3β and p38 in the whole cell lysates were analyzed by Western blot. (B) Untransfected Raw 264.7 cells or cells transfected with control or specific siRNA were stimulated with F. tularensis LVS (MOI = 10) for 24 h, and the level of TNF-α and IL-10 in the culture supernatants were analyzed by ELISA.
3.3. Effect of GSK3β on F. tularensis LVS-induced activation of NF-κB and CREB
NF-κB and CREB are important transcription factors that regulate many diverse cellular processes, including cytokine-mediated inflammation (Baeuerle, 1998; Blackwell and Christman, 1997; Platzer et al., 1999; Yabe et al., 2005). To understand the underlying cellular mechanisms responsible for the effect of GSK3β on the regulation of F. tularensis LVS-mediated cytokine production, we assessed how GSK3β influenced the activation of NF-κB and CREB. We first analyzed the activation of NF-κB and CREB in infected macrophages. Macrophages were infected with F. tularensis LVS at an MOI of 10 for various times, and the whole cell lysates were harvested and analyzed by Western blot for NF-κB and CREB activation. F. tularensis LVS induced rapid phosphorylation of NF-κB p65 and CREB (Fig. 4A). Maximal activation of NF-κB was observed at 10 to 30 min of stimulation. The phosphorylation level of CREB peaked at 30 min post infection and gradually diminished. We also prepared nuclear lysates and analyzed nuclear activation of NF-κB and CREB by TransAM assays. The results of the TransAM assays further confirmed the activation of nuclear NF-κB p65 and CREB in response to F. tularensis LVS stimulation and that the maximal DNA binding activity of both transcription factors occurred at 30 to 60 min (data not shown).
Figure 4.
(A) Activation of transcription factors NF-κB and CREB by F. tularensis LVS. Macrophages from C57BL/6 wt mice were stimulated with F. tularensis LVS (MOI = 10) for 0 to 120 min. The phosphorylation of NF-κB p65 (Ser536) and CREB (Ser133) were analyzed by Western Blotting. Total p38 in each sample was analyzed to ensure equal protein loading. Blots are representative of three independent experiments. (B) Effect of GSK3β inhibition on the nuclear translocation of NF-κB and CREB by F. tularensis LVS. Macrophages from C57BL/6 wt mice were pretreated with 20 mM LiCl or medium only for 1 h before stimulation with F. tularensis LVS (MOI = 10) for 30 or 60 min. Equal amounts of nuclear extracts were analyzed for NF-κB p65 and CREB activation using TransAM assay. Data are representative three separate experiments.
To investigate the functional role of GSK3β on F. tularensis LVS-mediated activation of NF-κB and CREB, macrophages were pretreated with LiCl for 1 h before infection with F. tularensis LVS for 30 – 60 min. Inhibition of GSK3β with LiCl resulted in a decrease in the activation of nuclear NF-κB p65 (Fig. 4B). In contrast, the nuclear DNA binding activity of CREB was increased when F. tularensis LVS-stimulated macrophages were pretreated with LiCl. These results indicate that GSK3β regulates F. tularensis LVS-induced cytokine production by differentially affecting NF-κB and CREB activities.
3.4. Inhibition of GSK3β confers a survival advantage in mice challenged with F. tularensis LVS
It has been suggested that the pronounced inflammatory response induced by F. tularensis infection is responsible for most of the tissue damage that occurs in tularemia (Cole et al., 2006). The role of GSK3β in the differential modulation of pro- and anti-inflammatory cytokine production by F. tularensis LVS-stimulated macrophages led us to assess the effects of GSK3β inhibition in mediating protection against F. tularensis LVS infection and in modulating the inflammatory response in vivo. In the first series of experiments, C57BL/6 wt mice were infected by the i.p. route with 2 × 104 CFU of F. tularensis LVS. One hour before or after infection, mice were given an i.p. injection of LiCl to inhibit GSK3β activity. Mice receiving LiCl 1 h before infection display a significant survival advantage over the infection-only control group (P = 0.0292), with a survival of 90% compared to the 60% survival of the infection-only group (Fig. 5A). A complete protection was observed when LiCl was given 1 h after infection, which was also significantly different from the infection-only control group (P = 0.0069). No significant difference in the percent survival was observed between mice receiving LiCl before or after infection (P = 0.3173). No lethality was seen in control mice receiving only a LiCl injection (data not shown).
Figure 5.
Inhibition of GSK3β confers a survival advantage in mice infected with F. tularensis LVS. C57BL/6 wt mice were immunized (i.p.) with different doses of F. tularensis LVS in 500 μl of PBS. For GSK3β inhibition, LiCl was given i.p. at 100 μg/g body weight 1 h before (LiCl + LVS) or after (LVS + LiCl) F. tularensis LVS infection. Mice were monitored daily over a 14-day period for survival. Results are expressed as Kaplan-Meier curves and P values were determined using a log rank test. The results show pooled data of three independent experiments (a total of 15 mice/group).
We next investigated the possible use of LiCl as a therapeutic method against F. tularensis infection. Mice were challenged (i.p.) with various doses of F. tularensis LVS and then given LiCl (i.p.) 1 h later. Mice infected with 5 × 104 CFU of F. tularensis LVS, which resulted in over 50% lethality in one week, were completely protected if LiCl was given 1 h after the infection (Fig. 5B). When mice were challenged with 2 × 105 CFU of F. tularensis LVS, LiCl treatment conferred a survival rate of more than 60% compared to a survival rate of less than 20% in the infection-only control mice (Fig. 5C). Although all mice died in one week after infection with 5 × 105 CFU of F. tularensis LVS, those receiving LiCl showed a prolonged survival, with a median time to death (MTD) of 5.5 days compared to a MTD of 3.8 days in control mice (Fig. 5D). These results demonstrate that inhibition of GSK3β by LiCl confers a survival advantage in mice infected with this bacterium.
3.5. Inhibition of GSK3β regulates the inflammatory response in mice challenged with F. tularensis LVS
We next sought to determine if inhibition of GSK3β by LiCl treatment affected the inflammatory response in vivo by assessing the serum cytokine profiles. Mice infected with 5 × 104 CFU of F. tularensis LVS exhibited a robust induction of serum IFN-γ (Fig. 6). An increase in serum IL-12p40, IL-6, TNF-α and IL-10 levels was also seen after infection. However, mice receiving LiCl after challenge with F. tularensis LVS showed a significant reduction in IFN-γ, IL-6, IL-12p40 and TNF-α at 6 or 24 h post infection. By 24 h post infection, the level of anti-inflammatory cytokine IL-10 in mice receiving LiCl was higher than that of infected only control mice; however, the difference was not statistical significant. A similar serum cytokine pattern was observed with other doses of the bacteria (data not shown). These findings indicate that targeting GSK3β in vivo can modulate the inflammatory response induced by F. tularensis LVS infection by down-regulating the production of pro-inflammatory cytokines, which in turn affords protection to mice against the infection.
Figure 6.
Inhibition of GSK3β suppresses the inflammatory response in mice infected with F. tularensis LVS. C57BL/6 wt mice were immunized (i.p.) with 5 × 104 CFU of F. tularensis LVS in 500 μl of PBS. For GSK3β inhibition, LiCl was given i.p. at 100 μg/g body weight 1 h before or after F. tularensis LVS infection. Serum samples were collected at 6, 24, 48 and 72 h after infection. The levels of IFN-γ, TNF-α, IL-6, IL-12p40, and IL-10 in the serum were determined by ELISA. Data represent the mean ± SEM of three separate experiments. Significant differences were seen at P < 0.05 (*), compared with the mice immunized with F. tularensis LVS only. The results show pooled data of three independent experiments (a total of 15 mice/group).
3.6. IFN-γ and the host outcome to F. tularensis LVS infection
IFN-γ has been shown to be crucial for the control of F. tularensis LVS infection (Duckett et al., 2005; Elkins et al., 2003). IFN-γ knockout mice have been shown to be highly susceptible to F. tularensis LVS infection when administered by the intradermal and intrapulmonary routes. The observed outcomes include a difficulty in clearing the organism from their livers; whereas, IFN-γ treatment of wt mice resulted in an enhanced resistance to infection (Chen et al., 2004; Collazo et al., 2006; Duckett et al., 2005; Hong et al., 2007). However, in the present study, we observed that an early and high level of serum IFN-γ induced by F. tularensis LVS correlated with the rapid death of infected animals. Mice challenged with 5 × 105 CFU of F. tularensis LVS, which had an MTD of 3.8 days, showed a maximal serum IFN-γ level 24 h after infection (Fig. 7A). However, mice challenged with 2 × 104 CFU of F. tularensis LVS that resulted in 60% survival by day 10, exhibited a peak serum IFN-γ level at 48 h after infection. Moreover, the mice that died in the latter group had significantly higher levels of serum IFN-γ at 24 h than the mice that survived (Fig. 7B). These results suggest that the production of IFN-γ contributes to the development of a fatal outcome of tularemia depending on the timing and the relative level of IFN-γ produced.
Figure 7.
IFN-γ and the host outcome after F. tularensis LVS infection. C57BL/6 wt mice were challenged (i.p.) with 2 × 104 or 5 × 105 CFU of F. tularensis LVS in 500 μl of PBS. Serum samples were collected at 6, 24, 48 and 72 h after the infection. The levels of IFN-γ in the serum were determined by ELISA. Data represent the mean ± SEM of three separate experiments. Significant differences were seen at P < 0.05 (*) between the groups challenged with the different doses of F. tularensis (A), and between the surviving and dead mice that were challenged with 2 × 104 of F. tularensis (B). The results show pooled data of three independent experiments (a total of 15 mice/group).
3.7. IFN-γ augments GSK3β activity in F. tularensis LVS-stimulated macrophages
The critical roles of IFN-γ and GSK3β in the outcome of the host’s response to F. tularensis LVS infection led us to consider a possible relationship between IFN-γ and F. tularensis LVS-induced GSK3β activity. IFN-γ has pleiotorpic effects on mononuclear phagocytes (Lorsbach et al., 1993). This cytokine can directly induce the synthesis of many cellular proteins and secretory products, e.g. MHC-II antigens and H2O2. It also has an indirect effect by amplifying the response to inflammatory stimuli such as LPS. Priming with IFN-γ has been shown to confer protection against Francisella by promoting phagolysosome fusion and impeding phagosomal escape (Santic et al., 2005). However, it is not clear how IFN-γ regulates F. tularensis-induced inflammatory responses. In the present study, we observed that IFN-γ significantly increased F. tularensis LVS-induced TNF-α, IL-6 and IL-12p40 production, either by treating macrophages with IFN-γ and F. tularensis LVS at the same time (Fig. 8) or by priming macrophages overnight with IFN-γ before F. tularensis LVS stimulation (data not shown). No significant difference was seen in IL-10 production between control macrophages and IFN-γ–treated macrophages following F. tularensis LVS stimulation (data not shown).
Figure 8.
The role of IFN-γ on F. tularenis-induced cytokine production. Macrophages from C57BL/6 wt mice were cultured with IFN-γ (200 U/ml) alone or in combination with F. tularensis LVS (MOI = 10) for 6 h. The levels of TNF-α, IL-6, and IL-12p40 in the culture supernatants were determined by ELISA. Results represent mean ± SD of three separate experiments. Values are significantly different from LVS-treated controls at P < 0.001 (***).
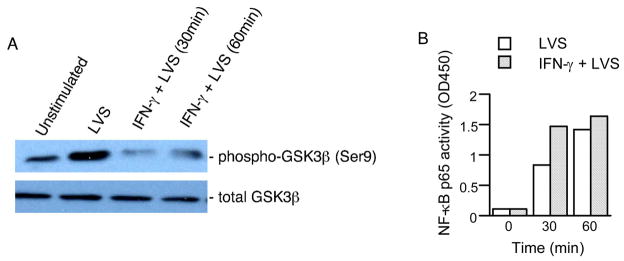
To assess the effect of IFN-γ on GSK3β activity, macrophages were untreated or pretreated with IFN-γ overnight, and then stimulated with F. tularensis LVS for 30 or 60 min. The phosphorylation of GSK3β in the whole cell lysates was analyzed by Western blot. Pretreatment of macrophages with IFN-γ inhibited F. tularensis LVS-induced phosphorylation of GSK3β (Fig. 9A). No phosphorylation of GSK3β was induced by IFN-γ alone. Since the activity of GSK3β is inhibited by serine-phosphorylation, these results suggest that IFN-γ treatment increased F. tularensis LVS-induced GSK3β activity.
Figure 9.
The role of IFN-γ on F. tularensis LVS-induced GSK3β (A) and NF-κB (B) activity. Macrophages from C57BL/6 wt mice were untreated or pretreated with IFN-γ (200 U/ml) overnight before stimulation with F. tularensis LVS (MOI = 10) for 30 or 60 min. Equal amounts of whole cell lysates or nuclear extracts were analyzed for GSK3β phosphorylation (A) or NF-κB p65 activation (B) using Western blot or TransAM assay, respectively. Data are representative three separate experiments.
In light of the above observations, we next sought to determine if IFN-γ affects F. tularensis-induced activation of NF-κB. Pretreatment of macrophages with IFN-γ alone did not induce the nuclear activation of NF-κB p65; whereas, priming of macrophages with IFN-γ augmented F. tularensis LVS-induced activation of NF-κB p65 (Fig. 9B).
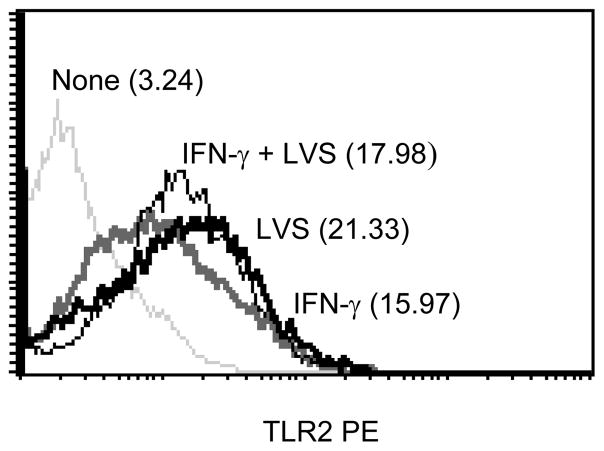
Lastly, since F. tularensis LVS-mediated cellular activation is via TLR2 signaling, we examined whether the enhancement of cytokine production and GSK3β activity was due to the up-regulation of TLR2 expression on macrophages by IFN-γ. Treatment of macrophages with IFN-γ or F. tularensis LVS alone induced an up-regulation of TLR2 expression; however, no synergistic effect was found when the cells were treated with both IFN-γ and F. tularensis LVS (Fig. 10). Taken together, these results suggest that IFN-γ treatment promotes macrophage responses to F. tularensis LVS by the up-regulation of cytokine production, the increased activation of GSK3β, and the enhanced nuclear activation of the transcription factor NF-κB.
Figure 10.
The role of IFN-γ on F. tularensis-induced TLR2 expression on macrophages. Macrophages from C57BL/6 wt mice were cultured with IFN-γ (200 U/ml) either alone or in combination with F. tularensis LVS (MOI = 10) for 24 h. The cells were stained with PE-conjugated anti-mouse TLR2 antibody and surface expression of TLR2 was determined by flow cytometric analysis. The values in the histograms are the MFI. The results are representative of three separate experiments.
4. Discussion
The host’s inflammatory response to Francisella is complex and requires the engagement of multiple cell signaling pathways downstream of TLR2 (Cole et al., 2008). Therefore, an understanding of these pathways is relevant for the development of therapeutic approaches aimed at regulating the inflammatory response, and thus limiting tissue damage. In the present study, we show that a restrained inflammatory cytokine response mediated by GSK3β, a downstream kinase of Akt, is central for protection of the host against F. tularensis LVS infection. GSK3β promoted the production of pro-inflammatory cytokines and dampened the production of anti-inflammatory cytokine IL-10, whereas inhibition of GSK3β by LiCl protected mice from lethal infection with F. tularensis LVS. Our results are in agreement with previous observations (Guha and Mackman, 2002; Martin et al., 2005) showing that GSK3β positively regulates E. coli LPS-induced NF-κB activity and pro-inflammatory cytokine production by human monocytic cells, whereas the upstream kinases PI3k/Akt negatively regulate these activities. Our results also support the findings that PI3k/Akt negatively regulates the inflammatory cytokine response of murine macrophages to F. tularensis LVS infection (Medina et al., 2007). Furthermore, PI3K deficiency has been associated with increased IL-12 production (Fukao and Koyasu, 2003; Fukao et al., 2002), whereas transgenic mice that express constitutively active Akt were protected from a lethal dose of LPS when compared with wild-type littermates (Bommhardt et al., 2004). However, our results differ from those of others who showed that over-expression of GSK3β inhibited TNF-α- and IL-1β-induced inflammatory gene expression by endothelial cells (Vines et al., 2006). Studies by Parsa et al. (Parsa et al., 2006) demonstrated that activation of Akt promoted a pro-inflammatory response to F. novicida in murine macrophages, whereas Rajaram et al. (Rajaram et al., 2006) further showed that mice expressing constitutively active Akt were protected against lethal doses of F. novicida, suggesting that Akt promoted pro-inflammatory cytokine production, which contributed to immunity against F. novicida. It appears that a number of factors could contribute to the discrepancies in these studies. Although it would seem unlikely that the different Francicella species used would bring about such dissimilar outcomes, it has nevertheless been reported that different Francisella strains induce distinct host responses (Kieffer et al., 2003; Nix et al., 2006; Thomas et al., 2007; Twine et al., 2006). Several investigators have shown that F. tularensis LVS induces substantial inflammatory cytokine production in vitro and in vivo (Cole et al., 2006; Golovliov et al., 1995; Katz et al., 2006; Stenmark et al., 1999), while others have reported that F. tularensis LVS failed to induce the production of inflammatory cytokines by murine dendritic cells or macrophages (Bolger et al., 2005; Bosio and Dow, 2005), or that F. tularensis LVS initially activates but subsequently down-regulates cytokine secretion in murine and human monocytic cells (Telepnev et al., 2005). It has recently been reported that the conditions used to culture F. tularensis including culture media, media components, temperature, and carbohydrate sources markedly influence the virulence of Francisella (Carlson et al., 2007; Nau, 2008), which ultimately affects the distinct signals received by the host cells. We have observed that the signal dependency of F. tularensis on TLR2 relies on the dose and the virulence of the bacteria (unpublished observation). Therefore, the cell-type specific function of Akt/GSK3β, the stimuli specific signals, and the specific experimental conditions could all contribute to the differences observed among these studies.
Although lithium has been the mainstay treatment for bipolar disorder, the mechanism of its therapeutic action remains uncertain (Jope, 2003). Lithium is a well-known GSK3β inhibitor (Klein and Melton, 1996; Phiel and Klein, 2001), it inhibits GSK3β activity both directly by competition for magnesium (Ryves and Harwood, 2001) and indirectly by increasing phosphorylation of serine (Zhang et al., 2003). In the present study, we have shown that LiCl inhibited GSK3β activity by increasing F. tularensis LVS-induced serine 9 phosphorylation. Moreover, LiCl inhibited F. tularensis LVS-induced inflammatory cytokine responses in vitro and in vivo, which likely contributed to the observed protection against F. tularensis LVS infection in mice treated with LiCl. However, it has been reported that treatment of Francisella with high concentrations of LiCl (0.12 M and 0.24 M) damages the bacterial surface structures and weakens the bacteria (Pavlovich and Tkacheva, 1990). This finding would suggest that the protection observed in our study could be due to an effect of LiCl on the bacteria, instead of an effect mediated by GSK3β. However, direct incubation of LiCl with F. tularensis LVS at the concentrations used in the present study resulted in no bacterial changes as assessed by growth and viability. Therefore, our results provide the first evidence that lithium confers protection of mice against F. tularensis LVS infection through GSK3β inhibition.
Our results also demonstrated that GSK3β positively regulates NF-κB activity and negatively regulates CREB activity. GSK3β is involved in the regulation of NF-κB activity where survival signals and inflammatory responses are thought to be involved (Hoeflich et al., 2000). Based on studies by Takada et al. (Takada et al., 2004), the down regulation of pro-inflammatory cytokines could be explained by a suppression of NF-κB activation as seen by the absence of GSK3β in mouse embryonic fibroblasts, in addition to inhibition of IκBα degradation, ERK1/2 phosphorylation and c-Jun N terminal kinase activation. In the present study, no difference was observed in ERK1/2 phosphorylation between macrophages treated with LiCl and non-treated control cells (data not shown); however, p65 NF-κB nuclear activity in macrophages treated with LiCl was indeed decreased. In a different study also using mouse embryonic fibroblasts lacking GSK3β, Steinbrecher et al. (Steinbrecher et al., 2005) were unable to show an effect in IKK activation or in the ability of the IKK complex to phosphorylate downstream proteins. However, they did show a decrease in NF-κB DNA binding ability at specific target sequences upon deletion of GSK3β or upon its inhibition. In LiCl-treated hepatocytes, Schwabe et al. (Schwabe and Brenner, 2002) observed that inhibition of GSK3 impaired NF-κB activity at the transcriptional level, but IκB degradation and NF-κB DNA binding activity was not affected.
IL-10 is a potent anti-inflammatory cytokine that mediates a feedback inhibition loop that limits inflammatory cytokine production (Hu et al., 2006). It inhibits multiple macrophage and dendritic cell effector functions and plays a critical role in limiting tissue injury during infection. F. tularensis LVS is a much weaker IL-10 inducer in human and mouse dendritic cells than other TLR2 agonist including Pam3Cys and peptidoglycan (Li et al., 2006). It has been shown that mice infected with F. tularensis LVS exhibit enhanced IL-10 mRNA only in the lungs at 48 and 72 h after i.p. or i.d. infection (Cole et al., 2006). Our results show that F. tularensis LVS induced minimal IL-10 production in vitro and in vivo. However, inhibition of GSK3β resulted in a significant increase in IL-10 production by macrophages. This is consistent with a previous report (Martin et al., 2005) and could be explained by an increase in CREB activation, a transcription factor that together with AP-1 is involved in IL-10 regulation (Grimes and Jope, 2001; Hu et al., 2006; Martin et al., 2005). Although we also saw an increase in serum IL-10 levels in mice treated with lithium and infected with F. tularensis LVS, the levels were not significantly different from those observed in the infected only mice. It is possible that unknown molecules are produced in vivo that limit the IL-10 response or that the IL-12 produced limited the IL-10 response in vivo since counter-regulatory events among these cytokines have been observed (Buelens et al., 1997; Zhang et al., 2005). Furthermore, IFN-γ has been shown to inhibit TLR2-induced IL-10 production by suppressing the activation of CREB and AP-1 (Hu et al., 2005). Thus, it is important to keep in mind that the integration of multiple signaling pathways is the essential end result of the crosstalk that takes place between many different factors that likely orchestrate the expression of specific genes.
The in vivo findings of the present study revealed an association between high levels of IFN-γ produced within 24 h after F. tularensis LVS infection and death of the animal. This outcome was somewhat surprising since IFN-γ has been shown to be required for protection of the host against F. tularensis LVS infection (Duckett et al., 2005). Furthermore, this cytokine has been known to prevent intracellular bacterial replication by interfering with bacterial escape from lysosomes (Kirimanjeswara et al., 2007). Moreover, depletion of IFN-γ producing cells such as CD4 or CD8 T cells or neutralization of IFN-γ in mice challenged with F. tularensis LVS results in death of the animal (Wayne Conlan et al., 2005; Wu et al., 2005). However, studies by Lindgren et al. (Lindgren et al., 2004) have shown that excessive levels of IFN-γ lead to an increased susceptibility to F. tularensis LVS infection in mice lacking reactive nitrogen and oxygen species. Furthermore, BALB/c mice are more susceptible to Burkholderia pseudomallei infection than C57BL/6 mice and exhibit acute disease and death within a week, due to the hyperproduction of IFN-γ, which correlated with high bacterial loads in different organs (Koo and Gan, 2006; Liu et al., 2002; Ulett et al., 2000). Therefore, although IFN-γ is absolutely required for host survival, hyperproduction of this cytokine seems to contribute to immune pathology and severe disease. It is also possible that the order in which macrophages encounter immune and inflammatory stimuli can profoundly influence the extent to which antimicrobial and cytolytic activity are induced in these cells (Lorsbach et al., 1993).
It was recognized early on that a key function of IFN-γ is to synergize with activating stimuli such as TLR ligands to enhance activation of pro-inflammatory pathways (Schroder et al., 2004). IFN-γ up-regulates TLR2 and TLR4 expression (Bosisio et al., 2002; Mita et al., 2001) and promotes NF-κB activation and DNA-binding kinetics upon LPS stimulation (Held et al., 1999). In accordance with these results, we showed an increased production of pro-inflammatory cytokines in response to F. tularensis LVS in the presence of IFN-γ. IFN-γ induced an up-regulation of TLR2 expression on macrophages, although it did not synergize with F. tularenisis LVS for an enhanced up-regulation of TLR2. It has been recently reported that IFN-γ alters TLR2 signaling by increasing GSK3 activity and suppressing MAPK activation, leading to a diminished IL-10 production (Hu et al., 2006). In the present study, we have shown that the addition of IFN-γ to murine macrophage cultures had no effect on IL-10 production following infection with F. tularensis. However, IFN-γ pretreatment promoted GSK3β activity and NF-κB activation upon F. tularensis LVS stimulation. Taken together, these results suggest the existence of a feedback loop between IFN-γ and GSK3β activity after F. tularensis LVS infection that contributes to the host inflammatory response.
To our knowledge, this is the first study that addresses the role of GSK3β in F. tularensis infection. The results of this study also reveal a possible mechanism for the detrimental effect of IFN-γ during F. tularensis LVS infection and suggest that inhibitors of GSK3β, such as lithium, may be useful in the treatment of tularemia.
Acknowledgments
We thank Gregory Harbor and DeLisa Whittaker for their excellent technical support. We also thank Dr. Richard S. Jope and Dr. Chuan-Ming Li for their valuable suggestions regarding this work. This work was supported by U. S. Public Health Service grant AI56460 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akira S, Takada K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA. IκB-NF-κB structures: at the interface of inflammation control. Cell. 1998;95:729–731. doi: 10.1016/s0092-8674(00)81694-3. [DOI] [PubMed] [Google Scholar]
- Blackwell TS, Christman JW. The role of nuclear factor-κB in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- Bolger CE, Forestal CA, Italo JK, Benach JL, Furie MB. The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secret proinflammatory cytokines. J Leukoc Biol. 2005;77:893–897. doi: 10.1189/jlb.1104637. [DOI] [PubMed] [Google Scholar]
- Bommhardt U, Chang KC, Swanson PE, Wagner TH, Tinsley KW, Karl IE, Hotchkiss RS. Akt decreases lymphocyte apoptosis and improves survival in sepsis. J Immunol. 2004;172:7583–7591. doi: 10.4049/jimmunol.172.12.7583. [DOI] [PubMed] [Google Scholar]
- Bosio CM, Dow SW. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J Immunol. 2005;175:6792–6801. doi: 10.4049/jimmunol.175.10.6792. [DOI] [PubMed] [Google Scholar]
- Bosisio D, Polentarutti N, Sironi M, Bernasconi S, Miyake K, Webb GR, Martin MU, Mantovani A, Muzio M. Stimulation of toll-like receptor 4 expression in human mononuclear phagocytes by interferon-γ: a molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood. 2002;99:3427–3431. doi: 10.1182/blood.v99.9.3427. [DOI] [PubMed] [Google Scholar]
- Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Human dendritic cell responses to lipopolysaccharide and CD40 ligation are differentially regulated by interleukin-10. Eur J Immunol. 1997;27:1845–1852. doi: 10.1002/eji.1830270805. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Carlson PEJ, Carroll JA, O’Dee DM, Nau GJ. Modulation of virulence factors in Francisella tularensis determines human macrophage responses. Microb Pathog. 2007;42:204–214. doi: 10.1016/j.micpath.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, KuoLee R, Shen H, Busa M, Conlan JW. Toll-like receptor 4 (TLR4) does not confer a resistance advantage on mice against low-dose aerosol infection with virulent type A Francisella tularensis. Microb Pathog. 2004;37:185–191. doi: 10.1016/j.micpath.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Clements DL, Lee B, Horwitz MA. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect Immun. 2005;73:5892–5902. doi: 10.1128/IAI.73.9.5892-5902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Frame S. The renaissance of GSK3. NAt Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- Cole LE, Elkins KL, Michalek SM, Qureshi N, Eaton LJ, Rallabhandi P, Cuesta N, Vogel SN. Immunologic consequences of Francisella tularensis live vaccine strain infection: role of the innate immune response in infection and immunity. J Immunol. 2006;176:6888–99. doi: 10.4049/jimmunol.176.11.6888. [DOI] [PubMed] [Google Scholar]
- Cole LE, Santiago A, Barry E, Kang TJ, Shirey KA, Roberts ZJ, Elkins KL, Cross AS, Vogel SN. Macrophage proinflammatory response to Francisella tularensis live vaccine strain requires coordination of multiple signaling pathways. J Immunol. 2008;180:6885–6891. doi: 10.4049/jimmunol.180.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LE, Shirey KA, Barry E, Santiago A, Rallabhandi P, Elkins KL, Puche AC, Michalek SM, Vogel SN. Toll-like receptor 2-mediated signaling requirements for Francisella tularensis live vaccine strain infection of murine macrophages. Infect Immun. 2007;75:4127–37. doi: 10.1128/IAI.01868-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazo CM, Sher A, Meierovics AI, Elkins KL. Myeloid differentiation factor-88 (MyD88) is essential for control of primary in vivo Francisella tularensis LVS infection, but not for control of intra-macrophage bacterial replication. Microb Infect. 2006;8:779–790. doi: 10.1016/j.micinf.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Duckett NS, Olmos S, Durrant DM, Metzger DW. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect Immun. 2005;73:2306–11. doi: 10.1128/IAI.73.4.2306-2311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugo L, Abdelrahman M, Murch O, Mazzon E, Cuzzocrea S, Thiemermann C. Glycogen synthase kinase-3beta inhibitors protect against the organ injury and dysfunction caused by hemorrhage and resuscitation. Shock. 2006;25:485–491. doi: 10.1097/01.shk.0000209545.29671.31. [DOI] [PubMed] [Google Scholar]
- Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microb Infect. 2003;5:135–42. doi: 10.1016/s1286-4579(02)00084-9. [DOI] [PubMed] [Google Scholar]
- Forsman M, Sandstrom G, Jaurin B. Identification of Francisella species and discrimination of type A and type B strains of F. tularensis by 16S rRNA analysis. Appl Environ Microbiol. 1990;56:949–955. doi: 10.1128/aem.56.4.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- Golovliov I, Sandstrom G, Ericsson M, Sjostedt A, Tarnvik A. Cytokine expression in the liver during early phase of murine tularemia. Infect Immun. 1995;63:534–538. doi: 10.1128/iai.63.2.534-538.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. CREB DNA binding activity is inhibited by glycogen synthase kinase-3β and facilitated by lithium. J Neurochem. 2001;78:1219–1232. doi: 10.1046/j.1471-4159.2001.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, Mackman N. The phophstidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- Held TK, Xiao W, Liang Y, Kalvakolanu DV, Cross AS. Gamma Interferon augments macrophage activation by lipopolysaccharide by two distinct mechanisms, at the signal transduction level and via an autocrine mechanism involving tumor necrosis factor alpha and interleukin-1. Infect Immun. 1999;67:206–212. doi: 10.1128/iai.67.1.206-212.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Ruble EA, Tsao M, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3• in cell survival and NF-•B activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Hong K, Wicksrum JR, Yeh H, Parmely MJ. Toll-like receptor 2 controls the gamma interferon response to Francisella tularensis by mouse liver lymphocytes. Infect Immun. 2007;75:5338–5345. doi: 10.1128/IAI.00561-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Paik PK, Chen J, Yarilina A, Kockeritz L, Lu TT, Woodgett JR, Ivashkiv LB. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24:563–74. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Hu X, Park-Min KH, Ho HH, Ivashkiv LB. IFN-gamma-primed macrophages exhibit increased CCR2-dependent migration and altered IFN-gamma responses mediated by Stat1. J Immunol. 2005;175:3637–47. doi: 10.4049/jimmunol.175.6.3637. [DOI] [PubMed] [Google Scholar]
- Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Biochem Scien. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- Jope RS, Johnson GVW. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Scien. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisho T, Akira S. Critical role of Toll-like receptors in host defense. Crit Rev Immunol. 2000;20:393–405. [PubMed] [Google Scholar]
- Katz J, Zhang P, Martin M, Vogel SN, Michalek SM. Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect Immun. 2006;74:2809–16. doi: 10.1128/IAI.74.5.2809-2816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TL, Cowley S, Nano FE, Elkins KL. Francisella novicida LPS has greater immunological activity in mice than F. tularensis LPS, and contributes to F. novicida murine pathogenesis. Microbes Infect. 2003;5:397–403. doi: 10.1016/s1286-4579(03)00052-2. [DOI] [PubMed] [Google Scholar]
- Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol. 2007;179:532–9. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo GC, Gan YH. The innate interferon gamma response of BALB/c and C57BL/6 mice to in vitro Burkholderia pseudomallei infection. BMC Immunol. 2006;7:19. doi: 10.1186/1471-2172-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Nookala S, Bina XR, Bina JE, Re F. Innate immune response to Francisella tularensis is mediated by TLR2 and caspase-1 activation. J Leukoc Biol. 2006;80:766–73. doi: 10.1189/jlb.0406294. [DOI] [PubMed] [Google Scholar]
- Lindgren H, Stenmark S, Chen W, Tarnvik A, Sjostedt A. Distinct roles of reactive nitrogen and oxygen species to control infection with the facultative intracellular bacterium Francisella tularensis. Infect Immun. 2004;72:7172–82. doi: 10.1128/IAI.72.12.7172-7182.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Koo GC, Yap EH, Chua KL, Gan YH. Model of differential susceptibility to mucosal Burkholderia pseudomallei infection. Infect Immun. 2002;70:504–11. doi: 10.1128/IAI.70.2.504-511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsbach RB, Murphy WJ, Lowenstein CJ, Snyder SH, Russell SW. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. J Biol Chem. 1993;268:1908–1913. [PubMed] [Google Scholar]
- Malik M, Bakshi CS, Sahay B, Shah A, Lotz SA, Sellati TJ. Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect Immun. 2006;74:3657–62. doi: 10.1128/IAI.02030-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–84. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina EA, Morris IR, Abplanalp AL, Berton MT. Toll-like receptor 2 signaling via the PI3-kinase/Akt pathway attenuates the macrophage inflammatory cytokine response to F. tularensis LVS infection. 2007 Tularemia workshop. 2007:220c. [Google Scholar]
- Mita Y, Dobashi K, Shimizu Y, Nakazawa T, Mori M. Toll-like receptor 2 and 4 surface expressions on human monocytes are modulated by inteferon-γ and macrophage colony-stimulating factor. Immunol Lett. 2001;78:97–101. doi: 10.1016/s0165-2478(01)00241-3. [DOI] [PubMed] [Google Scholar]
- Nau GJ. Enviromental adaption and Francisella pathogenesis. 2008 Tularemia Workshop. 2008:20. [Google Scholar]
- Nix EB, Cheung KK, Wang D, Zhang N, Burke RD, Nano FE. Virulence of Framcisella spp. in chicken embryos. Infect Immun. 2006;74:4809–4816. doi: 10.1128/IAI.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa KV, Ganesan LP, Rajaram MV, Gavrilin MA, Balagopal A, Mohapatra NP, Wewers MD, Schlesinger LS, Gunn JS, Tridandapani S. Macrophage pro-inflammatory response to Francisella novicida infection is regulated by SHIP. PLoS Pathog. 2006;2:e71. doi: 10.1371/journal.ppat.0020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovich NV, Tkacheva TI. Natural penicillin resistance of Francisella tularensis. Antibiot Khimioter. 1990;35:25–28. [PubMed] [Google Scholar]
- Phiel CJ, Klein PS. Molecular targets of lithium action. Annu Rev Pharmacol Toxicol. 2001;41:789–831. doi: 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- Platzer C, Fritsch E, Elsner T, Lehmann MH, Volk HD, Prosch S. Cyclic adenosine monophosphophate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur J Immunol. 1999;29:3098–3014. doi: 10.1002/(SICI)1521-4141(199910)29:10<3098::AID-IMMU3098>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Rajaram MV, Ganesan LP, Parsa KV, Butchar JP, Gunn JS, Tridandapani S. Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol. 2006;177:6317–24. doi: 10.4049/jimmunol.177.9.6317. [DOI] [PubMed] [Google Scholar]
- Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- Santic M, Molmeret M, Abu Kwaik Y. Modulation of biogenesis of the Francisella tularensis subsp. novicida-containing phagosome in quiescent human macrophages and its maturation into a phagolysosome upon activation by IFN-gamma. Cell Microbiol. 2005;7:957–967. doi: 10.1111/j.1462-5822.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- Santic M, Molmeret M, Klose KE, Abu Kwaik Y. Francisella tularensis travels a novel, twisted road within macrophages. Trends Microbiol. 2006;14:37–44. doi: 10.1016/j.tim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and function. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Brenner DA. Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283:G204–11. doi: 10.1152/ajpgi.00016.2002. [DOI] [PubMed] [Google Scholar]
- Scolari G, Vescovo M, Zacconi C, Vescovi F. Extraction and partial characterization of proteolytic activities from the cell surface of Lactobacillus helveticus Zuc2. J Dairy Sci. 2006;89:3800–3809. doi: 10.3168/jds.S0022-0302(06)72421-3. [DOI] [PubMed] [Google Scholar]
- Sjostedt A. Intracellular survival mechanisms of Francisella tularensis, a stealth pathogen. Microb Infect. 2006;8:561–567. doi: 10.1016/j.micinf.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Steinbrecher KA, Wilson W, III, CCP, Baldwin AS. Glycogen synthase kinase 3b functions to specify gene-specific, NF-κB-dependent transcription. Mol Cell Biol. 2005;25:8444–8455. doi: 10.1128/MCB.25.19.8444-8455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark S, Sunnemark D, Bucht A, Sjostedt A. Rapid local expression of interleukin-12, tumor necrosis factor alpha, and gamma interferon after cutaneous Francisella tularensis infection in tularemia-immune mice. Infect Immun. 1999;67:1789–1797. doi: 10.1128/iai.67.4.1789-1797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3• abrogates activation of I•B• kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factors. J Biol Chem. 2004;279:39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- Telepnev M, Golovliov I, Sjostedt A. Francisella tularensis LVS initially activates but subsequently down-regulates intracellular signaling and cytokine secretion in mouse monocytic and human peripheral blood mononuclear cells. Microb Pathog. 2005;38:239–247. doi: 10.1016/j.micpath.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Titball RW, Oyston PC, Griffin K, Waters E, Hitchen PG, Michell SF, Grice ID, Wilson JC, Prior JL. The immunologically distinct O antigens from Francisella tularensis subsepcies tularensis and Francisella novicida are both virulence determinants and protective antigens. Infect Immun. 2007;75:371–378. doi: 10.1128/IAI.01241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twine SM, Shen H, Kelly JF, Chen W, Sjostedt A, Conlan JW. Virulence comparison in mice of distinct isolates of type A Francisella tularensis. Microb Pathog. 2006;2006:133–138. doi: 10.1016/j.micpath.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulett GC, Ketheesan N, Hirst RG. Cytokine gene expression in innately susceptible BALB/c mice and relatively resistant C57BL/6 mice during infection with virulent Burkholderia pseudomallei. Infect Immun. 2000;68:2034–42. doi: 10.1128/iai.68.4.2034-2042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines A, Cahoon S, Goldberg I, Saxena U, Pillarisett S. Novel anti-inflammatory role for glycogen synthase kinase-3• in the inhibition of tumor necrosis factor-• and interleukin-1•-induced inflammatory gene expression. J Biol Chem. 2006;28:16985–16990. doi: 10.1074/jbc.M602446200. [DOI] [PubMed] [Google Scholar]
- Wayne Conlan J, Shen H, KuoLee R, Zhao X, Chen W. Aerosol-, but not intradermal-, immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an alphabeta T cell- and interferon gamma-dependent mechanism. Vaccine. 2005;23:2477–2485. doi: 10.1016/j.vaccine.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Wu TH, Hutt JA, Garrison KA, Berliba LS, Zhou Y, Lyons CR. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biobar A. Infect Immun. 2005;73:2644–2654. doi: 10.1128/IAI.73.5.2644-2654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe T, Sanagi T, Schwartz JP, Yamada H. Pigment epithelium-derived factor induces pro-inflammatory genes in neonatal astrocytes through activation of NF-kappa B and CREB. Glia. 2005;50:223–234. doi: 10.1002/glia.20171. [DOI] [PubMed] [Google Scholar]
- Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 in response to lithium. J Biol Chem. 2003;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- Zhang P, Martin M, Michalek SM, Katz J. Role of mitogen-activated protein kinases and NF-kappaB in the regulation of proinflammatory and anti-inflammatory cytokines by Porphyromonas gingivalis hemagglutinin B. Infect Immun. 2005;73:3990–8. doi: 10.1128/IAI.73.7.3990-3998.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]