Abstract
The anchored and secreted forms of the human immunodeficiency virus type 1 (HIV-1) 89.6 envelope glycoprotein, either complete or after deletion of the V3 loop, were expressed in a cloned attenuated measles virus (MV) vector. The recombinant viruses grew as efficiently as the parental virus and expressed high levels of the HIV protein. Expression was stable during serial passages. The immunogenicity of these recombinant vectors was tested in mice susceptible to MV and in macaques. High titers of antibodies to both MV and HIV-Env were obtained after a single injection in susceptible mice. These antibodies neutralized homologous SHIV89.6p virus, as well as several heterologous HIV-1 primary isolates. A gp160 mutant in which the V3 loop was deleted induced antibodies that neutralized heterologous viruses more efficiently than antibodies induced by the native envelope protein. A high level of CD8+ and CD4+ cells specific for HIV gp120 was also detected in MV-susceptible mice. Furthermore, recombinant MV was able to raise immune responses against HIV in mice and macaques with a preexisting anti-MV immunity. Therefore, recombinant MV vaccines inducing anti-HIV neutralizing antibodies and specific T lymphocytes responses deserve to be tested as a candidate AIDS vaccine.
The vast majority of the 40 million people currently infected by human immunodeficiency virus (HIV) are living in developing countries (77a). In these areas, mother-to-child transmission, including via breast-feeding, accounts for half a million infections every year, and most cases of sexual transmission occur in individuals under the age of 20 years. Therefore, developing a preventive pediatric HIV vaccine is a major goal in the fight against AIDS. Such a vaccine must be easy to produce on a large scale and at low cost in developing countries. It must be safe and able to induce protective immunity after one or two injections.
Vaccines developed from replicating live attenuated RNA viruses, such as Sabin poliovirus, Schwarz measles virus (MV), or the 17D strain of yellow fever virus, have a longstanding safety and efficacy record. They are produced on a large scale in most developing countries and can be distributed at very low cost. These vaccines induce strong cellular and humoral immune responses after a single injection and are particularly efficient at stimulating long-lasting memory B- and T cells. Although live attenuated simian immunodeficiency virus (SIV) protects macaques efficiently (18), a live attenuated HIV vaccine is not envisioned at present for safety reasons (4). Therefore, a number of recombinant viral vectors such as modified vaccinia virus Ankara, canarypox virus, and adenovirus have been evaluated in preclinical or clinical trials (45). However, these replication-defective vectors require several high-dose injections in order to induce and maintain efficient responses. We propose to explore the possibility of using live attenuated MV as a polyvalent AIDS vaccination vector.
MV vaccine induces a very efficient, life-long immunity after a single low-dose injection [104 50% tissue culture infective dose(s) (TCID50)] (26). Protection is mediated both by antibodies and by CD4+ and CD8+ T cells. The MV genome is very stable and reversion to pathogenicity has never been observed with this vaccine. MV replicates exclusively in the cytoplasm, ruling out the possibility of integration in host DNA. Furthermore, an infectious cDNA clone corresponding to the anti-genome of the Edmonston strain of MV, as well as a procedure to rescue the corresponding virus, has already been established (64). This cDNA has been adapted to create a vector to express foreign genes (63). It can accommodate up to 5 kb of foreign DNA and is genetically very stable (72, 78). Therefore, MV vaccine could be a vector to facilitate the induction of anti-HIV immunity. By taking advantage of the existing technology to produce and distribute large quantities of MV vaccine, recombinant MV-HIV could be used to mass immunize children and adolescents against both measles and AIDS. We describe here the production of live attenuated MV vaccines expressing different forms of clade B HIV89.6 envelope glycoprotein (Env), and the induction of immune responses by these vaccines.
Neutralizing anti-HIV antibodies are directed at the envelope glycoprotein and can contribute to the control of HIV spread (9, 43, 56). Broadly neutralizing antibodies have been detected in long-term nonprogressors (59). However, native gp120 is a poor inducer of cross-reactive neutralizing antibodies. As shown by X-ray crystallography, the variable V1 and V2 loops mask elements of the CD4 binding site, and the V2 and V3 loops mask the CD4-induced (CD4i) epitopes and the chemokine receptor binding site (38, 79, 81). Furthermore, some conserved epitopes can induce highly neutralizing antibodies, but they are buried in the three-dimensional structure of the envelope glycoprotein and become exposed only after binding to the receptor or coreceptor (52, 74, 75, 80). Neutralizing monoclonal antibodies (MAbs) have been obtained from patients' B cells (57). These are directed at gp41 linear or conformational epitopes (2F5) (52, 83) or at gp120 conformational epitopes (2G12, 17b, 48d, and b12) (39, 68, 75, 76). Used in synergy they can neutralize several primary isolates in vitro (44) and protect macaques against a mucosal challenge with simian/HIV (SHIV) (3). Although the role of neutralizing antibodies in the control of an established infection is unclear (61), several reports suggest that they could contribute to protective immunity (10, 45, 70). To be cross-reactive for primary isolates, antibodies must be directed at conserved epitopes that are critical for viral entry into host cells (24, 60, 77). However, in infected people, such antibodies appear slowly and are diluted in large quantities of antibodies directed at the accessible, highly variable gp120 loops (47, 58, 79). Most antibodies directed against the V3 loop are isolate specific and neutralize only genetically similar viruses (53). Likewise, the early neutralizing antibody response in SHIV-infected monkeys is strain specific and directed against epitopes of the V2 and V3 loops (23).
To favor the induction of cross-reactive neutralizing antibodies, we deleted the hypervariable V3 loop of the HIV89.6 gp160 (anchored) and gp140 (soluble) proteins in order to eliminate this “immunological decoy” and to expose more conserved epitopes (5, 30, 67). Indeed, the conserved elements near the chemokine receptor-binding site are poorly exposed in native gp120 (81). Also, the N-linked glycosylation of a conserved amino acid of the V3 loop modifies the loop structure and obstructs access to the conserved CD4 binding and CD4i sites of the gp120 from several HIV-1 isolates (42). Deleting the V3 loop could redirect the antibody response against the chemokine receptor binding site, therefore inducing broadly neutralizing antibodies (25). In addition, we replaced the V3 loop with the ELDKWAS sequence flanked on both sides by two alanines to maintain the native conformation of this gp41 conserved epitope normally buried in the native protein and targeted by the broadly neutralizing 2F5 MAb (52). We investigated the immunogenicity of native and ΔV3 EnvHIV89.6 mutants expressed in live attenuated MV. We found that MV-HIV recombinant viruses induced cross-reactive neutralizing antibodies, as well as gp120-specific CD4+- and CD8+-T-cell responses, after a single injection in CD46+/− alpha/beta interferon receptor−/− (IFN-α/βR−/−) mice. Furthermore, they induced anti-HIV antibodies in mice and macaques with preexisting anti-MV immunity.
MATERIALS AND METHODS
Plasmid constructions.
All MV recombinant plasmids were derived from plasmid p(+)MV, which carries the antigenomic MV tag Edmonston B sequence (64). Two additional transcription units (ATU) containing unique restriction sites for easy insertion of open reading frames have been introduced in p(+)MV (63): one downstream from the P gene (position 2) and the other one downstream from the H gene (position 3). Both plasmids (kindly provided by M. A. Billeter, University of Zurich, Zurich, Switzerland) were used for inserting the SHIV89.6p env open reading frames.
The envelope glycoproteins used in the present study were derived from SHIV89.6p, a chimeric SHIV with the tat, rev, vpu, and env genes of HIV-1 on a SIVmac239 background (65). The env gene is derived from a cytopathic primary HIV-1 isolate, 89.6, which is tropic for both macrophages and T cells (15). The env sequence was amplified from plasmid pSHIV-KB9 (NIH-AIDS Research & Reference Reagent Program) that had been cloned after passage of the original virus in vivo (34). The full-length (gp160) and secreted (gp140) env sequences were amplified by PCR (Pfu polymerase) with primers that contain unique BsiWI and BssHII sites for subsequent cloning in MV vectors (Fig. 1). A start and a stop codon were added to the ends of the genes. Nucleotides were also added after the stop codon in order to comply with the “rule of six,” which stipulates that the number of nucleotides of MV genome must be a multiple of six (11). Both gp160 and gp140 env fragments were cloned in pCR2.1-TOPO plasmid (Invitrogen, Groningen, The Netherlands) and sequenced.
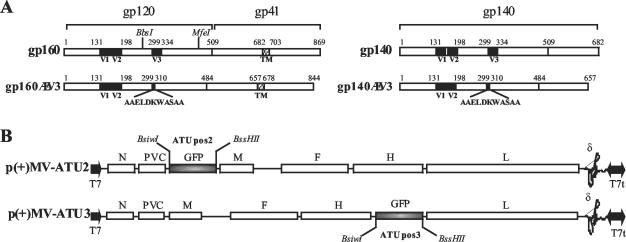
FIG. 1.
Constructions of recombinant MV-envHIV89.6. (A) Full-length gp160 and secreted gp140 HIV-1 Env proteins. Shaded boxes indicate V1, V2, and V3 regions; dashed boxes show the transmembrane domain. Amino acid positions are indicated. The BbsI and MfeI restriction sites used to introduce the ΔV3-AAELDKWASAA mutation are also indicated. (B) pMV(+) vectors with ATU containing a green fluorescent protein (GFP) gene in positions 2 and 3. The MV genes are indicated as follows: N, nucleoprotein; PVC, phosphoprotein and V C proteins; M, matrix; F, fusion; H, hemagglutinin; and L, polymerase. T7, T7 RNA polymerase promoter; T7t, T7 RNA polymerase terminator; ∂, hepatitis delta virus ribozyme.
The V3 loop-deletion mutants were generated by using two overlapping DNA fragments flanking the V3 sequence to be deleted (amino acids 299 to 334, Fig. 1A) and containing the sequence coding for the AAELDKWASAA peptide. These fragments were annealed by PCR, cloned in pCR2.1-TOPO plasmid, and sequenced. After digestion with BbsI and MfeI, the DNA fragment encoding the ΔV3-AAELDKWASAA mutation was introduced in place of the corresponding fragment in the gp160 and gp140 sequences already cloned in pCR2.1-TOPO plasmids (Fig. 1A). After BsiWI/BssHII digestion, the native and ΔV3 gp160 and gp140 sequences were introduced into the pMV vector in ATU positions 2 and 3 (Fig. 1B). The resulting plasmids were designated pMV2-gp140HIV, pMV2-gp160HIV, pMV3-gp140ΔV3HIV, and pMV2-gp160ΔV3HIV.
Cells.
Cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 5% fetal calf serum (FCS) for Vero cells (African green monkey kidney) or with 10% FCS and 1 mg of G418/ml for helper 293-3-46 cells (64) and for P4-CCR5 cells (HeLa-CD4-CXCR4-CCR5-HIVLTR-LacZ) (13).
Rescue of recombinant MV-EnvHIV89.6 viruses.
Recombinant MV-HIV viruses were recovered from plasmids by using the helper-cell-based rescue system described by Radecke et al. (64) and modified by Parks et al. (54). Human helper cells expressing T7 RNA polymerase and measles N and P proteins (293-3-46 cells [kindly provided by M. A. Billeter) were cotransfected with the pMV-EnvHIV plasmids (5 μg) and a plasmid expressing the MV L gene (pEMC-La, 20 ng [kindly provided by M. A. Billeter]). After overnight incubation at 37°C, the cells were heat shocked at 43°C for 3 h in fresh medium (54). Heat-shocked cells were incubated at 37°C for 2 days and then transferred onto a 70% confluent Vero cells layer (10-cm petri dishes). Syncytia appeared in Vero cells after 2 to 5 days of coculture. Single syncytia were harvested and transferred to Vero cells grown in 35-mm wells. The infected cells were expanded in 75- and 150-cm3 flasks. When syncytia reached 80 to 90% confluence, the cells were scraped into a small volume of OptiMEM (Gibco-BRL) and then frozen and thawed once. After centrifugation, the supernatant, which contained virus, was stored at −80°C.
Virus titration.
The titers of recombinant MV were determined by an endpoint limit dilution assay on Vero cells. The TCID50 values were calculated by the Kärber method (33).
Western blots.
Monolayers of Vero cells (T-25 flasks) were infected at a multiplicity of infection (MOI) of 0.05 with the recombinant viruses. When syncytia reached 80 to 90% confluence, cells were lysed in 150 mM NaCl, 50 mM Tris (pH 8), 1% NP-40, 0.5 mM phenylmethylsulfonyl fluoride, and 0.2 mg of Pefabloc (Interbiotech)/ml. Chromatin was removed by centrifugation, and the concentration of protein in the supernatant was determined by a Bradford assay. Proteins (50 μg) were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to cellulose membranes (Amersham Pharmacia Biotech). The blots were probed with a mouse monoclonal anti-HIV gp120 antibody (Chessie 13-39.1; NIH-AIDS Research & Reference Reagent Program) or with a monoclonal anti-MV N antibody (Chemicon, Temecula, Calif.). A goat anti-mouse immunoglobulin G (IgG)-horseradish peroxidase (HRP) conjugate (Amersham) was used as a secondary antibody. Peroxidase activity was visualized with an enhanced chemiluminescence detection kit (Pierce).
Animal immunization.
Mice susceptible for MV infection were obtained as described previously (50). FVB mice heterozygous for the CD46 MV receptor transgene (82) (kindly provided by F. Grosveld, Erasmus University, Rotterdam, The Netherlands) were crossed with 129Sv IFN-α/βR−/− mice (51) (kindly provided by M. Aguet, Swiss Institute for Experimental Cancer Research). The F1 progeny were screened by PCR, and the CD46+/− animals were crossed again with 129Sv IFN-α/βR−/− mice. IFN-α/βR−/− CD46+/− animals were selected and used for immunization experiments. Six-week-old CD46+/− IFN-α/βR−/− mice were inoculated intraperitoneally with 5 × 106 TCID50 of MV-HIV recombinant viruses. Control mice were immunized with 5 × 106 TCID50 of empty MV vector (EdB-tag MV). Mice were euthanized at 7 days or 1 month postinfection. Spleen and whole blood were collected.
Two colony-bred rhesus macaques (i.e., Macaca mulatta) that were seronegative for simian type D retrovirus, simian T-cell lymphotropic virus, simian immunodeficiency virus, and MV were vaccinated subcutaneously with 104 TCID50 of MV vaccine (Rouvax; Aventis Pasteur, Paris, France). The animals were boosted 1 year later with two injections of 5 × 106 TCID50 of MV2-gp140 recombinant virus separated by a 1-month interval. Blood samples were collected at different time points, and the samples were tested for the presence of anti-MV and anti-HIV antibodies.
Characterization of humoral immune responses.
Sera were collected 1 month after immunization and were heat inactivated. Anti-MV (Trinity Biotech) and anti-HIV Env (Sanofi Diagnostic Pasteur; Bio-Rad) antibodies were detected by using commercial enzyme-linked immunosorbent assay (ELISA) kits. An anti-mouse antibody-HRP conjugate (Amersham) was used as the secondary antibody. Titers were determined by limiting dilutions and calculated as the highest dilution of serum giving twice the absorbance of a 1/100 dilution of a mixture of control sera. The same ELISA kits were used for sera from macaque monkeys. An anti-monkey IgG secondary antibody was used to detect anti-HIV antibodies. Anti-MV antibodies were detected with an anti-human IgG in order to be able to calibrate the assay with standards supplied in the MV ELISA kit. Values were expressed in milli-international units per milliliter. A mixture of five samples from negative monkeys was used as the negative control. The titer of anti-ELDKWAS antibodies was determined by ELISA by using 96-well NeutrAvidin plates (Pierce) coated with the ELDKWAS biotinylated peptide (Neosystem; 5 μg/ml in 2 M NaHCO3-2 M Na2CO3 · H2O [pH 9.6]). Sera from mice immunized with standard MV were used as negative controls. Peptide-bound antibodies were detected by using an anti-mouse antibody-HRP conjugate.
HIV-1 neutralization assays.
Seroneutralization was tested against SHIV89.6p (A. M. Aubertin, Université Louis Pasteur, Strasbourg, France); the clade B HIV-1 primary isolates Bx08 (8; C. Moog, INSERM, Strasbourg, H. Fleury, Bordeaux, France) and 92US660, 92US714, and 92HT593 (NIH-AIDS Research & Reference Reagent Program); and the clade A primary isolate 3253 (G. Pancino, Institut Pasteur, Paris, France). These viruses were propagated on phytohemagglutinin-stimulated human peripheral blood mononuclear cells as described elsewhere (8). As a control virus that should not be neutralized by the test sera, we used an HIV-1LAI (vesicular stomatitis virus [VSV]) pseudotype in which HIV Env is replaced by VSV-G Env (7). HIV-1 neutralization assays were performed by using the P4-CCR5 indicator cell line (13). P4-CCR5 cells were seeded into 96-well plates (20,000 cells per well) and then incubated at 37°C in DMEM-10% FCS for 24 h. The medium was replaced with 50 μl of DMEM-10% FCS-DEAE dextran (10 μg/ml), and the cells were incubated at 37°C for 45 min. Viruses (2 to 5 ng of p24) were incubated with serial serum dilutions in 50 μl of culture medium at 37°C for 45 min, and the virus-serum mixtures were added to the cells in triplicate. After 48 h of incubation, the β-galactosidase activity was measured by using a chemiluminescence reporter gene assay (Roche). The mean percent neutralization for each serum dilution tested was calculated in comparison with the value recorded in wells containing control serum from nonimmunized mice at the same dilution.
To determine whether the additional ELDKWAS epitope elicited specific antibodies important for neutralization, we performed an antibody-ELDKWAS peptide competition assay. Sera from immunized mice were diluted in culture medium and preincubated for 1 h at room temperature with increasing concentrations (30 to 100 μg/ml) of ELDKWAS peptide (Neosystem). Neutralization assays were then performed as described above, and values were compared to those obtained without serum-peptide preincubation. An unrelated SIV Nef peptide was included as a negative control. To control that the ELDKWAS peptide did not directly alter the viruses, infection was also performed in the presence of increasing doses of peptide without preincubation with mice sera. Competition between 2F5 (25 μg/ml) and ELDKWAS peptide was used as a positive control.
Cellular immune responses.
Frozen splenocytes from immunized mice were thawed 18 h before functional assays and incubated in RPMI, 10% FCS, and 10 U of recombinant human interleukin-2 (rh-IL-2; Boehringer Mannheim). Their capacity to secrete IFN-γ upon specific stimulation was tested by enzyme-linked immunospot (ELISPOT) assays and flow cytometry. Cells were stimulated (18 to 36 h) by concanavalin A (5 μg/ml; Sigma) as a positive control, RPMI-IL-2 (10 U/ml) as a negative control, HIV-1 gp120 protein (1 μg/ml; AbCys), bovine serum albumin (BSA; 1 μg/ml; Sigma), or 5 × 105 TCID50 of EdB-tag MV. For ELISPOT assays, Multiscreen-HA 96-well plates were coated overnight at 4°C with 6 μg of anti-mouse IFN-γ (R4-6A2; Pharmingen)/ml in phosphate-buffered saline (PBS), washed, and then incubated with 100 μl of RPMI and 10% FCS for 1 h at 37°C. The medium was replaced by 100 μl of cell suspension (5 × 105 splenocytes per well in duplicate) and 100 μl of stimulating agent. After 2 h at 37°C, heated-FCS (10%) was added, and the plates were incubated for 18 to 36 h at 37°C. After a washing step, biotinylated anti-mouse IFN-γ antibody (XMG1.2; Pharmingen) was added (100 μl; 4 μg/ml in PBS-0.1% FCS), and the plates were incubated for 2 h at room temperature. Streptravidin-alkaline phosphatase conjugate (Roche) was used as secondary step. Spots were developed with BCIP/NBT (Promega) and counted (ELISpot Reader; Bio-Sys).
For flow cytometry assays, 5 × 105 splenocytes in 100 μl of RPMI medium were incubated in V-bottom 96-well plates with either 1 μg of HIV-1 gp120 (AbCys)/ml or 5 × 105 TCID50 of EdB-tag virus diluted in 100 μl of RPMI and 10 U of IL-2/ml. Control cells were incubated with RPMI with 10 U of IL-2/ml alone. After 2 h at 37°C, FCS was added (10%), and the plates were incubated overnight at 37°C. The medium was then replaced by 150 μl of RPMI and 10% FCS containing 10 U of rh-IL-2 and 10 μg of brefeldin A (Sigma)/ml. Cells were incubated for 4 h at 37°C, harvested, stained with anti-mouse CD8-APC (Pharmingen) and anti-mouse CD4-CyCr (Pharmingen) for 20 min at room temperature, washed with PBS-BSA (0.5%), and then fixed for 5 min at 37°C in CytoFix (Pharmingen). Cells were treated with 0.1% saponin (Sigma) in PBS-BSA (0.5%) and incubated for 30 min at room temperature with anti-mouse IFN-γ-PE (Pharmingen). Samples were analyzed by using a FACSCalibur cytometer (Becton Dickinson). The data were analyzed by using CellQuest software.
RESULTS
Recombinant MV expresses EnvHIV89.6 glycoproteins and replicates efficiently.
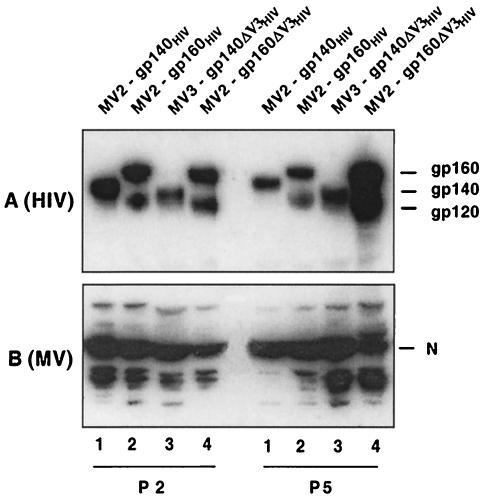
The anchored (gp160) and soluble (gp140) forms of the HIV Env glycoprotein (strain SHIV89.6p), with or without deletion of the V3 loop and insertion of an additional ELDKWAS epitope, were inserted into one of the ATU of the p(+)MV vector (Fig. 1). Recombinant viruses MV2-gp140, MV2-gp160, MV3-gp140ΔV3, and MV2-gp160ΔV3 were obtained after transfection of the plasmids into the 293-3-46 helper cell line and propagation in Vero cells [MV2 and MV3 refer to the site of insertion, at position 2 or 3 of the p(+)MV vector, respectively, of the EnvHIV89.6 construction]. Expression of the EnvHIV89.6 was analyzed by Western blotting of infected-cell lysates (Fig. 2) and immunofluorescence (not shown). The MV2-gp140 and MV2-gp160 viruses gave a high level of expression of the EnvHIV89.6 protein (Fig. 2A, lanes 1, 2, and 4). As expected, the MV2-gp160 and MV2-gp160ΔV3 viruses expressed the env gp160 precursor, as well as the cleaved gp120 protein (Fig. 2A, lanes 2 and 4). In contrast, the MV2-gp140 and MV3-gp140ΔV3 viruses expressed only the secreted, uncleaved gp140 form. The MV3-gp140ΔV3 virus expressed slightly lower levels of transgene than viruses of the MV2 series, as expected, due to the transcription gradient observed in MV expression (Fig. 2A, lane 3). Taken together, these results indicate that EnvHIV89.6 and the ΔV3 mutants were efficiently expressed and correctly matured. The recombinant MV were passaged five times on Vero cells, and the expression of the transgene was compared to that of the MV nucleoprotein. Figure 2 shows that EnvHIV89.6 expression was similar for passages 2 and 5, confirming the stability of transgene expression in this system.
FIG. 2.
Expression of HIV-189.6 envelope glycoproteins in recombinant MVs. gp160, gp140, and MV nucleoprotein (N) detected in lysates of Vero cells infected with MV-EnvHIV viruses. (A) HIV proteins were probed with mouse monoclonal anti-HIV gp120 antibody; (B) MV N protein was probed with monoclonal anti-MV N antibody.
The growth of MV-EnvHIV89.6 recombinant viruses was analyzed on Vero cells by using an MOI of 0.01 (Fig. 3). The growth of recombinant viruses was only slightly delayed compared to that of the standard EdB-tag MV rescued from p+(MV). Viruses expressing the secreted gp140 were less affected than viruses expressing the anchored gp160. The gp140ΔV3 recombinant grew at the same rate as control MV. The delay observed with viruses expressing the anchored gp160 may be due to a lower replication rate, to the larger size of the transgene, or to educed MV budding because of the insertion of gp160 at the surface of the infected cells. Nevertheless, the final yield of recombinant viruses was comparable to that of control MV, and peak titers of ca. 106 to 107 TCID50/ml were obtained routinely.
FIG. 3.
Growth kinetics of recombinant MV-EnvHIV89.6 viruses on Vero cells. Vero cells were infected with EdB-tag MV and MV- EnvHIV89.6 viruses at an MOI of 0.01. At each time point, cells were collected, and cell-associated virus titers were determined by using the TCID50 method on Vero cells.
Induction of humoral immune response to recombinant MV in susceptible mice.
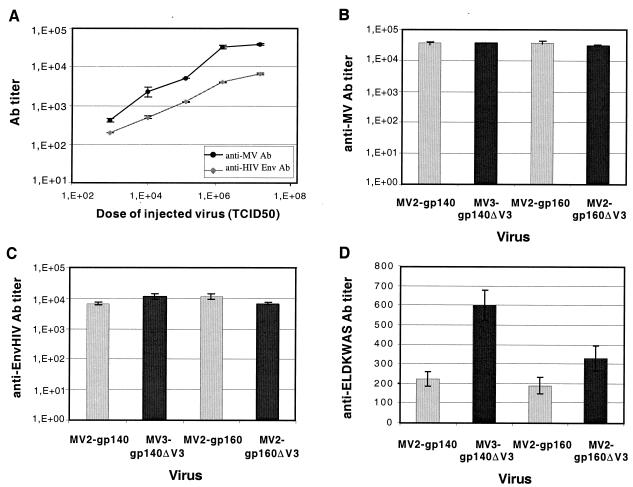
The immunogenicity of MV-EnvHIV89.6 viruses was tested in genetically modified mice expressing the human CD46 MV receptor and lacking the IFN-α/β receptor. Increasing doses of MV2-gp160 virus (103 to 107 TCID50) were tested in five groups of three mice. ELISAs were performed to detect the presence of anti-MV and anti-HIV antibodies in sera collected 1 month after immunization (Fig. 4A). Both anti-MV and anti-HIV antibody titers increased when the dose of recombinant MV increased. Since high anti-MV titers were obtained between 106 and 107 TCID50, mice were immunized with 5 × 106 TCID50 in all further experiments. At this dose, anti-MV antibody titers were sixfold higher than anti-HIV titers. One should keep in mind that immunization was against HIV Env only, whereas all MV proteins were expressed during infection. To compare the immunogenicity of the different EnvHIV89.6 constructs, four groups of six mice were inoculated intraperitoneally with various MV-EnvHIV89.6 viruses (Fig. 4B and C). All mice responded to MV (mean anti-MV titer = 5 × 104) and to HIV Env (mean anti-HIV titer = 8 × 103). No difference in anti-MV or anti-HIV titers was observed between the four constructs tested. Interestingly, expression from ATU position 2 or 3 of the MV vector did not affect the antibody response. Control mice that were immunized with empty MV (EdB-tag MV) raised similar anti-MV antibody titers (5 × 104) but remained negative for anti-HIV antibodies. Because the ΔV3 constructions expressed an additional ELDKWAS epitope, the antibody response against this gp41 epitope was examined separately by using a specific ELISA (Fig. 4D). The anti-ELDKWAS antibody titers were low for all of the different EnvHIV89.6 constructs. The ΔV3-ELDKWAS mutation caused only a twofold increase in anti-ELDKWAS antibody titers.
FIG. 4.
Anti-HIV and anti-MV antibody titers in IFN-α/βR−/− CD46+/− mice immunized with MV-EnvHIV89.6 viruses. (A) Anti-MV and anti-HIV antibody titers detected 28 days after injection of increasing doses of MV-gp160 (three mice per group). (B to D) Anti-MV (B), anti-HIV (C), and anti-ELDKWAS (D) antibody titers were detected in sera from animals 28 days after the injection of 5 × 106 TCID50 of MV-gp160/gp140 viruses (░⃞) and MV-gp160/gp140 ΔV3-ELDKWAS (▪) viruses (six mice per group). The results are expressed as the mean values ± the standard deviation.
MV-EnvHIV89.6 viruses induce neutralizing anti-HIV antibodies.
The capacity of sera from MV-EnvHIV89.6-immunized mice to neutralize either homologous SHIV89.6p virus or various heterologous primary HIV-1 isolates was tested by using a single-cycle virus infectivity assay on P4-CCR5 indicator cells (13). P4-CCR5 cells express the CD4, CXCR4, and CCR5 HIV-1 receptors and have been stably transfected with an HIV long terminal repeat LacZ construct. Therefore, these cells are susceptible to HIV-1 isolates and express β-galactosidase upon infection. The seroneutralization assay was validated by using a combination of anti-HIV immunoglobulin (HIVIG; 2.5 mg/ml) and MAbs (2F5 and 2G12, 25 μg/ml each) previously shown to synergistically neutralize primary HIV isolates (44). We also used sera from two infected patients at the same dilution previously shown to neutralize 90% of the infectivity of the Bx08 virus in a standard neutralization assay on human peripheral blood mononuclear cells (8). To take into account the possibility of nonspecific inhibition of HIV-1 infection by mice sera, the 100% level of infectivity for each HIV-1 isolate was determined in the presence of the same dilution of control sera from nonimmunized mice. Thus, the neutralizing activity (Table 1) is expressed as the percentage of reduction of infection obtained in the presence of the serum tested compared to that obtained with a negative control serum used at the same dilution. Sera from HIV-negative individuals were used as negative controls for MAb and human HIV sera, and sera from nonimmunized mice were used as controls for mice sera.
TABLE 1.
Neutralization of HIV-1 primary heterologous isolates by sera from MV-EnvHIV89.6-immunized micea
| Virus isolate (subtype) | % Reduction in infection in: |
Positive controls |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mouse serum (1:30) |
Mouse serum (1:60) |
MAbb (2F5/2G12/HIV-IG) | Human HIV serumc |
||||||||||
| MV | MV2 gp140 | MV3 gp140 ΔV3 | MV2 gp160 | MV2 gp160 ΔV3 | MV | MV2 gp140 | MV3 gp140 ΔV3 | MV2 gp160 | MV2 gp160 ΔV3 | 4 (1:50) | 33 (1:35) | ||
| SHIV 89.6 | <10 | 86 | >90 | >90 | >90 | <10 | 55 | 85 | 82 | >90 | >90 | 80 | 85 |
| Bx08 (B) | <10 | 36 | 76 | 80 | >90 | <10 | <10 | 55 | 50 | 73 | >90 | >90 | >90 |
| 92 US 660 (B) | <10 | 20 | 50 | 75 | 84 | <10 | <10 | 20 | 35 | 68 | >90 | 83 | 80 |
| 92 US 714 (B) | <10 | <10 | 33 | 71 | >90 | <10 | <10 | 20 | 45 | 74 | >90 | >90 | >90 |
| 92 HT 593 (B) | <10 | 25 | 45 | 38 | 66 | <10 | <10 | 25 | 20 | 60 | >90 | >90 | 68 |
| 3253 (A) | <10 | <10 | 43 | 76 | 80 | <10 | <10 | <10 | 20 | 70 | >90 | 65 | 45 |
| HIV-1LAI (VSV) | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
Sera were evaluated for neutralizing antibodies at two dilutions. Values are the percent reduction in infection of primary HIV isolates on P4-CCR5 cells in the presence of sera from vaccinated mice. The 100% infection value was determined for each HIV isolate in the presence of sera from nonimmunized mice. Boldface numbers represent a neutralization percentage of >80%. Determinations were made in triplicate for three mice per point and the standard deviations were <10%.
Mix of HIVIG (2.5 mg/ml) and MAbs 2F5 and 2G12 (25 μg/ml each).
Numbers correspond to the nomenclature used by Burrer et al. (8).
Table 1 shows that the HIVIG/2F5/2G12 combination and sera from HIV-infected patients, used as positive controls, neutralized clade B and A viruses equally well in this assay. No significant inhibition of the infection of any of the viral isolate tested was observed with sera from mice immunized with empty MV (EdB-tag), showing that anti-MV immunity did not neutralize HIV nonspecifically. An HIV-1LAI (VSV) pseudotype was used as a negative control that should not be neutralized by sera from MV-EnvHIV89.6-immunized mice. The infectivity of this VSV Env-pseudotyped HIV-1 was not inhibited by sera from MV-EnvHIV89.6-immunized mice, showing that the neutralization of HIV-1 primary isolates by these sera was due to antibodies specific for the HIV-1 envelope glycoprotein.
Serial dilutions of sera from MV-EnvHIV89.6-immunized mice were tested. The results obtained with 1:30 and 1:60 dilutions are presented in Table 1. Neutralizations with 1:120 dilutions were marginal except for the neutralization of SHIV89.6p. Antibodies induced by the four MV-EnvHIV89.6 viruses neutralized the homologous SHIV89.6p at both 1:30 and 1:60 dilutions. High neutralization activity (>90%) was observed except for sera from MV2-gp140-immunized mice. The ΔV3 mutants induced antibodies that neutralized SHIV89.6p slightly more efficiently. The gp160ΔV3 mutant elicited antibodies that neutralized 90% of infection by the SHIV89.6p at a 1:60 dilution and neutralized 50% of the infection at a 1:120 dilution. This suggests that the deletion of the V3 loop, known to contain type-specific neutralizing epitopes, had been compensated by the uncovering of other neutralizing epitopes.
The antibodies induced by the MV-EnvHIV89.6 recombinant also neutralized heterologous primary clade B isolates and a clade A virus. In each case, antibodies induced by the anchored gp160 were more neutralizing than antibodies induced by the secreted gp140. This result suggests that the gp160 protein was expressed by recombinant MV in a stable oligomeric form known to be more immunogenic. At the 1:60 dilution, none of the primary isolates could be neutralized by sera from MV2-gp140-immunized mice, whereas sera from MV2-gp160-immunized mice could still neutralize up to 50% of infectivity. The antibodies induced by the ΔV3-ELDKWAS EnvHIV89.6 neutralized heterologous viruses more efficiently than those induced by the native envelope. This was particularly striking with sera from MV2-gp160ΔV3-immunized mice at the 1:30 dilution, where neutralization reached 90% for most primary isolates tested. This neutralization was as efficient as that obtained with positive controls (human MAbs and sera).
The increased neutralizing activity of antibodies induced by the ΔV3-ELDKWAS mutants could be due either to the uncovering of the chemokine receptor binding site or to the addition of a second ELDKWAS gp41 epitope. To evaluate the role of the anti-ELDKWAS antibodies in neutralizing activity, we performed an antibody-ELDKWAS peptide competition experiment. Mice sera were preincubated with increasing concentrations of the ELDKWAS peptide (10 to 100 μg/ml) before the neutralization assay was performed (with the Bx08 clade B virus). The preincubation of viruses with peptide did not alter their infectivity. The neutralizing activity of the 2F5 MAb (100 μg/ml) was completely abolished in the presence of 100 μg of ELDKWAS peptide/ml but not in the presence of an irrelevant Nef peptide. On the other hand, no reduction of neutralization was observed when sera from mice immunized with MV2-gp140, MV2-gp160, or ΔV3-ELDKWAS mutants were incubated with the ELDKWAS peptide. Thus, the native forms of EnvHIV89.6 did not induce detectable levels of 2F5-type neutralizing antibodies, and the additional ELDKWAS epitope did not increase this level. Rather, deletion of the V3 immunodominant region has probably unmasked conserved critical epitopes on the envelope glycoprotein that induced neutralizing antibodies of broader specificity. However, grafting an additional ELDKWAS peptide in place of the V3 loop may have contributed to conformational changes of the gp120 leading to the induction of broader neutralizing antibodies.
Taken together, these results show that deleting the V3 loop of EnvHIV89.6 and expressing the construct with a MV vector allowed the induction of antibodies with cross-neutralizing activity against clade A and B HIV-1 primary isolates, after a single injection in mice.
Induction of cellular immune responses against recombinant MV.
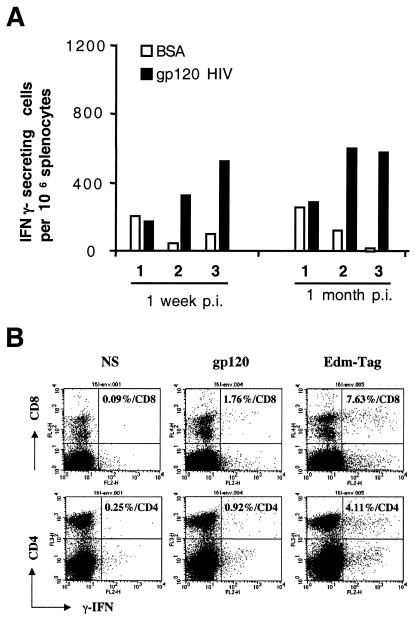
The capacity of splenocytes from vaccinated mice to secrete IFN-γ was tested by ELISPOT assays and flow cytometry. Both MV-specific and HIV-specific responses were studied in splenocytes collected 7 days or 1 month after immunization. HIV gp120 native protein was used to stimulate both CD4+ and CD8+ T cells since it has been shown that exogenous antigens can be presented by major histocompatibility complex class I molecules (32). Representative results obtained with splenocytes from mice immunized with MV2-gp160 are shown in Fig. 5. A significant number of HIV gp120-specific cells (up to 600/106 splenocytes) was detected by the ELISPOT assay 7 days or 1 month postinoculation (Fig. 5A). In a given mouse, the number of HIV gp120-specific spots corresponded to 15 to 20% of the number of MV-specific spots (not shown), indicating that MV vectors immunizes against the HIV proteins efficiently.
FIG. 5.
Characterization of MV- and HIV-specific T cells in mice immunized by MV2-gp160HIV89.6. (A) Enumeration of gp120-specific IFN-γ-ELISpots. Two groups of three mice were inoculated with 5 × 106 TCID50 of MV2-gp160 virus and then euthanized 7 days or 1 month postinoculation. ELISPOT assays were performed with splenocytes from immunized mice. Stimulation with HIV-gp120 purified protein (▪) or irrelevant BSA (□). (B) Fluorescence-activated cell sorting analysis of CD8+ (upper panel) and CD4+ (lower panel) T cells producing IFN-γ in mice splenocytes collected 7 days after immunization with 5 × 106 TCID50 of MV2-gp160. Ex vivo splenocytes were stimulated either with medium (left panel), HIV gp120 (middle panel), or EdB-tag MV (right panel). The percentages are given according to the total CD8+ and CD4+ counts.
The phenotype of MV- and HIVgp120-specific cells collected 7 days after immunization and stimulated in vitro with exogenous HIV Env protein as described above was determined by three-color cytofluorometry (Fig. 5B). The frequency of HIV gp120-specific T cells (middle panel) in the CD8+ and CD4+ subsets was 1.76% (mean for three mice = 1.69%) and 0.92% (mean for three mice = 0.76%), respectively. The frequency of MV-specific T cells in CD8+ and CD4+ subsets (right panel) was 7.63% (mean for three mice = 7.03%) and 4.11% (mean for three mice = 3.50%), respectively. These frequencies are very close to those obtained by ELISPOT since CD8+ and CD4+ cells correspond to 6 to 10% and 15 to 20%, respectively, of total splenocytes of CD46+/− IFN-α/βR−/− mice (not shown). These results show that a single inoculation of MV2-gp160 virus induced high levels of HIVgp120- and MV-specific CD8+ and CD4+ lymphocytes.
Boosting the anti-HIV response in animals with preexisting anti-MV immunity.
We first tested the possibility of boosting the anti-HIV response by performing a second injection of recombinant MV. Mice immunized with 5 × 106 TCID50 of MV2-gp140 recombinant virus were boosted 1 month after the first injection with a second injection of the same recombinant virus at the same dose. The mean anti-MV and anti-HIV antibody titers at the time of boosting were 5 × 104 and 8 × 103, respectively. These titers increased 1 month after the boosting to, respectively, 5 × 105 and 5 × 104. Thus, anti-MV and HIV responses can be boosted 10 times by a second injection of the same dose of vaccine given 1 month after the first one.
We then tested the ability of recombinant MV to induce anti-HIV antibodies in mice and monkeys in the presence of preexisting anti-MV immunity. Mice (three mice per point) were first immunized with 105 TCID50 of EdB-tag MV (without an HIV insert). High levels of anti-MV antibodies were induced (Fig. 6A). The titer decreased slightly after 2 months and remained stable for the following 9 months. The mice were then inoculated with 5 × 106 TCID50 of MV2-gp140 and boosted with the same dose 1 month later. The titer of anti-MV antibodies was increased 100 times, and high titers of anti-HIV antibodies (5 × 104) were induced. These titers were similar to those obtained after the immunization of naive animals with two injections.
FIG. 6.
Anti-MV and anti-HIV antibody titers in mice and macaques immunized with MV2-gp140HIV89.6 virus months after MV priming. (A) Mice (three per group) were vaccinated with 105 TCID50 of EdB-tag MV and then inoculated twice with 5 × 106 TCID50 of MV2-gp140 virus as indicated (arrows). (B) Cynomolgus macaques (animals 432 and 404) were vaccinated with Rouvax and were then inoculated twice with 5 × 106 TCID50 of MV2-gp140 virus as indicated (arrows).
The same experiment was performed with rhesus macaques (Fig. 6B). Two macaques were immunized with a standard dose (104 TCID50) of MV vaccine (Rouvax). High anti-MV antibody levels were induced and remained stable for 1 year. Macaques were then inoculated with 5 × 106 TCID50 of MV2-gp140 twice at 1-month intervals. Anti-MV titers were increased 150-fold after the first injection of MV-HIV, whereas the second injection had no or little effect. Anti-HIV antibodies were induced by the first MV2-gp140 injection despite the presence of preexisting anti-MV immunity. One month after the second MV2-gp140 injection, the anti-HIV antibody level had increased ∼10-fold and had reached titers similar to those obtained in mice. This level remained stable for the following 5 months.
Taken together, these results show that the presence of preexisting MV immunity in mice and nonhuman primates did not prevent the induction of antibodies to HIV Env protein after immunization by MV-EnvHIV89.6 recombinant virus.
DISCUSSION
The main goal of the present study was to test the ability of live attenuated MV-EnvHIV89.6 recombinant viruses to induce anti-EnvHIV89.6 immune responses in transgenic mice susceptible to MV. Our results show that such recombinants are genetically stable, express the HIV Env protein at high levels, and induce high titers of antibodies to both MV and the HIV Env constructs. The anti-HIV antibodies titers were ca. 15 to 20% of those of the anti-MV antibodies. This corresponds roughly to the ratio of HIV to MV proteins expressed by the recombinant viruses. A high level of MV- and HIV-specific CD8+ and CD4+ cells was also induced. As much as 7% of the total CD8+ T cells and 4% of the total CD4+ T cells were MV specific, and 1.7% of the total CD8+ T cells and 0.9% of the total CD4+ T cells were HIV specific. Hence, MV-EnvHIV89.6 recombinant viruses were able to elicit humoral and cellular immune responses against both MV and HIV Env.
An important aspect of our results is that the anti-HIV antibodies induced were neutralizing for the homologous SHIV89.6p virus, as well as for several heterologous clade A and clade B HIV-1 primary isolates. Antibodies induced by MV expressing the native gp140 or gp160 more efficiently neutralized homologous compared to heterologous viruses. The V3 loop deletion increased the level of antibodies neutralizing homologous SHIV89.6p, as well as heterologous primary HIV-1 isolates. The effect was more pronounced against heterologous viruses. This observation suggests that removing the V3 region may have exposed some immunological elements conserved among several HIV-1 isolates. For the neutralization of SHIV89.6p, this exposure probably compensated for the elimination of V3 loop type-specific epitopes. It was previously shown that vaccines expressing the ΔV3 mutants of HIV-1IIIB or HIV-1HIV89.6 envelope glycoprotein induced broader CD8+-T-cell activities than those induced by the wild-type counterparts (37). Hence, V3 loop deletion broadens both humoral and cellular immune responses.
Antibodies elicited by MV2-gp160ΔV3 neutralized heterologous isolates more efficiently than those induced by MV2-gp140ΔV3. Given that the neutralizing capacity of an antibody is linked to its ability to bind to the oligomeric envelope (69), the gp160ΔV3 expressed by MV appears to be a better antigenic mimic of the envelope glycoprotein trimeric complex present on virions than the gp140ΔV3 is. Even when expressed by a recombinant MV, gp140 does not seem able to form stable trimeric immunogenic complexes. Indeed, the immunogenicity of gp140 is increased after stabilization of oligomers either by an intramolecular disulfide bond (6) or by elimination of the gp120-gp41 cleavage site (5).
The broader neutralizing capacity of antibodies induced by MV2-gp160ΔV3-ELDKWAS was not due to the addition of a second ELDKWAS gp41 epitope, as shown by antibody-peptide competition experiments, but rather to the exposure of previously masked conserved neutralizing epitopes. Several groups have inserted the ELDKWAS epitope into various immunogenic molecules and failed to induce neutralizing antibodies (14, 21, 40). These studies suggest that the conformation of the grafted epitope is essential for the induction of neutralizing antibodies. A β-turn-like constraint was shown to be the most likely conformation of the ELDKWAS epitope recognized by the 2F5 neutralizing MAb (29). We inserted the short AAELDKWASAA sequence in place of the V3 loop, which is flanked by β-strands (38, 39), with the hope of mimicking the structure of the 2F5 epitope. Our results indicate that this was probably not achieved. Thus, as already suggested, the conformation of the 2F5 epitope must depend largely on flanking residues and the local conformation of the protein (83).
Several studies used variable loop deletions to reduce the immunodominant response and to expose conserved epitopes. The results of these studies were often contradictory. In some of them, deletion of the variable loops did not improve the induction of neutralizing antibodies (36, 41). This was the case when the immunogenicities of different V1-V2-, V3-, or V1-3-deleted envelopes (i.e., envelopes from which the V1-V2, V3, or V1-3 regions, respectively, had been deleted) were tested in rabbits injected with naked DNA (gp120, gp140, and gp160) (41) and in mice primed with gp160 recombinant vaccinia virus and boosted with soluble gp120 (36). In other cases, neutralizing antibodies with broad specificity were obtained after multiple injections of large amounts of soluble gp120 protein from which V1-3 had been deleted or after a DNA prime-protein boost regimen by using gp140 from which V2 had been deleted (5, 73). In our study, similar titers of antibodies able to neutralize heterologous isolates were obtained in mice after a single injection of MV-gp160 ΔV3-ELDKWAS. Several hypotheses may explain these discrepancies. First, epitope exposure and conformational flexibility of HIV-1 envelope are essential for the induction of cross-reactive neutralizing antibodies (49, 69). Hence, depending on the deleted region, the Env processing and its resulting structure, as well as epitope exposure, may be altered. For example, it was shown that deleting the V2 region of HIV-1SF162 Env did not directly increase the immunogenicity of the CD4-binding site but modified the antigenic structure of some already immunogenic regions and made additional regions immunogenic (73). Interestingly, these data suggest that the antibody composition and the relative ratio of each antibody are responsible for the differential ability of sera to neutralize HIV-1 isolates. Second, immunogenic determinants in gp120 can differ according to the virus strain (48). Actually, different HIV-1 strains were used in the different studies cited above. Third, different vaccination methods can induce different patterns of immune responses (12), especially regarding the induction of neutralizing antibodies (20). Good immunogenicity in our system is likely due to good levels of expression and maturation of HIV Env in cells infected by live recombinant MV, resulting to an efficient presentation to the immune system.
What are the advantages of MV among the existing viral vector systems developed for HIV immunization? Practical and logistics aspects have already been exposed. In addition, replicating live attenuated MV has the advantageous capacity to induce long-lasting immunity (27). Moreover, MV and HIV have several properties in common, including the fact that they both infect monocytes, macrophages, and dendritic cells (22, 28). Therefore, an MV vector that targets the HIV proteins in the same compartment as HIV itself may provide advantages, particularly in the induction of “danger signals” (46). This is considered to be one of the reasons for the protection induced by attenuated strains of pathogenic viruses. Indeed, live attenuated SIV provides strong protection against pathogenic SIV (18, 31), even if the correlates of protection remain unclear in this model (16). A live attenuated poliovirus vaccine has been engineered to express foreign genes (2, 62), and a recombinant Sabin poliovirus/SIV protected macaques from a virulent SIV challenge (17). However, poliovirus is less genetically stable than MV, and reversion to pathogenicity may occur (35). On the contrary, the Edmonston-derived live attenuated MV strains differ from the Edmonston wild-type isolate by at least 45 coding mutations (55) and from wild-type field isolates by even more mutations, making reversion almost impossible. VSV, another negative-strand RNA virus, has been made into a live attenuated vector expressing HIV proteins and is able to protect macaques from a SHIV challenge (66). However, whether VSV is safe for humans is unknown at this point, whereas live MV vaccines have a very long safety track record.
The presence of anti-MV immunity in nearly the entire adult human population would seem to restrict the use of MV recombinants to infants, an already worthy goal in any event. However, several studies showed that revaccinating previously immunized individuals results in a boost of anti-MV antibodies, suggesting that the attenuated live vaccine replicated and expressed its proteins in spite of preexisting immunity (19). Under such circumstances one might hope to be able to vaccinate adults against a foreign antigen with an MV recombinant. Indeed, our results demonstrate, in both mice and macaques, that high levels of anti-HIV neutralizing antibodies can be obtained in the presence of preexisting anti-MV immunity.
Various priming-boosting regimens, with combinations of naked DNA and viral vectors such as modified vaccinia virus Ankara (1) or adenovirus (71) in macaques, led to a good containment of viremia and CD4+ loss after a challenge with pathogenic SHIV89.6p. In the present study, we show that a single injection of MV-EnvHIV89.6 was able to induce humoral and cellular responses. It will be important to determine the ability of these recombinant MV-EnvHIV89.6 viruses, alone or in combination with recombinant MVs expressing other SHIV89.6p genes, to protect macaques against a SHIV89.6p challenge.
In conclusion, our study shows that a single injection of live attenuated MV vaccine expressing a gp160HIV89.6 with a deletion of V3 induces antibodies in mice that neutralize several primary HIV-1 isolates, as well as high levels of specific CD4+- and CD8+-T-cell responses. Anti-HIV antibodies were induced even in animals with preexisting anti-MV immunity. Although the correlates of immune protection against HIV-1 infection are still not completely understood, a preventive immunization should, ideally, induce both specific T lymphocytes and antibodies that neutralize primary isolates. The results presented here indicate that this might be possible by using an already existing live MV vaccine as a vector for expressing HIV proteins.
Acknowledgments
We thank Martin Billeter for kindly providing the p+(MV) plasmids and the MV rescue system, as well as for support and helpful discussions. We thank Hussein Naim for advice and helpful discussions. We also thank L. Sartorius for critical reading of the manuscript.
C.L. received a fellowship from the Agence Nationale pour la Recherche contre le SIDA (ANRS), and L.M. was supported by National Institute of Health (NIH) grant AI46007. This study was supported by institutional grants from the Pasteur Institute (PTR34) and CNRS and by grants from the ANRS (02004) and the NIH (AI46007).
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 2.Andino, R., D. Silvera, S. D. Suggett, P. L. Achacoso, C. J. Miller, D. Baltimore, and M. B. Feinberg. 1994. Engineering poliovirus as a vaccine vector for the expression of diverse antigens. Science 265:1448-1451. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 4.Baba, T. W., V. Liska, A. Khimani, N. Ray, P. Dailey, D. Penninck, R. Bronson, M. Greene, M. HM, M. LN, and R. Rprecht. 1999. Live attenuated, mulitiply deleted simian immunodeficiency viruses causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. [DOI] [PubMed] [Google Scholar]
- 5.Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borman, A. M., C. Quillent, P. Charneau, C. Dauguet, and F. Clavel. 1995. Human immunodeficiency virus type 1 Vif− mutant particles from restrictive cells: role of Vif in correct particle assembly and infectivity. J. Virol. 69:2058-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrer, R., D. Salmon-Ceron, S. Richert, G. Pancino, G. Spiridon, S. Haessig, V. Roques, F. Barre-Sinoussi, A. M. Aubertin, and C. Moog. 2001. Immunoglobulin G (IgG) and IgA, but also nonantibody factors, account for in vitro neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by serum and plasma of HIV-infected patients. J. Virol. 75:5421-5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton, D. 2002. Antibodies, viruses, and vaccines. Nat. Rev. Immunol. 2:706-713. [DOI] [PubMed] [Google Scholar]
- 10.Burton, D. 1997. A vaccine for HIV type 1: the antibody perspective. Proc. Natl. Acad. Sci. USA 94:10018-10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calain, P., and L. Roux. 1993. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering. RNA J. Virol. 67:4822-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charneau, P., G. Mirambeau, P. Roux, S. Paulous, H. Buc, and F. Clavel. 1994. HIV-1 reverse transcription: a termination step at the center of the genome. J. Mol. Biol. 241:651-662. [DOI] [PubMed] [Google Scholar]
- 14.Coeffier, E., J. Clement, V. Cussac, N. Khodaei-Boorane, M. Jehanno, M. Rojas, A. Dridi, M. Latour, R. El Habib, F. Barré-Sinoussi, M. Hofnung, and C. Leclerc. 2001. Antigenicity and immunogenicity of the HIV-1 gp41 epitope ELDKWA inserted into permissive sites of the MalE protein. Vaccine 19:684-693. [DOI] [PubMed] [Google Scholar]
- 15.Collman, R., J. W. Balliet, S. A. Gregory, H. Friedman, D. L. Kolson, N. Nathanson, and A. Srinivasan. 1992. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J. Virol. 66:7517-7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connor, R. I., D. C. Montefiori, J. M. Binley, J. P. Moore, S. Bonhoeffer, A. Gettie, E. A. Fenamore, K. E. Sheridan, D. D. Ho, P. J. Dailey, and P. A. Marx. 1998. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Virol. 72:7501-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crotty, S., C. J. Miller, B. L. Lohman, M. R. Neagu, L. Compton, D. Lu, F. X. Lu, L. Fritts, J. D. Lifson, and R. Andino. 2001. Protection against simian immunodeficiency virus vaginal challenge by using Sabin poliovirus vectors. J. Virol. 75:7435-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel, M., F. Kirchhoff, S. Czajak, P. Sehgal, and R. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 19.Dilraj, A., F. T. Cutts, J. F. de Castro, J. G. Wheeler, D. Brown, C. Roth, H. M. Coovadia, and J. V. Bennett. 2000. Response to different measles vaccine strains given by aerosol and subcutaneous routes to schoolchildren: a randomised trial. Lancet 355:798-803. [DOI] [PubMed] [Google Scholar]
- 20.Earl, P. L., L. S. Wyatt, D. C. Montefiori, M. Bilska, R. Woodward, P. D. Markham, J. D. Malley, T. U. Vogel, T. M. Allen, D. I. Watkins, N. Miller, and B. Moss. 2002. Comparison of vaccine strategies using recombinant env-gag-pol MVA with or without an oligomeric Env protein boost in the SHIV rhesus macaque model. Virology 294:270-281. [DOI] [PubMed] [Google Scholar]
- 21.Eckhart, L., W. Raffelberger, B. Ferko, A. Klima, M. Purtscher, H. Katinger, and F. Rüker. 1996. Immunogenic presentation of a conserved gp41 epitope of human immunodeficiency virus type 1 on recombinant surface antigen of hepatitis B virus. J. Gen. Virol. 77:2001-2008. [DOI] [PubMed] [Google Scholar]
- 22.Esolen, L. M., B. J. Ward, T. R. Moench, and D. E. Griffin. 1993. Infection of monocytes during measles. J. Infect. Dis. 168:47-52. [DOI] [PubMed] [Google Scholar]
- 23.Etemad-Moghadam, B., G. B. Karlsson, M. Halloran, Y. Sun, D. Schenten, M. Fernandes, N. L. Letvin, and J. Sodroski. 1998. Characterization of simian-human immunodeficiency virus envelope glycoprotein epitopes recognized by neutralizing antibodies from infected monkeys. J. Virol. 72:8437-8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrantelli, F., and R. M. Ruprecht. 2002. Neutralizing antibodies against HIV—back in the major leagues? Curr. Opin. Immunol. 14:495-502. [DOI] [PubMed] [Google Scholar]
- 25.Fouts, T., K. Godfrey, K. Bobb, D. Montefiori, C. V. Hanson, V. S. Kalyanaraman, A. DeVico, and R. Pal. 2002. Crosslinked HIV-1 envelope-CD4 receptor complexes elicit broadly cross-reactive neutralizing antibodies in rhesus macaques. Proc. Natl. Acad. Sci. USA 99:11842-11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilleman, M. 2002. Current overview of the pathogenesis and prophylaxis of measles with focus on practical implications. Vaccine 20:651-665. [DOI] [PubMed] [Google Scholar]
- 27.Hilleman, M. R. 2002. Overview: cause and prevention in biowarfare and bioterrorism. Vaccine 20:3055-3067. [DOI] [PubMed] [Google Scholar]
- 28.Hilleman, M. R. 1994. Vaccinology, immunology, and comparative pathogenesis of measles in the quest for a preventive against AIDS. AIDS Res. Hum. Retrovir. 10:10-12. [DOI] [PubMed]
- 29.Ho, J., K. MacDonald, and B. Barber. 2002. Construction of recombinant targeting immunogens incorporating an HIV-1 neutralizing epitope into sites of differing conformational constraint. Vaccine 20:1169-1180. [DOI] [PubMed] [Google Scholar]
- 30.Jeffs, S. A., C. Shotton, P. Balfe, and J. A. McKeating. 2002. Truncated gp120 envelope glycoprotein of human immunodeficiency virus 1 elicits a broadly reactive neutralizing immune response. J. Gen. Virol. 83:2723-2732. [DOI] [PubMed] [Google Scholar]
- 31.Johnson, R. P., J. D. Lifson, S. C. Czajak, K. S. Cole, K. H. Manson, R. Glickman, J. Yang, D. C. Montefiori, R. Montelaro, M. S. Wyand, and R. C. Desrosiers. 1999. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J. Virol. 73:4952-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jondal, M., R. Schirmbeck, and J. Reinmann. 1996. MHC class I-restricted CTL responses to exogenous antigens. Immunity 5:295-302. [DOI] [PubMed] [Google Scholar]
- 33.Kärber, G. 1931. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmak. 162:480-483. [Google Scholar]
- 34.Karlsson, G. B., M. Halloran, J. Li, I. W. Park, R. Gomila, K. A. Reimann, M. K. Axthelm, S. A. Iliff, N. L. Letvin, and J. Sodroski. 1997. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J. Virol. 71:4218-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kew, O., V. Morris-Glasgow, M. Landaverde, C. Burns, J. Shaw, Z. Garib, J. Andre, E. Blackman, C. J. Freeman, J. Jorba, R. Sutter, G. Tambini, L. Venczel, C. Pedreira, F. Laender, H. Shimizu, T. Yoneyama, T. Miyamura, H. Van der Avoort, O. M. S., D. Kilpatrick, S. Cochi, M. Pallansch, and C. De Quadros. 2002. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 296:356-359. [DOI] [PubMed] [Google Scholar]
- 36.Kim, Y. B., D. P. Han, C. Cao, and M. W. Cho. 2003. Immunogenicity and ability of variable loop-deleted human immunodeficiency virus type 1 envelope glycoproteins to elicit neutralizing antibodies. Virology 305:124-137. [DOI] [PubMed] [Google Scholar]
- 37.Kiszka, I., D. Kmieciak, J. Gzyl, T. Naito, E. Bolesta, A. Sieron, S. P. Singh, A. Srinivasan, G. Trinchieri, Y. Kaneko, and D. Kozbor. 2002. Effect of the V3 loop deletion of envelope glycoprotein on cellular responses and protection against challenge with recombinant vaccinia virus expressing gp160 of primary human immunodeficiency virus type 1 isolates. J. Virol. 76:4222-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwong, P. D., R. Wyatt, S. Majeed, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 2000. Structures of HIV-1 gp120 envelope glycoproteins from laboratory-adapted and primary isolates. Struct. Fold Des. 8:1329-1339. [DOI] [PubMed] [Google Scholar]
- 39.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang, X., S. Munshi, J. Shendure, Mark, M. Davies, D. Freed, D. Montefiori, and J. Shiver. 1999. Epitope insertion into variable loops of HIV-1 gp120 as a potential means to improve immunogenicity of viral envelope protein. Vaccine 17:2862-2872. [DOI] [PubMed] [Google Scholar]
- 41.Lu, S., R. Wyatt, J. F. Richmond, F. Mustafa, S. Wang, J. Weng, D. C. Montefiori, J. Sodroski, and H. L. Robinson. 1998. Immunogenicity of DNA vaccines expressing human immunodeficiency virus type 1 envelope glycoprotein with and without deletions in the V1/2 and V3 regions. AIDS Res. Hum. Retrovir. 14:151-155. [DOI] [PubMed] [Google Scholar]
- 42.Malenbaum, S. E., D. Yang, L. Cavacini, M. Posner, J. Robinson, and C. Cheng-Mayer. 2000. The N-terminal V3 loop glycan modulates the interaction of clade A and B human immunodeficiency virus type 1 envelopes with CD4 and chemokine receptors. J. Virol. 74:11008-11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mascola, J. R. 2002. Passive transfer studies to elucidate the role of antibody-mediated protection against HIV-1. Vaccine 20:1922-1925. [DOI] [PubMed] [Google Scholar]
- 44.Mascola, J. R., M. K. Louder, T. C. VanCott, C. V. Sapan, J. S. Lambert, L. R. Muenz, B. Bunow, D. L. Birx, and M. L. Robb. 1997. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J. Virol. 71:7198-7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mascola, J. R., and G. J. Nabel. 2001. Vaccines for the prevention of HIV-1 disease. Curr. Opin. Immunol. 13:489-495. [DOI] [PubMed] [Google Scholar]
- 46.Matzinger, P. 2002. The danger model: a renewed sense of self. Science 296:301-305. [DOI] [PubMed] [Google Scholar]
- 47.Moore, J. P., Y. Cao, D. D. Ho, and R. A. Koup. 1994. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J. Virol. 68:5142-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore, J. P., P. W. Parren, and D. R. Burton. 2001. Genetic subtypes, humoral immunity, and human immunodeficiency virus type 1 vaccine development. J. Virol. 75:5721-5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moulard, M., S. K. Phogat, Y. Shu, A. F. Labrijn, X. Xiao, J. M. Binley, M. Y. Zhang, I. A. Sidorov, C. C. Broder, J. Robinson, P. W. Parren, D. R. Burton, and D. S. Dimitrov. 2002. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc. Natl. Acad. Sci. USA 99:6913-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mrkic, B., J. Pavlovic, T. Rulicke, P. Volpe, C. J. Buchholz, D. Hourcade, J. P. Atkinson, A. Aguzzi, and R. Cattaneo. 1998. Measles virus spread and pathogenesis in genetically modified mice. J. Virol. 72:7420-7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 52.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Ruker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palker, T. J., M. E. Clark, A. J. Langlois, T. J. Matthews, K. J. Weinhold, R. R. Randall, D. P. Bolognesi, and B. F. Haynes. 1988. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc. Natl. Acad. Sci. USA 85:1932-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parks, C. L., R. A. Lerch, P. Walpita, M. S. Sidhu, and S. A. Udem. 1999. Enhanced measles virus cDNA rescue and gene expression after heat shock. J. Virol. 73:3560-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parks, C. L., R. A. Lerch, P. Walpita, H. P. Wang, M. S. Sidhu, and S. A. Udem. 2001. Comparison of predicted amino acid sequences of measles virus strains in the Edmonston vaccine lineage. J. Virol. 75:910-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parren, P. W., D. R. Burton, and Q. J. Sattentau. 1997. HIV-1 antibody—debris or virion? Nat. Med. 3:366-367. [DOI] [PubMed] [Google Scholar]
- 57.Parren, P. W., M. C. Gauduin, R. A. Koup, P. Poignard, P. Fisicaro, D. R. Burton, and Q. J. Sattentau. 1997. Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design. Immunol. Lett. 57:105-112. [DOI] [PubMed] [Google Scholar]
- 58.Parren, P. W., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13:S137-S162. [PubMed] [Google Scholar]
- 59.Pilgrim, A. K., G. Pantaleo, O. J. Cohen, L. M. Fink, J. Y. Zhou, J. T. Zhou, D. P. Bolognesi, A. S. Fauci, and D. C. Montefiori. 1997. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J. Infect. Dis. 176:924-932. [DOI] [PubMed] [Google Scholar]
- 60.Poignard, P., P. J. Klasse, and Q. J. Sattentau. 1996. Antibody neutralization of HIV-1. Immunol. Today 17:239-246. [DOI] [PubMed] [Google Scholar]
- 61.Poignard, P., R. Sabbe, G. R. Picchio, M. Wang, R. J. Gulizia, H. Katinger, P. W. Parren, D. E. Mosier, and D. R. Burton. 1999. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity 10:431-438. [DOI] [PubMed] [Google Scholar]
- 62.Racaniello, V. R., and D. Baltimore. 1981. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science 214:916-919. [DOI] [PubMed] [Google Scholar]
- 63.Radecke, F., and M. Billeter. 1997. Reverse genetics meets the nonsegmented negative-strand RNA viruses. Rev. Med. Virol. 7:49-63. [DOI] [PubMed] [Google Scholar]
- 64.Radecke, F., P. Spielhofer, H. Schneider, K. Kaelin, M. Huber, C. Dotsch, G. Christiansen, and M. A. Billeter. 1995. Rescue of measles viruses from cloned DNA. EMBO J. 14:5773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I. W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rose, N., P. Marx, A. Luckay, D. Nixon, W. Moretto, S. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 67.Sanders, R. W., L. Schiffner, A. Master, F. Kajumo, Y. Guo, T. Dragic, J. P. Moore, and J. M. Binley. 2000. Variable-loop-deleted variants of the human immunodeficiency virus type 1 envelope glycoprotein can be stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits. J. Virol. 74:5091-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saphire, E. O., P. W. Parren, R. Pantophlet, M. B. Zwick, G. M. Morris, P. M. Rudd, R. A. Dwek, R. L. Stanfield, D. R. Burton, and I. A. Wilson. 2001. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science 293:1155-1159. [DOI] [PubMed] [Google Scholar]
- 69.Sattentau, Q. J., and J. P. Moore. 1995. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J. Exp. Med. 182:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sattentau, Q. J., M. Moulard, B. Brivet, F. Botto, J. C. Guillemot, I. Mondor, P. Poignard, and S. Ugolini. 1999. Antibody neutralization of HIV-1 and the potential for vaccine design. Immunol. Lett. 66:143-149. [DOI] [PubMed] [Google Scholar]
- 71.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 72.Singh, M., R. Cattaneo, and M. A. Billeter. 1999. A recombinant measles virus expressing hepatitis B virus surface antigen induces humoral immune responses in genetically modified mice. J. Virol. 73:4823-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Srivastava, I. K., K. VanDorsten, L. Vojtech, S. W. Barnett, and L. Stamatatos. 2003. Changes in the immunogenic properties of soluble gp140 human immunodeficiency virus envelope constructs upon partial deletion of the second hypervariable region. J. Virol. 77:2310-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thali, M., C. Furman, D. D. Ho, J. Robinson, S. Tilley, A. Pinter, and J. Sodroski. 1992. Discontinuous, conserved neutralization epitopes overlapping the CD4-binding region of human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 66:5635-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thali, M., J. P. Moore, C. Furman, M. Charles, D. D. Ho, J. Robinson, and J. Sodroski. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 67:3978-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ugolini, S., I. Mondor, P. W. Parren, D. R. Burton, S. A. Tilley, P. J. Klasse, and Q. J. Sattentau. 1997. Inhibition of virus attachment to CD4+ target cells is a major mechanism of T-cell line-adapted HIV-1 neutralization. J. Exp. Med. 186:1287-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77a.United Nations Program on HIV/AIDs (UNAIDS). 2002. Report on the global HIV/AIDS epidemic. UNAIDS, Geneva, Switzerland.
- 78.Wang, Z., T. Hangartner, L. Cornu, A. Martin, M. Zuniga, M. Billeter, and H. Naim. 2001. Recombinant measles viruses expressing heterologous antigens of mumps and simian immunodeficiency viruses. Vaccine 19:2329-2336. [DOI] [PubMed] [Google Scholar]
- 79.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 80.Wyatt, R., J. Moore, M. Accola, E. Desjardin, J. Robinson, and J. Sodroski. 1995. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J. Virol. 69:5723-5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 82.Yannoutsos, N., J. N. Ijzermans, C. Harkes, F. Bonthuis, C. Y. Zhou, D. White, R. L. Marquet, and F. Grosveld. 1996. A membrane cofactor protein transgenic mouse model for the study of discordant xenograft rejection. Genes Cells 1:409-419. (Erratum, 1: 785.) [DOI] [PubMed] [Google Scholar]
- 83.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. O. Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]