Abstract
Rationale
Serotonin and especially serotonin 2A (5-HT2A) receptor signaling are important in the etiology and treatment of schizophrenia and affective disorders. We previously reported a novel 5-HT2A receptor effector, increased transglutaminase (TGase)-catalyzed transamidation, and activation of the small G protein Rac1 in A1A1v cells, a rat embryonic cortical cell line.
Objectives
In this study, we explore the signaling pathway involved in 5-HT2A receptor-mediated Rac1 transamidation.
Methods
A1A1v cells were pretreated with pharmacological inhibitors of phospholipase C (PLC) or calmodulin (CaM), and then stimulated by the 5-HT2A receptor agonist, 2,5-dimethoxy-4-iodoamphetamine (DOI). Intracellular Ca2+ concentration and TGase-modified Rac1 transamidation were monitored. The effect of manipulation of intracellular Ca2+ by a Ca2+ ionophore or a chelating agent on Rac1 transamidation was also evaluated.
Results
In cells pretreated with a PLC inhibitor U73122, DOI-stimulated increases in the intracellular Ca2+ concentration and TGase-modified Rac1 were significantly attenuated as compared to those pretreated with U73343, an inactive analog. The membrane-permeant Ca2+ chelator, BAPTA-AM strongly reduced TGase-catalyzed Rac1 transamidation upon DOI stimulation. Conversely, the Ca2+ ionophore ionomycin, at a concentration that induced an elevation of cytosolic Ca2+ to a level comparable to cells treated with DOI, produced an increase in TGase-modified Rac1 without 5-HT2A receptor activation. Moreover, the CaM inhibitor W-7, significantly decreased Rac1 transamidation in a dose-dependent manner in DOI-treated cells.
Conclusions
These results indicate that 5-HT2A receptorcoupled PLC activation and subsequent Ca2+ and CaM signaling are necessary for TGase-catalyzed Rac1 transamidation, and an increase in intracellular Ca2+ is sufficient to induce Rac1 transamidation.
Keywords: 5-HT2A receptor, Rac1, Transglutaminase, Transamidation, Phospholipase C, Calcium, Calmodulin, Serotonin, Small G proteins, A1A1v cells, Serotonylation
Introduction
Serotonin receptor signaling, especially 5-HT2A receptor signaling, has been implicated in schizophrenia and affective disorders including anxiety, depression, and posttraumatic stress disorder (Roth 1994; Weisstaub et al. 2006). However, the nature of the mechanisms underlying these disorders and their treatments has not been elucidated, hindering the development of better treatment approaches. We previously reported a novel signaling pathway for the 5-HT2A receptor system that has the potential to be involved in these disorders. Stimulation of 5-HT2A receptors causes transamidation of Ras-related C3 botulinum toxin substrate 1 (Rac1) in A1A1v cells, a rat cortical cell line (Dai et al. 2008). However, the underlying molecular mechanisms by which the 5-HT2A receptor signaling regulates transglutaminase (TGase)-catalyzed transamidation and activation of Rac1 are still unclear. TGases are a family of enzymes that catalyze the transamidation, esterification, and deamidation of peptide-bound glutamine residues. The transamidation reaction can result in the addition of a free low-molecular weight amine or the cross-linking of a protein-bound glutamine to a protein-bound lysine residue within or between proteins.
5-HT2A receptor activation can increase inositol 1,4,5-trisphosphate (IP3) via Gq/11-mediated activation of phospholipase C (PLC) (Conn and Sanders-Bush 1984). PLC plays an important role in intracellular signal transduction by hydrolyzing phosphatidylinositol 4,5-bisphosphate (PIP2), a membrane phospholipid, and thereby generating 2 second messengers: IP3 which diffuses through the cytosol and releases Ca2+ from intracellular endoplasmic reticulum (ER) stores and diacylglycerol (DAG). It has been reported that TGase activity can be significantly enhanced in response to increased intracellular Ca2+ through IP3 generation (Zhang et al. 1998). Moreover, serotonin-mediated activation of small G protein Cdc42 was dependent on PLC signaling pathways (Udo et al. 2005).
Rac1, one of the most extensively characterized members in the Rho family of small G proteins, was initially discovered as a substrate for ADP-ribosylation induced by the C3 component of botulinum toxin (Didsbury et al. 1989). Subsequently, Rac1 and its downstream effectors were identified as key signaling molecules in various cell functions, such as cytoskeleton reorganization, cell transformation, axonal guidance, and cell migration (Etienne-Manneville and Hall 2002). Since Rac1 is also an essential mediator in many pathological conditions such as Salmonella invasion, tumor cell migration, and retinal degeneration (Bourguignon et al. 2000; Brown et al. 2007), it is important to define cellular signaling pathways that lead to post-translational modifications and activation of Rac1.
Like all members of the Rho superfamily, Rac1 functions as a molecular switch, cycling between an inactive GDPbound state and an active GTP-bound state. Rho small G proteins can be activated by different signal transduction pathways initiated by extracellular factors such as plateletderived growth factor, insulin, or epidermal growth factor (Bishop and Hall 2000). Serotonin has also been shown to induce activation via TGase-catalyzed transamidation of small G proteins in neurons, platelets, aortic smooth muscle cells, and pancreatic beta cells (Dai et al. 2008; Guilluy et al. 2007; Paulmann et al. 2009; Walther et al. 2003). TGase catalyzes the transamidation between protein-bound glutamines and primary amines such as serotonin in a Ca2+-dependent manner (Folk and Chung 1985).
Calmodulin (CaM) has been shown to increase TGase enzymatic activity. In the presence of CaM, a membraneassociated erythrocyte TGase is activated at physiological and lower than physiological Ca2+ concentrations (Billett and Puszkin 1991). Moreover, in the presence of Ca2+, CaM enhanced TGase activity threefold in human platelets and the chicken gizzard (Puszkin and Raghuraman 1985). A CaM inhibitor prevented TGase-catalyzed cross-linking of huntingtin in cells co-transfected with mutant huntingtin and TGase (Zainelli et al. 2004). Taken together, we hypothesize that 5-HT2A receptor stimulated TGase-catalyzed Rac1 transamidation is dependent on PLC-mediated increases in intracellular Ca2+ and is regulated by CaM. To test this hypothesis, we examined the effects of PLC inhibition, CaM inhibition, and manipulation of intracellular Ca2+ by means of a Ca2+ ionophore or a chelating agent on TGase-modified Rac1 in response to 5-HT2A receptor activation in A1A1v cells. Knowing the molecular pathway involved in 5-HT2A receptor-mediated activation of TGase and subsequently Rac1 including the involvement of PLC, Ca2+, and CaM will allow for rationally choosing targets to selectively regulate activation of Rac1 independently of other second messengers activated by 5-HT2A receptors and probe the relevance of each pathway in the treatment and etiology of affective disorders and schizophrenia.
Methods
Cell culture
A1A1v cells, a rat cortical cell line, were grown on 100-mm2 plates coated with poly-L-ornithine (Sigma, St Louis, MO) and maintained in 5% CO2 at 33°C, in Dulbecco's modified Eagle medium (DMEM; Fisher Scientific, Pittsburgh, PA) containing 10% fetal bovine serum (FBS; Fisher Scientific, Pittsburgh, PA). Before each experiment, cells were maintained in DMEM with 10% charcoal-treated FBS for 48 h. Charcoal treatment of FBS reduces the concentration of serotonin in the media to approximately 3 nM (Unsworth and Molinoff 1992). Cells from passages 8–15 were used for all experiments.
Chemicals
The following chemicals were used in this study: (–)-1-(2,5-dimethoxy-4-iodoamphetamine HCl (DOI; Sigma, St Louis, MO), U73122 (Tocris Bioscience Ellisville, MO), U73343 (Sigma, St Louis, MO), BAPTA-AM (Invitrogen, Carlsbad, CA), ionomycin (Invitrogen, Carlsbad, CA), and W-7 hydrochloride (Tocris Bioscience Ellisville, MO).
Measurement of intracellular Ca2+ concentration
Cells were grown to 90% confluence in black-sided 96-well plates (Fisher Scientific, Pittsburgh, PA). Cells were washed twice with modified Kreb's medium (135 mM NaCl, 5.9 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 11.5 mM D-glucose, 11.6 mM Hepes, pH 7.3) and then incubated in the same medium with 5 µM Fura-2 AM (Molecular Probes, Carlsbad, CA), 0.1% bovine serum albumin, and 0.02% Pluronic F127 detergent for 60 min at 33°C in the dark. After loading, the cells were washed twice and incubated in the dark in modified Kreb's medium or pretreated with drugs for 30 min. Following 1 min of equilibration, the cells were stimulated with a single injection of DOI (resulting in a final concentration of 3 µM DOI), and the response was recorded for 3 min in 5-s intervals. Fura-2 fluorescence using 340- and 380-nm excitation and 510-nm emission was measured with a BioTek fluorescence plate reader. After background fluorescence was subtracted, the ratio of fluorescence at 340-nm excitation to that at 380-nm excitation was calculated and used as an index of the intracellular Ca2+ concentration (the 340/380 nm fluorescence ratio is positively correlated with the absolute values of intracellular Ca2+ concentration).
Immunoprecipitation of TGase-modified protein
A1A1v cells were harvested and lysed using lysis buffer A (25 mM Tris–HCl, pH 7.5, 250 mM NaCl, 5 mM EDTA, 1% Triton X-100, and 1:1,000 protease inhibitor cocktail (Sigma, St Louis, MO) containing 104 µM AEBSF, 0.08 µM aprotinin, 2 µM leupeptin, 4 µM bestatin, 1.5 µM pepstatin A and 1.4 µM E-64). Protein concentration was determined using the BCA Protein Assay kit (Pierce, Rockford, IL). Immunopurification of proteins containing TGase-catalyzed bonds was performed using 81D4 mAb (mouse IgM) prebound to Sepharose beads (Covalab, Lyon, France) using a protocol developed by Covalab and as described previously (Dai et al. 2008). Briefly 20 µl of sepharose-81D4 beads were washed three times in TBS/0.1% Tween 20 with gentle shaking for 15 min, followed by adding cell lysate containing 200 µg of protein (1 µg/µl) to the washed beads and incubating for 2 h at 37°C. After incubation, the pellets were washed four times in TBS/0.1% Tween 20 for 15 min. Then 20 µl of loading buffer (50 mM Tris–HCl, pH 6.8, 2% SDS, 0.1% bromophenol blue, 10% glycerol, and 5% β-mercaptoethanol) was added to the washed pellets followed by 5-min incubation at 90°C. The samples were then centrifuged at 9,000×g for 2 min and the supernatant was transferred and stored at −80°C until immunoblot analysis. It was previously reported that the 81D4 antibody is specific for the Nε-(γ-glutamyl) lysine isopeptide, i.e., the transamidation of a peptide bound lysine residue to a peptide-bound glutamate residue (el Alaoui et al. 1991). Several other substrates similar to the Nε-(γ-glutamyl) lysine isopeptide were found not to cross-react with the 81D4 antibody, however transamidation of a peptide bound lysine to a free low-molecular weight amine was not examined (el Alaoui et al. 1991). Our previous data suggest that the antibody also recognizes transamidation of a peptide bound lysine to serotonin (Dai et al. 2008).
Immunoblot
Immunoaffinity purified proteins and cell lysates were separated on 12% SDS-polyacrylamide gels and then electrophoretically transferred to nitrocellulose membranes. Membranes were then incubated in blocking buffer (5% non-fat dry milk, 0.1% Tween 20, ×1 TBS) for 1 h at room temperature. Membranes were incubated overnight at 4°C with primary antibodies on a shaker. Primary antibodies (Upstate Biotechnology, NY: anti-Rac1, mouse IgG, 1:700; anti-Na+/K+ ATPase, mouse IgG, 1: 10000; Millipore, Billerica, MA: anti-serotonin transporter (SERT) antibodies, rabbit, 1:1,000; Abcam, Cambridge, MA: anti-lactate dehydrogenase, goat antibody conjugated to HRP, 1:4,000) were diluted in antibody buffer (1% non-fat dry milk, 0.1% Tween 20, 1× TBS). The next day, membranes were washed with TBS/0.1% Tween 20 and then incubated with goat-antimouse or goat-anti-rabbit secondary antibody conjugated to HRP (Jackson ImmunoResearch, West Grove, PA) diluted in antibody buffer. Membranes were washed and signal was detected using enhanced chemiluminescence Western blotting detection reagents (Amersham Biosciences, Piscataway, NJ). Using Scion Image for Windows (Scion, Frederick, MD), immunoblots were quantified by calculating the integrated optical density (IOD) of each protein band on the film.
Statistical analyses
Data are presented as mean±the standard error of the mean (SEM) and analyzed by one- or two-way ANOVA. Post hoc tests were conducted using Bonferroni's multiple comparison tests. GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA) was used for all statistical analyses. A probability level of p<0.05 was considered to be statistically significant for all statistical tests.
Results
PLC inhibition
We previously reported that 5-HT2A receptor stimulation induced TGase-catalyzed Rac1 transamidation and activation in A1A1v cells (Dai et al. 2008). To test the dependence of Rac1 transamidation on PLC activation, cells were pretreated with 5 µM of the PLC inhibitor, U73122, or an inactive analog, U73343, for 30 min prior to the stimulation of 5-HT2A receptors by 3 µM DOI. U73122 inhibits both PLC-mediated hydrolysis of PIP2 to IP3 and the coupling of G protein-PLC activation. Changes in intracellular Ca2+ were monitored by the 340/380 nm fluorescence ratio of the Fura-2-loaded cells. DOI stimulation induced a robust increase (by at least 200%) in the intracellular Ca2+ concentration within cells pretreated with U73343, whereas PLC inhibition by U73122 reduced the intracellular Ca2+ response to DOI (Fig. 1a). In parallel experiments, TGase-modified Rac1 was evaluated by immunoprecipitation using the 81D4 antibody directed against the TGase-catalyzed covalent bond and immunoblots. There were significant increases in TGase-modified Rac1 within DOI-stimulated cells, but pretreatment with U73122 significantly attenuated DOI-induced Rac1 modification by TGase (Fig. 1b, c). These results suggest a specific role of PLC upstream of Rac1 transamidation in A1A1v cells. Two-way ANOVA indicates a significant main effect of DOI [F(1,8)=108.3; p<0.001] and U73122 [F(1,8)=29.99; p<0.001] on TGase-modified Rac1. There was also a significant interaction between DOI and U73122 [F(1,8)=17.40; p<0.01]. The ubiquitously expressed Na+/K+ ATPase is a well established plasma membrane marker, and its function underlies essentially all of mammalian cell physiology. To verify that the PLC inhibitor-mediated decrease in Rac1-transamidation by TGase was not due to U73122-induced cellular toxicity, cell lysates from the same experiment were examined on immunoblot with antibodies for Na+/K+ATPase and lactate dehydrogenase. There are no significant differences in Na+/K+AT Pase levels or lactate dehydrogenase among cells with different treatments (Fig. 1b). Similar measurements were used in all of the following experiments.
Fig. 1.
Effect of PLC inhibition on DOI-induced Ca2+ signals and TGase-modified Rac1 in A1A1v cells. a After pretreatment with 5 µM U73122 or U73343 (negative control) for 30 min, and stimulation with either 3 µM DOI or vehicle (Ve) for 15 min, changes in the fluorescence ratio (340/380 nm) were recorded from cells loaded with Fura-2. A representative example from three independent experiments conducted in triplicate is shown. Arrows indicate the time of drug application. b Upper, immunoprecipitation (IP) and immunoblot (IB) analyses revealed that U73122 pretreatment inhibited the increase in TGase-modified Rac1 upon DOI stimulation. Middle and lower, cells lysates from the same experiment were examined on IB with Na+/K+ ATPase and lactate dehydrogenase (LDH) antibodies respectively, which indicate that U73122 had no effect on cell viability. c Quantitation of effects of U73122 and DOI on TGase-modified Rac1 in three separate experiments. Data shown were the mean IOD±S.E. M., and they were normalized to U73343- and Ve- treated cells. Two-way ANOVA followed by Bonferroni test: one asterisk and three asterisks indicated p<0.05 and 0.001 as compared to U73343- and Ve-treated cells, respectively; one number sign indicated p<0.001 as compared to U73343- and DOI- treated cells
Ca2+ chelation
In order to determine if TGase-modified Rac1 specifically depends on an increase in intracellular Ca2+ upon 5-HT2A receptor activation, A1A1v cells were pretreated with increasing concentrations of the membrane-permeant Ca2+ chelator, BAPTA-AM (0, 5, 10, 20 µM) for 30 min, then the cells were challenged by DOI. Dose-dependent reductions in the DOI-mediated Ca2+ response were observed by measuring Fura-2 fluorescence (Fig. 2a). Since 20 µM BAPTA caused the most significant inhibition in Ca2+ increases, it was used in the parallel experiments to evaluate TGase-modified Rac1. DOI stimulation of cells without BAPTA-pretreatment significantly increases the amount of TGase-modified Rac1 about twofold compared with vehicle-stimulated cells, whereas pretreatment with BAPTA has a notable inhibitory effect on DOI-induced Rac1 modification by TGase (Fig. 2b, c), suggesting that an increase in intracellular Ca2+ is required for DOI-mediated Rac1 transamidation. Two-way ANOVA indicates a significant main effect of DOI [F(1,8)=37.09; p<0.001] and BAPTA [F(1,8)=9.85; p<0.05] on TGase-modified Rac1. There was also a significant interaction between DOI and BAPTA [F(1,8)=9.50; p<0.05].
Fig. 2.
Pre-incubation with a Ca2+ chelator attenuated the DOI-mediated elevation of cytosolic Ca2+ and TGase-modified Rac1. a Dynamic changes in the fluorescence ratio (340/380 nm) in Fura-2 loaded A1A1v cells. The Ca2+ response to exposure to 3 µM DOI was gradually reduced with the increasing concentrations of BAPTA. A representative example of three independent experiments conducted in triplicate is shown. b Upper, cells were pretreated with 20 µM BAPTA or the vehicle for BAPTA (Ve1) for 30 mins, and then stimulated with 3 µM DOI or the vehicle for DOI (Ve2) for 15 mins. TGase-modified Rac1 was detected by IP and IB analysis. BAPTA inhibited the increase in TGase-modified Rac1 in DOI- stimulated cells. Middle and lower, cells lysates from the same experiment were examined on IB with Na+/K+ATPase and LDH antibodies respectively. There was no significant difference among various treatments. c Quantitation of effects of BAPTA and DOI on TGase-modified Rac1 in three separate experiments. Data shown were the mean IOD±S.E. M., and they were normalized to Ve1- and Ve2- treated cells. Two-way ANOVA followed by Bonferroni test: one asterisk indicated p<0.001 as compared to Ve1- and Ve2- treated cells; one number sign and two number signs indicated p<0.05 and p<0.001 as compared to Ve1- and DOI- treated cells, respectively
Ca2+ ionophore
To determine if an increase in intracellular Ca2+ is sufficient to enhance Rac1 modification by TGase, a Ca2+ ionophore, ionomycin was used to produce an elevation of cytosolic Ca2+ concentration without 5-HT2A receptor activation. Ionomycin is an effective and specific Ca2+ carrier, so it has been used in studies of Ca2+ flux across plasma and ER membranes. To determine the concentration of ionomycin that raises the levels of cytosolic Ca2+ to levels comparable to 3 µM DOI (the concentration of DOI used to induce TGase-modified Rac1), serial dilutions of ionomycin (from 1,000 nM 12.5 nM) were tested for their ability to induce increases in intracellular Ca2+. As shown in Fig. 3a, 25 nM ionomycin was able to produce a comparable increase in cytosolic Ca2+ in comparison to 3 µM DOI, so this concentration was used in the parallel experiments to measure the effects on TGase-modified Rac1. After cells were treated with either 3 µM DOI or 25 nM ionomycin for 15 min, ionomycin mediated an increase in TGase-modified Rac1 to levels similar to those induced by DOI. One-way ANOVA indicated there is no significant difference in TGase-modified Rac1 between DOI- and ionomycin-treated cells. Taken together, these data suggest an increase in intracellular Ca2+ via an ionophore can mimic 5-HT2A receptor-induced cytosolic Ca2+ increases and is sufficient to induce TGase-catalyzed Rac1 transamidation.
Fig. 3.
The Ca2+ ionophore mimicked the DOI-induced increase in cytosolic Ca2+ and TGase-catalyzed transamidation of Rac1. a Fura-2-loaded cells were stimulated with either 3 µM DOI or different concentrations of ionomycin. The changes in the fluorescence ratio (340/380 nm) were recorded. A representative example from three independent experiments conducted in triplicate is shown. b Upper, cells were treated for 15 mins with 3 µM DOI, 25 nM ionomycin or vehicle (Ve). Ionomycin caused an increase in TGase-modified Rac1 comparable to that induced by DOI. Middle and lower, cell viability, as indicated by Na+/K+ATPase and LDH levels was not significantly different among the various treatments. c Quantitation of effects of ionomycin and DOI on TGase-modified Rac1 in three separate experiments. Data shown were the mean IOD±S.E.M., and were normalized to Ve-1-treated cells. One-way ANOVA followed by Bonferroni test: one asterisk indicates p<0.01 as compared to Ve-1-treated cells; one number sign indicates p<0.01 as compared DOI-treated cells
CaM inhibition
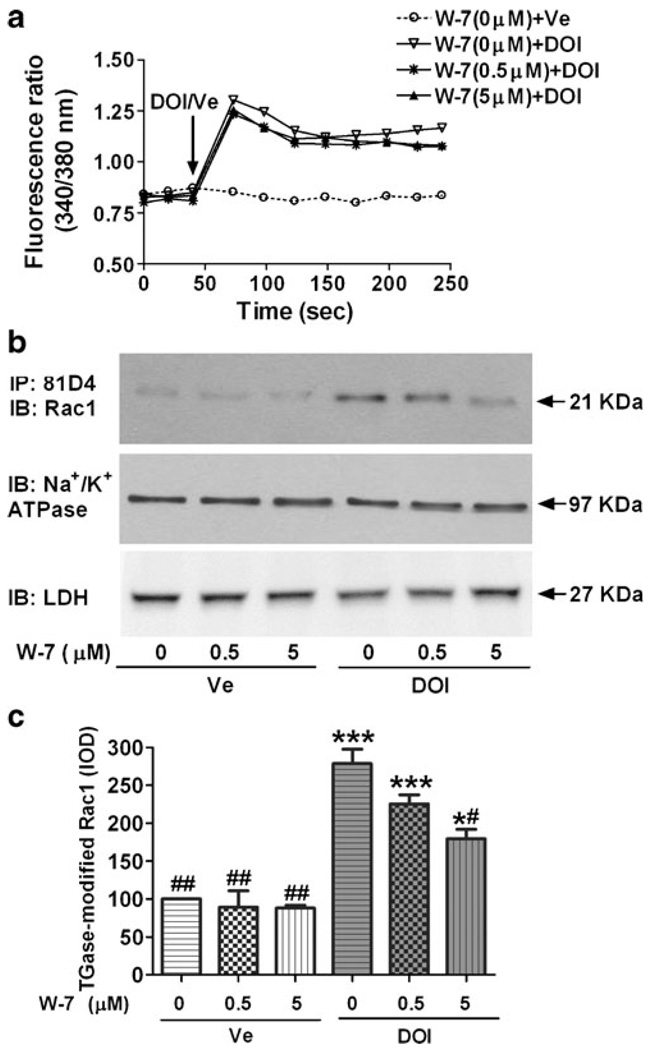
TGase is not only a Ca2+-dependent enzyme, but its activity is also positively regulated by CaM. Moreover, Ca2+ signaling in cells is mainly mediated by CaM, a potent Ca2+-binding protein. The CaM inhibitor, W-7, binds with high affinity to CaM in the presence of Ca2+ and thereby inhibits the activation of CaM-dependent enzymes. To test whether CaM modulates TGase-catalyzed transamidation of Rac1, cells were pre-incubated for 1 h with 0, 0.5, or 5 µM W-7 hydrochloride, and then stimulated with 3 µM DOI or vehicle for 15 min. We found that in DOI-stimulated cells, the levels of TGase-modified Rac1 were significantly higher than in vehicle-stimulated cells, whereas pretreatment with W-7 decreased DOI-stimulated Rac1 modification by TGase in a dose-dependent manner (Fig. 4). Two-way ANOVA indicates a significant main effect of DOI [F (1,12)=144.6; p<0.001] and W-7 [F(2,12)=8.152; p<0.01] on TGase-modified Rac1. There was also a significant interaction between DOI and W-7 [F(2,12)=5.01; p<0.05].
Fig. 4.
The CaM inhibitor W-7 caused a dose-dependent reduction in DOI-stimulated TGase-modified Rac1. a Pretreatment with the CaM inhibitor W-7 had no effect on DOI-stimulated Ca2+ release. Data shown from one representative experiment performed in triplicate and repeated two additional times. b Cells were pretreated with increasing concentrations of W-7 for 1 h and then stimulated with either 3 µM DOI or vehicle (Ve). TGase-modified Rac1, Na+/K+ATPase and LDH were measured by IP and/or IB. W-7 decreased the levels of TGase-modified Rac1 upon DOI stimulation, but did not significantly affect Na+/K+ATPase or LDH levels at the concentrations of W-7 applied. c Quantitation of effects of W-7 on TGase-modified Rac1 in three separate experiments. Data shown were the mean IOD±S.E.M., and were normalized to Ve-stimulated cells without W-7 pretreatment. Two-way ANOVA followed by Bonferroni test: one asterisk and three asterisks indicate p<0.05 and p<0.001 as compared to Ve-stimulated cells without W-7 pretreatment, respectively; one number sign and two number signs indicate p<0.01 and p<0.001 as compared to DOI-stimulated cells without W-7 pretreatment, respectively
To test whether CaM modulates 5-HT2A receptor-mediated Ca2+ release, cells were pre-incubated for 1.5 h with 0, 0.5, or 5 µMW-7 hydrochloride, and then stimulated with 3 µM DOI or vehicle for 3 min. We found pretreatment with W-7, did not alter DOI-stimulated Ca2+ release (Fig. 4a).
SERT expression in A1A1v cells
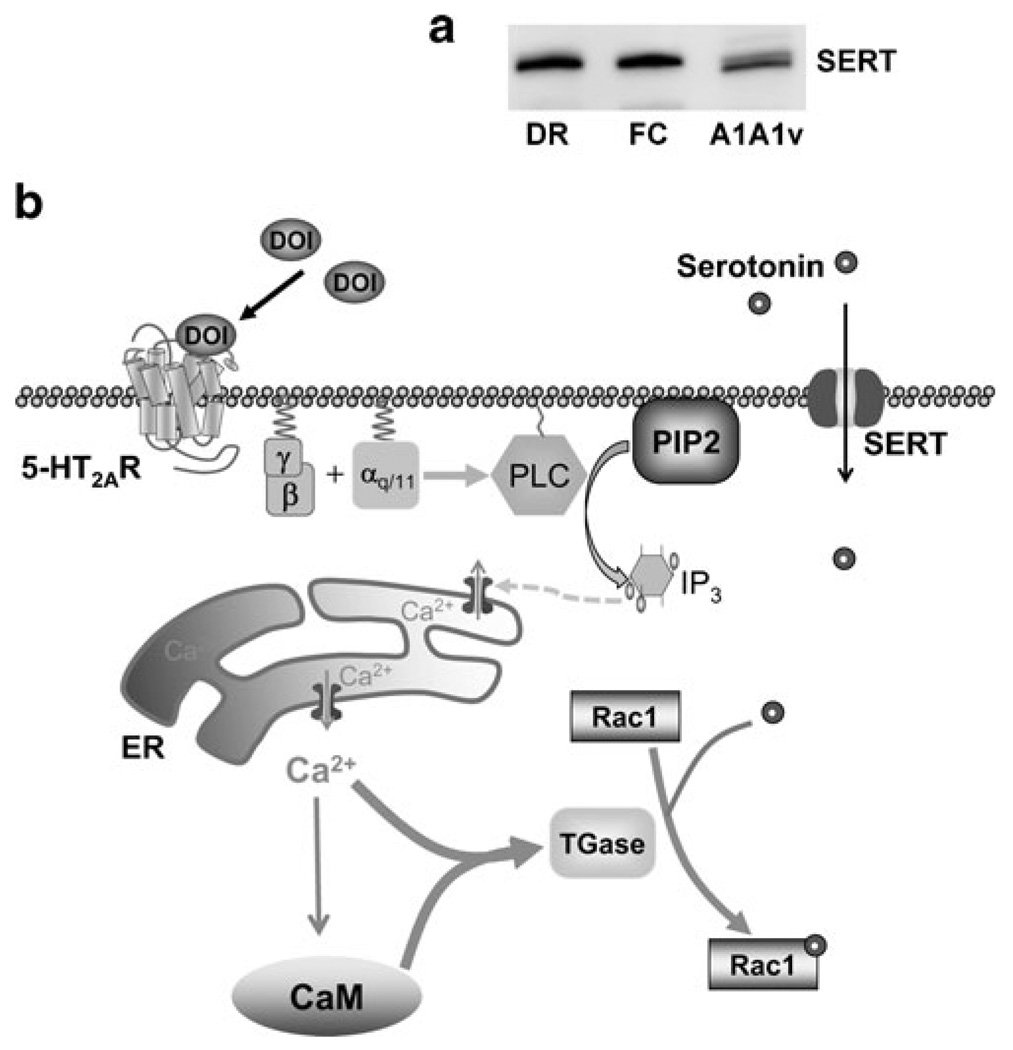
Western blots were prepared with the membrane fraction of rat dorsal raphe and frontal cortex and whole cell lysates from A1A1v cells. A single major band is detected with an antibody directed against the amino-terminus of SERT in A1A1v cells comparable to the band in rat dorsal raphe and frontal cortex which are used as positive controls (Fig. 5a).
Fig. 5.
a Western blot demonstrating the presence of SERT in A1A1v cells. A Western blot was prepared with 2.5 µg of the membrane fraction of rat dorsal raphe (DR) and frontal cortex (FC) and compared to 20 µg of lysate from A1A1v cells. b Schematic representation showing 5-HT2a receptor signal-mediated transamidation of Rac1 by TGase. DOI stimulates Gq/11 proteins-coupled 5-HT2a receptors, leading to the activation of PLC, which in turn hydrolyses PIP2 into IP3. IP3 releases Ca2+ from ER and results in the activation of Ca2+-dependent proteins, such as CaM and TGase. Positively regulated by both Ca2+ and CaM, TGase catalyzes transamidation of Rac1 to bioamines, such as serotonin (Dai et al. 2008) transported into the cytoplasm by SERT. Abbreviations: 5-HT2AR5-HT2a receptor; CaM, calmodulin; ER, endoplasmic reticulum; PLC, phospholipase C; PIP2, phophatidyl inositol-1,4-bisphosphate; IP3, inositol-1,4,5-triphosphate; SERT, serotonin transporter; TGase, transglutaminase; α, β, γ, G protein α,β,γ subunits
Discussion
Several lines of evidence indicate Gq/11-protein-coupled receptors, such as bradykinin or endothelin-1 receptor, are able to stimulate the activation of Rac1 (Clerk et al. 2001; van Leeuwen et al. 1999), but the mechanisms by which Rac1 becomes activated through these receptors remains poorly characterized. Nevertheless, it is worth noting that the activation of Rac1 is highly regulated by physiological stimuli, and aberrant activation occurs under many pathological conditions such as Salmonella invasion, tumor cell migration, and retinal degeneration (Bourguignon et al. 2000; Brown et al. 2007). Therefore, further studies that delineate signaling pathways involved in Rac1 activation may uncover new potential therapeutic targets for these diseases. We have previously demonstrated that the 5-HT2A receptor, another Gq/11-protein-coupled receptor, is responsible for TGase-catalyzed transamidation and activation of Rac1 in A1A1v cells based on the fact that selective 5-HT2A/2C receptor agonist, but not 5-HT1A receptor agonist, induces transamidation of Rac1 by TGase. Moreover, expression of 5-HT2C receptors is not detectable in this cell line and a selective 5-HT2A receptor antagonist blocks Rac1 transamidation (Dai et al. 2008). In the present study, we investigated the mechanisms involved in Rac1 transamidation in response to 5-HT2A receptor stimulation. Our experimental results identified that PLC-mediated increases in intracellular Ca2+ and subsequent CaM activation are required for 5-HT2A receptor-mediated Rac1 transamidation. 5-HT2A receptor stimulation activates PLC which catalyzes the hydrolysis of PIP2 into IP3. IP3 mobilizes Ca2+ from ER stores and thereby increases the intracellular Ca2+ and leads to the activation of CaM. Our data suggest that both Ca2+ and CaM activate TGase, which catalyzes transamidation of Rac1 to bioamines, such as serotonin (Fig. 5).
Except for the 5-HT3 receptor, which is a ligand-gated ion channel, the other 5-HT receptors belong to the G protein-coupled receptor (GPCR) superfamily. G proteins such as Gα12 and Gα13, have been shown to transduce signals from GPCR to activate Rho small G proteins (Suzuki et al. 2003). One of the guanine nucleotide exchange factors (GEFs) for Rho, p115RhoGEF, has served as a direct link between Gα12/Gα13 and Rho (Kozasa et al. 1998). However, the signaling pathways between 5-HT2A receptors and Rac1 transamidation and activation are not well understood. 5-HT2A receptor is primarily coupled to Gq/11 and activates various isoforms of PLC (Conn and Sanders-Bush 1984). It also induces the activation of phospholipase A2 and the release of arachidonic acid (Felder et al. 1990). Moveover, through interaction with ADP-ribosylation factor 1 via its C-terminal domain, 5-HT2A receptor is able to signal through the phospholipase D pathway (Robertson et al. 2003). Our results suggest that 5-HT2A receptor activation triggers TGase-catalyzed Rac1 transamidation predominantly through a pathway involving PLC (Fig. 1).
Although it has been reported that PLC activates Rac1 in a manner dependent on Iba1, a Ca2+-binding protein specifically expressed in macrophage/microglia (Kanazawa et al. 2002), Rac1 transamidation and activation in A1A1v cells, a neuronal cell line, could be mediated by other signaling pathways. Cleavage of PIP2 by PLC yields two important second messengers, IP3 and DAG, to initiate a variety of cellular functions. Since Ca2+ chelation reduces DOI-stimulated Rac1 transamidation to basal levels (Fig. 2), IP3-induced Ca2+ release rather than DAG signaling plays a pivotal role in TGase-catalyzed Rac1 transamidation. These results are in agreement with an earlier study suggesting that in thrombin- and collagen-stimulated human blood platelets, PLC activity and an increase in intracellular Ca2+ concentration were required for Rac1 activation, whereas PKC inhibition had no effect (Soulet et al. 2001). Increases in intracellular Ca2+ concentration upon 5-HT2A receptor stimulation are achieved by releasing intracellular Ca2+ stores and/or by activating Ca2+ channels, depending on the cell of interest. 5-HT2A receptor-induced contraction of rat aorta may require the concerted action of voltage-gated Ca2+ channels and phosphoinositide turnover (Nakaki et al. 1985). More interestingly, stimulation of 5-HT2A receptors on astrocytes caused Ca2+ influx through voltage independent Ca2+ channels which were dependent of the IP3 production and subsequent Ca2+ release from internal stores (Hagberg et al. 1998). Therefore, both Ca2+ mobilization from internal stores and influx of extracellular Ca2+ could contribute to the PLC/IP3-mediated intracellular Ca2+ increase in A1A1v cells.
CaM and TGase are Ca2+-dependent proteins. CaM activation is coupled to intracellular Ca2+ elevation and also correlates with the spatial pattern of increased Ca2+ (Hahn et al. 1992). Upon Ca2+ binding to the EF hand motifs of CaM, both N- and C-terminal domains adopt an open confirmation, exposing hydrophobic surfaces, which then interact with a variety of target proteins including TGase (Puszkin and Raghuraman 1985; Zainelli et al. 2004). TGases contain a cysteine in their active site that is unmasked only in the presence of Ca2+ (Hand et al. 1993) and Ca2+ activation of TGase is further regulated by other signal modulators such as GTP and CaM (Puszkin and Raghuraman 1985; Zainelli et al. 2004). In order for TGase to become receptive to the glutamine substrate, Ca2+ first must bind to TGase, inducing its conformational change which permits the glutamine substrate binding to the catalytic center (Folk 1983). A rise of Ca2+ concentration increases TGase activity in a dose-dependent manner in vitro and in cells (Shin et al. 2004; Siefring et al. 1978). Consistent with these results, the elevation of intracellular Ca2+ induced via a Ca2+ ionophore lead to increased TGase-catalyzed Rac1 transamidation (Fig. 3).
In addition to Ca2+, CaM also interacts with TGase and regulates its cross-linking activity. CaM has been shown to coimmunoprecipitate with TGase2 in transfected cells (Zainelli et al. 2004). CaM increased TGase activity in human erythrocyte (Billett and Puszkin 1991), platelets, and chicken gizzard (Puszkin and Raghuraman 1985). Interestingly, exogenous CaM with a binding site on the α-subunits of the hexadecameric phosphorylase kinase molecule (αβγδ)4, stimulates TGase-catalyzed incorporation of putrescine into the β- and γ-subunits, but inhibits incorporation of amines into the α-subunit (Nadeau and Carlson 1994). Some of the CaM antagonists, such as dansylcadaverine, inhibit TGase (Lorand et al. 1979) and its protein cross-linking ability. We have previously reported that a CaM antagonist or inhibitory CaM fragments, attenuated TGase-modified mutant huntingtin in cell and animal models of Huntington's disease (Dai et al. 2009; Dudek et al. 2009; Zainelli et al. 2004). CaM antagonists and Ca2+ chelators also have been shown to depress the motile progression of sperm cells possibly mediated by TGase-catalyzed cross-linking of the contractile proteins (Cariello and Nelson 1985). Our present results revealed that the CaM inhibitor, W-7, reduced TGase-modified-Rac1 in dose-dependent fashion (Fig. 4), indicating that TGase-catalyzed Rac1 transamidation, at least in part, was positively regulated by CaM. Moreover, in the presence of a 5-HT2a receptor agonist, CaM has been shown to interact with 5-HT2a receptors at the sites that are important for G protein coupling, suggesting a putative role for CaM in regulating 5-HT2a receptor function (Turner and Raymond 2005). However, since the CaM inhibitor had no effect on DOI-stimulated calcium release, the effect of CaM inhibition is not likely mediated by alterations in signaling at the 5-HT2A receptor but by modulation of TGase activity.
The significance of TGase in Rac1 transamidation was confirmed by TGase siRNA and a chemical inhibitor in our previous studies (Dai et al. 2008). In addition to small G proteins such as Rac1, RhoA, Rab3a, and Rab27a (Dai et al. 2008; Paulmann et al. 2009; Walther et al. 2003) that could undergo TGase-catalyzed transamidation to serotonin (serotonylation), more recently, cytoskeletal proteins including actin, myosin heavy chain, and filamin A have been identified as serotonylated proteins (Watts et al. 2009). However, serotonin may not be the only amine that could be incorporated in these proteins. Neuronal differentiation of SH-SY5Y cells induced by retinoic acid increases the expression/activation of TGases, resulting in transamidation of putrescine to RhoA and activation of RhoA (Singh et al. 2003). Moreover, spermidine and putrescine are suitable substrates for transglutaminase-catalyzed transamidation of RhoA in vitro (Schmidt et al. 2001). In addition to amine incorporation, another important TGase-catalyzed modification of small G proteins is deamidation of glutamine residues. However, this reaction has only been demonstrated by bacterial toxins, not in mammalian systems and transglutaminases. The site-specific deamidation of Gln53 and Gln61 of RhoA and Ras, respectively, by bacterial toxins with TGase activity inhibits the GTPase activity and renders small G proteins constitutively active (Lorand and Graham 2003).
In conclusion, our study provides important new insights into the signaling mechanisms underlying 5-HT2A receptor-induced Rac1 transamidation and activation by TGase in A1A1v cells, and highlights the necessity of PLC-mediated Ca2+ mobilization and CaM in TGase-catalyzed Rac1 transamidation. As an activating signal for both TGase and CaM, an elevation of intracellular Ca2+ is sufficient to induce Rac1 transamidation. Accumulating evidence suggests that Rho and Rab family of small G proteins are subject to TGase-mediated modification and activation. The essential role of Rac1 in neuronal development, especially in the regulation of dendritic spines (Corbetta et al. 2009; Tsai et al. 2009), axon formation and guidance (Ng et al. 2002), neurite outgrowth and differentiation (Li et al. 2002) is well characterized, and perturbations in Rac1 signaling have been associated with cognitive disorders such as mental retardation (Govek et al. 2005). Given the importance of Rac1 in various pathophysiological processes, the biological functions of Rac1 activation by TGase-catalyzed transamidation in neurons are worth exploring in more depth.
Acknowledgments
This work was supported by US Public Health Service [Grant MH068612].
Abbreviations
- DOI
2,5-Dimethoxy-4-iodoamphetamine
- CaM
Calmodulin
- DAG
Diacylglycerol
- ER
Endoplasmic reticulum
- GDIs
GDP-dissociation inhibitors
- GAPs
GTPase-activating proteins
- GEFs
Guanine nucleotide exchange factors
- IP3
Inositol 1,4,5-trisphosphate
- PIP2
Phophatidyl inositol-4,5-bisphosphate
- SERT
Serotonin transporter
- TGase
Transglutaminase
Contributor Information
Ying Dai, Neuroscience Program, Loyola University, School of Medicine, Maywood, IL, USA; and Department of Pharmacology and Toxicology, University of Kansas, School of Pharmacy, 1251 Wescoe Hall Drive, Lawrence, KS 66045, USA.
Nichole L. Dudek, Department of Pharmacology and Experimental Therapeutics, Loyola University Chicago, School of Medicine, Maywood, IL, USA
Qian Li, Department of Pharmacology and Toxicology, University of Kansas, School of Pharmacy, 1251 Wescoe Hall Drive, Lawrence, KS 66045, USA.
Nancy A. Muma, Email: nmuma@ku.edu, Department of Pharmacology and Toxicology, University of Kansas, School of Pharmacy, 1251 Wescoe Hall Drive, Lawrence, KS 66045, USA.
References
- Billett HH, Puszkin EG. The red cell membrane contains calmodulin-regulated crosslinking and proteolytic activity. Hematol Pathol. 1991;5:185–193. [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY, Zhu H, Shao L, Chen YW. CD44 interaction with tiam1 promotes Rac1 signaling and hyaluronic acid-mediated breast tumor cell migration. J Biol Chem. 2000;275:1829–1838. doi: 10.1074/jbc.275.3.1829. [DOI] [PubMed] [Google Scholar]
- Brown MD, Bry L, Li Z, Sacks DB. IQGAP1 regulates Salmonella invasion through interactions with actin, Rac1, and Cdc42. J Biol Chem. 2007;282:30265–30272. doi: 10.1074/jbc.M702537200. [DOI] [PubMed] [Google Scholar]
- Cariello L, Nelson L. Transglutaminase inhibitors, calmodulin antagonists, and calcium channel blockers: influence on Arbacia sperm motility. Gamete Res. 1985;11:261–270. [Google Scholar]
- Clerk A, Pham FH, Fuller SJ, Sahai E, Aktories K, Marais R, Marshall C, Sugden PH. Regulation of mitogen-activated protein kinases in cardiac myocytes through the small G protein Rac1. Mol Cell Biol. 2001;21:1173–1184. doi: 10.1128/MCB.21.4.1173-1184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Sanders-Bush E. Selective 5HT-2 antagonists inhibit serotonin stimulated phosphatidylinositol metabolism in cerebral cortex. Neuropharmacology. 1984;23:993–996. doi: 10.1016/0028-3908(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Corbetta S, Gualdoni S, Ciceri G, Monari M, Zuccaro E, Tybulewicz VL, de Curtis I. Essential role of Rac1 and Rac3 GTPases in neuronal development. FASEB J. 2009;23:1347–1357. doi: 10.1096/fj.08-121574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Dudek NL, Patel TB, Muma NA. Transglutaminase-catalyzed transamidation: a novel mechanism for Rac1 activation by 5-hydroxytryptamine2A receptor stimulation. J Pharmacol Exp Ther. 2008;326:153–162. doi: 10.1124/jpet.107.135046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Dudek NL, Li Q, Fowler SC, Muma NA. striatal expression of a calmodulin fragment improved motor function, weight loss, and neuropathology in the R6/2 mouse model of Huntington’s disease. J Neurosci. 2009;29:11550–11559. doi: 10.1523/JNEUROSCI.3307-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didsbury J, Weber RF, Bokoch GM, Evans T, Snyderman R. rac, a novel ras-related family of proteins that are botulinum toxin substrates. J Biol Chem. 1989;264:16378–16382. [PubMed] [Google Scholar]
- Dudek NL, Dai Y, Muma NA. Neuroprotective effects of calmodulin peptide 76–121aa: disruption of calmodulin binding to mutant Huntingtin. Brain Pathol. 2009;20(1):176–189. doi: 10.1111/j.1750-3639.2008.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Alaoui S, Legastelois S, Roch AM, Chantepie J, Quash G. Transglutaminase activity and N epsilon (gamma glutamyl) lysine isopeptide levels during cell growth: an enzymic and immunological study. Int J Cancer. 1991;48:221–226. doi: 10.1002/ijc.2910480212. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Felder CC, Kanterman RY, Ma AL, Axelrod J. Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc Natl Acad Sci USA. 1990;87:2187–2191. doi: 10.1073/pnas.87.6.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk JE. Mechanism and basis for specificity of transglutaminase-catalyzed epsilon-(gamma-glutamyl) lysine bond formation. Adv Enzymol Relat Areas Mol Biol. 1983;54:1–56. doi: 10.1002/9780470122990.ch1. [DOI] [PubMed] [Google Scholar]
- Folk JE, Chung SI. Transglutaminases. Methods Enzymol. 1985;113:358–375. doi: 10.1016/s0076-6879(85)13049-1. [DOI] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- Guilluy C, Rolli-Derkinderen M, Tharaux PL, Melino G, Pacaud P, Loirand G. Transglutaminase-dependent RhoA activation and depletion by serotonin in vascular smooth muscle cells. J Biol Chem. 2007;282:2918–2928. doi: 10.1074/jbc.M604195200. [DOI] [PubMed] [Google Scholar]
- Hagberg GB, Blomstrand F, Nilsson M, Tamir H, Hansson E. Stimulation of 5-HT2A receptors on astrocytes in primary culture opens voltage-independent Ca2+ channels. Neurochem Int. 1998;32:153–162. doi: 10.1016/s0197-0186(97)00087-9. [DOI] [PubMed] [Google Scholar]
- Hahn K, DeBiasio R, Taylor DL. Patterns of elevated free calcium and calmodulin activation in living cells. Nature. 1992;359:736–738. doi: 10.1038/359736a0. [DOI] [PubMed] [Google Scholar]
- Hand D, Perry MJ, Haynes LW. Cellular transglutaminases in neural development. Int J Dev Neurosci. 1993;11:709–720. doi: 10.1016/0736-5748(93)90060-q. [DOI] [PubMed] [Google Scholar]
- Kanazawa H, Ohsawa K, Sasaki Y, Kohsaka S, Imai Y. Macrophage/microglia-specific protein Iba1 enhances membrane ruffling and Rac activation via phospholipase C-gamma -dependent pathway. J Biol Chem. 2002;277:20026–20032. doi: 10.1074/jbc.M109218200. [DOI] [PubMed] [Google Scholar]
- Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- Li X, Saint-Cyr-Proulx E, Aktories K, Lamarche-Vane N. Rac1 and Cdc42 but not RhoA or Rho kinase activities are required for neurite outgrowth induced by the Netrin-1 receptor DCC (deleted in colorectal cancer) in N1E-115 neuroblastoma cells. J Biol Chem. 2002;277:15207–15214. doi: 10.1074/jbc.M109913200. [DOI] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Lorand L, Parameswaran KN, Stenberg P, Tong YS, Velasco PT, Jonsson NA, Mikiver L, Moses P. Specificity of guinea pig liver transglutaminase for amine substrates. Biochemistry. 1979;18:1756–1765. doi: 10.1021/bi00576a019. [DOI] [PubMed] [Google Scholar]
- Nadeau OW, Carlson GM. Zero length conformation-dependent cross-linking of phosphorylase kinase subunits by transglutaminase. J Biol Chem. 1994;269:29670–29676. [PubMed] [Google Scholar]
- Nakaki T, Roth BL, Chuang DM, Costa E. Phasic and tonic components in 5-HT2 receptor-mediated rat aorta contraction: participation of Ca++ channels and phospholipase C. J Pharmacol Exp Ther. 1985;234:442–446. [PubMed] [Google Scholar]
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L. Rac GTPases control axon growth, guidance and branching. Nature. 2002;416:442–447. doi: 10.1038/416442a. [DOI] [PubMed] [Google Scholar]
- Paulmann N, Grohmann M, Voigt JP, Bert B, Vowinckel J, Bader M, Skelin M, Jevsek M, Fink H, Rupnik M, Walther DJ. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009;7:e1000229. doi: 10.1371/journal.pbio.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puszkin EG, Raghuraman V. Catalytic properties of a calmodulin-regulated transglutaminase from human platelet and chicken gizzard. J Biol Chem. 1985;260:16012–16020. [PubMed] [Google Scholar]
- Robertson DN, Johnson MS, Moggach LO, Holland PJ, Lutz EM, Mitchell R. Selective interaction of ARF1 with the carboxy-terminal tail domain of the 5-HT2A receptor. Mol Pharmacol. 2003;64:1239–1250. doi: 10.1124/mol.64.5.1239. [DOI] [PubMed] [Google Scholar]
- Roth BL. Multiple serotonin receptors: clinical and experimental aspects. Ann Clin Psychiatry. 1994;6:67–78. doi: 10.3109/10401239409148985. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Goehring UM, Schirmer J, Uttenweiler-Joseph S, Wilm M, Lohmann M, Giese A, Schmalzing G, Aktories K. Lysine and polyamines are substrates for transglutamination of Rho by the Bordetella dermonecrotic toxin. Infect Immun. 2001;69:7663–7670. doi: 10.1128/IAI.69.12.7663-7670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Jeon JH, Kim CW, Cho SY, Kwon JC, Lee HJ, Choi KH, Park SC, Kim IG. Cell type-specific activation of intracellular transglutaminase 2 by oxidative stress or ultraviolet irradiation: implications of transglutaminase 2 in age-related cataractogenesis. J Biol Chem. 2004;279:15032–15039. doi: 10.1074/jbc.M308734200. [DOI] [PubMed] [Google Scholar]
- Siefring GE, Jr, Apostol AB, Velasco PT, Lorand L. Enzymatic basis for the Ca2+ -induced cross-linking of membrane proteins in intact human erythrocytes. Biochemistry. 1978;17:2598–2604. doi: 10.1021/bi00606a022. [DOI] [PubMed] [Google Scholar]
- Singh US, Pan J, Kao YL, Joshi S, Young KL, Baker KM. Tissue transglutaminase mediates activation of RhoA and MAP kinase pathways during retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Biol Chem. 2003;278:391–399. doi: 10.1074/jbc.M206361200. [DOI] [PubMed] [Google Scholar]
- Soulet C, Gendreau S, Missy K, Benard V, Plantavid M, Payrastre B. Characterisation of Rac activation in thrombin- and collagen-stimulated human blood platelets. FEBS Lett. 2001;507:253–258. doi: 10.1016/s0014-5793(01)02984-2. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Nakamura S, Mano H, Kozasa T. Galpha 12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc Natl Acad Sci USA. 2003;100:733–738. doi: 10.1073/pnas.0234057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Hayashi T, Harvey BK, Wang Y, Wu WW, Shen RF, Zhang Y, Becker KG, Hoffer BJ, Su TP. Sigma-1 receptors regulate hippocampal dendritic spine formation via a free radicalsensitive mechanism involving Rac1xGTP pathway. Proc Natl Acad Sci USA. 2009;106:22468–22473. doi: 10.1073/pnas.0909089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JH, Raymond JR. Interaction of calmodulin with the serotonin 5-hydroxytryptamine2A receptor. A putative regulator of G protein coupling and receptor phosphorylation by protein kinase C. J Biol Chem. 2005;280:30741–30750. doi: 10.1074/jbc.M501696200. [DOI] [PubMed] [Google Scholar]
- Udo H, Jin I, Kim JH, Li HL, Youn T, Hawkins RD, Kandel ER, Bailey CH. Serotonin-induced regulation of the actin network for learning-related synaptic growth requires Cdc42, N-WASP, and PAK in Aplysia sensory neurons. Neuron. 2005;45:887–901. doi: 10.1016/j.neuron.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Unsworth CD, Molinoff PB. Regulation of the 5-hydroxytryptamine1B receptor in opossum kidney cells after exposure to agonists. Mol Pharmacol. 1992;42:464–470. [PubMed] [Google Scholar]
- van Leeuwen FN, van Delft S, Kain HE, van der Kammen RA, Collard JG. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat Cell Biol. 1999;1:242–248. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 2003;115:851–862. doi: 10.1016/s0092-8674(03)01014-6. [DOI] [PubMed] [Google Scholar]
- Watts SW, Priestley JR, Thompson JM. Serotonylation of vascular proteins important to contraction. PLoS ONE. 2009;4:e5682. doi: 10.1371/journal.pone.0005682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Zainelli GM, Ross CA, Troncoso JC, Fitzgerald JK, Muma NA. Calmodulin regulates transglutaminase 2 cross-linking of huntingtin. J Neurosci. 2004;24:1954–1961. doi: 10.1523/JNEUROSCI.4424-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lesort M, Guttmann RP, Johnson GV. Modulation of the in situ activity of tissue transglutaminase by calcium and GTP. J Biol Chem. 1998;273:2288–2295. doi: 10.1074/jbc.273.4.2288. [DOI] [PubMed] [Google Scholar]