Abstract
Background
Basic lexical skills are hypotheseized to be relatively preserved in mild dementia but clinical studies have had inconsistent results.
Methods
More than 400 older Catholic nuns, priests, and brother recruited from groups across the United States completed annual evaluations for up to 15 years, died, and underwent brain autopsy. Each clinical evaluation included administration of a 20-item word reading test and a 15-item vocabulary test which were combined to form a composite measure of word knowledge. In a uniform neuropathologic examination, Alzheimer’s disease pathology was quantified with a composite index of plaques and tangles and the presence of gross and microscopic cerebral infarctions and Lewy bodies was recorded.
Results
Postmortem pathologic level of Alzheimer’s disease was linearly related to rate of decline in word knowledge. Decline was nearly fourfold faster at a relatively high level of pathology (75th percentile) compared to a relatively low level (25th percentile). Effects for word reading and vocabulary were similar. Gross cerebral infarctions and Lewy bodies were associated with accelerated decline in vocabulary but not in word reading.
Conclusion
Common chronic neurodegenerative conditions impair word knowledge in old age.
Keywords: Alzheimer’s disease, cerebrovascular disease, Lewy bodies, cognition, post mortem
Word knowledge is thought to be relatively well maintained in late life [1,2], especially when assessed with methods that minimize demands on other cognitive abilities [3,4]. Word knowledge tests of this sort, most notably the National Adult Reading Test [5], are also hypothesized to be performed normally by persons with mild dementia [5,6] and therefore to provide an index of cognitive ability prior to dementia onset. Research examining this hypothesis has been inconclusive, however. One problem is that most longitudinal studies have had few subjects (i.e., less than 100 [7–13]) and observation points (i.e., 2–3 per subject [7, 11–13]) making it difficult to characterize change in performance over time. In addition, the insidious onset and progression of most dementia syndromes make it difficult to reliably characterize mild dementia.
The present study examines the relation of dementia to change in word knowledge. It differs from previous research, however, in that dementia is defined by neuropathologic rather than clinical manifestations. Participants were older Catholic nuns, priests, and brothers from the Rush Religious Orders Study who had undergone annual testing of word knowledge and brain autopsy. In a uniform neuropathological examination, summary measures of plaques and tangles, gross and microscopic cerebral infarction, and Lewy bodies were obtained. We used mixed-effects models to characterize change in word knowledge and to test the hypothesis that decline in word knowledge is due in part to neurodegenerative disease.
METHODS
Participants
Subjects are older Catholic nuns, priests, and brothers participating in the Rush Religious Orders Study [14]. They all agreed to annual clinical evaluations and brain autopsy at death. The study was approved by the Institutional Review Board of Rush University Medical Center.
At the time of these analyses, 495 study participants had died and 463 (93.5%) of them underwent brain autopsy, the results of which were pending in 14. Of the remaining 449 individuals, 34 died with only one valid word knowledge score, leaving 415 with pathologic data and longitudinal clinical data. They had a mean age at death of 87.1 (SD=7.0). They had a mean of 17.9 years of education (SD=3.4) and 61.7% were woman. They died a mean of 6.4 months after the last assessment of word knowledge (SD=3.9) with a mean postmortem interval of 7.9 hours (SD=8.0).
Assessment of Word Knowledge
At each annual clinical evaluation, two measures of word knowledge were administered. A 20-item reading test with items from the National Adult Reading test [5] and subsequent modifications [15,16] required reading aloud words with atypical spelling-sound correspondence (e.g., epitome, impugn). A 15-item version [17] of Extended Range Vocabulary [18] required selecting the best synonym for each target word from five alternatives. Because the two tests loaded on a common factor in a previous factor analysis [17], we used a composite measure based on both of them as the primary outcome to minimize floor and ceiling artifacts and other forms of measurement error. Raw scores on each test were converted to z scores, using the baseline mean and SD from the entire cohort, and averaged to yield the composite. Further information on each test and the derivation of the composite measure of word knowledge is published elsewhere [17].
Neuropathological Assessment
A standard protocol was followed for brain removal (at Rush and 11 predetermined sites across the U.S.), tissue sectioning and preservation, and quantification of pathologic findings, as described in more detail elsewhere [19,20]. AD pathology was summarized with a composite measure based on counts of neuritic plaques, diffuse plaques, and neurofibrillary tangles in 4 brain regions (entorhinal cortex, midfrontal gyrus, middle temporal gyrus, inferior parietal gyrus) using a modified Bielschowsky sliver stain. All cerebral infarctions visible to the naked eye were noted and the age of each was estimated. The presence of chronic microscopic infarcts was determined using hematoxylin and eosin stain. Lewy bodies in the 6 brain regions (substantia nigra, cingulate cortex, entorhinal cortex, midfrontal gyrus, middle temporal gyrus, inferior parietal gyrus) were identified with antibodies to alpha-synuclein. Gross infarction, microscopic infarction, and Lewy bodies were each treated as present or absent in analyses.
Data Analysis
We used mixed-effects models [21] to characterize change in cognitive function and to test the relation of each pathologic index to rate of change. Each model included terms for time (in years since baseline), a given pathologic index, and the interaction of the index with time. The term for time indicates the mean change per year in word knowledge; the index term indicates its association with level of word knowledge; and the interaction indicates the association of the index with change in word knowledge. Models also included terms for the potentially confounding factors of age (at death), sex, and education plus their interactions with time. Initial models used a composite measure of word knowledge. Subsequent analyses used individual tests of word knowledge as outcomes and included multiple pathologic measures in a single model. Each person’s annual rate of change in word knowledge (estimated from a model without pathology) plotted against AD pathologic burden score (excluding 1 subject with an extreme burden score) was fitted with a function that uses robust locally linear fits [22].
RESULTS
Change in Word Knowledge
At baseline, scores on the composite measure of word knowledge ranged from a low of −3.17 to a high of 1.43 (mean =−0.09, SD=0.97, skewness=−0.76). Higher word knowledge score was associated with younger age (r=−.12, p=.018), more education (r=.47, p<.001), and female gender (t [413]=5.6, p<.001).
Word knowledge was assessed annually from study entry to death, with up to 16 observations per individual (mean=7.2, SD=3.3). To assess change in word knowledge, we constructed a mixed-effects model. This and all subsequent analyses controlled for the potentially confounding effects of age at death, sex, and education. In this analysis, there was a mean loss of 0.067 unit per year (SE=0.009, p<.001) in the composite measure of word knowledge.
Neurodegeneration and Word Knowledge
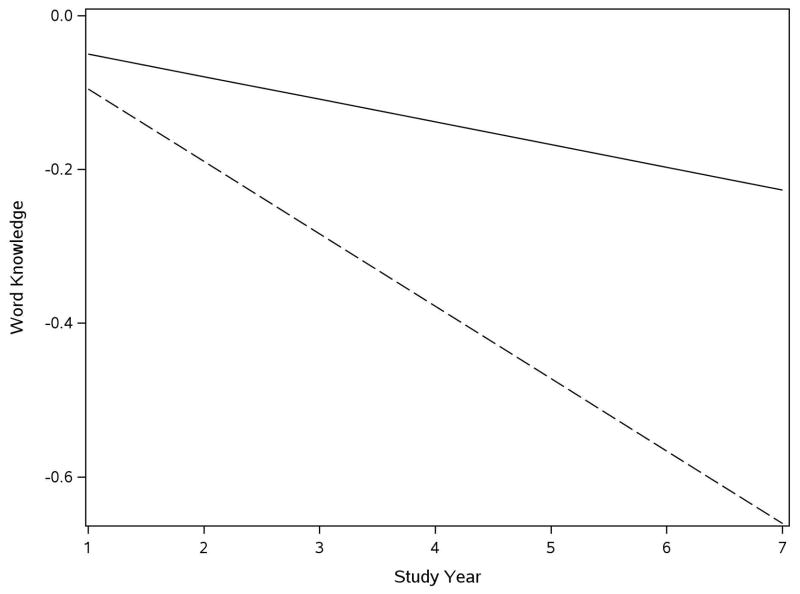
To test the hypothesis that neurodegenerative disease contributes to loss of word knowledge, we repeated the analysis of change in the word knowledge measure with terms added for postmortem measures of neurodegeneration and their interaction with time. We began with the composite measure of AD pathologic lesions which had a somewhat skewed distribution, with scores ranging from a low of 0 to a high of 4.38 (mean=0.62, SD=0.56, skewness=1.48). In the analysis (Table 1), word knowledge did not decline in the absence of postmortem evidence of AD, as shown by the term for time. AD pathologic burden was not related to level of word knowledge, but it was related to change. For each point on the composite measure of AD pathologic burden, annual decline in word knowledge score increased by 0.082 unit. Figure 1, which is based on this analysis, shows that the annual rate of word knowledge decline associated with a relatively high AD pathologic burden (score =0.92, 75th percentile, dashed line) was nearly four times faster than decline associated with a relatively low burden (score=0.13, 25th percentile).
Table 1.
Relation of postmortem measures of neurodegeneration to change in word knowledge*
| Word Knowledge | Reading | Vocabulary | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | (SE) | p | Estimate | (SE) | p | Estimate | (SE) | p | |
| Time | −0.011 | (0.012) | .363 | 0.097 | (0.046) | .033 | −0.106 | (0.044) | .017 |
| AD pathology | 0.025 | (0.073) | .740 | 0.375 | (0.358) | .295 | −0.139 | (0.269) | .606 |
| Time x AD pathology | −0.082 | (0.012) | <.001 | −0.366 | (0.050) | <.001 | −0.262 | (0.045) | <.001 |
| Time | −0.057 | (0.011) | <.001 | −0.154 | (0.043) | <.001 | −0.248 | (0.041) | <.001 |
| Gross infarction | 0.021 | (0.088) | .808 | 0.093 | (0.410) | .820 | 0.062 | (0.320) | .846 |
| Time x gross infarction | −0.046 | (0.016) | .003 | −0.039 | (0.062) | .528 | −0.167 | (0.057) | .003 |
| Time | −0.064 | (0.011) | <.001 | −0.137 | (0.043) | .001 | −0.274 | (0.041) | <.001 |
| Microscopic infarction | −0.131 | (0.091) | .151 | −0.448 | (0.421) | .288 | −0.574 | (0.329) | .087 |
| Time x microscopic infarction | −0.024 | (0.017) | .140 | −0.100 | (0.064) | .119 | −0.092 | (0.061) | .130 |
| Time | −0.062 | (0.010) | <.001 | −0.146 | (0.041) | <.001 | −0.276 | (0.038) | <.001 |
| Lewy bodies | −0.056 | (0.103) | .589 | −0.127 | (0.480) | .792 | −0.321 | (0.375) | .392 |
| Time x Lewy bodies | −0.059 | (0.019) | .001 | −0.124 | (0.073) | .091 | −0.159 | (0.068) | .020 |
From separate mixed-effects models adjusted for age at death, sex, education, and their interactions with time.
Figure 1.
Decline in word knowledge with low and high AD pathologic burden. Predicted 7-year course of change in word knowledge in persons with relatively low (25th percentile, solid line) and high (75th percentile, dashed line) Alzheimer’s disease pathologic burden.
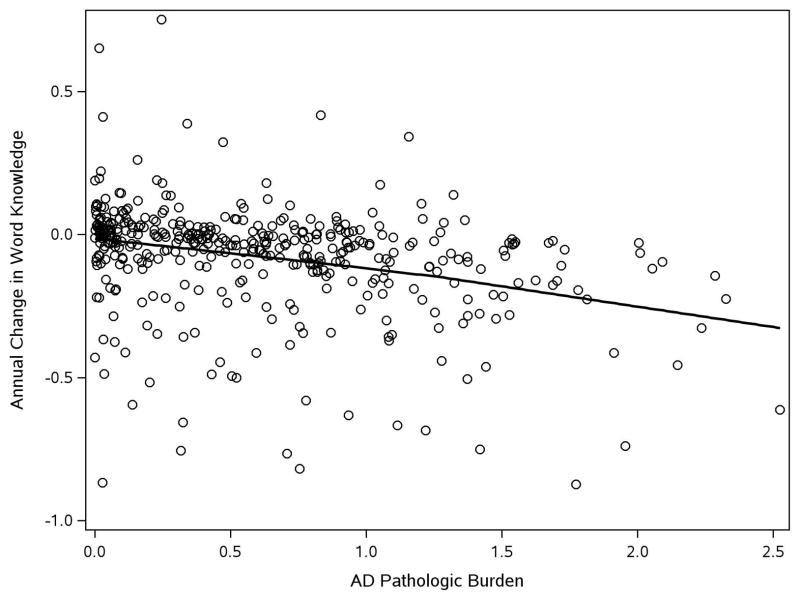
To further examine the contribution of AD to decline in word knowledge, we plotted the person-specific slopes derived from an age, sex, and education adjusted model against AD pathologic burden fitted with a locally reweighted linear smooth function (Figure 2). The figure suggests an approximately linear relationship of pathologic burden with rate of decline in word knowledge. To see if differences between the components of the word knowledge measure affected results, we repeated the analysis, first with the word reading test and then with the vocabulary test (Table 1). Higher AD pathologic level was associated with more rapid decline in both word reading and vocabulary, as shown by the interaction terms in each model.
Figure 2.
Relation of AD pathologic burden to decline in word knowledge. Predicted rate of decline in word knowledge as function of Alzheimer’s disease pathologic burden fitted with a locally reweighed linear smooth function.
Postmortem data on three other common neurodegenerative lesions was available in 383 persons: 140 (36.6%) had one or more gross cerebral infarctions, 118 (30.8%) had one or more microscopic cerebral infarctions, and 81 (21.6%) had at least one Lewy body. As shown in Table 1, gross infarction was associated with an 81% increase (0.046/0.057) in rate of decline in word knowledge, microscopic infarction had no effect, and Lewy bodies were associated with a 95% increase (0.059/0.062) in decline rate. When AD pathologic burden, gross infarction, and Lewy bodies were included in the same model, each was associated with more rapid decline in word knowledge (estimate for AD pathologic burden x time=−0.100, SE=−0.014, p<.001; estimate for gross infarction x time=−0.042, SE=0.014, p=.003; estimate for Lewy bodies x time=−0.055, SE=0.017, p=.001). In the absence of these lesions, there was no change in word knowledge (estimate for time =0.018, SE=0.014, p=.180).
In analyses using individual tests as outcomes, both gross infarction and Lewy bodies were associated with vocabulary decline, but neither was associated with decline in word reading though the Lewy body term was nearly significant (Table 1). When pathologic measures were simultaneously analyzed, results were comparable and in the absence of these lesions, reading score increased (estimate for time=0.111, SE=0.056, p=.048) and vocabulary score did not change (estimate for time=−0.006, SE=0.051, p=.902).
DISCUSSION
For up to 15 years, word knowledge was assessed annually in more than 400 older persons who subsequently died and underwent brain autopsy. Postmortem measures of plaques, tangles, cerebral infarction, and Lewy bodies were robustly related to rate of decline in word knowledge during the observation period. The results indicate that word knowledge declines in old age largely due to common neuropathologic lesions associated with late life dementia.
The fate of lexical knowledge in old age and mild dementia has been difficult to establish, as noted above. Word knowledge tests that minimize response demands have not been widely used in epidemiologic studies of older people without dementia [17,23]. Case-control studies of the effect of dementia on word knowledge are hard to interpret because lower premorbid cognitive ability is a risk factor for late life dementia [24]. Longitudinal studies of persons with clinically diagnosed dementia have, with some exceptions [7,13], suggested that word reading does decline in affected individuals [8–12] although the change has been minimal in some cases [8,11]. The present study differs from prior research in using pathological rather than clinical indicators of neurodegenerative disease. We found that neuropathologic measures of the lesions traditionally associated with late life dementia were robustly related to rate of decline in word knowledge.
Because word reading is hypothesized to be maintained in mild dementia, tests of word reading are often used to estimate premorbid cognitive ability in persons being evaluated for possible dementia. Contrary to this hypothesis, however, decline in word reading in this study was linearly related to pathologic level of AD. These data suggest that word reading and related measures of lexical knowledge have limited value as measures of premorbid cognitive ability in old age.
This study has several notable strengths. We used previously established measures of word knowledge and neurodegenerative disease in analyses. The availability of a mean of more than seven years of evenly spaced observations enhanced our ability to reliably capture individual differences in change in word knowledge. The high participation in follow-up and brain autopsy reduces the likelihood that attrition affected results. Results were consistent across multiple measures of word knowledge and neurodegeneration. An important limitation is the selected cohort; replication of these findings in other groups will be important.
Acknowledgments
We thank the hundreds of Catholic nuns, priests, and brothers who have participated in the Religious Orders Study; Julie Bach, MSW, and Traci Colvin, MPH, for study coordination; John Gibbons, MS, and Greg Klein, MS, for data management; Donna Esbjornson, MS, for statistical programming; and Priya Patel for preparing the manuscript.
FUNDING
Support for this project was provided by federal grants from the National Institute on Aging: P30AG10161 and R01AG15819.
Footnotes
COMPETING INTERESTS
None
References
- 1.Horn JL, Cattell RB. Age differences in fluid and crystallized intelligence. Acta Psychologica. 1967;26:107–129. doi: 10.1016/0001-6918(67)90011-x. [DOI] [PubMed] [Google Scholar]
- 2.Starr JM, Whalley LJ, Inch S, Shering PA. The quantification of the relative effects of age and NART-predicated IQ on cognitive function in healthy old people. Int J Geriatr Psychiatry. 1992;7:153–157. [Google Scholar]
- 3.Browles RP, Salthouse TA. Vocabulary test format and differential relations to age. Psychol Aging. 2008;23:366–376. doi: 10.1037/0882-7974.23.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verhaeghen P. Aging and vocabulary score: a meta-analysis. Psychol Aging. 2003;18:332–339. doi: 10.1037/0882-7974.18.2.332. [DOI] [PubMed] [Google Scholar]
- 5.Nelson HE. Windsor. NFER-Nelson; 1982. The National Adult Reading Test (NART): test manual. [Google Scholar]
- 6.Almkrist O, Tallberg I-M. Cognitive decline from estimated premorbid status predicts neurodegeneration in Alzheimer’s disease. Neuropsychol. 2009;23:117–124. doi: 10.1037/a0014074. [DOI] [PubMed] [Google Scholar]
- 7.Ashendorf L, Jefferson AL, Green RC, Stern RA. Test-retest stability on the WRAT-3 reading subtest in geriatric cognitive evaluations. J Clin Exp Neuropsychol. 2009;31:605–610. doi: 10.1080/13803390802375557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cockburn J, Keene J, Hope T, Smith P. Progressive decline in NART score with increasing dementia severity. J Clin Exp Neuropsychol. 2000;22:508–517. doi: 10.1076/1380-3395(200008)22:4;1-0;FT508. [DOI] [PubMed] [Google Scholar]
- 10.Taylor KI, Salmon DP, Rice VA, et al. Longitudinal examiniation of America National Adult Reading, Test (AMNART) performance in dementia of the Alzheimer’s type (DAT): validation and correction based on degree of cognitive decline. J Clin Exp Neuropsychol. 1996;18:883–891. doi: 10.1080/01688639608408309. [DOI] [PubMed] [Google Scholar]
- 11.Paque L, Warrington EK. A longitudinal study of reading ability in patients suffering from dementia. J Int Neuropsychol Soc. 1995;1:517–524. doi: 10.1017/s1355617700000643. [DOI] [PubMed] [Google Scholar]
- 12.Fromm D, Holland A, Nebes RD, Oakley MA. A longitudinal study of word-reading ability in Alzheimer’s disease: evidence from the National Adult Reading Test. Cortex. 1991;27:367–376. doi: 10.1016/s0010-9452(13)80032-9. [DOI] [PubMed] [Google Scholar]
- 13.O’Carroll RE, Prentice N, Murray C, van Beck M, Ebmeier KP, Goodwin GM. Further evidence that reading ability is not preserved in Alzheimer’s disease. Br J Psychiatry. 1995;167:659–662. doi: 10.1192/bjp.167.5.659. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RS, Bienias JL, Evans DA, Bennett DA. Religous Orders Study: overview and change in cognitive and motor speed. Aging Neuropsychol Cogn. 2004;11:280–303. [Google Scholar]
- 15.Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- 16.Grober E, Sliwinski M. Development and validation of model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- 17.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 18.Ekstrom RB, French JW, Harman HH, Kermen D. Manual for kit of factor-referenced cognitive tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- 19.Schneider JA, Aggarwal NT, Barnes LL, Boyle PA, Bennett DA. The neuropathology of older persons with and with dementia from community versus clinic cohorts. J Alzheimers Dis. 2009 Sept 11; doi: 10.3233/JAD-2009-1227. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer’s disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laird NB, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 22.Venables W, Ripley B. Modern applied statistics with S-Plus. New York: Springer-Verlag; 1994. [Google Scholar]
- 23.Korten AE, Henderson AD, Christenson H, et al. A prospective study of cognitive function in the elderly. Psychol Med. 1997;27:919–930. doi: 10.1017/s0033291797005217. [DOI] [PubMed] [Google Scholar]
- 24.McGurn B, Starr JM, Topfer JA, et al. Pronunciation of irregular words is preserved in dementia, validating premorbid IQ estimation. Neurology. 2004;62:1184–1186. doi: 10.1212/01.wnl.0000103169.80910.8b. [DOI] [PubMed] [Google Scholar]