Abstract
Members of the family Enterobacteriaceae including Klebsiella have re-emerged as major pathogens in solid organ transplantation. The recent appearance and dissemination of carbapenemase-producing Enterobacteriaceae in Europe and the northeastern United States represents a major challenge to the treatment of enteric gram-negative bacterial infections in immunocompromised patients; however, few reports have detailed the outcomes of such infections. Here we report two cases of Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella infections in orthotopic liver transplant recipients, which were the index case and initial secondary case for an outbreak of KPC-producing Enterobacteriaceae in our institution. In both instances, the pathogens were initially misidentified as being carbapenem sensitive, the infections recurred after cessation of directed therapy, and the patients ultimately succumbed to their infections.
MeSH Keywords: Adult; Cross infection/epidemiology; Cross Infection/microbiology*; Diseases Outbreaks*; DNA, Bacterial/genetics; Drug Resistance, Bacterial; Enterobacteriaceae Infections*/epidemiology; Enterobacteriaceae Infections*/microbiology; Enterobacteriaceae Infections*/mortality; Fatal Outcome; Humans; Klebsiella*/isolation & purification*; Klebsiella*/enzymology; Klebsiella*/genetics; Liver Transplantation*; Sepsis/microbiology*; beta-Lactam Resistance*; beta-Lactamases*/genetics
Introduction
Over the last decade, gram-negative bacilli have re-emerged as important pathogens in solid organ transplant (SOT) recipients (1). While numerous factors likely contributed to this epidemiologic shift, the resurgence of gram-negative pathogens has coincided with the dissemination of multidrug resistance in Enterobacteriaceae and nonfermentative bacilli. In Enterobacteriaceae, resistance to expanded-spectrum β-lactam antibiotics is usually conferred by plasmid-encoded extended spectrum β-lactamases (ESBLs), and ESBL-containing isolates are typically resistant to other classes of antibiotics. Since carbapenems are highly resistant to ESBL-mediated hydrolysis, these agents are preferred for the treatment of invasive infection caused by these organisms (2).
For that reason, the recent emergence of carbapenem-hydrolyzing β-lactamases (carbapenemases) in Enterobacteriaceae represents a concerning threat to the treatment of invasive enterobacterial infections. While carbapenemase-producing Enterobacteriaceae (CP-E) are rare in most parts of the world, certain geographic areas have seen significant outbreaks that may soon approach endemicity in some hospitals. In the northeastern United States and Israel, the plasmid encoded Ambler class A serine β-lactamase KPC (for Klebsiella pneumoniae carbapenemase) has been found in large institutional outbreaks of multidrug resistant Klebsiella and Enterobacter spp. (3–5). In southern Europe, the Ambler class B metallo-β-lactamase VIM has been associated with multiple genera outbreaks of gram-negative pathogens (6–8). Recent reports suggest that KPC-producing Enterobacteriaceae may be rapidly spreading across North America, while metallo-β-lactamase-producing Enterobacteriaceae are being reported in many locations worldwide (9–11). SOT recipients are vulnerable to infection by multidrug resistant bacteria due to their underlying end-stage organ failure combined with a major surgical procedure and the necessity of life long immunosuppression. In this report, we describe the clinical and microbiologic features of CP-E infection in two liver transplant (LT) recipients caused by KPC-producing Klebsiella.
Materials and Methods
Microbiology
Identification and antibiotic susceptibility testing was performed using the Vitek 2 system with the AST GN14 card (bioMérieux, Durham, NC). Minimum inhibitory concentrations (MICs) were determined by the microbroth dilution method in cation-adjusted Mueller-Hinton broth and the Kirby Bauer agar disk diffusion method, in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines (12). Modified Hodge testing was performed as previously described (13). Briefly, a Mueller-Hinton agar (MHA) plate was inoculated with a one-tenth dilution of the carbapenem sensitive strain Escherichia coli ATCC 25922 adjusted to a McFarland 0.5 standard. A 10-μg imipenem disk was then placed in the center of the plate and the scrutinized Klebsiella isolates were struck radially from the disk to the plate edge. Invagination of E. coli growth at interface between the Klebsiella streak and the imipenem zone of inhibition was read as a positive modified Hodge test (13).
DNA Techniques
Plasmid DNA was isolated from Klebsiella strains using the QIAGEN Plasmid Midi Kit (QIAGEN, Valencia, CA) using 60°C pre-warmed eluting buffer, as recommended by the manufacturer for large plasmid isolation. PCR amplification of the blaKPC gene was performed using previously reported primers and condition (14). blaKPC verification and allele identification was performed by bidirectional DNA sequencing (Biomolecular Research Facility, University of Virginia Health System).
Southern Analysis
Plasmid DNAs were subjected to electrophoresis on a 0.6% Pulse Field Certified Agarose (Bio-Rad Laboratories, Hercules, CA) gel using the following parameters: 0.5X TBE, 70 volts, 4°C, 20 hours. The gel was stained with ethidium bromide and migration distances of the DNA bands from the clinical isolates were compared to reference plasmid DNAs from E. coli strain V517. Plasmid DNA was transferred to Amersham Hybond N+ nylon membrane (GE Healthcare, Piscataway, NJ) by capillary transfer and hybridized with a 1009-bp DNA amplicon of blaKPC-2 labeled using the Amersham ECL Direct Nucleic Acid Labeling and Detection System (GE Healthcare) in accordance with the manufacturer’s instructions.
Pulse Field Gel Electrophoresis
Pulsed-field gel electrophoresis (PFGE) of XbaI-digested total DNA was performed using the PulseNet protocol for E. coli and other Enterobacteriaceae (15). PFGE profile analysis and dendrogram construction were performed using BioNumerics software (Applied Maths, Austin, TX).
Case Reports
Case 1
A 45-year-old man with decompensated liver disease resulting from hepatitis C virus infection and alcoholic cirrhosis underwent liver transplantation at our center in July 2007. The patient had been discharged from a Philadelphia hospital four days prior to LT after a nine day hospitalization for spontaneous bacterial peritonitis due to E. coli. LT using the piggyback technique with duct-to-duct biliary anastomosis was performed without complication; piperacillin-tazobactam (TZP) was given for perioperative antibiotic prophylaxis. Peritoneal fluid obtained during the transplant grew vancomycin resistant Enterococcus faecium (VRE). The patient was discharged on prophylactic immunosuppression (tacrolimus with trough levels of 5–10 ng/mL, mycophenolate mofetil (MMF), and a steroid taper), prophylactic trimethoprim-sulfamethoxazole (TMP-SMX), and a two week course of linezolid on postoperative day seven. Two weeks later he was readmitted to a Philadelphia hospital with increasing ascites and abdominal pain. His condition rapidly deteriorated and he was transferred to our hospital within 48 hours, where he was diagnosed with an anastomotic bile leak, peritonitis due to linezolid-resistant E. faecium (LRE) and Candida glabrata, and hepatic artery thrombosis. He underwent exploratory laparotomy and washout, ERCP with multiple biliary stent deployments, and unsuccessful attempts at hepatic artery stenting. Over the next two weeks additional complications arose including CMV hepatitis, Aspergillus fumigatus and ventilator-associated pneumonia, tacrolimus toxicity, acute kidney injury requiring renal replacement therapy, pancytopenia and luminal GI bleeding. Antimicrobial therapy during this period included tigecycline, ciprofloxacin, anidulafungin, voriconazole and ganciclovir; MMF was discontinued.
On hospital day 21, the patient became septic and Klebsiella pneumoniae grew from blood (strain Kpn1014), urine, abdominal drainage, and bronchial cultures. Antibiotic susceptibilities by Vitek 2 were notable for a meropenem MIC of ≤ 0.25 μg/mL (Table 1). LRE was also isolated from the blood. Meropenem and daptomycin were started and tigecycline was stopped. Despite initial improvement, the patient again developed septic shock and multiorgan dysfunction twelve days later while still receiving meropenem. K. pneumoniae (strain Kpn1016) was cultured from abdominal drain cultures, while Klebsiella oxytoca was isolated from both abdominal drain cultures (strain Kox1015) and respiratory secretions. The K. oxytoca isolate had a Vitek meropenem MIC of 1 μg/mL, and the K. pneumoniae isolate had a Vitek meropenem MIC of ≥ 16 μg/mL (Table 1). Imaging studies and a second exploratory laparotomy failed to identify any undrained purulent collections. Kox1015 was initially interpreted as meropenem susceptible; however, the elevated meropenem MIC in strain Kpn1016 raised concern for the presence of carbapenemase, which previously had not been observed in any clinical Enterobacteriaceae isolates at our medical center. Molecular studies confirmed the presence of blaKPC-2 in both Kpn1016 and Kox1015 (see below). Meropenem was discontinued and a two week course of colistimethate sodium (colistin) and tigecycline was given. Isolation of carbapenemase-producing Klebsiella triggered a hospital-wide epidemiology surveillance program and updated infection control training programs for transplant and ICU healthcare staff. The patient remained critically ill and the bile leak was not amenable to repair by interventional radiology or surgery. Over the next several months, he developed a large liver abscess and had several episodes of bacteremia with KPC-producing K. pneumoniae as well as Stenotrophomonas maltophilia, Enterococcus faecalis, and E. faecium. Multiple courses of culture-directed antibiotics, including combinations of colistin, tigecycline, amikacin, high dose TMP-SMX, and daptomycin, were given. On hospital day 115 (post-LT day 138), his clinical condition was deemed to be sufficiently stable to attempt re-LT as a final attempt for cure. The original graft was necrotic with several large abscesses. Intraoperatively the patient developed a severe coagulopathy and refractory hypotension despite massive resuscitation efforts, and he died several hours after graft reperfusion.
Table 1.
Carbapenem susceptibilities and carbapenemase characterization of the clinical Klebsiella isolates
| Patient | Isolate | Species | Modified Hodge test | blaKPC PCR | Source | Day of isolation1 | Duration of carbapenem therapy2 | meropenem MIC (μg/ml)3 |
ertapenem MIC (μg/ml)3 |
imipenem disk diffusion (mm) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitek 2 | Microbroth dilution | Vitek 2 | Microbroth dilution | |||||||||
| Case 1 | ||||||||||||
| Kpn1014 | K. pneumoniae | - | - | Blood | 0 | 0 | ≤ 0.25 | ≤ 0.25 | n/d | ≤ 0.5 | 28 | |
| Kox1015 | K. oxytoca | + | + | Drain | 12 | 10 | 1 | 8 | ≥ 8 | 16 | 16 | |
| Kpn1016 | K. pneumoniae | + | + | Drain | 12 | 10 | ≥ 16 | 4 | ≥ 8 | 8 | 17 | |
| Case 2 | ||||||||||||
| Kpn1017 | K. pneumoniae | + | + | Blood | 47 | 0 | 4 | 16 | 4 | 8 | 17 | |
Compared to the day of isolation of K. pneumoniae Kpn1014 from patient 1
Days of carbapenem therapy prior to culturing the isolate
Boldface values indicate intermediate or resistant susceptibilities
n/d, not determined
Case 2
A 64-year-old woman underwent LT for end-stage liver disease due to nonalcoholic steatohepatitis and hepatocellular carcinoma at our center in August 2007. LT using the piggyback technique with duct-to-duct biliary anastomosis was performed without complications, and she was admitted to the same surgical intensive unit as patient 1. TZP was given perioperatively. Prophylactic immunosuppression included tacrolimus (trough levels, 5–10 ng/mL), MMF and corticosteroids. Initial graft function was poor and she developed steroid-resistant acute cellular rejection, which responded to antithymocyte globulin. On postoperative day fourteen she developed a large pulmonary embolism with hemodynamic instability. Subsequent problems over the next month included GI bleeding, CMV viremia, Clostridium difficile colitis, VRE bacteremia, and acute kidney injury requiring renal replacement therapy. Antimicrobial therapy during this period included linezolid, cefepime, metronidazole, ganciclovir, and prophylactic TMP-SMX; she did not receive a carbapenem.
She slowly improved until post-LT day 45, when she developed septic shock and respiratory failure. K. pneumoniae was isolated from blood (strain Kpn1017) and urine cultures. The meropenem MIC was 4 μg/mL by Vitek 2, which was interpreted as susceptible. Meropenem was started and subsequent blood cultures were negative; however, she remained febrile and hypotensive requiring inotropic support. Five days later, Kpn1017 was confirmed to be modified Hodge test and blaKPC PCR positive, and meropenem was changed to colistin. She responded well to a two week course of colistin; unfortunately, graft function further deteriorated when K. pneumoniae bacteremia and septic shock recurred several weeks later. Due to her multiple medical problems, re-LT was not an option and she died within days of withdrawal of intensive medical care.
Microbiology
Carbapenem susceptibilities and genetic analysis of the Klebsiella isolates are presented in Table 1. Kpn1014, the initial K. pneumoniae isolate from patient 1, had a meropenem MIC ≤ 0.25 μg/mL by Vitek 2 and microbroth dilution and a zone diameter of 28 mm by disk diffusion. The isolate had a negative modified Hodge test and the blaKPC gene was not detected by PCR amplification (Fig. 1, Table 1). Kox1015, isolated from patient 1 after ten days of meropenem therapy, was found to have an MIC to meropenem of 1 μg/mL (susceptible) by Vitek 2 but 8 μg/mL (intermediate) by microbroth dilution (Table 1). Kpn1016 had an MIC to meropenem of ≥ 16 μg/mL (resistant) by Vitek 2 and 4 μg/mL (susceptible) by microbroth dilution (Table 1). Kpn1017 isolated from patient 2 thirty-five days after isolating Kpn1016 from patient 1, had an MIC to meropenem of 4 μg/mL (susceptible) by Vitek 2 and 16 μg/mL (resistant) by microbroth dilution (Table 1). On disk diffusion testing, the zones of inhibition for Kox1015, Kpn1016, and Kpn1017 (zone diameters 16–17 mm) were smaller than that for Kpn1014 but remained in the susceptible range. Recently, detection of CP-E by automated susceptibility testing systems has been shown to be greatly improved by using ertapenem rather than meropenem or imipenem as the class agent for carbapenems (16). Kox1015, Kpn1016, and Kpn1017 had an MIC to ertapenem of 4 μg/mL or greater (non-susceptible) by Vitek 2 and microbroth dilution (Table 1). Kox1015, Kpn1016, and Kpn1017 also had positive modified Hodge and blaKPC PCR tests (Fig. 1, Table 1). DNA sequencing confirmed that all isolates carried the blaKPC-2 allele.
Fig. 1.
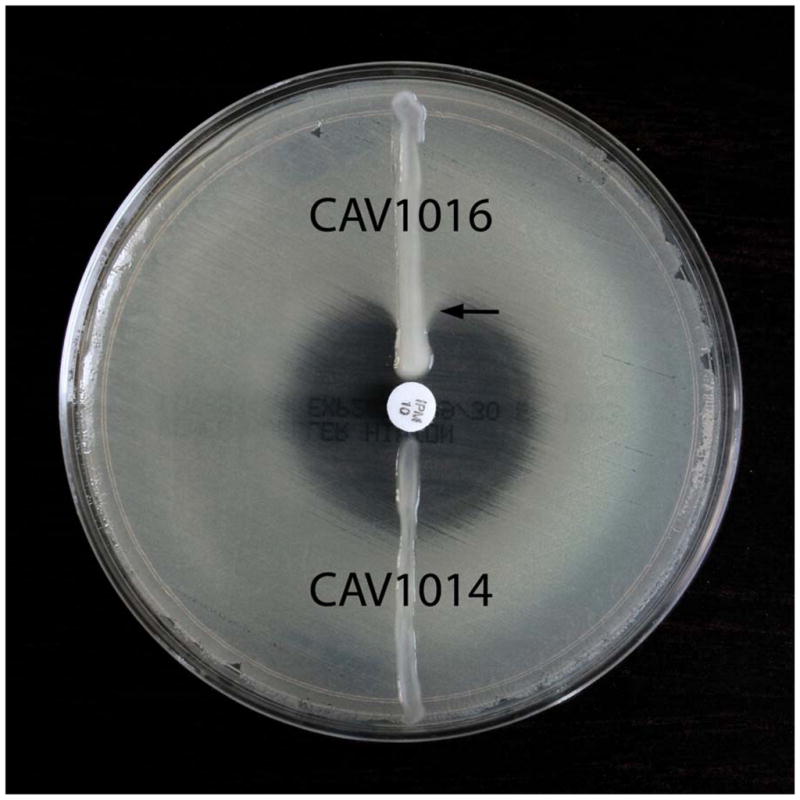
Modified Hodge tests of K. pneumoniae isolates from patient 1. A 10-μg imipenem disk was placed in the center of a MHA plate shortly after being inoculated with the carbapenem-sensitive strain E. coli ATCC 25922, and isolates Kpn1014 and Kpn1016 were struck radially from the disk to the plate edge. Kpn1014 is blaKPC-2-negative and shows a concentric zone of growth inhibition at its intersection with ATCC 25922, whereas invagination of ATCC 25922 growth into the imipenem zone of inhibition adjacent to Kpn1016 is indicative of carbapenemase production.
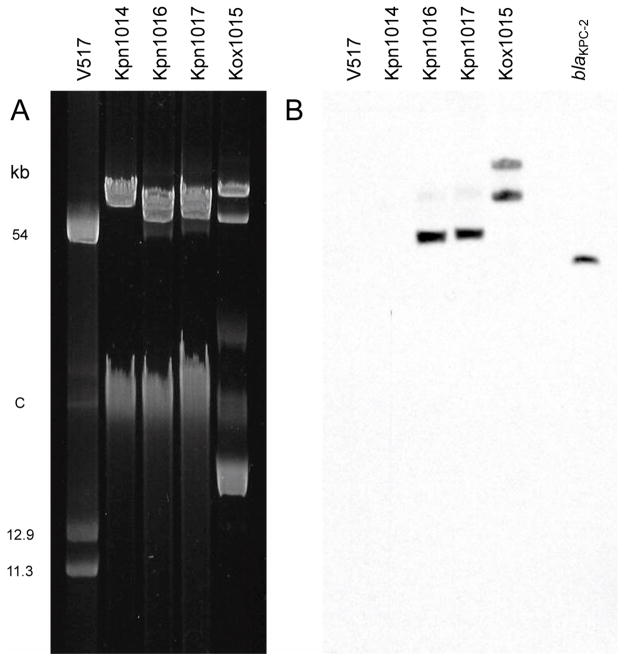
Plasmids were extracted from the Klebsiella isolates and separated by gel electrophoresis. As shown in Figure 2A, the plasmid profiles of Kpn1016 and Kpn1017 were identical, while those of Kpn1014 and Kox1015 differed by several bands. Southern blot analysis using a blaKPC specific probe confirmed the presence of blaKPC plasmids in strains Kox1015, Kpn1016, and Kpn1017 but not Kpn1014 (Fig. 2B). Kpn1016 and Kpn1017 had a single plasmid of 54-kb that hybridized to the blaKPC probe, while Kox1015 had two blaKPC-positive plasmid bands of approximately 80- and 110-kb.
Fig. 2.
(A) Plasmid DNA profiles of Klebsiella isolates from patient 1 (Kpn1014, Kpn1016, and Kox1015) and patient 2 (Kpn1017) resolved on a 0.6% agarose gel. E. coli V517 supercoiled plasmid standards (kb). C, chromosomal DNA. (B) Southern blot of the gel shown in panel A after hybridization with an 1009-bp blaKPC-2-specific probe. DNA sequence-confirmed blaKPC-2 PCR product (right-most lane) loaded one hour before completion of the electrophoresis served as the positive control.
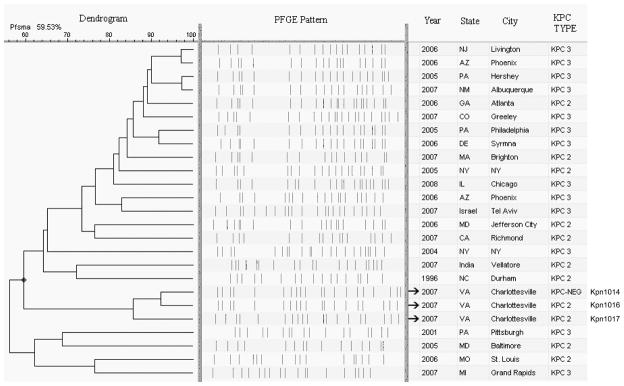
PFGE of the K. pneumoniae isolates revealed that Kpn1014 was closely related to Kpn1016, differing by a total of three bands, and that Kpn1016 and Kpn1017 belong to the same lineage (87% Dice similarity coefficient) (Fig. 3). Kpn1016 and Kpn1017 were not related (<60% Dice similarity coefficient) to other KPC-producing K. pneumoniae isolates in the CDC database, including archived strains obtained in 2005 from the Philadelphia hospital where patient 1 was hospitalized early in his course (data not shown).
Fig. 3.
Pulsed-field gel electrophoresis profiles and dendrogram of XbaI macrorestricted chromosomal DNA fragments of the K. pneumoniae isolates from patient 1 (Kpn1014, Kpn1016), patient 2 (Kpn1017), and 22 additional KPC-producing K. pneumoniae isolates.
Discussion
This report highlights several challenges regarding the diagnosis and treatment of CP-E infections. First, it demonstrates the difficulty in recognizing and verifying carbapenemase-production in Enterobacteriaceae by clinical microbiology laboratories. In the United States, the KPC enzyme has emerged in diverse array of Enterobacteriaceae, including K. pneumoniae, K. oxytoca, Enterobacter spp., E. coli, Citrobacter freundii, and Salmonella enterica (17). As seen in these cases, automated susceptibility testing can incorrectly report a KPC-producing organism as carbapenem sensitive (4, 16, 18). In hospitals with no prior experience with CP-E, initial recognition of an enteric carbapenemase producer may only occur due to the consideration of an astute clinician or microbiologist. Second, the cases confirm that the treatment of CP-E infections with carbapenemases is associated with clinical and/or microbiological failure. Erroneous assignment as carbapenem susceptible may have grave clinical consequences. Third, unrecognized, undrained or inaccessible collections may serve as a nidus for recurrent infection. Although not observed in these cases, re-treatment of CP-E infections has the potential to further select for resistance in these isolates.
Twelve cases of invasive CP-E infection, including the 2 presented herein, have been reported in the English language literature and are summarized in Table 2 (8, 19–23). The cases included 7 liver, 3 kidney, and one heart transplant recipient; the organ transplanted was not reported in one case. Thirteen Enterobacteriaceae were cultured: 9 K. pneumoniae, 2 K. oxytoca, and 2 Enterobacter cloacae. Nine of the twelve cases involved bloodstream infection. In North America all isolates (n=7) carried blaKPC, while in Europe the isolates (n=6), most from a single Italian hospital outbreak, carried blaVIM-1. This pattern generally reflects the current distribution of carbapenemases in Enterobacteriaceae, with a preponderance of KPC (serine) carbapenemases in North America and VIM metallo-β-lactamases in Europe. The crude mortality rate was 58%; six of the seven LT patients died. No clear relationship between the chosen antimicrobial regimen and survival could be determined.
Table 2.
Summary of clinical and microbiological characteristics of carbapenemase-producing Enterobacteriaceae infections in solid organ transplant recipients
| Patient | Ref No. | Age (y) | Locale | Transplant | Time after transplant | CP-E organism(s) isolated | Carbapenemase | Source | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | NR | Italy | Kidney | NR | K. pneumoniae | VIM-1 | Blood | TZP | Survived |
| 2 | 8 | NR | Italy | Kidney | NR | K. pneumoniae | VIM-1 | Blood, urine | MER | Survived |
| 3 | 8 | NR | Italy | Liver | NR | K. pneumoniae | VIM-1 | Blood | MER | Died |
| 4 | 8 | NR | Italy | Liver | NR | K. pneumoniae | VIM-1 | Blood, wound | AMK CIP LEV MER SXT TZP | Survived |
| 5 | 8 | NR | Italy | Liver | NR | K. pneumoniae | VIM-1 | Blood | AMK MER | Died |
| 6 | 19 | 44 | New York City | Kidney | 26 m | K. pneumoniae | KPC-2 | Blood | NR | Survived |
| 7 | 20 | 55 | Italy | Liver | 46 d | Enterobacter cloacae | VIM-1 | Pleural fluid, BAL, bile | AMK COL1 IMP RIF | Died |
| 8 | 21 | NR | Michigan | Heart | NR | K. oxytoca | KPC-2 | Sputum | COL2 GEN TGC | Survived |
| 9 | 22 | 57 | Pennsylvania | NR | NR | K. pneumoniae | KPC | Pleural fluid, BAL | GEN TGC | Died |
| 10 | 23 | 54 | Texas | Liver | 45 d | E. cloacae 3 | KPC-2 | Blood, wound | AMK CIP | Died |
| 11 | PR | 45 | Virginia | Liver | 55 d | K. pneumoniae, K. oxytoca | Both KPC-2 | Blood, sputum, abdominal drain, urine | AMK COL1 TGC | Died |
| 12 | PR | 64 | Virginia | Liver | 46 d | K. pneumoniae | KPC-2 | Blood | COL1 | Died |
Abbreviations: BAL, bronchoalveolar lavage; d, days; m, months; NR, not reported; PR, present report; y, years
Antimicrobial abbreviations: AMK, amikacin; CIP, ciprofloxacin; COL1, colistin parenteral; COL2, colistin inhaled; GEN, gentamicin; IPM, imipenem; LEV, levofloxacin; MER, meropenem; RIF, rifampin; SXT, trimethoprim-sulfamethoxazole; TGC, tigecycline; TZP, piperacillin-tazobactam
Also bacteremic with KPC-2 producing Pseudomonas putida
Several factors place SOT recipients at high risk for infection by CP-E. First, SOT recipients often receive prolonged courses of broad spectrum antibiotics, an enormous selective pressure for the acquisition of antimicrobial-resistant organisms. Determinants of resistance, including KPC and VIM carbapenemases, are often encoded on transposons or plasmids that carry suites of other resistance genes. Therefore, selective pressure occurs even with the use of unrelated classes of antibiotics. In this regard, it is instructive that patient 2 developed blaKPC-2-positive K. pneumoniae bacteremia without antecedent carbapenem therapy. Second, transplant recipients undergo numerous invasive therapeutic and diagnostic procedures. Technical complications, including early graft injury, as well as the intensity of immunosuppression, poor allograft function and rejection are particularly important risks for the development of nosocomial infections in the early post-transplant period (24, 25). Third, SOT patients have high rates of hospitalization, which increase the risk of becoming colonized with healthcare-associated multidrug resistant organisms like CP-E. Intra- and interhospital transfers are common amongst organ transplant recipients and may geographically expand opportunities for cross-colonization by drug-resistant organisms. We speculate that the index patient may have become colonized with blaKPC-2-containing Klebsiella during a hospitalization in Philadelphia, although PFGE failed to show genetic relatedness between his isolates and tested Philadelphia KPC-containing Klebsiella isolates. Alternatively, he may have acquired a strain during an earlier hospitalization that later served as a blaKPC-2-plasmid or transposon donor.
Infections in SOT patients and other immunocompromised hosts can provide sentinel warning of changes in pathogen trafficking and dissemination, both in the hospital and the community (26, 27). In this instance, the reported patients were the first cases of KPC-producing Enterobacteriaceae infection identified at our institution. A subsequent hospital-wide outbreak involving two dozen additional patients has been recognized and epidemiologically and/or microbiologically linked to the index cases, despite reinforced infection control education and monitoring efforts (A.M., H.C., K.C.H. and C.D.S., unpublished data). Similarly, in an Italian report describing an outbreak of VIM-1 producing Klebsiella pneumoniae bloodstream infections, the index case occurred in a kidney transplant recipient (8). These reports illustrate that SOT recipients can act not only as sentinels for new pathogens but may be conduits for their spread to new locations or facilities.
Unfortunately, the risk for continued CP-E dissemination will remain high due to suboptimal clinical laboratory recognition of carbapenem-resistance in enteric gram-negative bacilli (as demonstrated by the initial antibiotic susceptibility results in these cases), unrecognized “silent” fecal carriage of CP-E, and environmental contamination. While susceptibility testing systems that use ertapenem as the class agent for carbapenems have been shown to be vastly improved at detecting CP-E, they are not infallible (16). Other detection methods under investigation include the use of CHROMagar optimized for CP-E detection and the assessment of potentiation of carbapenem resistance by boronic acid compounds (28, 29). The optimal method for detection and screening for carbapenemase production in Enterobacteriaceae remains to be defined.
The optimal therapy for serious CP-E infection is also uncertain, and treatment of disease in SOT patients poses several additional challenges. Surgical debridement of devitalized tissue and drainage of collections remains a cornerstone for effective treatment of local infections. Reducing the level of immunosuppression may also offer the best opportunity for control of these infections, especially in cases where overimmunosuppression may have been an important contributing factor, such as the cases presented here. CP-E isolates are routinely resistant to many classes of antibiotics, often leaving polymyxins and tigecycline as the only remaining antimicrobials with in vitro activity against these organisms (30). In recent years, the polymyxins (colistin and polymyxin B) have been resurrected due to the emergence of carbapenem resistance in enteric and nonfermentative gram-negative bacilli. In the modern era, polymyxin-induced nephrotoxicity and neurotoxicity appears to be less common and severe compared to the historic experience (31). However, polymyxin toxicities remain important clinical considerations, and whether these toxicities are more frequent or severe in transplant patients receiving calcineurin inhibitors (with their associated nephrotoxicty and neurotoxicty) remains to be determined.
Another concern is the emergence of polymyxin resistance in CP-E. Rates of polymyxin resistance in KPC-producing K. pneumoniae reached 9–27% during recent outbreaks in New York, and an outbreak of colistin-resistant, metallo-β-lactamase-producing K. pneumoniae has been reported in a Greek intensive care unit (18, 32, 33). We believe that use of polymyxins for selective bowel decontamination prior to LT should be abandoned; not only is it doubtful that the practice reduces post-operative infections, but it is unambiguously linked to the development of colistin resistance (34–36).
Tigecycline is a new semisynthetic glycylcycline antibiotic that has in vitro activity against a broad array of nosocomial pathogens, including most Enterobacteriaceae, and is approved for the treatment of complicated skin and skin structure and complicated intra-abdominal infections (37). A recent survey of 104 CP-E isolates collected from medical centers located in North America, Europe and Latin America failed to identify a single strain that was tigecycline resistant (38). By contrast, 11.9% of the isolates were resistant to polymyxins. However, the efficacy of tigecycline to treat CP-E infection (especially endovascular infection) has been called into question, and treatment-associated increases in tigecycline MICs have been observed during therapy (22, 39). Furthermore, tigecycline is not active against Pseudomonas species and has reduced activity against genera of the tribe Proteeae (Proteus, Providencia, and Morganella).
In conclusion, carbapenem-resistant Enterobacteriaceae are perfectly suited to become important pathogens in SOT recipients. Once largely confined to certain geographic areas, these organisms are rapidly spreading to new institutions and locations around the world. Unfortunately, few new agents are in the pipeline that may have activity against these pan-resistant organisms. In what has been described as a “microbial perfect storm” of exploding drug resistance and neglected antibiotic development, pan-resistant CP-E and the specter of XDR (extreme drug resistance) Enterobacteriaceae represents an enormous threat to solid organ transplantation (40, 41). Strict infection control measures, aggressive antibiotic stewardship, and improved screening strategies will be needed to curtail their spread. Further research is urgently needed to determine the clinical impact and optimal therapeutic and prevention strategies for CP-E infection in organ transplantation.
Acknowledgments
We thank Joanne Carrol and Frankie Brewster for technical assistance and David C. Hooper (Massachusetts General Hospital) for providing us reference strain E coli V517.
Financial support:
Work in the Sifri lab is supported by a Howard Hughes Medical Institute Early Career Award.
Footnotes
Potential financial conflicts of interest:
None declared.
Substances: Anti-Bacterial Agents; Carbapenems; Polymyxins; tigecycline
References
- 1.Singh N, Wagener MM, Obman A, Cacciarelli TV, de Vera ME, Gayowski T. Bacteremias in liver transplant recipients: shift toward gram-negative bacteria as predominant pathogens. Liver Transpl. 2004;10:844–9. doi: 10.1002/lt.20214. [DOI] [PubMed] [Google Scholar]
- 2.Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–66. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 3.Bratu S, Landman D, Haag R, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med. 2005;165:1430–5. doi: 10.1001/archinte.165.12.1430. [DOI] [PubMed] [Google Scholar]
- 4.Bratu S, Landman D, Alam M, Tolentino E, Quale J. Detection of KPC carbapenem-hydrolyzing enzymes in Enterobacter spp. from Brooklyn, New York. Antimicrob Agents Chemother. 2005;49:776–8. doi: 10.1128/AAC.49.2.776-778.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leavitt A, Navon-Venezia S, Chmelnitsky I, Schwaber MJ, Carmeli Y. Emergence of KPC-2 and KPC-3 in carbapenem-resistant Klebsiella pneumoniae strains in an Israeli hospital. Antimicrob Agents Chemother. 2007;51:3026–9. doi: 10.1128/AAC.00299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giakkoupi P, Xanthaki A, Kanelopoulou M, et al. VIM-1 Metallo-beta-lactamase-producing Klebsiella pneumoniae strains in Greek hospitals. J Clin Microbiol. 2003;41:3893–6. doi: 10.1128/JCM.41.8.3893-3896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tato M, Coque TM, Ruiz-Garbajosa P, et al. Complex clonal and plasmid epidemiology in the first outbreak of Enterobacteriaceae infection involving VIM-1 metallo-beta-lactamase in Spain: toward endemicity? Clin Infect Dis. 2007;45:1171–8. doi: 10.1086/522288. [DOI] [PubMed] [Google Scholar]
- 8.Cagnacci S, Gualco L, Roveta S, et al. Bloodstream infections caused by multidrug-resistant Klebsiella pneumoniae producing the carbapenem-hydrolysing VIM-1 metallo-beta-lactamase: first Italian outbreak. J Antimicrob Chemother. 2008;61:296–300. doi: 10.1093/jac/dkm471. [DOI] [PubMed] [Google Scholar]
- 9.Yong D, Choi YS, Roh KH, et al. Increasing prevalence and diversity of metallo-beta-lactamases in Pseudomonas spp., Acinetobacter spp. and Enterobacteriaceae from Korea. Antimicrob Agents Chemother. 2006;50:1884–6. doi: 10.1128/AAC.50.5.1884-1886.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshpande LM, Jones RN, Fritsche TR, Sader HS. Occurrence and characterization of carbapenemase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program (2000–2004) Microb Drug Resist. 2006;12:223–30. doi: 10.1089/mdr.2006.12.223. [DOI] [PubMed] [Google Scholar]
- 11.Kitchel B, Rasheed J, Srinivasan A, McDougal K, Patel JB. Molecular epidemiology of KPC-producing Klebsiella pneumoniae in the United States. 48th Annual ICAAC/IDSA Annual Meeting; Washington, D.C. 2008. [Google Scholar]
- 12.Clinical and Laboratory Standards Institute (CLSI) Seventeenth Informational Supplement, 2008; CLSI document M100-S18. Clinical and Laboratory Standards Institute; Wayne, PA: 2008. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- 13.Lee K, Chong Y, Shin HB, Kim YA, Yong D, Yum JH. Modified Hodge and EDTA-disk synergy tests to screen metallo-beta-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2001;7:88–91. doi: 10.1046/j.1469-0691.2001.00204.x. [DOI] [PubMed] [Google Scholar]
- 14.Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45:1151–61. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Atlanta, GA: CDC; 2004. One-day (24–28 h) standardized laboratory protocol for molecular subtyping of Escherichia coli O157:H7, non-typhoidal Salmonella serotypes, and Shigella sonnei by pulsed field gel electrophoresis. [ http://www.cdc.gov/PULSENET/protocols/ecoli_salmonella_shigella_protocols.pdf] [Google Scholar]
- 16.Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol. 2007;45:2723–5. doi: 10.1128/JCM.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20:440–58. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bratu S, Mooty M, Nichani S, et al. Emergence of KPC-possessing Klebsiella pneumoniae in Brooklyn, New York: epidemiology and recommendations for detection. Antimicrob Agents Chemother. 2005;49:3018–20. doi: 10.1128/AAC.49.7.3018-3020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lomaestro BM, Tobin EH, Shang W, Gootz T. The spread of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae to upstate New York. Clin Infect Dis. 2006;43:e26–8. doi: 10.1086/505598. [DOI] [PubMed] [Google Scholar]
- 20.Tascini C, Urbani L, Biancofiore G, et al. Colistin in combination with rifampin and imipenem for treating a blaVIM-1 metallo-beta-lactamase-producing Enterobacter cloacae disseminated infection in a liver transplant patient. Minerva Anestesiol. 2008;74:47–9. [PubMed] [Google Scholar]
- 21.Rasheed JK, Biddle JW, Anderson KF, et al. Detection of the Klebsiella pneumoniae carbapenemase type 2 Carbapenem-hydrolyzing enzyme in clinical isolates of Citrobacter freundii and K. oxytoca carrying a common plasmid. J Clin Microbiol. 2008;46:2066–9. doi: 10.1128/JCM.02038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anthony KB, Fishman NO, Linkin DR, Gasink LB, Edelstein PH, Lautenbach E. Clinical and microbiological outcomes of serious infections with multidrug-resistant gram-negative organisms treated with tigecycline. Clin Infect Dis. 2008;46:567–70. doi: 10.1086/526775. [DOI] [PubMed] [Google Scholar]
- 23.Bennett JW, Herrera ML, Lewis JS, 2nd, Wickes BW, Jorgensen JH. KPC-2 producing Enterobacter cloacae and Pseudomonas putida co-infection in a liver transplant recipient. Antimicrob Agents Chemother. doi: 10.1128/AAC.00931-08. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellier C, Bert F, Durand F, et al. Risk factors for Enterobacteriaceae bacteremia after liver transplantation. Transpl Int. 2008;21:755–63. doi: 10.1111/j.1432-2277.2008.00673.x. [DOI] [PubMed] [Google Scholar]
- 25.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–14. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 26.Baden LR, Rubin RH. The sentinel chicken revisited: the impact of West Nile virus infection on transplant patients. Transplantation. 2004;77:356–7. doi: 10.1097/01.tp.0000101436.76588.d4. [DOI] [PubMed] [Google Scholar]
- 27.Rubin RH. The compromised host as sentinel chicken. N Engl J Med. 1987;317:1151–3. doi: 10.1056/NEJM198710293171809. [DOI] [PubMed] [Google Scholar]
- 28.Samra Z, Bahar J, Madar-Shapiro L, Aziz N, Israel S, Bishara J. Evaluation of CHROMagar KPC for rapid detection of carbapenem-resistant Enterobacteriaceae. J Clin Microbiol. 2008;46:3110–1. doi: 10.1128/JCM.00249-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doi Y, Potoski BA, Adams-Haduch JM, Sidjabat HE, Pasculle AW, Paterson DL. Simple Disk-Based Method for Detection of KPC-Type {beta}-Lactamase Using a Boronic Acid Compound. J Clin Microbiol. doi: 10.1128/JCM.01408-08. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodford N, Tierno PM, Jr, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York Medical Center. Antimicrob Agents Chemother. 2004;48:4793–9. doi: 10.1128/AAC.48.12.4793-4799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin Microbiol Rev. 2008;21:449–65. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bratu S, Tolaney P, Karumudi U, et al. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J Antimicrob Chemother. 2005;56:128–32. doi: 10.1093/jac/dki175. [DOI] [PubMed] [Google Scholar]
- 33.Antoniadou A, Kontopidou F, Poulakou G, et al. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: first report of a multiclonal cluster. J Antimicrob Chemother. 2007;59:786–90. doi: 10.1093/jac/dkl562. [DOI] [PubMed] [Google Scholar]
- 34.Hellinger WC, Yao JD, Alvarez S, et al. A randomized, prospective, double-blinded evaluation of selective bowel decontamination in liver transplantation. Transplantation. 2002;73:1904–9. doi: 10.1097/00007890-200206270-00009. [DOI] [PubMed] [Google Scholar]
- 35.O'Callaghan RJ, Rousset KM, Harkess NK, Murray ML, Lewis AC, Williams WL. Analysis of increasing antibiotic resistance of Klebsiella pneumoniae relative to changes in chemotherapy. J Infect Dis. 1978;138:293–8. doi: 10.1093/infdis/138.3.293. [DOI] [PubMed] [Google Scholar]
- 36.Grylack L, Neugebauer D, Scanlon JW. Effects of oral antibiotics on stool flora and overall sensitivity patterns in an intensive care nursery. Pediatr Res. 1982;16:509–11. doi: 10.1203/00006450-198207000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Noskin GA. Tigecycline: a new glycylcycline for treatment of serious infections. Clin Infect Dis. 2005;41 (Suppl 5):S303–14. doi: 10.1086/431672. [DOI] [PubMed] [Google Scholar]
- 38.Castanheira M, Sader HS, Deshpande LM, Fritsche TR, Jones RN. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-beta-lactamase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother. 2008;52:570–3. doi: 10.1128/AAC.01114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daly MW, Riddle DJ, Ledeboer NA, Dunne WM, Ritchie DJ. Tigecycline for treatment of pneumonia and empyema caused by carbapenemase-producing Klebsiella pneumoniae. Pharmacotherapy. 2007;27:1052–7. doi: 10.1592/phco.27.7.1052. [DOI] [PubMed] [Google Scholar]
- 40.Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–64. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 41.Paterson DL, Doi Y. A step closer to extreme drug resistance (XDR) in gram-negative bacilli. Clin Infect Dis. 2007;45:1179–81. doi: 10.1086/522287. [DOI] [PubMed] [Google Scholar]