Abstract
BACKGROUND
The human immunodeficiency virus (HIV) alters the presentation of pulmonary tuberculosis (PTB), but it remains unclear whether alterations occur at a CD4 cell threshold or throughout HIV infection.
OBJECTIVE
To better understand the relationship between CD4 count and clinical and radiographic presentation of PTB.
SETTING AND DESIGN
Initial presentations of culture-confirmed PTB patients evaluated at a Ugandan national TB referral center and an affiliated research unit were compared by HIV status and across 11 CD4 cell count strata: 0–50 to >500 cells/µl.
RESULTS
A total of 873 HIV-infected PTB cases were identified. Among HIV-infected PTB cases with CD4 < 50, 21% had a normal chest X-ray (CXR) vs. 2% with CD4 > 500, with a continuous trend across CD4 strata (test for trend, P < 0.001). All radiographic manifestations of PTB displayed significant trends across CD4 strata. HIV-infected vs. non-HIV-infected patients had no significant difference in CXR findings of miliary patterns or pleural effusion at CD4 > 100, normal CXR or fibrosis at CD4 > 150, adenopathy at CD4 > 250, and cavitation or upper lung disease at CD4 > 300. Twenty-three per cent of co-infected cases with CD4 < 50 and 1% with CD4 > 500 had negative acid-fast bacilli (AFB) smears, with a significant trend between (P < 0.001).
CONCLUSION
Variations in CXR appearance and AFB smear correlate with CD4 decline in significant, continuous trends.
Keywords: tuberculosis, HIV, CD4, chest X-ray
Approximately one third of the 33 million persons living with the human immunodeficiency virus (PLHIV) worldwide are infected with Mycobacterium tuberculosis.1 Of the estimated 700 000 HIV-infected people with active tuberculosis (TB), 85% live in sub-Saharan Africa, and TB is the leading cause of death among PLHIV in Africa.2–4 PLHIV are at higher risk of developing and of dying from TB compared to non-HIV-infected people.5,6 Shortening the time to pulmonary TB (PTB) diagnosis and treatment initiation is an important step in reducing TB-associated mortality and transmission.
One major challenge to diagnosing PTB is alteration of the presentation of PTB due to HIV infection. The problem is exacerbated in resource-limited settings without routine access to mycobacterial culture. Alteration in the clinical and radiographic presentation of PTB among HIV-infected persons has long been recognized.7,8 Multiple studies have demonstrated variation in the radiographic presentation of PTB by HIV infection status or CD4 cell count cut-offs.9–12
However, previous studies of PTB presentation in HIV-infected persons have generally dichotomized the CD4 cell count (e.g., above or below 200 cells/µl) to describe the level of immune suppression, or simply compared the clinical and chest X-ray (CXR) findings of PTB among HIV-infected and non-HIV-infected patients. In this study, we evaluated PTB presentation across a large range of CD4 strata in a cohort of HIV co-infected cases in Kampala, Uganda, to better understand the relationship between CD4 cell count and clinical and radiographic presentation of PTB.
METHODS
Subjects
We retrospectively evaluated sputum culture-confirmed cases of PTB seen at the National Tuberculosis and Leprosy Program (NTLP) clinic in Kampala, Uganda, and referred to the Case Western Reserve University Tuberculosis Research Unit (TBRU) in Kampala between 2004 and 2008. The NTLP clinic is both the national TB referral center for Uganda and the largest TB treatment clinic in Kampala. For study inclusion, patients had to be ≥15 years of age; have undergone an initial screening interview to capture demographic and clinical information, a physical examination, and a CXR; and to have sputum acid-fast bacilli (AFB) smear, mycobacterial culture and HIV antibody test results available from the time of initial presentation with PTB.
Radiographic screening
CXRs at the time of initial presentation to the NTLP were evaluated by the study radiologist, who was blinded to the subjects’ HIV status, AFB smear and culture results. The radiologist completed a standardized CXR evaluation form that included a rating of the extent of lung disease, as well as the presence or absence of the following: upper lung field infiltrates, lower lung field infiltrates, fibrosis, cavitary disease, miliary disease, adenopathy and pleural effusion.
TB diagnostic testing
AFB smear was performed at the NTLP with carbol fuchsin staining and interpreted using a standard scale to quantify bacilli per high power field, ranging from negative to 3+.13 Two sputum culture systems were used: a 7H10 solid media culture and a liquid culture using a BACTEC system (BD Diagnostic Systems, Sparks, MD, USA). Positive liquid cultures were evaluated by smear for AFB and, if positive, polymerase chain reaction (PCR) was used to distinguish M. tuberculosis from other mycobacteria. A patient was classified as having culture-confirmed PTB if at least one sputum sample grew one or more colonies of M. tuberculosis.
HIV testing/CD4 analysis
HIV antibody status was determined using the Determine HIV 1/2 rapid test (Inverness Medical, Princeton, NJ, USA), followed by confirmation of all positive results using the Clearview HIV 1/2 STAT-PAK rapid test (Inverness Medical). All HIV-infected subjects were referred for HIV treatment. We were unable to confirm whether or not subjects were taking antiretroviral therapy (ART) at the time of presentation. CD4 counts were determined for HIV-infected subjects at initial presentation to the NTLP using a FACSCalibur flow cytometer (BD).
Statistical methods
Symptoms, signs and CXR findings at the time of presentation were compared by HIV status and, among HIV-infected subjects, across 11 strata of absolute CD4 cell count from 0–50 to >500 cells/µl. Comparisons of signs and symptoms by HIV infection status were performed using the Pearson χ2 test, and by the Student’s t-test when comparing means. Variations in presentation across CD4 strata were evaluated graphically and by non-parametric tests for trend, using the method developed by Cuzick, an extension of the Wilcox rank-sum test.14
Multivariate logistic regression analysis was performed for the outcome of normal CXR. We included age, sex and a history of tobacco use in our logistic model, as all three variables may be independently associated with CXR abnormalities. Cough duration was included based on the finding that HIV infection was associated with a significantly shorter mean duration of cough (see Table 1) at the time of diagnosis. We chose to define immune status according to a CD4 cell count of ≤150 or >150 cells/µl among the HIV-infected in our logistic model, based on our study findings of the CD4 cell count threshold above which HIV-infected and non-HIV-infected PTB cases did not display significant differences in the proportion with normal CXR. All analyses were performed using Stata 10 software (Stata Corp, College Station, TX, USA).
Table 1.
Comparison of culture-confirmed cases of pulmonary tuberculosis by HIV infection status: symptoms, signs and chest X-ray findings
| HIV- positive |
HIV- negative |
||||
|---|---|---|---|---|---|
| Symptoms | n | % | n | % | P value |
| N | 873 | 1141 | |||
| Cough >3 weeks | 841 | 96 | 1091 | 96 | 0.713 |
| Duration of cough, mean, days | 99.22 | 112.81 | 0.001 | ||
| Subjective fever | 651 | 75 | 737 | 65 | <0.001 |
| Sweats | 582 | 67 | 669 | 59 | <0.001 |
| Weight loss (subjective) | 705 | 81 | 823 | 72 | <0.001 |
| Malaise | 159 | 18 | 150 | 13 | 0.002 |
| Arthralgias | 146 | 17 | 133 | 12 | 0.001 |
| Hemoptysis | 63 | 7 | 126 | 11 | 0.003 |
| Loss of appetite | 574 | 66 | 544 | 48 | <0.001 |
| Diarrhea | 59 | 7 | 29 | 3 | <0.001 |
| Productive sputum | 813 | 93 | 1072 | 94 | 0.068 |
| Signs | |||||
| Temperature >37.5°C | 321 | 37 | 363 | 32 | 0.019 |
| Abnormal chest examination | 630 | 72 | 898 | 79 | <0.001 |
| Abnormal lymph node exam | 252 | 29 | 142 | 12 | <0.001 |
| Oral thrush | 22 | 3 | 3 | 0 | 0.031 |
| AFB smear-positive | 734 | 84 | 1075 | 94 | <0.001 |
| Chest X-ray | (n = 848) | (n = 1085) | |||
| Normal X-ray | 71 | 8 | 26 | 2 | <0.001 |
| Cavitary disease | 338 | 40 | 705 | 65 | <0.001 |
| Miliary pattern | 35 | 4 | 21 | 2 | 0.004 |
| Fibrosis | 42 | 5 | 102 | 9 | <0.001 |
| Adenopathy | 62 | 7 | 21 | 2 | <0.001 |
| Pleural effusion | 73 | 9 | 67 | 6 | 0.04 |
| Upper lobe disease | 622 | 73 | 953 | 88 | <0.001 |
| Lower lobe disease | 571 | 67 | 863 | 80 | <0.001 |
HIV = human immunodeficiency virus; AFB = acid-fast bacilli.
Ethical approval
The Uganda National Council of Science and Technology, the Ugandan Joint Clinical Research Centre, and the University of California, San Francisco Committee on Human Research approved this study.
RESULTS
Patient population
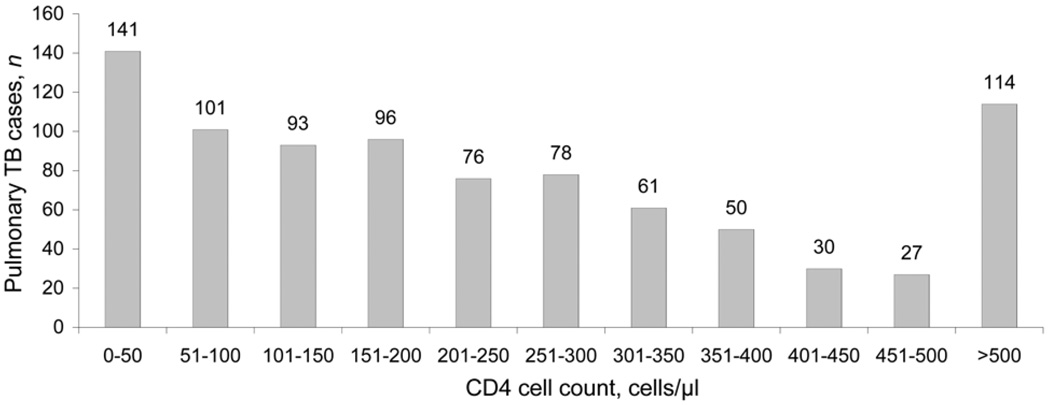
Among patients with suspected pulmonary TB evaluated at the NLTP clinic, 4911 were referred to the TBRU from September 2004 to July 2008 based on their willingness to participate in clinical research. Culture results were available for 2750 patients, of whom 2014 (73%) had a sputum culture positive for M. tuberculosis. Of the 2014 culture-positive sputum samples, 873 (43%) were TB-HIV co-infected. CD4 cell counts were obtained in 867 (99%) of the HIV-infected patients and were distributed across 11 strata (Figure 1). CXR results were available for 848 (97%) of the 873 HIV-infected and 1085 (95%) of the 1141 non-HIV-infected patients with PTB.
Figure 1.
Distribution of pulmonary tuberculosis cases by CD4 cell count in HIV-infected persons. TB = tuberculosis; HIV = human immunodeficiency virus.
Variation in PTB presentation by HIV status
In a comparison of PTB cases with and without HIV infection, baseline demographic characteristics were similar except that HIV-infected patients tended to be older and were more likely to be female, not to have attended college or technical school, and to self-identify as widowed, separated or divorced than non-HIV-infected patients (Table 2). HIV-infected patients with PTB differed significantly in their clinical and radiographic presentation from non-HIV-infected patients with PTB, with the exception of reporting cough for >3 weeks (Table 1).
Table 2.
Comparison of culture-confirmed cases of pulmonary TB by HIV infection status: demographic information
| HIV- positive |
HIV- negative |
||||
|---|---|---|---|---|---|
| Demographic | n | % | n | % | P value |
| N | 873 | 100 | 1141 | 100 | |
| Age, mean, years | 32 | 29 | <0.001 | ||
| Sex | <0.001 | ||||
| Men | 441 | 51 | 714 | 63 | |
| Women | 404 | 46 | 384 | 34 | |
| Marital status | <0.001 | ||||
| Single | 134 | 15 | 449 | 39 | |
| Currently married | 375 | 43 | 444 | 39 | |
| Divorced/separated/widowed | 332 | 38 | 193 | 17 | |
| Any higher education (university/technical institute) | 65 | 7 | 129 | 11 | 0.003 |
| Ever smoked | 238 | 27 | 272 | 24 | 0.09 |
| Smoke now | 56 | 6 | 73 | 6 | 0.54 |
| Currently drink alcohol | 180 | 21 | 249 | 22 | 0.505 |
| Heavy alcohol use | 62 | 7 | 85 | 7 | 0.931 |
| Injection drug use | 1 | 0 | 2 | 0 | 0.754 |
| Sex worker | 0 | 0 | 3 | 0 | 0.318 |
| History of TB treatment | 8 | 1 | 7 | 1 | 0.508 |
| History of isoniazid preventive therapy | 0 | 0 | 0 | 0 | 0.722 |
| History of close TB contact | 103 | 12 | 169 | 15 | 0.217 |
TB = tuberculosis; HIV = human immunodeficiency virus.
Influence of level of immune suppression on clinical presentation
Among the HIV-infected patients with PTB, decreasing CD4 cell count was significantly associated with an increase in the likelihood of having experienced subjective fever (test for trend, P < 0.001), weight loss (P = 0.001 for trend), malaise (P = 0.005 for trend), diarrhea (P = 0.001 for trend), and loss of appetite (P < 0.001 for trend). The proportion of HIV-infected patients with hemoptysis declined with decreasing CD4 count (P = 0.004 for trend), as did their mean duration of cough prior to presentation (P < 0.001 for trend). Decreasing CD4 cell count also correlated with an increasing likelihood of having an objectively measured fever (P <0.001 for trend) and a normal chest examination (P = 0.025 for trend).
AFB smear-negative disease
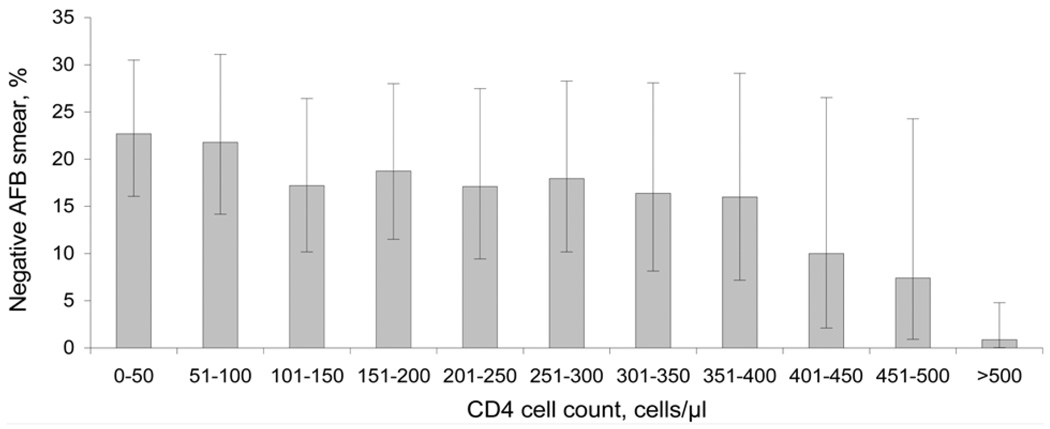
AFB smear-negative, culture-positive PTB was increasingly common with decreasing CD4 cell count (P < 0.001, for trend). Of 141 subjects with a CD4 < 50 cells/µl, 32 (23%) were AFB smear-negative, compared with only 1 of 114 (0.9%) with a CD4 > 500 cells/µl (Figure 2). As patients presented with lower CD4 counts, there was a significant decrease in high bacillary burden (3+ AFB) smears (P < 0.001 for trend) and an increase in low bacillary burden (1+ AFB) smears (P = 0.01).
Figure 2.
Proportion of HIV-infected patients with culture-confirmed pulmonary TB with negative AFB smear on initial presentation, by CD4 cell count. Error bars: 95% CIs (binomial exact) for the proportion of subjects with AFB smear-negative TB infection by CD4 strata. AFB = acid-fast bacilli; HIV = human immunodeficiency virus; TB = tuberculosis; CI = confidence interval.
Influence of level of immune suppression on chest X-ray findings
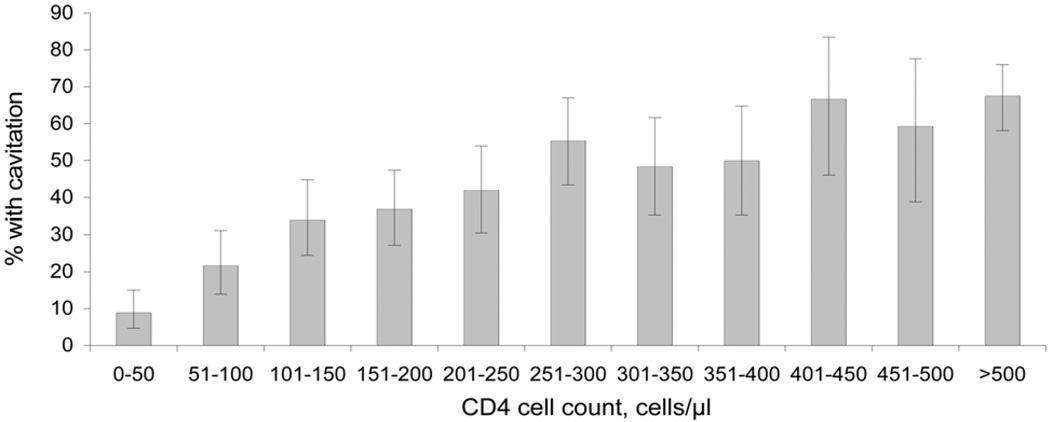
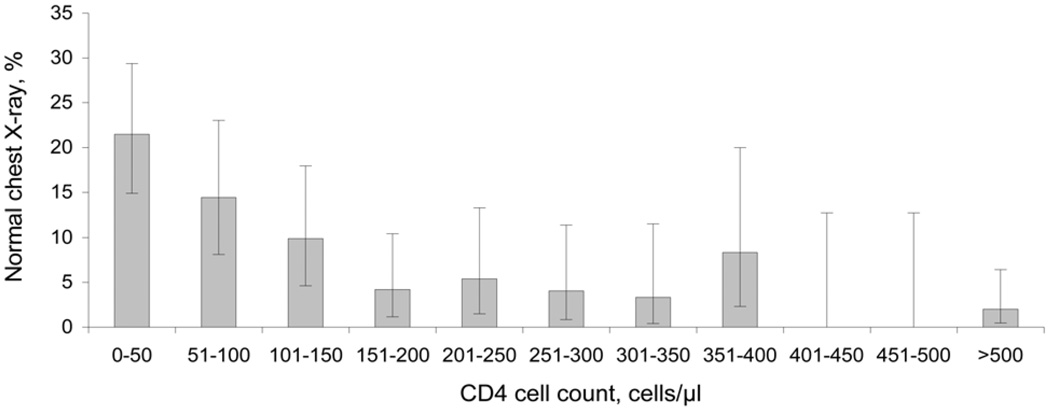
Among the HIV-infected patients with PTB, CXR findings varied with CD4 cell count. CXR findings of cavitary disease (P < 0.001 for trend), fibrosis (P = 0.001 for trend), and upper lobe disease (P < 0.001 for trend) were less likely to be present as CD4 cell count declined (Figure 3). Radiographic findings of normal CXR (P < 0.001 for trend), miliary disease (P = 0.02 for trend), adenopathy (P < 0.001 for trend) and pleural effusion (P = 0.03 for trend) were more likely to be present as CD4 cell count declined (Figure 4).
Figure 3.
Proportion of HIV-infected patients with culture-confirmed pulmonary TB with cavitation on chest X-ray at initial presentation, by CD4 cell count. Error bars: 95% CIs for the proportion of subjects with TB infection and cavitation on chest X-ray at initial presentation by CD4 strata. HIV = human immunodeficiency virus; TB = tuberculosis; CI = confidence interval.
Figure 4.
Proportion of HIV-infected patients with culture-confirmed pulmonary TB with a normal chest X-ray at initial presentation, by CD4 cell count. Error bars: 95% CIs for the proportion of subjects with TB infection and a normal chest X-ray at initial presentation by CD4 strata. HIV = human immunodeficiency virus; TB = tuberculosis; CI = confidence interval.
CD4 cell count thresholds above which HIV-infected and non-PTB-infected cases no longer differ in CXR presentation
The CD4 cell count above which there was no significant difference in the likelihood of a radiographic characteristic being present or absent in HIV-infected and non-HIV-infected patients with PTB varied by CXR finding. HIV-infected patients with PTB compared to non-HIV-infected patients had no significant difference in the CXR findings of a miliary pattern or a pleural effusion above a CD4 cell count of 100 cells/µl, a normal CXR or fibrosis on CXR at a CD4 cell count > 150 cells/µl, adenopathy on CXR at a CD4 cell count > 250 cells/µl, and cavitation and upper lung disease at a CD4 cell count > 300 cells/µl. Among HIV-infected patients with PTB and a CD4 cell count > 400 cells/µl, the proportion with a negative AFB smear (4%) was not significantly different from that of non-HIV-infected patients with PTB (6%, P = 0.35).
Predictors of a normal CXR at initial presentation among patients with PTB and HIV
In a multivariate logistic regression model to evaluate factors associated with having a normal CXR, the factors never having smoked, negative AFB smear and CD4 cell count < 150 cells/µl were all significantly associated with an increased likelihood of having a normal CXR, after adjusting for age, sex and cough duration (Table 3).
Table 3.
Predictors of a normal CXR at initial presentation among patients with pulmonary tuberculosis
| Characteristic | Normal CXR n/N |
% | Crude OR | Adjusted OR | 95%CI | P value |
|---|---|---|---|---|---|---|
| Immune status | ||||||
| CD4 ≤ 150 | 52/323 | 16 | 7.82 | 5.32 | 3.07–9.23 | <0.001 |
| CD4 > 150 | 19/525 | 4 | 1.53 | 1.05 | 0.54–2.04 | 0.897 |
| HIV-negative | 26/1085 | 2 | Reference | Reference | ||
| AFB smear-negative | ||||||
| Yes | 53/202 | 26 | 12.96 | 10.36 | 6.41–16.74 | <0.001 |
| No | 47/1760 | 3 | Reference | Reference | ||
| History of tobacco use | ||||||
| Yes | 15/490 | 3 | 0.50 | 0.36 | 0.18–0.74 | 0.005 |
| No | 84/1412 | 6 | Reference | Reference | ||
| Sex | ||||||
| Male | 52/1126 | 5 | 0.74 | 1.02 | 0.61–1.70 | 0.934 |
| Female | 48/780 | 6 | Reference | Reference | ||
| Age (for every 10-year increase) | NA | NA | 1.2 | 0.91–1.56 | 0.194 | |
| Cough duration (for every 30-day increase) | NA | NA | 0.98 | 0.92–1.05 | 0.613 |
CXR = chest X-ray; OR = odds ratio; CI = confidence interval; HIV = human immunodeficiency virus; AFB = acid-fast bacilli; NA = not applicable.
DISCUSSION
Our data show that, among HIV-infected patients, variations in CXR appearance and AFB smear at initial presentation with PTB occur continuously as patients present with lower CD4 counts. Previous studies have shown that AFB smear-positive PTB and the CXR findings of cavitary disease and upper lobe infiltrates are relatively less common in HIV-infected compared to non-HIV-infected persons.9,15–17 Radiographic characteristics such as mediastinal lymphadenopathy, pleural effusion, and lower lobe and miliary disease have been shown to be relatively more common in HIV-infected compared with non-HIV-infected persons,12,18 as has normal CXR.19 However, few studies have examined the relationship between level of immune suppression as measured by absolute CD4 cell count and the clinical and radiographic findings in HIV-infected patients with PTB across a spectrum of immune suppression rather than the dichotomous CD4 cut-off of greater or less than 200.12,18,20 Analysis of trends across multiple CD4 cell count strata shows that changes in CXR appearance of PTB occur in a continuous fashion across the range of CD4 counts rather than at a CD4 cell count threshold, and allows for a more nuanced understanding of how PTB presentation varies as patients present with lower CD4 cell counts. The continuous variation in CXR appearance of PTB across a wide range of CD4 cell counts suggests that CXR becomes increasingly insensitive for PTB diagnosis in HIV with advancing immunosuppression, even in patients with CD4 cell counts as high as 350 cells/µl.
Our findings reinforce some of the immense challenges in TB diagnosis faced by clinicians in resource-limited settings relying on symptoms, smear and/or CXR, and the importance of routine culture for TB diagnosis in HIV-infected patients. The limited sensitivity of symptoms in active case finding has been demonstrated. 21–23 In addition, symptoms at presentation among HIV-infected TB suspects are non-specific and in our population were exceedingly common, even in patients without PTB (data not shown). AFB smear is also insensitive in patients with HIV, and performed worst in patients with advanced immune suppression. Although our study population had a relatively low prevalence of AFB smear-negative disease (16% in HIV-infected patients overall compared to past estimates of 30–50%,11,15,24 likely a function of the NTLP’s role as a referral center), the trend indicates that in some settings, the prevalence of AFB smear-negative PTB in HIV-infected patients with advanced immune suppression may exceed 50%.
From the clinician’s point of view, our data suggest that CXRs cannot be used to rule out PTB in patients with advanced immunosuppression. A normal CXR at the time of presentation with PTB represents a major obstacle to PTB diagnosis. Our findings are consistent with previously published estimates of HIV-infected patients presenting with normal CXRs of approximately 2–22%.11,12,19,25 Along with limited sensitivity of CXR in patients with HIV infection is the problem of specificity, as the differential diagnosis for an abnormal CXR in an HIV-infected patient is broad and includes common diseases such as bacterial and fungal pneumonia. However, an abnormal CXR still remains a useful tool in PTB suspects, and the positive predictive value of CXR for PTB will likely remain high in settings with high TB prevalence. On the other hand, given the combination of nonspecific symptoms and a relatively high proportion of smear-negative PTB cases among culture-confirmed patients presenting with CD4 cell counts < 100, our finding of frequent normal CXRs in PTB patients with advanced HIV underscores the difficulty of diagnosing PTB in high HIV prevalence settings. Efforts to build capacity for routine sputum culture of TB suspects, HIV testing and earlier initiation of ART are likely to reduce the burden of unrecognized TB in communities with high HIV prevalence and TB incidence, until more sensitive, rapid, and point-of-care TB diagnostics are available.
Our study has several limitations. As the NTLP is a referral center for Uganda, it is likely that patients with PTB seen at the NTLP have more severe or clinically advanced PTB in comparison with a general TB population. Patients are often referred when community providers have a high suspicion for TB, and delays between referral and evaluation at the NTLP are common. These factors may select for smear-positive patients and explain the relatively low prevalence of AFB smear-negative PTB. Another potential limitation is our inability to confirm whether or not subjects were taking ART at the time of presentation, as immunologic restoration due to ART might impact the presentation of PTB independent of CD4 count. Based on a self-report of negative HIV status and using cross-referenced data from another ongoing clinical study of ART-naïve patients in this population, we were able to confirm that at least 61% of subjects were not taking ART. It is possible that our definition of ‘culture-confirmed’ using ≥1 colony-forming unit (cfu) of M. tuberculosis in sputum culture may have led to the misclassification of PTB cases. This case definition, in contrast to a definition requiring a higher colony count (e.g., ≥10 cfu), increases the risk that we included subjects with false-positive cultures in our PTB group. However, on reanalysis using a definition of ‘culture-confirmed’ of ≥10 cfu of M. tuberculosis in sputum culture, the results remained unchanged.
The strengths of our study include restricting our analysis to culture-confirmed cases of PTB and the availability of CD4 cell counts at the time of presentation with PTB. Few studies in sub-Saharan Africa have evaluated PTB presentation according to CD4 count in culture-confirmed PTB cases, and to our knowledge none have been as large.12,26 The large sample size allowed for evaluation of PTB presentation across a much higher resolution of CD4 strata than has been previously published, and for analysis of the relatively uncommon outcome of normal CXR in PTB.
Improvement in our understanding of the impact of declining CD4 cell count on the presentation of PTB allows clinicians to more accurately assess their patients’ likelihood of PTB and clarifies the limitations of current diagnostic tools among HIV-infected patients. Patients most in need of a rapid TB diagnosis, the most severely immune-suppressed, remain at highest risk for misdiagnosis given the poor performance of CXR, symptom screening and sputum microscopy in this group. Further research into developing more sensitive, rapid and point-of-care diagnostics for M. tuberculosis, as well as validation of their efficacy among HIV-infected patients with advanced immunosuppression, should be made an urgent priority.
Acknowledgements
The authors thank the study participants, as well as D Johnson, H Luzze and M Nsereko. This work was supported in part by the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (K-24 A151982 [Havlir]), the Traineeships in AIDS Prevention Studies, National Institute of Mental Health (T32 MH-19105-21 [Chamie]), and The PART study (Grant number AI051219-0607 [Whalen], Project Title: HIV/TB in Uganda: punctuated antiretroviral therapy) and the Case Western Reserve University Tuberculosis Research Unit (NIH contract no. HHSN 266200700022C/NO1-AI70022 and AI95383).
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Ansari NA, Kombe AH, Kenyon TA, et al. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–1998. Int J Tuberc Lung Dis. 2002;6:55–63. [PubMed] [Google Scholar]
- 3.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 4.Rana FS, Hawken MP, Mwachari C, et al. Autopsy study of HIV-1-positive and HIV-1-negative adult medical patients in Nairobi, Kenya. J Acquir Immune Defic Syndr. 2000;24:23–29. doi: 10.1097/00126334-200005010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Whalen CC, Chiunda A, Zalwango S, et al. Immune correlates of acute Mycobacterium tuberculosis infection in household contacts in Kampala, Uganda. Am J Trop Med Hyg. 2006;75:55–61. [PMC free article] [PubMed] [Google Scholar]
- 6.Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS (London, UK) 2001;15:143–152. doi: 10.1097/00002030-200101260-00002. [DOI] [PubMed] [Google Scholar]
- 7.Chaisson R, Schecter G, Theuer C, Rutherford G, Echenberg D, Hopewell PC. Tuberculosis in patients with the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1987;136:570–574. doi: 10.1164/ajrccm/136.3.570. [DOI] [PubMed] [Google Scholar]
- 8.Pitchenik A, Rubinson H. The radiographic appearance of tuberculosis in patients with the acquired immune deficiency syndrome (AIDS) and pre-AIDS. Am Rev Respir Dis. 1985;131:393–396. doi: 10.1164/arrd.1985.131.3.393. [DOI] [PubMed] [Google Scholar]
- 9.Asimos AW, Ehrhardt J. Radiographic presentation of pulmonary tuberculosis in severely immunosuppressed HIV-seropositive patients. Am J Emerg Med. 1996;14:359–363. doi: 10.1016/S0735-6757(96)90049-2. [DOI] [PubMed] [Google Scholar]
- 10.Keiper MD, Beumont M, Elshami A, Langlotz CP, Miller WT., Jr CD4 T lymphocyte count and the radiographic presentation of pulmonary tuberculosis. A study of the relationship between these factors in patients with human immunodeficiency virus infection. Chest. 1995;107:74–80. doi: 10.1378/chest.107.1.74. [DOI] [PubMed] [Google Scholar]
- 11.Perlman DC, el-Sadr WM, Nelson ET, et al. Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression. The Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). The AIDS Clinical Trials Group (ACTG) Clin Infect Dis. 1997;25:242–246. doi: 10.1086/514546. [DOI] [PubMed] [Google Scholar]
- 12.Post FA, Wood R, Pillay GP. Pulmonary tuberculosis in HIV infection: radiographic appearance is related to CD4+ T-lymphocyte count. Tubercle Lung Dis. 1995;76:518–521. doi: 10.1016/0962-8479(95)90527-8. [DOI] [PubMed] [Google Scholar]
- 13.The American Thoracic Society/Centers for Disease Control and Prevention. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 14.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 15.Lawson L, Yassin MA, Thacher TD, et al. Clinical presentation of adults with pulmonary tuberculosis with and without HIV infection in Nigeria. Scand J Infect Dis. 2008;40:30–35. doi: 10.1080/00365540701509899. [DOI] [PubMed] [Google Scholar]
- 16.Geng E, Kreiswirth B, Burzynski J, Schluger NW. Clinical and radiographic correlates of primary and reactivation tuberculosis: a molecular epidemiology study. JAMA. 2005;293:2740–2745. doi: 10.1001/jama.293.22.2740. [DOI] [PubMed] [Google Scholar]
- 17.Kassu A, Mengistu G, Ayele B, et al. Co-infection and clinical manifestations of tuberculosis in human immunodeficiency virus-infected and -uninfected adults at a teaching hospital, northwest Ethiopia. J Microbiol Immunol Infect. 2007;40:116–122. [PubMed] [Google Scholar]
- 18.Abouya L, Coulibaly IM, Coulibaly D, et al. Radiologic manifestations of pulmonary tuberculosis in HIV-1 and HIV-2-infected patients in Abidjan, Cote d’Ivoire. Tubercle Lung Dis. 1995;76:436–440. doi: 10.1016/0962-8479(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 19.Pepper T, Joseph P, Mwenya C, et al. Normal chest radiography in pulmonary tuberculosis: implications for obtaining respiratory specimen cultures. Int J Tuberc Lung Dis. 2008;12:397–403. [PubMed] [Google Scholar]
- 20.Lado Lado FL, Barrio Gomez E, Carballo Arceo E, Cabarcos Ortiz de Barron A. Clinical presentation of tuberculosis and the degree of immunodeficiency in patients with HIV infection. Scand J Infect Dis. 1999;31:387–391. doi: 10.1080/00365549950163842. [DOI] [PubMed] [Google Scholar]
- 21.Mtei L, Matee M, Herfort O, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005;40:1500–1507. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 22.Ayles H, Schaap A, Nota A, et al. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLoS ONE. 2009;4:e5602. doi: 10.1371/journal.pone.0005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimerling ME, Schuchter J, Chanthol E, et al. Prevalence of pulmonary tuberculosis among HIV-infected persons in a home care program in Phnom Penh, Cambodia. Int J Tuberc Lung Dis. 2002;6:988–994. [PubMed] [Google Scholar]
- 24.Jones B, Young S, Antoniskis D, Davidson P, Kramer F, Barnes P. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292–1297. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg SD, Frager D, Suster B, Walker S, Stavropoulos C, Rothpearl A. Active pulmonary tuberculosis in patients with AIDS: spectrum of radiographic findings (including a normal appearance) Radiology. 1994;193:115–119. doi: 10.1148/radiology.193.1.7916467. [DOI] [PubMed] [Google Scholar]
- 26.Mukadi Y, Perriens JH, St Louis ME, et al. Spectrum of immunodeficiency in HIV-1-infected patients with pulmonary tuberculosis in Zaire. Lancet. 1993;342:143–146. doi: 10.1016/0140-6736(93)91346-n. [DOI] [PubMed] [Google Scholar]