Abstract
New cone-beam computed tomographic (CBCT) mammography system designs are presented where the detectors provide high spatial resolution, high sensitivity, low noise, wide dynamic range, negligible lag and high frame rates similar to features required for high performance fluoroscopy detectors. The x-ray detectors consist of a phosphor coupled by a fiber-optic taper to either a high gain image light amplifier (LA) then CCD camera or to an electron multiplying CCD. When a square-array of such detectors is used, a field-of-view (FOV) to 20 × 20 cm can be obtained where the images have pixel-resolution of 100 µm or better. To achieve practical CBCT mammography scan-times, 30 fps may be acquired with quantum limited (noise free) performance below 0.2 µR detector exposure per frame. Because of the flexible voltage controlled gain of the LA’s and EMCCDs, large detector dynamic range is also achievable. Features of such detector systems with arrays of either generation 2 (Gen 2) or 3 (Gen 3) LAs optically coupled to CCD cameras or arrays of EMCCDs coupled directly are compared. Quantum accounting analysis is done for a variety of such designs where either the lowest number of information carriers off the LA photo-cathode or electrons released in the EMCCDs per x-ray absorbed in the phosphor are large enough to imply no quantum sink for the design. These new LA- or EMCCD-based systems could lead to vastly improved CBCT mammography, ROI-CT, or fluoroscopy performance compared to systems using flat panels.
Keywords: x-ray detectors, detector arrays, cone beam computed tomography, mammography, fluoroscopy, CT, electron multiplying CCD, light amplifiers, quantum accounting, detector mosaic
1. INTRODUCTION
1.1 Similarity of requirements for CBCT mammography and fluoroscopy detectors
Cone-beam computed tomographic (CBCT) mammography using a dedicated system may provide more diagnostic information than standard two-view projection mammography. If the total patient dose is kept approximately unchanged compared to two-view mammography and the duration of the diagnostic procedure is kept reasonable (10–30 sec) then to acquire the hundreds of images required for CBCT implies that the detector must have high frame rate capability and low detector noise to be quantum limited. These are features similar to good fluoroscopic detectors except that, for CBCT, the lag should also be minimal to enable good low-contrast resolution. Current flat panel detectors (FPD) have limitations on image quality due to large pixel size, excessive lag, and detector noise at low exposures; however, they are capable of rapid frame rates and are therefore employed in the new experimental CBCT mammography clinical systems [Boone 05] [Chen 05] [Tornai 05] [Ning 05] [Glick 05]. We have been developing different types of detectors based either on a light amplified (LA) CCD detector or an EMCCD that has all the desired characteristics of high speed and large dynamic range, but additionally has small pixels for higher spatial resolution, essentially no lag, and very low detector noise to enable quantum-limited performance at far lower exposures than FPDs. The only physical limitation has been the somewhat small field of view. To overcome this and provide the required field of view (FOV) of about 20 cm, we describe in detail the design of a dedicated CBCT mammography system composed of a square mosaic or array of 4 to 25 LA or EMCCD based detector modules with quantum-limited operation. We provide a quantum accounting analysis to demonstrate that these detectors can perform with no secondary quantum sinks and we review the high-resolution, high-sensitivity characteristics of these new detectors. Finally, we discuss the application of these high-resolution fluoroscopy detector arrays to ROI-CT where a region of the patient (whether centered on the rotational axis or off-center) is selected for closer examination with higher resolution and higher dose than surrounding regions.
1.2 Indirect vs. direct detector designs
A common requirement of mammography and of some ROI fluoroscopy interventional procedures is for high spatial resolution. Although direct flat panels are known to be capable of demonstrating superior spatial resolution compared to flat panel and other image detectors based on indirect x-ray converter phosphors, satisfying the additional requirements for high frame rate as well as high spatial resolution, while postulated [Ganguly 01] [Yamada 00], has been difficult to achieve and such systems have not been successfully developed at present. Rather, all the FPDs used for CBCT mammography and the high resolution rapid-sequence detectors, such as the ones our group has been developing, are based on indirect x-ray conversion in a CsI(Tl) phosphor.
1.3 Previous indirect CCD-based detector designs
Our group [Rudin 00] [Rudin 02] [Ganguly 03] and, previously, others [Herron 90] [Karellas 92] [Williams 99] have reported on the use of indirect detectors consisting of an x-ray sensitive phosphor layer to convert the x-ray energy into light that is coupled by a fiber optic plate (FOP) to a fiber-optic taper (FOT) which is in turn coupled through another FOP to a CCD camera for recording the image as indicated in Fig. 1.
Fig. 1.
Microangiographic detector (MA) design
Although such detectors are excellent for microangiography (MA) and micro CT applications because of their superior spatial resolution, they are not sensitive enough for fluoroscopy since there is insufficient gain with the possible exception of one group’s very large specialized CCDs [Vedanathan 04]. Generally, x-ray photon fluences for fluoroscopy are about two orders of magnitude lower than that for angiography. To achieve this increased gain, we built the detector indicated in Fig. 2 having a light amplifier (LA) with micro channel plates (MCPs). [Wu 05] [Rudin 02] We showed that these detectors had wide dynamic range, were capable of high frame rates, and had virtually no lag.
Fig. 2.
Microangiographic fluoroscope (MAF) design.
2. METHODS
Because of the basic similarity of component characteristics, the new detector designs presented here should have many of the attributes of the MAF such as high spatial resolution, high sensitivity, large dynamic range, real-time imaging capability with no lag. The goal in this work is to demonstrate that these positive features of the previously reported MAF can be extended to detector designs that are amenable to expansion of the imaging field of view without introducing secondary quantum sinks but maintaining quantum limited performance even at the low exposures characteristic of fluoroscopy.
2.1 Detector descriptions
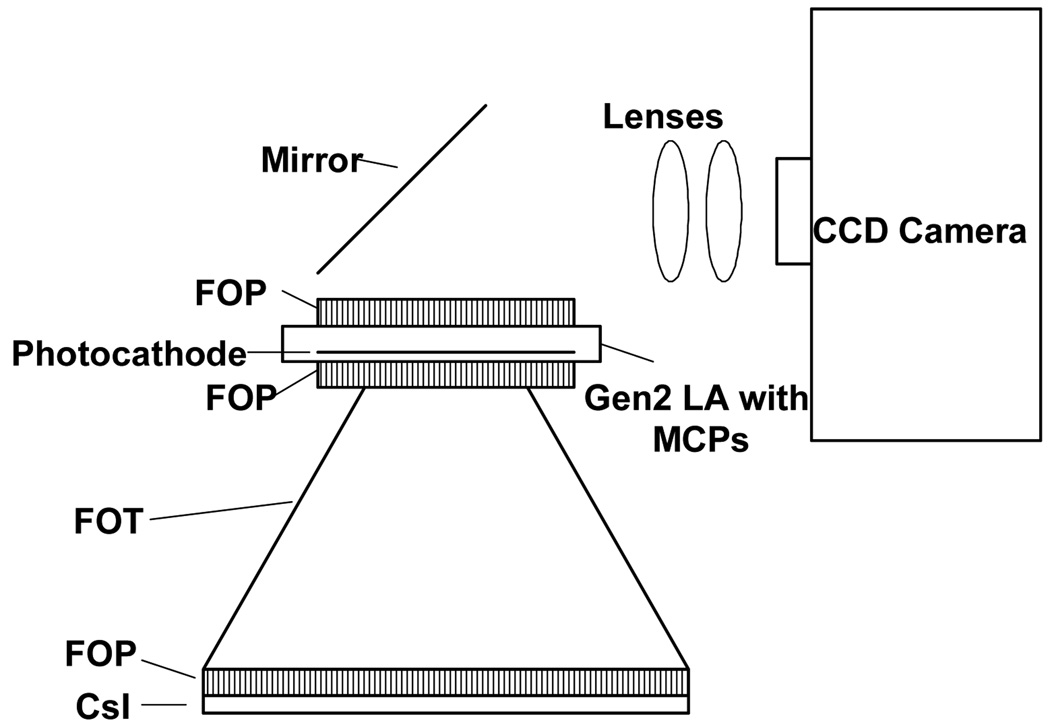
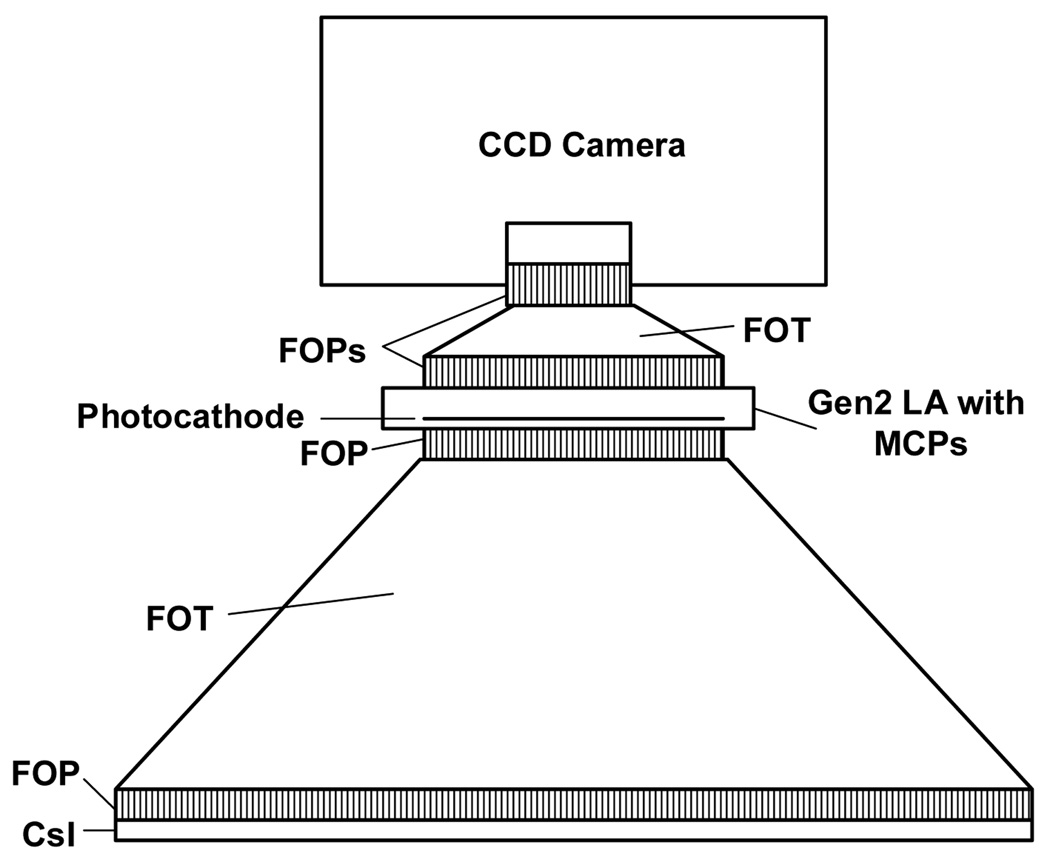
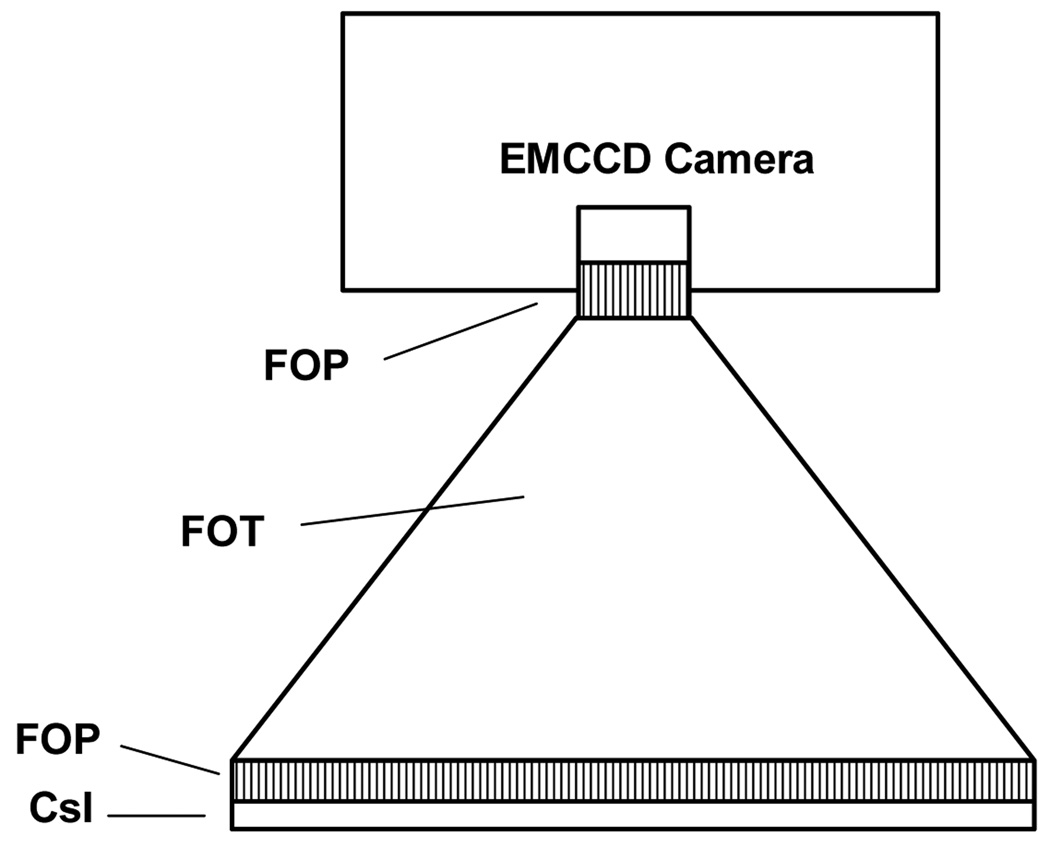
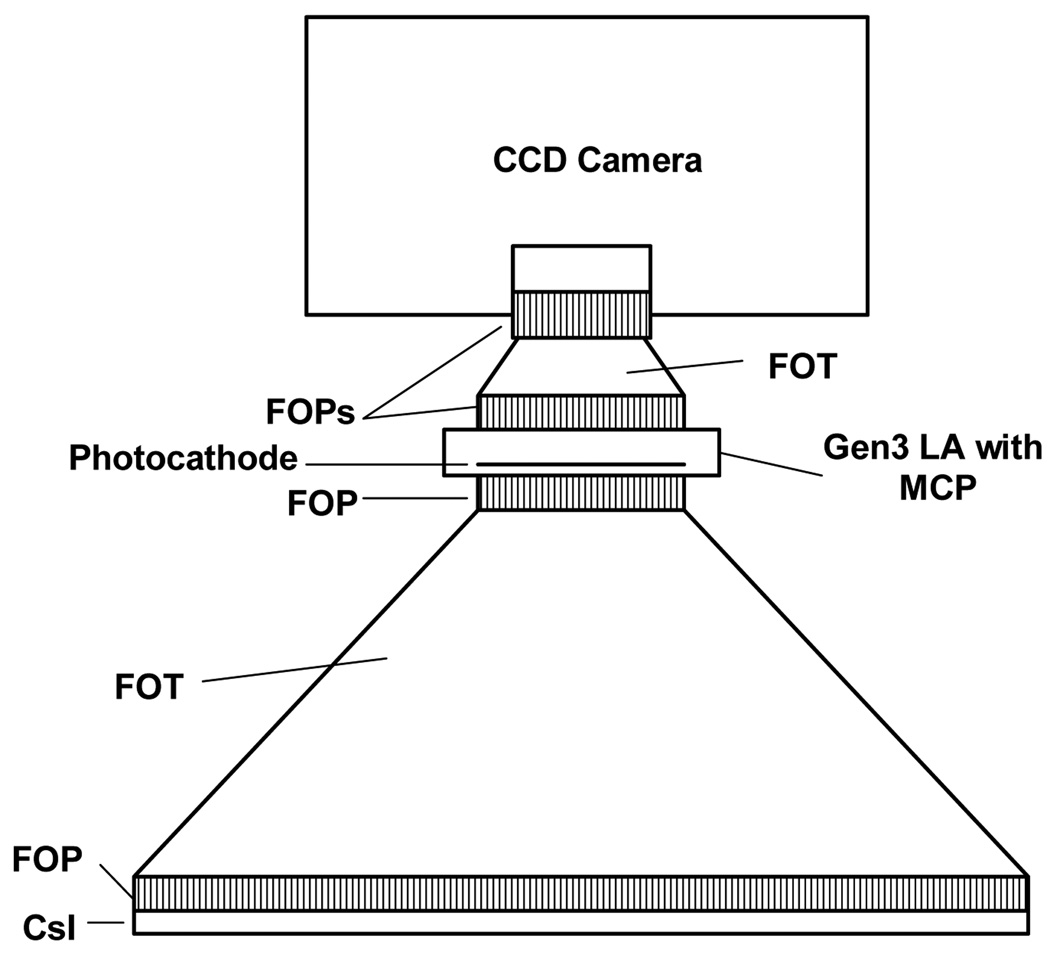
In this work, we present three different detector designs that have a number of components in common, especially at the front end. All three detector designs (Figs. 3–5) have a CsI(Tl) layer either on a FOP [Hamamatsu 97] or directly deposited on the large end of a FOT. Light from the scintillation, after going from the phosphor through the FOP and FOT, goes to another FOP which is the input window of either a light amplifier (LA) (Figs. 3 and 4) or an Electron Multiplying Charge Coupled Device (EMCCD) (Fig. 5) where the amplification occurs.
Fig. 3.
Schematic of Gen 2 LA having 2 MCPs fiber-optically coupled to a CCD.
Fig. 5.
Schematic of fiber-optically coupled EMCCD
Fig. 4.
Schematic of Gen 3 LA having 1 MCP fiber-optically coupled to a CCD.
2.1.1 New LA-CCD based detectors
The first two detector systems to be investigated have two different generations of LA’s with either one or two micro-channel plates (MCP) as integral parts. LA’s consist of a photocathode which converts a fraction of the incident light photons into electron information carriers which may be caused to enter and be amplified by an MCP. MCP’s are flat plates of glass with microscopic holes that have sides that are partially conducting so that when a high voltage is applied across the MCP, electrons that enter the holes are accelerated and caused to collide with the walls to free additional electrons that are in turn caused to collide further down the channel. In this way, gains of orders of magnitude can occur. When two such MCP’s are stacked as part of an LA assembly, gains of more than 5 orders can be achieved [Hamamatsu 05]. At the output end of the LA, the electrons are caused to bombard a phosphor which emits an amplified light distribution that can be imaged by a CCD sensor.
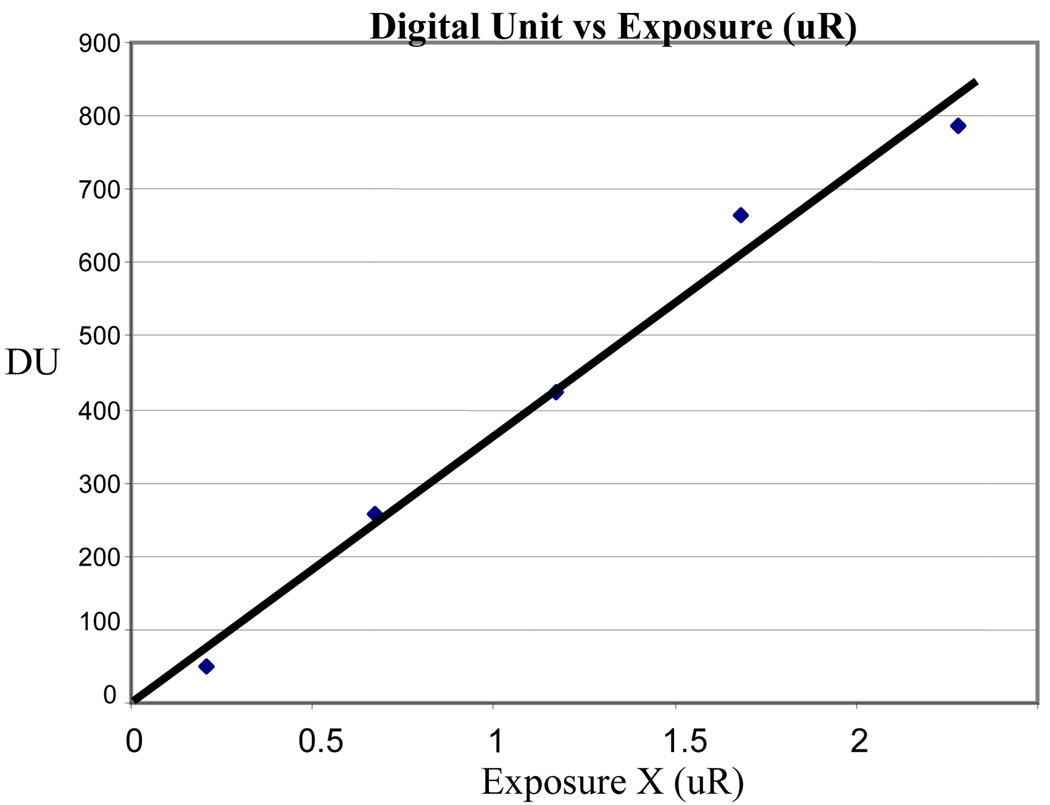
The generation 2 (Gen 2) LA while available in larger diameters has a rather poor light-to-electron quantum conversion efficiency, usually about 10% for light emitted by CsI(Tl). However, 4 cm diameter Gen 2 LA’s with integrated MCP’s are commercially available from multiple suppliers, and hence are the basis for the previous MAF (Fig. 2) as well as the first proposed new detector (Fig. 3). Because they are available with multiple MCP’s, there is some flexibility in the method useable for optically coupling the CCD sensor to the Gen 2 LA’s output. (See Fig. 2 or 3.) Fig. 6 demonstrates the MAF detector is able to record images with linearity down to values of 0.2 µR/frame. However, for the previous configuration (Fig. 2) using a mirror and lens to couple the LA output to the CCD, care has to be taken to avoid unstable and non-linear performance which may occur for high currents across the LA [Wu 05].
Fig. 6.
Linear response at low exposure for the Gen 2
LA – CCD detector using mirror and lens optical coupling.
The second detector design consists of a generation 3 (Gen 3) LA combined with a CCD sensor. Gen 3 LA’s, while having greater quantum efficiency of about 30%, are only available from multiple suppliers in smaller sizes up to 2.5 cm diameter and usually with up to one MCP. Gen 3 LA’s must then be more closely coupled with the CCD sensor, most probably using a fiber-optic conduit or by direct optical coupling to the FOT output (Fig. 4).
2.1.2 EMCCDs
The third detector design (Fig. 5) achieves the higher gain needed to overcome the low signal experienced during fluoroscopy with new sensors called Electron Multiplying Charge Coupled Devices (EMCCD) which have recently been reported for use in nuclear imaging for photon counting [de Vree 05] [Beekman 05]. In standard CCDs, a “bucket brigade” of charge derived from the exposure to light passes from pixel to pixel toward the output. For EMCCDs an additional row of special multiplying registers is inserted at the end before the final amplifier and A to D converter. For each of these registers, a somewhat higher switching voltage of 40–50 volts is used to cause a small gain of up to 1 to 2%; however, since there may be 512 or more of these in series after the row transfer, all the charge packets see this gain. For example, the resulting total amplification, for a 1.5% gain at each register is then 1.015512 = 2045. This is approximately 20× more than is needed for fluoroscopy. For radiographic mode where the signal starts out larger, the EMCCD multiplying registers may be bypassed, and the EMCCD would be made to perform as a standard CCD. To achieve larger fields of view, we propose to design an array of modules such that the phosphor layer for the assembled modules is contiguous just as in current XII and FPD imagers.
2.1.3 FOV considerations and detector arrays
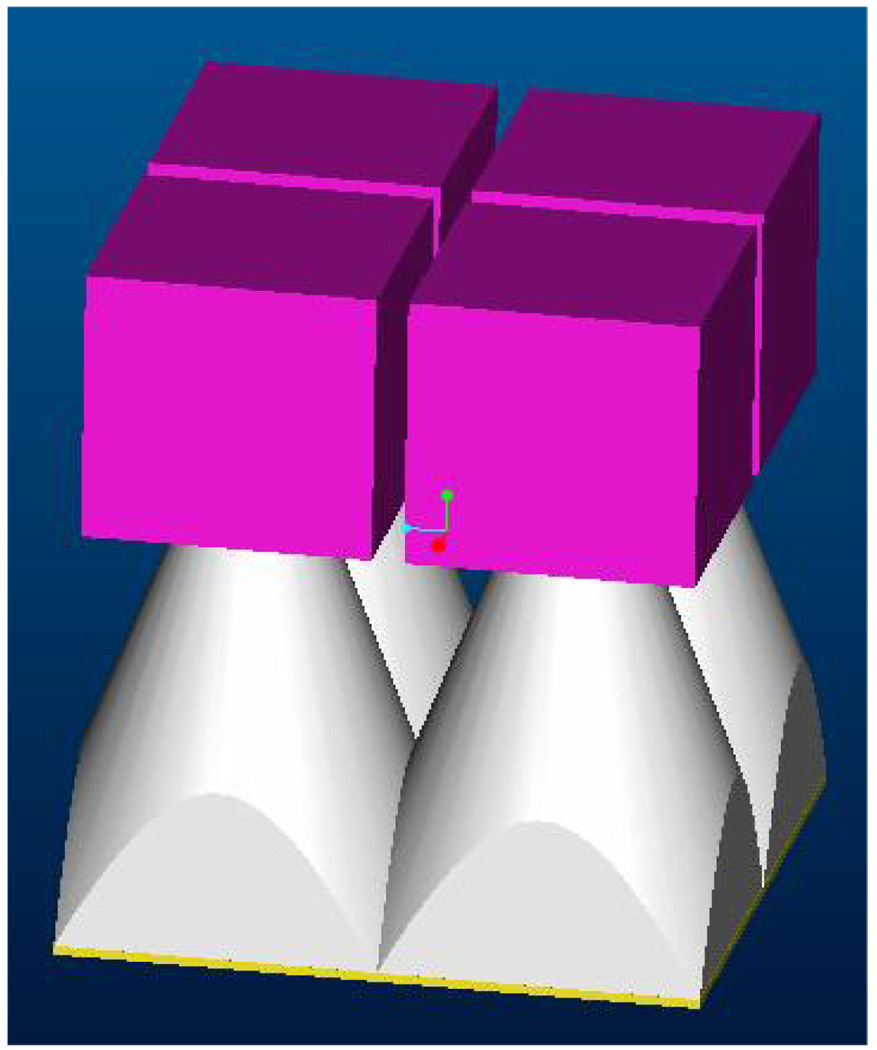
In a survey of breast diameters, the distribution peeked around 13–14 cm as the most frequent size in a range of 9–19 cm [Boone 04]. Hence, we have designed for a system FOV of about 20 cm. Although FPDs have a variety of disadvantages, one clear advantage is the adequate area, which is 30×40 cm in the case of the model 4030CB FPD that Varian has optimized specifically for CBCT. To achieve such FOVs using either LA or EMCCD based detector modules, we propose to combine these modules into square arrays as depicted in Fig 7. The fiber-optic tapers will be ground precisely so that there is minimal lost sensing-region at the boundary of adjacent modules. The use of mosaics of 2D self-scanning sensors, with or without light amplifiers, was previously proposed by our group [Rudin 81] [Rudin 83]. The use of arrays with non-amplified area sensors has also been investigated by a number of groups [Herron 90] [Stanton 99] [Allen 94] [Smith 98] and is the basis for the Bennett planar mammographic detector that underwent clinical trials but was never marketed.
Fig. 7.
Detector 2 × 2 array.
2.2 Means of evaluation
Both modeling and experimentally determining linear system analytical quantities such as MTF and DQE as functions of spatial frequency are generally accepted as a means for objectively evaluating x-ray detector system performance. However, prior to doing a full linear systems analysis and actually building the system, it is instructive during the preliminary design phase to evaluate signal to noise ratio (S/N) for both quantum noise and instrument noise to determine the weak points of the design. Quantum noise analysis may indicate a stage with too few information carriers for each absorbed x-ray resulting in possibly avoidable excess noise. Instrument noise will constrict the detector dynamic range and limit low-level sensitivity.
2.2.1 Quantum noise and quantum accounting
Quantum accounting is a method to keep track of the number of information carriers at each stage of the detector to determine if the number of such information carriers becomes so low as to cause additional quantum noise above that of the finite number of x-ray photons absorbed in the phosphor. At worst, if the number goes below unity, there is clearly a secondary quantum sink indicating severe problems with the design; however, some authors have advocated that the number of information carriers should not dip below 5 to 10 so that increased quantum noise does not substantially degrade the NEQ and DQE of the detector at higher spatial frequencies [Cunningham 00] [Williams 99].
For the three proposed detector designs discussed in this paper, the first few stages are virtually identical. Between the first stage where the x-ray photon energy is converted into multiple light photons at the cost of 18 eV per light photon [Rowland 00] and the photocathode of the LA or the EMCCD, all the stages contribute a fractional gain due primarily to optical coupling or transmission and electrical conversion losses. It is not always possible to obtain or determine exactly such gains for each of the optical components and the interfaces between these components either from manufacturer data or references in the literature, and some must be estimated. For a rigorous evaluation, the following losses should be considered:
Phosphor: number of the scintillation photons that stay in the fibers of the CsI(Tl) columnar structure, are reflected from the front surface and exit toward the first fiber optic face plate (FOP1)
Interface 1: fraction emitted from the phosphor and transmitted through to the FOP1 depending upon whether a Lambertian or collimated distribution occurs
FOP1: fraction transmitted by the FOP depending on its fill factor, absorption in the fiber core glass, and defects that could scatter the light
Interface 2: fraction transmitted from the FOP1 to the fiber optic taper (FOT) assuming coupling gel is used
FOT: fraction transmitted through the FOT depending on whether the light is Lambertian or collimated, the FOT fill factor, core absorption, fiber defects, core/clad refractive index matching, and, most important, the reduction in numerical aperture (NA) since it is inversely proportional to the square of the FOT minification [Kapany 67]
Interface 3: fraction transmitted from the FOT through to FOP2 nearest the LA or EMCCD
FOP2: fraction transmitted by the FOP2
Interface 4: fraction transmitted from FOP2 to the sensor
QE: quantum conversion efficiency for either the LA photocathode or the EMCCD
After this stage, the large gains of either the LA or the EMCCD increases the number of carriers sufficiently so that there should not be any further concern for a secondary quantum sink; however, one must consider the effect of gains of later stages, such as a mirror-lens optical coupling or an additional FOT2, on effective instrumentation noise discussed in the next section.
The estimation of gains may only be finally validated in total when a model-based DQE is compared to an experimentally measured DQE. Such a comparison was recently done by our group [Ganguly 03] and others [Williams 99]. Our comparison was done on the predecessor micro angiographic (MA) detector (Fig. 1) based on a phosphor convertor coupled to a CCD sensor with a FOT, but with no multiplying element such as an LA.
2.2.2 Instrument noise, S/N, and dynamic range of FPDs and CCD-based indirect detectors
For this work, we will compare the two new LA based designs: the previously built LA-CCD-mirror optics module (Fig. 2) and the new EMCCD based design with the previously described MA detector (Fig. 1). This will enable us to gauge the instrumentation noise of the new designs, which will limit the range of useful exposures.
In previous work on FPDs, the detector exposure at which the S/N was reduced by √2 (because the instrumentation noise and the quantum noise were equal) was determined from a plot of the S/N vs. exposure compared with a straight line representing an idealized detector with no instrumentation noise [Roos 04]. We designate this instrumentation noise equivalent exposure as INEE. For the Varian 4030CB FPD, the INEE was found to be 2.75 µR for the 194 µm pixel mode [Roos 04]. We model this instrumentation noise as a constant additive term in the following:
| (1) |
where Nexp is the measured total noise which is a combination of the quantum noise whose square is proportional to the signal which is in turn proportional by a constant k to the detector exposure, E, combined with the instrumentation noise equivalent exposure, INEE. The measured S/Nexp should obey
| (2) |
which can be used to determine INEE experimentally by fitting the (S/ Nexp)2 to the equivalent form:
| (3) |
where A is the constant k, characteristic of the slope of a straight line for large values of E, and the INEE value or B is found by subsequently fitting the curve at low values of E.
Using this method, the value of INEE for the MA (Fig. 1) was found to be <11 µR and for the MAF (Fig. 2) it is < 0.05 µR. Because the EMCCD-based design is largely similar to the MA, we can estimate the change in the INEE due to increased relative gain, hence sensitivity, for the EMCCD design from that for the MA by taking into account the gain increase due to the improved quantum efficiency and to the multiplication effect of the EMCCD. Similar comparisons apply to the MAF and new LA based designs as well when we account for the changes in gain of the LA and the mirror and lenses of the MAF and the direct optical coupling of the LA and CCD in the new designs. Since we must consider the intensity falling on the sensor, any minification factor, M, due to the lens system of the MAF or due to the tapers of the LA or EMCCD based systems will act as a minification gain in intensity. Thus for FOTs, the loss in numerical aperture as 1/M2 will be cancelled by the intensity minification gain of M2. This implies that the taper factor should not substantially affect the INEE. For this estimate, we will not consider any additional noise inherent to the LA and EMCCD or minor changes in optical component transmission but only consider the effects of quantum efficiency, electronic amplification, and CCD pixel size as they affect the exposure that is equivalent to the CCD camera readout noise. In this way, we can attempt to estimate the modified INEE from the better-studied MA design.
3. RESULTS AND DISCUSSSION
3.1 Quantum Accounting Evaluation
The results of the quantum accounting evaluation for the three proposed detectors are contained in Table 1. For all three detectors the first few stages are the same. We have assumed a 40 keV x-ray is absorbed with 18 eV required to create an average light photon [Rowlands 00]. The 0.8 factor for light transmitted through the columnar structure of the phosphor is estimated from the 0.83 [Rowlands 00] for the light remaining in the fiber and a small additional loss due to defects and coupling with the FOP. For losses in the FOP1, we have estimated the fill factor of the core glass of 0.85 as have others [Hajazi 97]. This area ratio is consistent with the linear dimension of the core to clad ratio of 12:1 or more, commonly provided by suppliers. The combination is somewhat in agreement with previous estimates up to this stage [Zhao 04]. If the phosphor were to be deposited directly on the FOT, we could eliminate the loss associated with FOT1. Assuming the use of good coupling gel at the interface from FOP1 to the FOT and assuming the inclusion of fill factor, reflections due to defects and a distribution somewhere between Lambertian and collimated with negligible absorption in the core glass (both of which have been demonstrated by others for other phosphor-fiber-optic couplings [Maidment 96]), we have estimated a combined loss factor of 0.7 × (1/M)2 for the FOT where M is the minification factor. We have used the manufacturer’s transmission estimates [INCOM 04] for a distribution between Lambertian and collimated. Similarly, we have combined any loss in the next interface to FOP2 with estimated transmission through FOP2 and interface to the sensor to yield another factor of 0.7. If the FOT were to be directly coupled to the sensor, the losses due to FOP2 could also be eliminated. We include both FOPs in the designs for ease of assembly and service. Finally, for the quantum efficiency for conversion of the remaining light to electrons for either the LAs or the EMCCD, we have estimated the values from manufacturer specifications [Hamamatsu 05] [TI 03]. For the minification factors chosen from module size considerations discussed in the next section, it can be seen that the resulting number of electrons/x-ray absorbed is in the desired range of 5–10. Because of the large gains of subsequent stages, these are not considered in Table 1. In parentheses, we have also indicated possible alternates in selections for the two LA designs. We can conclude that none of the designs should experience a secondary quantum sink.
Table 1.
Gain specifications for detectors and quantum accounting
| Parameter | Gen 2 LA / CCD | Gen 3 LA / CCD | EMCCD |
|---|---|---|---|
| Number of photons/(x-ray absorbed) (~40 keV; 18 eV/ph) | 2220 | 2220 | 2220 |
| Transmission to FOP1 | 0.8 | 0.8 | 0.8 |
| Transmission of FOP1 (incl. fill factor) | 0.85 | 0.85 | 0.85 |
| Transmission of FOT (incl. fill factor, distribution, fiber defects, and minification factor M) | 0.7/ M2 | 0.7/ M2 | 0.7/ M2 |
| Transmission through FOP2 (incl. loss into photo-sensitive layer) | 0.7 | 0.7 | 0.7 |
| FOT minification factor, M | 3.5X | 3.8X (5.0X) | 5X (8.4X) |
| QE (light ph to electrons) | 0.1 | 0.3 | 0.6 |
| Electrons/x-ray | 6.0 | 15.4 (8.9) | 17.8 (6.3) |
3.2 Instrument noise and sensitivity evaluation
3.2.1 FPDs
The primary limitation for some detector systems such as flat panel detectors (FPD) is excessive instrumentation noise rather than noise due to secondary quantum sinks. Such instrumentation noise could have a variety of causes (including dark current noise, other thermal noise, shot noise, readout noise, etc.) and has been estimated at approximately 1000 electrons per pixel per frame [Antonuk 00]. One group [Roos 04] has measured that for an FPD with 194 µm pixels, the instrumentation noise can be shown to be equivalent to the quantum noise of a 2.75 µR exposure for that FPD. We can use this value to check the number of electrons/(x-ray absorbed) for this device. Assuming approximately 2×105 x-ray photons/mm2 per mR with 90% absorbed in a 500 µm thick layer of CsI(Tl) for an 80 kVp spectrum (Table III of [Antonuk 00]), there would be 2×105 × 2.75 × 10−3 × (0.194)2 × 0.9 = 18.6 x-rays absorbed per pixel. If the standard deviation of this or 4.3 x-ray photons is equivalent to 1000 electrons, then we have 233 electrons/(x-ray photon). This loss of only about an order of magnitude below the number of light photons in the scintillation of an absorbed x-ray in the phosphor (top row of Table 1), includes light transmission losses, photo-sensor fill factor, and the light to electron conversion efficiency. This result implies no secondary quantum sink. However, clearly for fluoroscopic exposure levels, FPDs do not appear to be quantum limited but rather are instrumentation noise limited at low exposures below a few µR.
3.2.2 LA-CCD and EMCCD based detectors
In order for the MAF and any of the three proposed designs presented in this work to be potentially successful so as to enable fluoroscopic performance that can be used in a CBCT mammographic system, we must demonstrate the capacity to reduce the INEE by one to two orders of magnitude compared to the MA. As previously indicated, we will consider changes from the MA design for the optics, pixel size, and electronic gain.
For the MAF, we estimate the efficiency of the mirror and lens system to be as low as 0.2% compared to the light collection for the MA into the CCD; however, the relative intensity would be increased by the square of the minification gain, M ≈ 3, of the lens. The pixel-size reduction from 28 µm for the MA to 12 µm for the Dalsa 1M30 that we use in the MAF gives a total efficiency reduction of 0.002 × 9 × (12/28)2 ≈ 1/302. Combining this, with the additional factor of up to 100 in reduced dose per frame for fluoroscopy, results in a 3 × 104 gain requirement. The choice of the Gen 2 LAs with a 105 – 106 rated gain [Hamamatsu 05] thus appears to be necessary. (The quantum efficiency of 10% for the Gen 2 LA’s photocathode is assumed to be included in the overall LA gain estimate and the CCD quantum efficiency is assumed about the same for the MA and MAF. The actual gain depends on the details of light spectra and optical coupling, hence the gain could be somewhat lower than is rated.)
For the first proposed design of Fig. 3, the CCD is coupled more efficiently to the output of the Gen 2 LA with a second FOT rather than the MAF optics giving an efficiency factor determined by pixel size of (12/28)2 ≈ 1/5. With the fluoroscopy dose-reduction factor of up to 100, a total gain increase of about 5×102 is required, which is well with in the capability of Gen 2 LAs. For the second design of Fig. 4, the Gen 3 LAs provides a gain in the range of 103 – 104 [Hamamatsu 05] (assuming the 30% quantum efficiency or a factor of 3 increase compared to a Gen 2 LA is already included) and again this should be adequate for fluoroscopy requirements.
Finally, for the EMCCD design of Fig. 5, the increase in quantum efficiency from 20% for the MA’s CCD to 60% for the EMCCD (see Fig. 9 of reference [TI 03]) and the pixel size reduction from 28 µm to either 16 µm for the binned EMCCD or 8 µm for the unbinned EMCCD gives a gain change of (0.6/0.2) × (16/28) 2 ≈ 1 which, when combined with the fluoroscopy factor of 100, should be well within the range of up to 2000 for the EMCCD [TI 03] even if the unbinned mode with a factor of 400 is used.
3.3 Array and size considerations
Fig. 7 provides a schematic of an array of 2 × 2 modules of any one of the three different designs to form the total detector system. The FOV needed is estimated to be 20 cm × 20 cm. Only the phosphor layer, the FOTs, and a rectangular solid representing the remaining components including the LA-CCD combination or the EMCCD are indicated in the figure. Although (as mentioned in 2.1.3 above) other investigators including the present authors have previously explored arrays of FOT-CCD modules, we believe our designs are the first proposed arrays of detectors with a sufficiently large amplification component to enable achievement of images at both fluoroscopy and radiological exposure levels.
The minification factors indicated in Table 1 are selected to make the module dimensions as large as possible so as to minimize the number of modules needed to form the array with an adequate FOV. Table 2 indicates the detector module and array sizes for each of the three designs. For the Gen 2 LA – CCD design, the least minification factor of 3.5× is needed to achieve a 2 × 2 array with the required 20 cm FOV because these LAs are larger than that for the second design using Gen 3 LAs. The lower QE of the Gen 2 LA compared to the Gen 3 LA, however, results in a lower number of electrons per x-ray. For the first two designs, since the LAs are round in shape and the CCD sensor is square, we have accepted the loss in usable area of the LA by completely enclosing the square FOV of the CCD subtended at the plane of the LA. For 2 × 2 arrays, it may be possible to choose modifications to this design if some loss in CCD imaging area is accepted as a result of a lower minification factor selection to increase the number of electrons per x-ray. For the second design of the Gen 3 LA – CCD, because of the smaller size available, 2.5 cm diameter, a larger 3 × 3 array is indicated resulting in a more than adequate electron/x-ray ratio of 15.4 because of the improved QE for the Gen 3 LAs. Because 5 appears to be the largest minification factor that is standard for the FOT manufacturers, we have also indicated on the tables an alternate design for a 2 × 2 array in parentheses using that minification factor; however, the FOV would be restricted somewhat. In order to achieve the required 20 cm FOV, a minification factor of 5.6 would have had to be obtained with a consequent and still adequate 7.1 electron/x-ray ratio.
Table 2.
Detector and array size
| Parameter | Gen 2 LA / CCD | Gen 3 LA / CCD | EMCCD |
|---|---|---|---|
| Dimension (cm) | |||
| Circular diameter: LA | 4 | 2.5 | -- |
| Square side: CCD | 2.83 | 1.77 | 0.8 |
| Module size (cm) | 9.9 | 6.7 (8.9) | 4 (6.7) |
| Array | 2 X 2 | 3 X 3 (2 X 2) | 5 X 5 (3 X 3) |
| FOV | 20 | 20 (18) | 20 (20) |
The third detector based on EMCCDs appears to require either the largest array or greatest minification factor because of the small size of current EMCCDs. In the tables, a 5 × 5 array of modules is indicated, assuming the FOT minification ratio is no larger than 5. An alternative design listed in parentheses considers a much larger minification factor of 8.4, which would enable a 3 × 3 array to be used to achieve the desired 20 cm FOV. For both cases, the electron/x-ray ratio would appear to be acceptable for eliminating any secondary quantum sinks (Table 1) because of the superior QE of the EMCCDs for the light spectrum emitted by the CsI(Tl) phosphor.
3.4 Application to region of interest computed tomography (ROI-CT)
For clinical studies requiring superior images only in a small ROI, ROI-CT can be used. For CBCT mammography, this might be analogous to extra “mag” views done following standard screening planar mammography. In previous descriptions of ROI-CT [Chityala 05a] [Chityala 05b], two scans are taken: one at low resolution and low dose over the full field, and the other with the high-resolution ROI detector for high dose, but only over the ROI. The two scans would have to be aligned and grey-level or equalized before the mathematical back-projection. The higher dose ROI scan provides the high quality imagery, whereas the lower dose full-field scan enables low-artifact, accurate CBCT reconstruction.
If the ROI is off center of the CT rotational axis, the ROI beam-filter must move sinusoidally during the scan and the high-resolution ROI detector would have to move in synchrony sinusoidally with the ROI filter. Using the proposed high-resolution array, the ROI can be “moved” electronically and there is only need for one physical scan assuming there is adequate detector dynamic range. To extend the dynamic range, however, the full field-of-view exposure may have to be taken separately from the ROI exposure, while using greater LA or EMCCD gain.
An alternate scanning geometry is where the rotation is around the center of the ROI and where the bowtie filter for the larger FOV moves off axis sinusoidally. Reconstruction is then done by selecting appropriate rays from various views so as to backproject around a rotational axis through the ROI. In this case, the high-resolution ROI and ROI filter do not move. However, if the ROI is substantially distant from the center of the patient, such a rotation around an axis through the ROI may be less practical than the first ROI scanning method where the CT rotational axis remains at the center of the patient.
4. CONCLUSIONS
New x-ray detector designs consisting of a mosaic or array of modules having image amplifying components such as light amplifiers or EMCCDs were explored with regard to their possible application in fluoroscopy, CBCT mammography, and ROI-CT. These new detectors appear to have advantages over flat panel detectors in that they may provide higher spatial resolution, high sensitivity, wide dynamic range, negligible lag, high frame rate capability, and are quantum limited at low exposures since they have low additive instrumentation noise. These features, characteristic of the small field of view detectors previously reported by our group, may now be extended to medical imaging applications where larger field of views are expected. Work to develop prototypes of some of these advanced designs is continuing.
ACKNOWLEDGEMENTS
This work was supported in part by NIH grants R01NS43924 and R01EB02873, a grant from the UB2020 Interdisciplinary Research Development Fund (IRDF) of the University at Buffalo, and by an equipment grant from the Toshiba Medical Systems Corp.
REFERENCES
- Allen B. Application of CCDs to digital x-ray mammography; Proceedings from Medical Imaging 1994: Physics of Medical Imaging; 1994. pp. 264–273. [Google Scholar]
- Antonuk LE, Jee K-W, El-Mohri Y, Maolinbay M, Nassif S, Rong X, Zhao Q, Siewerdsen JH. Strategies to improve the signal and noise performance of active matrix flat-panel imager for diagnostic x-ray applications. Med Phys. 2000;27(2):289–306. doi: 10.1118/1.598831. [DOI] [PubMed] [Google Scholar]
- Beekman FJ, de Vree GA. Photon-counting versus an integrating CCD-based gamma camera: important consequences for spatial resolution. Phys. Med. Biol. 2005;50:N109–N119. doi: 10.1088/0031-9155/50/12/N01. [DOI] [PubMed] [Google Scholar]
- Boone JM, Shah N, Nelson TR. A comprehensive analysis of DgNCT coefficients for pendant-geometry cone-beam breast computed tomography. Medical Physics. 2004 Feb;31(2):314. doi: 10.1118/1.1636571. [DOI] [PubMed] [Google Scholar]
- Boone JM, Kwan A, Nelson TR, Shah N, Burkett G, Seibert JA, Lindfors KK, Roos G. Performance assessment of a pendant-geometry CT scanner for breast cancer detection. Medical Imaging 2005: Physics of Medical Imaging. In: Flynn MichaelJ., editor. Proceedings of SPIE. Vol. 5745. Bellingham, WA: SPIE; 2005. pp. 319–323. paper #38. [Google Scholar]
- Chen L, Shaw CC, Tu S, Altunbas MC, Wang T, Lai C, Liu X, Kappadath SC. Cone-beam CT breast imaging with a flat panel detector – a simulation study. Medical Imaging 2005: Physics of Medical Imaging. In: Flynn MichaelJ., editor. Proceedings of SPIE. Vol. 5745. Bellingham, WA: SPIE; 2005. pp. 943–951. [Google Scholar]
- Chityala R, Hoffmann KR, Rudin S, Bednarek DR. Region-of-interest (ROI) Computed Tomography: Combining dual resolution XRII images (abstract) Medical Physics. 2005 Jun;32(6):2056. MO-D-I-611-4. [Google Scholar]
- Chityala R, Hoffmann KR, Rudin S, Bednarek DR. ROI CT: a process to obtain high quality reconstructions with less dose (abstract). Scientific Program and 91st Scientific Assembly and Annual Meeting of RSNA; Nov. 27 – Dec. 2, 2005; Chicago. 2005. Nov, scientific educational poster. [Google Scholar]
- Cunningham IA. Handbook of Medical Imaging. Vol. 1. Bellingham, WA: SPIE Press; 2000. p. 131. Chapter 2. [Google Scholar]
- de Vree GA, Westra AH, Moody I, van der Have F, Ligtvoet KM, Beekman FJ. Photon-counting gamma camera based on an electron-multiplying CCD. IEEE Trans. on Nucl. Sci. 2005 Jun;52(3):580–588. [Google Scholar]
- Ganguly A, Rudin S, Bednarek DR, Hoffmann KR, Yang CJ, Wang Z. Direct conversion flat panel detector for region-of-interest angiography; Proceedings from Medical Imaging 2001: Physics of Medical Imaging, San Diego, CA; 2001. pp. 178–188. [Google Scholar]
- Ganguly A, Rudin S, Bednarek DR, Hoffmann KR, Kyprianou I. Micro angiography for Neuro vascular Imaging, Part 1: Experimental Measurements and Feasibility. Medical Physics. 2003 Nov;30(11):3018–3028. doi: 10.1118/1.1617549. [DOI] [PubMed] [Google Scholar]
- Ganguly A, Rudin S, Bednarek DR, Hoffmann KR. Micro angiography for Neuro vascular Imaging, Part 2: Cascade Model Analysis. Medical Physics. 2003 Nov;30(11):3029–3039. doi: 10.1118/1.1617550. [DOI] [PubMed] [Google Scholar]
- Glick SJ, Thacker S, Gong X. The importance of modeling normal mammographic structure in optimizing flat-panel CT breast imaging systems. Medical Imaging 2005: Physics of Medical Imaging. In: Flynn MichaelJ., editor. Proceedings of SPIE. Vol. 5745. Bellingham, WA: SPIE; 2005. pp. 847–859. [Google Scholar]
- Hejazi S, Trauernicht DP. System considerations in CCD-based x-ray imaging for digital chest radiography and digital mammography. Med. Phys. 1997;24:287. doi: 10.1118/1.598072. [DOI] [PubMed] [Google Scholar]
- Hamamatsu PhotonicsKK. Electron Tube Center, Japan, “Fiber optics plate with scintillator (FOS)” catalogue. 1997 [Google Scholar]
- Hamamatsu PhotonicsKK. Electron Tube Center, Japan, “Image intensifiers” brochure TII 0001E04. revised June 2005, http://sales.hamamatsu.com/assets/pdf/catsandguides/Image_Intensifier.pdf. [Google Scholar]
- Herron JM, Gur D, Daxon EG, Good WF, Shaw CC, Maitz GS, Boyer JW. Digital x-ray imaging with two-dimensional charge-coupled device (CCD) arrays; Proceedings from Medical Imaging 1990: Image Formation; 1990. pp. 472–478. [Google Scholar]
- BLS59-12 Fiber-optic Taper Data Sheet. last update: Nov. 2, 2004, INCOM Inc. http://www.incomusa.com/BLS59-12.html.
- Fiberoptics, Principles and Applications. New York: Academic Press; 1967. [Google Scholar]
- Karellas A, Harris Lisa, Liu H, Davis MA, D’Orsi CarlJ. Charge-coupled device detector: Performance and potential for small-field mammographic imaging application. Med. Phys. 1992;19:1015–1023. doi: 10.1118/1.596819. [DOI] [PubMed] [Google Scholar]
- Maidment A, Yaffe MJ. Analysis of signal propagation in optically coupled detectors for digital mammography: II. Lens and fibre optics. Phys Med Biol. 1996;41:475–493. doi: 10.1088/0031-9155/41/3/010. [DOI] [PubMed] [Google Scholar]
- Ning R, Concover DL, Yu Y, Lu X, Shiffhauer L, Cullinan J. Evaluation of flat panel detector based cone beam CT breast imaging with different sizes of breast phantoms. Proc SPIE Medical Imaging. 2005;Vol. 5745:626–636. paper #69. [Google Scholar]
- Roos PG, Colbeth RE, et al. Multiple gain ranging readout method to extend the dynamic range of amorphous silicon flat panel imagers. Proc SPIE Medical Imaging. 2004;Vol. 5368:139–149. paper #16. [Google Scholar]
- Rowlands JA, Yorkston J. Handbook of Medical Imaging. Vol. 1. Bellingham, WA: SPIE Press; 2000. pp. 244–247. Chapter 4. [Google Scholar]
- Rudin S, Bednarek DR, Wong R. Conical rotating aperture geometries in digital radiography. Digital Radiography; Proceedings of the Conference on Digital Radiography held at Stanford University; September 14 17, 1981; 1981. pp. 77–80. [Google Scholar]
- Rudin S, Bednarek DR, Wong R. Rapid scanning beam digital radiography. J. Imaging Technology (formerly J. Appl. Photog. Engin.) 1983 Dec;9(6):196–198. [Google Scholar]
- Rudin S, Bednarek DR, Yang C-YJ, Chattopadhyay A, Gopal A, Wu Y, Wang Z, Nazareth DP, Hoffmann KR. Region of interest (ROI) micro-angiography: imager development; Proceedings from Medical Imaging 2000: Physics of Medical Imaging, San Diego, CA. Paper #59. SPIE vol. 4682, pp. 344–354, 2002; 2000. pp. 534–541. [Google Scholar]
- Rudin S, Wu Y, Kyprianou I, Ionita C, Wang Z, Ganguly A, Bednarek DR. Microangiographic detector with fluoroscopic capability; Proceedings from Medical Imaging 2002: Physics of Medical Imaging, San Diego, CA, paper #36; 2002. pp. 344–354. [Google Scholar]
- Smith ST, Kim H, Swarnakar V, Jeong M, Wobschall DC. Parallel hardware architecture for CCD-mosaic digital mammography; Proceedings from Medical Imaging 1998: Medical Display, San Diego, CA; 1998. Feb, pp. 663–674. [Google Scholar]
- Stanton M, Phillips W, Stewart A, Smilowitz L, Williams MB, Simoni PU, Ingersoll C, McCauley T, Qian H. CCD-based detector for full-field digital mammography; Proceedings from Medical Imaging 1999: Physics of Medical Imaging, San Diego, CA; 1999. [Google Scholar]
- TC285SPD-30 1004x1002 Pixel IMPACTRON CCD Image Sensor. P. O. Box 655303, Dallas, Texas 75265: Pamplet published by Texas Instruments; 2003. [Google Scholar]
- Tornai MP, McKinley RL, Brzymialkiewicz CN, Madhav P, Cutler SJ, Crotty DJ, Bowsher JE, Samei E, Floyd CE. Design and Development of a Fully-3D Dedicated X-ray Computed Mammotomography System. Medical Imaging 2005: Physics of Medical Imaging. In: Flynn MichaelJ., editor. Proceedings of SPIE. Vol. 5745. Bellingham, WA: SPIE; 2005. pp. 189–197. [Google Scholar]
- Vedanthan S, Karellas A, Suryanarayanan S. Solid-state fluoroscopic imager for high-resolution angiography: Physical characteristic of an 8 cm × 8 cm experimental prototype. Medical physics. 2004;31(6):1462–1472. doi: 10.1118/1.1750992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MB, Simoni PU, Smilowitz L, Stanton M, Phillips W, Stewart A. Analysis of the detective quantum efficiency of a developmental detector for digital mammography. Med. Phys. 1999;26:2273–2285. doi: 10.1118/1.598741. [DOI] [PubMed] [Google Scholar]
- Wu Y, Rudin S, Bednarek DR. A prototype micro-angiographic fluoroscope and its application in animal studies; Proceedings from Medical Imaging 2005: Physics of Medical Imaging, San Diego, CA, paper #123; 2005. pp. 1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Takahashi A, Umazaki H, Honda M, Shiraishi K, Rudin S, Yang C-Y, Bednarek DR. Image quality evaluation of a new selenium-based flat panel digital X-ray detector system based on animal studies; Proceedings from Medical Imaging 2000: Physics of Medical Imaging, San Diego, CA. paper #46; 2000. pp. 429–436. [Google Scholar]
- Zhao W, Ristic G, Rowland JA. X-ray imaging performance of structured cesium iodide scintillators. Medical Physics. 2004;31(9):2594–2605. doi: 10.1118/1.1782676. See p. 2598. [DOI] [PubMed] [Google Scholar]