Abstract
Background
Bronchiolitis obliterans syndrome (BOS) is the major hurdle preventing long-term success in lung transplantation, and is the primary reason for the 50% 5-year survival. Recipient and perioperative risk factors have been investigated in BOS, but less is known about donor factors. Therefore, we investigated what donor factors are important in the development of BOS.
Methods
We performed a retrospective review of the United Network for Organ Sharing lung transplant database from 1987 to 2008. Lung transplant recipients had yearly follow-up. Donor factors were evaluated for their influence on BOS development. Kaplan-Meier plots of BOS-free survival were compared for each donor factor and a multivariate Cox proportional hazard model for BOS was created with donor factors.
Results
A total of 17,222 lung transplant recipients were identified; 6,991 recipients had sufficient follow-up BOS data. Of these recipients 57% (n = 3,984) developed BOS within 5 years. Recipients who received lungs from donors who were younger, without an active pulmonary infection, or those without current tobacco use had longer BOS-free survival. Recipients who received lungs with higher partial pressures of oxygen in arterial blood (PaO2) developed more BOS (p < 0.0001). Donor high PaO2, older age, and current tobacco use were independent predictors of BOS in lung transplant recipients.
Conclusions
Donor factors and donor management strategies are important contributors to development of recipient BOS. Identification of these factors may help limit BOS and may identify recipients at high risk. Surprisingly, high PaO2 in the donor is an independent predictor of BOS development.
Lung transplantation remains the best option for many patients with end-stage lung disease, but its long-term success remains limited by chronic allograft rejection, or bronchiolitis obliterans (BO). Bronchiolitis obliterans is pathologically seen as scarring and fibrosis of the small airways and may be temporally heterogeneous. Because of the difficulty detecting BO on transbronchial biopsies it is defined clinically by a declining forced expiratory volume in one second in the absence of another cause and termed bronchiolitis obliterans syndrome (BOS) [1]. Bronchiolitis obliterans syndrome is a leading cause of major morbidity and late mortality after lung transplantation [2]. Despite its high prevalence, reported to occur in 45% to 75% of lung transplant recipients (LTRs) within the first 5 years [3–9], risk factors for BOS remain unclear.
Several studies have investigated recipient risk factors involved in BOS development. Accepted risk factors include acute rejection, lymphocytic bronchitis-bronchiolitis, and ischemia-reperfusion injury [10–12]. Potential risk factors have also been reported and include cytomegalovirus infection, other infectious organisms, human leukocyte antigens (HLA) matching, and gastroesophageal reflux disease [10, 12].
Donor characteristics and donor management strategies have been less studied and are considered hypothetical risk factors [12]. Recognizing these donor factors could allow for more optimal donor selection or modification of their management with a resultant decrease in recipient BOS development. Therefore, in this study we investigated what donor factors are important in the development of BOS.
Patients and Methods
Data Source
The United Network for Organ Sharing (UNOS) provided transplant and follow-up information from the UNOS Standard Transplant Analysis and Research files for lung transplantations with all patient and center identifiers excluded. This study was reviewed by our Institutional Review Board and granted exemption from approval and consent (see Acknowledgments).
The UNOS lung transplant and follow-up data set is a prospectively collected database of every organ donation and transplantation in the United States since 1987. All LTRs had yearly follow-up. Over 400 donor, preoperative, intraoperative, and postoperative variables are contained within the UNOS dataset for LTRs. Each transplant center collects and reports data to UNOS based on data collection forms provided by UNOS.
Patient Population
The 17, 222 LTRs identified in the UNOS database were divided into recipients with BOS, those with an unknown BOS status, and recipients without BOS. The 10, 231 LTRs with unknown BOS status were excluded from the study. A LTR with BOS was defined as any recipient who had a single BOS event within the first 5 years after transplantation regardless of subsequent follow-up. A LTR without BOS was defined as any recipient who was BOS negative for 5 consecutive years after transplantation.
Variables Examined and Outcomes Measured
A retrospective review of all patients undergoing lung transplantation from 1987 to May 2008 was performed. All variables were included in the univariate analysis. In the multivariate analysis variables were excluded if greater than 50% of data was missing. Donor factors of interest were selected based on clinical relevance in the literature and lung transplant surgeon experience.
Relevant variables examined for each lung transplant recipient included the following: demographic factors (age, gender, race, etc), factors related to their pulmonary disease (diagnosis, oxygen requirement, etc), comorbidities, perioperative variables (transplant type, ischemic time, etc), postoperative outcomes, and complications (dialysis, airway dehiscence, pulmonary infection, acute rejection, etc). Pertinent donor factors included demographics, comorbidities, and pre-procurement workup (cytomegalovirus status, ABO status, infectious status, creatinine, cause of death, etc).
New variables were created to allow for simple comparisons between donor and recipient factors. These included the following: ABO match, gender match, HLA mismatch, A locus mismatch, old age (age ≥ 60 years old), high preoperative oxygen requirement in the recipient (≥ 75th percentile), long ischemic time (≥ 75th percentile), and high donor partial pressure of oxygen in arterial blood (Pao2) (≥ 75th percentile or ≥ 509 mm Hg).
The primary endpoint was the development of BOS within 5 years after lung transplantation. The influence of donor factors on the development of BOS was studied.
Statistical Analysis
Descriptive analyses comparing demographic data between LTRs with and without BOS were performed. In a univariate analysis the influence of each donor factor on the development of BOS was compared. Categoric variables were compared using the χ2 or Fischer exact test and continuous variables were compared with the 2-sided t test. Categoric data are reported as frequencies and percentages and a statistical significance of p less than 0.05 was used.
Kaplan-Meier curves of BOS-free survival by 5 years after transplantation were analyzed for each donor variable. A log-rank test was used to compare the influence of each donor factor on BOS-free survival. These differences were subsequently quantified with Cox proportional hazard ratios calculating the relative risk of each donor factor associated with the development of BOS after transplantation.
A multivariate model was created using a Cox proportional hazard model to identify donor factors that are independent predictors of BOS development in the recipient. The multivariate model was constructed using donor factors previously described in the literature or suspected based on clinical knowledge to be independent predictors of BOS development. Other donor factors were included if significant in the univariate analysis. The following donor factors were included: old age, high Pao2, HLA mismatch, active pulmonary infection, history of myocardial infarction, current tobacco, traumatic cause of death, and female gender. All statistical analysis was performed with SAS 9.1.3 (SAS Institute, Cary, NC) software.
Results
Baseline Demographics
From 1994 to May of 2008 17,222 patients underwent lung transplantation in the UNOS registry. Of this cohort, 10,231 recipients were excluded from the study because of unknown BOS status. The remainder of the cohort, 6,991 recipients, had a 57% (n = 3,984) prevalence of BOS. The mean age of this cohort was 46.1 ± 14.7 years old, with 52% women (n = 3,640).
The LTRs with BOS and without BOS varied on several characteristics (Table 1). There were small differences in peripheral vascular disease, history of malignancy, postoperative airway dehiscence, postoperative dialysis, and postoperative stroke. Larger differences were seen in age, diabetes, preoperative oxygen requirements, postoperative use of antibiotics, and antivirals. Not surprisingly, recipients who developed BOS were also more likely to have an episode of acute rejection prior to discharge. Because of a very large population, small differences between LTRs with and without BOS were statistically significant.
Table 1.
Baseline Recipient Demographics
| Variable | BOS Free at 5 Years (n = 3,007) (%) | BOS Within 5 Years (n = 3,984) (%) | p Valuea |
|---|---|---|---|
| Old age (≥60 years old) | 575 (19.1) | 903 (22.7) | 0.0003a |
| Gender (female) | 1,589 (52.8) | 2,051 (51.5) | 0.26 |
| Ethnicity | 0.05 | ||
| ABO status | 0.71 | ||
| CMV (IgG) | 565 (20.9) | 1,225 (33.1) | <0.0001a |
| Creatinine | 0.89 (0.72) | 0.94 (1.5) | 0.16 |
| Cerebrovascular disease | 9 (0.39) | 14 (0.44) | 0.13 |
| Diabetes | 152 (6.7) | 322 (10.1) | <0.0001a |
| Peripheral vascular disease | 27 (1.2) | 22 (0.69) | 0.001a |
| History of malignancy | 54 (1.8) | 89 (2.2) | <0.0001a |
| Crossmatch done | 2,496 (84.1) | 1,111 (69.1) | 0.67 |
| Diagnosis | <0.0001a | ||
| COPD | 1,174 (39.0) | 1,591 (39.9) | |
| Pulmonary fibrosis | 366 (12.2) | 593 (14.9) | |
| Cystic fibrosis | 527 (17.5) | 690 (17.3) | |
| Sarcoidosis | 77 (2.6) | 71 (1.8) | |
| Alpha 1 antitrypsin deficiency | 297 (9.8) | 302 (7.6) | |
| Pulmonary hypertension | 186 (6.2) | 180 (4.5) | |
| Bronchiectasis | 74 (2.5) | 78 (2.0) | |
| High O2 requirement (>75th percentile) | 409 (13.6) | 686 (17.2) | <0.0001a |
| Transplant type | |||
| Single | 1,575 (52.4) | 2,079 (52.2) | 0.87 |
| Double | 1,432 (47.6) | 1,904 (47.8) | |
| Long ischemic time (minutes) (>75th percentile) | 634 (21.1) | 846 (21.2) | 0.88 |
| Postoperative airway dehiscence | 12 (0.48) | 21 (0.61) | 0.02a |
| Postoperative dialysis | 51 (2.0) | 61 (1.8) | 0.01a |
| Postoperative stroke | 37 (1.5) | 42 (1.2) | 0.02a |
| Postoperative infection | 927 (36.9) | 1,422 (41.8) | <0.0001a |
| Postoperative antiviral treatment | 1,111 (37) | 2,091 (52.6) | <0.0001a |
| Acute rejection prior to discharge | 5 (41.7) | 85 (11.4) | 0.001a |
| Length of stay (days) | 21.5 (24.7) | 20.4 (24.7) | 0.43 |
Significance p < 0.05
BOS = bronchiolitis obliterans syndrome; CMV = cytomegalovirus; COPD = chronic obstructive pulmonary disease; IgG = immunoglobulin G.
Donor Factors Associated with BOS
There are several donor factors significantly associated with the development of BOS at 5 years (Tables 2; 3). The LTRs with BOS were more likely to receive lungs from older donors (≥ 60 years old), donors who were current smokers (< 6 months from donation), and those who had a history of myocardial infarction. A high donor Pao2 (≥ 509 mm Hg) at the time of procurement and an active pulmonary infection in the donor were also associated with the development of recipient BOS (Table 2). The HLA mismatch, A locus mismatch, and living donors were the only matched donor-recipient factors that significantly influenced BOS development (Table 3).
Table 2.
Univariate Analysis of Donor Factors Associated With BOS
| Variable | BOS Free At 5 Years (n = 3,307) (%) | BOS Within 5 Years (n = 3,984) (%) | p Valuea |
|---|---|---|---|
| Old age (≥ 60 years) | 686 (22.8) | 998 (25.1) | 0.03a |
| Gender (female) | 1,159 (38.5) | 1,440 (36.1) | 0.26 |
| Ethnicity | 0.07 | ||
| ABO status | <0.0001a | ||
| CMV status | 1,509 (51.8) | 2,183 (55.8) | 0.001a |
| Current tobacco use (< than 6 months from donation) | 518 (33) | 751 (45.4) | <0.0001a |
| History of smoking (≥ than 6 months from donation) | 648 (26.8) | 906 (26.7) | 0.09 |
| Creatinine | 1.2 (1.8) | 1.2 (1.4) | 0.03a |
| Hypertension | 297 (12.3) | 442 (13) | 0.67 |
| Diabetes | 48 (2) | 88 (2.6) | 0.32 |
| History of myocardial infarction | 12 (0.44) | 50 (1.4) | 0.0002a |
| History of tobacco use | 648 (26.7) | 906 (26.7) | 0.09 |
| History of IVDU | 23 (0.95) | 21 (0.78) | 0.58 |
| History of malignancy | 39 (1.6) | 53 (1.6) | 0.24 |
| Living donor | 68 (2.3) | 39 (0.98) | <0.0001a |
| Traumatic cause of death | 1,525 (57.1) | 2,097 (57.3) | 0.84 |
| Active pulmonary infection | 322 (11) | 569 (14.4) | <0.0001a |
| High Pao2 (≥ 75th percentile or ≥ 509 mm Hg) | 277 (9.2) | 580 (14.6) | <0.0001a |
Significance p < 0.05.
BOS = bronchiolitis obliterans syndrome; CMV = cytomegalovirus; IVDU = intravenous drug use; Pao2 = partial pressure of oxygen in arterial blood.
Table 3.
Univariate Analysis of Donor-Recipient Matched Factors Associated With BOS
| Variable | BOS Free At 5 Years (n = 3,007) (%) | BOS At 5 Years (n = 3,984) (%) | p Valuea |
|---|---|---|---|
| Gender match | 1,405 (58.0) | 2,579 (56.4) | 0.2 |
| ABO match | 0.7 | ||
| HLA mismatch | 0.03a | ||
| A locus mismatch | 0.0002a |
Significance p < 0.05.
BOS = bronchiolitis obliterans syndrome; HLA = human leukocyte antigens.
Risk of Developing BOS by Donor Factor
The Kaplan-Meier curves of BOS-free survival demonstrated that recipients who received lungs from donors with a high Pao2 had significantly less BOS-free days (Fig 1). Donors with an active pulmonary infection, older donor age, history of myocardial infarction and current tobacco use also had significantly less BOS-free days (Fig 2). Living donors had greater BOS-free days than cadaveric donors. Human leukocyte antigen mismatch and A locus mismatch were also significantly associated with less BOS-free survival (Table 4).
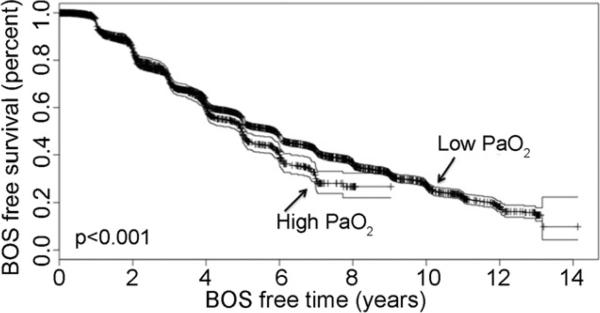
Fig 1.
Kaplan-Meier bronchiolitis obliterans (BOS)-free survival for donor hyperoxia. (Pao2 = partial pressure of oxygen in arterial blood.)
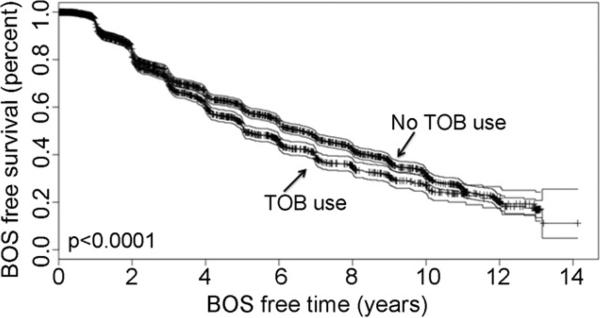
Fig 2.
Kaplan-Meier bronchiolitis obliterans (BOS)-free survival for donor current tobacco use (TOB).
Table 4.
Risk of BOS by Donor Factor
| Variable | K-M Log-rank Test | Relative Risk | 95% Confidence Intervals | p Valuea |
|---|---|---|---|---|
| Gender match | 0.33 | 0.97 | 0.91-1.03 | 0.33 |
| Female gender | 0.06 | 0.94 | 0.88-1.00 | 0.06 |
| Old age (≥ 60 years old) | <0.0001a | 1.11 | 1.04-1.2 | 0.004a |
| Current tobacco use (< 6 months from donation) | <0.0001a | 1.43 | 1.3-1.57 | <0.0001a |
| Living donor | <0.0001a | 0.53 | 0.39-0.73 | <0.0001a |
| DR locus mismatch | 0.29 | 1.04 | 0.98-1.1 | 0.16 |
| HLA mismatch | 0.02a | 1.08 | 1.03-1.1 | 0.01a |
| A locus mismatch | 0.0001a | 1.13 | 1.07-1.09 | <0.0001a |
| High Pao2 (≥ 75th percentile or ≥ 509 mm Hg) | <0.0001a | 1.34 | 1.23-1.46 | <0.0001a |
| History of myocardial infarction | <0.0001a | 1.94 | 1.46-2.56 | <0.0001a |
| Active pulmonary infection | <0.0001a | 1.25 | 1.14-1.36 | <0.0001a |
Significance p < 0.05.
BOS = bronchiolitis obliterans syndrome; HLA = human leukocyte antigens; K-M = Kaplan-Meier; Pao2 = partial pressure of oxygen in arterial blood.
A high Pao2 in the donor inferred an increased risk of BOS in the recipient by 34% (relative risk [RR] 1.34, 95% confidence interval [CI] 1.23 to 1.46, p < 0.0001). Similarly, an active pulmonary infection in the donor increased the risk of BOS in the recipient by 25% (RR 1.25, 95% CI 1.14 to 1.36, p < 0.0001) and current tobacco use in the donor increased risk of BOS by 43% in the recipient (RR 1.43, 95% CI 1.3 to 1.57, p < 0.0001). Older age, history of myocardial infarction, HLA mismatch, and A locus mismatch all demonstrated a significant increase in the relative risk of BOS (Table 4). A living donor was protective against BOS by 47% (RR 0.53, 95% CI 0.39 to 0.73, p < 0.0001).
Independent Predictors of BOS
The multivariate model identified three independent predictors of BOS after lung transplantation (Table 5). High donor Pao2 (hazard ratio [HR] 1.38, 95% CI 1.11 to 1.73, p = 0.005) and current tobacco use (HR 1.33, 95% CI 1.18 to 1.49, p < 0.0001) were independent predictors of BOS in the recipient after lung transplantation. Donor old age was also an important predictor of BOS (HR 1.16, 95% CI 1.002 to 1.33, p = 0.047). Donor active pulmonary infection and HLA mismatch were not found to be independent predictors of BOS (Table 5).
Table 5.
Multivariate Cox Proportional Hazards Model for Independent Predictors of BOS
| Variable | Hazard Ratio | 95% Confidence Interval | p Valuea |
|---|---|---|---|
| High Pao2 (≥ 75th percentile or ≥ 509 mm Hg) | 1.38 | 1.11-1.73 | 0.005a |
| Donor old age (≥ 60 years old) | 1.16 | 1.002-1.33 | 0.047a |
| Donor history of myocardial infarction | 1.79 | 1.00-3.19 | 0.05 |
| Donor active pulmonary infection | 1.04 | 0.88-1.23 | 0.63 |
| Donor traumatic cause of death | 1.0 | 0.87-1.14 | 0.97 |
| HLA mismatch | 1.08 | 0.98-1.18 | 0.14 |
| Current tobacco use (< 6 months from donation) | 1.33 | 1.18-1.49 | <0.0001a |
Significance p < 0.05
BOS = bronchiolitis obliterans syndrome; HLA = human leukocyte antigens; Pao2 = partial pressure of oxygen in arterial blood.
Comment
Bronchiolitis obliterans syndrome is a serious complication after lung transplantation occurring in more than 50% of LTRs. It has been the major hurdle and the key limitation to long-term success after lung transplantation. While many recipient and perioperative risk factors have been implicated in the development of BOS, donor factors have not been well investigated. The studies that do exist often report conflicting information, which is frequently due to small sample sizes and single institution studies.
In this current study, 3,984 LTRs (57%) developed BOS. This study augments the literature because it is a large multi-institutional review of donor factors that contribute to recipient BOS development. We found several donor factors to be associated with the development of BOS including donor hyperoxia, older age, active pulmonary infection, and current tobacco use (Table 4). Donor hyperoxia, older age, and current tobacco use were the only three factors found in multivariate analysis to be independent predictors of BOS (Table 5).
Donor hyperoxia is a surprising risk factor for BOS, as higher Pao2 in the donor should suggest a better quality of lungs. One standard criterion for lung donation is a Pao2 greater than 300 mm Hg on a fraction of inspired oxygen of 100% and 5 of positive end-expiratory pressure. In our study, a high Pao2 only became associated with increased development of BOS at levels greater than a Pao2 of 509 mm Hg. In these donors, hyperoxia led to a 34% increase in the relative risk of BOS after lung transplantation and recipients who received lungs from donors with hyperoxia had less BOS-free days (Fig 1). Most importantly, donor hyperoxia was found to be independently predictive of BOS in the recipient. Previous studies examining the influence of donor factors on the development of BOS in LTRs did not identify donor hyperoxia as a potential risk factor. Many recognize hyperoxia as a cause of acute lung injury [13] and reactive oxygen species are believed to play a significant role in the pathogenesis of hyperoxia-induced lung injury [14]. Therefore, donor hyperoxia may cause an increase in reactive oxygen species and lead to subclinical lung injury. We speculate that these very high Pao2s may be caused from ventilator strategies of aggressive recruitment causing barotrauma. Barotrauma in the donor lung has been identified as one cause of lung injury after transplantation [15, 16]. Haniuda and colleagues [16] reported that donor lung storage at high lung volumes or a high-inspired oxygen fraction increases pulmonary capillary permeability. Our conclusions are preliminary. Unfortunately, the UNOS database does not collect data on other pertinent donor variables such as ventilator settings and arterial blood gases. We recognize that donor hyperoxia is a very complex issue. The relationship between donor hyperoxia and BOS may instead be related to an over-reliance on Pao2 as the decisive factor for a suitable lung donor, while overlooking other significant abnormal donor characteristics. Further studies on the donor and ventilator management are necessary.
Our findings suggest that older donor age is associated with increased BOS development in lung transplant recipients. The relationship between donor age and BOS development has been previously investigated with conflicting results. Fischer and colleagues [17] compared 49 LTRs from older donors (> 50 years old) with 244 LTRs from younger donors. They reported no difference in survival (up to 60 months) in LTRs who received lungs from older donors, but they did not specifically address BOS. Also, while Fischer and colleagues defined older age as greater than 50 years old, we defined older age as 60 years old or greater. We found that older donor age was an important independent predictor of BOS and LTRs who received lungs from older donors had less BOS-free days. Consistent with our current study, De Perrot and colleagues [18] reported that the use of older donors (> 60 years old) was associated with significantly worse 10-year survival, predominantly due to BOS [18].
A significant smoking history in the donor is believed to increase both early and late complications after lung transplantation [1]. Oto and colleagues [19] compared 77 LTRs who received lungs from donors with a smoking history to 84 LTRs who received lungs from donors with no smoking history. They found that donor smoking history conferred worse early outcomes after lung transplantation with no difference in 3-year survival and BOS. On the other hand, in our study current tobacco use in the donor was not only associated with increased risk of BOS (Fig 2) in the recipient, but it was also found to be a significant independent predictor of BOS after lung transplantation on multivariate analysis. In our study, donor tobacco use was divided into those donors with a history of tobacco use versus current tobacco users. This division may account for the difference in our findings from Oto and colleagues. The implications of continued tobacco use by the donor maybe comparable with continued tobacco use prior to pulmonary surgery. Nakagawa and colleagues [20] reported a decrease in the number of postoperative complications after pulmonary surgery as the smoke-free period lengthened. In a review by Theadom and colleagues [21] the number of postoperative complications in past smokers was significantly less than in the current smokers and they found that a benefit may be gained from longer periods of preoperative smoking cessation.
In our multivariate analysis donor hyperoxia, older age, and current tobacco use by the donor were found to be independent predictors of BOS in the recipient. These findings are unique in that these donor factors had not been previously identified in the literature. It is important to consider ventilator management in the donor and the implications of donor characteristics on their recipient. Understanding the role of these donor factors in the development of BOS may lead to new preventative and treatment strategies for BOS. However, we do not believe that these donor factors should be used to change or limit the allocation of donor lungs to potential recipients. Donor organ shortage continues to be the most common cause of death for patients with endstage lung disease and the donor organ pool needs to be expanded, not limited.
Limitations
This study is inherently limited by its retrospective nature. We acknowledge the fact that the UNOS data-set is collected by individual centers and that each center has an independent method of interpreting the variables measured by UNOS and patient outcomes. However, UNOS has made strong efforts to limit such confounders with standardized data collection forms. It is also likely that the errors that do occur are equally distributed among all transplant centers. We also recognize that it is also possible that other factors that were not collected by UNOS may influence BOS. Donors and recipients in the UNOS database represent heterogeneous groups and we cannot know for sure that higher risk donors were not used for higher risk recipients. This is an inherent limitation with using a large multi-institutional database; however, one may argue that given the very large population in this database it could be assumed the various donor-recipient matches would occur equally and randomly. Despite these limitations, the UNOS dataset does allow us to study one of the largest populations of LTRs, which is often one of the major limitations of single-center studies.
A limitation of the multivariate model is that it only considers the role of donor factors (except HLA mismatch) in the development of BOS. Pertinent recipient variables in the UNOS database (cytomegalovirus mismatch, acute rejection, etc) were poorly collected and therefore not included in the analysis to prevent any bias and inaccurate conclusions. Although donor factors clearly play a role in BOS development, they only represent one part of the complex interaction between immunologic and nonimmunologic factors leading to BOS in the recipient. Therefore, it is an incomplete model of BOS but it does provide insight into the importance of donors in BOS development after lung transplantation.
Conclusion
In this study we have identified that donor factors are involved in the development of BOS after lung transplantation. Most importantly, donor hyperoxia, older donor age, and current tobacco use in the donor are independent predictors of BOS. These donor factors will help us understand the mechanism involved in the development of BOS and may improve survival after lung transplantation.
We do not recommend limiting lung donation from these donors. We can make adjustments in donor ventilator management to prevent hyperoxia-induced lung injury. Protective donor ventilator management is one simple change that may have a significant impact on survival in LTRs.
Acknowledgments
This work was supported in part by Health Resources and Services Administration contract 234-20050370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does it mention trade names, commercial products, or organizations imply endorsement by the U.S. Government.
DISCUSSION
DR G. ALEXANDER PATTERSON (St. Louis, MO): Sara, I enjoyed that presentation very much. It was very clearly presented, the manuscript is excellent, and I appreciate you sending it to me. I think this is actually very extraordinarily important work. It suffers from all the caveats of the UNOS (United Network for Organ Sharing) data registry reports and papers manufactured from that data. However, to its credit, the UNOS database is a mandatory database unlike the STS (Society of Thoracic Surgeons) or many other types of registries. So in that sense it is very important. As I mentioned to you earlier, I am a little skeptical about the quality of data from the early years of that registry or this experience, and I wonder if you looked at just the last 10 years, you would still have a large number of patients, and I don't know that it would necessarily change the outcome, but I think it would be a little bit more reliable data.
And the other question I have is, why did you do your analysis after 5 years? Plenty of patients would have died or developed severe BOS (bronchiolitis obliterans syndrome) prior to that time. I am not sure it would have influenced your outcomes. It wasn't clear to me exactly why you did the data mining at 5 years.
In your analysis, did you separate bilateral from single lung recipients?
DR HENNESSY: Thank you for your very kind comments, Dr Patterson. With regards to your first question, center dependent data collection is of course one limitation of the UNOS database; however, UNOS uses preset data collection forms in attempts to limit some of the bias and errors in such large multicenter databases. Many of the variables that we looked at, particularly BOS and donor hyperoxia, along with several other donor factors, actually started data collection in 1994. Therefore, although we looked at the entire UNOS database, our main variables of interest limited patients to the last 15 years of lung transplantation. We excluded patients that were missing any significant data in those variables. Your suggestion, however, is very valid. I think it would be worthwhile to go back and repeat this study limiting it to the last 10 years to validate our findings since so much has changed in lung transplantation.
With regards to your second question, we focused on our primary endpoint, BOS at 5 years, because we felt that at this time point we would capture a large number of recipients that developed BOS. As you know, 45% to 75% of lung transplant recipients develop BOS by 5 years after transplantation and it is the leading cause of mortality. However, many recipients never develop BOS. What is special about this group of patients? Why they survive so long and never develop BOS versus those who do is of significant interest to us.
Another reason to choose a 5-year endpoint was to prevent inclusion of patients that died before 5 years from another cause, as these patients may not have had the chance to develop BOS that would have otherwise.
In response to your third question, we did not do a subanalysis focusing on bilateral versus single lung transplantation, but we did, of course, include type of in our univariate analysis, and that was not statistically significant between the two groups.
DR PATTERSON: Just a comment. We have been interested in our own program about the impact of donor factors and inflammatory events that occur that are more donor-related than recipient and how those things impact on subsequent development of BOS, and I think it is pretty clear that injured lungs, for whatever reason, are more apt to develop BOS. Now, there are certain practical practicalities here and certain logistics. It is kind of hard to ask a donor to stop smoking before they become a donor and you can't really do much about the donor age, but you certainly can impact in terms of ventilatory management. Is there any way of finding out those patients who had very high Pao2s [partial pressure of oxygen, arterial], over 500? Those obviously were 100% gases. Is there any way to know how long the donor was ventilated at a 100%?
DR HENNESSY: Unfortunately, the UNOS database did not collect any specific variables on how the donors’ ventilators were managed. It is something that we plan on looking at in the future with our own lung transplants and donors. We will also implement protective ventilator management to determine if those changes make a difference in outcomes.
DR PATTERSON: Well, you may be able to get the data from your own center, because they record that; if they are going to do an oxygen challenge, they record the timing of the vent changes. You might be able to capture that. With a few OPOs (organ procurement organization) you might be able to capture that information.
DR HENNESSY: Well, it is certainly something that we will look into for the future. Thank you so much.
DR JOSHUA R. SONETT (New York, NY): That was excellent. I enjoyed the presentation. I agree with Dr Patterson and your group that we have to really look at the donor recipient interaction more on many different levels, and we probably should push UNOS to collect better donor data. When you try to look at the donor data through UNOS, it is really Spartan; peak inspira-tory pressures and full ventilatory support parameters, timing of the gases, that we could probably, since it is there, get better data for us all to look at later.
In terms of your conclusions, I am a little bit suspicious. One is that the hyperoxia, for the most part, is going to be a transitory gas. I think most OPOs do the 100% gas and then go back down on the Fio2 (fraction of inspired oxygen), thus reducing continued hyperoxia. And then a lot of lungs, no matter how hard you flog them will not generate Pao2 of 500, and it is the lungs with the Pao2s of 200 to 300, my guess is those are the lungs that are having the most aggressive ventilatory management and resultant barotrauma and other issues, and they are not just generating a Pao2 of 500. So something about the lungs in the cohort you looked at might have been wrong with them, like maybe subtle emphysema. I know when I get a donor with a Pao2 of over 500, I am awfully suspicious they have some emphysema or smoking history and I usually get a CT, because we don't get that many donors with Pao2s of 500. You may be selecting a cohort already with some subtle problems, because it is few and far between that our donors have Pao2s of 500, frankly, or the very young donors, which we also know are a risk factor for problems like acute graft failure. Thanks.
DR JOHN V. CONTE (Baltimore, MD): A related question that I thought of when I read your abstract is how can we sort out the hyperoxia of the donor at that point and the hyperoxia of the newly transplanted lung, because oftentimes when the patients are in the operating room, they are ventilated with high oxygen concentrations, and do you think that that also may have played a role in the development of OB (obliterative bronchiolitis), assuming that the hyperoxia is a factor in the development of OB?
DR HENNESSY: I think that is definitely a valid point. Unfortunately, we didn't look specifically at the Pao2s of the recipients in the operating room or soon after their transplantation, and that would be difficult to tease out, at least with the data that we have as it is. It is something that is important to us and we could look at this as a single institution study. Hyperoxia, of course, is known to cause a significant amount of lung injury, that has been well described in the literature, and I think probably both donor and recipient factors are important.
Footnotes
Presented at the Fifty-sixth Annual Meeting of the Southern Thoracic Surgical Association, Marco Island, FL, Nov 4–7, 2009.
References
- 1.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 2.Verleden GM. Chronic allograft rejection (obliterative bronchiolitis). Semin Respir Crit Care Med. 2001;22:551–8. doi: 10.1055/s-2001-18427. [DOI] [PubMed] [Google Scholar]
- 3.Burton CM, Carlsen J, Mortensen J, Andersen CB, Milman N, Iversen M. Long-term survival after lung transplantation depends on development and severity of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2007;26:681–6. doi: 10.1016/j.healun.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant. 1998;17:1255–63. [PubMed] [Google Scholar]
- 5.Lama R, Santos F, Alvarez A, et al. Analysis of lung transplant recipients surviving beyond 5 years. Transplant Proc. 2005;37:1523–5. doi: 10.1016/j.transproceed.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Meyers BF, Lynch J, Trulock EP, Guthrie TJ, Cooper JD, Patterson GA. Lung transplantation: a decade of experience. Ann Surg. 1999;230:362–71. doi: 10.1097/00000658-199909000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reichenspurner H, Girgis RE, Robbins RC, et al. Stanford experience with obliterative bronchiolitis after lung and heart-lung transplantation. Ann Thorac Surg. 1996;62:1467–73. doi: 10.1016/0003-4975(96)00776-X. [DOI] [PubMed] [Google Scholar]
- 8.Trulock EP, Edwards LB, Taylor DO, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult lung and heart-lung transplantation report–2006. J Heart Lung Transplant. 2006;25:880–92. doi: 10.1016/j.healun.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Vermuelen KM, van der Bij W, Erasmus ME, TenVergert EM. Long-term health-related quality of life after lung transplantation: different predictors for different dimensions. J Heart Lung Transplant. 2007;26:188–93. doi: 10.1016/j.healun.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Belperio JA, Weigt SS, Fishbein MC, Lynch JP., 3rd Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009;6:108–21. doi: 10.1513/pats.200807-073GO. [DOI] [PubMed] [Google Scholar]
- 11.Fiser SM, Tribble CG, Long SM, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73:1041–8. doi: 10.1016/s0003-4975(01)03606-2. [DOI] [PubMed] [Google Scholar]
- 12.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant. 2002;21:271–81. doi: 10.1016/s1053-2498(01)00360-6. [DOI] [PubMed] [Google Scholar]
- 13.Scott AI, Sharples LD, Stewart S. Bronchiolitis obliterans syndrome: risk factors and therapeutic strategies. Drugs. 2005;65:761–71. doi: 10.2165/00003495-200565060-00004. [DOI] [PubMed] [Google Scholar]
- 14.Valentine VG, Gupta MR, Walker JE, et al. Effect of etiology and timing of respiratory tract infections on development of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2008;28:163–9. doi: 10.1016/j.healun.2008.11.907. [DOI] [PubMed] [Google Scholar]
- 15.Aoe M, Okabayashi K, Cooper JD, Patterson GA. Hyperinflation of canine lung allografts during storage increases reperfusion pulmonary edema. J Thorac Cardiovasc Surg. 1996;112:94–102. doi: 10.1016/s0022-5223(96)70182-4. [DOI] [PubMed] [Google Scholar]
- 16.Haniuda M, Hasegawa S, Shiraishi T, Dresler CM, Cooper JD, Patterson GA. Effects of inflation volume during lung preservation on pulmonary capillary permeability. J Thorac Cardiovasc Surg. 1996;112:85–93. doi: 10.1016/s0022-5223(96)70181-2. [DOI] [PubMed] [Google Scholar]
- 17.Fischer S, Gohrbandt B, Struckmeier P, et al. Lung transplantation with lungs from donors fifty years of age and older. J Thorac Cardiovasc Surg. 2005;129:919–25. doi: 10.1016/j.jtcvs.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 18.De Perrot M, Waddell TK, Shargall Y, et al. Impact of donors aged 60 years or more on outcome after lung transplantation: results of an 11-year single-center experience. J Thorac Cardiovasc Surg. 2007;133:525–31. doi: 10.1016/j.jtcvs.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 19.Oto T, Griffiths AP, Levvey B, et al. A donor history of smoking affects early but not late outcome in lung transplantation. Transplantation. 2004;78:599–606. doi: 10.1097/01.tp.0000131975.98323.13. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa M, Tanaka H, Tsukuma H, Kishi Y. Relationship between the duration of the preoperative smoke free period and the incidence of postoperative pulmonary complications after pulmonary surgery. Chest. 2001;120:705–10. doi: 10.1378/chest.120.3.705. [DOI] [PubMed] [Google Scholar]
- 21.Theadom A, Cropley M. Effects of preoperative smoking cessation on the incidence and risk of intraoperative and postoperative complications in adult smokers: a systemic review. Tobacco Control. 2006;15:352–8. doi: 10.1136/tc.2005.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]