Abstract
We have previously developed replicon vectors derived from the Australian flavivirus Kunjin that have a unique noncytopathic nature and have been shown to direct prolonged high-level expression of encoded heterologous genes in vitro and in vivo and to induce strong and long-lasting immune responses to encoded immunogens in mice. To facilitate further applications of these vectors in the form of virus-like particles (VLPs), we have now generated a stable BHK packaging cell line, tetKUNCprME, carrying a Kunjin structural gene cassette under the control of a tetracycline-inducible promoter. Withdrawal of tetracycline from the medium resulted in production of Kunjin structural proteins that were capable of packaging transfected and self-amplified Kunjin replicon RNA into the secreted VLPs at titers of up to 1.6 × 109 VLPs per ml. Furthermore, secreted KUN replicon VLPs from tetKUNCprME cells could be harvested continuously for as long as 10 days after RNA transfection, producing a total yield of more than 1010 VLPs per 106 transfected cells. Passaging of VLPs on Vero cells or intracerebral injection into 2- to 4-day-old suckling mice illustrated the complete absence of any infectious Kunjin virus. tetKUNCprME cells were also capable of packaging replicon RNA from closely and distantly related flaviviruses, West Nile virus and dengue virus type 2, respectively. The utility of high-titer KUN replicon VLPs was demonstrated by showing increasing CD8+-T-cell responses to encoded foreign protein with increasing doses of KUN VLPs. A single dose of 2.5 × 107 VLPs carrying the human respiratory syncytial virus M2 gene induced 1,400 CD8 T cells per 106 splenocytes in an ex vivo gamma interferon enzyme-linked immunospot assay. The packaging cell line thus represents a significant advance in the development of the noncytopathic Kunjin virus replicon-based gene expression system and may be widely applicable to the basic studies of flavivirus RNA packaging and virus assembly as well as to the development of gene expression systems based on replicons from different flaviviruses.
Subgenomic replicon vectors of positive-strand RNA viruses offer great potential as gene expression vectors and for development of vaccines (12). They possess a number of unique advantages over other viral vector systems, which are used for vaccine development. These are the following: (i) high-level expression of encoded heterologous proteins due to efficient amplification of replicon RNA; (ii) no DNA intermediates and replication exclusively in the cell cytoplasm, thereby eliminating any problems associated with integration into the cell genome; (iii) a single infection cycle, and thus, replicon RNA cannot escape from cells they are delivered to, eliminating any possibility of uncontrolled virus spread in vaccinees; and (iv) the relative ease of constructing replicon-based vaccines.
Kunjin virus (KUN) is a member of the Japanese encephalitis subgroup of the genus Flavivirus in the family Flaviviridae (41). Unlike the majority of other human pathogens from the Flavivirus genus, KUN appears to be naturally attenuated in the human population, with infections nearly always asymptomatic. KUN is endemic only in the northern parts of Australia and neighboring islands, with no preexisting immunity in the rest of the world. In addition, KUN is genetically stable, and its epidemiology and virus replication strategy are well understood (38), making KUN an attractive candidate for vaccine vector development. The KUN genome consists of a positive-polarity single-stranded RNA encoding a large polyprotein that is cotranslated and posttranslationally cleaved into three structural proteins, i.e., core (C), premembrane (prM), and envelope (E), and seven nonstructural proteins, i.e., NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 (3, 13). We previously constructed the first flavivirus replicons based on KUN cDNA by deleting the majority of the genomic region encoding structural genes and retaining only the first 20 codons of the C gene and the last 22 codons of the E gene (14). KUN replicons have been extensively used as vectors for expression of heterologous genes in vitro and in vivo and for the development of vaccines (1, 9, 14, 16, 36, 37). Replicons have also recently been developed for other flaviviruses, e.g., dengue type 2 virus (DEN2) (27, 28), West Nile virus (WN) (33), yellow fever virus(25), and most recently for tick-borne encephalitis virus (TBE) (5). The unique feature of flavivirus replicons is their low cytopathicity, which allows prolonged expression of encoded heterologous genes (1, 9, 14, 27, 28, 33, 36, 37).
The packaging system developed previously for production of KUN replicon virus-like particles (VLPs) (16) required two consecutive electroporations, first with KUN replicon RNA and then, after a delay, with a cytopathic Semliki Forest virus (SFV) replicon RNA encoding KUN structural genes (SFV-MEC105). This procedure produced a single harvest of VLPs with titers reaching 1 × 106 to 5 × 106 VLPs per ml (36) or a total of 2.5 × 106 to 7.5 × 106 VLPs per 106 transfected cells. KUN replicons have also been developed for delivery in plasmid DNA form (37). Our recent comparative analyses of vaccine potential of KUN replicons delivered as plasmid DNA, as naked RNA, and as VLPs showed a significantly better induction of immune responses to an encoded immunogen after VLP delivery than with other delivery modalities (1, 9). However, the rather cumbersome procedure required for KUN replicon VLP production and the relatively low titers of harvested VLPs complicates the current and future studies on evaluation of KUN replicon vectors and makes large-scale commercial manufacture of KUN replicon VLP-based vaccines unattractive. We therefore developed a simplified and more efficient system for production of KUN replicon VLPs by generating a tetracycline-inducible BHK cell line with a stably integrated KUN structural gene cassette that allows production and multiple harvesting of high titers of KUN replicon VLPs for up to 10 days after transfection with KUN replicon RNA. This cell line was also shown to be capable of packaging other flavivirus replicon RNAs.
Establishment of the tetracycline-inducible BHK cell line, tetKUNCprME, capable of packaging KUN replicon RNA into VLPs.
To our knowledge, no stable cell lines simultaneously expressing all three flavivirus structural proteins have been reported to date. We have previously generated a Vero cell line stably expressing the KUN C protein; however, the level of expression was low (40). Previous attempts to generate a stable cell line continuously expressing all three KUN structural genes under control of separate promoters (expressing C, prM, and E separately), using standard (noninducible) DNA expression vectors, resulted in great instability of expression, producing only 10 to 20% positively expressing cells after a few cell passages (A. N. Varnavski and A. Khromykh, unpublished data). Attempts to use these cell lines to produce KUN replicon VLPs resulted in very low VLP titers (Varnavski and Khromykh, unpublished).
Several inducible systems for regulating transgene activity have been described for many cell types (32). Of these, the tetracycline system (6, 42) holds the greatest appeal. The system is commercially available from Clontech in Tet-On and Tet-Off formulations, allowing induction of gene expression either by addition or by the removal of tetracycline (http://www.clontech.com/techinfo/manuals/PDF/PT3001-1.pdf). In view of the intended use of KUN replicon VLPs in vaccine or protein production applications, it was logical to choose the Tet-Off system in order to avoid the presence of antibiotic in VLP preparations. To generate a packaging cell line that allows tetracycline-inducible expression of the KUN structural gene cassette (CprME), we first established a BHK cell line, BHK-Tet-Off, stably expressing the tetracycline transactivator. Thus, BHK21 cells were transfected with pEF-tTA-IRESpuro plasmid DNA (kindly provided by Rick Sturm, University of Queensland), a derivative of pEFIRES-P (10) containing a sequence coding for the tetracycline transactivator (Fig. 1A). Two days following transfection, the antibiotic puromycin (Sigma) was added at a concentration of 10 μg/ml for selection of cell clones. Five cell clones were isolated and cultured successfully from this transfection. These clones were then analyzed for induction of expression by transfection with the plasmid, pTRE2luciferase (Clontech), in the presence (0.5 μg/ml) or absence of doxycycline (an antibiotic of the same spectrum as tetracycline but with higher specific activity and a longer half-life). Established BHK-Tet-Off cell clones demonstrated various degrees of induction of expression and background levels (results not shown). Two BHK-Tet-Off cell clones displaying the highest fold induction of luciferase expression compared to uninduced cells were used to establish a stable BHK cell line expressing the KUN structural gene cassette CprME. The cells were transfected with pTRE2CprME-IRESNeo plasmid DNA (Fig. 1A) constructed by subcloning the KUN CprME gene cassette and the encephalomyocarditis virus internal ribosomal entry site-neomycin phosphotransferase gene cassette (IRESNeo) into the pTRE2 vector (Clontech, North Ryde, Australia). Transfected cells were subjected to selection with 0.5 mg of Geneticin (G418)/ml in medium that also contained 10 μg of puromycin/ml and 0.5 μg of doxycycline/ml. To select the most efficient packaging cell line, a number of cell clones conferring resistance to G418 and puromycin were electroporated with KUN replicon RNA (RNAleu) (1) and cultured without doxycycline to determine whether they were able to produce infectious KUN replicon VLPs. The titers of infectious VLPs (in infectious units [IU] per milliliter) present in harvested culture fluids (CFs) were determined by infection of Vero cells followed by immunofluorescence analysis with anti-NS3 antibodies as described previously (15, 39). Four cell clones, i.e., #A3, #A8, #E1, and #E5, were capable of VLP production, with efficiencies varying from 5 × 104 to 2 × 108 IU per ml at 53 h after RNA electroporation. The most efficient cell clone, #A8, producing 2 × 108 IU of VLPs/ml, was designated tetKUNCprME and used in all further studies. The identity of the KUN CprME sequence encoded in the mRNA produced in tetKUNCprME cells to that of the wild-type KUN CprME sequence was confirmed by sequencing the entire CprME region after reverse transcription-PCR amplification of total RNA isolated from tetKUNCprME cells. No nucleotide changes from the sequence present in the plasmid DNA pTRE2CprME-IRESNeo were found.
FIG. 1.
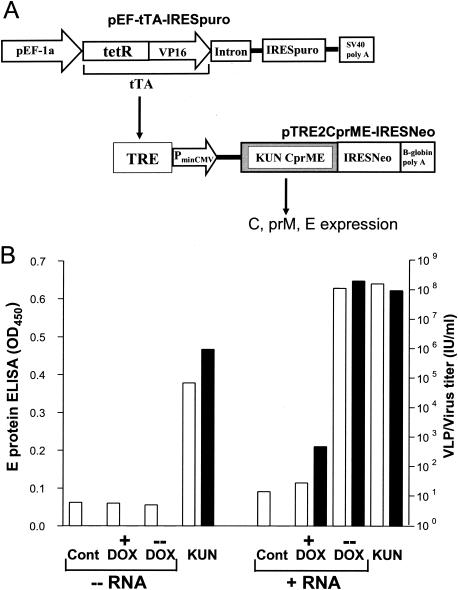
Generation and characterization of stable packaging cell line tetKUNCprME. (A) Schematic representation of the plasmid constructs used for generation of the stable packaging cell line tetKUNCprME. The pEF-tTA-IRESpuro plasmid was used to generate a first stable BHK cell line, BHK-Tet-Off, continuously expressing the tetracycline transactivator (tTA) from the human elongation factor 1α promoter (pEF-1a). tetKUNCprME, expressing KUN structural genes C, prM, and E (KUN CprME) from the tetracycline-inducible cytomegalovirus promoter (PminCMV), was established by transfection of pTRE2CprME-IRESNeo plasmid DNA into BHK-Tet-Off cells and selection or cells growing in the presence of G418 and puromycin (see the text). In uninduced tetKUNCprME cells, doxycycline (DOX) (a form of tetracycline with higher specific activity) binds to tTA and prevents its binding to the tetracycline-responsive element (TRE) and subsequent activation of CprME mRNA transcription from the cytomegalovirus promoter. To induce expression of KUN CprME genes, DOX is removed from the medium, resulting in the release of tTA, its binding to TRE, and activation of CprME mRNA transcription from the cytomegalovirus promoter. tetR, Tet repressor protein; VP16, herpes simplex virus VP16 activation domain; IRES, EMCV internal ribosome entry site; puro, puromycin N-acetyltransferase; Neo, neomycin resistance gene; SV40 poly A, SV40 transcription terminator/poly(A) signal; β-globin poly A, β-globin transcription terminator/poly(A) signal. (B) Production of secreted E protein and VLPs in induced and uninduced tetKUNCprME cells in the presence and absence of KUN replicon RNA. -- RNA graph (left part) shows the results of an experiment without replicon RNA transfection; + RNA graph (right part) shows the results of another experiment with electroporation of RNAleu. Results are shown for tetKUNCprME cells either electroporated with KUN replicon RNA (+ RNA) or not electroporated (- RNA) and maintained for 48 h in the medium with (+) or without (-) 0.5 μg of doxycycline/ml. Detection of secreted KUN E protein (white bars) by antigen capture ELISA and determination of VLP titers (black bars) (in infectious units per milliliter) by infectivity assay on Vero cells were performed as described in the text. Negative controls in both experiments (Cont) were culture fluids from normal BHK cells. The titers of KUN positive controls (KUN) used in each experiment were determined by plaque assay on BHK cells.
CprME expression and optimization of production of secreted KUN replicon VLPs in tetKUNCprME cells.
To examine levels of secreted KUN proteins and KUN VLPs in the culture fluid of tetKUNCprME cells, we used an antigen capture enzyme-linked immunosorbent assay (ELISA) (11). CFs collected from induced and uninduced tetKUNCprME cells that were cultured for 48 h prior to analysis showed no detectable levels of KUN E protein in both CF samples (Fig. 1B). However, when the cells were electroporated with RNAleu replicon RNA, a dramatic increase in ELISA readings was noticed by 45 h after RNA electroporation in the CF sample from induced cells, while only a marginal increase in ELISA readings was detected in the CF sample from uninduced cells (Fig. 1B). When VLPs in these CF samples were titrated on Vero cells, the titers of VLPs correlated well with the ELISA results (Fig. 1B). For example, ∼500 IU of VLPs per ml detected in the CF samples collected from uninduced cells produced an ELISA reading of an optical density at 450 nm of ∼0.11, while 2.1 × 108 IU of VLPs per ml in the CF sample from induced cells gave an ELISA reading of ∼0.63 (Fig. 1B). The observed dramatic increase in the levels of secreted E protein and VLPs after transfection with KUN replicon RNA is in accord with the current model of processing of flavivirus structural proteins and virus assembly, which requires cleavage by viral NS2B-3 protease (expressed from replicon RNA) at the cleavage site in C-prM junction for the proper cleavage of the prM signal sequence by cellular signal peptidase and secretion of prM and E particles (22).
In order to optimize VLP production, studies were performed with the harvesting of culture fluid and the removal of doxycycline from the media at different time points. Following electroporation of KUN replicon RNA (RNAleu), medium containing doxycycline (0.5 μg/ml) was added to the cells for a further 16 or 30 h and then replaced with fresh medium without doxycycline. A 60-mm2-diameter dish of electroporated cells was maintained continually without doxycycline for comparison. The culture fluid was harvested from each dish at 53 and 68 h postelectroporation and examined by infectivity assay with Vero cells. The results showed that the optimal time for removal of doxycycline to induce CprME expression for VLP production was immediately after RNA electroporation (data not shown). A delay in the removal of doxycycline from the medium resulted in a substantial decrease in the amount of VLPs produced.
To determine the optimal VLP harvesting protocol and the ability of tetKUNcprME cells to produce high levels of VLPs encoding various heterologous genes, KUN replicon RNA RNAleu and replicon RNAs encoding different heterologous genes, such as murine polytope (RNAleuMpt), HIV-1 gag (KUNgag), puromycin acetyltransferase (repPAC), puromycin acetyltransferase, β-galactosidase (repPACβ-gal), and green fluorescence protein (repGFP) (1, 9, 14, 24, 36, 37) were electroporated into tetKUNCprME cells. VLPs were harvested at different times after RNA electroporation, and the medium was replaced with fresh medium every time VLPs were harvested to allow multiple harvesting of VLPs (Table 1). Nearly all the VLP titers from day 3 onwards after electroporation were in the range of 107 to 109 IU per ml, and they remained high even in the third or fourth consecutive harvests up to 10 days after transfection, depending on the nature of the replicon RNA and the VLP harvesting protocol (Table 1). The total production of VLPs from the initially transfected 3 × 106 tetKUNCprME cells using the most optimal VLP harvesting protocol reached 5.4 × 1010 infectious particles (repPACβ-gal RNA experiment 2 in Table 1) and was in the range from 1.1 × 109 to 1.3 × 1010 infectious particles per 3 × 106 electroporated cells when other harvesting protocols and different KUN replicon RNAs were used (Table 1).
TABLE 1.
Production of secreted KUN replicon VLPs encoding different heterologous genes in the tetKUNCprME packaging cell line
| VLP type | Titer of VLPs (IU/ml)c
|
Total VLP production per 3 × 106 cellsd | ||||||
|---|---|---|---|---|---|---|---|---|
| 2d | 3d | 4d | 5d | 6d | 8d | 10d | ||
| RNAleuMPta | 3.1 × 107 | 5.5 × 107 | 3.8 × 108 | — | 2.9 × 108 | 1.3 × 108 | — | 5.3 × 109 |
| KUNgaga | 1 × 107 | 3.9 × 107 | 1.2 × 108 | 1.6 × 107 | — | — | — | 1.1 × 109 |
| RNAleua | 1.8 × 108 | 1.9 × 108 | — | 2.5 × 106 | — | — | — | 2.2 × 109 |
| repGFPa | 1.6 × 108 | 2.6 × 108 | 3.7 × 108 | 2 × 108 | — | — | — | 5.0 × 109 |
| repPACa | — | — | 1.6 × 108 | — | 2.2 × 108 | — | 1.9 × 108 | 6.7 × 109 |
| repPACβ-gala exp 1 | 4 × 105 | — | 1.1 × 108 | — | 2.3 × 108 | — | — | 3.4 × 109 |
| repPACβ-galb exp 2 | 1.2 × 106 | — | 1.6 × 109 | — | 1.1 × 109 | — | — | 5.4 × 1010 |
| repPACβ-galb exp 3 | 5 × 106 | — | 1.3 × 108 | — | 1.8 × 108 | 3.3 × 108 | — | 1.3 × 1010 |
Cells (3 × 106) were electroporated with ≈20 μg of RNA, seeded onto one 10-cm culture dish, and incubated in different volumes of medium and for different times prior to harvesting VLPs. Six (RNAleuMPt, KUNgag, and RNAleu), five (repGFP), or 10 (repPACβ-gal exp 1) ml or of medium in each dish were used for initial VLP harvest and to replace harvested VLPs to allow further VLP production and harvests. For repPAC, 10 ml of medium was harvested and replaced at days 4 and 6 and 15 ml of medium was harvested at day 10. exp, experiment.
Electroporated cells (3 × 106) were seeded onto two 10-cm culture dishes, and cells in each dish were incubated in 10 ml of medium that was replaced with 10 ml of fresh medium at each indicated harvest day.
—, VLPs were not harvested at this time and the medium remained unchanged until the next harvest; d, days.
Total VLP production was calculated by combining amounts of VLPs obtained in each harvest.
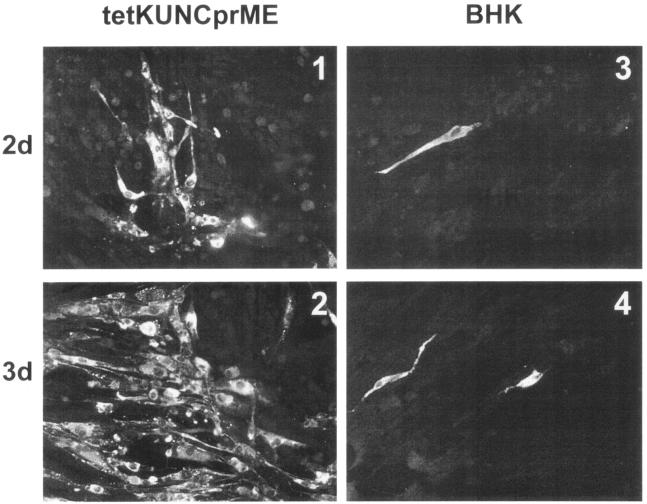
To examine whether KUN replicon VLPs can be amplified by spread in tetKUNCprME cells but not in normal BHK cells, the cells were infected with RNAleuMpt VLPs at a low multiplicity of infection (MOI) (0.1) and incubated in the medium without doxycycline. Immunofluorescence (IF) analysis of infected cells with KUN anti-NS3 antibodies showed a significant increase in the size of positive cell foci from day 2 to day 3 postinfection (Fig. 2, panels 1 and 2), demonstrating amplification and spread of VLPs in tetKUNCprME cells. In contrast, only individual positive cells were detected in infected normal BHK21 cells at both day 2 and day 3 after VLP infection (Fig. 2, panels 3 and 4). In a separate experiment, an approximately 10-fold increase in VLP titers from day 3 to day 5 of incubation after infection of tetKUNCprME cells with 0.1 MOI of RNAleuMpt VLPs was detected (results not shown), thus further confirming amplification of VLPs by spread in the packaging cells. This relatively modest increase (10-fold) of VLP titer observed from day 3 to day 5 of infection could be due to the impaired ability of newly infected aged (3- to 5-day-old confluent) BHK cells to support efficient KUN RNA replication and not necessarily represent inefficient spread of VLPs. We have previously observed a lower efficiency of KUN RNA replication in aged BHK cells compared to that in actively dividing BHK cells in many experiments (data not shown). It is, however, evident from the results of prolonged production of VLPs (Table 1) that in those aged (confluent) BHK cells where the replication of KUN RNA has already been established, it continues to replicate and is packaged into secreted VLPs for a long period of time.
FIG. 2.
Amplification and spread of KUN replicon VLPs in tetKUNCprME cells. Coverslips of tetKUNCprME and BHK21 cells were infected with 0.1 MOI of RNAleuMpt VLPs and analyzed by IF with KUN anti-NS3 antibodies at 2 and 3 days after infection.
The results convincingly demonstrate that the tetKUNCprME cell line is able to produce substantially larger (∼1,500-fold) amounts of KUN replicon VLPs compared to our previously published protocol using the cytopathic SFV replicon for expression of KUN structural genes (36). Most recently a paper describing the generation of a CHO cell line stably expressing tick-borne encephalitis (TBE) prME genes and its use for packaging of TBE replicon RNA deleted in prME genes has been published (5). The highest titer of secreted TBE replicon VLPs obtained in prME-expressing CHO cells was 5 × 107 IU/ml, about 30-fold lower than the highest titer of KUN replicon VLPs obtained in tetKUNCprME cells (1.6 × 109 IU/ml) (Table 1). Moreover, the total maximum amount of TBE replicon VLPs produced per 106 transfected cells was ∼108 IU, which is about 180-fold less than that obtained for KUN replicon VLPs (1.8 × 1010 IU) (Table 1). It is difficult, however, to do any further comparison of the packaging efficiencies between these two systems in view of the differences in cell lines used (CHO for TBE and BHK for KUN), replicon RNAs (with core gene for TBE and without core gene for KUN), electroporation conditions (i.e., number of transfected cells, RNA quantities not reported for TBE RNA, and electroporator settings), and protocols for harvesting VLPs. Clearly, both TBE and KUN replicon packaging cell lines represent a substantial improvement over our previously published KUN replicon RNA packaging protocol (16, 36). tetKUNCprME cells, however, offer the flexibility of inducible expression, apparently higher titers, continuous harvesting, and higher total amounts of produced replicon VLPs. In addition, tetKUNCprME cells were capable of packaging replicon RNAs from different flaviviruses (see below).
Stable expression of KUN structural proteins in tetKUNCprME cells.
To determine the stability of expression of the KUN CprME genes, tetKUNCprME cells were cultured for 12 passages without puromycin and G418 and then electroporated with KUN replicon RNA (RNAleu) to determine the efficiency of VLP production. Doxycycline was present in the medium during passaging to ensure suppression of CprME expression. tetKUNCprME cells that were cultured for 12 passages in the presence of all three antibiotics, i.e., puromycin, G418, and doxycycline, were electroporated in parallel to compare VLP production efficiency. Doxycycline was removed from the medium immediately after electroporation of a replicon RNA to induce expression of CprME and enable VLP production. Titers of VLPs collected at 48 h after replicon RNA transfection from cells that were maintained under puromycin and G418 selection during passaging were similar to the titers of VLPs collected at the same time from cells that were maintained without puromycin and G418 selection (2.2 × 106 and 1.7 × 106 IU/ml, respectively). Although the VLP titers in this particular experiment were lower than in the majority of the other packaging experiments, the results clearly demonstrate the stability of expression of KUN structural proteins in tetKUNCprME cells after at least 12 passages in the absence of antibiotic selection and thus indicate stable integration of the KUN structural gene cassette into the cell genome.
Absence of infectious KUN in replicon VLP preparations.
The presence of overlapping sequences in the C-terminal region of the C gene and the N-terminal region of the E gene of KUN replicon RNA and of CprME mRNA produced in tetKUNCprME cells may potentially promote homologous recombination that may lead to production of infectious KUN in VLP preparations. In our previously developed packaging system, we eliminated any possibility of this potential recombination by separating expression of the C gene and prM and E genes from two different mRNAs produced from the SFV replicon vector (16). However, our numerous complementation experiments with KUN RNAs (for a summary, see reference 17) as well as complementation experiments with yellow fever virus RNAs (20, 21), where extended regions of complementarity were present between defective and helper RNAs, failed to detect infectious viruses that could have been generated by homologous recombination. To examine whether any recombined replication-competent KUN was produced during production of KUN replicon VLPs in tetKUNCprME cells, CFs harvested at 2 days after transfection with RNAleu RNA were used to infect Vero cells grown on coverslips. The infected cells were incubated for 5 days and examined for expression of E protein by immunofluorescence. The tissue culture fluid from the infected coverslips was then passaged again on fresh cultures of Vero cells for a further 5 days and examined by IF with anti-E antibodies. No E-positive cells were detected in both passages (results not shown). Parallel labeling with anti-NS3 antibodies showed numerous positive cells in the first passage but no positive cells in the second passage (results not shown), demonstrating that VLPs deliver replicon RNA only in the first round of infection. Similarly, packaging of TBE replicon RNA in CHO cells stably expressing prM and E genes failed to produce any replicating TBE virus even after several passages in the packaging cell line, despite the overlap in viral genomic sequences between prM and E mRNA and replicon RNAs (5).
Additional evidence of the absence of infectious KUN in VLP preparations was sought by the most sensitive method for virus detection, intracranial injection of suckling mice. Groups of 10 2- to 3-day-old BALB/c suckling mice were inoculated intracranially with 4 × 106 IU of KUN-MPt VLPs or with 1 PFU of wild-type KUN (strain MRM61C) as a positive control. All 10 mice injected with 1 PFU of wild-type KUN developed paralysis of the hind legs at 4 days postinoculation and had to be sacrificed. In contrast, all VLP-injected mice remained healthy and demonstrated normal development for the duration of the experiment (21 days). These in vitro and in vivo results with KUN replicon VLPs and the in vitro results with TBE replicon VLPs (5) clearly demonstrate that production of flavivirus replicon VLPs in packaging cells expressing continuous structural gene cassettes does not lead to the generation of any recombinant infectious virus despite the presence of overlapping regions. In comparison, 108 IU of Sindbis virus replicon VLPs produced in a BHK packaging cell line expressing a continuous Sindbis virus structural gene cassette, contained ∼105 PFU of infectious viruses generated by recombination (30). Splitting the structural genes into two separate expression cassettes in the packaging cell line appeared to remove contamination with infectious viruses to an undetectable level, but at the same time, it reduced the titers of replicon VLPs to 5 × 106 to 1 × 107 VLPs per ml (30).
Packaging of WN and dengue virus replicons.
To examine whether tetKUNCprME cells can be used to package replicon RNAs derived from other flaviviruses, we used replicon RNAs from a closely related New York 99 strain of WN and from a distantly related DEN2. The WN replicon construct Replicon, with a deletion of greater than 92% of the structural region, was described previously (33). The DEN2 replicon constructs pDENΔCprME and pDENΔprME were derived from the plasmid pDVWS601, which contains a full-length cDNA clone corresponding to the genome of the New Guinea C strain of DEN2 (31) by creating large in frame deletions in the structural region. The deletions were designed so that pDENΔCprME retained only the first 27 codons of the C gene and the last 24 codons of the E gene, while pDENΔprME retained the entire C gene, the first 7 codons of the prM gene, and the last 24 codons of the E gene.
Electroporation of WN replicon RNA into tetKUNCprME cells resulted in detection of ∼70 to 80% cells positive in IF analysis with cross-reacting KUN NS3 antibodies and production of 7 × 107 IU of secreted VLPs/ml by 4 days postelectroporation. Electroporation of KUN replicon RNA RNAleu performed in the same experiment resulted in detection of ∼80 to 90% NS3-positive cells and production of 108 IU of VLPs/ml by day 4 postelectroporation. In a separate experiment, electroporation of DENΔCME or DENΔME replicon RNAs into tetKUNCprME cells resulted in ∼80 and 95% cells, respectively, that were positive in IF analysis with cross-reacting KUN NS3 antibodies. Transfection of KUN replicon RNA RNAleu in the same experiment resulted in ∼95% NS3-positive cells. The titers of secreted VLPs produced in CF at 2 days after transfection with DENΔME and DENΔCprME replicon RNAs were 8 × 104 and 1.8 × 105 IU/ml, respectively. The KUN replicon RNA in the same experiment produced VLPs with a titer of 2.2 × 107 IU/ml. The VLP titers both for DEN2 and for KUN replicons in this experiment did not increase with extension of the incubation periods to 3 and 6 days.
The successful generation of chimeric flaviviruses by replacing structural genes from one virus with those from other flaviviruses (8, 26, 29) demonstrates that structural proteins from one flavivirus are capable of packaging RNA of another flavivirus when they are expressed in cis from the same RNA molecule. Our results represent the first demonstration of packaging of different flavivirus replicon RNAs by the KUN structural proteins provided in trans. Given very high homology between KUN and NY99 strain of WN virus (19, 23) and their relatively similar replication efficiencies (13, 23, 34), the similar packaging efficiencies of KUN and WN replicon RNAs observed here are not surprising. The ∼100-fold-lower packaging efficiency of DEN2 replicon RNAs compared to that of KUN replicon RNA could be attributed to a number of factors, including significant sequence differences between these two viruses and lower replication efficiencies of dengue viruses in general. Previous experiments with full-length infectious DEN2 cDNA showed relatively inefficient production of secreted DEN2 virus directly after RNA transfection into BHK cells (7). Although we did not compare the efficiencies of replication of DEN2 and KUN replicon RNAs in tetKUNCprME cells, it is likely that replication of DEN2 replicon RNAs would be less efficient than that of KUN replicon RNA, leaving less RNA available for packaging. Optimal packaging may also require specific interactions between RNA and core protein of the same virus; however, no signals or motifs in flavivirus RNA or core protein that determine specificity of packaging have yet been defined. The packaging system developed in this study is likely to contribute to future studies of packaging signals and increase understanding of how flavivirus virions are assembled and secreted.
Immunization with higher doses of KUN replicon VLPs prepared in tetKUNCprME cells improves CD8+-T-cell responses to encoded immunogens.
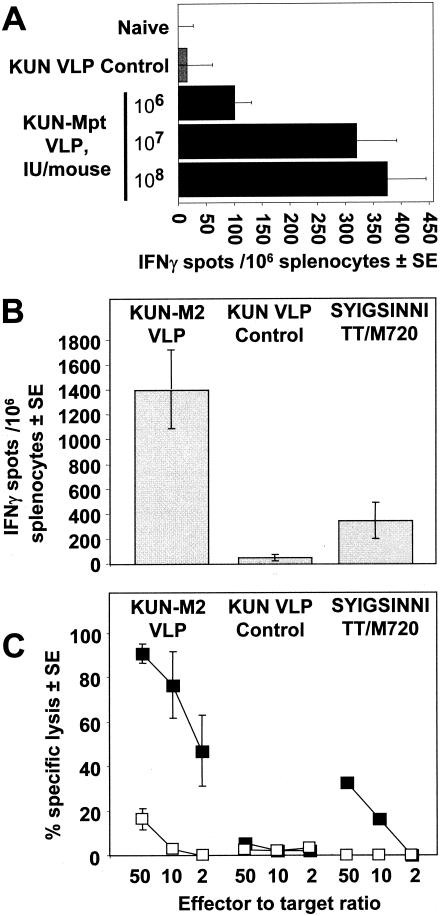
The packaging cell line allowed production of KUN replicon VLPs with ∼100-fold-higher titers, thus enabling testing of increasing doses of VLPs in immunization experiments. A 10-fold increase in the dose of KUN replicon VLPs encoding murine polytope (KUN-Mpt VLPs) from 106 to 107 IU of VLPs induced threefold to fourfold more SIINFEKL epitope-specific CD8 T cells as measured by an ex vivo gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay (Fig. 3A). A further 10-fold increase from 107 to 108 IU of VLPs resulted in only a marginal increase in the number of SIINFEKL-specific CD8 T cells induced (Fig. 3A). In a separate experiment, BALB/c mice were immunized once with 2.5 × 107 IU of KUN VLPs encoding the respiratory syncytial virus (RSV) M2 gene. A KUN replicon encoding the RSV M2 gene was constructed by cloning into the RNAleu vector (1) a DNA fragment containing an RSV M2 cDNA sequence that was prepared by reverse transcription and PCR amplification of RNA isolated from cells infected with the RSV A2 isolate (kindly provided by Paul Young, University of Queensland). Highly potent CD8+-T-cell responses specific for the RSV M2 epitope, SYIGSINNI, were generated, with ELISPOT analysis showing an average of 1,400 spots per 106 splenocytes (Fig. 3B, KUN-M2 VLP) and a standard chromium release showing more than 45% specific lysis after effectors were diluted to an effector/target ratio of 2:1 (Fig. 3C, KUN-M2 VLP). These responses exceeded those reported following vaccination with a replication-competent recombinant vaccinia virus encoding RSV M2 (2, 18, 35). As expected, a control KUN VLP failed to induce significant specific responses (Fig. 3B and C, KUN VLP control), and a peptide-vaccine formulated with SYIGSINNI peptide induced severalfold-lower responses (Fig. 3B and C, SYIGSINNI/TT/M720).
FIG. 3.
CD8-T-cell responses in mice immunized with high-titer KUN VLP replicons. (A) C57BL/6 mice (n = 4 per group) were immunized intraperitoneally with phosphate-buffered saline (Naive), 108 IU of KUN VLPs not encoding a recombinant antigen (KUN VLP Control), or the indicated dose of KUN VLPs encoding the murine polytope (2) (KUN-Mpt VLP). After 2 weeks, splenocytes were removed and analyzed for H-2kb-restricted SIINFEKL-specific responses by IFN-γ ELISPOT. (B and C) BALB/c mice (n = 3 per group) were immunized intraperitoneally with 2.5 × 107 IU of KUN VLPs encoding respiratory syncytial virus matrix 2 protein (KUN-M2 VLP), 2.5 × 107 IU of KUN VLP not encoding a recombinant antigen (KUN VLP Control), or subcutaneously with a peptide vaccine containing the H-2Kd-restricted RSV M2 epitope, SYIGSINNI, formulated with tetanus toxoid in Montanide ISA 720 (SYIGSINNI/TT/M720) as described previously (4) After 2 weeks splenocytes were removed and analyzed for SYIGSINNI-specific responses by IFN-γ ELISPOT (B) and by standard chromium release assay (C) (black squares, P815 target cells sensitized with SYIGSINNI peptide; white squares, P815 target cells without peptide) as described previously (1).
In summary, we have generated a stable packaging cell line allowing production of large amounts of high-titer secreted KUN replicon virus-like particles free of infectious virus and have demonstrated that immunization with these particles induced a potent immune response to the encoded immunogen. The packaging cell line thus should prove to be useful for the manufacture of KUN replicon-based vaccines. In addition, the packaging cell line was also capable of packaging replicons from other flaviviruses and should prove to be useful in basic studies on flavivirus RNA packaging and virus assembly and in the development of gene expression systems based on different flavivirus replicons.
Acknowledgments
The work was supported by grant no. 1 R21 AI053579-01A1 from the National Institutes of Health and grant no. 142911 from the National Health and Medical Research Council of Australia. M.J. is a Wellcome Trust Advanced Fellow.
REFERENCES
- 1.Anraku, I., T. J. Harvey, R. Linedale, J. Gardner, D. Harrich, A. Suhrbier, and A. A. Khromykh. 2002. Kunjin virus replicon vaccine vectors induce protective CD8+ T-cell immunity. J. Virol. 76:3791-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aung, S., Y. W. Tang, and B. S. Graham. 1999. Interleukin-4 diminishes CD8+ respiratory syncytial virus-specific cytotoxic T-lymphocyte activity in vivo. J. Virol. 73:8944-8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coia, G., M. D. Parker, G. Speight, M. E. Byrne, and E. G. Westaway. 1988. Nucleotide and complete amino acid sequences of Kunjin virus: definitive gene order and characteristics of the virus-specified proteins. J. Gen. Virol. 69:1-21. [DOI] [PubMed] [Google Scholar]
- 4.Elliott, S. L., S. Pye, T. Le, L. Mateo, J. Cox, L. Macdonald, A. A. Scalzo, C. A. Forbes, and A. Suhrbier. 1999. Peptide based cytotoxic T-cell vaccines; delivery of multiple epitopes, help, memory and problems. Vaccine 17:2009-2019. [DOI] [PubMed] [Google Scholar]
- 5.Gehrke, R., M. Ecker, S. W. Aberle, S. L. Allison, F. X. Heinz, and C. W. Mandl. 2003. Incorporation of tick-borne encephalitis virus replicons into virus-like particles by a packaging cell line. J. Virol. 77:8924-8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gualano, R. C., M. J. Pryor, M. R. Cauchi, P. J. Wright, and A. D. Davidson. 1998. Identification of a major determinant of mouse neurovirulence of dengue virus type 2 using stably cloned genomic-length cDNA. J. Gen. Virol. 79:437-446. [DOI] [PubMed] [Google Scholar]
- 8.Guirakhoo, F., R. Weltzin, T. J. Chambers, Z. X. Zhang, K. Soike, M. Ratterree, J. Arroyo, K. Georgakopoulos, J. Catalan, and T. P. Monath. 2000. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J. Virol. 74:5477-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvey, T. J., I. Anraku, R. Linedale, D. Harrich, J. Mackenzie, A. Suhrbier, and A. A. Khromykh. 2003. Kunjin replicon vectors for human immunodeficiency virus vaccine development. J. Virol. 77:7796-7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobbs, S., S. Jitrapakdee, and J. C. Wallace. 1998. Development of a bicistronic vector driven by the human polypeptide chain elongation factor 1alpha promoter for creation of stable mammalian cell lines that express very high levels of recombinant proteins. Biochem. Biophys. Res. Commun. 252:368-372. [DOI] [PubMed] [Google Scholar]
- 11.Hunt, A. R., R. A. Hall, A. J. Kerst, R. S. Nasci, H. M. Savage, N. A. Panella, K. L. Gottfried, K. L. Burkhalter, and J. T. Roehrig. 2002. Detection of West Nile virus antigen in mosquitoes and avian tissues by a monoclonal antibody-based capture enzyme immunoassay. J. Clin. Microbiol. 40:2023-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khromykh, A. A. 2000. Replicon-based vectors of positive strand RNA viruses. Curr. Opin. Mol. Ther. 2:555-569. [PubMed] [Google Scholar]
- 13.Khromykh, A. A., and E. G. Westaway. 1994. Completion of Kunjin virus RNA sequence and recovery of an infectious RNA transcribed from stably cloned full-length cDNA. J. Virol. 68:4580-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khromykh, A. A., and E. G. Westaway. 1997. Subgenomic replicons of the flavivirus Kunjin: construction and applications. J. Virol. 71:1497-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khromykh, A. A., M. T. Kenney, and E. G. Westaway. 1998. trans-Complementation of flavivirus RNA polymerase gene NS5 by using Kunjin virus replicon-expressing BHK cells. J. Virol. 72:7270-7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khromykh, A. A., A. N. Varnavski, and E. G. Westaway. 1998. Encapsidation of the flavivirus Kunjin replicon RNA by using a complementation system providing Kunjin virus structural proteins in trans. J. Virol. 72:5967-5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khromykh, A. A., P. L. Sedlak, and E. G. Westaway. 2000. cis- and trans-acting elements in flavivirus RNA replication. J. Virol. 74:3253-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulkarni, A. B., H. C. Morse III, J. R. Bennink, J. W. Yewdell, and B. R. Murphy. 1993. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J. Virol. 67:4086-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanciotti, R. S., J. T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K. E. Volpe, M. B. Crabtree, J. H. Scherret, R. A. Hall, J. S. MacKenzie, C. B. Cropp, B. Panigrahy, E. Ostlund, B. Schmitt, M. Malkinson, C. Banet, J. Weissman, N. Komar, H. M. Savage, W. Stone, T. McNamara, and D. J. Gubler. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333-2337. [DOI] [PubMed] [Google Scholar]
- 20.Lindenbach, B. D., and C. M. Rice. 1997. trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J. Virol. 71:9608-9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindenbach, B. D., and C. M. Rice. 1999. Genetic interaction of flavivirus nonstructural proteins NS1 and NS4A as a determinant of replicase function. J. Virol. 73:4611-4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindenbach, B. D. and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In B. N. Fields, D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology, 4th ed. Lippincott-Roven, New York, N.Y.
- 23.Liu, W. J., H. B. Chen, and A. A. Khromykh. 2003. Molecular and functional analyses of Kunjin virus infectious cDNA clones demonstrate the essential roles for NS2A in virus assembly and for a nonconservative residue in NS3 in RNA replication. J. Virol. 77:7804-7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, W. J., P. L. Sedlak, N. Kondratieva, and A. A. Khromykh. 2002. Complementation analysis of the flavivirus Kunjin NS3 and NS5 proteins defines the minimal regions essential for formation of a replication complex and shows a requirement of NS3 in cis for virus assembly. J. Virol. 76:10766-10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molenkamp, R., E. A. Kooi, M. A. Lucassen, S. Greve, J. C. Thijssen, W. J. Spaan, and P. J. Bredenbeek. 2003. Yellow fever virus replicons as an expression system for hepatitis C virus structural proteins. J. Virol. 77:1644-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monath, T. P., I. Levenbook, K. Soike, Z. X. Zhang, M. Ratterree, K. Draper, A. D. Barrett, R. Nichols, R. Weltzin, J. Arroyo, and F. Guirakhoo. 2000. Chimeric yellow fever virus 17D-Japanese encephalitis virus vaccine: dose-response effectiveness and extended safety testing in rhesus monkeys. J. Virol. 74:1742-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pang, X., M. Zhang, and A. I. Dayton. 2001. Development of Dengue virus type 2 replicons capable of prolonged expression in host cells. BMC Microbiol. 1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pang, X., M. Zhang, and A. I. Dayton. 2001. Development of dengue virus replicons expressing HIV-1 gp120 and other heterologous genes: a potential future tool for dual vaccination against dengue virus and HIV. BMC Microbiol. 1:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pletnev, A. G., M. Bray, J. Huggins, and C. J. Lai. 1992. Construction and characterization of chimeric tick-borne encephalitis/dengue type 4 viruses. Proc. Natl. Acad. Sci. USA 89:10532-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polo, J. M., B. A. Belli, D. A. Driver, I. Frolov, S. Sherrill, M. J. Hariharan, K. Townsend, S. Perri, S. J. Mento, D. J. Jolly, S. M. Chang, S. Schlesinger, and T. W. Dubensky, Jr. 1999. Stable alphavirus packaging cell lines for Sindbis virus and Semliki Forest virus-derived vectors. Proc. Natl. Acad. Sci. USA 96:4598-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pryor, M. J., J. M. Carr, H. Hocking, A. D. Davidson, P. Li, and P. J. Wright. 2001. Replication of dengue virus type 2 in human monocyte-derived macrophages: comparisons of isolates and recombinant viruses with substitutions at amino acid 390 in the envelope glycoprotein. Am. J. Trop. Med. Hyg. 65:427-434. [DOI] [PubMed] [Google Scholar]
- 32.Rossi, F. M., and H. M. Blau. 1998. Recent advances in inducible gene expression systems. Curr. Opin. Biotechnol. 9:451-456. [DOI] [PubMed] [Google Scholar]
- 33.Shi, P. Y., M. Tilgner, and M. K. Lo. 2002. Construction and characterization of subgenomic replicons of New York strain of West Nile virus. Virology 296:219-233. [DOI] [PubMed] [Google Scholar]
- 34.Shi, P. Y., M. Tilgner, M. K. Lo, K. A. Kent, and K. A. Bernard. 2002. Infectious cDNA clone of the epidemic West Nile virus from New York City. J. Virol. 76:5847-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simmons, C. P., T. Hussell, T. Sparer, G. Walzl, P. Openshaw, and G. Dougan. 2001. Mucosal delivery of a respiratory syncytial virus CTL peptide with enterotoxin-based adjuvants elicits protective, immunopathogenic, and immunoregulatory antiviral CD8+ T cell responses. J. Immunol. 166:1106-1113. [DOI] [PubMed] [Google Scholar]
- 36.Varnavski, A. N., and A. A. Khromykh. 1999. Noncytopathic flavivirus replicon RNA-based system for expression and delivery of heterologous genes. Virology 255:366-375. [DOI] [PubMed] [Google Scholar]
- 37.Varnavski, A. N., P. R. Young, and A. A. Khromykh. 2000. Stable high-level expression of heterologous genes in vitro and in vivo by noncytopathic DNA-based Kunjin virus replicon vectors. J. Virol. 74:4394-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westaway, E. G., J. M. Mackenzie, and A. A. Khromykh. 2002. Replication and gene function in Kunjin virus. Curr. Top. Microbiol. Immunol. 267:323-351. [DOI] [PubMed] [Google Scholar]
- 39.Westaway, E. G., J. M. Mackenzie, M. T. Kenney, M. K. Jones, and A. A. Khromykh. 1997. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 71:6650-6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westaway, E. G., A. A. Khromykh, M. T. Kenney, J. M. Mackenzie, and M. K. Jones. 1997. Proteins C and NS4B of the flavivirus Kunjin translocate independently into the nucleus. Virology 234:31-41. [DOI] [PubMed] [Google Scholar]
- 41.Westaway, E. G., M. A. Brinton, S. Gaidamovich, M. C. Horzinek, A. Igarashi, L. Kaariainen, D. K. Lvov, J. S. Porterfield, P. K. Russell, and D. W. Trent. 1985. Flaviviridae. Intervirology 24:183-192. [DOI] [PubMed] [Google Scholar]
- 42.Yin, D. X., L. Zhu, and R. T. Schimke. 1996. Tetracycline-controlled gene expression system achieves high-level and quantitative control of gene expression. Anal. Biochem. 235:195-201. [DOI] [PubMed] [Google Scholar]