Abstract
Prophylactic hepatitis C virus (HCV) vaccine trials with human volunteers are pending. There is an important need for immunological end points which correlate with vaccine efficacy and which do not involve invasive procedures, such as liver biopsies. By using a multicomponent DNA priming-protein boosting vaccine strategy, naïve chimpanzees were immunized against HCV structural proteins (core, E1, and E2) as well as a nonstructural (NS3) protein. Following immunization, exposure to the heterologous HCV 1b J4 subtype resulted in a peak of plasma viremia which was lower in both immunized animals. Compared to the naïve infection control and nine additional historical controls which became chronic, vaccinee 2 (Vac2) rapidly resolved the infection, while the other (Vac1) clearly controlled HCV infection. Immunization induced antibodies, peptide-specific gamma interferon (IFN-γ), protein-specific lymphoproliferative responses, IFN-γ, interleukin-2 (IL-2), and IL-4 T-helper responses in both vaccinees. However, the specificities were markedly different: Vac2 developed responses which were lower in magnitude than those of Vac1 but which were biased towards Th1-type cytokine responses for E1 and NS3. This proof-of-principle study in chimpanzees revealed that immunization with a combination of nonstructural and structural antigens elicited T-cell responses associated with an alteration of the course of infection. Our findings provide data to support the concept that the quality of the response to conserved epitopes and the specific nature of the peripheral T-helper immune response are likely pivotal factors influencing the control and clearance of HCV infection.
The hepatitis C virus (HCV) is the etiologic agent of non-A non-B hepatitis, the leading cause of chronic liver infections. Chronic HCV infection is correlated with the risk of developing liver cirrhosis and hepatocellular carcinoma (1). It is estimated that there are more than 170 million chronic carriers worldwide (44). To date, there is neither a prophylactic vaccine nor a satisfactory therapeutic treatment (27), and although routine testing of blood products for HCV has reduced posttransfusion infection in the Western world, not all of the routes of transmission are known, and new cases are still accumulating.
The development of an HCV vaccine has been problematic due to the logistics and the ethical restrictions in the numbers and use of chimpanzees, the only species other than Homo sapiens that is susceptible to chronic HCV infection. Furthermore, essential information regarding the immune correlates of protective immunity is still lacking. Although important proof-of-principle ex vivo neutralization studies have been undertaken with chimpanzees (14), neutralizing antibodies are not easily attained and are considered insufficient for viral clearance or the prevention of reinfection (9, 13). The study of immune responses induced in individuals and chimpanzees with acute resolved versus chronic infections has so far revealed only an emerging picture of potential protective immune responses following the establishment of active viral infection. In addition to innate host immunity (38), the importance of cytotoxic T lymphocytes (CTLs) (9, 12, 18, 43, 46), gamma interferon (IFN-γ)-producing T cells homing into the liver (36), and T-helper (Th) responses (4, 10, 20, 26) in the control of HCV infection has been demonstrated. However, the correlates of prophylactic vaccine protection following parenteral immunization are expected to be largely extrahepatic prior to HCV exposure. We set out to correlate such peripheral immune responses with vaccine efficacy in order to identify peripheral immune readouts that are likely to be indicative of a desirable vaccine-induced response. Such end points should be readily measurable in human volunteers without necessitating invasive liver biopsies.
A limited number of vaccine efficacy studies have been performed with chimpanzees. Immunization with E1 and E2 proteins protected five out of seven animals from infection after a low-dose homologous challenge (with exactly the same clone being used for immunization and challenge) (8) but not against heterologous challenge. In another study, DNA expressing E2 was used to immunize two chimpanzees. Following homologous challenge, both vaccinees became infected but resolved the infection (15). However, none of these studies provided insights into the nature of the vaccine-induced immune correlates of clearance. In addition, HCV presents a high degree of variability (35). Multiple genotypes coexist worldwide, and in addition, HCV circulates as a quasispecies within an individual (28). This heterogeneity provides HCV with the capacity to escape vaccine- or infection-induced immune responses (7).
In an effort to maximize protection against heterologous challenge and to reduce the likelihood of vaccine escape, we used a multicomponent vaccine strategy to identify four structural as well as nonstructural proteins (E1, E2, core, and NS3 proteins) as potential targets for virus-neutralizing antibodies and T-cell responses. The core and NS3 proteins are more conserved antigens and therefore may elicit immune responses that target a broad range of virus variants. NS3 constitutes a key antigen, as NS3-specific Th responses have been linked to viral clearance (10), and CTLs have been described in the context of therapy-linked resolution of infection (37, 40). Our immunization strategy consisted of DNA priming followed by subunit boosting with the aim of inducing both humoral and cellular responses of the broadest possible magnitude to multiple antigens (30). We report the correlation of peripheral cellular immune responses with the control or resolution of nonhomologous 1b HCV infections in chimpanzees.
MATERIALS AND METHODS
Animals.
Three naïve chimpanzees (Pan troglodytes verus) were chosen as subjects for this study based on the observations of nine historical controls that were infected with the J4 1b challenge stock and became chronically infected (23, 31, 32; unpublished data; J. Bukh, personal communication). Following ethical committee approval, the animals were housed under special conditions for the ethical care of chimpanzees (39). They were mature animals and in good physical health. Hematological and clinical values, including liver enzymes such as serum alanine transaminase (ALT) and gamma-glutamyl transpeptidase (γ-GT), were monitored at regular intervals in an independent hospital laboratory.
Vaccine antigens and reagents.
For the priming of the immune response, four DNA plasmids were constructed. They expressed truncated forms of E1 and E2 glycoproteins of a European 1b strain (GenBank accession no. A48711) and the core and NS3 proteins of the HCV 1a strain H (GenBank accession no. M67463) (22). Coding sequences were amplified by PCR and cloned into the pCI plasmid vector (Promega, Lyon, France). The following boundaries were used: for the core protein, amino acids (aa) 1 to 191 (pCMVC2) (41); for E1, aa 134 to 328 (pCIE1t); for E2, aa 340 to 674 (pCIE2t); and for NS3, aa 1027 to 1657 (pCINS3) (3). The assessment of antigen production was performed as described previously (16, 17, 41). The constructs were used for the immunization of mice and elicited strong humoral as well as cellular immune responses (3, 41; G. Inchauspé, unpublished data). For DNA immunization, the DNA plasmids were purified with an EndoFree Plasmid Giga kit (QIAGEN, Courtaboeuf, France).
Three recombinant proteins derived from the European 1b sequence were used in the booster immunizations: E1 (aa 192 to 326), E2 (aa 412 to 715), and core (aa 1 to 191). In addition, a fusion protein representing two NS3 regions (aa 1071 to 1084 and 1181 to 1465) of a different 1b isolate was used. E1 and E2 proteins were derived from a mammalian cell culture, core was derived from Saccharomyces cerevisiae, and NS3 was expressed in Escherichia coli. All proteins were over 95% pure as estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were absorbed on alum (Superfos, Frederikssund, Denmark), yielding a formulation of 50 μg of protein/ml on 0.13% alum. The antigenicity and immunogenicity of the recombinant proteins were controlled with sera from chronic HCV patients and immunized mice, respectively.
Fifteen-mer peptides with overlaps of 7 aa covering the core (aa 1 to 191) and NS3 (aa 1028 to 1651) were used for enzyme-linked immunospot (ELISPOT) assays. The peptides were divided into peptide pools (pp) as follows: the core pp was aa 1 to 199 and NS3 pp 1 was aa 1028 to 1154, pp 2 was aa 1148 to 1274, pp 3 was aa 1268 to 1402, pp 4 was aa 1395 to 1530, and pp 5 was aa 1524 to 1659. The peptides were based on the Shimothono 1b strain, having a high degree of homology to the vaccine (91% identity with the DNA vaccine and 93% identity with the recombinant protein) and to the challenge strain (96% identity).
Immunizations and challenges.
The animals were immunized with DNA constructs at weeks 0, 8, and 16. For each immunization, 1 mg of each vector was prepared in saline and administered intramuscularly (900 μg) and intradermally (100 μg). Immune responses were boosted intramuscularly with 50 μg of each recombinant protein at weeks 24, 29, 33, and 45. The control animal was immunized with the empty vectors and with the adjuvant alone. The two immunized animals are subsequently referred to as Vac1 and Vac2.
All animals were challenged at week 49 by an intravenous administration of 25 50% chimpanzee infectious doses of the HCV J4 virus stock (generous gift from R. H. Purcell, National Institutes of Health, Bethesda, Md.) diluted in autologous preimmune plasma. HCV J4 is of the genotype 1b. The percentages of amino acid sequence heterogeneity between the inoculum-derived proteins and the recombinant proteins or vaccine DNAs are shown in Table 1.
TABLE 1.
Heterogeneity of the amino acid sequences between vaccine DNA plasmids or recombinant proteins and the inoculum J4 sequences
| Sequences compared | Heterogeneity with indicated inoculum J4 sequencea
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Core (191 aa)b
|
E1 (192 aa)
|
E2 (363 aa)
|
NS3 (631 aa)
|
|||||
| % | No. of heterologous aa/total no. | % | No. of heterologous aa/total no. | % | No. of heterologous aa/total no. | % | No. of heterologous aa/total no. | |
| DNA vector products | 2.1 | 4/191 | 2.6 | 5/194c | 12.6 | 42/334d | 9.4 | 59/630 |
| Proteins | 2.1 | 4/191 | 5.9 | 8/135 | 13.5 | 41/304 | 4.7 | 14/299 |
Results are percentages of heterogeneity relative to the inoculum sequence
Numbers in parentheses indicate the total length of the protein sequence, and numbers of heterologous amino acids out of the total number used in the vaccines.
DNA plasmid also codes for part of the C terminus of core as a signal sequence for E1.
DNA plasmid also codes for part of the C terminus of E1 as a signal sequence for E2.
Blood sampling and liver biopsies.
Blood samples were collected regularly during the study using aseptic techniques (Vacutainer systems; Becton Dickinson). Peripheral blood mononuclear cells (PBMC) from heparinized blood were isolated by lymphocyte separation medium density gradient centrifugation (Organon Teknika, Os, The Netherlands). PBMC were used fresh for the assessment of the cellular immune responses by ELISPOT assay or were cryopreserved at −135°C. As strict ethical rules limited the types of invasive procedures that could be performed on the chimpanzees, only three double-pass liver biopsies were permitted during the study, and these were taken prior to challenge and 8 weeks and 18 months after challenge. The last liver biopsy was taken in order to assess the final outcome of the HCV infection in these three animals.
Viral parameters.
Quantitative HCV PCRs for the determination of HCV viral load were performed on serum and liver tissue in two different laboratories by using a nested-PCR and a quantitative reverse transcription-PCR assay (Amplicor HCV monitor 2.0; Roche Diagnostic Systems, Branchburg, N.J.).
Humoral immune responses.
Antibodies specific for HCV proteins were detected in serum by using a research line immunoassay (INNO-LIA HCV antibody IV, prototype version; Innogenetics, Ghent, Belgium), including core, E1, E2, NS3, NS4A, NS4B, and NS5A HCV antigens. Antibody titers against the protein immunogens were estimated by enzyme-linked immunosorbent assay with a sequential dilution of serum samples and by determining the dilution that resulted in a signal that was two times above the recognized standard background level of the assay.
Cellular immune responses.
The lymphoproliferation assay was performed with cryopreserved PBMC as described previously (2). The quantification of specific cytokine-secreting cells was performed by using an ELISPOT assay (U-Cytech, Utrecht, The Netherlands) as described previously (2). Briefly, for both tests, freshly isolated or cryopreserved PBMC were stimulated with concanavalin A, recombinant viral proteins, and peptides (individually or in pools) at concentrations of 5 μg/ml or with medium alone. Results of lymphoproliferation were expressed as the mean counts of triplicate determinations minus the mean counts of the negative control ± standard deviation. The results of the ELISPOT assays were expressed as the mean numbers of spots of triplicate assays per 1 million cells minus the mean number of spots obtained with the medium-cultured cells (also in triplicate assays). Results were considered positive when the mean spot number was at least two times greater than the mean spot number obtained in the negative-control wells (medium alone). When the counts were below this limit, counts were recorded as 0. For the depletion assay, CD4+ or CD8+ cells were depleted prior to the assay with Dynabeads (Dynal, Skøyen, Norway), and thereafter the cells were used in the ELISPOT assay.
Immunological events in the liver.
The real-time PCR was performed by using primers specific to interleukin-2 (IL-2), IFN-γ, tumor necrosis factor alpha (TNF-α), and glyceraldehyde phosphate dehydrogenase (GAPDH). Real-time PCR runs were performed in 96-well optical reaction plates, each containing 1× PCR master mix (Applied Biosystems), a 0.3-pmol/ml concentration of each forward primer, reverse primer, and 6-carboxyfluorescein-labeled probe, and 2 μl of template DNA (chimpanzee cDNA; human cDNA for IL-2-, TNF-α-, and GAPDH-positive controls; and cloned IFN-γ plasmid DNA for the IFN-γ-positive control), in a final volume of 25 μl, for 40 cycles with an ABI 7700 Prism sequence detection system (Applied Biosystems). The default 7700 cycle conditions were as follows: 2 min at 50°C and then 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Results were analyzed using Sequence Detection System software (version 1.9; Applied Biosystems).
RESULTS
Postexposure course of HCV infection.
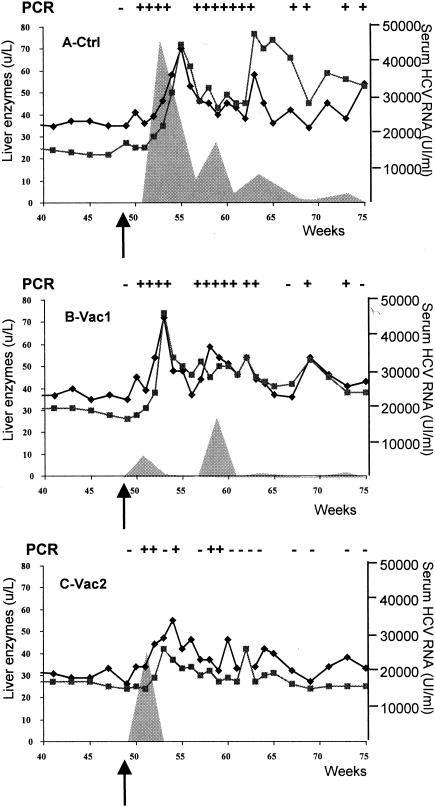
As shown in Fig. 1A, a high peak of RNA titers (46,990 IU/ml) developed in the control animal at 4 weeks postexposure (p.e.). Thereafter, the viral load decreased but remained positive throughout the 26-week follow-up period. Liver enzymes showed a transient elevation at 6 weeks p.e. Thereafter, liver enzymes continued fluctuating at elevated levels (especially γ-GT [>50 U/liter]) up to week 75 (Fig. 1). During the subsequent 2 years, ALT levels were controlled at a few time points and ranged from 42 to 85 U/liter. The liver was still positive by HCV nested PCR at 18 months p.e. (data not shown).
FIG. 1.
Courses of infection in the control chimpanzee (Ctrl) (A) and in immunized chimpanzees Vac1 (B) and Vac2 (C). All animals were challenged at week 49 after immunization (arrow). PCR results with plasma for HCV RNA are indicated above each graph (−, negative; +, positive). Quantitative HCV RNA concentrations are shown as shaded areas (right y axis). The levels of the liver enzymes ALT and γ-GT are also plotted (black diamonds and black squares, respectively; left y axis).
Compared to the control animal, Vac1 developed a lower peak of serum HCV RNA titers at 2 weeks p.e. (6,970 UI/ml) and 9 weeks p.e. (17,300 UI/ml) (Fig. 1B). Thereafter, viremia remained at low or undetectable levels. Liver enzymes increased 4 weeks p.e. and then returned to near-normal levels with transient fluctuations during the follow-up (≤50 U/liter for both ALT and γ-GT) (Fig. 1B). Over a 2-year period, ALT levels ranged from 45 to 59 U/liter. At 18 months p.e., HCV RNA was detectable in the liver (data not shown).
The second vaccinee, Vac2, had a transient peak of viremia of 24,580 IU/ml at 2 weeks p.e., which quickly declined to levels below detection (Fig. 1C). After week 61 (week 11 p.e.), both nested and quantitative reverse transcription-PCRs remained consistently negative for HCV RNA (Fig. 1C). Liver enzyme levels showed a slight elevation (55 U/liter for ALT at week 5 p.e.) but thereafter remained within normal limits (between 30 and 40 U/liter) (Fig. 1C). After a period of 15 months with liver enzyme levels consistently within normal ranges (ALT at 30 U/liter and γ-GT between 24 and 26 U/liter), an analysis of the liver biopsy at 18 months p.e. revealed that the liver of Vac2 was negative for HCV RNA.
Humoral responses against non-vaccine-encoded HCV antigens were assessed p.e. by using the line immunoassay as an independent assessment of primary infection. In all animals, antibody responses were developed against NS4B (data not shown). Interestingly, this response was more rapid and stronger in Vac2 than in the control and Vac1. No responses against NS4A and NS5A were detected (data not shown).
In summary, these data indicate that immunization accelerated viral clearance (Vac2) or controlled viremia levels (Vac1). Vac2 completely and rapidly resolved the infection within 4 weeks, a remarkable result that was not previously observed in the nine chimpanzees that were experimentally infected with HCV 1b J4 and became chronically infected (23, 31, 32; unpublished data; J. Bukh, personal communication).
Immune responses specific to core antigen.
In order to identify the specific nature of the vaccine-induced immune responses that allowed Vac2 to resolve the infection, we comparatively dissected the vaccine-specific humoral and cellular immune responses of all animals prior to and following challenge.
Unexpectedly, the antibody responses to core antigen were low or undetectable in all animals prior to, as well as after, challenge (data not shown). Antibody to core appeared only after the last protein booster and were comparable in both vaccinees (antibody titer of 194 for Vac1 and 168 for Vac2 before challenge and 200 for Vac1 and 500 for Vac2 after challenge). Low-level core-specific lymphoproliferation (4,655 cpm for Vac1 and 3,888 cpm for Vac2 at week 33 postimmunization) as well as low numbers of IL-2- and IL-4-secreting cells were detected in both vaccinees. The control animal remained negative in all assays. After the stimulation of the cells with the core protein, no IFN-γ secretion was detected in any of the animals at any time point. In addition, IFN-γ secretion that was specific to a peptide pool covering the entire core protein was not detected in any animal prior to challenge. These results strongly suggest that the vaccine was not able to induce core-specific CD8+-T-cell responses in either of the two vaccinees.
The fact that the immune responses induced with the core protein were weak was rather unexpected, as core antigen is known to induce B- and T-cell responses in the great majority of HCV-infected individuals and because anti-core antibodies and specific T-cell responses were readily detectable in mice immunized with the same plasmid or recombinant protein (41). The total absence of peripheral core-specific IFN-γ secretion argues against the presence of and a role for core-specific responses in the control of HCV infection.
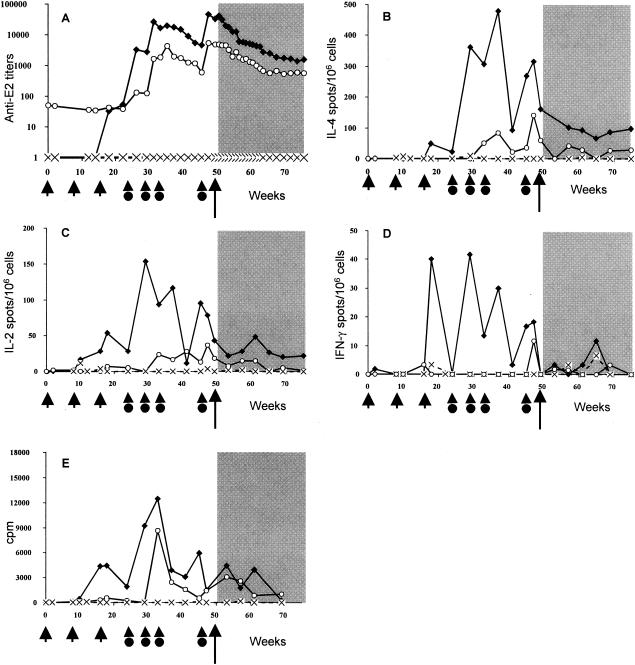
Immune responses specific to E1 antigen.
Vac1 and Vac2 elicited strong immune responses specific to the E1 antigen, predominantly following the second or third protein immunization (Fig. 2). As seen in Fig. 2A and B, the high antibody titers and IL-4 production levels were comparable between the two vaccinees. Vac2 developed marked lymphoproliferation responses (up to 14,155 cpm for Vac2 versus 4,747 cpm for Vac1 at 33 weeks postimmunization) (Fig. 2E) that were associated with high numbers of IL-2-producing cells prior to, as well as after, challenge. In addition, moderate but specific IFN-γ production was induced in Vac2 but was absent in Vac1 (Fig. 2D). None of these specific responses could be detected in the control animal at any time point.
FIG. 2.
Kinetics of anti-E1 immune responses elicited in Vac1 (▪), Vac2 (○), and the control animal (×) by DNA priming and protein boosting (indicated by short arrows and arrowheads with circles, respectively) and after HCV challenge (long arrow, shaded area). The results for antibody titers (A), IL-4-producing cells (B), IL-2-producing cells (C), IFN-γ-producing cells (D), and lymphoproliferation assays (E) are displayed.
These data suggest that a Th1-oriented E1-specific cellular response (IL-2 and IFN-γ) was beneficial, given the correlation with the resolution of infection in Vac2.
Immune responses specific to E2 antigen.
In contrast to E1-specific responses, E2-specific responses were detected early: during or shortly after DNA immunization (IL-4, IL-2, and IFN-γ) and after the first protein booster injection (antibody) (Fig. 3). The immune response to the E2 antigen that was observed in Vac1 was more rapid and effective than that in Vac2. High antibody titers (up to 45,161 at week 47 postimmunization) (Fig. 3A), a vigorous lymphoproliferation response (Fig. 3E), and high numbers of antigen-specific IL-4-, IL-2-, and IFN-γ-secreting cells were induced in Vac1 (Fig. 3B to D). In contrast, 10-fold-lower anti-E2 antibody titers (5,443 at week 47) (Fig. 3A) and low IL-4 and IL-2 responses (Fig. 3B and C) were observed for Vac2. Also, no IFN-γ was secreted following the stimulation of the PBMC with E2 antigen in Vac2 (Fig. 3D). No specific responses could be detected in the control animal at any time point tested. These results suggest that the strong E2-specific immune response induced in Vac1 was not sufficient to prevent or induce the clearance of heterologous HCV infection.
FIG. 3.
Kinetics of anti-E2 immune responses elicited in Vac1 (▪), Vac2 (○), and the control animal (×) by DNA priming and protein boosting (indicated by short arrows and arrowheads with circles, respectively) and after HCV challenge (long arrow, shaded area). Results for antibody titers (A), IL-4-producing cells (B), IL-2-producing cells (C), IFN-γ-producing cells (D), and lymphoproliferation assays (E) are displayed.
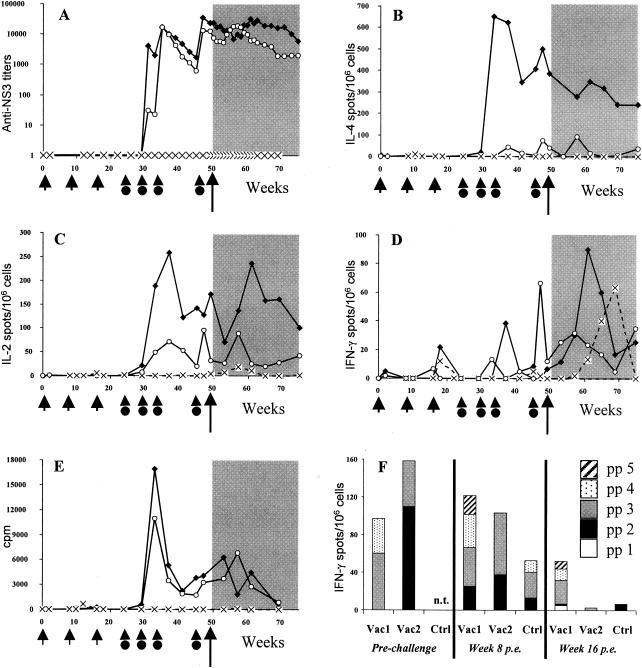
Immune responses specific to NS3 antigen.
The majority of the NS3-specific responses were detected after the second protein immunization (Fig. 4). Both vaccinees elicited a strong humoral immune response to NS3 (Fig. 4A). However, only Vac1 presented a very high number of NS3-specific IL-4-producing cells (up to 652 spots/106 cells) (Fig. 4B). NS3-specific IL-2-producing cells and proliferative responses were detected in both animals but were higher in Vac1 at all time points tested (Fig. 4C and E). On the other hand, Vac2 developed a low NS3-specific IFN-γ response prior to challenge (Fig. 4D) which was reciprocally associated with very weak IL-4 production (Fig. 4B). This finding suggests that, in contrast to Vac1, Vac2 developed a cellular immune response to NS3 antigen which was skewed towards a Th1-like profile. An anamnestic response was observed in both vaccinees, relating mainly to the detection of IL-2 and IFN-γ. The production of IFN-γ was observed in the control animal after challenge but not until week 60. (Fig. 4D). No other specific responses, including lymphoproliferation, were detected in the control animal at any time point tested (Fig. 4E).
FIG. 4.
Kinetics of anti-NS3 immune responses elicited in Vac1 (▪), Vac2 (○), and the control animal (Ctrl) (×) by DNA priming and protein boosting (indicated by short arrows and arrowheads with circles, respectively) and after HCV challenge (long arrow, shaded area). Results for antibody titers (A), IL-4-producing cells (B), IL-2-producing cells (C), IFN-γ-producing cells (D), and lymphoproliferation assays (E) are displayed. (F) Results of IFN-γ ELISPOT assay with pools of 15-mer peptides covering the NS3 sequence (pp 1, aa 1028 to 1154; pp 2, aa 1148 to 1274; pp 3, aa 1268 to 1402; pp 4, aa 1395 to 1530; and pp 5, aa 1524 to 1659). Assays were performed prior to challenge (weeks 37 and 47 for Vac1 and week 47 for Vac2) and 8 and 16 weeks p.e. n.t., not tested.
Since cellular immune responses to NS3 correlated to a resolution of HCV infection in humans (10, 11, 34, 40), we undertook a detailed analysis of the NS3-specific IFN-γ responses in the three animals by using overlapping peptides covering the NS3 region. As a first step, we assessed the peptide-specific IFN-γ responses by using five peptide pools as illustrated in Fig. 4F. Prior to challenge, the peptide-specific IFN-γ responses were directed to three different pp: while Vac1 responded to pp 3 and 4, Vac2 responded to pp 2 and 3 (Fig. 4F). In the acute phase of infection following exposure (week 8 p.e.), Vac1 responded to two additional pools, while Vac2 remained responsive to the same pools as it did prior to challenge (pp 2 and 3). By week 16 p.e., responses to NS3 in peripheral blood had declined in Vac2 and the control but persisted in Vac1 (Fig. 4F). These observations suggest that Vac2 had developed effective responses to epitopes that were sufficient to accelerate the clearance of the infection and that Vac1 was less capable of containing the infection and developed responses to additional epitopes.
We further examined the peptide specificity of the IFN-γ responses within pp 2 and 3 (Table 2). No IFN-γ production was detected in the control when individual peptides were used in the assay, although this animal reacted to pp 2 and 3, suggesting that the sensitivity is lower when individual peptides are used. Prior to challenge (week 47), Vac1 recognized three peptides within pp 3, while Vac2 recognized three additional peptides within both pools (Table 2). Postchallenge (week 8 p.e.), the number of epitopes inducing IFN-γ production remained greater for Vac2 than for Vac1.
TABLE 2.
Responses to individual NS3 peptides from pp2 (first four peptides listed) and pp3 (last four peptides listed) that consistently induced IFN-γ-producing cells in at least one of the animals
| Peptide location (aa) | Peptide sequencea | IFN-γ productionb
|
||||
|---|---|---|---|---|---|---|
| Prechallenge
|
Postchallenge
|
|||||
| Vac1 | Vac2 | Vac1 | Vac2 | Ctrl | ||
| 1156 | RPVSYLKGSSGGPLLc | — | <20 | — | — | — |
| 1172 | PSGHVVGIFRAAVCTc | — | — | — | <20 | — |
| 1252 | RVLNPSVAATLGFGAd | — | <20 | <20 | <20 | — |
| 1268 | MSKAHGIEPNIRTGVc | — | — | — | <20 | — |
| 1364 | EVALSNTGEIPFYGKc | — | <20 | — | — | — |
| 1372 | EIPFYGKAIPIEAIK | 20-39 | >40 | — | 20-39 | — |
| 1380 | IPIEAIKGGRHLIFC | 20-39 | 20-39 | — | — | — |
| 1388 | GRHLIFCHSKKKCDEc,d | 20-39 | <20 | — | <20 | — |
The overlapping sequences are underlined.
Results shown are the number of IFN-γ spots per million cells. —, no IFN-γ production detected at week 47 (prechallenge) and week 8 postchallenge. The major histocompatability complex class I typing of the chimpanzees (Patr class I) is as follows: for Vac1, A−, B*0201, C*0601, and A*0601, B*0101, C*0401; for Vac2, A*0401, B*1401, C*0203 and A*1401, B*16011, C*0502; and for the control animal, A*0601, B*0301, C*0901 and A*0401, B*0101, C*0401.
Peptides covering previously described human CD8 epitopes.
Peptides covering previously described human CD4 epitopes.
In an attempt to characterize the region which induced IFN-γ production in both vaccinees (peptides 1372 and 1388), we performed a depletion ELISPOT assay prior to challenge. When CD8+ cells were depleted, peptides 1372 and 1388 induced IFN-γ production in Vac1 and Vac2, but IFN-γ production was abolished when CD4+ cells were removed (data not shown), demonstrating that the responses to these peptides are CD4+ restricted.
In conclusion, marked IL-4 responses were induced for all four antigens in Vac1. Furthermore, Vac1 also exhibited high numbers of IL-2-producing cells specific for the E2 and NS3 antigens. In contrast, high numbers of IL-2-producing cells specific for the E1 antigen were induced in Vac2, and the last protein booster induced a low IFN-γ response to the E1 and NS3 antigens in Vac2. This response peaked just prior to challenge and targeted more NS3 peptides than did the response in Vac1. This NS3-specific response was skewed towards a Th1-type profile.
Liver biopsy tissue was carefully analyzed to determine if the virus-specific peripheral immune responses correlated with the elevation of cytokine production in the liver. The analysis of cytokine mRNA levels in the liver biopsies prior to exposure and 8 weeks and 18 months p.e. revealed that there was no detectable production of IFN-γ, TNF-α, and IL-2 in the livers of any of the three animals, despite the presence of HCV-specific IFN-γ- and IL-2-producing cells in peripheral blood cells at 8 weeks p.e. (Fig. 2 to 4).
DISCUSSION
A specific objective of this study was to perform a proof-of-principle preclinical immunogenicity and efficacy study with chimpanzees by using noninvasive immune readout analyses, such as the ELISPOT assay, which could be standardized for pending human clinical trials. Most HCV studies of chimpanzees to date have involved multiple liver biopsies that would not readily be applied to wide-scale phase I and II prophylactic vaccine trials with humans. We set out to determine if peripheral blood-based ELISPOT assays could be used for this purpose and if they would provide meaningful data with regard to the potential immune correlates of HCV vaccine efficacy prior to virus exposure. Furthermore, human phase III HCV prophylactic vaccine trials are likely to involve exposure to heterologous HCV variants. This is the first study of this kind with chimpanzees. Only two HCV vaccine efficacy studies of chimpanzees have been published to date, and both were restricted to homologous challenges (8, 15). The present study, although it involved a limited number of chimpanzees, shows that vaccine protection from chronic HCV infection with a heterologous 1b subtype is feasible. This study constitutes a proof-of-concept study in the field of HCV vaccine development, as it is not only the first vaccine cross-protection study but also the first multicomponent priming-boosting HCV vaccine regimen to be applied effectively. Our study identifies important peripheral and noninvasive immunological end points and provides additional data supporting the importance of a Th1-like cellular immune response to nonstructural viral proteins in the control of HCV infection.
Anti-E1 and -E2 antibodies were readily detected in both vaccinees. No major differences were observed in the kinetics of antibody appearance in the immunized animals. Antibody titers were high for both vaccinees, arguing against a decisive role played by such antibodies in the clearance of infection observed for Vac2. These observations reinforce those of Cooper et al. (9), who demonstrated, using liver biopsies, that the clearance of HCV infection in chimpanzees correlated with T-cell, but not with B-cell, HCV-specific responses.
Vac2 developed lower antibody titers and lower IL-4 responses than Vac1, and it was striking that both the kinetics and vigor of the IFN-γ and IL-2 responses generated that were specific for E1 and NS3 differed considerably in the two animals. The peak production in Vac2 for both antigens was detected 2 weeks prior to challenge, while for Vac1, such a response was either absent (E1) or developed more than 15 weeks prior to challenge (NS3) (Fig. 2D and 4D). A tempting interpretation is that a greater number of antigen-specific Th1-like lymphocytes were present at the time of challenge, which facilitated a more efficient CTL response or a homing to the liver that eliminated the infected cells (36). Both chimpanzees acquired memory-type responses for IFN-γ-producing T cells, as was indicated by the recall response observed following challenge. The kinetics of this response was more rapid in Vac2. IFN-γ can also have a direct antiviral effect (19, 36). Thus, E1- and NS3-specific IFN-γ-producing T cells, present at early stages of infection, may have been key players in efficient vaccine-induced immunity, corroborating the results of several studies with humans and chimpanzees (11, 25, 29, 34, 36, 40). It is interesting that the analysis of immunological events in the liver biopsies did not reveal additional information. The role of liver-expressed cytokines in HCV infection is still being debated. Detection of IFN-γ in liver biopsies was recently reported to correlate with the control of HCV infection (36), while another group demonstrated that there was no distinct pattern of liver IFN-γ production with respect to infection outcome (38). In this context, the restriction on the number of liver biopsies probably limited the relevance of this analysis in our study, as peaks of immune responses in the liver may have been missed. This suggests that it will be extremely difficult, if not impossible, to use cytokine mRNA production in the liver as a relevant end point in human clinical trials and emphasizes the importance of thorough immunological assays on PBMC.
Remarkably, both Vac1 and the control animal developed IFN-γ responses that were specific for additional pp after challenge, compared with responses prior to challenge. This suggests that HCV replication was permitted in Vac1 and the control animal, which mounted new and broader responses; however, this was limited in Vac2 due to the early control of HCV infection. A similar phenomenon has been observed with CTL responses in chimpanzees and humans, which tend to wane following HCV resolution but expand in persistent infection (9, 45). We chose to analyze the fine specificity of the IFN-γ responses within pp 2 and 3. In this assay, a reaction was measured to eight peptides for Vac2, but only four of them induced IFN-γ production in Vac1. Interestingly, the peptides recognized by Vac2 covered several highly conserved CD8+ epitopes (aa 1163 to 1177, 1169 to 1177, 1261 to 1270, 1267 to 1275, 1359 to 1367, 1395 to 1403, and 1396 to 1404), as well as the critical CD4+ T-cell epitopes (aa 1251 to 1259 and aa 1388 to 1407) previously described to induce CD8+ responses in the periphery or in liver in humans (5, 6, 10, 21, 24, 33, 46). In particular, Vac2 elicited IFN-γ responses, prior to, as well as after challenge, to peptides 1252 to 1266 and 1388 to 1403, comprising the critical human CD4+-T-cell epitopes 1251 to 1259 and 1388 to 1407 that were previously identified in patients with self-limiting HCV infection (10).
Finally, the overall lack of response observed in the control chimpanzee was intriguing. Following experimental infection, chimpanzees are known to develop weaker immune responses than HCV-infected patients (42). The characteristics of the immune responses following experimental infection with the subtype 1b strain HC-J4 used here have not yet been reported, since the vast majority of HCV vaccine efficacy studies of chimpanzees have been based on challenges with subtype 1a strains. In such scenarios, between 30 and 70% of the animals become chronic carriers. The 1b isolate used in this study (HC-J4) is particularly aggressive compared to all other strains evaluated to date for chimpanzees. All of the nine naïve animals infected with this strain and for which the outcome is known have developed chronic HCV infection accompanied by elevated liver enzyme levels (23, 31, 32; unpublished data; J. Bukh, personal communication). Our control chimpanzee accurately reflected the pattern of chronic p.e. progression of HCV infection that is associated with the HC-J4 inoculum (Fig. 1A). The two immunized animals had patterns that contrasted with that of the naïve animal, as one rapidly cleared and the other controlled this heterologous infection. Although our study was limited to two vaccinees, the prevention of evolution to chronicity after HC-J4 challenge may be relevant in light of the high chronicity rate of this strain. It is of interest that one of the strongest immune correlates of resolved HCV infection in humans has been CD4+ Th1-type responses to multiple antigens (29) and especially to the NS3 protein (11). Our findings from clinically applicable ELISPOT assays confirm and extend the role of strong Th1-type responses in HCV prophylactic vaccine-induced immunity. These findings concerning heterologous-HCV vaccine-induced immunity will need to be extended and confirmed in larger trials. In addition, a refinement of this vaccine strategy is needed to improve its efficacy and to support the possible key roles played by NS3 and E1, as well as to determine the correct balance of Th1- and Th2-driven effector responses that facilitate the clearance of HCV infection.
Acknowledgments
This work was supported in part by the European Community, Biotechnology contract no. BIO4 CT 980357, contract HCVacc cluster no. QLK2-CT-1999-00356 within the fifth framework program, and also by the Association pour la Recherche sur le Cancer.
We are grateful to H. Bazin for his support in the very early stage of the conception of this project. We thank Ronald Bontrop and Natasja de Groot for Patr class I typing and our colleagues for technical support, helpful discussion, and advice.
REFERENCES
- 1.Armstrong, G. L., M. J. Alter, G. M. McQuillan, and H. S. Margolis. 2000. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology 31:777-782. [DOI] [PubMed] [Google Scholar]
- 2.Balla-Jhagjhoorsingh, S. S., P. Mooij, P. J. ten Haaft, W. M. Bogers, V. J. Teeuwsen, G. Koopman, and J. L. Heeney. 2001. Protection from secondary human immunodeficiency virus type 1 infection in chimpanzees suggests the importance of antigenic boosting and a possible role for cytotoxic T cells. J. Infect. Dis. 184:36-143. [DOI] [PubMed] [Google Scholar]
- 3.Brinster, C., S. Muguet, Y. C. Lone, D. Boucreux, N. Renard, A. Fournillier, F. Lemonnier, and G. Inchauspe. 2001. Different hepatitis C virus nonstructural protein 3 (Ns3)-DNA-expressing vaccines induce in HLA-A2.1 transgenic mice stable cytotoxic T lymphocytes that target one major epitope. Hepatology 34:1206-1217. [DOI] [PubMed] [Google Scholar]
- 4.Cerny, A., and F. V. Chisari. 1999. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology 30:595-601. [DOI] [PubMed] [Google Scholar]
- 5.Cerny, A., J. G. McHutchison, C. Pasquinelli, M. E. Brown, M. A. Brothers, B. Grabscheid, P. Fowler, M. Houghton, and F. V. Chisari. 1995. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J. Clin. Investig. 95:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, K. M., N. H. Gruener, S. Southwood, J. Sidney, G. R. Pape, F. V. Chisari, and A. Sette. 1999. Identification of HLA-A3 and -B7-restricted CTL response to hepatitis C virus in patients with acute and chronic hepatitis C. J. Immunol. 162:1156-1164. [PubMed] [Google Scholar]
- 7.Chang, K. M., B. Rehermann, J. G. McHutchison, C. Pasquinelli, S. Southwood, A. Sette, and F. V. Chisari. 1997. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J. Clin. Investig. 100:2376-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choo, Q. L., G. Kuo, R. Ralston, A. Weiner, D. Chien, G. Van Nest, J. Han, K. Berger, K. Thudium, C. Kuo, J. Kansopon, J. McFarland, A. Tabrizi, K. Ching, B. Moss, L. B. Cummins, M. Houghton, and E. Muchmore. 1994. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc. Natl. Acad. Sci. USA 91:1294-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper, S., A. L. Erickson, E. J. Adams, J. Kansopon, A. J. Weiner, D. Y. Chien, M. Houghton, P. Parham, and C. M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10:439-449. [DOI] [PubMed] [Google Scholar]
- 10.Diepolder, H. M., J.-T. Gerlach, R. Zachoval, R. M. Hoffmann, M.-C. Jung, E. A. Wierenga, S. Scholz, T. Santantonio, M. Houghton, S. Southwood, A. Sette, and G. R. Pape. 1997. Immunodominant CD4+ T-cell epitope within nonstructural protein 3 in acute hepatitis C virus infection. J. Virol. 71:6011-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diepolder, H. M., R. Zachoval, R. M. Hoffmann, E. A. Wierenga, T. Santantonio, M. C. Jung, D. Eichenlaub, and G. R. Pape. 1995. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet 346:1006-1007. [DOI] [PubMed] [Google Scholar]
- 12.Erickson, A. L., Y. Kimura, S. Igarashi, J. Eichelberger, M. Houghton, J. Sidney, D. McKinney, A. Sette, A. L. Hughes, and C. M. Walker. 2001. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity 15:883-895. [DOI] [PubMed] [Google Scholar]
- 13.Farci, P., H. J. Alter, D. C. Wong, R. H. Miller, S. Govindarajan, R. Engle, M. Shapiro, and R. H. Purcell. 1994. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc. Natl. Acad. Sci. USA 91:7792-7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farci, P., A. Shimoda, D. Wong, T. Cabezon, D. De Gioannis, A. Strazzera, Y. Shimizu, M. Shapiro, H. J. Alter, and R. H. Purcell. 1996. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc. Natl. Acad. Sci. USA 93:15394-15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forns, X., P. J. Payette, X. Ma, W. Satterfield, G. Eder, I. K. Mushahwar, S. Govindarajan, H. L. Davis, S. U. Emerson, R. H. Purcell, and J. Bukh. 2000. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology 32:618-625. [DOI] [PubMed] [Google Scholar]
- 16.Fournillier, A., E. Depla, P. Karayiannis, O. Vidalin, G. Maertens, C. Trépo, and G. Inchauspé. 1999. Expression of noncovalent hepatitis C virus envelope E1-E2 complexes is not required for the induction of antibodies with neutralizing properties following DNA immunization. J. Virol. 73:7497-7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fournillier, A., I. Nakano, L. Vitvitski, E. Depla, O. Vidalin, G. Maertens, C. Trepo, and G. Inchauspe. 1998. Modulation of immune responses to hepatitis C virus envelope E2 protein following injection of plasmid DNA using single or combined delivery routes. Hepatology 28:237-244. [DOI] [PubMed] [Google Scholar]
- 18.Gruner, N. H., T. J. Gerlach, M. C. Jung, H. M. Diepolder, C. A. Schirren, W. W. Schraut, R. Hoffmann, R. Zachoval, T. Santantonio, M. Cucchiarini, A. Cerny, and G. R. Pape. 2000. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J. Infect. Dis. 181:1528-1536. [DOI] [PubMed] [Google Scholar]
- 19.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 20.He, X. S., B. Rehermann, F. X. Lopez-Labrador, J. Boisvert, R. Cheung, J. Mumm, H. Wedemeyer, M. Berenguer, T. L. Wright, M. M. Davis, and H. B. Greenberg. 1999. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. USA 96:5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibe, M., T. Sakaguchi, K. Tanaka, S. Saito, S. Yokota, T. Tanaka, K. Shimotohno, Y. Chujoh, Y. Shiratori, M. Omata, K. Miwa, and M. Takiguchi. 1998. Identification and characterization of a cytotoxic T cell epitope of hepatitis C virus presented by HLA-B*3501 in acute hepatitis. J. Gen. Virol. 79:1735-1744. [DOI] [PubMed] [Google Scholar]
- 22.Inchauspe, G., S. Zebedee, D. H. Lee, M. Sugitani, M. Nasoff, and A. M. Prince. 1991. Genomic structure of the human prototype strain H of hepatitis C virus: comparison with American and Japanese isolates. Proc. Natl. Acad. Sci. USA 88:10292-10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojima, M., T. Osuga, F. Tsuda, T. Tanaka, and H. Okamoto. 1994. Influence of antibodies to the hypervariable region of E2/NS1 glycoprotein on the selective replication of hepatitis C virus in chimpanzees. Virology 204:665-672. [DOI] [PubMed] [Google Scholar]
- 24.Koziel, M. J., D. Dudley, N. Afdhal, A. Grakoui, C. M. Rice, Q. L. Choo, M. Houghton, and B. D. Walker. 1995. HLA class I-restricted cytotoxic T lymphocytes specific for hepatitis C virus. Identification of multiple epitopes and characterization of patterns of cytokine release. J. Clin. Investig. 96:2311-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lechmann, M., H. G. Ihlenfeldt, I. Braunschweiger, G. Giers, G. Jung, B. Matz, R. Kaiser, T. Sauerbruch, and U. Spengler. 1996. T- and B-cell responses to different hepatitis C virus antigens in patients with chronic hepatitis C infection and in healthy anti-hepatitis C virus-positive blood donors without viremia. Hepatology 24:790-795. [DOI] [PubMed] [Google Scholar]
- 26.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang, T. J., B. Rehermann, L. B. Seeff, and J. H. Hoofnagle. 2000. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann. Intern. Med. 132:296-305. [DOI] [PubMed] [Google Scholar]
- 28.Martell, M., J. I. Esteban, J. Quer, J. Genescà, A. Weiner, R. Esteban, J. Guardia, and J. Gómez. 1992. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J. Virol. 66:3225-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Missale, G., R. Bertoni, V. Lamonaca, A. Valli, M. Massari, C. Mori, M. G. Rumi, M. Houghton, F. Fiaccadori, and C. Ferrari. 1996. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J. Clin. Investig. 98:706-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mooij, P., and J. L. Heeney. 2001. Rational development of prophylactic HIV vaccines based on structural and regulatory proteins. Vaccine 20:304-321. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto, H., M. Kojima, S. Okada, H. Yoshizawa, H. Iizuka, T. Tanaka, E. E. Muchmore, D. A. Peterson, Y. Ito, and S. Mishiro. 1992. Genetic drift of hepatitis C virus during an 8.2-year infection in a chimpanzee: variability and stability. Virology 190:894-899. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto, H., S. Mishiro, H. Tokita, F. Tsuda, Y. Miyakawa, and M. Mayumi. 1994. Superinfection of chimpanzees carrying hepatitis C virus of genotype II/1b with that of genotype III/2a or I/1a. Hepatology 20:1131-1136. [PubMed] [Google Scholar]
- 33.Rehermann, B., K.-M. Chang, J. McHutchison, R. Kokka, M. Houghton, C. M. Rice, and F. V. Chisari. 1996. Differential cytotoxic T-lymphocyte responsiveness to the hepatitis B and C viruses in chronically infected patients. J. Virol. 70:7092-7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen, H. R., C. Miner, A. W. Sasaki, D. M. Lewinsohn, A. J. Conrad, A. Bakke, H. G. Bouwer, and D. J. Hinrichs. 2002. Frequencies of HCV-specific effector CD4+ T cells by flow cytometry: correlation with clinical disease stages. Hepatology 35:190-198. [DOI] [PubMed] [Google Scholar]
- 35.Simmonds, P., E. C. Holmes, T. A. Cha, S. W. Chan, F. McOmish, B. Irvine, E. Beall, P. L. Yap, J. Kolberg, and M. S. Urdea. 1993. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J. Gen. Virol. 74:2391-2399. [DOI] [PubMed] [Google Scholar]
- 36.Thimme, R., J. Bukh, H. C. Spangenberg, S. Wieland, J. Pemberton, C. Steiger, S. Govindarajan, R. H. Purcell, and F. V. Chisari. 2002. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc. Natl. Acad. Sci. USA 99:15661-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomson, M., M. Nascimbeni, M. B. Havert, M. Major, S. Gonzales, H. Alter, S. M. Feinstone, K. K. Murthy, B. Rehermann, and T. J. Liang. 2003. The clearance of hepatitis C virus infection in chimpanzees may not necessarily correlate with the appearance of acquired immunity. J. Virol. 77:862-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Akker, R., M. Balls, J. W. Eichberg, J. Goodall, J. L. Heeney, A. D. Osterhaus, A. M. Prince, and I. Spruit. 1993. Chimpanzees in AIDS research: a biomedical and bioethical perspective. J. Med. Primatol. 22:390-392. [PubMed] [Google Scholar]
- 40.Vertuani, S., M. Bazzaro, G. Gualandi, F. Micheletti, M. Marastoni, C. Fortini, A. Canella, M. Marino, R. Tomatis, S. Traniello, and R. Gavioli. 2002. Effect of interferon-alpha therapy on epitope-specific cytotoxic T lymphocyte responses in hepatitis C virus-infected individuals. Eur. J. Immunol. 32: 144-154. [DOI] [PubMed] [Google Scholar]
- 41.Vidalin, O., A. Fournillier, N. Renard, M. Chen, E. Depla, D. Boucreux, C. Brinster, T. Baumert, I. Nakano, Y. Fukuda, P. Liljestrom, C. Trepo, and G. Inchauspe. 2000. Use of conventional or replicating nucleic acid-based vaccines and recombinant Semliki Forest virus-derived particles for the induction of immune responses against hepatitis C virus core and E2 antigens. Virology 276:259-270. [DOI] [PubMed] [Google Scholar]
- 42.Walker, C. M. 1997. Comparative features of hepatitis C virus infection in humans and chimpanzees. Springer Semin. Immunopathol. 19:85-98. [DOI] [PubMed] [Google Scholar]
- 43.Ward, S., G. Lauer, R. Isba, B. Walker, and P. Klenerman. 2002. Cellular immune responses against hepatitis C virus: the evidence base 2002. Clin. Exp. Immunol. 128:195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wasley, A., and M. J. Alter. 2000. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin. Liver Dis. 20:1-16. [DOI] [PubMed] [Google Scholar]
- 45.Wedemeyer, H., X. S. He, M. Nascimbeni, A. R. Davis, H. B. Greenberg, J. H. Hoofnagle, T. J. Liang, H. Alter, and B. Rehermann. 2002. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 169:3447-3458. [DOI] [PubMed] [Google Scholar]
- 46.Wong, D. K., D. D. Dudley, N. H. Afdhal, J. Dienstag, C. M. Rice, L. Wang, M. Houghton, B. D. Walker, and M. J. Koziel. 1998. Liver-derived CTL in hepatitis C virus infection: breadth and specificity of responses in a cohort of persons with chronic infection. J. Immunol. 160:1479-1488. [PubMed] [Google Scholar]