Abstract
Background
Cerebrospinal fluid (CSF) white blood cell (WBC) counts for neonates and young infants are usually interpreted based on values reported in reference texts or handbooks. However, current reference texts either present normal CSF parameters without citation or cite studies with significant limitations.
Objective
To determine accurate, age-specific reference values for CSF WBC counts in a large population of neonates and young infants.
Methods
This cross-sectional study included patients ≤56 days who had a lumbar puncture performed in the emergency department from January 1, 2005 – June 30, 2007. Patients were excluded from analysis for conditions suspected to cause CSF pleocytosis including traumatic lumbar puncture, serious bacterial infection, congenital infection, seizure, and presence of a ventricular shunt. Children who tested positive for enterovirus in the CSF by polymerase chain reaction were also excluded. Two-sample Wilcoxon rank-sum tests were used to compare median CSF WBC values of those with negative enterovirus testing to those without enterovirus testing.
Results
380 (36%) of 1064 patients met inclusion criteria; 54% were male, 15% were preterm, and 39% presented during enterovirus season. The median CSF WBC count was significantly higher in infants ≤28 days old (3/mm3, 95th percentile: 19/mm3) than in infants 29–56 days old (2/mm3, 95th percentile: 9/mm3, p<0.001). In both age groups, infants with a negative enterovirus PCR had a higher upper bound of the 95% confidence interval of the mean values compared with infants who did not have enterovirus testing performed.
Conclusions
We determined age specific CSF WBC reference values in a large cohort of neonates and young infants that can be used to accurately interpret the results of lumbar punctures in this population.
Keywords: cerebrospinal fluid; infant, newborn; lumbar puncture; reference values
INTRODUCTION
Lumbar puncture and analysis of cerebrospinal fluid (CSF) aids clinicians in identifying patients with meningitis or encephalitis and, therefore, requires knowledge of accurate reference values for white blood cell (WBC) counts. Determining normal CSF values for young infants and neonates is challenging. It is unethical to evaluate healthy children with a painful, potentially harmful procedure at a time when they cannot provide either written consent or verbal assent. Therefore, reference values must be based on children who are not truly normal; generally, values are determined from infants who undergo lumbar puncture for suspicion of meningitis.
Reference literature is widely available to guide clinicians interpreting the results of a lumbar puncture. Textbooks and handbooks of general pediatrics,1–3 infectious diseases,4, 5 hospital medicine,6–8 emergency medicine,9, 10 neonatology11 and neurology12, 13 outline normal CSF parameters. These parameters are based on other reference texts or on studies with important limitations. Previous studies determined reference values based on children considered healthy after initial evaluation for infection of the central nervous system. However, few patients ≤56 days of age have been studied, and different ranges of reference values have been established as authors have used varying exclusion criteria. These past studies included children with traumatic lumbar puncture,14–20 seizures,17 sepsis,21 congenital infections19, 22 and very low birth weights.23
Most previous work was also conducted at a time when molecular tools were not routinely used in clinical practice.15–26 Polymerase chain reaction (PCR) tests to detect viral genomes allow more accurate identification of infected neonates and young infants. Only one study excluded those children with CSF positive for enterovirus by PCR.27 The objective of our study was to determine clinically relevant CSF WBC reference values in a large cohort of children less than or equal to 56 days of age with strict exclusion criteria and the incorporation of results of PCR tests.
METHODS
Study design and setting
This cross-sectional study was performed at the Children’s Hospital of Philadelphia (Philadelphia, PA), an urban, tertiary care children’s hospital. The Committees for the Protection of Human Subjects of The Children’s Hospital of Philadelphia approved this study with a waiver of informed consent.
Review of literature
A systematic review of literature defining normal CSF WBC counts was carried out through an Ovid Medline search of all work published prior to September 1, 2008. The initial search term was “cerebrospinal fluid,” which was then combined with “white blood cell” or “leukocytosis,” and either “neonate,” “infant” or “newborn.” Limits included “humans” and “English language.” Titles and abstracts of 300 articles were reviewed for relevance: 5 studies were found to be relevant.17, 21, 23, 25, 27 Nine additional studies were identified during review of the references of textbooks and published studies.14–16, 18–20, 22, 24, 26
Study population
Infants less than or equal to 56 days of age were eligible for inclusion if they had a lumbar puncture performed as part of their emergency department evaluation between January 1, 2005 and June 30, 2007. Children in this age range were selected as they routinely undergo lumbar puncture when presenting with fever at our institution.28, 29 Patients undergoing lumbar puncture in the emergency department were identified using two different data sources to ensure accurate identification of all eligible infants: 1.) Emergency department computerized order entry records identified all infants with cerebrospinal fluid testing (including CSF gram stain, culture, cell count, glucose or protein) performed during the study period, and 2.) Clinical Virology Laboratory records identified all infants in whom CSF herpes simplex virus or enterovirus PCR testing was performed. Medical records of infants identified by these two sources were reviewed to determine study eligibility.
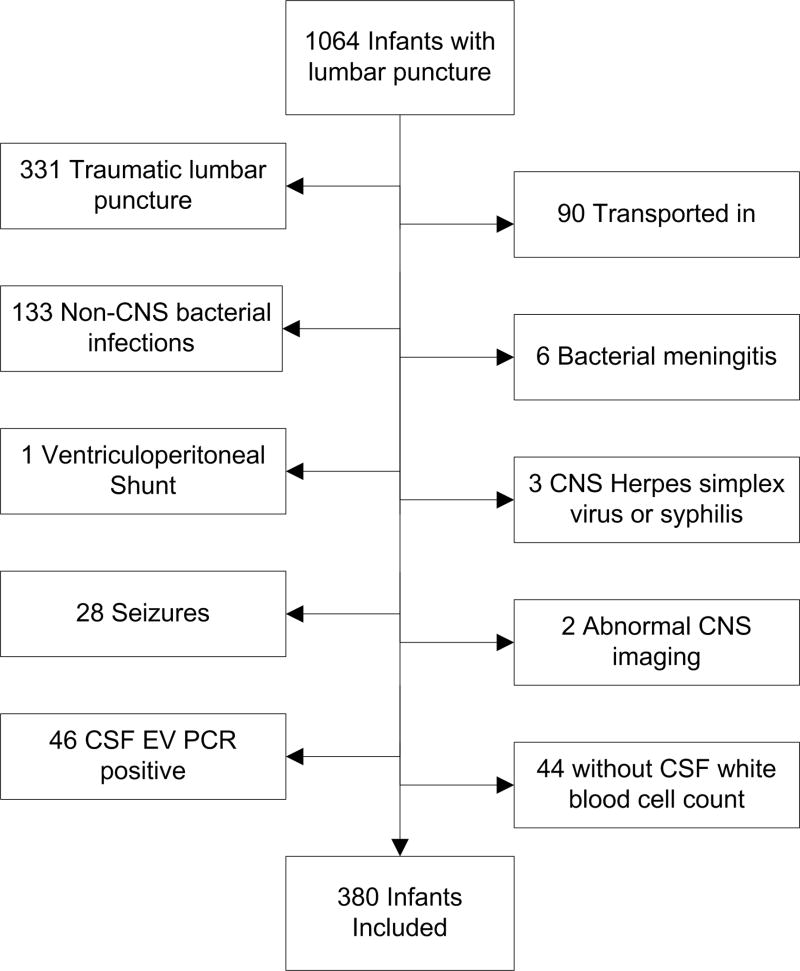
Figure 1 outlines major exclusion criteria used to derive the reference group. Patients were excluded sequentially if the lumbar puncture was traumatic or a condition known or suspected to cause CSF pleocytosis was present. In a traumatic lumbar puncture, the presence of red blood cells in the CSF alters WBC counts, and adjustment formulas cannot reliably approximate the actual values.30–33 Conditions known or suspected to cause CSF pleocytosis include stroke, hydrocephalus, seizure on presentation, ventricular shunt or previous intracranial infection, congenital infection, herpes simplex virus meningoencephalitis, and bacterial meningitis.34–36 Patients with serious bacterial illness including bacteremia, urinary tract infection, osteomyelitis, septic arthritis, pneumonia and bacterial gastroenteritis were also excluded as studies have identified CSF pleocytosis with non-central nervous system infections.36–38 Infants may have met more than one of the exclusion criteria.
Figure 1.
Infants ≤56 days of age presenting to an emergency department with an indication for lumbar puncture and indication for exclusion from the study population.
The remaining infants were divided based on whether or not testing for enterovirus was performed in the CSF by polymerase chain reaction and, if performed, whether the test result was positive or negative. Details of our approach to enterovirus PCR testing have been published previously.39 As viral meningitis can cause CSF pleocytosis, patients with a positive CSF enterovirus PCR were excluded from the reference group.40, 41 While previous studies have examined preterm infants separately from term infants, CSF WBC counts are influenced by postnatal rather than postgestational age.42 Our primary analysis, therefore, combined preterm and term infants into a single group.
Study definitions
Prematurity was defined as a gestational age of less than 37 weeks. Fever was defined as presence of temperature ≥ 38.0°C. “History of fever” was defined as report of tactile temperature or recorded temperature greater than 38.0°C at home. Traumatic lumbar puncture was defined as the presence of 500 or more red blood cells/mm3 in the CSF.43 Seizure was defined by clinical description of the event within 48 hours of presentation, or by an electroencephalogram demonstrating epileptiform activity. Bacterial meningitis was defined as the isolation of a bacterial pathogen from the CSF. As CSF sterilization may occur after antibiotic administration,44 if antibiotics were administered prior to lumbar puncture, bacterial meningitis was defined as bacteria on CSF Gram stain with low CSF glucose or elevated CSF protein values. Bacteremia was defined as isolation of a known bacterial pathogen from blood culture; isolates that reflected commensal skin flora such as coagulase-negative staphylococci were considered contaminants.
Urinary tract infection was defined as growth of a single known pathogen meeting 1 of 3 criteria: 1) ≥1000 colony-forming units (cfu)/mL for urine cultures obtained by suprapubic aspiration, 2) ≥50,000 cfu/mL from a catheterized specimen, or 3) ≥10,000 cfu/mL from a catheterized specimen in association with a positive urinalysis.45 Positive urinalysis was defined as one or more of the following: 1) trace or greater result for leukocyte esterase and/or nitrite on dipstick, 2) >9 WBC per high-power field on standard microscopic examination of centrifuged urine, or 3) >10 WBC/mm3 by hemocytometer count of uncentrifuged urine.46, 47
Osteomyelitis was defined as the growth of pathogenic bacteria from blood, bone or subperiosteal aspirate culture in a patient with fever and localized tenderness, edema or erythema overlying the suspected site of bony infection and compatible radiologic findings. Septic arthritis was defined as the growth of pathogenic bacteria from synovial fluid or blood culture in a patient with purulent joint fluid or positive results of Gram staining of joint fluid.48 Bacterial pneumonia was defined as a new discrete infiltrate on chest radiograph with growth of a respiratory bacterial pathogen from blood culture.28 Enterovirus season was defined as June 1–October 31 in each year.
Data collection
Data were abstracted from the medical records of study patients and entered onto a standardized data collection form. Information collected included demographics, vital signs, clinical and historical findings on presentation, birth history, empiric antibiotic use, imaging studies performed, and results of laboratory testing.
Data analysis
Data were analyzed using STATA/SE, version 10 (Stata Corporation, College Station, Texas). The primary variable considered was CSF WBC count. Continuous variables were described using mean, median, interquartile range (IQR), 90th and 95th percentile values and compared using the Wilcoxon rank-sum test.
RESULTS
Of 1064 infants identified for the study, 380 (36%) met inclusion criteria [Figure 1]. Most patients (54%) were male; 15% were preterm, 39% presented during enterovirus season and 80% had fever on presentation. Forty-three (11.3%) infants received antibiotics prior to lumbar puncture. The median length of hospital stay was 2 days [Table 1].
Table 1.
Characteristics of eligible infants.*
| 0–28 Days (N=142) | 29–56 Days (N=238) | |
|---|---|---|
| Male Gender | 82 (58%) | 125 (52%) |
| White | 47 (34%) | 64 (29%) |
| Nonhispanic | 136 (96%) | 215 (90%) |
| Fever on presentation | 92 (65%) | 213 (90%) |
| Preterm | 22 (15%) | 35 (15%) |
| Presentation during EV season | 66 (46%) | 82 (34%) |
| Peripheral WBC Median* | 10.7×103/mm3 (8.2–14.2) | 9.3×103/mm3 (7.2–12.7) |
| Admitted | 140 (98.6%) | 168 (70.5%) |
| Median length of Hospital Stay* | 2 days (2–3) | 2 days (1–2.5) |
Values presented as number (percent) or median (interquartile range).
Abbreviations: EV, enterovirus. WBC, white blood cell.
Infants 0–28 days of age had a median CSF WBC of 3/mm3 with a 95th percentile value of 19/mm3 whereas infants ages 29–56 days of age had a statistically lower median CSF WBC count of 2/mm3 with a 95th percentile value of 9/mm3 (p<0.001) [Table 2]. Within age groups, we subsequently compared patients who tested negative for enterovirus by PCR with those who did not have enterovirus PCR testing performed. In the 0–28 days of age group, infants who had negative CSF enterovirus PCR had a median CSF WBC of 4/mm3 [Table 3]. This value was significantly higher than those who did not have enterovirus testing performed within enterovirus season or outside of enterovirus season. [Figure 2a]. In the 29–56 days of age group, infants who had their CSF tested for enterovirus with a negative result had a median CSF WBC of 2.5/mm3 [Table 3]. This value was higher than the median CSF WBC count of those who did not have enterovirus testing performed within enterovirus season, though the differences were not statistically significant [Figure 2b]. In both age ranges, there were no significant differences between infants evaluated within or outside of enterovirus season when enterovirus CSF PCR testing was not performed.
Table 2.
Cerebrospinal fluid white blood cell counts†
| 0–28 Days (n=142) | 29–56 Days (n=238) | |
|---|---|---|
| Mean value (standard deviation) | 9.2 (32.1) | 3.1 (5.0) |
| Upper bound of 95% confidence interval of the mean value | 14.5 | 3.8 |
| Median value* | 3 | 2 |
| 90th percentile value | 12 | 6 |
| 95th percentile value | 19 | 9 |
| Interquartile range | 2–6 | 1–3 |
All units are/mm3
Difference in median values p<0.001, Wilcoxon Rank-Sum test
Table 3.
Cerebrospinal fluid white blood cell counts stratified by enterovirus testing and season for patients a.) 0–28 days of age and b.) 29–56 days of age.
Table 3a. Infants Ages 0–28 Days of Age†
| EV Negative All Seasons (N=37) | CSF EV PCR Not Sent EV Season (N=35) | CSF EV PCR Not Sent Not EV Season (N=70) | |
|---|---|---|---|
| Mean value (SD) | 12.6 | 4.8 | 9.5 |
| Upper bound of 95% confidence interval of the mean value | 23.8 | 6.8 | 18.7 |
| Median value | 4 | 3* | 3** |
| 90th percentile value | 14 | 15 | 11.5 |
| 95th percentile value | 78 | 19 | 19 |
| Interquartile range | 3–8 | 1–6 | 1–4 |
All units are/mm3
p=0.034 (compared to median value of patients who tested negative for enterovirus)
p=0.009 (compared to median value of patients who tested negative for enterovirus), Wilcoxon Rank-Sum test.
Abbreviations: CSF, cerebrospinal fluid. SD, standard deviation. EV, enterovirus. PCR, polymerase chain reaction.
Figure 2.
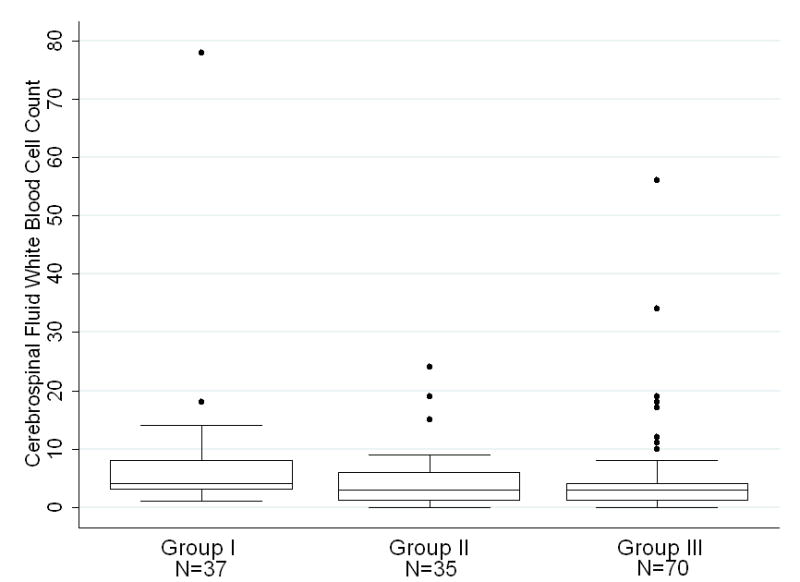
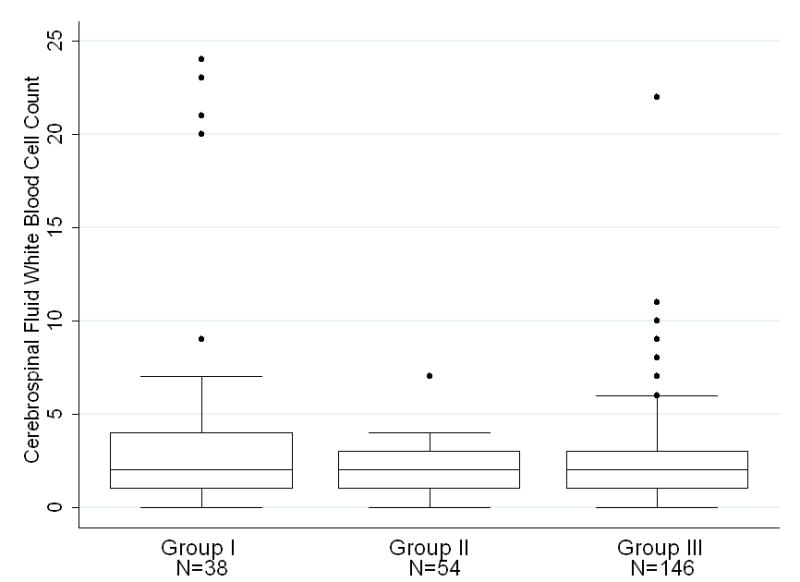
Figure 2a. Patients ages 0–28 days stratified by CSF EV PCR status and season. Group I represents neonates who tested negative for enterovirus by PCR during all seasons. Group II represents neonates who were not tested for enterovirus, during enterovirus season. Group III represents neonates who were not tested for enterovirus outside of enterovirus season. On each box plot, the box spans the interquartile range (IQR), the horizontal line through the box is the median value, and the whiskers denote outlying values, defined by convention as 1.5 IQRs lower than the first quartile and 1.5 IQRs higher than the third quartile. Dots represent individual patient values that fall beyond 1.5 IQRs. Data from two patients with CSF WBC values of 320/mm3 and 198/mm3 are not shown for clarity of presentation.
Figure 2b. Patients ages 29–56 days stratified by CSF EV PCR status and season. Group I represents infants who tested negative for enterovirus by PCR during all seasons. Group II represents infants who were not tested for enterovirus, during enterovirus season. Group III represents infants who were not tested for enterovirus outside of enterovirus season. On each box plot, the box spans the interquartile range (IQR), the horizontal line through the box is the median value, and the whiskers denote outlying values, defined by convention as 1.5 IQRs lower than the first quartile and 1.5 IQRs higher than the third quartile. Dots represent individual patient values that fall beyond 1.5 IQRs. Data from two patients with CSF WBC values of 34/mm3 and 43/mm3 are not shown for clarity of presentation.
We also compared CSF WBC counts between infants with and without fever. Among infants ≤28 days of age, differences in the median CSF WBC count values between infants with fever (median, 3/mm3; 95th percentile, 19/mm3) and without fever (median, 4/mm3; 95th percentile, 18/mm3) were not statistically significant (p=0.27, Wilcoxon rank-sum test). Among infants 29–56 days of age, the median CSF WBC count was lower in infants with fever (median, 2/mm3; 95th percentile, 9/mm3) compared with infants without fever (median, 3/mm3; 95th percentile, 20/mm3) (p=0.04, Wilcoxon rank-sum test), though the latter group included only 25 infants.
Several infants had modestly elevated CSF WBC counts without an identifiable cause. A patient with a CSF WBC count of 320/mm3 did not have sufficient CSF obtained for enterovirus PCR but had a mononuclear cell predominance (i.e., 40% lymphocytes and 33% monocytes) and was discharged with a diagnosis of probable aseptic meningitis. A patient with a CSF WBC count of 198/mm3 had a negative CSF enterovirus PCR and was treated for presumed bacterial meningitis, although all cultures were negative. Two patients with CSF WBC counts of 43/mm3 and 34/mm3 had negative CSF enterovirus PCR and were discharged home after two days.
The 57 preterm infants had a median CSF WBC of 3/mm3. Those ages 0–28 days (N=22) had a median CSF WBC of 3/mm3 and a 95th percentile of 19/mm3, which was not statistically different (p=0.58, Wilcoxon rank-sum test) than the comparable term group. Those ages 29–56 days (N=35) had a median CSF WBC of 2/mm3 with a 95th percentile of 7/mm3, which was not statistically different than term 29–56 day old infants (p=0.43, Wilcoxon rank-sum test).
DISCUSSION
Our study establishes reference values for cerebrospinal fluid white blood cell counts in neonates and young infants to bring literature up to date at a time when molecular tools are commonly used in clinical practice. Previous studies have based reference ranges on those infants with an indication for a lumbar puncture, as it is not ethical to test healthy infants. However, those studies have had small sample sizes and have included patients with conditions often associated with CSF pleocytosis, including traumatic lumbar puncture, seizure, and congenital infections.
Our study was designed to address the limitations of previous studies that are frequently cited by reference texts. Sarff et al18 enrolled 87 term children less than 10 days of age; while all of the infants had sterile urine, blood and CSF, the exclusion criteria for a traumatic lumbar puncture was defined as “gross blood.” CSF red blood cell counts up to 45,000/mm3 are documented by Sarff et al, obscuring the validity of those reference values. Portnoy and Olson17 enrolled 131 children under 3 months of age; while enrolled infants had negative CSF bacterial and viral cultures, infants with bacteremia, urinary tract infections and seizures were included. Additionally, traumatic lumbar puncture was not specified as an exclusion criterion. Bonadio et al24 applied strict exclusion criteria including only those infants with negative blood, urine, and CSF cultures, yet had a small population with only 75 infants under 8 weeks of age enrolled.24 Ahmed et al27 also used strict exclusion criteria and was the first study to incorporate PCR testing, but only enrolled patients under 30 days of age. Our approach overcomes many of the limitations of previous studies to establish reference values for infants who routinely undergo lumbar puncture. The values established in this study are based on the largest population of neonates and young infants identified to date, and we incorporated results of CSF enterovirus PCR testing to accurately define an upper limit of normal for use by clinicians.
For infants 0–28 days of age, the reference values that we observed are a median value of 3/mm3 with a 90th percentile value of 12/mm3. In this age range, Bonadio et al documented median values of 8.5/mm3 with a 90th percentile value of 22/mm3. The values proposed by Bonadio may be higher due to a smaller sample size, the inability to integrate PCR testing, and use of a higher CSF red blood cell count cut-off value (i.e., >1000 red blood cells/mm3) to define traumatic lumbar puncture.24 Our work confirms the values found by Ahmed et al in 108 infants <30 days of age, which determined a median CSF WBC of 4/mm3 with a 90th percentile value of 11/mm3.27 The 95th percentile value in our study was 19/mm3; Ahmed et al did not report comparable data.
As CSF WBC counts are age dependent,24 it is imperative to examine each age group independently. Infants 29–56 days of age must also have age-dependent reference values, yet reference CSF WBC counts for this population have only been specifically studied once in a cohort of 40 patients with a median CSF WBC value of 4.5/mm3 and a 90th percentile value of 15/mm3.24 Viral PCR was not used at that time, and the sample size was insufficient to guide practice. The values established in our study can now be used for this patient population.
The observation that those patients who tested negative for enterovirus by PCR had consistently higher median CSF WBC values than those who were not tested was unforeseen. Ideally, those with negative enterovirus testing represent the noninfected neonates and most closely approximate normal. However, as this was a retrospective study, those with enterovirus testing may merely represent a group with a CSF WBC count on the higher end of normal whose CSF was sent for enterovirus PCR testing because of physician concern for infection. Alternatively, the higher CSF WBC counts of these patients may in fact represent viral infections, such as parechovirus, that are not yet part of routine testing.49 As our understanding of viral pathogens evolves, our value for the upper limit of normal for CSF WBC counts may also change. Finally, while a statistical difference exists between infants with negative enterovirus testing and infants who did not have testing done, the differences are small and the clinical management of both groups is likely the same.
Several patients had CSF WBC counts far outside the range most clinicians would consider “normal.” While we assume that CSF WBC counts such as 320/mm3 and 198/mm3 represent pathology, we chose to include these patients because the aim of our study was to define ranges of normal and none of these patients had identifiable conditions that warranted exclusion. With that in mind, outliers were included in analysis and the 90th and 95th percentile values are documented to guide physicians in using these reference values.
This study had several limitations. First, not all young infants and neonates had enterovirus testing, and viral culture of the CSF was not performed. Therefore, patients without testing may have viral meningitis, and those with negative testing may have another virus not identified with PCR tests routinely used at our institution. The misclassification of infants with aseptic meningitis as uninfected may lead to an overestimate of the upper bound of the reference values determined in this study. However, many infants in the first few months of life with documented viral infection of the central nervous system lack CSF WBC abnormalities,41, 50, 51 mitigating the impact of such misclassification. Second, certain patients received antibiotics prior to lumbar puncture, leaving open the rare possibility that a child with bacterial meningitis was included in the study population; this approach could falsely elevate our reference values. This possibility is unlikely because bacterial meningitis is rare and we incorporated results of CSF Gram stain, glucose and protein into our definition of meningitis in patients receiving antibiotics prior to lumbar puncture.
Third, this was an observational study so physicians selected which infants underwent lumbar puncture. As it is routine practice in our emergency department to perform a lumbar puncture on all febrile infants, it is likely that most febrile infants 56 days of age or younger had a lumbar puncture regardless of presentation, and our population does not represent only ill-appearing patients. Fourth, this is a single center study. If different viral pathogens have a different propensity to cause CSF WBC elevations, then generalizability of our study results may be influenced by the variability of causative viral pathogens by region. Fifth, this study includes preterm infants who are categorized by postnatal age; while these infants demonstrate an age dependant decline in CSF WBC counts which is not statistically different from term infants, it is not known if changes in their physiology are identical to term infants. Finally, though our study objective was to define age-specific reference values for CSF WBC counts in neonates and young infants, we recognize that infants with CSF WBC counts within the reference ranges defined in this study may still have serious infection.
CSF WBC counts are routinely measured in ill neonates. The age-dependent reference values presented in this study serve to guide clinicians in determining when the CSF WBC count of an ill neonate is above the upper limits of normal.
Table 3b.
Cerebrospinal fluid white blood cell counts stratified by enterovirus testing and season for patients a.) 0–28 days of age and b.) 29–56 days of age.
Infants Ages 29–56 Dates of Age†
| EV Negative All Seasons (N=38) | CSF EV PCR Not Sent EV Season (N=54) | CSF EV PCR Not Sent Not EV Season (N=146) | |
|---|---|---|---|
| Mean value (SD) | 6.8 | 2.2 | 2.5 |
| Upper bound of 95% confidence interval of the mean value | 10.3 | 2.7 | 3.0 |
| Median value | 2.5 | 2* | 2** |
| 90th percentile value | 23 | 4 | 6 |
| 95th percentile value | 34 | 7 | 7 |
| Interquartile range | 1–7 | 1–3 | 1–3 |
All units are/mm3
p<0.16 (compared to median value of patients who tested negative for enterovirus)
p<0.12 (compared to median value of patients who tested negative for enterovirus), Wilcoxon Rank-Sum test.
Abbreviations: CSF, cerebrospinal fluid; SD, standard deviation; EV, enterovirus; PCR, polymerase chain reaction
Abbreviations Used in Manuscript
- CSF
Cerebrospinal fluid
- IQR
Interquartile range
- PCR
Polymerase chain reaction
- WBC
White blood cell count
Footnotes
Financial Disclosures: Dr. Kestenbaum received support from the Clinical Neurosciences Scholars Track at the University of Pennsylvania School of Medicine. Dr. Shah received support from the National Institute of Allergy and Infectious Diseases (K01 AI73729) and the Robert Wood Johnson Foundation under its Physician Faculty Scholar Program.
References
- 1.Behrman RE, Kliegman R, Jenson HB. Nelson Textbook of Pediatrics. 17. Philadelphia: Saunders; 2004. [Google Scholar]
- 2.McMillan JA, Feigin RD, DeAngelis C, Jones MD. Oski’s pediatrics: principles & practice. 4. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 3.Robertson J, Shilkofski N, editors. Johns Hopkins: The Harriet Lane Handbook: A Manual for Pediatric House Officers. 17. Philadelphia: Elsevier Mosby; 2005. [Google Scholar]
- 4.Feigin RD, Cherry JD, Demmler GJ, Kaplan SL. Textbook of Pediatric Infectious Diseases. 5. Philadelphia: Saunders; 2004. [Google Scholar]
- 5.Remington JS, Klein JO. Infectious Diseases of the Fetus and Newborn Infant. 6. Philadelphia: Elsevier Saunders; 2006. [Google Scholar]
- 6.Frank G, Shah SS, Catallozzi MC, Zaoutis LB. The Philadelphia Guide: Inpatient Pediatrics. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 7.Perkin RM, Swift JD, Newton DA, Anas NG. Pediatric Hospital Medicine: Textbook of Inpatient Management. Philadelphia; London: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 8.Zaoutis LB, Chiang VW. Comprehensive pediatric hospital medicine. Philadelphia: Mosby Elsevier; 2007. [Google Scholar]
- 9.Baren JM, Brennan JA, LB, Rothrock SG. Pediatric Emergency Medicine. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 10.Fleisher GR, Ludwig S, Henretig F. Textbook of Pediatric Emergency Medicine. 5. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 11.Taeusch HW, Ballard RA. Avery’s diseases of the newborn. 7. Philadelphia: Saunders; 1998. [Google Scholar]
- 12.Menkes JH, Sarnat HB. Child Neurology. 6. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 13.Swaiman KF, Ashwal S. Pediatric Neurology: Principles and Practice. 3. St. Louis: Mosby; 1999. [Google Scholar]
- 14.Stewart D. The normal cerebro-spinal fluid in children. Archives of Disease in Childhood. 1928:96–108. doi: 10.1136/adc.3.14.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Widell S. On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo-encephalitis. Acta Paediatr Suppl. 1958;47:1–102. [PubMed] [Google Scholar]
- 16.Naidoo BT. The cerebrospinal fluid in the healthy newborn infant. S Afr Med J. 1968;42:933–5. [PubMed] [Google Scholar]
- 17.Portnoy JM, Olson LC. Normal cerebrospinal fluid values in children: another look. Pediatrics. 1985;75:484–7. [PubMed] [Google Scholar]
- 18.Sarff LD, Platt LH, McCracken GH., Jr Cerebrospinal fluid evaluation in neonates: comparison of high-risk infants with and without meningitis. J Pediatr. 1976;88:473–7. doi: 10.1016/s0022-3476(76)80271-5. [DOI] [PubMed] [Google Scholar]
- 19.Roberts MH. The spinal fluid in the new-born. JAMA. 1925;85:500–3. [Google Scholar]
- 20.Wolf H, Hoepffner L. The cerebrospinal fluid in the newborn and premature infant. World Neurol. 1961;2:871–8. [PubMed] [Google Scholar]
- 21.Nascimento-Carvalho CM, Moreno-Carvalho OA. Normal cerebrospinal fluid values in full-term gestation and premature neonates. Arq Neuropsiquiatr. 1998;56:375–80. doi: 10.1590/s0004-282x1998000300005. [DOI] [PubMed] [Google Scholar]
- 22.Fielkow S, Reuter S, Gotoff SP. Cerebrospinal fluid examination in symptom-free infants with risk factors for infection. J Pediatr. 1991;119:971–3. doi: 10.1016/s0022-3476(05)83058-6. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez AF, Kaplan SL, Mason EO., Jr Cerebrospinal fluid values in the very low birth weight infant. J Pediatr. 1990;116:971–4. doi: 10.1016/s0022-3476(05)80663-8. [DOI] [PubMed] [Google Scholar]
- 24.Bonadio WA, Stanco L, Bruce R, Barry D, Smith D. Reference values of normal cerebrospinal fluid composition in infants ages 0 to 8 weeks. Pediatr Infect Dis J. 1992;11:589–91. doi: 10.1097/00006454-199207000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Ancel A, Garcia-Alix A, Salas S, Del Castillo F, Cabanas F, Quero J. Cerebrospinal fluid leucocyte counts in healthy neonates. Arch Dis Child Fetal Neonatal Ed. 2006;91:F357–8. doi: 10.1136/adc.2005.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pappu LD, Purohit DM, Levkoff AH, Kaplan B. CSF cytology in the neonate. Am J Dis Child. 1982;136:297–8. doi: 10.1001/archpedi.1982.03970400015004. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed A, Hickey SM, Ehrett S, et al. Cerebrospinal fluid values in the term neonate. Pediatr Infect Dis J. 1996;15:298–303. doi: 10.1097/00006454-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Baker MD, Bell LM. Unpredictability of serious bacterial illness in febrile infants from birth to 1 month of age. Arch Pediatr Adolesc Med. 1999;153:508–11. doi: 10.1001/archpedi.153.5.508. [DOI] [PubMed] [Google Scholar]
- 29.Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics. 1999;103:627–31. doi: 10.1542/peds.103.3.627. [DOI] [PubMed] [Google Scholar]
- 30.Mayefsky JH, Roghmann KJ. Determination of leukocytosis in traumatic spinal tap specimens. Am J Med. 1987;82:1175–81. doi: 10.1016/0002-9343(87)90221-x. [DOI] [PubMed] [Google Scholar]
- 31.Rubenstein JS, Yogev R. What represents pleocytosis in blood-contaminated (“traumatic tap”) cerebrospinal fluid in children? J Pediatr. 1985;107:249–51. doi: 10.1016/s0022-3476(85)80137-2. [DOI] [PubMed] [Google Scholar]
- 32.Bonadio WA, Smith DS, Goddard S, Burroughs J, Khaja G. Distinguishing cerebrospinal fluid abnormalities in children with bacterial meningitis and traumatic lumbar puncture. J Infect Dis. 1990;162:251–4. doi: 10.1093/infdis/162.1.251. [DOI] [PubMed] [Google Scholar]
- 33.Greenberg RG, Smith PB, Cotten CM, Moody MA, Clark RH, Benjamin DK., Jr Traumatic lumbar punctures in neonates: test performance of the cerebrospinal fluid white blood cell count. Pediatr Infect Dis J. 2008;27:1047–51. doi: 10.1097/INF.0b013e31817e519b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidley JW, Simon RP. Postictal pleocytosis. Ann Neurol. 1981;9:81–4. doi: 10.1002/ana.410090116. [DOI] [PubMed] [Google Scholar]
- 35.Haslam RHA. Neurologic Evaluation. In: Kleigman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson Textbook of Pediatrics. 18. Philadelphia: Saunders Elsevier; 2007. [Google Scholar]
- 36.Carraccio C, Blotny K, Fisher MC. Cerebrospinal fluid analysis in systemically ill children without central nervous system disease. Pediatrics. 1995;96:48–51. [PubMed] [Google Scholar]
- 37.Adler-Shohet FC, Cheung MM, Hill M, Lieberman JM. Aseptic meningitis in infants younger than six months of age hospitalized with urinary tract infections. Pediatr Infect Dis J. 2003;22:1039–42. doi: 10.1097/01.inf.0000100576.99266.07. [DOI] [PubMed] [Google Scholar]
- 38.Shah SS, Zorc JJ, Levine DA, Platt SL, Kuppermann N. Sterile cerebrospinal fluid pleocytosis in young infants with urinary tract infections. J Pediatr. 2008;153:290–2. doi: 10.1016/j.jpeds.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 39.Maus MV, Posencheg MA, Geddes K, et al. Detection of echovirus 18 in human breast milk. J Clin Microbiol. 2008;46:1137–40. doi: 10.1128/JCM.01991-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rorabaugh ML, Berlin LE, Heldrich F, et al. Aseptic meningitis in infants younger than 2 years of age: acute illness and neurologic complications. Pediatrics. 1993;92:206–11. [PubMed] [Google Scholar]
- 41.Mulford WS, Buller RS, Arens MQ, Storch GA. Correlation of cerebrospinal fluid (CSF) cell counts and elevated CSF protein levels with enterovirus reverse transcription-PCR results in pediatric and adult patients. J Clin Microbiol. 2004;42:4199–203. doi: 10.1128/JCM.42.9.4199-4203.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mhanna MJ, Alesseh H, Gori A, Aziz HF. Cerebrospinal fluid values in very low birth weight infants with suspected sepsis at different ages. Pediatr Crit Care Med. 2008;9:294–8. doi: 10.1097/01.PCC.0b013e31816c6e12. [DOI] [PubMed] [Google Scholar]
- 43.Mazor SS, McNulty JE, Roosevelt GE. Interpretation of traumatic lumbar punctures: who can go home? Pediatrics. 2003;111:525–8. [PubMed] [Google Scholar]
- 44.Kanegaye JT, Soliemanzadeh P, Bradley JS. Lumbar puncture in pediatric bacterial meningitis: defining the time interval for recovery of cerebrospinal fluid pathogens after parenteral antibiotic pretreatment. Pediatrics. 2001;108:1169–74. [PubMed] [Google Scholar]
- 45.Zorc JJ, Levine DA, Platt SL, et al. Clinical and demographic factors associated with urinary tract infection in young febrile infants. Pediatrics. 2005;116:644–8. doi: 10.1542/peds.2004-1825. [DOI] [PubMed] [Google Scholar]
- 46.Hoberman A, Wald ER, Penchansky L, Reynolds EA, Young S. Enhanced urinalysis as a screening test for urinary tract infection. Pediatrics. 1993;91:1196–9. [PubMed] [Google Scholar]
- 47.Shaw KN, McGowan KL, Gorelick MH, Schwartz JS. Screening for urinary tract infection in infants in the emergency department: which test is best? Pediatrics. 1998;101:E1. doi: 10.1542/peds.101.6.e1. [DOI] [PubMed] [Google Scholar]
- 48.Shah SS, Shofer FS, Seidel JS, Baren JM. Significance of extreme leukocytosis in the evaluation of febrile children. Pediatr Infect Dis J. 2005;24:627–30. doi: 10.1097/01.inf.0000168753.60433.e2. [DOI] [PubMed] [Google Scholar]
- 49.Verboon-Maciolek MA, Krediet TG, Gerards LJ, de Vries LS, Groenendaal F, van Loon AM. Severe neonatal parechovirus infection and similarity with enterovirus infection. Pediatr Infect Dis J. 2008;27:241–5. doi: 10.1097/INF.0b013e31815c1b07. [DOI] [PubMed] [Google Scholar]
- 50.King RL, Lorch SA, Cohen DM, Hodinka RL, Cohn KA, Shah SS. Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger. Pediatrics. 2007;120:489–96. doi: 10.1542/peds.2007-0252. [DOI] [PubMed] [Google Scholar]
- 51.Sawyer MH, Holland D, Aintablian N, Connor JD, Keyser EF, Waecker NJ., Jr Diagnosis of enteroviral central nervous system infection by polymerase chain reaction during a large community outbreak. Pediatr Infect Dis J. 1994;13:177–82. doi: 10.1097/00006454-199403000-00002. [DOI] [PubMed] [Google Scholar]