Abstract
Transmissible spongiform encephalopathies arise as a consequence of infection of the central nervous system (CNS) by prions. Spreading of the infectious agent through the peripheral nervous system (PNS) may represent a crucial step toward CNS neuroinvasion, but the modalities of this process have yet to be clarified. Here we provide further evidence that PNS glial cells are likely targets for infection by prions. Glial cell clones originating from dorsal root ganglia of transgenic mice expressing ovine PrP (tgOv) and simian virus 40 T antigen were found to be readily infectible by sheep scrapie agent. This led us to establish two stable cell lines that exhibited features of Schwann cells. These cells were shown to sustain an efficient and stable replication of sheep prion based on the high level of accumulation of abnormal PrP and infectivity in exposed cultures. We also provide evidence for abnormal PrP deposition in peripheral neuroglial cells from scrapie-infected tgOv mice and sheep. These findings have potential implications in terms of designing new cell systems permissive to prions and of peripheral pathobiology of prion infections.
Prions are a class of proposed proteinaceous infectious agents that cause neurodegenerative diseases known as transmissible spongiform encephalopathies (TSEs). They include Creutzfeldt-Jakob disease (CJD) and kuru in humans, scrapie in sheep and goats, bovine spongiform encephalopathy in cattle, and chronic wasting disease in wild cervids. A hallmark of TSEs is the accumulation in nervous and other tissues of PrPsc, a misfolded form of the cellular glycoprotein PrPc (for reviews, see references 14 and 43). There is ample experimental evidence that the conversion of PrPc into PrPsc plays a pivotal role in both the prion replication process and induced neurodegenerative disorders. PrPsc production can be monitored by the detection of PrP species resistant to proteolytic digestion (PrPres) and is the sole marker specific to prion infection available to date. It has been firmly established from in vivo and cell culture experiments that expression of PrPc is indispensable, albeit not sufficient, for the infection to proceed (8, 11, 16, 46).
Infectious forms of TSE can be experimentally transmitted through oral absorption or peripheral inoculation, and such entryways are assumed to be operative in naturally occurring diseases (for a review, see reference 60). How the causative agent is conveyed from peripheral entry sites to its targets within the central nervous system (CNS) is a major issue that remains incompletely elucidated. There is now good evidence that the lymphoreticular system (LRS) and the peripheral nervous system (PNS) may both play a crucial role in this process (8, 20, 30, 31, 35, 38, 44). Spleen and gut lymphoid tissues are early sites of prion accumulation or replication in peripherally infected rodents and naturally infected sheep. Prion accumulation in the spleen and development of CNS disease are clearly impaired in mice with various immunodeficiencies. However, several observations argue for an LRS-independent spread of prions. Indeed, neuroinvasion following peripheral infection requires PrP expression in a sessile compartment that cannot be reconstituted by bone marrow adoptive transfer (8). Conversely, neuroinvasion can take place in mice that do not express PrP in the LRS (44). Direct transport along peripheral nerves was first suggested from intranerval inoculation experiments in mice (29). Spatiotemporal analyses of pathological PrP accumulation in orally inoculated hamsters have involved both the sympathetic and parasympathetic systems, with the earliest sites of PrP deposition being PNS ganglia and CNS areas neuroanatomically connected to the vagus or splanchnic nerves (7, 41). The pattern of PrP deposition in subclinically, naturally TSE-infected sheep and mule deer was reported to be consistent with spreading along the same pathways (53, 56). A transgenetic approach in mice identified sympathetic nervous fibers innervating lymphoid tissues as a potentially rate-limiting component for prion neuroinvasion (22). Sciatic nerve infection of hamsters (5) and of PrP-overexpressing mice (21) as well as intralingual inoculation of hamsters (4) led to an efficient CNS neuroinvasion despite a late infection of LRS and provided further support for a retrograde transport of the infectivity within the PNS.
While a variety of cell types, including follicular dendritic cells and B lymphocytes, have already been implicated in prion expansion within the LRS (for a review, see reference 38), the identification of the PNS cells involved in prion spreading has not made much progress. Not without reason, the neurons are regarded as prime candidates for such a role, and prions are commonly assumed to be transported through an axonal pathway (4, 21, 41). However, as pointed out by some investigators, a possible implication of glial cells, in particular of myelin-forming Schwann cells, should also be considered (1, 18, 22, 23, 34).
As an approach to better identify PNS cells able to sustain prion propagation, we have generated cell clones from dorsal root ganglia of transgenic mice expressing ovine PrP (tgOv) (58) and examined their permissiveness to infection by natural sheep scrapie agent. Here we show that two independent clones with features of Schwann cells are highly permissive to prion infection. We also provide evidence that peripheral neuroglial cells from scrapie agent-infected tgOv mice and sheep accumulate PrPsc, thus further arguing that prion spread within the PNS compartment may involve cellular players other than neurons.
MATERIALS AND METHODS
Transgenic mice.
The transgenic animals used to derive stable mouse cell clones expressing ovine PrP were obtained by crossing the following two parental lines: the tg301+/− line, which expresses the Val136-Arg154-Gln171 high-susceptibility allele of ovine PrP from a bacterial artificial chromosome construct (125 kb) under an endogenous PrP-null background (tgOv) (58), and a line which carries a transgene specifying the simian virus 40 (SV40) T antigen (Tag) under control of the vimentin promoter (52), which was introduced on the same mouse PrP-null background (PrP0/0 mouse Zürich I) (11).
DRG primary cultures and stable cell clones.
Mouse dorsal root ganglia (DRG) and fragments of adjacent peripheral nerves were collected all along the spinal cord and incubated with collagenase (2,500 U/ml; Sigma) for 30 min at 37°C prior to gentle mechanical dissociation (37). Dissociated cells resuspended in feeding medium (see below) were subjected to a 4-h preplating step on poly-dl-ornithine-coated six-well plate dishes, so as to deplete the culture in fast adherent cells such as fibroblasts. Preplated cells were cultured in poly-dl-ornithine-coated wells with Dulbecco's minimal essential medium and F-12 (3:1) plus 10% fetal calf serum as a feeding medium. They were expanded for one or two subpassages by 1:3 splitting at ∼10 days postseeding. Stable cell clones were derived through low-density plating of DRG cultures from 6-week-old transgenic mice expressing SV40 Tag (see above). Established cell clones were propagated in noncoated plates with a weekly 1:10 splitting.
Immunofluorescence analysis for PrPc and cell markers.
To detect PrPc and the SV40 Tag, cells were fixed for 10 min in phosphate-buffered saline (PBS) containing 4% paraformaldehyde plus 4% sucrose, permeabilized for 4 min with 0.2% Triton X-100, incubated with 4F2 antibody for PrPc or anti-SV40 Tag antibody (BD Biosciences), and then incubated with anti-mouse immunoglobulin G (IgG) fluorescein isothiocyanate antibodies. Immunocharacterization of the cell clones was performed with markers for S100 (Dako, Glostrup, Denmark), p75-NGFR (mouse IgG1 monoclonal anti-human antibody, 1:200; Sigma), GFAP (polyclonal rabbit anti-cow antibody, 1:100; Dako), and GalC (mouse IgG3 hybridoma) (48). For immunolabeling, cell monolayers were fixed for 10 min in 2% paraformaldehyde and rinsed three times with PBS. The cells were then incubated with primary antibodies for 30 min at room temperature, rinsed, and incubated with the respective secondary antibodies. The preparations were analyzed with the DMRD Leica fluorescent microscope. Data for each marker are the averages ± standard errors of the means of a minimum of 1,500 cell counts and are expressed as percentages of the total population labeled with Hoechst 33342 (5 μg/ml; Sigma).
Sheep scrapie agent.
The 127 strain was used as the main source of infectious agent. This strain was obtained by propagation in tgOv mice of a natural sheep scrapie isolate supplied by the Veterinary Laboratory Agency (Weybridge, United Kingdom), as already described (58). Infectious inocula were prepared by using terminally diseased mouse brain homogenized in 5% glucose (10% wt/vol). The infectious titer of the brain material used was determined through end limit dilution in the tg301+/− line by the Kärber method. In a few experiments (data not shown), infection was realized by using an inoculum prepared from the original sheep brain.
Infection of cell cultures.
Confluent cultures established in 12-well plates were allowed to incubate with 1 ml of culture medium containing 2.5% (wt/vol) of infectious brain homogenate for 4 days, unless stated otherwise. After removal of the inoculum, the cultures were rinsed with PBS and then allowed to recover for 24 to 48 h in fresh medium prior to trypsinization and seeding in two 25-cm2 flasks (passage p1). Subsequently, the cultures were passaged once per week with a 1:10 splitting.
Western blotting.
Cell cultures at confluence were lysed in 0.5% sodium deoxycholate, 0.5% Triton X-100, and 50 mM Tris-HCl (10 min at 4°C). The lysates were clarified and stored at −20°C. Samples to be analyzed for PrPc were methanol precipitated. For PrPres analysis (57), lysates were treated for 30 min at 37°C with proteinase K (PK) (2 μg of PK/500 μg of protein) and then 4 mM Pefabloc was added. Sedimentable material was collected by centrifugation at 13,000 rpm (Eppendorf centrifuge) for 20 min at room temperature, resuspended in denaturing buffer containing 2-mercaptoethanol, and analyzed by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis and immunoblotting. PrP signals were revealed by using biotinylated 2D6 antibody (49), unless stated otherwise, and an enhanced chemiluminescence detection system (Amersham Pharmacia).
Cell blotting.
A previously described procedure (9) was used, with only slight modifications, for cell blotting. The main steps comprised (i) transfer of cell monolayers grown on coverslips onto nitrocellulose membranes soaked in lysis buffer (0.5% deoxycholate, 0.5% Triton X-100, 150 mM NaCl, and Tris-HCl [pH 7.5]), (ii) incubation for 1 h at 37°C in lysis buffer plus 20 μg of PK/ml, (iii) brief denaturation with 3 M guanidine thiocyanate in 10 mM Tris HCl (pH 8.0), and (iv) blocking of the blot and PrP detection with 8G8 antibody (33) at 1/3,000 and an enhanced chemiluminescence system.
In situ detection of PrPsc in cultured cells.
The following two different previously described techniques were used: (i) immunoperoxidase detection applied to trypsinized, paraffin-embedded cells (57) and (ii) immunofluorescence detection applied to fixed cell monolayers permeabilized with 0.5% Triton X-100 and then treated with 3 M guanidine-HCl (54).
Mouse bioassay.
The infectivity associated with MovS cell-infected cultures was assayed in tgOv mice. For preparation of cell extracts, cells scraped into PBS were pelleted and then resuspended in sterile 5% glucose solution. The samples were freeze-thawed and sonicated before intracerebral inoculation (20 μl). Cultures in duplicate were used to determine the number of inoculated cells. A clinical survey of diseased animals and brain PrPres analysis were performed as described previously (58).
Cell grafting experiment.
Dissociated, uninfected MovS6 cells (p14) were counted, pelleted into medium without serum, and inoculated (3 × 106 cells/mouse in 20 μl) into the brains of scrapie-diseased tgOv mice. No immunosuppressive treatment was applied to the recipient animals, since Mov cells were derived from the same mouse line. At 4 days or a few minutes postinoculation, mice were sacrificed and their brains were dissociated to get primary cultures by using the same method as described above for DRG cultures but without a preplating step. Cultures issued from grafted brains presented fast-growing colonies, which were scraped 5 days postplating, individually subcultured for 1 week, and then tested for the presence of PrPres and SV40 Tag. Primary cultures from control, nongrafted diseased brains showed sparse cells and developed very slowly.
PrPsc immunodetection in DRG.
DRG from intraperitoneally inoculated tgOv mice and ewes naturally affected with scrapie were isolated and formalin fixed before being paraffin embedded. PrPsc immunolabeling and double labeling for GFAP and PrPsc were performed as previously described (2) with 8G8 antibody (1/2,000) and a polyclonal rabbit antiserum (1/2,000; Dako) for PrPsc and GFAP detection, respectively. For mouse tissues in which PrPc is overexpressed, a PK treatment (5 μg/ml in TBS, 10 min at 37°C) was performed prior to immunohistochemistry. Nonspecific immunolabeling was checked by performing serum controls in which the primary antibody was either omitted or replaced with normal rabbit or purified IgG2a. DRG from mock-infected mice and scrapie-free sheep were used as negative controls.
RESULTS
Nonneuronal cell clones derived from DRG are readily infectible by scrapie agent.
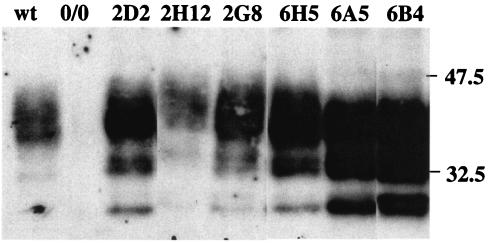
Stable cell clones were established from DRG of 6-week-old mice, with glial cell-enriched primary cultures as starting material. Ovine PrPc-expressing mice (58) crossed with mice expressing SV40 Tag were used as donor animals (52) (see Materials and Methods). While cultures lacking the Tag oncogene ceased proliferating by ∼6 passages, the cultures so obtained divided rather actively, making it feasible to isolate stable clonal cell populations. Seven cell clones were derived from two ovine PrP-expressing donors, and two clones were derived from one donor expressing no PrP (listed in Table 1). All of the clones carrying the tgOv transgene expressed PrP, as established by immunoblotting analysis (Fig. 1 and data not shown). While showing a similar distribution of the major PrPc glycoforms with predominant diglycosylated species, the different clones differed in their levels of expression. The clones 2D2 (MovS2) and 6H5 (MovS6) expressed six- and eightfold more than the clones 2H12 and 2A4, which expressed PrP at physiological levels (compared to primary glial cell cultures from wild-type mice). The individual expression levels were found to be remarkably stable upon subpassaging (data not shown).
TABLE 1.
Infection by sheep prion of cell clones derived from DRG of mice transgenic for ovine PrPa
| Ovine PrP transgenec | Cell clone
|
PrPres detection at p.i. passage no.b:
|
|||
|---|---|---|---|---|---|
| Designation | Morphology | 1 | 2 | 3 | |
| + | 2D2 (MovS2) | Fusiform | + | + | + |
| + | 2H12 | Fusiform | − | − | + |
| + | 2G8 | Bipolar, then fusiform | − | + | + |
| + | 2A4 | Large, pavimentous | − | − | − |
| + | 6H5 (MovS6) | Bipolar, spindle shaped | + | + | + |
| + | 6A5 | Bipolar, spindle shaped | − | + | + |
| + | 6B4 | Bipolar, spindle shaped | − | − | + |
| − | 9G9 (MS0/0) | Bipolar, spindle shaped | − | − | − |
| − | 9F10 | Bipolar, then fusiform | − | − | − |
Primary glial cell-enriched cultures established from three donors, designated 2, 6, and 9 (SV40 Tag+/− and mouse PrP0/0 background).
Subpassaging at a weekly 1:10 split ratio. +, PrPres detected; −, PrPres not detected.
Presence (+) or absence (−) of PrP transgene.
FIG. 1.
PrPc expression in neuroglial cell clones. Six clones derived from DRG of ovine PrP transgenic mice (Table 1) were analyzed for PrPc with 4F2 antibody. A Western blot performed on lysates from cultures at 11 to 15 passages postexplantation of the indicated clones and from primary glial cell cultures of C57BL6 (wild type [wt]) or PrP0/0 (0/0) mice is shown (10 μg of protein/lane, equivalent to 3 × 104 cells). Molecular masses (in kilodaltons) are indicated to the right of the blot.
Following exposure to the scrapie inoculum, immunoblotting analysis revealed the early appearance (≤3 subpassages) of PK-resistant material (PrPres) in most of the PrP-expressing clones, including the low expressing clone 2H12 (Table 1). The dynamics of accumulation upon subpassaging differed among the clones, without obvious correlation with their respective PrPc expression levels (e.g., PrPres detection in the highest expresser clones 6A5 and 6B4 was consistently delayed compared to that in other clones) (Fig. 1 and Table 1). As already noted in earlier studies (12, 57), the PK-resistant species were shorter in size than in the inoculum (see Fig. 5, lanes 1 to 3). Moreover, no PrPres could be detected in the PrP0/0 clones and in the morphologically distinct clone 2A4, ruling out any contribution of residual inoculum to the PrPres signal in PrPc-expressing cultures.
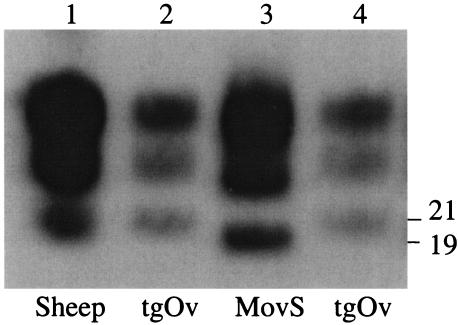
FIG. 5.
Reversible change of the molecular profile of sheep scrapie agent propagated in MovS cells. PK-digested material (50 μg) derived from brain tissues (500 μg) or a cell culture (105 cells) infected by the same source of scrapie agent was analyzed by Western blotting (2D6 antibody). The original PG127 scrapie isolate (lane 1) was passaged once in tgOv mice (lane 2) and then propagated for 9 subpassages in MovS6 cells (lane 3) and reinoculated into tgOv mice (lane 4). Molecular masses (in kilodaltons) are indicated to the right of the figure.
As indicated in Table 1, the infectible clones fell into two categories according to cell morphology. Part of them exhibited a bi- or tripolar spindle shape, closely resembling that described for cultured Schwann cells (10, 15). The others were comprised of cells that were more fusiform in shape. Because two initially Schwann-like clones (2G8 and 9F10) subsequently acquired a fusiform shape, the latter phenotype may have resulted from a stabilization under a less differentiated state.
Schwann cell markers are expressed in prion-infectible, nonneuronal cell clones.
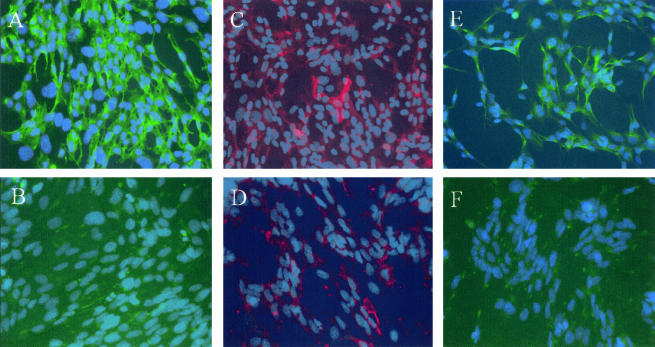
Altogether, these findings provided evidence that prion infection can initiate in cultured, nonneuronal PNS-derived cells. Two cell clones representative of either above-mentioned phenotype, MovS2 and MovS6 (Table 1), were retained for further characterization. Immunofluorescence was performed with antibodies directed to relevant Schwann cell markers (27) such as p75-NGFR and GFAP for the nonmyelinating phenotype and GalC for the promyelinating phenotype to determine Schwann cell identity. As shown in Fig. 2, MovS2 did not express p75-NGFR or GFAP; however, a minor population expressed the promyelinating marker GalC (10% ± 1%). MovS6 stained for p75-NGFR (85% ± 1%), GFAP (15% ± 10%), and GalC (15% ± 1%). Staining with antibodies against Thy-1.2, a fibroblast marker, was not found in either S2 or S6 cultures. These data indicated that cells of the MovS6 clone have more characteristics of Schwann cells than those of the MovS2 clone.
FIG. 2.
Expression of Schwann cell markers in MovS cells. Immunohistochemical characterization of MovS6 (A, C, and E) and MovS2 cells (B, D, and F). p75-NGFR (A and B) is expressed by MovS6 cells but not MovS2 cells. GalC (C and D) is expressed by a minority of cells in both clones. GFAP (E and F) is not expressed by MovS2 but is expressed by a subpopulation of MovS6 cells.
MovS cells sustain an efficient and stable propagation of sheep scrapie infectious agent.
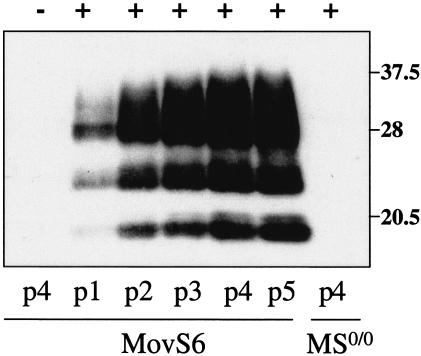
Figure 3 shows the kinetics of PrPres accumulation in MovS6 cells following exposure to the scrapie inoculum. Typically, PrPres was detected from the first passage on, and reached a plateau at four or five passages postinfection (p.i.). MovS2 cultures led to similar observations in terms of PrPres level and accumulation rate (data not shown). Infection of MovS2 and MovS6 cells was fairly reproducible, as nearly all the experiments carried out so far turned out to be successful (22 of 23 and 6 of 6, respectively). Subcloning (9, 45) was found to not be required for maintaining the infected state. Strikingly, one persistently infected MovS2 cell culture was propagated for more than 100 passages and still accumulated PrPres in large amounts (data not shown).
FIG. 3.
Dynamics of PrPres accumulation in MovS6 cells following exposure to sheep prion. PK-treated cell lysates (500 μg of protein) from cultures at the indicated passage p.i. (p1 to p5) were analyzed by Western blotting with 2D6 antibody. MovS6 and MS0/0 cultures (12th subpassage) were incubated for 4 days with 2.5% brain homogenate prepared from scrapie-diseased (+) or control (−) tgOv mice. MOI, ∼20 ID50 U/cell.
In situ detection was performed to evaluate the proportion of PrP-converting cells in steady-state infected cultures. Figure 4 shows the accumulation of PrP observed in paraffin-embedded and PK-treated (Fig. 4, top) or guanidine-treated (Fig. 4, bottom) MovS2 cultures. Both approaches revealed that PrP conversion involved most cells, i.e., 60 to 90%, respectively. Similar results were obtained for MovS6 cells (data not shown). Notwithstanding the marked accumulation seen in many cells, no obvious adverse effect could be observed in long-term-infected cultures.
FIG. 4.
In situ detection of PrPsc in MovS2 cells infected with sheep prion. (Top) Slices of paraffin-embedded, mock-infected (left) and infected cells (13 passages p.i.) (right) were PK treated and immunolabeled with 8G8 antibody and peroxidase (see Materials and Methods). PrPres deposits are visible at the intracellular and plasma membrane (inset) levels. (Bottom) Fixed cell monolayers were treated with guanidine thiocyanate and immunolabeled with ICSM18 antibody. In infected cultures (panels 2 and 3), most cells show a punctuate fluorescence not seen in mock-infected cultures (panel 1) and are thus assumed to reflect abnormal PrP accumulation. Magnifications, ×200 and ×400.
Cell-associated infectivity in MovS cultures was determined by bioassay in tgOv mice (Table 2). A 100% attack rate was observed in the mice inoculated with cell extracts from steady-state infected cultures of either clone. All mice inoculated with exposed MS0/0 cells remained healthy, thus showing the lack of residual input infectivity at 4 passages p.i. in glial cell cultures. The diseased mice presented neurological disorders typical of TSE, and all brains tested (12 of 12) were PrPres positive by Western blotting (Fig. 5). MovS cultures were highly infectious, as 1 × 106 to 3 × 106 cells killed inoculated mice even faster than 2 mg of diseased tgOv mice brain (58), thus indicating an infectivity level of ≥6 log 50% infective dose (ID50) U in the inoculated cell extracts (see Materials and Methods).
TABLE 2.
Bioassay in ovine PrP transgenic micea of cell extracts from MovS cultures exposed to sheep prion and then subpassaged as indicated
| Cell line | Inoculumb
|
Incubation timec | |
|---|---|---|---|
| No. of passages p.i. | No. of cells/mouse | ||
| MovS2 | 13 | 3 × 106 | 58 ± 0.9 (6/6) |
| 26 | 5 × 105 | 74.7 ± 1.8 (6/6) | |
| 26 | 5 × 104 | 80 ± 1.3 (7/7) | |
| MovS6 | 9 | 3 × 106 | 55 ± 0.4 (6/6) |
| 9 | 1 × 104 | 68 ± 0.9 (6/6) | |
| MS0/0d | 4 | 3 × 106 | >220 (0/5) |
The tg301 mouse line overexpressing the PrPVRQ allele about eight-fold (58).
Animals were inoculated intracerebrally with freeze-thawed cell suspension.
Mean number of days to terminal scrapie disease ± standard error of the mean. Values in parentheses are numbers of ill mice/numbers of inoculated mice.
Expressing no PrP (clone 9G9) (Table 1).
Mov cells can be infected at a low MOI or by grafting in infected brain.
The susceptibility of these cells to scrapie infection was further investigated through two kinds of experiments. First, Mov cultures were inoculated with serial dilutions of scrapie inoculum and PrP conversion was monitored for up to 5 subpassages p.i. As shown in Fig. 6A, PrPres-positive MovS6 cultures could be observed following exposure to a 125-ng equivalent of a tgOv brain pool titrating at ∼109 ID50 U/g (two independent experiments), meaning that as few as ∼2 × 10−4 ID50 U per cell is sufficient to initiate the infection. An ∼5-fold-higher multiplicity of infection (MOI) was required to infect MovS2 cells (data not shown).
FIG. 6.
Infection of MovS cells by exposure to low infectious dose in culture or by in vivo infection within the brain of a scrapie-diseased mouse. (A) PrPres detection (ICSM18 antibody) in MovS6 cultures serially passaged (p1 to p5) following a 6-day incubation with 0.5 ml of 2.5% infectious tgOv brain homogenate at the indicated dilutions. (B) Cell blot assay on MovS2 cells recovered from the brain of infected tgOv mice drafted with uninfected cells. Two tgOv mice at the onset of the clinical phase were inoculated intracerebrally with 3 × 106 cells, and 4 days later their brains were removed and explanted into culture. Cell colonies formed within 5-day-old primary cultures were scraped, individually seeded on coverslips, and analyzed for PrPres 1 week later (8G8 antibody). Cell colonies derived from infected brain inoculated with Mov cells just before explantation into culture (−) or from a persistently infected culture (+) were used as negative and positive controls, respectively.
Second, uninfected MovS2 cells were inoculated into the brain of infected tgOv mice at the onset of the disease, where they were allowed to reside for 4 days before isolation and expansion in culture. Such cultures comprised a majority of Tag-positive cells (data not shown), consistent with the high proliferative rate of Mov cells versus adult brain primary cells. A cell blot analysis revealed the accumulation of PrPres in these cultures, whereas parallel cultures derived from an infected brain inoculated with Mov cells just prior to explantation remained PrPres negative (Fig. 6B). From this result, it was concluded that a significant proportion of Mov cells had been successfully infected in vivo.
Peripheral glial cells from scrapie agent-infected mice and sheep do accumulate abnormal PrP.
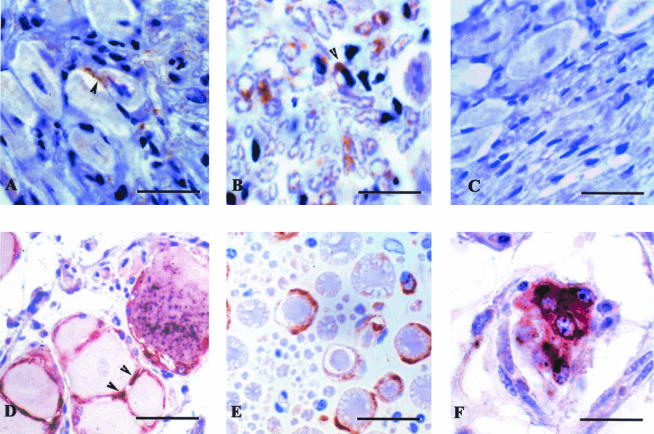
To see whether infection of neuroglial cells would naturally occur in the tgOv mouse model, DRG were collected from intraperitoneally infected animals with end-stage disease (∼90 days p.i.) and the nature of PrP-converting cells in this tissue was examined. Glial cell-enriched cultures were established and found to contain ≥80% cells positively stained for S100 (data not shown). Western blot analysis performed early after explantation showed that PrPres was present from passage 0 to 2 (Fig. 7). PrPres accumulation was confirmed by cell blot assay and shown to involve numerous foci evenly distributed in the culture, consistent with an infection of glial cells ab initio. Alternatively, glial cells may have become infected in contact with cocultured neurons, which represented about 10% of the primary cells but disappeared from the first subpassage onward. To clarify this point, immunohistochemical analysis was performed on DRG collected from similarly infected mice. It revealed an intracellular accumulation of PK-resistant PrP in neurons and in two morphologically distinct types of glial cells: (i) satellite glial cells, which have an intimate contact with neurons (Fig. 8A), and (ii) Schwann cells ensheathing axons (Fig. 8B). The PrPsc distribution on sections was heterogeneous, with a percentage of positive glial cells and of neurons varying from 1 to 30% and 10 to 40%, respectively. Although PrPsc-positive satellite cells adjacent to negative neurons were observed (Fig. 8A), most PrPsc-positive satellite cells were surrounding positive neurons. No labeling was observed in mock-infected DRG sections (Fig. 8C).
FIG. 7.
Detection of PrPres in PNS glial cells derived from infected tgOv mice. Primary cultures were established from DRG of terminally diseased animals inoculated by the peritoneal route or of control animals (−). PK-resistant PrP was revealed in cell lysates (Western blotting) (left) or monolayers (cell blotting) (right) from cultures at the indicated passage level (p0, 10 days postexplantation). A cell blot on a monolayer from a persistently infected Mov culture (+) is shown for comparison. Molecular mass (in kilodaltons) is indicated on the left.
FIG. 8.
PrPsc accumulation in DRG from scrapie-diseased mice and sheep. DRG from intraperitoneally inoculated tgOv mice (A and B) and naturally scrapie-diseased sheep (D to F) were formalin fixed, paraffin embedded, and immunolabeled for PrPsc (8G8 antibody). PrPsc deposits involve both neurons and glial cells: (i) satellite cells in panels A (diaminobenzidine [DAB] revelation, brown end product; bar, 15 μm) and D (5-bromo-4-chloro-3-indolylphosphate [BCIP]-nitroblue tetrazolium [NBT] revelation, black end product; bar, 25 μm) and (ii) axon ensheathing Schwann cells in panels B and E (DAB revelation; bar, 25 μm). Double labeling for PrPsc (NBT-BCIP revelation, black end product) and GFAP (3-amino-9-ethylcarbazole revelation, red end product) applied to sheep DRG shows PrPsc in GFAP-positive satellite cells (panel D) and interstitial, isolated Schwann cells (panel F; bar, 15 μm). No PrP labeling is seen in DRG from mock-infected mice (panel C; bar, 15 μm).
In DRG from naturally scrapie-affected sheep, PrPsc accumulation was identified in neurons (Fig. 8E) and in Schwann cells ensheathing axons. PrPsc-GFAP double labeling clearly demonstrated abnormal prion protein accumulation in both satellite glial cells (Fig. 8D) and in isolated, interstitial Schwann cells (Fig. 8F).
DISCUSSION
A new cell system highly permissive to infection by sheep prion.
Most of the currently available TSE cell culture systems, which include N2A, PC12, and GT1 (45, 50, 51), consist of established rodent cell lines found to be permissive to rodent-adapted prions. In an effort to establish cell systems that would enable an ex vivo propagation of prions naturally infecting humans and animals, we have explored several strategies based on the genetic engineering of various cell types for expression of ovine PrP (36). This led us to develop the Rov system, consisting of rabbit cells that inducibly express the VRQ allele of ovine PrP and are highly and stably susceptible to sheep scrapie agent (57).
In the present study, we demonstrate that mouse PNS-derived glial cells expressing ovine PrP can also sustain an active propagation of sheep prion, thus leading to the introduction of a novel permissive system called Mov. The starting observation was that cell clones derived from DRG of triple transgenic mice expressing PrP VRQ and immortalizing SV40 Tag under a mouse PrP-null background were easy to establish and readily infectible by exposure to scrapie inoculum. Two lines found to exhibit features of Schwann cells, MovS2 and MovS6, were studied in more detail. Actually, very few cell lines of this type have been described (10, 59), reflecting the fact that Schwann cells in mouse species do not tend to immortalize spontaneously, in contrast to what has been reported for rat species (39).
Several lines of evidence indicate that efficient prion multiplication can take place in MovS2 or MovS6 cell lines. First, exposure to the scrapie inoculum led to an early and strong accumulation of PK-resistant PrP, which involved a majority of cells in steady-state infected cultures. Second, such cultures were highly infectious and produced up to 2 ID50 U/cell, as assessed by bioassay in tgOv mice. Upon reinoculation to mice, the PrPres resumed its original banding pattern in mouse brain, consistent with our finding that ex vivo propagation of this strain does not alter its biological phenotype (unpublished data). This observation further underlines the fact that the cell context in which prion replication takes place may affect not only the glycoform ratio but also the size of the PrPsc or PrPres species produced (12). Third, MovS cells could be infected by grafting into the brains of clinically affected mice and by exposure to low doses of the inoculum. Interestingly, the PrPres accumulation in such cultures was delayed but eventually reached levels similar to those in higher-MOI-exposed cultures. This may suggest that the PrPres-producing cells increased in number during subcultivation. All together, our data point to the view that neuroglial cells may easily enter an infected state but also ensure cell-to-cell propagation of the infectious agent. Additional studies are being performed to address the issue of whether cell-to-cell spreading does actually take place and, if so, whether adjacent (28) or distant cells are primarily involved. MovS cultures consist of very actively dividing cells that can be maintained in a persistent, long-term infected state without the need for subcloning (9, 45). Thus, both postmitotic cells, such as neurons and follicular dendritic cells, regarded as primary targets for prion replication, and highly proliferative cells may efficiently replicate prions.
During the course of this study, the MSC-80 mouse Schwann cell line was reported to sustain replication of the Chandler mouse-adapted strain (18). The original cell line can display a well differentiated phenotype and produce myelin when associated with axons in vivo (10). Although the PrPc expression level was similar to that of N2a cells, infected MSC-80 cultures were found to accumulate PrPres in markedly smaller amounts (18). A titer of ∼104 ID50 U/g of cell extract was determined by bioassays in tga20 mice, indicative of a relatively low efficiency of replication. For comparison, a titer of ≥108 ID50/g was calculated for MovS cultures on the basis of 109 cells/g of extract.
The above-mentioned characteristics make the PNS-derived glial cells an attractive TSE cell system which could be exploited for the propagation of prions affecting species other than sheep, for which permissive cell systems are still lacking. The Schwann-like stable cell clones derived from PrP0/0 mice established during this study could be of interest in this regard. Whether these MS0/0 cells can be genetically engineered to express various PrP constructs, thus providing a versatile ex vivo system for studies on prion infection, is currently being examined. An alternative strategy would consist of deriving MS-like cell clones from transgenic animals that express human or bovine PrP.
Schwann cells as players in TSE infection.
The observation that, in this and another study (18), cultured PNS neuroglial cells were able to sustain active prion replication further questions their possible involvement in TSE peripheral pathogenesis. The rate of infectivity spreading in peripheral nerves was estimated to be around 1 mm/day (21), i.e., roughly similar to that determined in the spinal cord (4, 6). However, at the cellular level, the mode of transport of prions within the PNS remains most obscure. While established mechanisms of axonal transport, either active (fast) or passive were considered at first (4, 29, 41), an alternative process of prion propagation has progressively emerged, involving a domino-like effect via a PrPc-paved cell chain (1, 8, 21, 34). Hence, it is not unreasonable to speculate that Schwann cells may be an integral part of such a chain.
Recently, Schwann cells in the mouse sciatic nerve were reported to express PrPc, as well as green fluorescent protein, from a transgene under the control of PrP natural regulatory sequences (18). In a detailed study also performed on mice, weak, most frequently negative PrPc immunostaining was found on satellite cells while axon-associated Schwann cells were often strongly positive (19). Intriguingly, Schwann cells associated with unlabeled axons were similarly immunonegative. In this work, PrPc expression was found to be a general feature of glia-like cell clones. The MSC-80 Schwann cells, derived from wild-type mice, were also shown to express PrPc at the cell surface (18). Thus, although information regarding PrPc expression in peripheral glial cells in vivo remains rather scarce to date, it seems likely that natural regulatory sequences can promote the expression of PrPc in peripheral neuroglial cells.
There is, however, only limited evidence to date that peripheral glial cells can actually be infected in vivo. A general outcome of immunohistological analyses focused on the PNS is that direct observation of PrP deposition is infrequent and often restricted to terminally affected individuals. In a detailed study performed on orally infected hamsters, only scant deposition along a few axons of autonomous nerves was visualized (41). PrPres accumulation has been detected by Western blotting on the sciatic nerves of terminally diseased hamsters (5) and neuron-specific enolase promoter transgenic mice (44), but no immunohistological data are available. There are only scattered observations indicative of an infection of glial cells in the PNS compartment. Immunolabeling for pathological PrP has been mentioned to involve satellite cells in DRG of experimentally infected hamsters (40) and sheep (23), enteric ganglia of naturally infected sheep (26) and deer (53), and trigeminal ganglia of CJD-affected patients (24). In addition, an adaxonal pattern of PrP deposition was described for nerve fibers adjacent to ganglia from infected sheep (23) and humans (25), suggestive of an involvement of myelin-forming Schwann cells.
Our present data provide more evidence arguing for infection of peripheral neuroglial cells by sheep scrapie agent in vivo. Satellite and Schwann cells with intracellular PrPres staining were observed within DRG removed from peripherally scrapie agent-infected tgOv mice. Consistent with these immunohistological data, glial cell-enriched, primary cultures established from the same tissue were found to contain infected cells. Extending these investigations to natural scrapie, we found clear evidence for PrP deposition in DRG GFAP-positive glial cells from diseased sheep. This result would also suggest that the ability to infect PNS glial cells is merely a feature of the scrapie agent rather than of a strain since the natural case isolate exhibited a quite distinct phenotype from that of the cell-cultured strain when transmitted to tgOv mice (58).
In conclusion, this study provided clear evidence that prions, like other pathogens that target peripheral nerves, such as herpes simplex virus (55), Mycobacterium leprae (47), and Trypanosoma cruzi (13), can replicate actively in PNS glial cells. The role of these cells in the pathogenesis of TSE infections deserves closer investigation in several respects. Neuroglial cells appear to be potential reservoirs of infectivity in peripheral tissues such as ganglia, nerves, gut, and muscles. Whether or not infection of such cells may play a significant role in the peripheral transport of prions is still an open issue that now has to be challenged in vivo, including through transgenetic approaches. Finally, the question arises as to whether infection of PNS glial cells might be damaging in itself. Although PNS is usually not involved in neurodegenerative changes in prion diseases, demyelinating peripheral neuropathy has been observed in sporadic or familial cases of Creutzfeldt-Jakob disease (references 3, 17, 32, and 42 and references therein). One logical approach toward the development of a prophylactic intervention against TSE in humans would be to eliminate the movement of prions through the PNS. A deeper understanding, at the cellular level, of the mode of spreading of prions in this compartment would certainly represent an important step toward this goal.
Acknowledgments
We are indebted to Denise Paulin (Jussieu University, Paris, France) for making the Tag transgenic mice available to us. We are most grateful to Simon Hawke (Imperial College, London, United Kingdom) for antibody ICSM18, Jacques Grassi (SPI/CEA, Saclay, France) for antibodies 4F2 and 8G8, and Piotr Tolpiko (ENS, Paris, France) and Roger Morris (King’s College London, London, United Kingdom) for helpful discussions. We also acknowledge Rachid Essalmani for mouse genotyping and José Costa and Marthe Hudrisier for animal care.
This work was supported by grants from the European Union (BIOTEC PL976064) and from the French government (GIS Infections à Prion).
REFERENCES
- 1.Aguzzi, A. 1997. Neuro-immune connection in spread of prions in the body? Lancet 349:742-743. [DOI] [PubMed] [Google Scholar]
- 2.Andreoletti, O., P. Berthon, E. Levavasseur, D. Marc, F. Lantier, E. Monks, J. M. Elsen, and F. Schelcher. 2002. Phenotyping of protein-prion (PrPsc)-accumulating cells in lymphoid and neural tissues of naturally scrapie-affected sheep by double-labeling immunohistochemistry. J. Histochem. Cytochem. 50:1357-1370. [DOI] [PubMed] [Google Scholar]
- 3.Antoine, J. C., J. L. Laplanche, J. F. Mosnier, P. Beaudry, J. Chatelain, and D. Michel. 1996. Demyelinating peripheral neuropathy with Creutzfeldt-Jakob disease and mutation at codon 200 of the prion protein gene. Neurology 46:1123-1127. [DOI] [PubMed] [Google Scholar]
- 4.Bartz, J. C., A. E. Kincaid, and R. A. Bessen. 2003. Rapid prion neuroinvasion following tongue infection. J. Virol. 77:583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartz, J. C., A. E. Kincaid, and R. A. Bessen. 2002. Retrograde transport of transmissible mink encephalopathy within descending motor tracts. J. Virol. 76:5759-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beekes, M., E. Baldauf, and H. Diringer. 1996. Sequential appearance and accumulation of pathognomonic markers in the central nervous system of hamsters orally infected with scrapie. J. Gen. Virol. 77:1925-1934. [DOI] [PubMed] [Google Scholar]
- 7.Beekes, M., P. A. McBride, and E. Baldauf. 1998. Cerebral targeting indicates vagal spread of infection in hamsters fed with scrapie. J. Gen. Virol. 79:601-607. [DOI] [PubMed] [Google Scholar]
- 8.Blattler, T., S. Brandner, A. J. Raeber, M. A. Klein, T. Voigtlander, C. Weissmann, and A. Aguzzi. 1997. PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain. Nature 389:69-73. [DOI] [PubMed] [Google Scholar]
- 9.Bosque, P. J., and S. B. Prusiner. 2000. Cultured cell sublines highly susceptible to prion infection. J. Virol. 74:4377-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boutry, J. M., J. J. Hauw, A. Gansmuller, N. Di-Bert, M. Pouchelet, and A. Baron-Van Evercooren. 1992. Establishment and characterization of a mouse Schwann cell line which produces myelin in vivo. J. Neurosci. Res. 32:15-26. [DOI] [PubMed] [Google Scholar]
- 11.Bueler, H., A. Aguzzi, A. Sailer, R. A. Greiner, P. Autenried, M. Aguet, and C. Weissmann. 1993. Mice devoid of PrP are resistant to scrapie. Cell 73:1339-1347. [DOI] [PubMed] [Google Scholar]
- 12.Caughey, B., G. J. Raymond, D. Ernst, and R. E. Race. 1991. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J. Virol. 65:6597-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuenkova, M. V., F. B. Furnari, W. K. Cavenee, and M. A. Pereira. 2001. Trypanosoma cruzi trans-sialidase: a potent and specific survival factor for human Schwann cells by means of phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl. Acad. Sci. USA 98:9936-9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collinge, J. 2001. Prion diseases of humans and animals: their causes and molecular basis. Annu. Rev. Neurosci. 24:519-550. [DOI] [PubMed] [Google Scholar]
- 15.Dubois-Dalcq, M., B. Rentier, A. Baron, N. van Evercooren, and B. W. Burge. 1981. Structure and behavior of rat primary and secondary Schwann cells in vitro. Exp. Cell Res. 131:283-297. [DOI] [PubMed] [Google Scholar]
- 16.Enari, M., E. Flechsig, and C. Weissmann. 2001. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc. Natl. Acad. Sci. USA 98:9295-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esiri, M. M., W. I. Gordon, J. Collinge, and J. S. Patten. 1997. Peripheral neuropathy in Creutzfeldt-Jakob disease. Neurology 48:784. [DOI] [PubMed] [Google Scholar]
- 18.Follet, J., C. Lemaire-Vieille, F. Blanquet-Grossard, V. Podevin-Dimster, S. Lehmann, J. P. Chauvin, J. P. Decavel, R. Varea, J. Grassi, M. Fontes, and J. Y. Cesbron. 2002. PrP expression and replication by Schwann cells: implications in prion spreading. J. Virol. 76:2434-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford, M. J., L. J. Burton, R. J. Morris, and S. M. Hall. 2002. Selective expression of prion protein in peripheral tissues of the adult mouse. Neuroscience 113:177-192. [DOI] [PubMed] [Google Scholar]
- 20.Glatzel, M., and A. Aguzzi. 2000. Peripheral pathogenesis of prion diseases. Microbes Infect. 2:613-619. [DOI] [PubMed] [Google Scholar]
- 21.Glatzel, M., and A. Aguzzi. 2000. PrP(C) expression in the peripheral nervous system is a determinant of prion neuroinvasion. J. Gen. Virol. 81:2813-2821. [DOI] [PubMed] [Google Scholar]
- 22.Glatzel, M., F. L. Heppner, K. M. Albers, and A. Aguzzi. 2001. Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron 31:25-34. [DOI] [PubMed] [Google Scholar]
- 23.Groschup, M. H., M. Beekes, P. A. McBride, M. Hardt, J. A. Hainfellner, and H. Budka. 1999. Deposition of disease-associated prion protein involves the peripheral nervous system in experimental scrapie. Acta Neuropathol. (Berlin) 98:453-457. [DOI] [PubMed] [Google Scholar]
- 24.Guiroy, D. C., S. K. Shankar, C. J. Gibbs, Jr., J. A. Messenheimer, S. Das, and D. C. Gajdusek. 1989. Neuronal degeneration and neurofilament accumulation in the trigeminal ganglia in Creutzfeldt-Jakob disease. Ann. Neurol. 25:102-106. [DOI] [PubMed] [Google Scholar]
- 25.Hainfellner, J. A., and H. Budka. 1999. Disease associated prion protein may deposit in the peripheral nervous system in human transmissible spongiform encephalopathies. Acta Neuropathol. (Berlin) 98:458-460. [DOI] [PubMed] [Google Scholar]
- 26.Heggebo, R., L. Gonzalez, C. M. Press, G. Gunnes, A. Espenes, and M. Jeffrey. 2003. Disease-associated PrP in the enteric nervous system of scrapie-affected Suffolk sheep. J. Gen. Virol. 84:1327-1338. [DOI] [PubMed] [Google Scholar]
- 27.Jessen, K. R., and R. Mirsky. 2002. Signals that determine Schwann cell identity. J. Anat. 200:367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanu, N., Y. Imokawa, D. N. Drechsel, R. A. Williamson, C. R. Birkett, C. J. Bostock, and J. P. Brockes. 2002. Transfer of scrapie prion infectivity by cell contact in culture. Curr. Biol. 12:523-530. [DOI] [PubMed] [Google Scholar]
- 29.Kimberlin, R. H., S. M. Hall, and C. A. Walker. 1983. Pathogenesis of mouse scrapie. Evidence for direct neural spread of infection to the CNS after injection of sciatic nerve. J. Neurol. Sci. 61:315-325. [DOI] [PubMed] [Google Scholar]
- 30.Kimberlin, R. H., and C. A. Walker. 1988. Incubation periods in six models of intraperitoneally injected scrapie depend mainly on the dynamics of agent replication within the nervous system and not the lymphoreticular system. J. Gen. Virol. 69:2953-2960. [DOI] [PubMed] [Google Scholar]
- 31.Klein, M. A., R. Frigg, E. Flechsig, A. J. Raeber, U. Kalinke, H. Bluethmann, F. Bootz, M. Suter, R. M. Zinkernagel, and A. Aguzzi. 1997. A crucial role for B cells in neuroinvasive scrapie. Nature 390:687-690. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs, T., Z. Aranyi, I. Szirmai, and P. L. Lantos. 2002. Creutzfeldt-Jakob disease with amyotrophy and demyelinating polyneuropathy. Arch. Neurol. 59:1811-1814. [DOI] [PubMed] [Google Scholar]
- 33.Krasemann, S., M. H. Groschup, S. Harmeyer, G. Hunsmann, and W. Bodemer. 1996. Generation of monoclonal antibodies against human prion proteins in PrP0/0 mice. Mol. Med. 2:725-734. [PMC free article] [PubMed] [Google Scholar]
- 34.Kunzi, V., M. Glatzel, M. Y. Nakano, U. F. Greber, F. Van Leuven, and A. Aguzzi. 2002. Unhampered prion neuroinvasion despite impaired fast axonal transport in transgenic mice overexpressing four-repeat tau. J. Neurosci. 22:7471-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lasmezas, C. I., J. Y. Cesbron, J. P. Deslys, R. Demaimay, K. T. Adjou, R. Rioux, C. Lemaire, C. Locht, and D. Dormont. 1996. Immune system-dependent and -independent replication of the scrapie agent. J. Virol. 70:1292-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laude, H., D. Vilette, A. Le Dur, F. Archer, S. Soulier, N. Besnard, R. Essalmani, and J. L. Vilotte. 2002. New in vivo and ex vivo models for the experimental study of sheep scrapie: development and perspectives. C. R. Acad. Sci. Ser. III 325:49-57. [DOI] [PubMed] [Google Scholar]
- 37.Lindsay, R. M., C. J. Evison, and J. Winter. 1991. Culture of adult mammalian peripheral neurons, p. 1-17. In J. Chad and H. Weal (ed.), Cellular neurobiology: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 38.Mabbott, N. A., and M. E. Bruce. 2001. The immunobiology of TSE diseases. J. Gen. Virol. 82:2307-2318. [DOI] [PubMed] [Google Scholar]
- 39.Mathon, N. F., D. S. Malcolm, M. C. Harrisingh, L. Cheng, and A. C. Lloyd. 2001. Lack of replicative senescence in normal rodent glia. Science 291:872-875. [DOI] [PubMed] [Google Scholar]
- 40.McBride, P. A., and M. Beekes. 1999. Pathological PrP is abundant in sympathetic and sensory ganglia of hamsters fed with scrapie. Neurosci. Lett. 265:135-138. [DOI] [PubMed] [Google Scholar]
- 41.McBride, P. A., W. J. Schulz-Schaeffer, M. Donaldson, M. Bruce, H. Diringer, H. A. Kretzschmar, and M. Beekes. 2001. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J. Virol. 75:9320-9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neufeld, M. Y., J. Josiphov, and A. D. Korczyn. 1992. Demyelinating peripheral neuropathy in Creutzfeldt-Jakob disease. Muscle Nerve 15:1234-1239. [DOI] [PubMed] [Google Scholar]
- 43.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Race, R., M. Oldstone, and B. Chesebro. 2000. Entry versus blockade of brain infection following oral or intraperitoneal scrapie administration: role of prion protein expression in peripheral nerves and spleen. J. Virol. 74:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Race, R. E., B. Caughey, K. Graham, D. Ernst, and B. Chesebro. 1988. Analyses of frequency of infection, specific infectivity, and prion protein biosynthesis in scrapie-infected neuroblastoma cell clones. J. Virol. 62:2845-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raeber, A. J., A. Sailer, I. Hegyi, M. A. Klein, T. Rulicke, M. Fischer, S. Brandner, A. Aguzzi, and C. Weissmann. 1999. Ectopic expression of prion protein (PrP) in T lymphocytes or hepatocytes of PrP knockout mice is insufficient to sustain prion replication. Proc. Natl. Acad. Sci. USA 96:3987-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rambukkana, A., G. Zanazzi, N. Tapinos, and J. L. Salzer. 2002. Contact-dependent demyelination by Mycobacterium leprae in the absence of immune cells. Science 296:927-931. [DOI] [PubMed] [Google Scholar]
- 48.Ranscht, B., P. A. Clapshaw, J. Price, M. Noble, and W. Seifert. 1982. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc. Natl. Acad. Sci. USA 79:2709-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rezaei, H., D. Marc, Y. Choiset, M. Takahashi, G. Hui Bon Hoa, T. Haertle, J. Grosclaude, and P. Debey. 2000. High yield purification and physico-chemical properties of full-length recombinant allelic variants of sheep prion protein linked to scrapie susceptibility. Eur. J. Biochem. 267:2833-2839. [DOI] [PubMed] [Google Scholar]
- 50.Rubenstein, R., R. I. Carp, and S. M. Callahan. 1984. In vitro replication of scrapie agent in a neuronal model: infection of PC12 cells. J. Gen. Virol. 65:2191-2198. [DOI] [PubMed] [Google Scholar]
- 51.Schatzl, H. M., L. Laszlo, D. M. Holtzman, J. Tatzelt, S. J. DeArmond, R. I. Weiner, W. C. Mobley, and S. B. Prusiner. 1997. A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J. Virol. 71:8821-8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz, B., P. Vicart, C. Delouis, and D. Paulin. 1991. Mammalian cell lines can be efficiently established in vitro upon expression of the SV40 large T antigen driven by a promoter sequence derived from the human vimentin gene. Biol. Cell 73:7-14. [DOI] [PubMed] [Google Scholar]
- 53.Sigurdson, C. J., T. R. Spraker, M. W. Miller, B. Oesch, and E. A. Hoover. 2001. PrP(CWD) in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J. Gen. Virol. 82:2327-2334. [DOI] [PubMed] [Google Scholar]
- 54.Taraboulos, A., D. Serban, and S. B. Prusiner. 1990. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J. Cell Biol. 110:2117-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taus, N. S., and W. J. Mitchell. 2001. The transgenic ICP4 promoter is activated in Schwann cells in trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J. Virol. 75:10401-10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Keulen, L. J., B. E. Schreuder, M. E. Vromans, J. P. Langeveld, and M. A. Smits. 2000. Pathogenesis of natural scrapie in sheep. Arch. Virol. Suppl. 16:57-71. [DOI] [PubMed] [Google Scholar]
- 57.Vilette, D., O. Andreoletti, F. Archer, M. F. Madelaine, J. L. Vilotte, S. Lehmann, and H. Laude. 2001. Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proc. Natl. Acad. Sci. USA 98:4055-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vilotte, J.-L., S. Soulier, R. Essalmani, M.-G. Stinnakre, D. Vaiman, L. Lepourry, J. C. Da Silva, N. Besnard, M. Dawson, A. Buschmann, M. Groschup, S. Petit, M.-F. Madelaine, S. Rakatobe, A. Le Dur, D. Vilette, and H. Laude. 2001. Markedly increased susceptibility to natural sheep scrapie of transgenic mice expressing ovine PrP. J. Virol. 75:5977-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watabe, K., T. Fukuda, J. Tanaka, H. Honda, K. Toyohara, and O. Sakai. 1995. Spontaneously immortalized adult mouse Schwann cells secrete autocrine and paracrine growth-promoting activities. J. Neurosci. Res. 41:279-290. [DOI] [PubMed] [Google Scholar]
- 60.Weissmann, C., M. Enari, P. C. Klohn, D. Rossi, and E. Flechsig. 2002. Transmission of prions. Proc. Natl. Acad. Sci. USA 99(Suppl. 4):16378-16383. [DOI] [PMC free article] [PubMed] [Google Scholar]