Abstract
Introduction
In this in-vitro study, we aimed to compare the residual monomers in composites beneath brackets bonded to enamel, using a light-emitting diode (LED) or a halogen unit, and to compare the residual monomers in the central to the peripheral areas of the composite.
Methods
Twenty bovine teeth preserved in 0.1% thymol were used in this study. Ten teeth were used to standardize the thickness of the composite film, since different thicknesses would cause different absorbance of light. Brackets were bonded to 10 bovine incisors, with the halogen light (n = 5) and the LED (n = 5). The brackets were debonded, and the remaining composite on the enamel surface was sectioned in 2 regions: peripheral (0.8 mm) and central, resulting in 2 subgroups per group: central halogen (n = 5), peripheral halogen (n = 5), central LED (n = 5), and peripheral LED (n = 5). The spectrometric analysis in the infrared region was used to measure the free monomers with the attenuated total reflectance method.
Results
Normal distribution was tested by using the Kolmogorov-Smirnov test. Data were compared by 2-way analysis of variance (ANOVA) at P <0.05. The LED group showed fewer residual monomers than did the halogen group (P = 0.014). No differences were found among the regions (P = 0.354), and there were no interactions between light type and region (P = 0.368).
Conclusions
LED leaves less residual monomer than does the halogen light, even with half of the irradiation time; there were no differences between the central and peripheral regions, and no interaction between light type and region.
The halogen light is the most commonly used source of polymerization of dental materials, with known efficiency. Halogen bulbs produce light when electric energy heats a small tungsten filament to a high temperature.1
Mills2 proposed the use of light-emitting diode (LED) technology for the polymerization of light-initiated dental materials to overcome the shortcomings of halogen light-curing units. LEDs use junctions of doped semiconductors to generate light instead of the hot filaments in halogen bulbs. LEDs have a lifetime over 10,000 hours and undergoes little degradation of output over this time. LEDs require no filters to produce blue light, resists shock and vibration, and saves energy to operate. The longer lifespan of LEDs and more consistent light output compared with halogen bulb technology indicate that it should have a great use in dentistry.1,3–5
For these reasons, LED technology is closer to the ideal of a light-curing unit, which in theory should have the following characteristics: light power of at least 300 mW per square centimeter, a narrow spectrum of light emitted (around the wavelength that camphorquinone reacts, with peak absorption at 468 nm), low heat generation, sterilized active point, and silent cooling fan or no fan.6,7
The efficiency of a light-curing unit is closely related to its lighting potency: the higher the luminous density irradiated, the more free radicals involved in the light-curing process, and the more effective the cure of the composite.3–5 The increase in luminous intensity is the key to diminish irradiation time.8
Although the shear bond strength test is more widely used and practical,1,9–15 evaluation of residual monomers through infrared spectroscopy is important in the results of physical test referents to dental materials.16–18 It is a sensitive method that identifies monomers in cured adhesive; monomers are responsible for increased bonding failures8 and can also cause adverse biologic effects, showing estrogenic characteristics.19
The aims of this study were to quantify the residual monomers in the composite films beneath the brackets photo-initiated by LED and halogen lights, and to verify any difference in the polymerization of the central and the peripheral areas according to the light source used.
MATERIAL AND METHODS
Twenty bovine teeth preserved in 0.1% thymol were used after approval by the Ethics Board of State University of Rio de Janeiro. Ten teeth were used to standardize the thickness of the composite film, since different thicknesses would allow different absorbance of light. The brackets were bonded by the same operator (F.A.R.C) without previous enamel conditioning to facilitate removal of the composite film. For debonding, the brackets were bent with pliers, and the films were removed from the dental surface with a surgical blade.
The thickness of each film was measured 3 times with digital calipers. The data were compared statistically by analysis of variance (ANOVA), and there were no statistically significant differences (P = 0.702). Thus, it can be assumed that the thicknesses of the films were standardized.
The other 10 teeth were cleaned with a mixture of water and pumice in a rubber-polishing cup for 15 seconds, thoroughly rinsed with water for 15 seconds, and dried with an oil-and moisture-free airstream. Each tooth was etched with 37% phosphoric acid gel for 15 seconds, rinsed, and dried again.
Transbond XT (3M Unitek, Monrovia, Calif) primer was applied to each tooth, thinned with a gentle stream of air, and cured according to the manufacturer’s recommendations. All brackets were central incisor metal brackets, Edgewise Standard (Morelli, Sorocaba, São Paulo, Brazil) because they have plan bases and are larger than the others, resulting in a longer path for the light to the center of the base, simulating a critical clinic situation. The brackets were bonded to the enamel by using Transbond XT adhesive paste (3M Unitek).
The sample was divided into 2 groups of 5 teeth according to the type of light used to photo-initiate the polymerization process. The procedures for the halogen group were 20 seconds of light exposure 10 seconds at each proximal surface with the Ortholux XT Visible Light Curing Unit (3M Unitek) with light intensity of 400 mW per square centimeter. The procedures for the LED group were 10 seconds of light exposure, 5 seconds in each proximal surface, with the Ortholux LED Curing Light (3M Unitek) with light intensity of 800 mW per square centimeter.
The bonding procedures were performed in the same environment to prevent different luminous intensities that could interfere with the results. The distance of the curing light to the bracket was minimal; once the tooth was isolated, it allowed close positioning of the active point to the bracket-tooth interface. A surge protector was used to prevent input energy variations. The light intensity was measured before and after the experiments to determine whether there was any change, by using an Optilux Radiometer (SDS, Kerr, Danbury, Conn) that measures the energy emitted between 400 and 500 nm.
The samples were identified by group and numbered in the sequence of bonding. They were debonded in the same sequence, minimizing the discrepancy between the bonding and debonding times to standardize the residual polymerization time of the samples. After bonding, the samples were stored in black plastic containers to prevent light and additional irradiation on the composite films. The containers were stored for 24 hours in an incubator at 37°C to simulate the temperature of the human mouth.
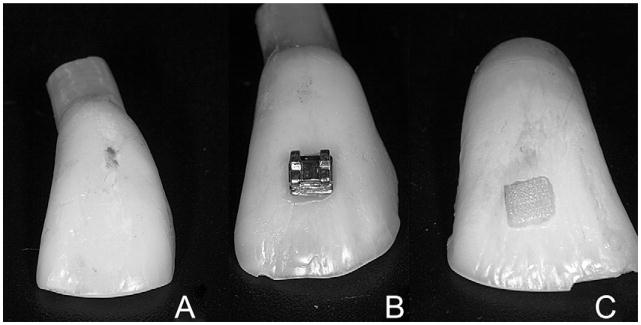
Polymerized composite films were obtained by removing the brackets by their deformation with pliers and preserving the whole film on the dental surface (Fig 1). Digital calipers were used to mark margins of 0.8 mm in the composite, scratching the film with 1 active point and maintaining the other in the external margin of the composite.
Fig 1.
A, Bovine tooth; B, bonded bracket; C, composite film preserved in the dental surface after bracket removal.
Segmentation of the composite was performed through the marked margins with a low-speed diamond bur, making 2 subgroups from each group: central region of the photo-initiated films with the halogen light (CH), peripheral region of the photo-initiated films with the halogen light (PH), central region of the photo-initiated films with the LED (CL), and peripheral region of the photo-initiated films with the LED (PL).
The composite films of each subgroup were mashed into a fine powder, which was weighted by a precision scale to separate an amount between 0.0011 and 0.0012 g from each subgroup.
Infrared spectroscopy was used with the attenuated total reflectance technique. The obtained dust was analyzed in the center of the crystal of the universal accessory for attenuated total reflectance samples, which is part of the Spectrum appliance (PerkinElmer, Salem, Mass). The samples were evaluated in the region of 650 to 4000 cm−1 for 32 scans, with a resolution of 4 cm−1 and pressed by a pistol with a constant tension.
The monomers of Transbond XT are the bisphenol-A-glycidyl methacrylate (Bis-GMA) and the triethylene glycol dimethacrylate (TEGDMA). Both show double bonds in the extremes of the monomer molecule. The unsaturation generates an absorbance of energy from the infrared beam in the region of wave number 1640 cm−1, so that with greater band intensity, more unsaturation and more monomers are present.20 In this study, the spectra were generated according to the transmittance (%T), so that with higher values, fewer monomers were present.
Statistical analysis
The Kolmogorov-Smirnov test was used to verify the normal distribution of the sample. To check for statistical differences among the results, 2-way ANOVA was used, with region as the dependent factor and light type as the independent factor. A significance level of P ≤ 0.05 was considered.
RESULTS
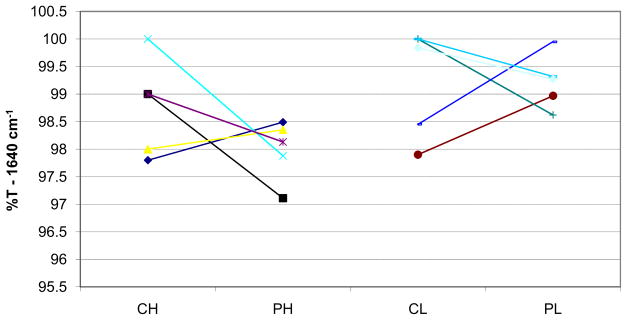
The mean values of band intensity in the wave number 1640 cm−1 were 98.76 ± 0.89 for the CH group, 97.92 ± 0.54 for the PH group, 99.23 ± 0.99 for the CL group, and 99.22 ± 0.49 for the PL group. The statistical analysis showed no significant difference among the central and peripheral regions (P = 0.354). There was a significant difference between light types; the LED group had less residual monomer than did the halogen group (P = 0.014). The interaction between light type and region was not statistically significant (P = 0.368). Mean values and standard deviations are shown in Table I. Table II shows the 2-way ANOVA values, and Figure 2 shows data dispersion.
Table I.
Descriptive statistics: values of transmittance (%T) in the region of wave number 1640 cm−1
| CH | PH | CL | PL | |
|---|---|---|---|---|
| Mean value | 98.76 | 97.92 | 99.23 | 99.22 |
| SD | 0.89 | 0.54 | 0.99 | 0.49 |
Table II.
Results of 2-way ANOVA with the region as the dependent factor and light type as the independent factor
| Source | Type III sum of squares | df | Mean square | F | P |
|---|---|---|---|---|---|
| Tests of within-subjects effects | |||||
| Region | 0.761 | 1 | 0.761 | 0.969 | 0.354 |
| Region* group | 0.714 | 1 | 0.714 | 0.910 | 0.368 |
| Error (region) | 6.279 | 8 | 0.785 | ||
| Tests of between-subjects effects | |||||
| Intercept | 195244.608 | 1 | 195244.608 | 527527.734 | 0.000 |
| Group | 3.664 | 1 | 3.664 | 9.899 | 0.014 |
| Error | 2.961 | 8 | 0.370 | ||
Fig 2.
Dispersion of the %T values in the region of wave number 1640 cm−1.
DISCUSSION
We used bovine teeth, which have chemical, histologic, and anatomic similarities to human teeth, to reproduce the clinical procedure.21–23 Although bovine teeth have been previously used in adhesive studies, the residual monomer evaluations done by Eliades et al,16,19 Kauppi and Combe,8 and Niepraschk et al24 to simulate the clinical situations of orthodontic bonding used other substrates than enamel. These studies cannot be directly compared with our study because they used other substrates to reproduce the refraction index and the topography of human dental enamel.
The adhesive volume quantifications described by Eliades et al in 199516 and 200019 are essential in studies with photo-polymerization, because it influences the thickness of the composite beneath the brackets and alters the entrance of light to photo-initiate the polymerization process. To ensure that the entrance of light was the same, in this study, in addition to comparative quantification of the volumes, we measured 10 composite films. They were previously separated and measured 3 times each by the same operator who executed the bonding, with no significant intraobserver difference.
The methods used by Eliades et al16,19 and Kauppi and Combe8 allowed quantitative analyses of the composite degree of cure. For this purpose, they assessed the absorbance (%A) of the material not polymerized relative to the polymerized one in the spectrum region to measure the percentage of monomer conversion obtained or the degree of cure.
Walton and Lorimer25 described the degree of polymerization as related to the number of “mers” (less repetitive unit of a polymeric molecule) in the polymeric chain. They stated that this can only be evaluated through methods of measuring the molecular weight. Although the molecular weight is closely related to the mechanical properties of the polymers and the infrared region test identifies the unsaturated or unreacted monomer, it can be concluded that the degree of polymerization is high when there is a high conversion. This relationship between the degrees of monomer conversion and polymerization is only possible because the polymerization process of the dental composites is done by a free radical polyaddition reaction, which is characterized as forming polymers with high molecular weight and low degree of monomer conversion at the beginning of the polymerization reaction.
In this study, the residual monomer was measured by evaluating the band intensity at 1640 cm−1 in the infrared region spectrum, once the monomers’ double bonds absorb energy from the infrared beam in this region.20 Because of this, it was possible to detect the residual monomer. The %T value represents the remaining amount of energy of the infrared beam after it passed through the sample, so it can be assumed, with the higher values evaluated, that fewer monomers are present. Previous studies evaluated the degree of cure (%DC), measuring the absorbance of energy (%A) in the same region (1640 cm−1). These studies describe a ratio between the nonpolymerized material (%A) values and the polymerized one, aiming to measure the percentage of monomer conversion obtained or the degree of cure.
Considering the above statements, it is important to understand that both methods (%T and %DC) can identify the presence of nonreacted monomers, but the second one relates a nonpolymerized sample to a polymerized one, achieving with this a ratio described as degree of cure, that should not be confused with degree of polymerization.25
Kauppi and Combe8 reported that subpolymerized adhesive should be avoided, since it is a factor responsible for failure in bonding, in addition to the possibility of adverse biologic effects, although this is still controversial and based on in-vitro studies.8
The biologic effects of the monomers not reacted are still unclear.19 Gioka et al26 affirmed that the residual monomer from self-polymerized or photo-polymerized composites did not show cytotoxic effects in in-vitro evaluations, although they had a little cytostatic effect. Kostoryz et al27 evaluated, in an in-vitro study, metabolites of Bis-GMA and bisphenol F diglycidyl ether (BFDGE) and concluded that those do not have mutagenic or estrogenic effects; they are also less cytotoxic than the no metabolized monomers. Nomura et al28 studied the estrogenicity of monomers and initiation agents and concluded that canforoquinone and Bis-GMA did not show estrogenic effects in vitro. Hashimoto et al29 studied the estrogenic effects in vitro of bisphenol-A, Bis-GMA, TEGDMA, methylmethacrylate, hydroxyethyl methacryIate, dibutyl phthalate, butyl benzyl phthalate, butylbutyleneglycol phthalate, di-2-ethylhexyl phthalate, and dioctyl phthalate and concluded that only bisphenol-A and butyl benzyl phthalate showed estrogenic effects in the concentrations tested.
Compared with conventional lights, the light produced by an LED has a narrower spectral distribution,30 which can justify the results obtained by Stahl et al,31 Cacciafesta et al,10 Dunn and Bush,11 and Mills.2 These authors found no significant differences in polymerization depth, resistance to compression, flexural resistance, and shear resistance of restoring and bonding composites polymerized with LED when compared with halogen lights, even for LEDs with luminous intensity 7 times lower than the halogen light.
Our results showed significant differences between the lights used, with the LED leaving less residual monomer compared with the halogen light (P = 0.014), even when used for half of the irradiation time. This could have happened because the LED evaluated in this study had a higher luminous intensity than the halogen source and because LEDs have a narrower spectral distribution of light.
Regarding the regions, no significant difference was found (P = 0.354). It was expected that the central regions would show more residual monomer than the peripheral, once the cure reduces when the depth increases.11 A possible explanation for this finding is that the central region receives light, even if attenuated, from both sides (mesial and distal), and this amount of irradiance might be similar to the peripheral region, which receives closer irradiance on light of 1 side and a second weakened beam from the other.
No interaction between light type and region was found (P = 0.368); this means that the light type effect is independent of the region, and the region effect is independent of light type.
These results should be analyzed carefully. Although the statistical analysis showed differences between the light types, it might not be clinically significant, since the means and the standard deviations were close.
These findings differ from those of Niepraschk et al,24 who reported a higher degree of cure (%DC) of composite films polymerized for 20 seconds with the Trilight halogen (3M ESPE, Seefeld, Germany) compared with those polymerized for 10 seconds with the Cure LED (Spring Health Products, Norristown, Pa). However, these results cannot be directly compared with ours because those authors used different models of light sources and different evaluation methods.
CONCLUSIONS
The following can be concluded under the conditions of this in-vitro study.
LED (Ortholux LED) leaves less residual monomer compared with the halogen light (Ortholux XT QTH), even when used for half the irradiation time.
There are no differences in residual monomers between the central and peripheral regions.
No interaction was found between light type and region.
Footnotes
The authors report no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.Usumez S, Buyukyilmaz T, Karaman AI. Effect of light-emitting diode on bond strength of orthodontic brackets. Angle Orthod. 2004;74:259–63. doi: 10.1043/0003-3219(2004)074<0259:EOLDOB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Mills RW. Blue light emitting diodes—another method of light curing? Br Dent J. 1995;178:169. doi: 10.1038/sj.bdj.4808693. [DOI] [PubMed] [Google Scholar]
- 3.Burgess JO, Walker RS, Porche CJ, Rappold AJ. Light curing—an update. Compend Contin Educ Dent. 2002;23:889–92. [PubMed] [Google Scholar]
- 4.Duke ES. Light-emitting diodes in composite resin photopolymerization. Compend Contin Educ Dent. 2001;22:722–5. [PubMed] [Google Scholar]
- 5.Jandt KD, Mills RW, Blackwell GB, Ashworth SH. Depth of cure and compressive strength of dental composites cured with blue light emitting diodes (LEDs) Dent Mater. 2000;16:41–7. doi: 10.1016/s0109-5641(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 6.Shortall AC, Harrington E. Guidelines for the selection, use, and maintenance of visible light activation units. Br Dent J. 1996;181:383–7. doi: 10.1038/sj.bdj.4809265. [DOI] [PubMed] [Google Scholar]
- 7.Sze SM. Physics of semiconductor devices. New York: Wiley-Interscience; 1981. pp. 608–19. [Google Scholar]
- 8.Kauppi MR, Combe EC. Polymerization of orthodontic adhesives using modern high-intensity visible curing lights. Am J Orthod Dentofacial Orthop. 2003;124:316–22. doi: 10.1016/s0889-5406(03)00402-5. [DOI] [PubMed] [Google Scholar]
- 9.Bishara SE, Ajlouni R, Oonsombat C. Evaluation of a new curing light on the shear bond strength of orthodontic brackets. Angle Orthod. 2003;73:431–5. doi: 10.1043/0003-3219(2003)073<0431:EOANCL>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Cacciafesta V, Sfondrini MF, Jost-Brinkmann PG, Boehme A. Light-emitting diode technology for orthodontic bonding. J Clin Orthod. 2002;36:461–5. [PubMed] [Google Scholar]
- 11.Dunn WJ, Bush AC. A comparison of polymerization by light-emitting diode and halogen-based light curing units. J Am Dent Assoc. 2002;133:335–41. doi: 10.14219/jada.archive.2002.0173. [DOI] [PubMed] [Google Scholar]
- 12.Dunn WJ, Taloumis LJ. Polymerization of orthodontic resin cement with light-emitting diode curing units. Am J Orthod Dentofacial Orthop. 2002;122:236–41. doi: 10.1067/mod.2002.123949. [DOI] [PubMed] [Google Scholar]
- 13.Silta YT, Dunn WJ, Peters CB. Effect of shorter polymerization times when using the latest generation of light-emitting diodes. Am J Orthod Dentofacial Orthop. 2005;128:744–8. doi: 10.1016/j.ajodo.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Swanson T, Dunn WJ, Childers DE, Taloumis LJ. Shear bond strength of orthodontic brackets bonded with light-emitting diode curing units at various polymerization times. Am J Orthod Dentofacial Orthop. 2004;125:337–41. doi: 10.1016/j.ajodo.2003.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Türkkahraman H, Küçükesmen HC. Orthodontic bracket shear bond strengths produced by two high-power light-emitting diode modes and halogen light. Angle Orthod. 2005;75:854–7. doi: 10.1043/0003-3219(2005)75[854:OBSBSP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Eliades T, Eliades G, Brantley WA, Johnston WM. Polymerization efficiency of chemically cured visible light-cured orthodontic adhesives: degree of cure. Am J Orthod Dentofacial Orthop. 1995;108:294–301. doi: 10.1016/s0889-5406(95)70024-2. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier MA, Stangel I, Ellis TH, Zhu XX. A new method for quantifying the intensity of the C = C band of dimethacrylate dental monomers in their FTIR and Raman spectra. Biomaterials. 2005;26:6440–8. doi: 10.1016/j.biomaterials.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 18.Stansbury JW, Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater. 2001;17:71–9. doi: 10.1016/s0109-5641(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 19.Eliades T, Eliades E, Bradley TG, Watts DC. Degree of cure of orthodontic adhesives with various polymerization initiation modes. Eur J Orthod. 2000;22:395–9. doi: 10.1093/ejo/22.4.395. [DOI] [PubMed] [Google Scholar]
- 20.Rueggeberg FA, Hashinger DT, Fairhurst CW. Calibration of FTIR conversion analysis of contemporary dental resin composites. Dent Mater. 1990;6:241–9. doi: 10.1016/S0109-5641(05)80005-3. [DOI] [PubMed] [Google Scholar]
- 21.Leicestesr H. Biochemistry of the teeth. St Louis: Mosby; 1949. pp. 13–102. [Google Scholar]
- 22.Nakamichi I, Iwaku M, Fusayama T. Bovine teeth as possible substitutes in the adhesion test. J Dent Res. 1983;62:1076–81. doi: 10.1177/00220345830620101501. [DOI] [PubMed] [Google Scholar]
- 23.Oesterle LJ, Shellhart WC, Belanger GK. The use of bovine enamel in bonding studies. Am J Orthod Dentofacial Orthop. 1998;114:514–9. doi: 10.1016/s0889-5406(98)70171-4. [DOI] [PubMed] [Google Scholar]
- 24.Niepraschk M, Rahiotis C, Bradley TG, Eliades T, Eliades G. Effect of various curing lights on the degree of cure of orthodontic adhesives. Am J Orthod Dentofacial Orthop. 2007;132:381–4. doi: 10.1016/j.ajodo.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Walton D, Lorimer P. Polymers. New York: Oxford University Press; 2000. pp. 1–91. [Google Scholar]
- 26.Gioka C, Bourauel C, Hiskia A, Kletsas D, Eliades T, Eliades G. Light-cured or chemically cured orthodontic adhesive resins? A selection based on the degree of cure, monomer leaching, and cytotoxicity. Am J Orthod Dentofacial Orthop. 2005;127:413–9. doi: 10.1016/j.ajodo.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Kostoryz EL, Eick JD, Glaros AG, Judy BM, Welshons WV, Burmaster S, et al. Biocompatibility of hydroxylated metabolites of BISGMA and BFDGE. J Dent Res. 2003;82:367–71. doi: 10.1177/154405910308200508. [DOI] [PubMed] [Google Scholar]
- 28.Nomura Y, Ishibashi H, Miyahara M, Shinohara R, Shiraishi F, Arizono K. Effects of dental resin metabolites on estrogenic activity in vitro. J Mater Sci Mater Med. 2003;14:307–10. doi: 10.1023/a:1022923713892. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto Y, Moriguchi Y, Oshima H, Nishikawa J, Nishihara T, Nakamura M. Estrogenic activity of chemicals for dental and similar use in vitro. J Mater Sci Mater Med. 2000;11:465–8. doi: 10.1023/a:1013009006522. [DOI] [PubMed] [Google Scholar]
- 30.Crawford MG, Holonyak JN, Kish JFA. In pursuit of the ultimate lamp. Sci Am. 2001;2:62–7. [Google Scholar]
- 31.Stahl F, Ashworth SH, Jandt KD, Mills RW. Light-emitting diode (LED) polymerization of dental composites: flexural properties and polymerization potential. Biomaterials. 2000;21:1379–85. doi: 10.1016/s0142-9612(00)00029-6. [DOI] [PubMed] [Google Scholar]