Abstract
Upon binding to the poliovirus receptor (PVR), the poliovirus 160S particles undergo a conformational transition to generate 135S particles, which are believed to be intermediates in the virus entry process. The 135S particles interact with host cell membranes through exposure of the N termini of VP1 and the myristylated VP4 protein, and successful cytoplasmic delivery of the genomic RNA requires the interaction of these domains with cellular membranes whose identity is unknown. Because detergent-insoluble microdomains (DIMs) in the plasma membrane have been shown to be important in the entry of other picornaviruses, it was of interest to determine if poliovirus similarly required DIMs during virus entry. We show here that methyl-β-cyclodextrin (MβCD), which disrupts DIMs by depleting cells of cholesterol, inhibits virus infection and that this inhibition was partially reversed by partially restoring cholesterol levels in cells, suggesting that MβCD inhibition of virus infection was mediated by removal of cellular cholesterol. However, fractionation of cellular membranes into DIMs and detergent-soluble membrane fractions showed that both PVR and poliovirus capsid proteins localize not to DIMs but to detergent-soluble membrane fractions during entry into the cells, and their localization was unaffected by treatment with MβCD. We further demonstrate that treatment with MβCD inhibits RNA delivery after formation of the 135S particles. These data indicate that the cholesterol status of the cell is important during the process of genome delivery and that these entry pathways are distinct from those requiring DIM integrity.
The process of genome delivery for poliovirus is initiated by the virus capsid binding to its cognate cell surface receptor, the poliovirus receptor (PVR/CD155) (29). The PVR, besides being a binding site on the cell surface for the virus, also functions as a catalyst to facilitate an energy-requiring conformational transition of the poliovirus capsid (40). This conformational transition changes the sedimentation coefficient of the virion from 160S to 135S and results in exposure of the N termini of VP1 and of VP4 sequences on the poliovirus capsid surface (16, 40).
Evidence is consistent with the model that the 135S particle is an entry intermediate which becomes an 80S empty capsid upon delivery of the RNA genome into cells. The 135S particle is capable of binding to liposomes upon exposure of the VP1 N termini, possibly in conjunction with the myristoylated VP4 protein (16). In addition, upon interacting with cellular membranes, the 135S particle is able to infect cells in a PVR-independent manner (10, 20). Amino acid substitutions in the VP1 N termini generate viruses with altered RNA delivery kinetics, demonstrating that this VP1 membrane-binding region may also be important in vivo for RNA uncoating and/or penetration (9, 22). Other studies show that upon formation of the 135S particle, exposure of VP4 sequences leads to the localization of VP4 to the membranes of infected cells and that the VP4 sequence also regulates the infectivity of the virus (11). Mutations in the VP4 sequences affect both the ability of the virus to deliver RNA after 135S particle formation and the interactions of the virus with lipid bilayers to form ion channels.
Although the domains of the 135S particle (such as VP4 and N termini of VP1) that mediate these membrane interactions during virus entry have been studied, neither the lipid composition nor the identity of the membrane involved at this stage is known. Recently, the entry of two other picornaviruses, echovirus 1 (25) and echovirus 11 (37), has been shown to require cholesterol-rich detergent-insoluble membrane microdomains (DIMs) at the cell surface. Because the interaction of poliovirus with cellular membranes is important during virus entry, it is of interest to investigate whether DIMs are important for viral entry.
DIMs in the plasma membrane can be disrupted by treatment with agents that bind, sequester, or deplete membrane cholesterol (36). Due to its ability to sequester cholesterol in its hydrophobic pocket, the cholesterol-depleting agent methyl-β-cyclodextrin (MβCD) disrupts DIMs by removing cholesterol from the membranes (8, 21, 41) and has been used to show that DIMs are important for the entry of other viruses (14, 25, 27, 37). We report here that poliovirus infection is inhibited by treating cells with MβCD. This inhibition can be partially compensated by replenishing cholesterol levels in the cells, suggesting that the effect of MβCD treatment on virus infection is through its ability to remove cholesterol. However, in contrast to echovirus 1 and 11, neither poliovirus nor PVR localize during entry to the DIMs, which would be disrupted upon MβCD treatment. We also demonstrate that MβCD treatment prevents infection by inhibiting RNA delivery into host cells at a stage after the receptor-mediated conformational transition to form 135S particles. These results suggest that poliovirus entry is not dependent on DIM integrity and that the entry pathway(s) leading to cytoplasmic delivery of the viral genome is affected by the cholesterol environment at the cell surface.
MATERIALS AND METHODS
Cells.
HeLa-S3 cells were maintained in suspension (3 × 105 to 4 × 105 cells/ml) in Joklik's modified minimal essential medium (S-MEM) supplemented with 7.5% horse serum and 1 mM MEM sodium pyruvate. For monolayer growth, cells were transferred into Dulbecco's modified Eagle's (DME) medium supplemented with 5% fetal calf serum (FCS), 50 U of penicillin/ml, 50 μg of streptomycin/ml, and 1 mM sodium pyruvate (DME-5% FCS) and grown in a 5% CO2 incubator. Hep2c cells, obtained from the American Type Culture Collection, were maintained in DME-10% FCS. PVR-enhanced green fluorescent protein (EGFP)-expressing CHO cells were generously provided by X. Ning. These CHO cells stably express, via retrovirus-mediated transduction, a PVR-EGFP fusion construct in which sequences encoding EGFP are fused to the C terminus of the alpha form of PVR. PVR-EGFP-positive CHO cells were maintained in F12 medium supplemented with 10% FCS and 800 μg of G418/ml.
Viruses.
Poliovirus serotype 1 Mahoney strain was the parental wild-type virus for all studies. Viral stocks were propagated in HeLa cells as previously described (30). 135S particles were generated from purified 160S particles as described earlier (10). A recombinant poliovirus that expresses EGFP (polio-EGFP) was generated by a strategy similar to those described earlier (1, 26). pPVM1-EGFP cDNA contains the EGFP gene inserted in frame upstream of the P1 region separated by a 3C cleavage site. Seed stocks of polio-EGFP virus were generated from coupled vaccinia virus-poliovirus cDNA transfections using DOTAP transfection methods instead of electroporation (35). Vaccinia virus-T7-infected cells were transfected with 10 μg of pPVM1-EGFP cDNA using 45 μg of DOTAP liposomal transfection reagent (Roche, Indianapolis, Ind.) and transferred to plates (106 cells/ml). Cells were harvested at 12 h posttransfection and lysed in reticulocyte standard buffer (10 mM Tris-HCl [pH 7.4], 10 mM NaCl, 1.5 mM MgCl2)-1% NP-40 at 4°C. Virus was concentrated from the postnuclear supernatant by centrifugation at 50,000 rpm for 1 h at 4°C in a Beckman 70.1 Ti rotor and partially purified by 15-to-30% sucrose density gradients. Fractions constituting the 160S virus peaks were identified using a parallel gradient of [35S]methionine-labeled poliovirus. These fractions were pooled and dialyzed against phosphate-buffered saline (PBS), and the working stocks of polio-EGFP (passage 2 [P2]) were generated from this seed stock in a single low-multiplicity passage. The low-multiplicity-of-infection (MOI) cells were harvested when the majority of cells showed green fluorescence, and virus was released from infected cell pellets by three cycles of freeze-thaw and used without further purification. Typical polio-EGFP stocks had titers of 2 × 108 PFU/ml. Stocks beyond the P2 passage often contained viruses which had lost the EGFP sequence from the viral genome. Therefore, the P2 passage was used for all experiments conducted here. Vesicular stomatitis virus (VSV) and VSV-expressing GFP were obtained from M. Whitt (University of Tennessee, Memphis) and were propagated by low-multiplicity passages on BHK cells.
Measurement of cholesterol levels.
HeLa cells (107 cells/200 μl of PBS) were lysed by three cycles of freeze-thaw followed by sonication in a water bath sonicator (3 bursts of 20 s at room temperature). Cholesterol was extracted from cell lysates by adding chloroform (400 μl) and methanol (400 μl) to the sonicated cell lysate (100 μl). The bottom (chloroform) layer was collected and evaporated under vacuum. The residual cholesterol was dissolved in ethanol and assayed using the Infinity cholesterol estimation kit (Sigma Diagnostics, St. Louis, Mo.). Using this colorimetric assay, the absorbance (490 nm) versus cell number is linear with cell numbers varying over 2 orders of magnitude. To determine the percentage of cholesterol remaining after MβCD treatment, the cell number equivalent to the measured absorbance was obtained from a standard curve and divided by the total number of cells used for cholesterol extraction and multiplied by 100.
MβCD treatment of cells.
For cholesterol removal, cell monolayers were washed with PBS and incubated for 1 h at 37°C with serum-free DME in the absence (control cells) or presence (treated cells) of 5 mM MβCD (Sigma). For cholesterol replenishment, cholesterol (0.25 mM, final concentration) was added to DME containing 2.5 mM MβCD and was filter sterilized. To replenish cellular cholesterol levels, MβCD-treated cells (see above) were incubated for 1 h in cholesterol-MβCD-containing medium at 37°C. After 1 h, the medium was removed and the cells were washed to remove the MβCD. Following either cholesterol depletion or replenishment, the cells were processed as required for each experiment.
Poliovirus infection of control, MβCD-treated, and MβCD-cholesterol-treated cells.
Poliovirus was bound to monolayers of control, MβCD-treated, or MβCD-cholesterol-replenished cells for 30 min at room temperature. Unbound virus was removed by washing with PBS, and the infection was initiated by the addition of serum-free DME, prewarmed to 37°C. Cells were harvested at various times postinfection (p.i.), and the cell pellet was lysed by three cycles of freeze-thaw for plaque assays or used to isolate total RNA for reverse transcriptase PCR (RT-PCR) analyses. When viral replication was inhibited, guanidine hydrochloride (GuHCl; 200 μg/ml) was added at 30 min p.i. and maintained throughout the course of infection.
Infectious center assays.
Control or MβCD-treated cell monolayers were infected with poliovirus (160S or 135S particles) or VSV. At 1 h p.i., the medium was removed and the cells were harvested by trypsinization. Cells were washed twice with DME-5% FCS (prewarmed to 37°C), resuspended in DME-5% FCS, and counted. Known numbers of cells were plated on confluent monolayers of HeLa cells for 2 h, and an agar overlay was added. The resultant number of plaques was counted after 36 to 48 h. The percentage of infected cells was determined as the average number of plaques (in triplicate or more) divided by the theoretical number of cells added to each plate.
Flow cytometry.
Polio-EGFP-infected control or MβCD-treated cell monolayers were harvested at 5 to 6 h p.i. by trypsinization. The infected cell pellets were washed with PBS and fixed in PBS containing 2% paraformaldehyde. Parallel cultures of uninfected MβCD-treated and untreated cells were used as negative controls. Fixed cells were analyzed for EGFP fluorescence using a Becton-Dickinson FACSCalibur. For analysis of cells infected with 135S virus, the fixed infected cells, after saponin-mediated permeabilization, were stained with anti-3D monoclonal antibodies (1:100 dilution) and goat anti-mouse secondary antibodies conjugated to fluorescein isothiocyanate (1:200 dilution; Caltag). Infected cells, stained similarly with an isotype control primary antibody and fluorescein isothiocyanate-conjugated goat anti-mouse secondary antibodies, were used as negative controls.
RNA extraction and RT-PCR.
RNA was isolated from harvested cells using RNAZol B (Tel-Test, Friendswood, Tex.) according to the manufacturer's instructions. RNA from 105 cell equivalents was analyzed by RT-PCR using a MasterAmp RT-PCR kit (Epicentre Technologies, Madison, Wis.). Random hexamers were added to prime the reverse transcription reaction, and resultant cDNAs were PCR amplified using primers E1 and E2 specific for the 5′ untranslated region of poliovirus (13) or using primers specific for human β-actin (Stratagene, Palo Alto, Calif.). PCR products were resolved on 1.5% agarose gels.
Binding assays.
35S-labeled poliovirus was bound to control or MβCD-treated cell monolayers at various multiplicities for 60 min at 4°C. The cells were washed with chilled PBS to remove unbound virus and harvested by scraping into PBS, and the cell-associated radioactivity was quantitated by scintillation counting.
Fractionation of detergent-insoluble and -soluble membranes.
Cells were fractionated into detergent-soluble and -insoluble fractions as described previously (32). Briefly, control or MβCD-treated HeLa or similarly treated PVR-EGFP-expressing CHO cells (107 cells/150-mm plate) were infected with poliovirus (MOI = 5). At various times p.i., the cells were harvested by scraping into PBS (at 4°C) and centrifuged at 1,000 × g. Cell pellets were lysed in 500 μl of TNE (10 mM Tris, 150 mM NaCl, 5 mM EDTA)-1% TX-100 at 4°C. The cell lysate was fractionated by centrifugation at 13,000 × g for 10 min at 4°C. The resultant pellet contained DIMs. The supernatant contained the cytosol and detergent-soluble membranes. The pellet was resuspended in 500 μl of TNE-0.5% sodium dodecyl sulfate. Fractions were analyzed for the presence of various viral and cellular proteins by Western blot analysis.
Western blot analysis.
Fractions were resolved on 12.5% polyacrylamide gels and were transferred to nitrocellulose or polyvinylidene difluoride membranes. The membranes were blocked in TBST (10 mM Tris-Cl [pH 7.5], 150 mM NaCl, 0.1% Tween 20) containing 5% nonfat milk (TBST-milk) for 1 h at room temperature. CD55 and EGFP were detected as follows. The membranes were incubated overnight at 4°C with CD55 (1:5,000 dilution; Santa Cruz Biotechnology Inc., Santa Cruz, Calif.) or EGFP (1:250 dilution; Clontech, Palo Alto, Calif.) antibodies in TBST-milk. Horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (1:20,000 dilution; Gibco) were added for 1 h at room temperature. After washing the membranes with TBST for 10 min after each antibody incubation, the blots were developed using the Renaissance Western Blot Chemiluminescence Reagent Plus (NEN, Boston, Mass.). VP1 and VP4 were detected as follows. VP1 (1:10,000 dilution) and VP4 (1:2,000 dilution) polyclonal primary antibodies were incubated overnight with the membranes in TBST-milk (7, 24). Blots were washed with TBST and were subsequently incubated with alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (1:7,500 dilution; Boehringer Mannheim) for 30 min at room temperature. The blots were developed using Nitro Blue Tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (Fisherbiotech, Fair Lawn, N.J.). GM1 was detected as follows: horseradish peroxidase-labeled β-subunit of cholera toxin (10 μg/ml; Sigma) was added for 1 h at room temperature in TBST-milk. The blot was developed using Renaissance Western Blot Chemiluminescence Reagent Plus (NEN).
RESULTS
MβCD treatment inhibits poliovirus infection.
MβCD treatment of HeLa cells reduced the levels of cellular cholesterol by 90% compared to levels found in control untreated cells (Fig. 1A). Repeated exposures with MβCD did not lead to further reductions in cellular cholesterol levels, indicating that the 10% residual levels of cholesterol represented threshold levels of cholesterol which could not be extracted by further MβCD treatment. In addition, when MβCD-treated cells subsequently were maintained for 12 additional hours in serum-free medium, no restoration of cellular cholesterol levels was observed (Fig. 1B). This is consistent with previous studies, which showed that the effects of MβCD treatment on cellular cholesterol levels were long-lasting relative to the length of a single poliovirus replication cycle (4 to 6 h) (15). Consequently, to examine the effects on viral infection due to cholesterol removal by MβCD treatment, it should be sufficient to incubate the cells with MβCD and subsequently remove the MβCD prior to initiating infection. The kinetics of viral growth were compared in untreated control and MβCD-treated cells infected at high multiplicity over the period of a single-step growth cycle (Fig. 1C). As expected in untreated control cells the virus grew rapidly, with titers increasing by 3 orders of magnitude between 2 and 5 h p.i.; maximal titers were achieved within 4 to 6 h p.i. In contrast, virus growth was inhibited in MβCD-treated cells; titers at 8 h p.i. in MβCD-treated cells were reduced by 1 log compared to titers in untreated cells.
FIG. 1.
MβCD treatment inhibits the kinetics of poliovirus growth. (A) MβCD treatment reduces cellular cholesterol levels. Cholesterol levels were measured from equal numbers of untreated HeLa cells or HeLa cells after one or two cycles of MβCD treatment. (B) Effects of MβCD treatment on cellular cholesterol levels are long lasting. Cholesterol was extracted from equivalent numbers of untreated cells, MβCD-treated cells, and MβCD-treated cells that were grown for 12 h in serum-free medium. (C) Virus infection (MOI = 5) of MβCD-treated (♦) and untreated control (○) HeLa cells. Infected cells were harvested at the indicated times, and viral titers were measured by plaque assays.
The reduced titers from MβCD-treated cells could be due to virus replication being inhibited throughout the entire cell population or to infection occurring in only a fraction of the MβCD-treated cell population. To distinguish between these possibilities, infection of MβCD-treated cells was analyzed in infectious center assays (Fig. 2A). Following MβCD treatment, only 17% of the plated cells formed plaques. In contrast, virtually all of the cells from the untreated control population (80 to 90%) generated plaques. Similar results were observed in MβCD-treated and untreated control cells that were infected with polio-EFGP (Fig. 2B). This recombinant poliovirus expresses EGFP during replication, and the resultant fluorescence in the infected cells can be detected by 3 to 4 h p.i. Flow cytometric analyses showed that by 5 h p.i., 94% of the infected control cells were positive for EGFP fluorescence, whereas in MβCD-treated cells only 15% were EGFP positive. Thus, the infectious center assays and flow cytometric analyses both indicated that MβCD treatment reduced the frequency of infected cells within the HeLa cell population. With repeated treatments with MβCD, the percentage of the MβCD-treated cell population that remained permissive to viral infection was unchanged, suggesting the presence of an MβCD-nonresponsive subpopulation. Similar results were observed with infected control and MβCD-treated HEp2c cells (Fig. 2C and D) and with MβCD-treated Vero cells and a stably transfected, PVR-expressing line of CHO cells which were poliovirus permissive (data not shown). To exclude the possibility that the inhibitory effects of MβCD treatment on poliovirus infection were due to nonspecific toxicities or drastic alterations in cell physiology, the percentages of VSV-infected control and MβCD-treated HeLa cells were measured (Fig. 2E and F) (14, 28). The percentages of VSV-infected cells were similar in MβCD-treated or untreated cells. Thus, MβCD treatment appeared to reduce the percentage of cells that were permissive for poliovirus infection. Because MβCD treatment depletes cells of cholesterol, these results imply that modulation of cellular cholesterol levels affects the frequency of cells susceptible to viral infection.
FIG. 2.
MβCD treatment reduces the frequency of poliovirus-infected cells. MβCD-treated or control cells were infected at high multiplicities (MOI = 5) with either poliovirus (A to D) or VSV (E and F) and analyzed in infectious center assays (A, C, and E) or by flow cytometry (B, D, and F). For flow cytometry, histograms shown are of poliovirus-infected untreated (no fill) and MβCD-treated (grey filled) cells. The percentage of cells with fluorescent intensities above those of uninfected, MβCD-treated, or untreated cells is indicated in the insert table. (A and B) Poliovirus-infected HeLa cells; (C and D) poliovirus-infected Hep2c cells; (E and F) VSV-infected HeLa cells.
If the effects of MβCD on virus infection were due to the removal of cellular cholesterol, then cholesterol replenishment in MβCD-treated cells might counter the effects of the initial depletion. Although MβCD treatment extracts cholesterol from cellular membranes, MβCD when previously complexed to cholesterol acts as a cholesterol donor to cells (8). Thus, cells were initially incubated with MβCD to deplete cellular cholesterol levels and subsequently incubated with MβCD-cholesterol complexes to replenish cellular cholesterol levels. Treatment with MβCD-cholesterol complexes partially restored cellular cholesterol levels to ∼50% of the original value (Fig. 3A). To determine whether the increased cholesterol levels had an effect on viral infection, virus yields from infected MβCD-cholesterol-treated cells were compared with those from infected MβCD-treated and untreated cells (Fig. 3B). The effect of the partial cholesterol replacement on virus titers was to partially restore the yield of virus to ∼50% of that from untreated control cells. To determine if this effect was due to increased burst sizes from the 10 to 15% of MβCD-treated cells that remained permissive for viral infection or due to an increased numbers of cells able to support infection, the infected cells were analyzed by flow cytometry (Fig. 3C). Upon cholesterol replenishment, the percentage of GFP-positive cells in the infected population increased. The increased percentage of permissive cells was also confirmed by infectious center assays (data not shown). These results strongly suggest that increasing cellular cholesterol levels resulted in increased numbers of cells permissive to poliovirus infection and that MβCD-mediated reduction of cellular cholesterol levels leads to inhibition of virus infection.
FIG. 3.
Cholesterol replenishment reverses the effects of MβCD treatment. (A) Cholesterol levels were measured from equal numbers of control cells, MβCD-treated cells, or MβCD-treated cells which were subsequently incubated with MβCD-cholesterol (MβCD-Chol) complexes. (B) Control, MβCD-treated, and MβCD-cholesterol-treated cells were infected with poliovirus (MOI = 5) and harvested at 6 h p.i. Titers were measured, and percentage yields were determined with titers from infected untreated (control) cells normalized to 100%. (C) Flow cytometry analysis of infected untreated control (dotted line), MβCD-treated (solid line), and MβCD-cholesterol complex-treated (grey filled) cells. Cells were infected with polio-EGFP (MOI = 5), harvested at 5 h p.i., and analyzed by flow cytometry. The range of positive fluorescent intensities above that of uninfected cells is shown. The percentage of GFP-positive cells is shown in the insert table.
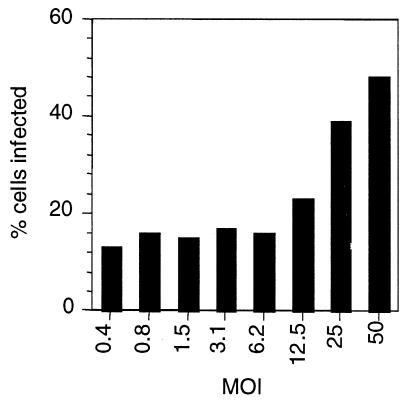
Other studies showed that the inhibition seen upon MβCD treatment is multiplicity dependent (Fig. 4). At multiplicities below 6, a constant fraction of cells (approximately 15%) remained permissive to poliovirus infection after MβCD treatment, whereas at multiplicities greater than 6 increasing percentages of the cell population were infected. The inhibitory effects of MβCD treatment were less pronounced at very high MOIs, suggesting that infection at very high multiplicities could counter the inhibition due to cholesterol depletion.
FIG. 4.
MβCD inhibition of virus infection is multiplicity dependent. Control and MβCD-treated cells were infected with polio-EGFP at varying multiplicities, and the percentages of GFP-positive cells were measured by flow cytometry. Because the fraction of infected cells in the control untreated population changed with MOI, the percentage of infected cells is the percentage of GFP-positive cells in MβCD-treated samples divided by the percentage of GFP-positive cells in the control untreated samples.
MβCD inhibition of poliovirus infection is not due to disruption of DIM integrity.
MβCD-mediated removal of cholesterol leads to disruption of cholesterol-rich DIMs (commonly known as lipid rafts) in plasma membranes. Thus, if poliovirus normally localized to these membrane microdomains during entry, disruption of DIMs could inhibit viral infection. DIMs can be distinguished to some extent by their resistance to solubilization by detergents (such as Triton X-100) at low temperatures, and this property was used to isolate DIMs and detergent-soluble fractions from MβCD-treated and untreated cells. To validate the DIM fractionation strategy, the distributions of a known DIM-associated protein, CD55, and a DIM marker ganglioside, GM1, in detergent-soluble and -insoluble fractions, were examined (Fig. 5A). As expected, both CD55 and GM1 localized to the DIM fractions and not to the detergent-soluble fraction. In addition, as reported previously, MβCD treatment of cells prior to membrane fractionation did not alter the localization of CD55 and GM1; CD55 and GM1 remained localized to the detergent-insoluble fraction (17, 21, 34).
FIG. 5.
PVR and poliovirus localize to the detergent-soluble membrane fractions. (A) Detergent-soluble (S) and -insoluble (I) membranes were isolated from MβCD-treated (+) or untreated (−) cells and analyzed by Western blotting for the presence of CD55 and the ganglioside GM1. (B) Detergent-soluble (S) and -insoluble (I) membranes were isolated from MβCD-treated (+) or untreated (−) infected cells expressing the EGFP-tagged PVR at 0 and 30 min p.i. Similar membrane fractions were also isolated from infected MβCD-treated cells. The isolated fractions were analyzed by Western blotting to detect capsid proteins VP1 and VP4 using capsid-specific polyclonal antibodies or to detect PVR using anti-EGFP antibody.
DIMs and soluble membrane fractions were isolated from control and MβCD-treated cells after virus binding, and the presence of poliovirus and/or its receptor was examined in these fractions (Fig. 5B). In uninfected cells, PVR was found not in the DIM fractions but in the detergent-soluble membrane fractions (data not shown). Upon virus binding and throughout the period of virus entry (0 to 30 min p.i.), PVR remained associated with the detergent-soluble membrane fractions; no PVR was detected in the DIM fractions. Moreover, consistent with its localization to detergent-soluble fractions, localization of the PVR (in the presence or absence of bound virus) was unaffected by MβCD-mediated disruption of DIMs.
Analyses of the location of the viral capsid proteins in these fractions yielded similar results. At the start of infection when virus was bound to cells via its receptor, the poliovirus capsid proteins, VP1 and VP4, localized to the soluble membrane fractions from both control and MβCD-treated cells. These proteins remained associated with the soluble membrane fractions throughout the early times p.i. after 135S particle was formed and insertion of VP4 into the membranes had occurred (11). Similar fractionation of cells infected with 35S-labeled poliovirus indicated that only background levels (1 to 3% of total cell-associated radioactivity) of the input virus were associated with DIM fractions from untreated control cells, and this fraction of the cell-associated label was unchanged after MβCD treatment (data not shown). Collectively, these results suggested that neither the PVR nor the poliovirus capsid proteins selectively localize to DIMs during infection. Thus, it is unlikely that MβCD inhibits viral infection by disrupting DIMs.
MβCD treatment inhibits poliovirus entry after formation of 135S particles.
MβCD inhibited viral infection in the majority of the cells. Consequently, although a fraction of the cell population (<20%) remained permissive to poliovirus infection after MβCD treatment, it was possible to determine the stage of virus infection that was affected by MβCD treatment in the majority of the cells. Virus yields were maximally reduced when cells were treated with MβCD prior to the start of infection; the percentage of infected cells and total virus yields were reduced by 80 to 90% (Fig. 6A). Interestingly, addition of MβCD at 10 min or later after the start of infection had little to no effect on virus yields or the frequencies of infected cells. Because MβCD was only effective in inhibiting infection when added prior to or at the start of infection, these data suggest that a very early stage in the virus infection process was sensitive to MβCD treatment.
FIG. 6.
MβCD inhibition occurs during early stages of poliovirus infection. (A) MβCD (5 mM) was added to HeLa cells at the indicated times before or during the course of viral infection (MOI = 5) for a period of 1 h. The percentage of GFP-positive cells (▪) was measured by flow cytometry at 5 h p.i. Viral titers (░⃞) were determined by plaque assays of cell-associated virus at 6 h p.i. Virus yield at 6 h p.i. from infected untreated control cells was considered 100%. (B) At the indicated multiplicities, 35S-labeled poliovirus was bound to control (▪) or MβCD-treated (░⃞) HeLa cell monolayers for 1 h, and the cell-associated radioactivity was measured by scintillation counting. (C) Control or MβCD-treated CHO cells were infected with 135S virus particles (MOI = 5). The percentages of infected cells and resultant virus yields at 6 h were measured by infectious center assays (▪) and plaque assays on HeLa cells (░⃞). The titer from control untreated cells was considered 100%.
The entry phases of poliovirus infection can be artificially divided into two stages. The first stage requires PVR binding and PVR-mediated conformational transition in the virus capsid to form the 135S particle. The second stage occurs in a PVR-independent manner and allows delivery of RNA into the cells by the 135S particle (10, 20). MβCD treatment did not affect either the ability of the virus to bind to cells (Fig. 6B; Fig. 5B, T = 0 lanes) or the stability of the 160S virus particle (data not shown). To determine if MβCD affected virus infection after the formation of 135S particle, the yields and the percentages of infected cells were determined after infection was initiated using in vitro-generated 135S virus particles (Fig. 6C). As seen for infections with the 160S form of the virus, total virus yields from infection with 135S particles and the frequency of infected cells were also reduced as a result of MβCD treatment. These data indicate that MβCD treatment affects poliovirus infection at a stage(s) after the 160S-135S conformational transition.
Formation of the 135S particle is followed by delivery of RNA into the host cells. Viral RNA delivery was examined in MβCD-treated and untreated cells at various times p.i. by RT-PCR (Fig. 7A). Consistently, strong positive signals were seen at times later in MβCD-treated cells than in control cells. The strength of the signal is dependent on RNA replication as well as RNA delivery. Thus, the presence of RNA signals in MβCD-treated cells was clearly due in part to viral replication, which was occurring in the small fraction of MβCD-treated cells that remained permissive for viral infection. However, the issue is whether, in the majority of cells which were MβCD responsive, MβCD affected RNA delivery and/or RNA replication. To distinguish between its potential effects on RNA delivery versus RNA replication, MβCD was added at 30 min p.i., a time when RNA delivery should have already occurred in all cells but viral replication would be initiating (Fig. 7B). No significant delays in the appearance of RT-PCR signals were detected between MβCD-treated and control cells. In contrast, when GuHCl, a known inhibitor of polioviral RNA synthesis, was added at 30 min p.i., no RNA products were obtained. These data indicate that MβCD treatment does not inhibit overall viral RNA replication or translation but inhibits delivery of the viral genome into the cell. Consistent with RNA delivery being inhibited is the fact that the majority of MβCD-treated cells when infected with polio-EGFP failed to express EGFP by flow cytometry (Fig. 2 and 3). Using MβCD-cholesterol complexes to replenish cellular cholesterol levels in MβCD-treated cells caused a partial restoration of RNA signals to earlier times (Fig. 7C) and was consistent with data (Fig. 3) showing that an increased fraction of cells becomes permissive when cholesterol levels are replenished. Thus, cytoplasmic delivery of the viral genome during entry appears to be affected by the cholesterol status of the cell.
FIG. 7.
MβCD inhibits RNA delivery during virus entry. (A) RNA was extracted at various times p.i. from control cells or cells pretreated with MβCD and analyzed by RT-PCR using primers specific for viral RNA or β-actin mRNA sequences. (B) MβCD or GuHCl was added to infected cells at 30 min p.i. RNA was extracted at various times and analyzed by RT-PCR. (C) Control cells or cells pretreated with MβCD or MβCD-cholesterol complexes were infected with poliovirus (MOI = 5). At various times p.i., RNA was extracted and analyzed by RT-PCR to detect viral RNA or β-actin mRNA.
DISCUSSION
The data show that MβCD treatment inhibits poliovirus entry at a stage of RNA delivery after receptor binding and the formation of the 135S particle. Previously, cholesterol depletion by MβCD was shown to affect the entry of other nonenveloped viruses, echovirus 1 (25) and echovirus 11 (37), as well as simian virus 40 (6, 27). The receptors for each of these viruses, α2β1 integrin, CD55, and major histocompatibility complex class I, respectively, all localize to cholesterol-rich DIM domains prior to or as a result of virus attachment. These receptors therefore enable the viruses to access caveolin-dependent endocytic pathways which occur from these domains. Removal of cholesterol by MβCD disrupts these pathways, thereby affecting entry of these viruses. We show here that PVR and poliovirus do not localize to DIMs during virus entry. Therefore, in contrast to these other nonenveloped viruses, disruption of the integrity of DIMs by cholesterol depletion is unlikely to explain the inhibitory effects of MβCD on poliovirus entry. In addition, other data indicate that poliovirus infection can still occur even in the presence of a dominant-negative dynamin (12; E. S. Mittler, J. M. Bergelson, and J. M. Hogle, personal communication), which inhibits both caveolin- and clathrin-dependent endocytosis (18, 19, 33). These data collectively argue that poliovirus can enter cells via DIM-independent, caveolin-independent pathways.
Potential effects of cholesterol on virus entry.
Inhibition of viral infection occurs as cholesterol content decreases due to MβCD treatment. This correlation, coupled with the ability to reverse this inhibition by increasing cellular cholesterol levels, strongly suggests that it is the effects of cholesterol depletion per se which inhibit poliovirus entry. Consistent with this view, modulation of cellular cholesterol content by inhibiting cholesterol biosynthesis (rather than removal by MβCD) also negatively impacts poliovirus infection (31).
During poliovirus entry, the interaction of the virus particle with cellular membranes is critical (11). Being a key component of the host cell membranes, cholesterol could inhibit RNA delivery potentially by several different mechanisms. Although poliovirus entry does not require localization to DIMs on the cell surface, cholesterol may affect virus entry by altering the interaction of the virus particle with host cell membranes. Cholesterol levels are important for maintaining membrane fluidity, and its removal can reduce lateral diffusion within the membrane. This reduction in fluidity could perhaps affect migration of PVR within the membranes. During virus infection, the data here show that PVR and PVR-virus complexes do not migrate to DIMs during virus entry. However, the in vivo stoichiometry of PVR binding to 160S particles is not known. Thus, upon initial virus binding to a single PVR on the cell surface, local recruitment of additional PVR molecules may possibly be required to catalyze the 160S-to-135S conformational transition. Although the kinetics of 135S formation appear to be similar overall in MβCD-treated and control cells (data not shown), decreases in membrane fluidity may inhibit this local recruitment of additional PVR molecules and thus contribute to the MβCD-mediated inhibition seen when infection is initiated using 160S virus particles. However, differences in receptor recruitment resulting from cholesterol-mediated alterations in membrane fluidity cannot explain the MβCD-mediated inhibition of infection by 135S particles (Fig. 6C), which is PVR independent.
Poliovirus interactions with lipid bilayers also lead to the formation of ion channels, and the ability of the virus to form ion channels in lipid bilayers correlates with its ability to deliver RNA during entry into host cells (11, 39). The lipid environment, including cholesterol levels, is known to affect the electrical properties of other ion channels (3, 23; reference 2 and references therein). Although the virus is able to form ion channels in non-cholesterol-containing lipid bilayers (11, 39), the cholesterol content of membranes could affect the electrical properties of these virus-induced ion channels, or the channels formed on cellular membranes during entry may require the presence of cholesterol for successful cytoplasmic delivery of the genome.
Cholesterol depletion by MβCD treatment also could affect poliovirus entry by affecting cellular signaling pathways. MβCD treatment of several cell lines, such as Cos-1 cells and NIH 3T3 cells, results in the activation of mitogen-activated protein kinase pathways through activation of phosphatidylinositol-3 kinase (4). Removal of cholesterol in these cell types also causes activation of the epidermal growth factor signaling pathway due to ligand-independent dimerization of the epidermal growth factor receptor (5). It is possible that such signaling pathways are also induced as a result of MβCD treatment in the cell types used in this study. Other studies have indicated that cholesterol removal results in an inhibition of cellular signaling pathways (38). Modulation of these or other unknown signaling pathways may be deleterious to the process of viral RNA delivery.
MβCD-resistant subpopulation of cells.
A small population of cells (∼15%) remains infectible after MβCD treatment. For a number of reasons, we feel that this population is not due to a technical artifact. The size of the MβCD-resistant population does not decrease after repeated treatments with MβCD, suggesting that the existence of this population is not due to incomplete removal of cholesterol during the initial treatment with MβCD. Although infection at high multiplicities (MOI > 6) can overcome the inhibitory effects of MβCD treatment, approximately 15% of the cells are MβCD resistant even when cells are infected at low multiplicities (MOI = 0.4). Thus, it is unlikely that this resistant cell population represents the small fraction of cells at each MOI which statistically might be infected at high multiplicity. Although the number of GFP+ events is significantly lower in MβCD-treated cells, the range of fluorescent intensities seen in this population is similar to that in infected control cells (Fig. 2). Similarly, the size of the plaques from MβCD-treated and untreated control cells seen in the infectious center assays are similar (data not shown). These latter sets of data suggest that the susceptible subpopulation present in the MβCD-treated samples appears to be fully permissive for viral infection and that the burst size from the infected MβCD-treated cells is similar to that from control cells. Several other explanations are possible in accounting for the existence of this MβCD-nonresponsive fraction. There appears to be a threshold level of cellular cholesterol (approximately 10 to 15% of original) which remains even after repeated MβCD treatments. Therefore, it is possible that a certain fraction of cells (comprising 10 to 15% of the total population) is resistant to the action of MβCD, retaining most of its cholesterol and consequently remaining susceptible to virus infection. An equally possible alternative explanation may be that multiple virus entry pathways exist within the cell which can be distinguished by their sensitivity to MβCD. In this latter scenario, the virus gains entry into the resistant cells by employing alternative, MβCD-insensitive pathways that are yet to be deciphered.
Acknowledgments
This work was supported by Public Health Service grant AI42390 from the National Institute of Allergy and Infectious Diseases.
We thank X. Ning for providing the CHO-PVR-EGFP cells and pPVM1-EGFP cDNA.
REFERENCES
- 1.Andino, R., D. Silvera, S. D. Suggett, P. L. Achacoso, C. J. Miller, D. Baltimore, and M. B. Feinberg. 1994. Engineering poliovirus as a vaccine vector for the expression of diverse antigens. Science 265:1448-1451. [DOI] [PubMed] [Google Scholar]
- 2.Bastiaanse, E. M., K. M. Hold, and A. Van der Laarse. 1997. The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovasc. Res. 33:272-283. [DOI] [PubMed] [Google Scholar]
- 3.Chang, H. M., R. Reitstetter, R. P. Mason, and R. Gruener. 1995. Attenuation of channel kinetics and conductance by cholesterol: an interpretation using structural stress as a unifying concept. J. Membr. Biol. 143:51-63. [DOI] [PubMed] [Google Scholar]
- 4.Chen, X., and M. D. Resh. 2001. Activation of mitogen-activated protein kinase by membrane-targeted Raf chimeras is independent of raft localization. J. Biol. Chem. 276:34617-34623. [DOI] [PubMed] [Google Scholar]
- 5.Chen, X., and M. D. Resh. 2002. Cholesterol depletion from the plasma membrane triggers ligand-independent activation of the epidermal growth factor receptor. J. Biol. Chem. 277:49631-49637. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Y., and L. C. Norkin. 1999. Extracellular simian virus 40 transmits a signal that promotes virus enclosure within caveolae. Exp. Cell Res. 246:83-90. [DOI] [PubMed] [Google Scholar]
- 7.Chow, M., and D. Baltimore. 1982. Isolated poliovirus capsid protein VP1 induces a neutralizing response in rats. Proc. Natl. Acad. Sci. USA 79:7518-7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christian, A. E., M. P. Haynes, M. C. Phillips, and G. H. Rothblat. 1997. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 38:2264-2272. [PubMed] [Google Scholar]
- 9.Couderc, T., F. Delpeyroux, H. Le Blay, and B. Blondel. 1996. Mouse adaptation determinants of poliovirus type 1 enhance viral uncoating. J. Virol. 70:305-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curry, S., M. Chow, and J. M. Hogle. 1996. The poliovirus 135S particle is infectious. J. Virol. 70:7125-7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danthi, P., M. Tosteson, Q.-H. Li, and M. Chow. 2003. Genome delivery and ion channel properties are altered in VP4 mutants of poliovirus. J. Virol. 77:5266-5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17:4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egger, D., L. Pasamontes, M. Ostermayer, and K. Bienz. 1995. Reverse transcription multiplex PCR for differentiation between polio- and enteroviruses from clinical and environmental samples. J. Clin. Microbiol. 33:1442-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Empig, C. J., and M. A. Goldsmith. 2002. Association of the caveola vesicular system with cellular entry by filoviruses. J. Virol. 76:5266-5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis, S. A., J. M. Kelly, J. McCormack, R. A. Rogers, J. Lai, E. E. Schneeberger, and R. D. Lynch. 1999. Rapid reduction of MDCK cell cholesterol by methyl-beta-cyclodextrin alters steady state transepithelial electrical resistance. Eur. J. Cell Biol. 78:473-484. [DOI] [PubMed] [Google Scholar]
- 16.Fricks, C. E., and J. M. Hogle. 1990. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J. Virol. 64:1934-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gousset, K., W. F. Wolkers, N. M. Tsvetkova, A. E. Oliver, C. L. Field, N. J. Walker, J. H. Crowe, and F. Tablin. 2002. Evidence for a physiological role for membrane rafts in human platelets. J. Cell. Physiol. 190:117-128. [DOI] [PubMed] [Google Scholar]
- 18.Henley, J. R., H. Cao, and M. A. McNiven. 1999. Participation of dynamin in the biogenesis of cytoplasmic vesicles. FASEB J. 13(Suppl. 2):S243-S247. [DOI] [PubMed] [Google Scholar]
- 19.Henley, J. R., E. W. Krueger, B. J. Oswald, and M. A. McNiven. 1998. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, Y., J. M. Hogle, and M. Chow. 2000. Is the 135S poliovirus particle an intermediate during cell entry? J. Virol. 74:8757-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilangumaran, S., and D. C. Hoessli. 1998. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 335:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkegaard, K. 1990. Mutations in VP1 of poliovirus specifically affect both encapsidation and release of viral RNA. J. Virol. 64:195-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levitan, I., A. E. Christian, T. N. Tulenko, and G. H. Rothblat. 2000. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J. Gen. Physiol. 115:405-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, Q., A. G. Yafal, Y. M. Lee, J. Hogle, and M. Chow. 1994. Poliovirus neutralization by antibodies to internal epitopes of VP4 and VP1 results from reversible exposure of these sequences at physiological temperature. J. Virol. 68:3965-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marjomaki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of echovirus 1 in caveolae. J. Virol. 76:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller, S., and E. Wimmer. 1998. Expression of foreign proteins by poliovirus polyprotein fusion: analysis of genetic stability reveals rapid deletions and formation of cardiovirus-like open reading frames. J. Virol. 72:20-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norkin, L. C. 1999. Simian virus 40 infection via MHC class I molecules and caveolae. Immunol. Rev. 168:13-22. [DOI] [PubMed] [Google Scholar]
- 28.Popik, W., T. M. Alce, and W. C. Au. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4+ T cells. J. Virol. 76:4709-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Racaniello, V. R. 2001. Picornaviridae: the viruses and their replication, p. 685-722. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 30.Reynolds, C., D. Birnby, and M. Chow. 1992. Folding and processing of the capsid protein precursor P1 is kinetically retarded in neutralization site 3B mutants of poliovirus. J. Virol. 66:1641-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez, V. A., and M. T. Tosteson. 2003. Cholesterol depletion reduces poliovirus infectivity and viral protein synthesis in HeLa cells. Biophys. J. 84:514a. [Google Scholar]
- 32.Rousso, I., M. B. Mixon, B. K. Chen, and P. S. Kim. 2000. Palmitoylation of the HIV-1 envelope glycoprotein is critical for viral infectivity. Proc. Natl. Acad. Sci. USA 97:13523-13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid, S. L., M. A. McNiven, and P. De Camilli. 1998. Dynamin and its partners: a progress report. Curr. Opin. Cell Biol. 10:504-512. [DOI] [PubMed] [Google Scholar]
- 34.Semac, I., C. Palomba, K. Kulangara, N. Klages, G. van Echten-Deckert, B. Borisch, and D. C. Hoessli. 2003. Anti-CD20 therapeutic antibody rituximab modifies the functional organization of rafts/microdomains of B lymphoma cells. Cancer Res. 63:534-540. [PubMed] [Google Scholar]
- 35.Simons, J., A. Rogove, N. Moscufo, C. Reynolds, and M. Chow. 1993. Efficient analysis of nonviable poliovirus capsid mutants. J. Virol. 67:1734-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 37.Stuart, A. D., H. E. Eustace, T. A. McKee, and T. D. Brown. 2002. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J. Virol. 76:9307-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stulnig, T. M., M. Berger, T. Sigmund, H. Stockinger, V. Horejsi, and W. Waldhausl. 1997. Signal transduction via glycosyl phosphatidylinositol-anchored proteins in T cells is inhibited by lowering cellular cholesterol. J. Biol. Chem. 272:19242-19247. [DOI] [PubMed] [Google Scholar]
- 39.Tosteson, M. T., and M. Chow. 1997. Characterization of the ion channels formed by poliovirus in planar lipid membranes. J. Virol. 71:507-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsang, S. K., B. M. McDermott, V. R. Racaniello, and J. M. Hogle. 2001. Kinetic analysis of the effect of poliovirus receptor on viral uncoating: the receptor as a catalyst. J. Virol. 75:4984-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yancey, P. G., W. V. Rodrigueza, E. P. Kilsdonk, G. W. Stoudt, W. J. Johnson, M. C. Phillips, and G. H. Rothblat. 1996. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J. Biol. Chem. 271:16026-16034. [DOI] [PubMed] [Google Scholar]