Abstract
The induction of lytic replication of the Epstein-Barr virus (EBV) completely arrests cell cycle progression, in spite of elevation of S-phase cyclin-dependent kinase (CDK) activity, thereby causing accumulation of hyperphosphorylated forms of retinoblastoma (Rb) protein (A. Kudoh, M. Fujita, T. Kiyono, K. Kuzushima, Y. Sugaya, S. Izuta, Y. Nishiyama, and T. Tsurumi, J. Virol. 77:851-861, 2003). Thus, the EBV lytic program appears to promote specific cell cycle-associated activity involved in the progression from G1 to S phase. We have proposed that this provides a cellular environment that is advantageous for EBV productive infection. Purvalanol A and roscovitine, inhibitors of S-phase CDKs, blocked the viral lytic replication when cells were treated at the early stage of lytic infection, while well-characterized inhibitors of enzymes, such as mitogen-activated protein kinase, phosphatidylinositol 3-kinase, and protein kinase C, known to be involved in BZLF1 gene expression did not. Inhibition of CDK activity resulted in the accumulation of the hypophosphorylated form of Rb protein and inhibition of expression of EBV immediate-early and early proteins. Cycloheximide block-and-release experiments clearly demonstrated that even in the presence of enough amounts of the BZLF1 protein, purvalanol A blocked expression of lytic viral proteins at transcription level. Furthermore, reporter gene experiments confirmed that BZLF1-induced activation of early EBV promoters was impaired in the presence of the CDK inhibitor. We conclude here that the EBV lytic program promotes specific cell cycle-associated activity involved in the progression from G1 to S phase because the S-phase-like cellular environment is essential for the expression of immediate-early and early genes supplying the viral replication proteins and hence for lytic viral replication.
The Epstein-Barr virus (EBV) is a B-lymphotropic gammaherpesvirus which is a causative agent of infectious mononucleosis known to be closely associated with several human cancers, including Burkitt's lymphoma and nasopharyngeal carcinoma, as well as lymphoproliferative disorders (16). Although infection by EBV occurs in most individuals, it is usually asymptomatic. The life cycle is quite distinct from those of other herpesviruses, such as herpes simplex virus type 1 (HSV-1) or cytomegalovirus (CMV), for which full lytic replication can be accomplished by infection of certain cell types. Such an efficient lytic replication system, however, does not exist for EBV. The virus specifically infects resting B lymphocytes, inducing the continuous proliferation of B cells (16), and the resulting lymphoblastoid cell lines (LCLs) express a limited number of EBV gene products, including six nuclear proteins (EBNA-1, EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, and EBNA-LP), three membrane proteins (LMP-1, LMP-2A, and LMP-2B), EBV-encoded small RNAs (EBER1 and EBER2), and transcripts from the BamHI A region. They activate quiescent B cells to enter the cell cycle, maintain continuous proliferation, and prevent cells from undergoing apoptosis. The viral genome is maintained as covalently closed circular plasmids forming nucleosomal structures with histone proteins in the nuclei of the LCLs. The number of copies is maintained at 10 to 50 per cell, to be duplicated once during each cell division cycle by the host cellular DNA replication machinery (66).
Lytic replication differs from the latent amplification state in that multiple rounds of replication are initiated within oriLyt (23), and the replication process has a greater dependence on EBV-encoded replication proteins (18). EBV encodes seven viral replication genes. The BZLF1 protein acts as an oriLyt-binding protein as well as the immediate-early transactivator (50). The BALF5 gene encodes the DNA polymerase (Pol) catalytic subunit (59), and the BMRF1 gene encodes the DNA Pol accessory subunit (55-57). A single-stranded DNA-binding protein is encoded by the BALF2 gene (58, 61). The remaining three proteins encoded by the BBLF4, BSLF1, and BBLF2/3 genes form a tight complex (19, 67) and are predicted to act as helicase, primase, and helicase-primase-associated proteins, respectively, based on sequence homology to the HSV-1 UL5, UL52, and UL8 genes (18). These viral replication proteins, other than the BZLF1 protein, conceivably work together at replication forks to synthesize leading and lagging strands of the concatemeric EBV genome. Upon reactivation, the two key EBV immediate-early lytic genes, BZLF1 and BRLF1, are expressed. These genes encode transactivators that activate viral and certain cellular promoters and lead to an ordered cascade of viral gene expression: activation of early gene expression followed by the lytic cascade of viral genome replication and late gene expression (16). In the viral productive cycle, the EBV genome is amplified more than 100-fold. Intermediates of viral DNA replication are found as large head-to-tail concatemeric molecules, probably resulting from rolling-circle DNA replication (23), which are subsequently cleaved into unit length genomes and packaged into virions in the nucleus.
The BZLF1 protein mediates the switch between the latent and lytic forms of EBV infection and alone is sufficient to activate the EBV lytic cascade (23). It has been demonstrated that it inhibits host cell proliferation by causing cell cycle arrest in G0/G1 in several epithelial tumor cell lines lacking the EBV genome (7, 43). Such G0/G1 arrest was found to result from induction of the tumor suppressor protein p53 and the cyclin-dependent kinase (CDK) inhibitors p21WAF-1/CIP-1 and p27KIP-1, followed by accumulation of the hypophosphorylated form of retinoblastoma (Rb) protein (7). Recently, it has been reported that the BZLF1 protein by itself activates E2F-1, cyclin E, and Cdc25A, which are involved in cell cycle progression in telomerase-immortalized human keratinocytes and primary tonsil keratinocytes but not in human fibroblasts (35). Furthermore, the other immediate-early transactivator BRLF1 protein can induce contact-inhibited, quiescent human fibroblasts to enter the S phase with dramatic increase in the level of E2F-1 (53). E2F-1 activates the transcription of many proteins involved in cellular DNA synthesis and cell cycle progression (1) and probably the transcription of the EBV DNA Pol gene as well (33). The available data suggest that E2F activity is required for lytic viral DNA replication.
In a previous study seeking to characterize cellular circumstances suitable for lytic replication in LCLs, we established a tetracycline-inducible expression system for the BZLF1 protein in the B95-8 B-lymphoblastoid cell line, which is latently infected with EBV and possesses normal p53 pathway (15, 30). The system is simple and highly efficient for conditional induction of the lytic replication program in the absence of any other external stimuli. As a result, it could be clearly demonstrated that induction of the BZLF1 protein results in arrest of cell cycle progression and inhibition of replication of cellular DNA simultaneous with induction of that for lytic viral DNA. Unexpectedly, although Tet-BZLF1/B95-8 cells harbor functional and responsive p53 and retain the ability to activate checkpoint pathways (30), the levels of p53 and the CDK inhibitors p21WAF-1/CIP-1, p27KIP-1, and p16INK4a were found to be constant before and after induction of the lytic program, in contrast to the results reported by Cayrol and Flemington (7). Rather, activation of S-phase-promoting CDK, i.e., cyclin E/A-CDK2, and accumulation of the hyperphosphorylated form of Rb protein were observed. Therefore, we speculated that the late G1 or early S-arrested state induced by productive EBV infection provides a cellular environment that is advantageous for EBV replication in that metabolic precursors (i.e., nucleotides) are abundantly available for viral replication. Since cellular DNA synthesis is inhibited, there is no competition for viral access to these precursors.
Cellular CDKs comprise a family of serine-threonine protein kinases whose activity is regulated by association with specific cyclin partners, phosphorylation, and interaction with CDK inhibitors such as p21waf1/cip1, p27kip1, p16ink4a, and p15ink4b, whose expression is itself tightly controlled. With regard to the involvement of CDKs in DNA synthesis, activation of CDK-2 is necessary for expression of many cellular DNA replication proteins and CDK-2 phosphorylates and activates several of the cellular proteins that are required for DNA synthesis. Among the CDK2 targets, the Rb protein is most important for cell cycle progression. The present paradigm for phosphorylation regulation of Rb protein is as follows (6, 24, 27, 29). During early G1, Rb is phosphorylated by cyclin D-CDK4/6, leading to cyclin E expression. Near the end of G1, it becomes maximally hyperphosphorylated by cyclin E/CDK2, allowing cells to enter into S phase, and the hyperphosphorylated state is maintained by cyclins A/E-CDK2. In a previous study, Kudoh et al. found that hyperphosphorylated Rb proteins accumulate as lytic EBV infection proceeds (30). In particular, CDK2 phosphorylation of Ser612 in Rb continued to increase. Based on these observations, we speculated that cell cycle-related factors normally active in uninduced cells in the late G1 or early S phase may be required for lytic replication of EBV. If this is indeed the case, inhibition of their activity should block viral replication. To test this hypothesis, we measured the effects on viral replication of two recently described CDK inhibitors, purvalanol A and roscovitine (22, 36). Both drugs inhibit CDK2 and CDK1 but not CDK4 or CDK6 or a large number of other kinases. They compete with ATP for binding to the ATP-binding pocket of the target CDKs, and all known effects of purvalanol A and roscovitine on cells can be attributed to the resultant inhibition of kinase activity of CDK2 and CDK1 (17, 21, 36, 51).
In the present study, we found that both purvalanol A and roscovitine block EBV lytic replication while well-characterized inhibitors of other protein kinases, such as mitogen-activated protein (MAP) kinase, phosphatidylinositol (PI) 3-kinase, and protein kinase C, do not. The effects of purvalanol A can be shown to be due to inhibition of expression of viral immediate-early and early proteins, including the replication proteins encoded by the BALF2, BALF5, BMRF1, and BBLF2/3 genes. We conclude that the EBV lytic program promotes specific cell cycle-associated activity involved in the progression from G1 to S phase, because S-phase CDK activity appears to be essential for the expression of viral immediate-early and early lytic proteins and hence for lytic viral replication.
MATERIALS AND METHODS
Materials.
Purvalanol A was purchased from Calbiochem (San Diego, Calif.) and roscovitine was obtained from Promega (Madison, Wis.), with stock solutions being prepared at concentrations of 10 mM in dimethyl sulfoxide (DMSO). Both are selective inhibitors of CDKs and have the following characteristics: purvalanol A, 2-(1-R-isopropyl-2-hydroxyethylamino)-6-(3-chloroanilino)-9-isopropylpurine, molecular weight of 388.9, 50% inhibitory concentrations (IC50s) of 4 nM for CDK1/cyclin B, 70 nM for CDK2/cyclin A, 35 nM for CDK2/cyclin E, and 75 nM for CDK5/p35 (22); roscovitine, 2-(1-d,l-hydroxymethylpropylamino)-6-benzylamino-9-isopropylpurine, molecular weight of 354.4, IC50s of 65 nM for CDK1/cyclin B, 700 nM for CDK2/cyclin A, 700 nM for CDK2/cyclin E, and 200 nM for CDK5/p35. Roscovitine exhibits reduced sensitivity towards related kinases, including ERK1 and ERK2 (IC50s of 30 and 14 μM, respectively) and does not significantly affect the activities of other protein kinases, including CDK4/cyclin D and CDK6/cyclin E, even at a concentration of 100 μM (3, 36). LY-294002, mevinolin, bisindolylmaleimide I, and cycloheximide were purchased from Sigma. LY-294002 [2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one], a specific PI 3-kinase inhibitor, was dissolved in DMSO at a concentration of 20 mM. Mevinolin, a drug that specifically inhibits MAP kinase (ERK1 and ERK2) but not CDKs, was diluted in ethanol to a concentration of 20 mM. Bisindolylmaleimide I {2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)-maleimide} (molecular weight, 412.5), a broad-spectrum protein kinase C inhibitor that is structurally similar to staurosporine, was stored in DMSO at a concentration of 10 mM. It shows high selectivity for protein kinase C (Ki = 10 nM) and may inhibit protein kinase A at a much higher concentration (Ki = 2 μM) (54). Cycloheximide, a specific inhibitor of protein synthesis which works by preventing translocation of ribosomes, was diluted in ethanol to a concentration of 50 mg/ml.
Stocks of all drugs were aliquoted and kept at −20°C until use. The final concentration of each drug used is indicated in the figure legends.
Cells.
Kudoh et al. previously reported that the exogenous BZLF1 protein is conditionally expressed under the control of a tetracycline-regulated promoter in Tet-BZLF1/B95-8 cells, a marmoset B-cell line latently infected with EBV (30). Tet-BZLF1/B95-8 cells and Tet-BZLF1/Akata cells were maintained in RPMI medium supplemented with 1 μg of puromycin/ml, 250 μg of hygromycin B/ml, and 10% tetracycline-free fetal calf serum at 37°C in a humidified atmosphere containing 5% CO2. To induce lytic EBV replication, the tetracycline derivative doxycycline was added to the culture medium at a final concentration of 1 to 5 μg/ml.
Establishment of Tet-BZLF1/Akata cells.
Akata cells, human EBV-positive Burkitt's lymphoma cells, were infected with the stocks of recombinant retrovirus, rv-BZLF1 and rv-rtTA, as described previously (30). Clones resistant to puromycin and hygromycin B were isolated by limiting dilution and checked for expression of the BZLF1 and BALF2 proteins with doxycycline by Western blot analysis.
Establishment of Tet-BZLF1/HeLa.
The plasmid pCMSCV-RevTRE(hyg)-BZLF1 (30) was transfected into HeLa Tet-on cells expressing rtTA (Clontech). One day later, cells were plated in selective medium containing 250 μg of hygromycin B/ml and 100 μg of G418/ml. Several clones expressing BZLF1 protein with doxycycline were isolated, and one was used for the present study. The Tet-BZLF1 HeLa cell was cultured in Dulbecco's modified Eagle's medium containing 10% tetracycline-free fetal calf serum, hygromycin B (250 μg/ml), and G418 (100 μg/ml). To induce BZLF1 protein expression, doxycycline was added to the culture medium at a final concentration of 5 μg/ml.
Transfections and CAT assays.
Approximately 5 × 105 Tet-BZLF1/HeLa cells were plated in 60-mm-diameter dishes. Cells were transfected with 2 μg of pBMRF1-CAT or pBHRF1-CAT (8, 68) by using Lipofectamine with Plus reagent (Invitrogen) according to the manufacturer's instructions. Media were replaced at 16 h posttransfection with fresh media containing or free of purvalanol A (20 μM). Twenty hours posttransfection, doxycycline was added to a final concentration of 5 μg/ml or not. Forty-eight hours after addition of doxycycline, cells were harvested and treated with Reporter lysis buffer (Promega) for 15 min. After incubation, the samples were heated at 60°C for 10 min to inactivate endogenous deacetylase activity. Chloramphenicol acetyltransferase (CAT) activity was assayed by incubating each sample with n-butyryl coenzyme A and [14C]chloramphenicol at 37°C for 3 h according to the manufacturer's instructions (Promega). The reaction product, n-butyryl [14C]chloramphenicol, was selectively extracted with 300 μl of xylene. The xylene phase was mixed with scintillation cocktail, and the radioactivity of the product was measured with a scintillation counter.
Quantification of viral DNA synthesis during lytic replication.
Tet-BZLF1/B95-8 cells were incubated with or without 1 μg of doxycycline/ml in the presence or absence of the indicated drugs and harvested at the indicated times. Total DNAs from a total of 3.5 × 106 Tet-BZLF1/B95-8 cells were purified with a DNeasy Tissue kit (Qiagen) and quantified. Diluted amounts of DNA samples were dot-blotted on a Hybond-N membrane (Amersham) and hybridized with a 32P-labeled DNA fragment consisting of a portion of the EBV EBNA1 coding region (EBV nucleotides 109110 to 109379). Signal intensity was quantified with an Image guider (BAS2500; Fujifilm). A standard curve for viral DNA quantification was obtained from serial dilutions of a plasmid, p500, which harbors an EBNA1 coding region. The copy numbers of viral genome per cell were determined from the standard curve and serial dilutions of DNA from Raji cells in which about 45 copies of viral genome per cell are present (11). DNA from BJAB cells was also used as a negative control.
Quantification of viral and host RNA transcripts during lytic replication.
Tet-BZLF1/B95-8 cells were incubated with 5 μg of doxycycline/ml in the presence or absence of the indicated drugs and harvested at the indicated times. Total RNA was isolated from a total of 107 cells with an RNeasy mini kit (Qiagen). The RNA concentration was measured spectrophotometrically, assuming that 1 A260 is equal to a concentration of 40 μg/ml. Samples were stored at −80°C without detectable signs of degradation. RNA samples (5 μg) were size-fractionated on a 1.2% formaldehyde-agarose gel in morpholinepropanesulfonic acid (MOPS) running buffer and transferred to a Hybond-N+ membrane (Amersham). The membrane filters were hybridized at 65°C with 32P-labeled probes obtained by PCR amplification of each viral and cellular DNA with the following primers: BZLF1, 5′-caccgggaccgatccagcct-3′ and 3′-atcgatgttaacaagcttg-5′; BRLF1, 5′-gttccagactatggtctcgt-3′ and 3′-tatccaaggctgttcaggtt-5′; BALF5, 5′-ccccaactccagatgctcaagggt-3′ and 3′-agcagatccatgaccagg-5′; BMRF1, 5′-cgggatccatggaaaccactcagactctc-3′ and 3′-gtggtacaahagcgttcgcttaagg-5′; 36B4, 5′-ctggagaaactgctgcctcatatccg-3′ and 3′-ccaaatcccatatcctcgtccga-5′. Signal intensity was quantified with an Image guider (BAS2500; Fujifilm). The levels of the specific viral and cellular gene transcripts were plotted and compared with those prior to treatment with the drugs.
Protein preparation.
Tet-BZLF1/B95-8 cells were harvested at the indicated times postinduction with or without doxycycline, washed with phosphate-buffered saline (PBS), and treated with lysis buffer (0.02% sodium dodecyl sulfate [SDS], 0.5% Triton X-100, 300 mM NaCl, 20 mM Tris-HCl [pH 7.6], 1 mM EDTA, 1 mM dithiothreitol, 10 μg of leupeptin/ml, 5 μg of pepstatin A/ml) for 20 min on ice. Samples were centrifuged at 18,000 × g for 10 min at 4°C, and the clarified cell extracts were measured for protein concentrations by using a Bio-Rad protein assay kit.
Immunoblot analysis.
Twenty micrograms of proteins was loaded in each lane for SDS-10% polyacrylamide (acrylamide/bisacrylamide ratio = 29.2:0.8) gel electrophoresis (PAGE). To separate phosphorylated forms of Rb proteins, proteins were subjected to SDS-7.5% PAGE (acrylamide/bisacrylamide ratio = 60:1) and then transferred to polyvinylidene difluoride membranes. The membranes were washed with blotting buffer (1× PBS containing 0.1% Tween 20), blocked for 60 min in blotting buffer containing 10% low-fat powdered milk, washed once with blotting buffer, and incubated at room temperature for 60 min with primary antibodies in blotting buffer containing 5% low-fat powdered milk. They were then further washed in blotting buffer and incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 60 min. The target proteins were detected with an enhanced chemiluminescence detection system (Amersham). Images were processed by LumiVision PRO (Aisin/Taitec, Inc.) with a cooled charge-coupled device (CCD) camera and assembled using an Apple G4 computer with Adobe Photoshop 5.0. Signal intensities were quantified with a LumiVision image analyzer. The system used in this study features a cooled CCD camera that has a 16 bit = 65,535 grayscale wide dynamic range. The accuracy of the quantitative analysis was enhanced up to 100 times compared with the standard quantitative analysis scanning X-ray film.
Antibodies.
Preparation and specificities of the anti-BZLF1 protein-specific, anti-BALF2 protein-specific, anti-BALF5 protein-specific, and anti-BBLF2/3 protein-specific polyclonal antibodies were described previously (59, 60, 67). An anti-BMRF1 protein-specific monoclonal antibody, clone name R3, was purchased from Chemicon, and an anti-BRLF1-specific antibody was obtained from Argen, Inc. The anti-Rb-specific monoclonal antibody was purchased from Becton Dickinson Transduction Laboratories and the anti-phospho-Rb (Ser612)-specific monoclonal antibody was the kind gift of Katsuyuki Tamai (MBL, Inc.). Anti-PCNA antibody was commercially obtained from MBL, Inc.
RESULTS
In a recent study, Kudoh et al. isolated Tet-BZLF1/B95-8 cells in which the exogenous BZLF1 protein was conditionally expressed under the control of a tetracycline-regulated promoter (30). More than 80% of Tet-BZLF1/B95-8 cells became positive for BZLF1 and BMRF1 proteins 48 h after doxycycline annexation. The copy number of the viral genome in the cells was significantly amplified after 24 h postinduction. Thus, the system is simple and highly efficient for conditional induction of the lytic replication program in the absence of any other external stimuli, allowing use for further analyses.
S-phase CDK inhibitors purvalanol A and roscovitine completely inhibit lytic EBV replication.
The previous study by Kudoh et al. indicated that the EBV lytic program promotes post-restriction point characteristics, including high CDK2 activity and hyperphosphorylation of the Rb protein (30). This finding suggests the possibility that S-phase CDK activity or free E2F released from the Rb-E2F complex due to hyperphosphorylation of the Rb protein is required for lytic EBV replication. If this were the case, then inhibition of CDK2 activity, which would thereby prevent phosphorylation of Rb protein, would result in inhibition of EBV productive infection. Roscovitine (36) and purvalanol A (22) are the 2,6,9-trisubstituted purine-derived pharmacological CDK inhibitors which have been best characterized so far. Both drugs inhibit CDKs 1 and 2 with high potency, but they do not inhibit CDKs 4 or 6 or a number of other kinases.
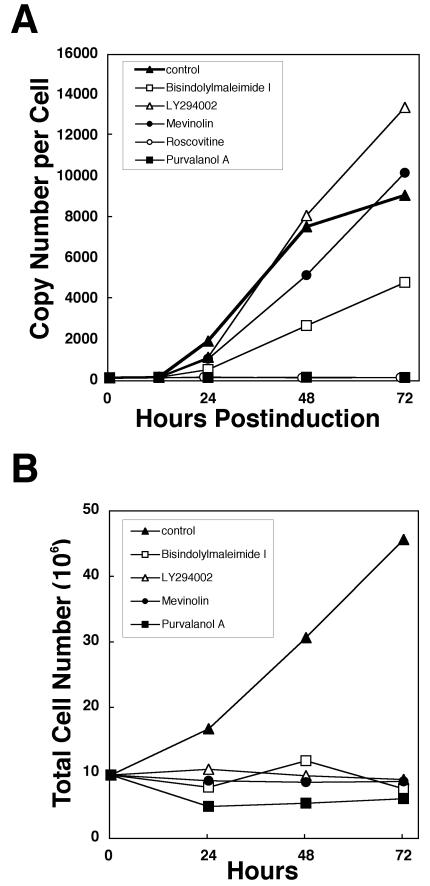
To clarify the effects of purvalanol A and roscovitine on the progression of EBV lytic replication, Tet-BZLF1/B95-8 cells were treated with doxycycline in the presence or absence of either drug. Total DNA was extracted from the cells, and the EBV DNA-specific signal was quantitated. The copy number of the EBV genome per Tet-BZLF1/B95-8 cell was calculated to be about 40 before treatment with doxycycline. In the absence of kinase inhibitors, the copy number of the viral DNA was amplified to more than 9,000 per cell after 72 h postinduction. As shown in Fig. 1A, 15 μM purvalanol A and 50 μM roscovitine blocked lytic replication of EBV almost completely throughout the 72-h period. No obvious cytotoxic effects were observed by microscopic evaluation when uninduced Tet-BZLF1/B95-8 cells were treated with concentrations of purvalanol A up to 50 μM (data not shown).
FIG. 1.
(A) Specific inhibition of EBV lytic replication by the CDK inhibitors roscovitine and purvalanol A. Tet-BZLF1/B95-8 cells were cultured in the presence of 1 μg of doxycycline/ml with drug-free medium (control) or media containing each indicated drug and harvested at the indicated times. The drug concentrations used in the experiments were as follows: 10 μM bisindolylmaleimide I, 15 μM LY-294002, 20 μM mevinolin, 50 μM roscovitine, and 15 μM purvalanol A. To determine viral DNA synthesis, slot blot assays for viral DNA were performed as described in Materials and Methods. Signal intensity was quantified with an Image guider (Fujifilm), and the copy numbers of viral genome per cell were calculated. (B) Effect of bisindolylmaleimide I, LY-294002, mevinolin, and purvalanol A on the proliferation of Tet-BZLF1/B95-8 cells. Tet-BZLF1/B95-8 cells were seeded in flasks (107 cells/flask) with drug-free medium (control) or media containing each indicated drug. The numbers of cells were counted with a hemocytometer at the indicated times, and the data were plotted in the graph. The drug concentrations used in the experiments were the same as those described for panel A.
The specificities of purvalanol A and roscovitine have been evaluated so extensively that these drugs are presently used to confirm the involvement of CDKs in biological processes (4, 17, 21, 36, 46, 47, 49, 51). However, it remained a theoretical possibility that our findings with purvalanol A and roscovitine were the consequence of a block of another cellular protein serine/threonine kinase(s) such as protein kinase C, PI 3-kinase, or MAP kinase. These are known to be activated on the occasion of a switch from latent to lytic infection of EBV, leading to expression of the BZLF1 protein in vivo (9, 20). Indeed, roscovitine also inhibits MAP kinase (ERK1 and -2) (although ∼20- and ∼10-fold less efficiently, respectively, than for CDKs). We also investigated whether these kinases are required for lytic EBV replication in the Tet-on expression system. As shown in Fig. 1A, mevinolin, a drug that specifically inhibits ERK1 and -2 but not CDKs (14), did not inhibit EBV lytic replication at a concentration of 20 μM. Similarly, the PI 3-kinase inhibitor LY-294002 did not block the lytic replication at all at a concentration of 15 μM. Further, there appeared to be a moderate inhibition of EBV lytic infection with bisindolylmaleimide I, a broad-spectrum protein kinase C inhibitor, at a concentration of 10 μM. Since protein kinase C increases early in lytic cycle promoter activity (32), the addition of protein kinase C inhibitor to the culture appears to hinder the productive replication moderately. As shown in Fig. 1B, purvalanol A, mevinolin, LY-294002, and bisindolylmaleimide I did prevent cell proliferation completely at the concentrations utilized.
Thus, lytic EBV replication was inhibited only by inhibitors of S-phase CDKs and not by those of PI 3-kinase, MAP kinase, and protein kinase C.
Inhibition of lytic EBV replication by purvalanol A is not mediated by irreversible drug-induced cytotoxicity.
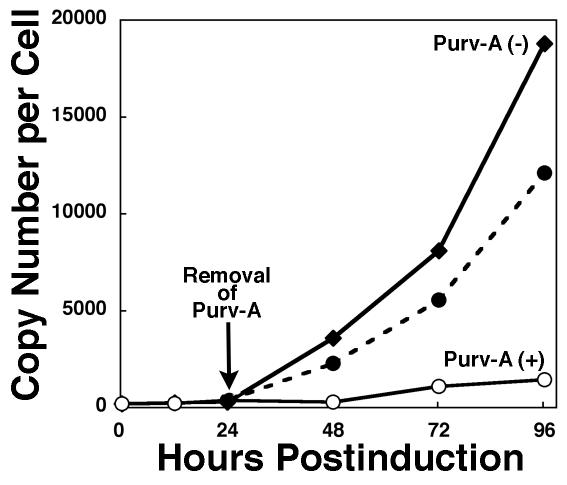
Although purvalanol A and roscovitine did not induce detectable cytopathology, as determined by microscopic evaluation, at the concentrations used to block EBV replication (data not shown), it remained a possibility that more subtle but irreversible toxic effects of these drugs may have compromised the ability of cells to support lytic EBV replication. To determine whether this was the case, we performed a purvalanol A reversal experiment. Purvalanol A was selected for these experiments because it inhibits EBV replication efficiently at a lower concentration than does roscovitine. Tet-BZLF1/B95-8 cells treated with doxycycline were cultured in the presence of 15 μM purvalanol A for 24 h, and the medium was replaced with purvalanol A-free fresh medium. At the indicated times, cells were harvested and the copy numbers of viral genome DNA were calculated by a standard Southern blot hybridization assay (Fig. 2). When the medium was replaced with fresh medium containing purvalanol A, the copy number of the viral genome per cell did not increase. In contrast, after release from the purvalanol A block, resumption of EBV lytic replication was evident in the cells (Fig. 2).
FIG. 2.
Inhibition of EBV lytic replication by purvalanol A is reversible. Tet-BZLF1/B95-8 cells were cultured with doxycycline (1 μg/ml) in the presence (○, •) or absence (♦) of purvalanol A (15 μM). Aliquots of cells treated with purvalanol A were also transferred to purvalanol A-free fresh medium at 24 h postinduction (dashed line). This medium change is indicated by the arrow. Cells were harvested at the indicated times, and viral DNA synthesis was determined by slot blot assay as described in Materials and Methods.
We conclude from these experiments that purvalanol A-induced inhibition of EBV lytic replication is not mediated by irreversible drug-induced toxicity in Tet-BZLF1/B95-8 cells.
Purvalanol A prevents cell proliferation in inverse proportion to the phosphorylation state of Rb protein.
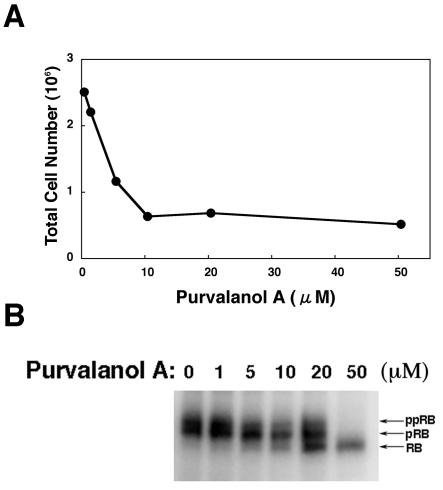
As shown in Fig. 3A, proliferation of Tet-BZLF1/B95-8 cells was inhibited by the CDK inhibitor purvalanol A at concentrations greater than 5 μM. Since the compound inhibits CDK2 and CDK1 activity selectively, the phosphorylation states of Rb protein in Tet-BZLF1/B95-8 cells were drastically changed as the concentration of purvalanol A was increased in the medium (Fig. 3B), i.e., the hypophosphorylated form of Rb protein was increased in a dose-dependent manner (Fig. 3B). Cell growth inhibition by purvalanol A appeared to be in proportion to the ratio of the hypophosphorylated to hyperphosphorylated forms of the Rb protein (compare Fig. 3A and B). More than 90 percent of the Tet-BZLF1/B95-8 cells treated with purvalanol A were in G1 phase by fluorescence-activated cell sorter analysis (data not shown). Collectively, these observations indicate that the cellular condition of G1 phase induced by purvalanol A is not favorable for induction of lytic replication of EBV.
FIG. 3.
(A) Dose dependence of purvalanol A effects on the proliferation of Tet-BZLF1/B95-8 cells. Tet-BZLF1/B95-8 cells were seeded in 35-mm-diameter petri dishes (1.0 × 106 cells/dish). The cells were incubated for 48 h in RPMI 1640 medium containing the indicated concentrations of purvalanol A. The cells were harvested, and total cell numbers were counted with a hemocytometer. (B) Effects of purvalanol A on the phosphorylation state of Rb protein. Cells were cultured in the presence of indicated concentrations of purvalanol A and harvested after 48 h. Clarified cell lysates were prepared, separated by SDS-7.5% PAGE, and applied for Western blot analyses with anti-Rb protein polyclonal antibody. pRB and ppRB are slower-migrating hyperphosphorylated forms of Rb protein. The faster-migrating band is the hypophosphorylated form of Rb protein and is designated as RB.
Purvalanol A and roscovitine inhibit EBV lytic replication when added until 9 h postinduction.
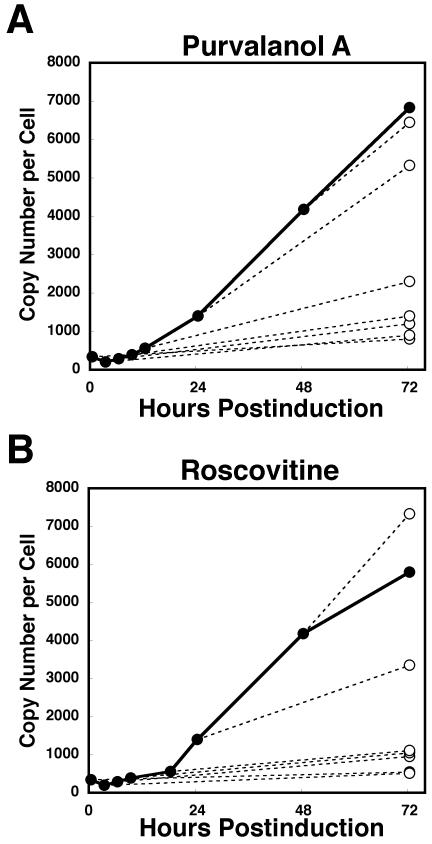
To determine whether CDKs are required for essential EBV functions that occur after expression of the BZLF1 protein, we evaluated the effects on viral replication of adding purvalanol A at selected times after annexation of doxycycline. Two recognized cellular targets of purvalanol A are CDK-1 and CDK-2. Since CDK-2 is involved in cellular DNA replication (12), we determined whether CDK-2 might also participate in viral DNA replication directly. If this were the case, then purvalanol A or roscovitine would inhibit the lytic phase of viral DNA replication even in the presence of viral replication proteins. To test this hypothesis, after addition of doxycycline to the culture medium, either purvalanol A or roscovitine was added at the indicated times. Cells were harvested at 72 h posttreatment with doxycycline, and the copy number of EBV genomes was calculated (Fig. 4). No CDK inhibitor was added to control cultures. As shown in Fig. 4, the addition of purvalanol A or roscovitine immediately after doxycycline treatment resulted in an almost total block in EBV lytic replication throughout the 72-h period. The addition of purvalanol A or roscovitine at 3, 6, and 9 h postinduction had nearly identical inhibitory effects on viral replication (Fig. 4). However, viral genome synthesis by lytic EBV replication was not markedly inhibited when purvalanol A was added to the culture medium 24 h postannexation, although there was a moderate inhibition of viral genome synthesis at 24 h postinduction with roscovitine (Fig. 4). Until 24 h postinduction, viral replication proteins were expressed at near plateau levels sufficient for synthesis of the viral genome in Tet-BZLF1/B95-8 cells (Fig. 5B, lanes 5 and 6). These results strongly suggest that cellular CDKs sensitive to drugs are not directly involved in the lytic phase of viral DNA replication after viral replication proteins are already expressed but rather affect viral function(s) at early stages of the lytic replication.
FIG. 4.
The CDK inhibitors purvalanol A and roscovitine inhibit EBV lytic replication when added at least until 9 h postinduction. Tet-BZLF1/B95-8 cells were treated with 1 μg of doxycycline/ml for 72 h. Purvalanol A (15 μM) (A) or roscovitine (50 μM) (B) was added at the indicated times (0, 3, 6, 9, 24, and 48 h) postinduction, and the cells were harvested at 72 h postinduction and viral DNA synthesis was determined by slot blot assay as described in Materials and Methods (dashed lines, ○). Signal intensity was quantified with an Image guider (Fujifilm). An additional series of Tet-BZLF1/B95-8 cells with productive infection was incubated continuously in CDK inhibitor-free medium and harvested at the indicated times (control; •).
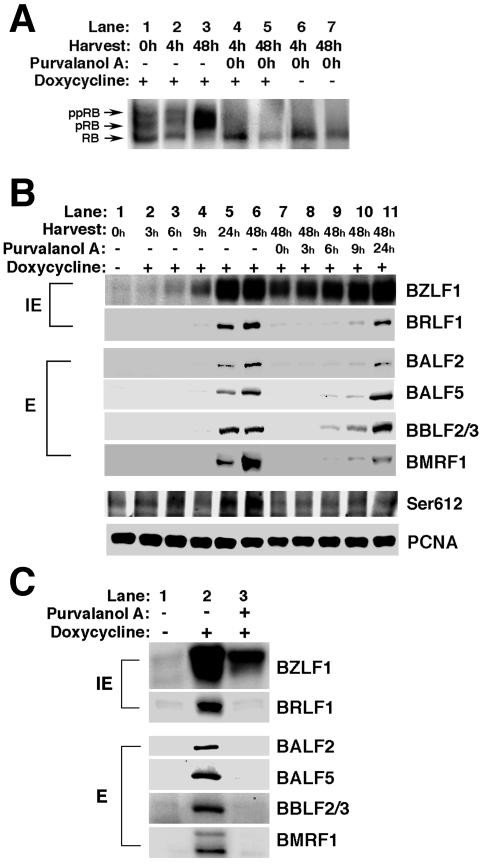
FIG. 5.
Effects of purvalanol A on the expression of lytic viral proteins and phosphorylation of Rb protein during EBV lytic infection. (A) Tet-BZLF1/B95-8 cells were treated with (+) or without (−) 5 μg of doxycycline/ml for 48 h and purvalanol A (20 μM) was added to the culture immediately thereafter (0 h). The cells were harvested at the indicated times, and clarified cell lysates were prepared. Phosphorylation states of the Rb protein were analyzed by Western blotting with anti-Rb protein monoclonal antibody. pRB and ppRB are slower-migrating hyperphosphorylated forms of Rb protein. The faster-migrating band is the hypophosphorylated form of Rb protein and is designated as RB. (B) Purvalanol A (20 μM) was added to the culture of doxycycline-treated Tet-BZLF1/B95-8 cells at 0, 3, 6, 9, and 24 h postinduction, and the cells were harvested at the indicated times. Clarified cell lysates were separated by SDS-PAGE and applied for Western blot analyses with each antibody as indicated on the right. The proteins were detected with an enhanced chemiluminescence detection system (Amersham). Images were processed by LumiVision PRO (Aisin/Taitec, Inc.) with a cooled CCD camera and assembled using an Apple G4 computer with Adobe Photoshop 5.0. Signal intensity was quantified with a LumiVision image analyzer as described in Materials and Methods. IE, immediate-early proteins; E, early proteins. (C) Tet-BZLF1/AKATA cells were treated with (+) or without (−) 3 μg of doxycycline/ml and cultured for 48 h in the absence or presence of purvalanol A (5 μM). Lane 1 shows the sample prepared when the cells were treated with doxycycline at time zero. The cells were harvested, and clarified cell lysates were prepared and applied for Western blot analyses with each antibody as indicated on the right.
Accumulation of viral immediate-early and early proteins is significantly reduced in the presence of purvalanol A.
Since purvalanol A inhibits lytic EBV replication when added at least until 9 h after induction of the lytic program (Fig. 4A), we hypothesized that it may inhibit an (relatively) early stage of viral function. Lytic viral DNA synthesis requires previous immediate-early and early gene expression for supply of viral replication proteins and consequently occurs later in the infection cycle. To determine whether viral gene expression was impaired by purvalanol A, the following experiments were designed. Tet-BZLF1/B95-8 cells were treated with doxycycline in the presence or absence of purvalanol A, and total cell extracts were prepared at the indicated times postinduction. As shown in Fig. 5A, the phosphorylation states of Rb protein drastically changed in Tet-BZLF1/B95-8 cells treated with doxycycline as well as untreated cells (Fig. 3). In the absence of purvalanol A, almost all Rb protein became hyperphosphorylated at 48 h postinduction, as was observed previously (30). Ser612 of Rb protein is known to be preferentially phosphorylated by cyclin E/A-CDK2 (52). As shown in Fig. 5B, lane 6, phosphorylation of Ser612 in Rb protein was conspicuous. In contrast, with the addition of purvalanol A, Rb protein was hypophosphorylated within 4 h after treatment and this continued throughout 48 h postinduction in both the doxycycline-treated and untreated Tet-BZLF1/B95-8 cells (Fig. 5A, compare lane 1 with lanes 4 and 5 or lanes 6 and 7). Also, phosphorylation of Ser612 in Rb protein was clearly inhibited by purvalanol A treatment (Fig. 5B, compare lane 6 with lane 7). On the other hand, levels of PCNA (as a control) were constant in the presence or absence of purvalanol A (Fig. 5B).
As shown in Fig. 5B, lane 7, the BZLF1 protein was expressed in the presence of purvalanol A, although the level was somewhat reduced compared with that in the absence of the drug. It should be noted that, since the BZLF1 protein itself activates expression of its own gene (2), the total amount is a sum of the Tet-on and endogenously expressed protein. Thus, it appears that the former conditional expression is not markedly affected by purvalanol A.
Expression of the other immediate-early protein, BRLF1, and early viral proteins such as the BALF2, BALF5, BBLF2/3, and BMRF1 proteins was, however, greatly inhibited by purvalanol A treatment, whereas the cellular protein PCNA was largely unaffected (Fig. 5B, compare lane 6 with lane 7). As expected, cells in purvalanol A-free medium synthesized viral immediate-early as well as early proteins during the same period (Fig. 5B, lane 6). Also, when cells treated with doxycycline were overlaid with purvalanol A-containing medium at 3 h postannexation, viral protein synthesis was significantly prevented for 48 h (Fig. 5B, lane 8). When purvalanol A was added to the culture at 9 h postinduction, BRLF1, BALF2, BALF5, BBLF2/3, and BMRF1 proteins were expressed to some extent but at low levels. At 9 h posttreatment with doxycycline in the absence of purvalanol A, the levels of the BRLF1, BALF2, BALF5, BBLF2/3, and BMRF1 proteins were very low (Fig. 5B, lane 4). Since viral immediate-early and early proteins were synthesized largely during the first 24 h of induction (Fig. 5B, lane 5), relatively large amounts of viral replication proteins had already been expressed when purvalanol A was added at 24 h postinduction (Fig. 5B, compare lane 6 with lane 11). Purvalanol A had no effects on lytic viral replication 24 h postinduction as shown in Fig. 4A. Thus, purvalanol A does not inhibit lytic EBV replication when added after viral replication proteins are already expressed. These results led us to the conclusion that purvalanol A blocks an (relatively) early stage of viral productive infection due to impairment of viral immediate-early and/or early gene expression. We speculate that the cellular environment in which the Rb protein is underphosphorylated is inappropriate for viral transcription since under such conditions the level of free E2F transcription factor may be very low.
To confirm whether purvalanol A impairs viral immediate-early and/or early gene expression in other EBV latently infected B cells, we examined the effect of the drug in Akata cells, an EBV-positive B-cell line derived from Burkitt's lymphoma. It has previously been reported that induction of lytic replication by anti-immunoglobulin G treatment in Akata cells resulted in hyperphosphorylation of Rb protein, as is the case with Tet-BZLF1/B95-8 cells (33). We also constructed Tet-BZLF1/Akata cells in which the exogenous BZLF1 protein is conditionally expressed under the control of a tetracycline-regulated promoter. As shown in Fig. 5C, lane 2, treatment of the Tet-BZLF1/Akata cells with doxycycline resulted in a significant expression of EBV lytic proteins at 48 h postinduction. In contrast, in the presence of purvalanol A, expression of lytic viral proteins besides the BZLF1 protein was hardly observed (Fig. 5C, lane 3). Thus, it was confirmed that inhibition of S-phase CDK activity impairs viral immediate-early and early gene expression in Akata cells as well as in B95-8 cells.
Purvalanol A inhibits expression of the BRLF1 and viral early proteins even in the presence of sufficient BZLF1 protein.
It is technically difficult to evaluate the direct effects of purvalanol A on viral early gene expression by adding the drug at different times after induction because of the overlap in the times of expression of EBV immediate-early and early proteins. The effects of purvalanol A on specific stages of the EBV replication cycle can be determined, however, by blocking the progress of lytic infection by using cycloheximide to inhibit translation of the BZLF1 transcripts and then releasing the block in the presence or absence of purvalanol A. Using the cycloheximide block-and-release experiment, we examined the effects of addition of purvalanol A on the expression of the other immediate-early (BRLF1 protein) and early viral proteins when high levels of the BZLF1 protein had already been expressed (Fig. 6). Cycloheximide is a general inhibitor of translation; hence, during infections in the presence of this drug, the BZLF1 transcripts accumulate but are not translated. Since viral early promoters are not activated, viral early transcripts and proteins are not synthesized and consequently EBV lytic replication does not occur. Further, cycloheximide inhibition is reversible. Thus, if S-phase CDK activity is required for EBV replication functions that occur after accumulation of sufficient BZLF1 protein, purvalanol A would inhibit expression of early viral proteins when added after the reversal of a cycloheximide block.
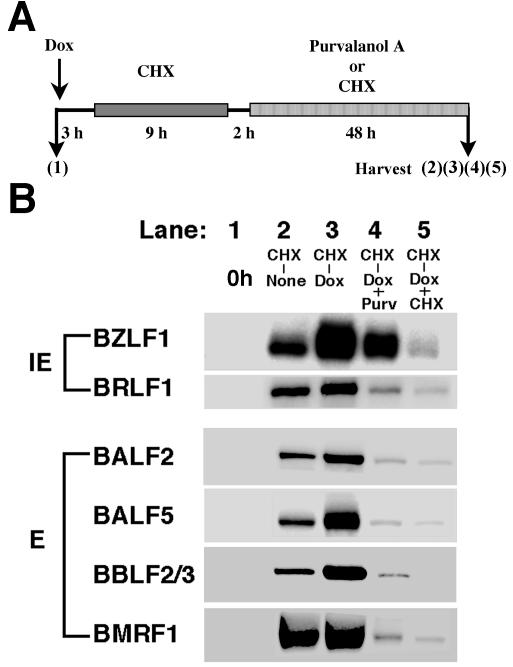
FIG. 6.
Effects of purvalanol A on expression of EBV immediate-early and early proteins after release from a cycloheximide block. (A) Illustration of the experimental protocol. Tet-BZLF1/B95-8 cells were treated with doxycycline (1 μg/ml). Three hours later, 50 μg of cycloheximide (CHX)/ml was added to the culture. The cells were incubated for 9 h and then removed from the CHX block by replacing the medium with drug-free medium and culturing for a further 2 h. Subsequently, the medium was replaced with one containing no drug (CHX-None), 1 μg of doxycycline/ml (CHX-Dox), 1 μg of doxycycline/ml plus 15 μM purvalanol A (CHX-Dox+Purv), or 1 μg of doxycycline/ml plus 50 μg of cycloheximide/ml (CHX-Dox+CHX), and the cells were cultured for 48 h and harvested (lanes 2, 3, 4, and 5 of panel B, respectively; lane 1 of panel B represents the sample prepared when the cells were treated with doxycycline at time zero). (B) Clarified lysates were prepared and applied for Western blot analyses with each specific antibody as indicated on the left. The proteins were detected by an enhanced chemiluminescence detection system (Amersham). Images were processed by LumiVision PRO (Aisin/Taitec, Inc.) with a cooled CCD camera and assembled using an Apple G4 computer with Adobe Photoshop 5.0. Signal intensity was quantified with a LumiVision image analyzer as described in Materials and Methods. IE, immediate-early proteins; E, early proteins.
To test this possibility, cycloheximide was added to culture medium of the Tet-BZLF1/B95-8 cells at 3 h postinduction (Fig. 6A), because within 3 h postinduction, no expression of the BZLF1 protein was detected and the expression level of the BZLF1 protein in this case was higher than that seen with simultaneous treatment with cycloheximide and doxycycline (data not shown). Medium was removed 9 h later, and the cells were washed twice with PBS and overlaid with cycloheximide-free fresh medium followed by incubation for 2 h to accumulate sufficient amounts of the BZLF1 proteins in cells. After that, no drug (control), 50 μg of cycloheximide/ml, or 15 μM purvalanol A was added to the culture medium containing doxycycline. At 48 h after the change of medium, cells were harvested, and the cellular extracts were prepared, followed by Western blot analysis (Fig. 6).
Before the Tet-BZLF1/B95-8 cells were treated with doxycycline, no lytic viral protein was detected (Fig. 6B, lane 1). After the cycloheximide block, the cells were cultured in purvalanol A-free medium with doxycycline and several EBV early proteins as well as the BRLF1 protein were fully expressed (Fig. 6B, lane 3). Figure 6B, lane 2, shows the results when the cells were cultured in purvalanol A-free medium without doxycycline after cycloheximide block. Although the level of the BZLF1 protein was low compared with that in the case of lane 3, it should be noted that the expression level was enough for the expression of other immediate-early and early proteins. In contrast, expression of the BRLF1 protein and early viral proteins was drastically prevented in cells released from the 9-h cycloheximide block into purvalanol A-containing medium with doxycycline, although relatively large amounts of the BZLF1 protein accumulated (Fig. 6B, compare lane 4 and lane 2). When cycloheximide was added in place of purvalanol A, the expression level of the BZLF1 was low and there were very low expression levels of lytic viral proteins (Fig. 6B, lane 5). As the BRLF1 protein is also an important transactivator of early genes (33, 41), inhibition of its synthesis by purvalanol A may provide another plausible explanation as to why viral early gene expression is also inhibited. Thus, the inhibitory effect of purvalanol A on EBV replication after reversal of a cycloheximide block could be the result of inhibition of expression of the viral replication proteins.
Purvalanol A inhibits EBV immediate-early and early gene expression at the transcription level.
To determine whether purvalanol A inhibits viral gene expression at the transcription level in the presence of a sufficient amount of the BZLF1 protein, we measured the levels of representative viral immediate-early (BZLF1 and BRLF1), early (BALF5 and BMRF1), and cellular housekeeping gene (36B4) transcripts accumulated during the 48 h after the release of the cycloheximide block in the presence of purvalanol A. Total RNAs were extracted from the cells, and the levels of the specific viral and cellular gene transcripts relative to those before treatment with the drugs were compared by Northern blot analyses (Fig. 7A).
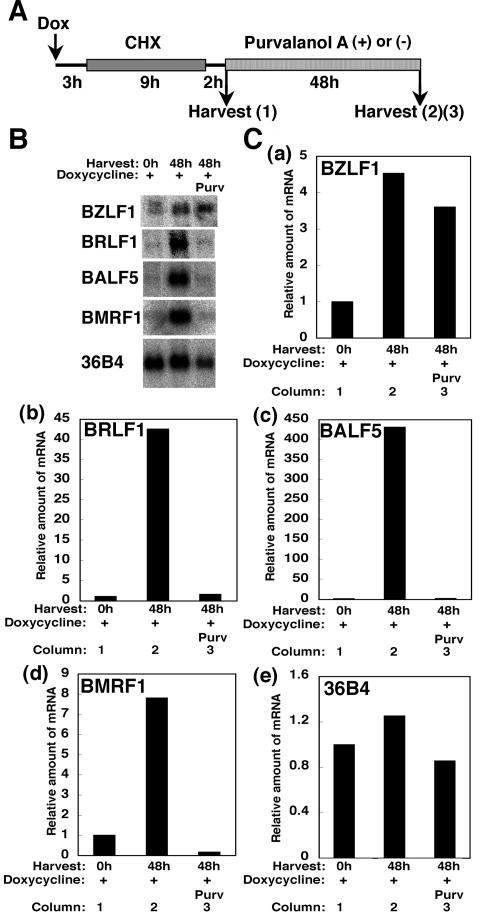
FIG. 7.
Effects of purvalanol A on transcription of EBV immediate-early and early genes after release from a cycloheximide block. (A) Illustration of the experimental protocol. Tet-BZLF1/B95-8 cells were treated with doxycycline (1 μg/ml). At 3 h thereafter, 50 μg of cycloheximide (CHX)/ml was added to the culture. The cells were incubated for 9 h and then removed from the CHX block by replacing the medium with drug-free medium and culturing for a further 2 h. Subsequently, the medium was replaced with fresh medium containing 1 μg of doxycycline/ml and cultured for 48 h in the absence or presence of 15 μM purvalanol A [(2) and (3), respectively] and then harvested. As a control, cells were harvested before 48 h cultivation [(1)]. (B) Total RNA was extracted and applied for Northern blot analyses as described in Materials and Methods. (C) Levels of BZLF1-, BRLF1-, BALF5-, BMRF1-, and 36B4-specific transcripts (panels a to e, respectively, in Fig. 7C) were measured by Northern hybridization with 32P-labeled probes obtained by PCR amplification of each viral and cellular DNA. Signal intensity was quantitated with an Image guider (BAS2500; Fujifilm). The levels of the specific viral and cellular gene transcripts relative to the pretreatment values were calculated and plotted in the graphs.
It must be noted that the BZLF1 transcripts had already accumulated during the first 9-h cycloheximide block by doxycycline annexation (Fig. 7B, lane 1). As shown in Fig. 7B, when the lytic program-induced cells were released from the cycloheximide block into fresh medium containing only doxycycline, the level of BZLF1 gene transcripts increased fivefold during the 48-h cultivation (Fig. 7C, panel a, column 2). Since the BZLF1 protein itself activates expression of its own gene (2), the total amount of the BZLF1 transcripts would be the sum of the doxycycline-induced transcripts and the endogenously expressed one. When purvalanol A-containing medium was overlayed 2 h after release of the 9-h cycloheximide block, the BZLF1 gene transcripts also accumulated.
Next, the levels of other lytic transcripts were measured. When the cells were released into fresh medium containing only doxycycline after release of the cycloheximide block, BRLF1, BALF5, and BMRF1 transcripts accumulated more than 42-, 430-, and 8-fold, respectively, compared to prerelease levels (Fig. 7C), as expected. After release of the cycloheximide block into purvalanol A-containing medium, the levels of the BRLF1, BALF5, and BMRF1 transcripts were very low.
In contrast to that of viral transcripts, the levels of a cellular housekeeping gene (36B4) transcript were not significantly affected following release of the cycloheximide block into cycloheximide- or purvalanol A-containing medium relative to what occurred upon release into drug-free medium (Fig. 7C, panel e).
Based on a comparison of the results presented in Fig. 6 and 7, although the level of the BZLF1 protein synthesized in cells released from the 9-h cycloheximide block into purvalanol A-containing medium was higher than in cells released into drug-free medium (Fig. 6B, compare lane 2 with lane 4), viral early transcript accumulation was significantly impaired by purvalanol A under these conditions (Fig. 7C). We conclude that purvalanol A inhibits transcription of immediate-early and early genes even in the presence of sufficient levels of the BZLF1 protein.
Purvalanol A inhibits BZLF1-induced activation of early EBV promoters pBMRF1 and pBHRF1.
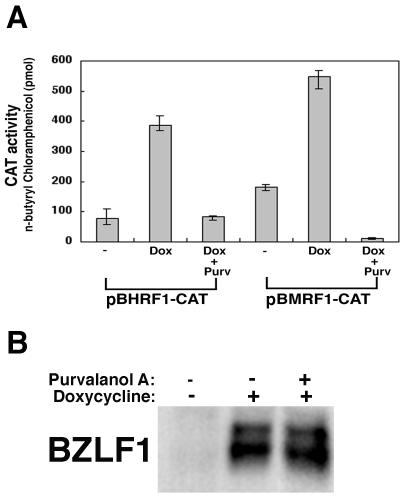
To determine whether the BZLF1 transactivator function is impaired in purvalanol A-treated cells lacking EBV genome, we performed reporter gene transfection studies with Tet-BZLF1/HeLa cells by using CAT gene constructs driven by two different early EBV promoters: the BMRF1 promoter and the BHRF1 promoter. The BMRF1 promoter drives expression of the BMRF1 gene product, whereas the BHRF1 promoter is within oriLyt. Each of these promoters has been previously shown to be directly bound and transcriptionally activated by the BZLF1 protein (8, 68). In Tet-BZLF1/HeLa cells, the exogenous BZLF1 protein is conditionally expressed under the control of a tetracycline-regulated promoter. As shown in Fig. 8B, the BZLF1 protein was conditionally expressed in the Tet-BZLF1/HeLa cells by the addition of doxycycline in the presence or absence of purvalanol A. As shown in Fig. 8A, the BZLF1 protein produced a stimulatory activation in the constitutive expression of the BMRF1 and BHRF1 promoters in the absence of purvalanol A. However, it was clearly demonstrated that purvalanol A had an inhibitory effect on the BZLF1-induced transcriptional activation of early EBV promoters pBMRF1 and pBHRF1 even in the presence of the BZLF1 protein (Fig. 8A). Further, purvalanol A appeared to inhibit the basal activity of the BMRF1 promoter in HeLa cells (Fig. 8A, compare the left and right columns of the pBMRF1-CAT samples). We speculate that the BHRF1 and BMRF1 promoters might contain binding sites of S-phase CDK-responsive cellular transcription factor(s).
FIG. 8.
Purvalanol A prevents BZLF1-induced transcriptional activation of the early EBV promoters pBMRF1 and pBHRF1. Tet-BZLF1/HeLa cells were transfected with 2 μg of pBMRF1-CAT or pBHRF1-CAT and media were replaced with fresh media containing or free of purvalanol A (20 μM) at 16 h posttransfection. Twenty hours posttransfection, doxycycline was added to a final concentration of 5 μg/ml or not. Forty-eight hours after addition of doxycycline, cells were harvested and some of them were used for a CAT enzyme assay. (A) CAT activity was assayed by using the Promega CAT enzyme assay system. The total amount of n-butyryl [14C]chloramphenicol (in picomoles) was measured and plotted on the graph. Data represent averages from three independent experiments. (B) The rest of the cells were treated with lysis buffer, and clarified cell lysates were prepared. Clarified cell lysates were separated by SDS-10% PAGE and applied for Western blot analyses with the BZLF1 protein-specific antibody. The proteins were detected by an enhanced chemiluminescence detection system (Amersham). The slower-migrating band is a phosphorylated form of the BZLF1 protein. The faster-migrating band is the hypophosphorylated form.
DISCUSSION
The lack of elevation of the cellular CDK inhibitors p21CIP1/WAF1 and p27KIP-1, and instead the accumulation of hyperphosphorylated forms of Rb protein in EBV productive infection-induced LCLs, indicated that the p21CIP1/WAF1- and p27KIP-1-sensitive CDKs might play an important role in EBV productive replication. The results of a previous report (30) prompted us to ask whether EBV lytic infection could specifically be inhibited by chemical drugs that function similarly to p21CIP1/WAF1 and p27KIP-1. Several purine derivative drugs, including roscovitine and purvalanol A, that inhibit specific types of CDK activity have recently been described (21, 37). At low concentrations, their inhibitory effects are highly specific for CDK1/cyclin B, CDK2/cyclin A, and CDK2/cyclin E and none can significantly inhibit CDK4 and CDK6 activities. In this study, it was revealed that these chemical CDK inhibitors block EBV lytic replication significantly, providing evidence that G1 is an unsuitable period in the cell cycle for EBV productive infection and confirming the requirement for the environment of the late G1/S boundary or beyond.
Although the mechanism by which CDK2 and/or CDK1 activate EBV gene expression is unknown at the present time, it has been shown that transcription of the EBV DNA Pol is activated by the E2F transcription factor (33). There are several putative E2F-binding motifs in the promoters of viral genes such as BRLF1 (42), BALF5 (33), BALF4, BRRF2, BVRF1, and BPLF1 (A. Kudoh and T. Tsurumi, unpublished results). Also, E2F putative binding sites have been identified within the promoter of the cellular transcription factor Sp1 (40) and E2F exerts an additive effect on its overexpression. Within the EBV lytic gene promoters are many Sp1 binding sites. CDK2 and/or CDK1 act in part by phosphorylating the Rb protein, which results in the release of the transcription factor E2F (39), which can then transcriptionally activate genes that are required for efficient lytic EBV DNA synthesis. EBV DNA replication is not completely independent of cellular replication proteins; e.g., DNA ligase I and FEN 1 may be required. It is conceivable that the activities of these cellular replication proteins may be promoted under S-phase conditions, when energy generation and other resources may support viral replication.
Purvalanol A induces a reversible arrest in progression through the G1 and G2 phases of the cell cycle. Its inhibitory effects are highly specific for CDK1, CDK2, and CDK5. Although CDK5 is a member of the CDK family of cell cycle regulators with important roles in neuronal differentiation, it has no observed function in the regulation of cell proliferation. Its protein kinase activity is only detected in postmitotic neurons. Although influence on CDK7 and CDK9 has not previously been determined, it has been reported recently that roscovitine inhibits CDK7 and CDK9 but not CDK8 (45, 63). The target of CDK7 and -9 is known to be the C-terminal domain of RNA Pol II, which regulates gene transcription. Villerbu et al., however, have reported that purvalanol A does not inhibit transcription under cell-free conditions and that the level of the CDK inhibitory protein p21(WAF1/CIP1) is increased in cells incubated with purvalanol A (62). Remarkably, it has been demonstrated that roscovitine inhibits HSV-1 transcription but not cellular transcription (which requires CDK7 and CDK9) (31, 38, 47, 49), and in this study, purvalanol A did not inhibit the conditional expression of the BZLF1 protein with doxycycline. Thus, purvalanol A does not cause a general inhibition of gene expression and the specificity of action of the drug is supported by the selective inhibition of the phosphorylation of CDK substrates such as Rb protein and cyclin E.
The potential applications of CDK inhibitors have been explored for infections with HSV (47-49), CMV (4), human T-cell leukemia virus type 1 (64), and human immunodeficiency virus (63). Like EBV, these viruses might have evolved various means to perturb the cell cycle to optimize the cellular conditions in favor of their own replication. Although attempts have been made to identify viruses resistant to the CDK inhibitor, almost no resistant HSV-1 or CMV strains could be isolated (4, 47). However, we cannot preclude the possibility that, in addition to cellular CDKs, the inhibitor could inhibit any of the EBV-encoded kinases. Indeed, the EBV BGLF4-encoded protein kinase mediates hyperphosphorylation of cellular elongation factor 1δ (EF-1δ). The BGLF4 protein and CDK1 target the same phosphorylation site (Ser133) in EF-1δ (28). Given that the BGLF4 protein may have the potential to phosphorylate Rb protein followed by release of free E2F, cellular CDKs and viral protein kinase might act to phosphorylate Rb protein synergistically. However, at least cellular CDK activity would be essential for EBV lytic replication since the viral protein kinase(s) belongs to an early or late protein group and is not expressed in the early stage of EBV productive infection.
The present findings clearly demonstrated the requirement for CDK activity in the replication of EBV. Thus, inhibition of CDK activity by drugs blocks EBV replication. We hypothesized that CDK2 in particular may be required to phosphorylate the Rb protein, allowing for release of E2F and subsequently transcription of cellular and/or viral genes required for EBV replication. To investigate this hypothesis further, it will be necessary to determine whether upon phosphorylation of Rb protein, subsequent release of E2F enhances synthesis of viral immediate-early and early proteins. Alternatively, CDK2 activity may also play another role in EBV productive replication. CDK2 activity has been reported to be involved in the initiation of cellular DNA synthesis at origins of replication (25). It is entirely possible that CDK2 activity may also play a similar role in initiating EBV lytic replication. Purvalanol A-sensitive CDKs might possibly regulate BZLF1-mediated transactivation of EBV immediate-early and early genes, as the CDK activity clearly plays a role in BZLF1-mediated transactivation based on the data shown in Fig. 6 and 7. There is a possibility that purvalanol A-sensitive CDKs might directly phosphorylate the BZLF1 protein, which, in turn, is required for its function, and/or regulate downstream effectors of the BZLF1 protein. It should be noted that roscovitine impaired the activities of immediate-early proteins from human CMV (HCMV) and HSV-1 (5, 10, 46), thus supporting one hypothesis that purvalanol A might directly inhibit BZLF1 function. However, when the BZLF1 protein was expressed in Tet-BZLF1/HeLa cells with doxycycline, purvalanol A did not inhibit the phosphorylation of the protein (Fig. 8B). Also, the fact that inhibition of CDK activity by purvalanol A blocks EBV lytic replication sometime after the BZLF1 gene expression, but prior to initiation of viral DNA synthesis, supports the former hypothesis.
A setting similar to the cellular environment evoked by EBV lytic replication has been observed for HCMV infection. A number of studies have revealed that productive HCMV infection stimulates arrested cells to enter the cell cycle (5, 13, 34, 44). Recent studies have confirmed these early observations and demonstrated that productively infected cells traverse the cell cycle through at least late G1 with induction of cyclin E/CDK2 activity and hyperphosphorylation of Rb protein (4, 5, 26, 65). HCMV infection reduces the abundance of two CDK2 inhibitors (p21WAF1/CIP1 and p27KIP1). In cells stimulated to traverse the cell cycle by serum growth factors, cyclin E/CDK2 acts in part by phosphorylating pRB protein, which results in the release of the transcription factor E2F. These findings suggest an important role for S-phase CDK activity in HCMV replication as well as the lytic phase of EBV DNA replication.
Acknowledgments
We thank Y. Nishikawa and T. Yoshida for technical assistance, K. Tamai for providing the antibody specific for the Ser612 phosphorylation site of the Rb protein, and S. Kenney for sending us the reporter gene constructs pBMRF1-CAT and pBHRF1-CAT.
This work was supported by grants-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, Culture and Technology of Japan (14021138 and 12470073 to T.T.).
REFERENCES
- 1.Adams, P. D., and W. G. Kaelin, Jr. 1995. Transcriptional control by E2F. Semin. Cancer Biol. 6:99-108. [DOI] [PubMed] [Google Scholar]
- 2.Adamson, A. L., and S. C. Kenney. 1998. Rescue of the Epstein-Barr virus BZLF1 mutant, Z(S186A), early gene activation defect by the BRLF1 gene product. Virology 251:187-197. [DOI] [PubMed] [Google Scholar]
- 3.Borgne, A., and L. Meijer. 1996. Sequential dephosphorylation of p34(cdc2) on Thr-14 and Tyr-15 at the prophase/metaphase transition. J. Biol. Chem. 271:27847-27854. [DOI] [PubMed] [Google Scholar]
- 4.Bresnahan, W. A., I. Boldogh, P. Chi, E. A. Thompson, and T. Albrecht. 1997. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology 231:239-247. [DOI] [PubMed] [Google Scholar]
- 5.Bresnahan, W. A., I. Boldogh, E. A. Thompson, and T. Albrecht. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224:150-160. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso, M. C., H. Leonhardt, and B. Nadal-Ginard. 1993. Reversal of terminal differentiation and control of DNA replication: cyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell 74:979-992. [DOI] [PubMed] [Google Scholar]
- 7.Cayrol, C., and E. K. Flemington. 1996. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J. 15:2748-2759. [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, Y. N., D. L. Dong, G. S. Hayward, and S. D. Hayward. 1990. The Epstein-Barr virus Zta transactivator: a member of the bZIP family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J. Virol. 64:3358-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darr, C. D., A. Mauser, and S. Kenney. 2001. Epstein-Barr virus immediate-early protein BRLF1 induces the lytic form of viral replication through a mechanism involving phosphatidylinositol-3 kinase activation. J. Virol. 75:6135-6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davido, D. J., D. A. Leib, and P. A. Schaffer. 2002. The cyclin-dependent kinase inhibitor roscovitine inhibits the transactivating activity and alters the posttranslational modification of herpes simplex virus type 1 ICP0. J. Virol. 76:1077-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decaussin, G., V. Leclerc, and T. Ooka. 1995. The lytic cycle of Epstein-Barr virus in the nonproducer Raji line can be rescued by the expression of a 135-kilodalton protein encoded by the BALF2 open reading frame. J. Virol. 69:7309-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DePamphilis, M. L. (ed.). 1996. DNA replication in eukaryotic cells. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 13.Dittmer, D., and E. S. Mocarski. 1997. Human cytomegalovirus infection inhibits G1/S transition. J. Virol. 71:1629-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley, D. T., L. Pang, S. J. Decker, A. J. Bridges, and A. R. Saltiel. 1995. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 92:7686-7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrell, P. J., G. J. Allan, F. Shanahan, K. H. Vousden, and T. Crook. 1991. p53 is frequently mutated in Burkitt's lymphoma cell lines. EMBO J. 10:2879-2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields, B. N., D. M. Knipe, P. M. Howley, and D. E. Griffin. 2002. Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 17.Fischer, P. M., and D. P. Lane. 2000. Inhibitors of cyclin-dependent kinases as anti-cancer therapeutics. Curr. Med. Chem. 7:1213-1245. [DOI] [PubMed] [Google Scholar]
- 18.Fixman, E. D., G. S. Hayward, and S. D. Hayward. 1995. Replication of Epstein-Barr virus oriLyt: lack of a dedicated virally encoded origin-binding protein and dependence on Zta in cotransfection assays. J. Virol. 69:2998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii, K., N. Yokoyama, T. Kiyono, K. Kuzushima, M. Homma, Y. Nishiyama, M. Fujita, and T. Tsurumi. 2000. The Epstein-Barr virus Pol catalytic subunit physically interacts with the BBLF4-BSLF1-BBLF2/3 complex. J. Virol. 74:2550-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao, X., K. Ikuta, M. Tajima, and T. Sairenji. 2001. 12-O-tetradecanoylphorbol-13-acetate induces Epstein-Barr virus reactivation via NF-κB and AP-1 as regulated by protein kinase C and mitogen-activated protein kinase. Virology 286:91-99. [DOI] [PubMed] [Google Scholar]
- 21.Gray, N., L. Detivaud, C. Doerig, and L. Meijer. 1999. ATP-site directed inhibitors of cyclin-dependent kinases. Curr. Med. Chem. 6:859-875. [PubMed] [Google Scholar]
- 22.Gray, N. S., L. Wodicka, A. M. Thunnissen, T. C. Norman, S. Kwon, F. H. Espinoza, D. O. Morgan, G. Barnes, S. LeClerc, L. Meijer, S. H. Kim, D. J. Lockhart, and P. G. Schultz. 1998. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 281:533-538. [DOI] [PubMed] [Google Scholar]
- 23.Hammerschmidt, W., and B. Sugden. 1988. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell 55:427-433. [DOI] [PubMed] [Google Scholar]
- 24.Harbour, J. W., and D. C. Dean. 2000. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14:2393-2409. [DOI] [PubMed] [Google Scholar]
- 25.Jackson, P. K., S. Chevalier, M. Philippe, and M. W. Kirschner. 1995. Early events in DNA replication require cyclin E and are blocked by p21CIP1. J. Cell Biol. 130:755-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jault, F. M., J. M. Jault, F. Ruchti, E. A. Fortunato, C. Clark, J. Corbeil, D. D. Richman, and D. H. Spector. 1995. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J. Virol. 69:6697-6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato, J. 1999. Induction of S phase by G1 regulatory factors. Front. Biosci. 4:D787-D792. [DOI] [PubMed] [Google Scholar]
- 28.Kawaguchi, Y., K. Kato, M. Tanaka, M. Kanamori, Y. Nishiyama, and Y. Yamanashi. 2003. Conserved protein kinases encoded by herpesviruses and cellular protein kinase cdc2 target the same phosphorylation site in eukaryotic elongation factor 1δ. J. Virol. 77:2359-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krude, T., M. Jackman, J. Pines, and R. A. Laskey. 1997. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell 88:109-119. [DOI] [PubMed] [Google Scholar]
- 30.Kudoh, A., M. Fujita, T. Kiyono, K. Kuzushima, Y. Sugaya, S. Izuta, Y. Nishiyama, and T. Tsurumi. 2003. Reactivation of lytic replication from B cells latently infected with Epstein-Barr virus occurs with high S-phase cyclin-dependent kinase activity while inhibiting cellular DNA replication. J. Virol. 77:851-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam, L. T., O. K. Pickeral, A. C. Peng, A. Rosenwald, E. M. Hurt, J. M. Giltnane, L. M. Averett, H. Zhao, R. E. Davis, M. Sathyamoorthy, L. M. Wahl, E. D. Harris, J. A. Mikovits, A. P. Monks, M. G. Hollingshead, E. A. Sausville, and L. M. Staudt. 2001. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biol. 2:research0041.1-0041.11. [Online.] [DOI] [PMC free article] [PubMed]
- 32.Laux, G., U. K. Freese, R. Fischer, A. Polack, E. Kofler, and G. W. Bornkamm. 1988. TPA-inducible Epstein-Barr virus genes in Raji cells and their regulation. Virology 162:503-507. [DOI] [PubMed] [Google Scholar]
- 33.Liu, C., N. D. Sista, and J. S. Pagano. 1996. Activation of the Epstein-Barr virus DNA polymerase promoter by the BRLF1 immediate-early protein is mediated through USF and E2F. J. Virol. 70:2545-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu, M., and T. Shenk. 1996. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J. Virol. 70:8850-8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauser, A., E. Holley-Guthrie, A. Zanation, W. Yarborough, W. Kaufmann, A. Klingelhutz, W. T. Seaman, and S. Kenney. 2002. The Epstein-Barr virus immediate-early protein BZLF1 induces expression of E2F-1 and other proteins involved in cell cycle progression in primary keratinocytes and gastric carcinoma cells. J. Virol. 76:12543-12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meijer, L., A. Borgne, O. Mulner, J. P. Chong, J. J. Blow, N. Inagaki, M. Inagaki, J. G. Delcros, and J. P. Moulinoux. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243:527-536. [DOI] [PubMed] [Google Scholar]
- 37.Meijer, L., S. Leclerc, and M. Leost. 1999. Properties and potential-applications of chemical inhibitors of cyclin-dependent kinases. Pharmacol. Ther. 82:279-284. [DOI] [PubMed] [Google Scholar]
- 38.Nelson, P. J., I. H. Gelman, and P. E. Klotman. 2001. Suppression of HIV-1 expression by inhibitors of cyclin-dependent kinases promotes differentiation of infected podocytes. J. Am. Soc. Nephrol. 12:2827-2831. [DOI] [PubMed] [Google Scholar]
- 39.Nevins, J. R. 1992. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science 258:424-429. [DOI] [PubMed] [Google Scholar]
- 40.Nicolas, M., V. V. Noe, and C. J. Ciudad. 2003. Transcriptional regulation of the human SP1 gene promoter by SP-family members, NF-Y and E2F. Biochem. J. 371:265-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinlivan, E. B., E. A. Holley-Guthrie, M. Norris, D. Gutsch, S. L. Bachenheimer, and S. C. Kenney. 1993. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 21:1999-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ragoczy, T., and G. Miller. 2001. Autostimulation of the Epstein-Barr virus BRLF1 promoter is mediated through consensus Sp1 and Sp3 binding sites. J. Virol. 75:5240-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez, A., E. J. Jung, Q. Yin, C. Cayrol, and E. K. Flemington. 2001. Role of c-myc regulation in Zta-mediated induction of the cyclin-dependent kinase inhibitors p21 and p27 and cell growth arrest. Virology 284:159-169. [DOI] [PubMed] [Google Scholar]
- 44.Salvant, B. S., E. A. Fortunato, and D. H. Spector. 1998. Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription. J. Virol. 72:3729-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schang, L. M. 2002. Cyclin-dependent kinases as cellular targets for antiviral drugs. J. Antimicrob. Chemother. 50:779-792. [DOI] [PubMed] [Google Scholar]
- 46.Schang, L. M., A. Bantly, M. Knockaert, F. Shaheen, L. Meijer, M. H. Malim, N. S. Gray, and P. A. Schaffer. 2002. Pharmacological cyclin-dependent kinase inhibitors inhibit replication of wild-type and drug-resistant strains of herpes simplex virus and human immunodeficiency virus type 1 by targeting cellular, not viral, proteins. J. Virol. 76:7874-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schang, L. M., J. Phillips, and P. A. Schaffer. 1998. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J. Virol. 72:5626-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 2000. Roscovitine, a specific inhibitor of cellular cyclin-dependent kinases, inhibits herpes simplex virus DNA synthesis in the presence of viral early proteins. J. Virol. 74:2107-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 1999. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J. Virol. 73:2161-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schepers, A., D. Pich, and W. Hammerschmidt. 1996. Activation of oriLyt, the lytic origin of DNA replication of Epstein-Barr virus, by BZLF1. Virology 220:367-376. [DOI] [PubMed] [Google Scholar]
- 51.Sielecki, T. M., J. F. Boylan, P. A. Benfield, and G. L. Trainor. 2000. Cyclin-dependent kinase inhibitors: useful targets in cell cycle regulation. J. Med. Chem. 43:1-18. [PubMed] [Google Scholar]
- 52.Suzuki, S., K. Tamai, and S. Yoshida. 2002. Enzyme-linked immunosorbent assay for distinct cyclin-dependent kinase activities using phosphorylation-site-specific anti-pRB monoclonal antibodies. Anal. Biochem. 301:65-74. [DOI] [PubMed] [Google Scholar]
- 53.Swenson, J. J., A. E. Mauser, W. K. Kaufmann, and S. C. Kenney. 1999. The Epstein-Barr virus protein BRLF1 activates S phase entry through E2F1 induction. J. Virol. 73:6540-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toullec, D., P. Pianetti, H. Coste, P. Bellevergue, T. Grand-Perret, M. Ajakane, V. Baudet, P. Boissin, E. Boursier, F. Loriolle, et al. 1991. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 266:15771-15781. [PubMed] [Google Scholar]
- 55.Tsurumi, T. 1993. Purification and characterization of the DNA-binding activity of the Epstein-Barr virus DNA polymerase accessory protein BMRF1 gene products, as expressed in insect cells by using the baculovirus system. J. Virol. 67:1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsurumi, T., T. Daikoku, R. Kurachi, and Y. Nishiyama. 1993. Functional interaction between Epstein-Barr virus DNA polymerase catalytic subunit and its accessory subunit in vitro. J. Virol. 67:7648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsurumi, T., T. Daikoku, and Y. Nishiyama. 1994. Further characterization of the interaction between the Epstein-Barr virus DNA polymerase catalytic subunit and its accessory subunit with regard to the 3′-to-5′ exonucleolytic activity and stability of initiation complex at primer terminus. J. Virol. 68:3354-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsurumi, T., J. Kishore, N. Yokoyama, M. Fujita, T. Daikoku, H. Yamada, Y. Yamashita, and Y. Nishiyama. 1998. Overexpression, purification and helix-destabilizing properties of Epstein-Barr virus ssDNA-binding protein. J. Gen. Virol. 79:1257-1264. [DOI] [PubMed] [Google Scholar]
- 59.Tsurumi, T., A. Kobayashi, K. Tamai, T. Daikoku, R. Kurachi, and Y. Nishiyama. 1993. Functional expression and characterization of the Epstein-Barr virus DNA polymerase catalytic subunit. J. Virol. 67:4651-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsurumi, T., A. Kobayashi, K. Tamai, H. Yamada, T. Daikoku, Y. Yamashita, and Y. Nishiyama. 1996. Epstein-Barr virus single-stranded DNA-binding protein: purification, characterization, and action on DNA synthesis by the viral DNA polymerase. Virology 222:352-364. [DOI] [PubMed] [Google Scholar]
- 61.Tsurumi, T., H. Yamada, T. Daikoku, Y. Yamashita, and Y. Nishiyama. 1997. Strand displacement associated DNA synthesis catalyzed by the Epstein-Barr virus DNA polymerase. Biochem. Biophys. Res. Commun. 238:33-38. [DOI] [PubMed] [Google Scholar]
- 62.Villerbu, N., A. M. Gaben, G. Redeuilh, and J. Mester. 2002. Cellular effects of purvalanol A: a specific inhibitor of cyclin-dependent kinase activities. Int. J. Cancer 97:761-769. [DOI] [PubMed] [Google Scholar]
- 63.Wang, D., C. de la Fuente, L. Deng, L. Wang, I. Zilberman, C. Eadie, M. Healey, D. Stein, T. Denny, L. E. Harrison, L. Meijer, and F. Kashanchi. 2001. Inhibition of human immunodeficiency virus type 1 transcription by chemical cyclin-dependent kinase inhibitors. J. Virol. 75:7266-7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, L., L. Deng, K. Wu, C. de la Fuente, D. Wang, K. Kehn, A. Maddukuri, S. Baylor, F. Santiago, E. Agbottah, S. Trigon, M. Morange, R. Mahieux, and F. Kashanchi. 2002. Inhibition of HTLV-1 transcription by cyclin dependent kinase inhibitors. Mol. Cell. Biochem. 237:137-153. [DOI] [PubMed] [Google Scholar]
- 65.Wiebusch, L., and C. Hagemeier. 2001. The human cytomegalovirus immediate early 2 protein dissociates cellular DNA synthesis from cyclin-dependent kinase activation. EMBO J. 20:1086-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yates, J. L., and N. Guan. 1991. Epstein-Barr virus-derived plasmids replicate only once per cell cycle and are not amplified after entry into cells. J. Virol. 65:483-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yokoyama, N., K. Fujii, M. Hirata, K. Tamai, T. Kiyono, K. Kuzushima, Y. Nishiyama, M. Fujita, and T. Tsurumi. 1999. Assembly of the Epstein Barr virus BBLF4, BSLF1 and BBLF2/3 proteins and their interactive properties. J. Gen. Virol. 80:2879-2887. [DOI] [PubMed] [Google Scholar]
- 68.Zhang, Q., Y. Hong, D. Dorsky, E. Holley-Guthrie, S. Zalani, N. A. Elshiekh, A. Kiehl, T. Le, and S. Kenney. 1996. Functional and physical interactions between the Epstein-Barr virus (EBV) proteins BZLF1 and BMRF1: effects on EBV transcription and lytic replication. J. Virol. 70:5131-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]