Abstract
The present study examines BPA pharmacokinetics in neonatal rats following s.c. injection or oral delivery of 10μg BPA/kg BW and compares susceptibility to estrogen-induced prostate intraepithelial neoplasia (PIN) following either exposure route. Serum BPA in PND3 rats was measured using HPLC-MS-MS. Free and total BPA at Cmax were 1.77 and 2.0 ng/ml, respectively following injection and 0.26 and 1.02 ng/ml, respectively following oral exposure. The AUC0-2 for free and total BPA was 4.1-fold and 1.8-fold greater, respectively, in s.c. versus oral delivery. While exposure route affected BPA metabolism, internal dosimetry following s.c. injection of 10μg BPA/kg BW is similar to BPA levels observed in humans. Prostates from aged rats given s.c. or oral BPA neonatally and T+E implants as adults exhibited nearly identical, heightened susceptibility to PIN incidence and score as compared to neonatal oil-controls. These findings on prostate health are directly relevant to humans at current BPA exposure levels.
Keywords: BPA, bisphenol A, prostate, pharmacokinetics, prostate intraepithelial neoplasia
1.1 INTRODUCTION
Bisphenol A (BPA) is a high volume (> 3 million tons/year) synthetic monomer used in the production of polycarbonate plastics, epoxy resins that line food and beverage cans, and innumerous common household and consumer products [http:www.bisphenol-a.org 2007]. Although polymeric forms are relatively stable, water-soluble BPA monomers are released when exposed to heat, acidic pH, after repeated use and over time, leaching into consumable liquids and foods and bioaccumulating in environments worldwide [1–3, 4 ]. Consequently, the majority of humans tested (>93%) have detectable levels of BPA in their system [5, 6] with highest levels found in infants and children [7–10]. Since BPA is rapidly metabolized and excreted with a half-life < 6 hours, this indicates that humans are being continuously exposed to BPA [11].
Health concerns regarding human exposures to BPA stem from its estrogenic properties. While affinity for nuclear estrogen receptors (ERα and ERβ) is low relative to natural estradiol [12], BPA has activational capacity equivalent to estradiol for membrane associated ERs [13, 14] and can rapidly activate membrane-initiated ER signaling at low doses [15]. While the topic remains highly controversial, there is a growing body of evidence that BPA has adverse effects on multiple hormone responsive tissues at environmentally relevant doses [16]. It is of particular note that the majority of low-dose BPA effects have been observed during the developmental period when sensitive organs are most susceptible to reprogramming by steroidal exposures.
Estrogens play a physiologic role during normal prostate gland development and inappropriate levels or timing of estrogenic exposures during early life can reprogram the gland and predispose to prostate neoplasia with aging [17–19]. In 2006, our laboratory was the first to demonstrate that transient, early-life exposure to BPA at low-doses increased susceptibility to adult-onset precancerous lesions and hormonal carcinogenesis. Specifically, subcutaneous (s.c.) injection of BPA at 10 μg/kg BW to neonatal Sprague-Dawley (SD) rats on postnatal days (PND) 1, 3 and 5 significantly increased the incidence and score of adult estrogen-induced prostate intraepithelial neoplasia (PIN), the precursor lesion for prostate cancer, as to compared to control rats [20]. This model of heightened sensitivity to hormonal carcinogenesis is highly relevant to humans in that relative estradiol levels increase in the aging male and may contribute to prostate disease risk [21]. Furthermore, our study identified an epigenetic underpinning for early life reprogramming by BPA with multiple genes exhibiting life-long alterations in DNA methylation patterns and gene transcription. In 2008, the National Toxicology Program (NTP) released a report that concluded there is “some concern for BPA exposure in fetuses, infants and children at current human exposures based on effects in the prostate gland” [16], based in part on this work with our rat model. More recently, the FDA adopted these concerns by the NTP and called for further studies to provide additional information and clarify present uncertainties about the risks of BPA.
A number of valid issues were raised by the NTP with regards to our initial studies on BPA effects on the prostate gland. Among them was the s. c. route of exposure that was employed and the inherent differences in BPA metabolism in rodents and humans, both of which may affect the internal dosimetry of the free, biologically active form of BPA. It is currently believed that the majority of BPA intake in humans occurs through oral ingestion and is thus subjected to hepatic first-pass metabolism, also referred to as presystemic Phase II metabolism [22]. In adults, free BPA is rapidly metabolized through glucuronidation to an inactive form by the liver detoxifying enzyme UDP-glucuronosyltransferase isoform, UGT2B1 [23]. Since UGT2B1 is highly efficient, ~99% of orally ingested BPA by adults is metabolized to BPA-glucuronide prior to entry into the general circulation [22]. In contrast, BPA delivery via s.c. injection will enter the circulation prior to liver glucuronidation, thus initial exposure levels of free BPA by this route may be markedly higher than current human circulating levels. This must be taken into consideration when evaluating BPA toxicity and its relevance to human health. However, it is also known that liver UGT2B1 expression is absent during fetal life in rats and is low during the immediate neonatal period [24], thus it is possible that BPA exposures through the oral versus s.c. injection route may result in circulating free BPA levels that are not dissimilar from one another.
Another issue that has been raised is the species difference in BPA metabolism between rodents and humans with rodents having a slower clearance rate as well as deconjugation of bound BPA by gut β-glucuronidases and enterohepatic recirculation [16]. Based on this fact, it has been argued that adverse effects observed in rodent models may not be relevant for human diseases [16, 22, 25]. Ideally, animal studies used to evaluate the potential health effects of BPA should be designed so that internal levels of free BPA are similar between the model system employed and that observed in human circulation.
In this context, the objectives of the present study were threefold. First, we sought to determine the internal dosimetry of total and free BPA levels in neonatal male SD rat sera immediately following s.c. injection of 10 μg BPA/kg BW as used in our prior studies so that they can be directly compared to values currently observed in the human population. Second, we aimed to compare the internal BPA levels after s.c. injection to those following oral exposure at the same applied dosage in matched littermates. Finally, we wanted to directly compare the prostate lesions observed at 7 months of age after a 16 week exposure to androgen-supported, elevated estradiol in rats neonatally treated with vehicle, oral or s.c. injection of 10 μg BPA/kg BW. Our findings reveal significant differences in internal free BPA levels between the two routes of exposure; however, the s.c. injection of a BPA depot delivers free BPA levels similar to those reported in multiple studies for the human fetus and newborn males [11]. Furthermore, elevations in estrogen-driven PIN incidence and score are observed in the ventral and lateral rat prostate lobes following neonatal BPA treatments irrespective of route of exposure. Together, these findings support the relevance of our rat model for developmental BPA exposures to human potential prostate health risks as a function of early-life BPA exposures.
1.2 MATERIALS and METHODS
1.2.1 Animals
All animal treatments were approved by the Animal Use Committee at UIC. Sprague Dawley rats (Hsd:SD®™) were purchased from Harlan Industries, Inc. (Indianapolis, IN). It is noteworthy that SD rats used in previous BPA studies from our laboratories [20] were obtained from the now defunct vendor, Zivic-Miller Laboratories (Pittsburgh, PA) who maintained a closed colony of SD rats for 40 years using natural birthing conditions without selection for large litter size. For the present studies, we chose to return to the original SD™ lineage created by the Sprague-Dawley Company in 1925 which was bought by Harlan Industries, Inc. in 1980. The renamed Hsd:SD®™ rats have been maintained without selection for large litter size or other character traits.
Timed pregnant female rats were shipped on gestation day 14 and immediately transferred to strict housing conditions. Rooms were maintained at 21°C with 50% relative humidity and a 14L:10D schedule. Care was taken to avoid all polycarbonate and epoxy resin contact to the animals and tissue samples. To avoid BPA leaching from polycarbonate plastic, all rats were housed in new polysulfone solid-bottom cages and double-deionized water was supplied from glass bottles. Animals were fed ad libitum a soy-free, phytoestrogen-reduced diet (Zeigler Reduced Rodent Diet 2, Ziegler Bros, Inc., Gardners, PA) with <12 ppm phytoestrogens as determined by HPLC. To avoid variability, a single feed lot was purchased for the entire study, packaged in 25-pound bags and stored at −30°C. Pregnant dams were monitored and the day of birth was designated PND 0. The pups were sexed by ano-genital distance and each litter size was culled to 10 pups by removing or adding female pups as necessary.
1.2.2 BPA dosing for comparison of BPA levels in oral vs s.c. injection exposures
Since our neonatal BPA studies employ dosing of rat pups on PND 1, 3 and 5, we examined BPA pharmacokinetics in PND 3 male rats as a mid-point to evaluate free (active) and total (free + glucuronidated) BPA levels following oral versus s.c. injection of 10 μg/kg BW, the dose employed in our earlier studies [20]. The experimental design is schematized in Figure 1A. Powdered BPA, obtained from the National Toxicology Program for common use in all NIEHS-funded BPA studies, was dissolved in 95% ethanol (EtOH) and solubilized in α-tocopherol stripped corn oil (MP Biomedicals LLC, Solon, OH) at a final administered concentration of 10 μg/ml (0.02% final EtOH volume). Solutions were made and stored using glass containers and any plastic products used for the collection and storage of blood and sera samples were polypropylene. Twenty pregnant rats were used to obtain 180 male pups for use in this study with males from each litter evenly divided between the two routes of BPA exposure. On PND 3, each pup was weighed and a precise volume of BPA solution from the same preparation was calculated for accurate delivery of 10 μg/kg BW to each pup via either the s.c. or oral route. Oral BPA exposure was performed through gentle feeding with a pipette tip to the neonatal rats which readily drank the solution. The s.c. injection of BPA was made using a glass Hamilton #705 microliter syringe (Hamilton Co., Reno, NV) with a 26 G needle to deliver a ~ 8–10 μl depot in the nape of the neck. The puncture site was held with gentle pressure for 60 sec to prevent leakage. Pups were killed by decapitation at 0.5, 1 and 2 hr after dosing and blood was collected and serum separated using a Microvette Capillary Blood Collection System (Sarstedt, Newton, SC). Sera from 8–10 pups at each time point and route of exposure were pooled and frozen at −80°C until BPA analysis within 6 weeks from collection. Three to five separate sample pools at each time point for both oral and injection exposure routes were used for BPA quantitation.
Figure 1.
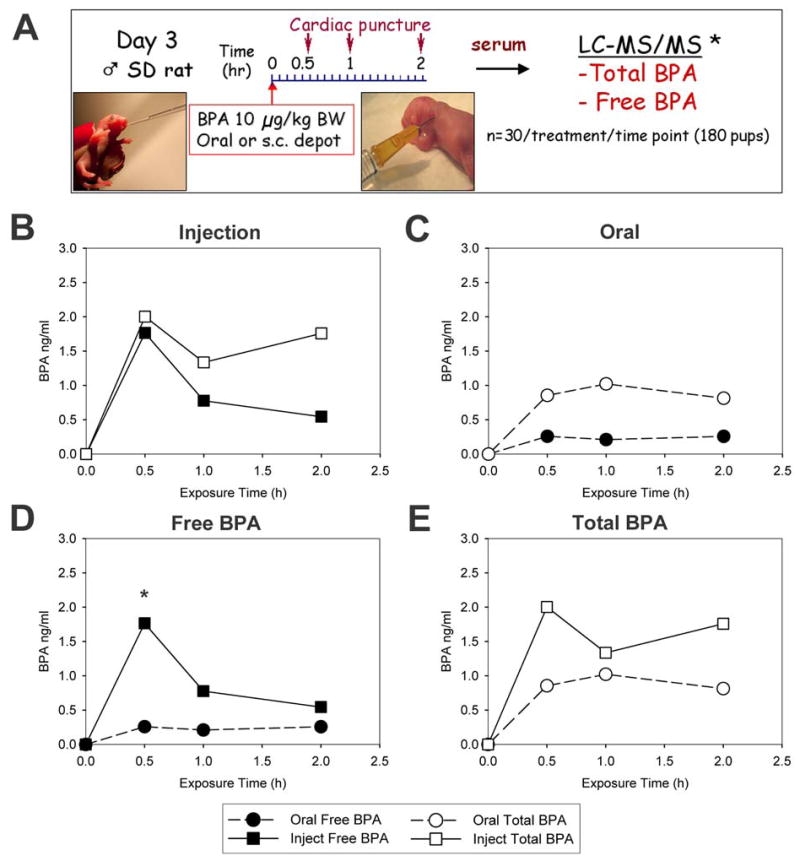
A: Experimental design for BPA pharmacokinetic study. B: Total BPA (open squares) and free BPA (closed squares) levels (ng/ml) in PND 3 male rats at 0.5, 1 and 2 hrs following s.c. injection of a BPA-oil depot at 10 μg/kg BW. C: Total BPA (open circles) and free BPA (closed circles) levels (ng/ml) in PND 3 male rats at 0.5, 1 and 2 hrs following oral ingestion of BPA in oil at 10 μg/kg BW. D: Comparison of free BPA levels (ng/ml) during first 2 hrs after s.c. injection (solid squares) and oral ingestion (solid circles) of BPA at dose of at 10 μg/kg BW. * denotes p < 0.05 for 0.5 hr values between injection versus oral exposure. E: Comparison of total BPA levels (ng/ml) during first 2 hrs after s.c. injection (open squares) and oral ingestion (open circles) of BPA at dose of at 10 μg/kg BW.
1.2.3 BPA Analytical method
After thawing at room temperature, sera from each pool was separated into two 500 μl aliquots by transfer into two 15 ml polypropylene tubes with 5 ng of deuterated-bisphenol A (d-BPA) added as an internal standard. One aliquot was used for measurement of freely available BPA (active) and the other for analysis of free plus glucuronidated-BPA, the main bound form of BPA in serum (inactive). For free BPA analysis, 3 ml of ethyl ether was added to the samples, shaken for 30 min in an orbital shaker, centrifuged at 4000 rpm for 5 min and the ethyl ether layer was transferred to a clean polypropylene tube using glass pipettes. Extraction from the residue was repeated twice more and the ethyl ether layers combined, concentrated to near-dryness under a gentle stream of nitrogen and reconstituted with 0.5 ml of methanol. For total BPA analysis, (free + glucuronidated form), the second serum aliquot from each sample was directly digested with 1 ml of glucuronidase (2 μl /ml) at 37°C for 12 hours followed by extraction with ethyl ether 3 times as described above. The final extracts were concentrated and the solvent reconstituted with 0.5 ml of methanol. Standards of BPA used for calibration were also prepared in methanol.
BPA levels in samples were quantified using high-performance liquid chromatography (HPLC) coupled with API 2000 electrospray triple-quadrupole mass spectrometer (ESI-MS/MS) as previously described [26] with minor modifications. Analyte separation and detection were carried out using an Agilent 1100 series HPLC interfaced with an Applied Biosystems API 2000 electrospray MS/MS (Applied Biosystems, Foster City, CA). Ten μL of the extract was injected onto an analytical column (Betasil® C18, 100 × 2.1 mm column; Thermo Electron Corporation, Waltham, MA) connected to a Javelin® guard column (Betasil® C18, 20 × 2.1 mm). The mobile phase was comprised of methanol and water at a gradient starting from 25% methanol to 99% methanol in 4 minutes and held for 10 minutes before it was reversed to initial condition. The flow rate and the column temperature were 300 μL/min and 25°C respectively. The MS/MS was operated in the electrospray negative ion mode. Instrumental parameters were optimized to transmit the [M-H]− ion before fragmentation to one or more product ions. Cone voltage and collision energies were 30 V and 25 V, respectively. Capillary voltage was 4.5 KV, and desolvation temperature was 400°C. Data was acquired using multiple reaction monitoring for the transitions of 227>212 for BPA and 241>223 for d-BPA.
Quality assurance and quality control parameters include validation of the method by spiking d-BPA into the sample matrices and passing through the entire analytical procedure to calculate recoveries of BPA. A procedural blank containing milli-Q water in place of serum was analyzed with the biological samples to check for interferences or laboratory contamination. The limit of detection was 0.05 ng/mL which was calculated as twice that of the valid “lowest acceptable calibration standard”. A curve point was deemed valid if 1) it was back calculated to be within 30% of the theoretical value when evaluated versus the 1/x weighted curve, and 2) the peak area of the standard was at least 2 times greater than the surrogate matrix blank. Trace levels of BPA present in blanks (<0.01 ng) were subtracted from sample values for determining the concentrations in samples. The recoveries of d-BPA spiked into samples were 58%±11%. Reported concentrations were corrected for the recoveries of surrogate standard. The native BPA standard spiked to selected sample matrices and passed through the entire analytical procedure yielded a mean recovery of 84% (range: 62%–114%). Quantification was based on an external calibration curve prepared by injecting 10 μL of 0.02, 0.05, 0.1, 0.2, 0.5, 1, 5, 10, and 50 ng/mL standards. Free and total BPA levels were plotted over time and the area under curve for 0–2 hr (AUC0-2) was determined using SigmaPlot 9.01 which integrates AUC using the trapezoidal rule. Free and total BPA values are expressed as the mean ± SEM at each time point for oral and injection exposures and Cmax was considered the highest serum level. Statistical analysis included ANOVA followed by Tukey-Kramer multiple comparison tests and p < 0.05 was considered significant.
1.2.4 Prostatic Response to Neonatal BPA following Oral vs Subcutaneous Injection Exposures
Newborn male pups were assigned to one of three neonatal treatment groups with 15–25 pups/group: 1) tocopherol stripped corn oil vehicle as controls, 2) oral exposure to BPA at 10 μg/kg BW on PND 1, 3 and 5, or 3) s.c. depot injection of BPA at 10 μg/kg BW on PND 1, 3 and 5 (Figure 2A). Solution preparation and dosing methods were as described above. To avoid litter effects, male pups within each litter were randomly assigned to a treatment and tattooed for permanent identification. The pups were weaned at PND 21 and siblings were housed two/cage. At PND 90, a 16 week exposure to testosterone plus estradiol-17β (T+E) was initiated to drive PIN lesions in the prostate lobes. Rats from each neonatal treatment group were given implants of Silastic capsules (Dow Corning, Midland, MI; i.d. 1.5 mm, o.d.2.0 mm) packed with estradiol-17β (one 1 cm tube) and testosterone (two 2 cm tubes) for 16 weeks (replaced after 8 weeks). These T+E capsule lengths result in testosterone at 1.72 ± 0.14 ng/ml (i.e. normal T levels for aging male rats) and estradiol-17β at 164 ± 58 pg/ml serum (mean ± SEM) which is 3-fold higher than normal estradiol levels in our untreated aging male rats. Previous studies have shown a 25–45% PIN incidence in the separate prostate lobes of Sprague-Dawley rats following a 16 week T+E exposure in adulthood [20, 27]. At 28 weeks of age, the animals were sacrificed by decapitation and the prostatic-urethral complex was removed in total, fixed in methacarn (BBC Biochemical, Mt Vernon, WA) for 48–72 hr, rinsed and stored in 70% EtOH until histological processing.
Figure 2.
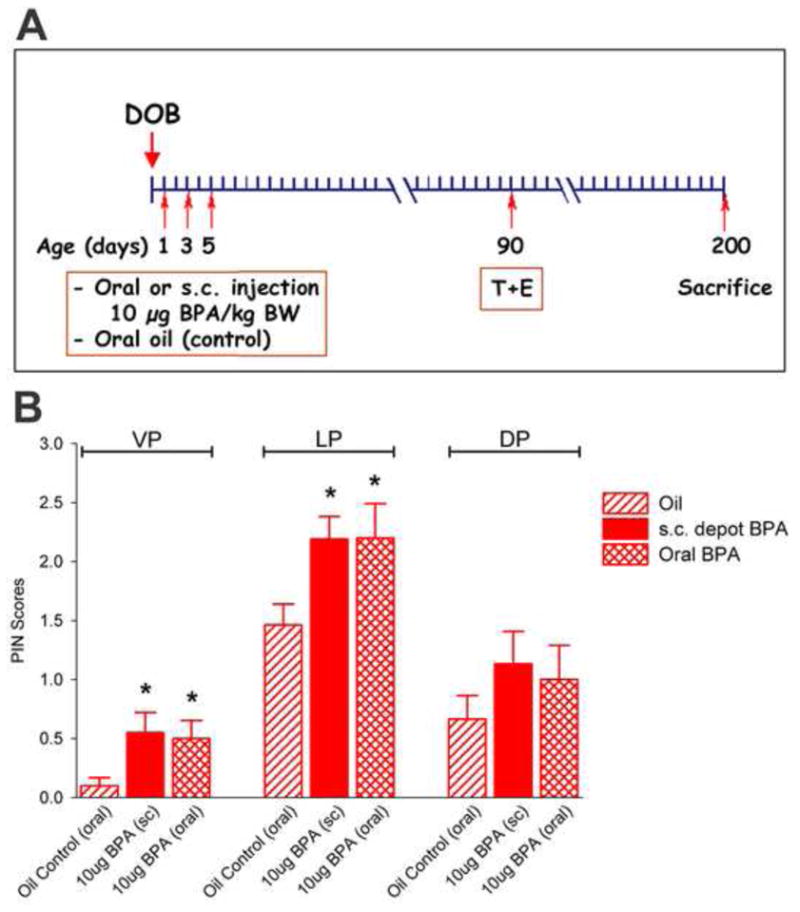
A: Experimental design to compare prostate response to 16 weeks of T+E treatment as adults in rats exposed neonatally to oil, oral BPA (10 μg/kg BW) or s.c. injection of BPA (10 μg/kg BW) on PND 1, 3 and 5. B: PIN scores in the ventral (VP), lateral (LP) and dorsal (DP) prostate lobes collected on day 200 of life in the three neonatal treatment groups. * denotes p < 0.05 versus oil control for the separate prostate lobes.
1.2.5 Histopathology
The fixed prostatic tissues were dehydrated through an ascending series of 70–100% ethanol, 100% xylene and paraffin embedded with the separate ventral (VP), lateral (LP) and dorsal (DP) prostate lobes mounted along one plane surrounding the urethral region. Coronal sections of this complex permitted viewing of prostate structures and ducts along their proximal-distal longitudinal axis. Three to four serial sections (4μm) were made at four levels of the tissue block 150 μm apart to permit pathologic analysis along the tissue depth. A minimum of 12–16 sections were analyzed for each tissue. The sections were coded to prevent reader bias and stained with hematoxylin and eosin. Each lobe was scored in a blinded fashion for the presence of prostatic intraepithelial neoplasia (PIN), epithelial and stromal hyperplasia and inflammatory infiltration. PIN lesions were characterized by the presence of nuclear atypia (enlarged and elongated nuclei, hyperchromasia, prominent nucleoli) with or without aberrant cellular piling and ductal formation [28]. Regions of aberrant cell piling and cribiform pattern without nuclear atypia were scored as atypical hyperplasia. PIN lesions were graded on a scale of 0–3 with 0= no atypia, 1= low-grade PIN, 2= focal high-grade PIN (HGPIN) and 3= extensive HGPIN. For PIN lesions, the incidence and the mean PIN score per treatment group were determined. Incidences of the separate prostate lesions in each lobe were analyzed by Chi-square and PIN scores were analyzed by ANOVA followed by Fischer’s exact test with significance accepted at P<0.05.
1.3 RESULTS
1.3.1 BPA pharmacokinetics in neonatal rats: oral vs subcutaneous exposure
Since previous studies from our laboratory had shown prostate gland reprogramming and increased carcinogenic susceptibility following a s.c. injection of 10 μg BPA/kg BW on PND 1, 3 and 5, we determined the levels of free BPA and total BPA (free+ glucuronidated) in the serum immediately following this exposure as a direct measure of circulating BPA available to the tissues in our animal model. A total of 90 PND 3 male rats were given a single s.c. injection of 10 μg BPA/kg BW and blood was collected at 0.5, 1 and 2 hr (n=30/time point). Serum concentrations of free and total BPA over time are shown in Figure 1B and pharmacokinetic parameters are provided in Table 1. Cmax levels seen at 0.5 hr were 1.77 and 2.00 ng/ml for free and total BPA, respectively. At this early time point, 88% of total BPA was in the free bioavailable form. Levels of free BPA dropped quickly to 0.70 and 0.54 ng/ml at 1 hr and 2 hr, respectively, constituting 52% and 31% of total BPA respectively. Unlike free BPA levels, the total BPA level declined at 1 hr and rose at 2 hr to levels similar to those observed at 0.5 hr.
Table 1.
Pharmacokinetic parameters after exposure of neonatal rats to 10μg/kg BW of Bisphenol A by oral and s.c. injection routes.
| s.c. Injection | Oral | |
|---|---|---|
| Free BPA | ||
| Cmax (ng/ml) | 1.77 ± 0.63 * | 0.26 ± 0.04 |
| Cmax (nM) | 7.73 ± 4.80 | 1.13 ± 0.31 |
| AUC0-2 | 1.738 | 0.416 |
| Total BPA (free & glucuronide bound) | ||
| Cmax (ng/ml) | 2.00 ± 1.00 | 1.02 ± 0.30 |
| Cmax (nM) | 8.77 ± 4.38 | 4.48 ± 1.32 |
| AUC0-2 | 2.883 | 1.601 |
| % Free | ||
| 0.5 hr | 88% | 29% |
| 1 hr | 52% | 21% |
| 2 hr | 31% | 31% |
Values represent mean ± S.E.M.
P < 0.05 vs oral Cmax
For direct comparison of BPA levels after s.c. injection to concentrations attained after oral administration, littermates were exposed to the same BPA oil-based solution via the oral route. Serum levels of free BPA reached a Cmax at 0.5 hr of 0.26 ng/ml while total BPA Cmax was reached at 1 hr at 1.02 ng/ml (Figure 1C and Table 1). Both free and total BPA levels held relatively constant over the first 2 hours after oral exposure. Free BPA constituted 29, 21 and 31% of total BPA levels at 0.5, 1 and 2 hr, respectively which supports first-pass metabolism following oral exposure, albeit at levels markedly lower than the adult rat [29, 30].
Comparison of circulating free and total BPA levels between the two routes of exposure are shown in Figure 1D and E, respectively. Since the s.c. injection route resulted in an initial peak of free BPA that was not seen after oral exposure, there was an initial 7-fold higher level of bioavailable BPA in the s.c. injected rats compared to orally exposed rats (P<0.05). However, this bolus was rapidly metabolized and the difference in serum free BPA levels at 1 and 2 hr were not statistically different between the two groups. Overall, the AUC0-2 for free BPA during the first 2 hrs after exposure was 4.14-fold higher in the s.c. injected pups as compared to the orally fed pups. Interestingly, total BPA levels were relatively similar following the two different exposure routes with AUC0-2 values 1.8-fold greater in the s.c. injection pups as compared to the orally fed pups.
It is important to note that the LC-MS-MS methodology and laboratory procedures employed for BPA measurements in the present study have undergone a rigorous quality control process that includes d-BPA recovery in each sample with recovery corrections for final values, recovery of native BPA spiked in test sample matrix, and procedural blanks that showed trace BPA levels < 0.01 ng/ml. Furthermore, robust analysis has demonstrated the lack of background BPA contamination from products used for blood collection, sera storage and instrumentation used for HPLC-tandem MS [26]. Thus suggestions that reported values may be a result of procedural noise or product contamination will not be applicable to the current dataset.
1.3.2 Prostatic response to neonatal BPA: Oral versus subcutaneous exposure
Male rats neonatally exposed to 10 μg BPA/kg BW through either s.c. injection or oral exposure on PND 1, 3 and 5 were evaluated for their susceptibility to prostatic lesions induced by 16 weeks of adult exposure to a constant, physiologic level of testosterone and 3-fold elevation in circulating estradiol-17β. The ventral (VP), lateral (LP) and dorsal (DP) prostate lobes were histologically assessed in a blinded manner at 7 months of age for the presence of PIN, atypical hyperplasia, epithelial hyperplasia and inflammatory cell infiltration (Figure 3) and incidence rates are presented in Table 2. The PIN lesions were scored on a scale of 0 to 3 as described in Methods and average PIN scores were calculated for each lobe and treatment (Figure 2B). T+E treatment to adult rats given oil neonatally (controls) exhibited PIN, hyperplasia and inflammatory cell infiltration with incidence varying between the separate lobes. As previous observed [20], the LP was the most sensitive lobe to adult T+E with high incidence of PIN (64%), hyperplasia (59–70%) and inflammation (47%). Similar to our previous study, the VP exhibited the least sensitivity to adult hormone exposure with incidence of 18% PIN, 12–35% hyperplasia and 18% inflammatory cell infiltration. Average PIN scores in the neonatal-oil, adult T+E rats were 0.15 in VP, 1.47 in LP and 0.67 in DP.
Figure 3.
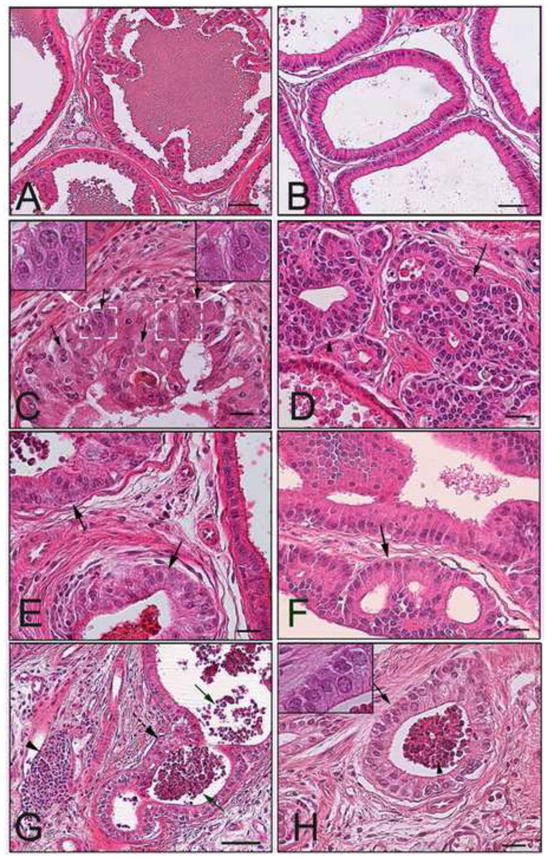
Prostate lobes of day 200 rats treated neonatally with oil (A-B), oral BPA at 10 μg/kgBW (B-C, I) or s.c. injection of BPA at 10 μg/kgBW (E-G) plus T+E as adults. A: Normal histologic region observed in LP lobe of rat treated with neonatal oil and adult T+E showing normal ductal architecture and intraluminal secretions. B: Normal histologic region found in VP lobe of rat treated with neonatal oil and adult T+E shows a single cell layer of columnar epithelium and limited stromal tissue. C: LP lobe from rat given neonatal oral BPA and adult T+E exhibiting high-grade PIN. Arrows point to some of the nuclei with prominent nucleoli which is seen throughout the acinus. Note variable nuclear size and shape and aberrant piling as compared to normal epithelium in adjacent acini in upper left. D: VP lobe from rat given neonatal oral BPA and adult T+E exhibiting adenoma (arrow) and atypical hyperplasia (arrowhead) within crowded acini. E: LP lobe from rat given neonatal s.c. BPA plus adult T+E exhibiting high-grade PIN similar (arrows) and a thickened stromal cell compartment. F: VP lobe from rat given neonatal s.c. BPA and adult T+E showing adenoma ((arrows) and epithelial hyperplasia. G: LP lobe from rat given neonatal s.c. BPA plus adult T+E contains inflammatory cell infiltration. Solid arrows point to intraluminal leukocytes, arrowhead marks stromal lymphocytes and dashed arrow identifies proliferative reaction in the epithelial layer. H: LP lobe from rat given neonatal oral BPA plus adult T+E contains acini with intraluminal leukocytes (arrowhead) and epithelial piling (arrow) characteristic of reactive proliferation to the immune cells. Bar denotes 50 μm in A, B and G, 40 μm in C-F, H and insets are at 100× magnification.
Table 2.
Incidence of prostatic lesions at 7 months in Sprague Dawley rats treated neonatally with oil or BPA via s.c or oral route of exposure and with T+E as adults.
| PIN | Atypical Hyperplasia | Epithelial Hyperplasia | Inflammatory Cells | |
|---|---|---|---|---|
| Ventral | ||||
| Oil | 18% | 12% | 35% | 18% |
| BPA: s.c. | 40% | 45% | 90%† | 20% |
| BPA: oral | 40% | 50% | 70%† | 0% |
| Lateral | ||||
| Oil | 64% | 59% | 70% | 47% |
| BPA: s.c. | 100%* | 42% | 58% | 89%** |
| BPA: oral | 90%* | 50% | 50% | 90%** |
| Dorsal | ||||
| Oil | 33% | 7% | 33% | 33% |
| BPA: s.c. | 47% | 35% | 59% | 29% |
| BPA: oral | 66% | 22% | 22% | 33% |
P < 0.01 vs oil control;
P < 0.02 vs oil control;
P < 0.05 vs oil control
Rats treated neonatally with BPA by either route of exposure exhibited nearly identical, augmented responses to T+E induced prostate lesions (Figure 2B, Figure 3 and Table 2). In the VP, mean PIN scores rose from 0.15 in oil-controls to 0.55 and 0.50 in neonatal BPA-treated rats via s.c. injection and oral exposure, respectively (P<0.05). While the incidence of PIN, atypical hyperplasia and epithelial hyperplasia increased in the VP in response to neonatal BPA as compared to vehicle-treated controls, the difference reached significance only for epithelial hyperplasia which rose to 70% (P<0.01). Examples of adenoma formation due to extensive cribiform patterning and epithelial hyperplasia in the VP are shown in Figure 3D and F as compared to normal regions in control VPs (Figure 3B). In the LP, PIN scores rose from 1.47 in oil controls to 2.19 and 2.20 in the s.c. injection and oral BPA exposed rats, respectively (P<0.05) while the PIN incidence rose from 64% in control rats to 100% and 90% in the same BPA treated rats, respectively (P<0.02) (Figure 3A, C, E). While there was no effect of BPA on atypical hyperplasia or epithelial hyperplasia over that seen with neonatal oil, there was a marked increase (P<0.05) in the incidence of inflammatory cell infiltration in the LP, primarily stromal T-cells and intraluminal neutrophils (Figure 3G-H). It deserves mention that the PIN lesions in the present study were scored separate from inflammatory reaction of epithelial cells typically observed in the immediate vicinity of immune cells. Although the DP exhibited a similar trend in PIN scores and incidence as a function of neonatal BPA treatment, the effects in the DP were not statistically significant for any parameter.
1.4 DISCUSSION
1.4.1 BPA pharmacokinetics following subcutaneous and oral exposures in the neonatal SD™ male rat
Our previous studies provided evidence for a direct link between developmental low-dose BPA exposure and heightened susceptibility to prostate carcinogenesis with aging [20]. In order to use that toxicity data for informed decisions regarding potential risks in humans, it is essential to determine the internal BPA dosimetry in our animal model. Using a highly sensitive, validated LC-MS-MS analytical method [26] with improved sensitivity, the present findings establish the internal exposure to free (bioactive) BPA and total BPA during the first two hours following s.c. BPA delivery in our neonatal SD rats and directly compare these values to internal doses following an equivalent BPA exposure through the oral route. Following s.c. injection of a BPA-oil depot, as performed in our laboratory at a dose of 10 μg/kg BW, a Cmax of 1.77 ng/ml of free BPA is observed at 0.5 hr which rapidly falls to 0.54 ng/ml at 2 hr. Although the subcutaneous route is not the typical manner in which humans are exposed to BPA, the final internal dosimetry determines biological effectiveness. Importantly, this transient internal dose of free BPA following s.c. exposure is within the range of chronic internal BPA levels reported for humans from routine environmental exposure including sera of pregnant women and children, amniotic fluid, fetal tissues and breast milk [9, 11, 26, 31–33]. Thus the present results provide support that our previous and current findings on BPA and prostate health are directly relevant to the human population with regards to current exposure levels.
Analysis of pharmacokinetic data following s.c. injection of BPA at 10 μg/kg BW shows that at 0.5 hr, 88% of total BPA is in the free, unconjugated form which confirms that the s.c. route bypasses hepatic first-pass metabolism at this early age. The % free BPA rapidly decreases to 31% by 2 hr indicating that BPA entering the circulation by a non-oral route is glucuronidated by UGT2B1 expressed in the neonatal liver, albeit at rates that are markedly lower than observed in the adult rat [30, 34]. In contrast to free BPA, total BPA levels remain relatively constant over the first two hours postdosing which may reflect the slow release of BPA from the subcutaneous oil depot in the nape of the neck over time. In addition, this may reflect enterohepatic recirculation and glucuronidation of bound BPA that was deconjugated by gut β–glucouronidases, as has been reported to occur in rats [16].
The BPA pharmacokinetics were markedly different when exposed through the oral route in neonatal male rats. Free BPA did not peak at 0.5 hr resulting in a 7-fold lower initial Cmax at 0.26 ng/ml as compared to the s.c. route. The free BPA level was 29% of total BPA at 0.5 hr which confirms glucuronidation at this early age as a result of hepatic first-pass metabolism by UGT2B1 [34]. Interestingly, the free and total BPA remained relatively constant during the first 2 hours postdosing by the oral route which may be a result of slow release and absorption of BPA from the oil-based solution in the G-I tract. It is noteworthy that the % free BPA remains at 31% at 2 hr which is the same level seen following s.c. injection at 2 hr and suggests an equilibrium between free and bound at this early time point regardless of exposure route which may be a reflection of immaturity in enzymatic capacities in the liver and gut. It has been established that UGT2B1 expression and enzymatic activity is lower at PND3 in rats and does not reach adult capacity until PND21 [29, 34]. Due to differences in metabolism between the two routes of exposure, the AUC0-2 is 4.1-fold higher for free BPA when the solution is administered s.c as compared to oral exposure. In contrast, total BPA levels are relatively similar for the initial 2 hr post-dosing with the AUC0-2 1.8-fold higher in the s.c. route which is not unexpected since both groups received identical amounts of BPA. The higher total BPA levels in the s.c. route as compared to oral exposure suggests that the entire amount of BPA through the oral route may not have entered the circulation in the neonatal pups but instead been passed through the gut into the feces as has been reported following oral exposures in rats (35).
The present findings on free and total BPA levels after s.c. and oral routes of exposure are strikingly different from values recently published by Doerge and colleagues [30] and this deserves discussion. In that study, the vast majority of BPA was conjugated at 0.5 hr post-dosing with 6.6% and 20% available as free BPA at Cmax as compared to 29% and 88% as free BPA herein for oral and s.c. injection exposures, respectively, in PND 3 rats. The % free (or parent) BPA in the present study more closely aligns with the 76% parent BPA at Cmax following s.c. injection and 8% parent BPA after oral exposure in adult rats, when taking into account the 4–5 fold lower UGT2B1 activity in PND3 rats compared to adults [35]. The Cmax for free BPA following oral exposure at 100 μg/kg BW in the Doerge study was 6.64 ng/ml and if linearity is applicable down to 10 μg/kg BW used in our study, the free BPA levels are within 2-fold range of the free BPA values following oral exposure in the present study. The differences in measured levels between the two oral exposure studies lie in the quantitation of total BPA levels which are 10-fold higher in the Doerge study after correcting for dosage differences. The greatest discrepancies between the two studies, however, lie in the levels of BPA achieved following s.c. injection where values for free BPA are 13-fold higher while total BPA values are 58-fold higher in the Doerge study as compared to the present results, after correcting for dosage differences. It is interesting to note that in the Doerge study, the total BPA values following s.c. injection show a 10-fold higher Cmax and a 6-fold higher AUC compared to oral exposure which is puzzling given that the equivalent BPA doses were administered by each route of exposure. In contrast, there is only a 2-fold difference in total BPA Cmax and AUC between s.c. injection and oral exposure in the present study which is similar to a previous 2-fold difference in AUC of plasma total radioactivity following s.c. versus oral delivery of 14C-BPA in adult rats [35]. While it is clear that BPA absorption varies with route of BPA administration in these and other studies, it is uncertain why the uptake differences show greater divergence in the Doerge study. There are procedural differences between the two studies that may explain some, but not all, of the discrepant findings. First, in the Doerge study, DMSO was used as a solvent and water as the vehicle for BPA delivery as opposed to EtOH as solvent and oil as vehicle in the present study and BPA release and absorption can differ with these variables [36, 37]. It is possible that relative to an oil depot, BPA is more rapidly released from water resulting in different absorption kinetics, particularly with the s.c. route which would explain the extreme peak after s.c. injection in the Doerge study. While both studies describe the injection route as subcutaneous, ours is delivered under the loose skin in the nape of the neck while Doerge describes their delivery as subcapsular. Thirdly, the doses used were 10-fold higher in the Doerge study and although they observe linearity between 50–200 μg/kg BW in adult female rats, metabolism may be alinear at the lower doses and neonatal time points used in our study. Finally, different SD rat substrains were used in the two studies. The SD rats used by Doerge at the NCTR are a closed colony CD-SD rat substrain obtained from Charles River Laboratories in 1972 [38]. This is a critical issue since the Charles River CD® (SD) substrain is well-characterized for metabolic aberrations including obesity, insulin resistance and estrogen insensitivity as a function of trait selection for large litter size (see [39] for detailed review). Large differences between the SD rat substrains have been observed in multiple endocrine studies and it is deserves repeating that since not all SD rats are the same, their lineage must be carefully considered when interpreting data [39]. Further, dramatic rat strain differences in xenobiotic metabolism have been noted including hepatic Phase I (CYPs) and Phase II (UGTs) enzymes that will have a profound effect on xenobiotic metabolism [39]. Thus it is possible that SD substrain differences in metabolic enzymes may be responsible, in part, for the different results observed in the present study that used Hsd:SD®™ rats to examine BPA pharmacokinetics.
The present findings show similarities as well as differences from another study that directly compared BPA pharmacokinetics following oral or s.c. injections administration in PND3 female mice [40]. The measured free BPA levels are relatively comparable following oral exposure after calculating for dosage differences between the two studies. Further, total BPA levels were similar between the two routes of exposure in the female mice as was observed herein for male rats. In contrast, the 7-fold higher peak levels of free BPA at 0.5 hr in the s.c. injected rat pups as compared to the oral exposure in our study was not observed in the neonatal mice where free BPA levels were only 1.5–2 fold higher in the injection group during the first hour. These differences may reflect species differences in BPA pharmacokinetics as well as experimental design and methodologies that differ between the studies.
1.4.2 Heightened prostate lobe susceptibility to adult tumorigenesis as a function of oral or s.c. injection of BPA during neonatal period
Results of the present study confirm our previously reported observation of significantly increased sensitivity to adult estrogen-induced PIN lesions by early-life exposure to an environmentally relevant dose of BPA during the critical neonatal period. Importantly, we observed no difference in the prostatic responses to 10 μg BPA/kg BW when delivered by either the s.c. or oral exposure routes. Since the prostate pathology was scored by an experienced reader who was blinded to the treatment group and tabulated by an independent investigator, the findings represent an unbiased assessment of the prostatic carcinogenic response to adult hormone treatments. Tissues were scored for the presence and severity of PIN lesions based on nuclear changes that include enlarged size, amorphous shape, hyperchromasia and presence of prominent nucleoli with pathologies confirmed on adjacent sections. While other characteristics were typically observed in PIN regions including aberrant cell piling, cribiform patterning and cytoplasmic vacuoles, nuclear atypia was necessary for a PIN classification. Using this diagnostic criteria, early life exposure to 10 μg BPA/kg BW augmented the adult prostatic response to T+E induced lesions irrespective of route of exposure with significantly higher PIN scores in the VP and LP but not DP as compared to controls. It is important to note that our prior study found elevated rates of epithelial cell proliferation and apoptosis in the PIN lesions of rats treated neonatally with BPA and T+E as adults [20]. This is a critical issue since the consensus report from the Mouse Models of Human Cancer Consortium Prostate Biology Committee cites alterations in apoptosis and proliferation within rodent PIN lesions as evidence of a relevant precancerous lesion with similarity to human high-grade PIN lesions, considered the precursor lesions to prostate cancer [28]. Thus we propose that the PIN lesions observed in the neonatal BPA-exposed rat model are relevant precancerous lesions that model human prostate carcinogenesis.
The lobe-specific responses observed herein have a few noteworthy differences from our original studies which may be a function of the different SD lineages used. It is known that closed breeding within the SD strain can cause biological drift that includes alterations in hormone sensitivities [39]. Our previous BPA study used SD rats from Zivic-Miller Laboratories (Pittsburgh, PA) while SD rats from Harlan (Hsd:SD®™) were used in the present study. The DP response to BPA was less robust in the Harlan Hsd:SD®™ rats than previously observed with Zivic-Miller SD rats leading to elevations in PIN scores in the present study that were not significantly different from oil-treated control rats. In contrast, the LP response to BPA was more robust with Hsd:SD®™ rats in terms of PIN scores of greater severity than previously observed. Further, a high incidence of inflammatory cell infiltration was consistently found in the LP in response to BPA, an effect that was not previously observed. Early-life BPA exposure was reported to induce persistent immunological effects including augmented TH2 cytokine production in adult mice [41]. It is known that chronic inflammatory cell infiltration in the prostate leads to proliferative reactions in the epithelium and is suspected to play a role in the process of carcinogenesis itself [42, 43]. The involvement of the immune axis in BPA-treated rats and the role of inflammatory cells in prostate carcinogenesis is an area of active investigation in several laboratories and the specific interactions between BPA, estradiol, inflammatory responses and prostatic carcinogenesis warrants detailed examination in future studies.
1.5 CONCLUSIONS
While the route of BPA exposure affected the initial metabolism of the parent BPA compound in the present study, it is important to keep in mind that the actual serum level of free BPA represents what is biologically available to the developing tissues and is the dose metric most relevant to mechanism of action and human health. Herein we have identified the internal dosimetry of free, bioactive BPA in neonatal rats which peaks at 1.77 ng/ml at 0.5 hr following s.c. injection of a BPA-oil depot and is similar to agylcone BPA concentrations measured in the developing human fetus and newborn. Thus despite differences in BPA metabolism, clearance and excretion mechanisms that diverge between rodents and humans and despite differences in BPA pharmacokinetics in route of exposure, the s.c. delivery of BPA employed by our laboratory provides an internal dose and tissue bioavailability that models internal human levels. This addresses the NTP-CERHR Panel conclusion that developmental “injection studies, unless proved otherwise, would produce irrelevantly high internal doses of the active parent compound and would tend to produce false positive effects from the point of view of the human oral situation” [16] by demonstrating that early-life delivery of BPA by this route, as used in our studies that show increased susceptibility to prostate carcinogenesis, has direct relevance to BPA levels currently observed in the human population. Furthermore, direct comparison of prostate gland susceptibility to estrogen-induced carcinogenesis following delivery of 10 μg/kg BW BPA by s.c. injection versus the oral exposure routes reveals nearly identical responses of the separate prostate lobes to this challenge. These later findings indicate that despite the metabolic differences during the first hour post-dosing and the four fold higher free BPA AUC0-2 with s.c. injection compared to oral exposures, the active BPA levels are sufficient and similar enough to reprogram the prostate towards heightened susceptibility to rising estradiol levels with aging. These findings support the proposal that exposures to BPA during fetal and neonatal life may increase the risk of carcinogenic events during adult life in the human population.
Acknowledgments
The authors gratefully acknowledge Danping Hu for technical assistance and Maarten Bosland, PhD, DVM for pathology consultation. Supported by NIH grant ES-015584 with supplemental NIH funding from American Recovery and Reinvestment Act.
Abbreviations
- AUC
Area under the curve
- BPA
bisphenol A
- BW
Body weight
- T+E
testosterone plus estradiol implant
- Cmax
maximal concentration
- DP
dorsal prostate lobe
- ER
estrogen receptor
- FDA
Federal Drug Agency
- LP
lateral prostate lobe
- NCTR
National Center for Toxicological Research, FDA
- NTP
National Toxicology Panel
- PIN
prostate intraepithelial neoplasia
- PND
postnatal day
- ppm
parts per million
- s.c.
subcutaneous
- SD
Sprague-Dawley
- UDP
uridine diphosphate-glucuronide
- UGT
uridine diphosphate-glucuronsyl transferase
- VP
ventral prostate lobe
Footnotes
Conflict of Interests:
The authors declare that there are no conflicts of interest.
Contributor Information
Gail S. Prins, Email: gprins@uic.edu.
Shuk-mei Ho, Email: Shuk-mei.ho@uc.edu.
Kurunthachalam Kannan, Email: kkannan@wadsworth.org.
References
- 1.Sajiki J, Yonekubo J. Leaching of bisphenol A (BPA) to seawater from polycarbonate plastic and its degradation by reactive oxygen species. Chemosphere. 2003;51:55–62. doi: 10.1016/s0045-6535(02)00789-0. [DOI] [PubMed] [Google Scholar]
- 2.Kawahata H, Ohta H, Inoue M, Suzuki A. Endocrine disrupter nonylphenol and bisphenol A contamination in Okinawa and Ishigaki Islands, Japan--within coral reefs and adjacent river mouths. Chemosphere. 2004;55:1519–1527. doi: 10.1016/j.chemosphere.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 3.Teuten E, Saquing J, Knappe D, et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc Lond B Biol Sci. 2009;364:2027–45. doi: 10.1098/rstb.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nam SH, Seo YM, Kim MG. Bisphenol A migration from polycarbonate baby bottle with repeated use. Chemosphere. 2010;79:949–952. doi: 10.1016/j.chemosphere.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Envir Hlth Prospect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Envir Hlth Prospect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuroda N, Kinoshita Y, Sun Y, et al. Measurement of bisphenol A levels in human blood serum and ascitic fluid by HPLC using a fluorescent labeling reagent. J Pharm Biol Anal. 2003;30:1743–49. doi: 10.1016/s0731-7085(02)00516-2. [DOI] [PubMed] [Google Scholar]
- 8.Lee YJ, Ryu HY, Kim HK, et al. Maternal and fetal exposure to bisphenol A in Korea. Repro Toxicology. 2008;25:413–19. doi: 10.1016/j.reprotox.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 9.Calafat AM, Weuve J, Ye X, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Envir Hlth Perspect. 2009;117:639–44. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eddington AN, Ritter L. Predicting plasma concentrations of bisphenol A in children younger than 2 years of age after typical feeding schedules, using a physiologically based toxicokinetic model. Envir Hlth Perspect. 2009;117:645–52. doi: 10.1289/ehp.0800073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Repro Toxicology. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- 13.Quesada I, Fuentes E, Viso-Leon MC, Ripoll C, Nadal A. Low doses of the endocrine disruptor bisphenol-A and the native hormone 17β-estradiol rapidly activate the transcription factor CREB. The FASEB Journal. 2002;16:1671–1673. doi: 10.1096/fj.02-0313fje. [DOI] [PubMed] [Google Scholar]
- 14.Zsarnovszky A, Le HH, Wang HS, Belcher SM. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology. 2005;146:5388–5296. doi: 10.1210/en.2005-0565. [DOI] [PubMed] [Google Scholar]
- 15.Wetherill YB, Akingbemi BT, Kanno J, et al. In vitro molecular mechanisms of bisphenol A action. Repro Toxicology. 2007;24:178–98. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Shelby M. National Toxicology Program-CERHR monograph on the potential human reproductive and developmental effects of Bisphenol A. NIH Publication No. 08-5994. 2008 [PubMed] [Google Scholar]
- 17.Prins GS. Developmental estrogenization of the prostate gland. In: Naz RK, editor. Prostate: Basic and Clinical Aspects. Chapter 10. Boca Raton: C. R. C. Press; 1997. pp. 247–265. [Google Scholar]
- 18.Prins GS, Huang L, Birch L, Pu Y. The role of estrogens in normal and abnormal development of the prostate gland. Annals of the New York Academy of Sciences. 2006;1089:1–13. doi: 10.1196/annals.1386.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prins GS, Birch L, Tang WY, Ho SM. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod Toxicol. 2007;23:374–382. doi: 10.1016/j.reprotox.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho SM, Tang WY, Belmonte J, Prins GS. Developmental exposure estradiol and bisphenol A (BPA) increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant (PDE4D4) in the rat prostate. Canc Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocrine Reviews. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 22.Dekant W, Völkel W. Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol. 2008;228:118–134. doi: 10.1016/j.taap.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Yokota H, Iwano H, Endo M, et al. Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem J. 1999;340:405–409. [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto J, Yokota H, Yuasa A. Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ Health Perspect. 2002;110:193–196. doi: 10.1289/ehp.02110193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.EFSA and Panel on Food Additives F, Processing Aids and Materials in Contact with Foods (AFC) Toxicokinetics of Bisphenol A - Scientific Opinion of the Panel on Food additives, Flavourings, Processing aids and Materials in Contact with Food (AFC) EFSA Journal. 2008:6. doi: 10.2903/j.efsa.2008.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padmanabhan V, Siefert K, Ransom S, et al. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatology. 2008;28:258–263. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosland MC, Ford H, Horton L. Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd:SD rats treated with a combination of testosterone and estradiol-17β or diethylstilbestrol. Carcinogenesis. 1995;16:1311–1317. doi: 10.1093/carcin/16.6.1311. [DOI] [PubMed] [Google Scholar]
- 28.Shappell S, Thomas D, Roberts R, et al. Prostate pathology of genetically engineered mice: Definitions and classification. The consensus report from the Bar Harbor Meeting of the Mouse Models of Human Cancer Consortium Prostate Biology Committee. Canc Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 29.Domoradzki JY, Thornton CM, Pottenger LH, et al. Age and dose dependency of the pharmacokinetics and metabolism of bisphenol A in neonatal sprague-dawley rats following oral administration. Toxicol Sci. 2004;77:230–242. doi: 10.1093/toxsci/kfh054. [DOI] [PubMed] [Google Scholar]
- 30.Doerge D, Twaddle NC, Vanlaningham M, Fischer JW. Pharmacokinetics of bisphenol-A in neonatal and adult Sprague-Dawlet rats. Toxicology and Applied Pharmacology. 2010;246 doi: 10.1016/j.taap.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Envir Hlth Prospect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calafat AM, Ye X, Silva MJ, Kuklenyik Z, Needham LL. Human exposure assessment to environmental chemicals using biomonitoring. In J Androl. 2006;29:166–171. doi: 10.1111/j.1365-2605.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- 33.Ye X, Tao LJ, Needham LL, Calafat AM. Automated on-line column switching HPLC MS/MS method for measuring environmental phenols and parabens in serum. Talanta. 2008;76:865–71. doi: 10.1016/j.talanta.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto J, Yokota H, Yuasa A. Developmental increases in rat hepatic microsomal UDP-glucuronyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ Hlth Persp. 2002;110:193–196. doi: 10.1289/ehp.02110193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pottenger LH, Domoradzki JY, Markham DA, Hansen SC, Cagen SZ, Waechter JMJ. The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Tox Sci. 2000;54:3–18. doi: 10.1093/toxsci/54.1.3. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe E, Sudo R, Takahashi M, Hayashi M. Evaluation of absorbability of poorly water soluble drugs: Validity of the use of additives. Biol Pharm Bull. 2000;23:838–843. doi: 10.1248/bpb.23.838. [DOI] [PubMed] [Google Scholar]
- 37.Hu XL, Peng JF, Liu JF, Jiang GB, Jönsson JA. Evaluating the impacts of some environmentally relevant factors on the availability of bisphenol A with negligible-depletion SPME. Chemosphere. 2006;65:1935–41. doi: 10.1016/j.chemosphere.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 38.Latendresse JR, Bucci TJ, Olson G, et al. Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats. Repro Toxicology. 2009;28:342–353. doi: 10.1016/j.reprotox.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Spearow JL. Reviewer’s Appendix to the EPA White Paper on Species/Stock/Strain in Endocrine Disruptor Assays, Contract # 68-W-01-023, Assignment #4-5, Task #16. White Paper | Endocrine Disruptor Screening Program | US EPA. 2007 http://www.epa.gov/endo/pubs/program/whitepaper.htm.
- 40.Taylor JA, Welshins WV, vom Saal FS. No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24 h after administration in neonatal female mice. Repro Toxicology. 2008;25:169–176. doi: 10.1016/j.reprotox.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan H, Takamoto M, Sugane K. Exposure to Bisphenol A prenatally or in adulthood promotes T(H)2 cytokine production associated with reduction of CD4CD25 regulatory T cells. Envir Hlth Perspect. 2008;116:514–19. doi: 10.1289/ehp.10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeMarzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate implications for prostatic carcinogenesis. Amer J Path. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernoulli J, Yatkin E, Laakso A, et al. Histopathological evidence for an association of inflammation with ductal pin-like lesions but not with ductal adenocarcinoma in the prostate of the noble rat. Vol. 68. 2008. p. 7. [DOI] [PubMed] [Google Scholar]


