Abstract
Objective
The present study was undertaken to investigate the pharmacokinetics of metronidazole in pregnant patients with bacterial vaginosis.
Methods
Twenty patients received metronidazole (Flagyl,® Pfizer, 235 East 42nd Street, NY, NY 10017) oral dose 500 mg twice a day for 3 consecutive days. Pharmacokinetic analyses of metronidazole were performed after a single oral dose on the morning of the day 4.
Results
Although absolute estimates of metronidazole total body exposure were highest in women during early term pregnancy, weight-corrected estimates of exposure maximum plasma drug concentration (Cmax, ) and the area under the plasma concentration-versus-time curve (AUC0-12), along with apparent oral clearance and distribution volume, were not significantly different between women at early, middle, and late stages of pregnancy and were in the range of reported values for nonpregnant patients receiving a similar dose.
Conclusions
The pharmacokinetic profile of metronidazole did not change at the different time points assessed during pregnancy.
Keywords: Bacterial vaginosis, pharmacokinetics, metronidazole, pregnancy
INTRODUCTION
Bacterial vaginosis accounts for nearly 40% of vaginal infections during pregnancy [1].It is associated with such obstetric complications as preterm labor and delivery [2–6], premature rupture of membranes [3,4,7], chorioamnionitis [3], and postpartum endometritis [8,9]. For several decades, the drug of choice for treatment of bacterial vaginosis has been metronidazole, an agent of the nitroimidazole antibiotic family [10].Its efficacy for the treatment of symptomatic bacterial vaginosis ranges between 80% and 90% [11–14], it can be given during all stages of pregnancy if indicated, and it is well tolerated by pregnant women [15]. The drug crosses the placenta and can be found in the fetal tissue, cord blood, and amniotic fluid in a high concentrations [16].
Two early studies performed during pregnancy after a single intravenous dose of metronidazole [17] and after single or multiple oral doses administered between the 8th and 14th weeks of pregnancy [18] revealed that the pharmacokinetic parameters of metronidazole in pregnant women resemble those of healthy volunteers. However, pregnancy-induced increases in the plasma volume which could affect drug distribution reach their maximum at about 34 weeks of gestation.
The use of metronidazole in treating bacterial vaginosis and its obstetric complications throughout pregnancy, coupled with the dependence of antimicrobial efficacy on achievable concentrations, warrants the need for additional data on metronidazole disposition during the middle and late stages of gestation. The aim of this investigation was to estimate the pharmacokinetics of metronidazole in patients at different stages of pregnancy, including early (10–14 weeks), middle (22–26 weeks), and late term (34–38 weeks).
METHODS
Subjects
Pregnant females with a diagnosis of bacterial vaginosis whose providers recommended treatment with metronidazole were eligible for enrollment in this study. To participate, they had to be 18 years of age or older, pregnant with singletons, and in a pregnancy window of 10–14 weeks, 22–26 weeks, or 34–38 weeks. Women were excluded from participation if there was anemia with a hematocrit of less than 28% and with a prior history or current medical examination consistent with the presence of clinically significant alterations in hepatic, renal, or gastrointestinal function. Decisions about diagnosis and treatment were made by the women’s own healthcare providers and were independent of participation in this study. Participants were considered eligible on medication use, comedication, smoking habits, intake of caffeine (eg, coffee, cola, tea), and daily use of prenatal vitamins.
All procedures involving human subjects were conducted according to the declaration of Helsinki and its actual amended version, the International Conference on Harmonization–Good Clinical Practices (ICH-GCP) guidelines. All women were enrolled with written informed consent under a protocol that was reviewed and approved by the University of Texas Medical Branch Institutional Review Board.
Study protocol
All subjects received metronidazole 500 mg orally twice daily for 3 consecutive days. Metronidazole was supplied as 250 mg tablets of Flagyl®. The pharmacokinetics of metronidazole were studied on each patient after a single oral dose on the morning of day 4. Blood samples were taken from the antecubital vein immediately prior to dosing and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 hours post ingestion. The plasma was centrifuged immediately and stored in polypropylene tubes at −70°C until analysis. Urine samples were collected over the same 12-hour interval at 0, 0–2, 2–4, 4–6, 6–8, 8–10, and 10–12 hours and stored at −70°C until analysis.
High performance liquid chromatography (HPLC) method
The HPLC system consisted of a 1525 binary HPLC pump, a 2489 dual λ absorbance detector, and a 717 autosampler controlled by a Millennium chromatography manager (Waters, Milford, MA, USA). An isocratic elution was performed on a reverse phase C18 column (Symmetry 4.6 mm×150 mm), 5 μm particle size, plus phenomenex guard cartridge. The mobile phase was a degassed and filtered (0.45 μm; Millipore) mixture of acetonitrile: water (7:93, v/v) adjusted to pH 6.5 using acetic acid. The flow-rate was 1 mL/min, and the UV detector wavelength was set at 317 nm. The retention time for metronidazole was 7.8 minutes under the described conditions.
Calibration curves were constructed by spiking known amounts of standards into blank plasma or urine. The plasma and urine standard curves for metronidazole were found to be linear in the range of 0.2–25 μg/mL and 2.5–200 μg/mL, respectively. The R-squared value was greater than 0.99.
Six replicates of the quality control (QC) samples at each concentration level were used to evaluate the intra-day precision and accuracy. Two replicates of the QC samples at each concentration level from 3 separate batches were used to evaluate the interday precision and accuracy. In plasma, the intra- and interassay mean precision was between 1.40% and 4.30%, and the mean accuracy was between -4.37% and 0.42% for metronidazole. In urine, the intra- and inter-assay mean precision ranged from 0.95% to 2.58%, and the mean accuracy was between −2.56% and −0.63%.
The recovery of metronidazole from the 3 different matrices was determined at 3 QC levels by comparing the peak area of samples that had been spiked with metronidazole prior to precipitation using perchloric acid with samples prepared using the standard solution in water. The average recovery of metronidazole from human plasma and urine was greater than 95%.
Plasma and urine sample preparation
Plasma samples were thawed at room temperature and vortexed, then centrifuged for 5 minutes at 6000g at approximately 4°C. To 250 μL of plasma, 7 μL of 70% perchloric acid were added. Samples were then vortexed (20 seconds) and centrifuged at 8000g for 10 minutes. Then the supernatant was collected and transferred to autosampler vials, and 25 μL was injected into the HPLC.
Urine samples were prepared by adding 5μL of 35% perchloric acid and 250 μL of urine (standard, quality control, or patient samples) to a 1.5-mL microcentrifuge tube. Each tube was vortex-mixed and then centrifuged at 8,000 × g for 5 minutes. An aliquot of 10 μL of supernatant was injected into HPLC system.
Pharmacokinetic analysis
Pharmacokinetic analyses were conducted using Kinetica software version 5.0 (Thermo Scientific, Waltham, MA). All data were evaluated using a model independent approach. Plasma concentration-versus-time data were curve fit using a peeling algorithm to generate initial polyexponential parameter estimates. Final estimates of the terminal elimination rate constant (Ke) were determined from an iterative, nonlinear least squares regression algorithm. Individual maximum plasma drug concentration (Cmax) and time to Cmax (Tmax) were determined by visual inspection of the plasma concentration-versus-time profile. The area under the plasma concentration-versus-time curve at steady-state (AUCss) was determined using the log-linear trapezoidal rule. For participants whose plasma sampling was truncated prior to 12 hours, C12 was extrapolated from the best fit-curve, and the AUC to 12 hours (AUC0-12) was calculated by summation of AUC0-n +AUCn-12 where n represents the last measured time point. Apparent total plasma clearance (CL/F) and steady-state volume of distribution (Vdss/F) were calculated from the AUCss.
Statistical analysis
Metronidazole pharmacokinetic data for the study cohort were examined using standard descriptive statistics. One-way analysis of variance was used to compare calculated pharmacokinetic parameters between dosing cohorts. Univariable analysis of variance and nonlinear regression techniques were used to evaluate the relationship between demographic variables and pharmacokinetic parameter estimates. All analyses were performed in SPSS version 12.0 (SPSS, Chicago, IL), and P-values less than 0.05 were considered to indicate statistical significance.
RESULTS
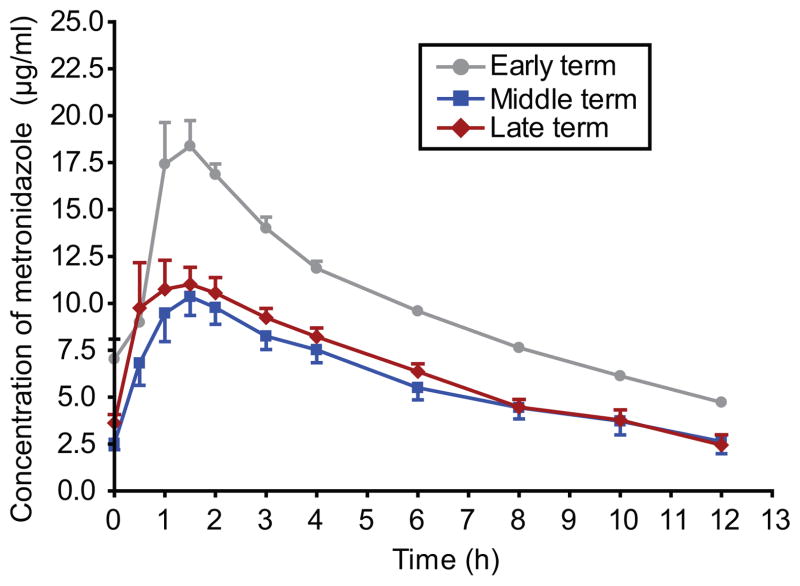
Twenty pregnant women volunteered to participate in this study; 2 in the early term group and 9 each in the middle and late term groups, respectively. The relevant patient characteristics are summarized in Table 1. The mean plasma metronidazole concentration-versus-time profiles at different stages of pregnancy following oral metronidazole administration are presented in Figure 1. On average, the concentrations of metronidazole in females early in the course of their pregnancies appeared higher over the course of the entire dosing interval as compared with women in the middle or later stages of pregnancy. However, these women received a slightly higher dose when corrected for weight (7.8±0.7 mg/kg vs. 7.0±2.2 mg/kg and 6.3±1.9 mg/kg in middle and late stage pregnancy, respectively).
Table I.
Characteristics of the study population women during pregnancy. Values were expressed as mean±SD unless specified otherwise.
| Characteristics | Value |
|---|---|
| No. of subjects | 20 |
| Maternal age (y) | 23.3±3.3 |
| Weight (kg), n=20 | 80.8±28.6 |
| 10–14 weeks, n=2 | 60.2, 67.8 |
| 22–26 weeks, n=9 | 80.7±36.1 |
| 34–38 weeks, n=9 | 84.7±23.5 |
| Cigarette smoking, n (%) | 1 (5%) |
| Co-medication, n (%) | 8 (40%) |
| Prenatal vitamins use, n (%) | 16 (80%) |
| Ethnicity, n (%) | |
| Caucasian | 5 (25%) |
| Hispanic | 9 (45%) |
| African-American | 6 (30%) |
Figure 1.
Metronidazole plasma concentration-time profiles in early (circles), middle (squares), and late (diamonds) pregnancy after per os administration of 500 mg Flagyl®. Results were mean±SEM.
Table 2 summarizes the pharmacokinetic parameters of metronidazole at early, middle, and late term in pregnancy. With the exception of 1 woman in late term who experienced a peak concentration at 3 hours, Tmax in the remaining participants occurred between 1 to 2 hours. Maximum plasma concentrations in early term females trended higher than those observed in middle or late stage pregnancy (P =0.072); however, the small sample size in this cohort precluded the ability to identify a statistically significant difference. In contrast, AUC was significantly different between the cohorts (P =0.005). When adjusted for the weight-normalized dose received by the participants, however, no estimate of exposure was significantly different between the cohorts.
Table II.
Metronidazole pharmacokinetic values (mean±SD) at different terms in pregnancy after per os administration 500 mg Flagyl®.
| Pregnant women |
|||
|---|---|---|---|
| Early terma (n = 2) | Middle term (n = 9) | Late term (n = 9) | |
| Cmax (μg/mL) | 17.0, 19.7 | 11.7±3.1 | 14.2±4.2 |
| Cmax (μg/mL, per mg/kg dose) | 2.3, 2.4 | 1.8±0.7 | 2.4±1.2 |
| Tmax (h) | 1.5 | 1.3±0.4 | 1.3±0.8 |
| AUC0-12 (μg h/mL) | 117.4, 121.8* | 69.2±19.9 | 79.2±14.7 |
| AUC0-12 (μg h/mL, per mg/kg dose) | 14.7, 15.9 | 10.8±4.7 | 13.4±5.0 |
| Vd (mL/kg) | 517, 558 | 793±291 | 717±334 |
| CL/F (mL/h/kg) | 62.8, 68.2 | 110.0±53.9 | 82.2±27.0 |
| t1/2 (h) | 5.2, 6.2 | 5.4±1.8 | 6.2±2.3 |
| Urinary elimination, % (0–12 h)b | 8.4, 14.7 | 7.5±1.8 | 5.7±2.9 |
Data presented as minimum and maximum values
Data represent only those participants with complete urine collections (n=2 early, n=4 middle, n=4 late
P<0.01 compared to middle and late term.
Among the remaining pharmacokinetic parameters, no significant differences were observed between women at early, middle, and late stages of pregnancy. Interestingly, nearly 40% of the variability in both apparent oral clearance and half-life could be accounted for when race was included in a covariate model with pregnancy stage (African-American<Hispanic<Caucasian). However, the parameters independently were not statistically significant. Similarly, we observed no significant differences among the pharmacokinetic parameters of participants reporting concurrent caffeine intake (eg, coffee, cola, and tea). The limited sample size precluded similar comparisons for other concomitant medications.
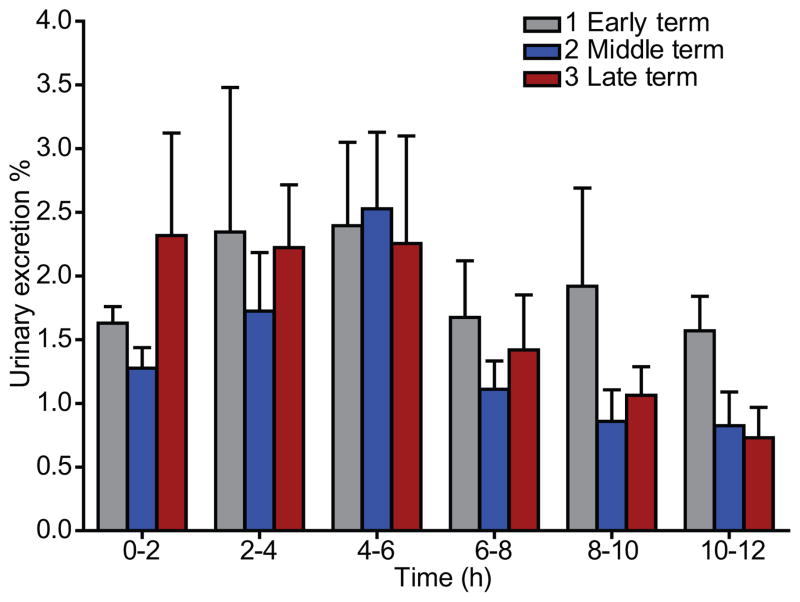
The different time intervals of urine excretion of metronidazole are shown in Figure 2. The maximum urinary excretions (the greatest percentage of dose recovered in the urine collected in different time intervals) of metronidazole were observed at 4–6 hours. The cumulative urinary excretions of metronidazole over 12 hours in early, middle, and late terms of pregnant women were 11.5%, 7.5%, and 5.7%, respectively (Table 2); however, these values reflect excretion data for only half of the participants from whom a complete urine collection was available.
Figure 2.
Metronidazole urinary excretion-time profiles in early (1), middle (2), and late (3) pregnancy after per os administration 500 mg Flagyl®. Results were mean±SEM.
DISCUSSION
Earlier studies by Visser [17] and Amon [18] revealed that pharmacokinetic parameters of metronidazole in pregnant women did not differ significantly from parameters determined in nonpregnant women. However, in the investigation conducted by Amon et al, the pregnant women enrolled in the study were between 8 and 14 weeks of gestation, whereas in the study by Visser et al, gestational ages of the women were not reported. Given the unique pharmacologic properties of the drug (eg, extremely low plasma protein binding [19,20], rapid distribution into body tissues), we hypothesized that the pharmacokinetic parameters of metronidazole would differ during middle and late pregnancy as compared with the nonpregnant state because of increases in mean plasma volume by 1.1 to 1.5 L toward the third trimester [21].
The data obtained in this investigation revealed that plasma concentrations of metronidazole during the early period of pregnancy (10–14 weeks of gestation) (Figure 1), and consequently the AUC0-12, were higher than in women during middle (22–26 weeks) and late (34–38 weeks) gestation. However, after adjustment for the weight normalized dose, maximal plasma drug concentration and AUC0-12 at early stage did not differ from their values determined at middle or late stages (Table 2). In addition, the other pharmacokinetic parameters Vd, CL/F, and t1/2, did not significantly differ between groups, suggesting that the extent of drug distribution and clearance was not affected by different gestation periods.
The small sample size of early term females in this study precluded our ability to adequately assess differences in the pharmacokinetic parameters derived from this population with those of women in the mid- and late-stages of pregnancy. Additionally, this study did not include nonpregnant controls. However, we were able to compare these data with data from historical controls [20,22–24]. The pharmacokinetic parameters obtained in this investigation for the early period of pregnancy agreed with the parameters obtained for the same gestational age group reported by Amon [20]. Several of the pharmacokinetic parameters were in the range of values reported for nonpregnant patients after a 500 mg oral dose of metronidazole (eg, Cmax: 9.8–13 μg/ml), Tmax: 0.25–4 hours, urinary excretion: 6–18%). However, total body exposure was slightly lower, distribution volume slightly greater, and half-life slightly faster than reported for nonpregnant patients (AUC: 105–159 mg h/L, Vd: 610–690 L/kg, t1/2: 7.0–8.8 hours) [22–24], indicating that pharmacokinetic parameters for metronidazole may be affected by the physiological changes coincident with pregnancy and gestation.
The minimum inhibitory concentrations (MIC50) of metronidazole for the most commonly involved species in bacterial vaginosis [25,26] are Gardnerella vaginalis, 8 μg/mL; Mobiluncus, 16 μg/mL; and Bacteroides and Prevotella species, 1–2 μg/mL [10]. In this investigation, all patients received 500 mg metronidazole orally twice a day for 4 days for treatment of bacterial vaginosis. The peak plasma levels averaging 13.5±4.0μg/mL (range 5.6 to 20.6μg/mL) were achieved in all pregnant patients within 1–2 hours after administration, and the lowest plasma concentration 12 hours after ingestion was 2.95±1.41μg/mL (range 1.2 to 5.0μg/mL). Therefore, 500 mg of metronidazole twice a day per os should yield adequate plasma levels for treatment of most anaerobic infections associated with bacterial vaginosis during pregnancy.
Acknowledgments
This work was supported by grant U10HD047891 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Obstetrical Pharmacology Research Unit Network (OPRU), to G. Hankins.
References
- 1.Sobel JD. Vaginitis in Adult Women. Obstet Gynecol Clin North Am. 1990;17:851–79. [PubMed] [Google Scholar]
- 2.Eschenbach DA, Gravett MG, Chen KC, Hoyme UB, Holmes KK. Bacterial vaginosis during pregnancy. An association with prematurity and postpartum complications. Scand J Urol Nephrol. 1984;86 (suppl):213–22. [PubMed] [Google Scholar]
- 3.Gravett MG, Nelson HP, Derouen T, Critchlow C, Eschenbach DA, Holmes KK. Independent Associations of Bacterial Vaginosis and Chlamydia-Trachomatis Infection with Adverse Pregnancy Outcome. JAMA. 1986;256:1899–903. [PubMed] [Google Scholar]
- 4.Kurki T, Sivonen A, Renkonen OV, Savia E, Ylikorkala O. Bacterial Vaginosis in Early-Pregnancy and Pregnancy Outcome. Obstet Gynecol. 1992;80:173–7. [PubMed] [Google Scholar]
- 5.Riduan JM, Hillier SL, Utomo B, Wiknjosastro G, Linnan M, Kandun N. Bacterial Vaginosis and Prematurity in Indonesia -Association in Early and Late Pregnancy. Am J Obstet Gynecol. 1993;169:175–8. doi: 10.1016/0002-9378(93)90157-e. [DOI] [PubMed] [Google Scholar]
- 6.Meis PJ, Goldenberg RL, Mercer B, Moawad A, Das A, McNellis D, Johnson F, Iams JD, Thom E, Andrews WW. The Preterm Prediction Study - Significance of Vaginal Infections. Am J Obstet Gynecol. 1995;173:1231–5. doi: 10.1016/0002-9378(95)91360-2. [DOI] [PubMed] [Google Scholar]
- 7.Mcdonald HM, Oloughlin JA, Jolley P, Vigneswaran R, Mcdonald PJ. Prenatal Microbiological Risk-Factors Associated with Preterm Birth. Br J Obstet Gynaecol. 1992;99:190–6. doi: 10.1111/j.1471-0528.1992.tb14497.x. [DOI] [PubMed] [Google Scholar]
- 8.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The Association of Occult Amniotic-Fluid Infection with Gestational-Age and Neonatal Outcome Among Women in Preterm Labor. Obstet Gynecol. 1992;79:351–7. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Newton ER, Prihoda TJ, Gibbs RS. A Clinical and Microbiologic Analysis of Risk-Factors for Puerperal Endometritis. Obstet Gynecol. 1990;75:402–6. [PubMed] [Google Scholar]
- 10.Schwebke JR. Metronidazole - Utilization in the Obstetric and Gynecologic Patient. Sex Transm Dis. 1995;22:370–6. [PubMed] [Google Scholar]
- 11.Jerve F, Berdal TB, Bohman P, Smith CC, Evjen OK, Gjønnaess H, Gaasemyr M, Hausken L, Hesla K, Hoftvedt E. Metronidazole in the Treatment of Non-Specific Vaginitis (Nsv) Br J Vener Dis. 1984;60:171–4. doi: 10.1136/sti.60.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eschenbach DA, Critchlow CW, Watkins H, Smith K, Spiegel CA, Chen KC, Holmes KK. A Dose-Duration Study of Metronidazole for the Treatment of Nonspecific Vaginosis. Scand J Infect Dis. 1983:73–80. [PubMed] [Google Scholar]
- 13.Blackwell A, Fox A, Phillips I, Barlow D. Metronidazole in treatment of non-specific vaginitis. Clinical and microbiological findings in ten patients given 2 grams of metronidazole. Scand J Infect Dis. 1983;40(suppl):103–6. [PubMed] [Google Scholar]
- 14.Koumans EH, Markowitz LE, Hogan V. Indications for therapy and treatment recommendations for bacterial vaginosis in nonpregnant and pregnant women: A synthesis of data. Clin Infect Dis. 2002;35:S152–72. doi: 10.1086/342103. [DOI] [PubMed] [Google Scholar]
- 15.Amon I. Placental-Transfer of Metronidazole. J Perinat Med. 1985;13:97–8. doi: 10.1515/jpme.1985.13.2.97. [DOI] [PubMed] [Google Scholar]
- 16.Amon I, Huller H, Amon K. Proc VIIth Int Congr Chemotherapy, 1971. Munich: Urban & Schwarzenberg; 1972. Metronidazole pharmacokinetics in non-pregnant women, gravidae and women in childbed; pp. 111–112. [Google Scholar]
- 17.Visser AA, Hundt HKL. The Pharmacokinetics of A Single Intravenous Dose of Metronidazole in Pregnant Patients. J Antimicrob Chemother. 1984;13:279–83. doi: 10.1093/jac/13.3.279. [DOI] [PubMed] [Google Scholar]
- 18.Amon I, Amon K, Huller H. Pharmacokinetics and Therapeutic Efficacy of Metronidazole at Different Dosages. Int J Clin Pharmacol Biopharm. 1978;16:384–6. [PubMed] [Google Scholar]
- 19.Schwartz DE, Jeunet F. Comparative Pharmacokinetic Studies of Ornidazole and Metronidazole in Man. Chemotherapy. 1976;22:19–29. doi: 10.1159/000221906. [DOI] [PubMed] [Google Scholar]
- 20.Amon I, Amon K, Franke G, Mohr C. Pharmacokinetics of Metronidazole in Pregnant-Women. Chemotherapy. 1981;27:73–9. doi: 10.1159/000237958. [DOI] [PubMed] [Google Scholar]
- 21.Hytten FE, Leitch I. The physiology of human pregnancy. Oxford: Brackwell Scientific Publications; 1971. [Google Scholar]
- 22.Bergan T, Arnold E. Pharmacokinetics of Metronidazole in Healthy Adult Volunteers After Tablets and Suppositories. Chemotherapy. 1980;26:231–41. doi: 10.1159/000237911. [DOI] [PubMed] [Google Scholar]
- 23.Houghton GW, Thorne PS, Smith J, Templeton R, Collier J. Comparison of the Pharmacokinetics of Metronidazole in Healthy Female Volunteers Following Either A Single Oral Or Intravenous Dose. Br J Clin Pharmacol. 1979;8:337–41. doi: 10.1111/j.1365-2125.1979.tb04715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loft S, Døssing M, Poulsen HE, Sonne J, Olesen KL, Simonsen K, Andreasen PB. Influence of Dose and Route of Administration on Disposition of Metronidazole and Its Major Metabolites. Eur J Clin Pharmacol. 1986;30:467–73. doi: 10.1007/BF00607962. [DOI] [PubMed] [Google Scholar]
- 25.Hill GB. The Microbiology of Bacterial Vaginosis. Am J Obstet Gynecol. 1993;169:450–4. doi: 10.1016/0002-9378(93)90339-k. [DOI] [PubMed] [Google Scholar]
- 26.Hillier SL. Diagnostic Microbiology of Bacterial Vaginosis. Am J Obstet Gynecol. 1993;169:455–9. doi: 10.1016/0002-9378(93)90340-o. [DOI] [PubMed] [Google Scholar]