Abstract
The inhibition of UVB-induced immunosuppression by dietary grape seed proanthocyanidins (GSPs) has been associated with the induction of interleukin (IL)-12 in mice and we now confirm that GSPs do not inhibit UVB-induced immunosuppression in IL-12p40 knock out (IL-12-KO) mice and that treatment of these mice with recombinant IL-12 restores the inhibitory effect. To characterize the cell population responsible for the GSPs-mediated inhibition of UVB-induced immunosuppression and the role of IL-12 in this process, we used an adoptive transfer approach. Splenocytes and draining lymph nodes were harvested from mice that had been administered dietary GSPs (0.5-1.0%, w/w), exposed to UVB, and sensitized by application of DNFB onto the UVB-exposed skin. CD8+ and CD4+ T cells were positively selected and transferred into naïve mice that were subsequently challenged by application of DNFB on the ear skin. Naïve recipients that received CD8+ T cells from GSPs-treated, UVB-irradiated donors exhibited full contact hypersensitivity (CHS) response. Naïve mice that received CD4+ suppressor T cells from GSPs-treated, UVB-exposed mice were able to mount a CHS response after sensitization and subsequent challenge with DNFB. On culture, the CD8+ T-cells from GSPs-treated, UVB-exposed mice secreted higher levels (5-8 fold) of Th1 cytokines than CD8+ T cells from UVB-irradiated mice not treated with GSPs. CD4+ T cells from GSPs-treated, UVB-exposed mice secreted significantly lower levels (80-100%) of Th2 cytokines than CD4+ T cells from UVB-exposed mice not treated with GSPs. These data suggest that GSPs inhibit UVB-induced immunosuppression by stimulating CD8+ effector T cells and diminishing regulatory CD4+ T cells.
Keywords: Contact hypersensitivity, Interleukin, Proanthocyanidins, Ultraviolet radiation
Introduction
The immunosuppressive effects of solar ultraviolet (UV) radiation, particularly UVB (290-320 nm), are well recognized. This is most clearly evident in terms of the effects of UV radiation in inhibiting the contact hypersensitivity (CHS) response to contact sensitizers, which is considered to be a prototypic T-cell-mediated immune response (1, 2). Experimental evidence indicates that UV-induced immunosuppression is a risk factor for skin cancer development in mice and clinical evidence suggests that it is a risk factor in humans (3, 4). Organ transplant recipients and chronically immunosuppressed patients living in regions of intense sun exposure are at greater risk of developing skin cancer and that the increase in risk correlates strongly with cumulative UV exposure (5, 6). It has been reported that UV-induced immunosuppression enables the outgrowth of transplanted epithelial skin cancers and melanomas in mice (7, 8). T cells from UV-irradiated mice that have been activated by antigen exposure can transfer suppression to normal recipients as indicated by enhanced growth of UV-induced skin tumors (9). Conversely, animals with an enhanced immune response exhibit a lower risk of skin tumor development on chronic UV exposure (10). Collectively, these observations suggest that protection from UV-induced immunosuppression may be an important strategy in the management of skin cancer.
Grapes (Vitis vinifera) are rich in polyphenols with approximately 60-70% of these polyphenols being found in the grape seeds. The seeds contain a larger fraction of proanthocyanidins, which are composed of dimers, trimers, tetramers and oligomers of monomeric catechins or epicatechins (11-13). These grape seed proanthocyanidins (GSPs) have been shown to have anti-oxidant (14, 15), anti-inflammatory and anti-skin carcinogenic (16, 17) activities. We have shown that supplementation of a control AIN76A diet with GSPs inhibits UV-induced skin tumor development in terms of tumor incidence, tumor multiplicity and tumor growth in mice (17). GSPs also inhibit the UVB-induced suppression of the CHS response to the contact sensitizer, 2,4-dinitrofluorobenzene (DNFB) in C3H/HeN mice through a mechanism that is associated with the induction of interleukin (IL)-12 (18). IL-12 is a heterodimeric cytokine composed of two disulfide-bonded protein chains p35 and p40 (19, 20) that has been shown to have anti-tumor activity in a variety of tumor models (21-23) and to play a role in bridging innate and adaptive immune responses (24). IL-12 plays a critical role in CHS as in vivo neutralization of IL-12 inhibits the induction of CHS and also induces hapten-specific tolerance. If IL-12 is injected in mice i.p. between UV-irradiation and hapten application, it can prevent UV-induced immunosuppression (25).
Although dietary GSPs inhibit UVB-induced immunosuppression in mice, the molecular mechanisms underlying this inhibitory effect of GSPs are not yet clearly understood. Here, we report that analysis of the effects of GSPs in an IL-12p40 knock out (IL-12 KO) mouse model verify that inhibition of UVB-induced immunosuppression by GSPs is mediated through stimulation of IL-12. To characterize the cell populations responsible for GSPs-induced inhibition of UVB-induced immunosuppression, we investigated whether GSPs affect the development of CD8+ effector T cells and/or CD4+ regulatory T cells, which have been shown to play critical roles in CHS responses. For this purpose, CD8+ and CD4+ T cells from spleens and lymph nodes were positively selected from UVB-irradiated and DNFB sensitized mice that received GSPs in the diet. The T-cell subpopulations were transferred into naïve mice that were subsequently sensitized and/or challenged with DNFB and the CHS response determined. The effects of dietary GSPs on the synthesis of Th1 and Th2 cytokines by the CD8+ and CD4+ T cells were also evaluated.
Materials and Methods
Animals
Female C3H/HeN mice of 6-7 weeks of age were purchased from Charles River Laboratories (Wilmington, MA). The IL-12p40 KO mice on a C3H/HeN background were generated and bred in our Animal Resource Facility, as described previously (26). The mutation in the p40 protein chain of IL-12 in IL-12 KO mice completely eliminates the synthesis of biologically active IL-12 (26). The animal protocol used in this study was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
UVB irradiation
The clipper shaved backs of the mice were UVB irradiated using a band of four FS20 UVB lamps (Daavlin, UVA/UVB Research Irradiation Unit, Bryan, OH) equipped with an electronic controller to regulate UV dosage, as described earlier (15, 17). The UV lamps emit UVB (280-320 nm; ≈80% of total energy) and UVA (320-375 nm; ≈20% of total energy) with UVC emission being insignificant. The majority of the resulting wavelengths of UV radiation are in the UVB (290-320 nm) range with a peak emission at 314 nm.
Grape seed proanthocyanidins (GSPs) and dietary supplementation
The purified GSPs were obtained from the Kikkoman Corporation (Tokyo, Japan) and the chemical composition has been described earlier (17, 18). Briefly, GSPs contain approximately 89% proanthocyanidins, with dimers (6.6%), trimers (5.0%), tetramers (2.9%) and oligomers (74.8%), and GSPs are stable for at least 2 years when refrigerated at 4°C. Experimental diets containing GSPs (0.5, 1.0%, w/w) were prepared in pellet form in the AIN76A powdered control diet by TestDiet (Richmond, IN) using the GSPs that we provide for this purpose. The dose of GSPs in the diet was selected based upon its significant chemopreventive effect on photocarcinogenesis in mice (17). Dietary administration of GSPs was started at least 1 week before UVB-irradiation of mice and continued until the termination of the experiment.
Assessment of CHS response
Only the clipper shaved backs of the mice were exposed to UVB radiation (150 mJ/cm2) for 4 consecutive days. Twenty-four hours after the last UV exposure, the mice were sensitized by painting 25 μl of 0.5% DNFB in acetone: olive oil (4:1, v/v) on the UVB-irradiated clipper shaved skin site. The CHS response was elicited 5 days later by challenging both surfaces of the right ear of each mouse with 20 μl of 0.2% DNFB in acetone: olive oil (4:1, v/v). The ear swelling was measured 24 h after the challenge using an engineers micrometer (Mitutoyo, Tokyo, Japan) and was compared with the ear thickness just before the challenge. Mice that received the same dose of DNFB for skin sensitization as well as challenge on ear skin but were not UV irradiated served as a positive control, whereas the non-UVB-irradiated mice which received only ear challenge with DNFB served as a negative control. To determine the chemopreventive effect of GSPs against UV-induced immunosuppression, GSPs were given in the diet of the mice in the separate groups. During UV exposure of the mice, the ears of the mice were protected from the UV irradiation by opaque black tape, which was removed after exposure. The backs of the mice that were not UV-irradiated were also shaved to maintain the identical regimen. The UV-induced suppression of CHS was determined as described previously (18, 27). Each treatment group had five mice, and each experiment was repeated at least twice.
To determine whether treatment of mice with GSPs leads to a long-term immunity, the CHS experiment was extended. After measuring the right ear swelling response to challenge with DNFB (primary challenge), the mice were rested for 4 weeks and were fed a standard diet without GSPs. The mice were then re-challenged (secondary challenge) on the left ear skin with the DNFB and the ear swelling response measured.
Adoptive transfer of the CHS response
For the adoptive transfer experiments, donor mice were exposed to UVB radiation with or without treatment with GSPs, and DNFB-sensitized 24 h after the last UV exposure as described above. Five days after sensitization, the mice were sacrificed and single-cell suspensions prepared from the spleens and regional lymph nodes. In one set of experiments, the unfractionated cells (5 × 107) in 200 μL of PBS were injected i.v. into each recipient mouse. The mice were sensitized 24 h later by epicutaneous application of DNFB on the shaved abdominal skin and challenged with DNFB on the ear skin 5 days after sensitization. Ear thickness was measured before and 24 h after challenge. In a second set of experiments, purified CD8+ T cells (8× 106) were injected i.v. into the recipients. In this experiment the mice were challenged with DNFB immediately after injection of the cells. In a third set of experiments, purified CD4+ T cells (8× 106) were injected i.v. into recipient mice, which were sensitized 24 h later, sensitized by epicutaneous application of DNFB on the shaved abdominal skin and challenged with DNFB on the ear skin 5 days after sensitization, and ear swelling determined before and 24 h after the challenge. Groups of naïve mice, which were not sensitized but were ear challenged, served as negative controls.
Purification of T-cell subpopulations
In vitro purification of CD4+ and CD8+ T cells from single-cell suspensions of the spleens and lymph nodes of the sensitized mice from various treatment groups was performed using rat anti-mouse CD4 or anti-CD8 monoclonal antibody and the MACS system following the manufacturer's instructions (Miltenyi Biotech, Inc.). The efficiency of positive-selection of each T-cell subpopulation was examined by flow cytometry (EPICS XL, Coulter, Miami, FL) using specific antibodies to target cells. The efficiency of purification was >95%.
In vivo treatment with recombinant IL-12 or anti-IL-12 antibody
To verify the role of GSPs-induced IL-12 on GSPs-induced protection from UVB-induced suppression of the CHS response in mice, anti-IL-12 antibody was diluted in sterile endotoxin-free saline and injected i.p.. The mice received two doses (500 ng each) one at 24 h and one at 3 h before DNFB sensitization. Control mice were injected i.p. with equal volumes of rat IgG1 (isotype control for the anti-IL-12) in saline. IL-12 KO mice were injected i.p. with recombinant murine IL-12 (1000 ng/100 μL PBS) or an equal volume of PBS 3 h before DNFB sensitization, as standardized previously (18, 28).
In vitro stimulation of T cells by bone marrow-derived dendritic cells and measurement of Th1 and Th2 cytokine production
Mice were UVB irradiated with and without GSPs treatment and sensitized with DNFB 24 h after the last UV exposure, as detailed above. The mice were sacrificed 5 days later, the spleens and draining lymph nodes collected, single-cell suspensions prepared and the CD8+ and CD4+ T-cell subpopulations purified as described above. Bone marrow-derived dendritic cells (BMDC) were prepared from naïve mice and used for in vitro stimulation of primed T cells as described earlier (29). Purified CD8+ and CD4+ T cells (2× 106/mL) prepared from the different treatment groups were stimulated with the BMDC (2× 105/mL) and the culture supernatants collected 48 h later. After centrifugation, the supernatants were analyzed for Th1 (IL-2, IFNγ) and Th2 (IL-4 and IL-10) cytokines using cytokine-specific ELISA kits (BioSource International, Inc.).
Statistical analysis
The difference between experimental groups for CHS response and the levels of cytokines were analyzed using the Student's t test. A p value <0.05 was considered significant.
Results
Prevention of UV-induced suppression of the CHS response by GSPs requires IL-12
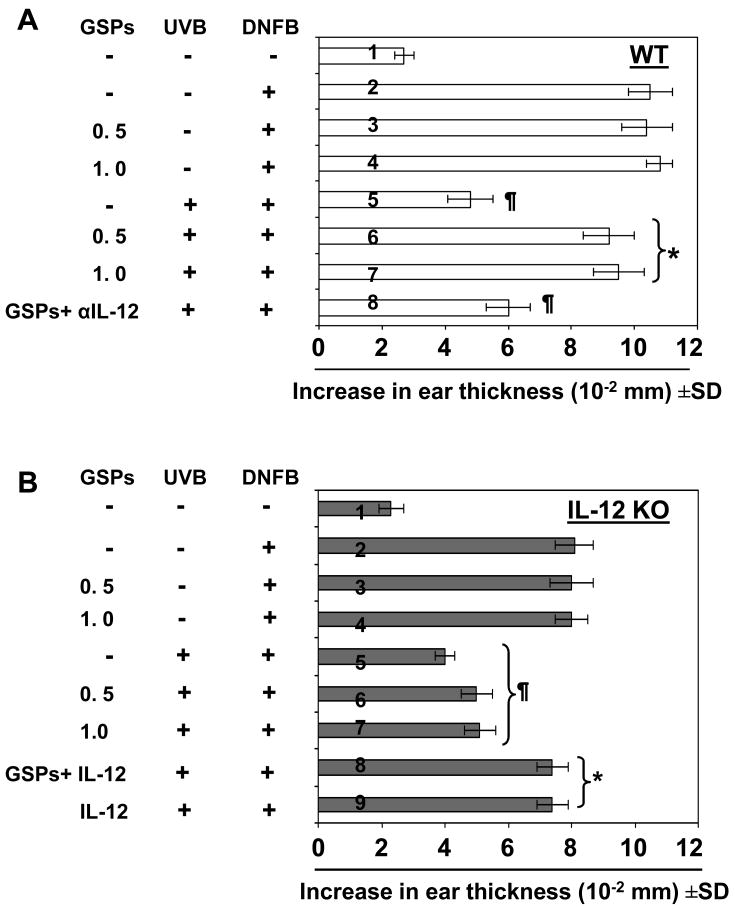
We have shown previously that dietary GSPs prevent UV-induced suppression of CHS in C3H/HeN (WT) mice and that this ability of GSPs to prevent immunosuppression is associated with the stimulation of an increase in the levels of IL-12 (18). To further verify whether the prevention of UV-induced immunosuppression by GSPs requires or is mediated through IL-12, the CHS response was assessed in IL-12 KO mice and their WT (C3H/HeN) counterparts. Application of DNFB to the UV-exposed skin did not induce significant sensitization in either WT (Fig. 1A, 5th bar) or IL-12 KO mice (Fig 1B, 5th bar), but did induce sensitization in the positive control group which were not exposed to UV radiation (2nd bar). Treatment with GSPs (0.5 and 1.0%, w/w) prevented the UV-induced suppression of the CHS response in WT mice as demonstrated by significant ear swelling upon ear challenge (Fig 1A, 6th and 7th bar). In contrast, the UV-exposed IL-12 KO mice remained unresponsive to the DNFB challenge despite treatment with GSPs (Fig 1B, 6th and 7th bar), indicating that the immunopreventive effect of GSPs on UV-induced suppression of CHS may require or be mediated through IL-12. Importantly, treatment with GSPs in the absence of UVB irradiation did not affect the DNFB-induced CHS response (Fig. 1A, 1B, 3rd and 4th bar).
Figure 1.
Prevention of UVB-induced suppression of the CHS response by GSPs requires IL-12. Dietary GSPs prevent UVB-induced suppression of the CHS response in wild-type mice but not in IL-12 KO mice. Wild-type (C3H/HeN) (Panel A) or IL-12 KO C3H/HeN (Panel B) mice that received either a standard diet or a diet supplemented with GSPs (0.5% or 1.0%, w/w) and exposed to UVB radiation (150 mJ/cm2) on four-consecutive days, sensitized to DNFB and the CHS response to application of DNFB on ear skin (challenge) assessed by measurement of ear swelling response 24 h later, as described in the Materials and Methods. Treatment group number 1 in each panel indicates that mice were not sensitized with DNFB but only challenged with DNFB in ear skin. One group of wild-type mice were injected with anti-IL-12 antibody (Panel A, 8th bar), and one group of IL-12 KO mice (Panel B, 8th and 9th bar) were injected with 1,000 ng of IL-12, 3 h before DNFB sensitization. The change in ear thickness is reported in millimeter (mm ×10-2) as the mean ± SD, with n=5 per group. The experiment was repeated once with similar results. *Significant sensitization versus UVB exposure in the absence of GSPs treatment (5th bar), P<0.001; ¶Significant inhibition versus the positive control of sensitization in the absence of UVB irradiation or GSPs treatment (2nd bar), P<0.005.
To further verify that prevention of UV-induced suppression of the CHS response by GSPs requires IL-12, WT mice were treated with anti-IL-12 monoclonal antibody i.p. 24 and 3 h before DNFB sensitization. The injection of GSPs-treated WT mice with anti-IL-12 antibody significantly reversed the protective effect of GSPs on the UV-induced suppression of CHS (Fig 1A, 8th bar; P<0.005) as compared to the GSPs-treated mice (6th and 7th bar). Moreover, the injection of IL-12 KO mice with recombinant IL-12 3 h before DNFB sensitization resulted in an enhanced ear swelling response (Fig. 1B, 8th and 9th bar).
GSPs stimulate long-term immunity in UVB-irradiated mice
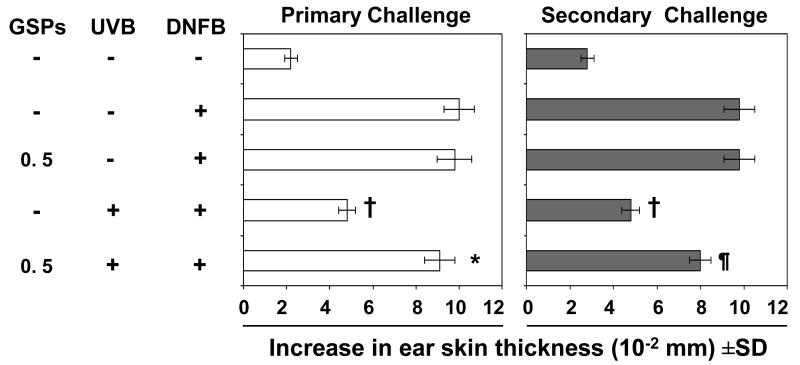
To determine whether treatment of mice with GSPs induces long-term effects on the immune responses in UVB-irradiated mice, the mice that had been subjected to the CHS protocol (Fig. 2, left panel) were rested for 4 weeks after primary challenge with DNFB and were provided a standard diet without GSPs supplementation during this period of time. As shown in Figure 2 (right panel), those mice that had been given GSPs in their diet before the primary challenge (Fig. 2, left panel) exhibited similar CHS response after secondary challenge with DNFB on the ear skin. These data suggest that dietary GSPs have the ability to protect the mice against UVB-induced immunosuppression for a considerable period of time after cessation of consumption of GSPs.
Figure 2.
GSPs protect against UVB-induced immunosuppression and does so even after cessation of treatment. Mice (C3H/HeN) that received a standard diet or a GSPs-supplemented diet were UVB-irradiated, sensitized through the UVB-irradiated skin, challenged by application of DNFB on the right ear (primary challenge), and the ear skin swelling measured 24 h later. The CHS response after primary challenge of the UVB-exposed mice that did not receive GSPs was significantly lower than the CHS response of the mice that were not UVB-irradiated, whereas mice that received GSPs in the diet before and during the CHS protocol mounted a CHS response to the primary DNFB challenge that was comparable to the response in the mice that were not UVB-irradiated. When the mice were rested for 4 weeks after primary challenge, then further challenged with DNFB (secondary challenge) on the left ear similar results were obtained. The change in ear swelling response in each group is reported as mean ± SD, n=5 per group. The experiment was repeated once with similar observations. *Significant increase versus UVB exposure in the absence of GSPs treatment, P<0.001; ¶Significant increase versus UVB in the absence of GSPs treatment, P<0.005; †Significant inhibition versus the positive control (unirradiated mice) (2nd bar from the top), P<0.001.
Prevention of UVB-induced suppression of CHS by GSPs is transferable via T cells
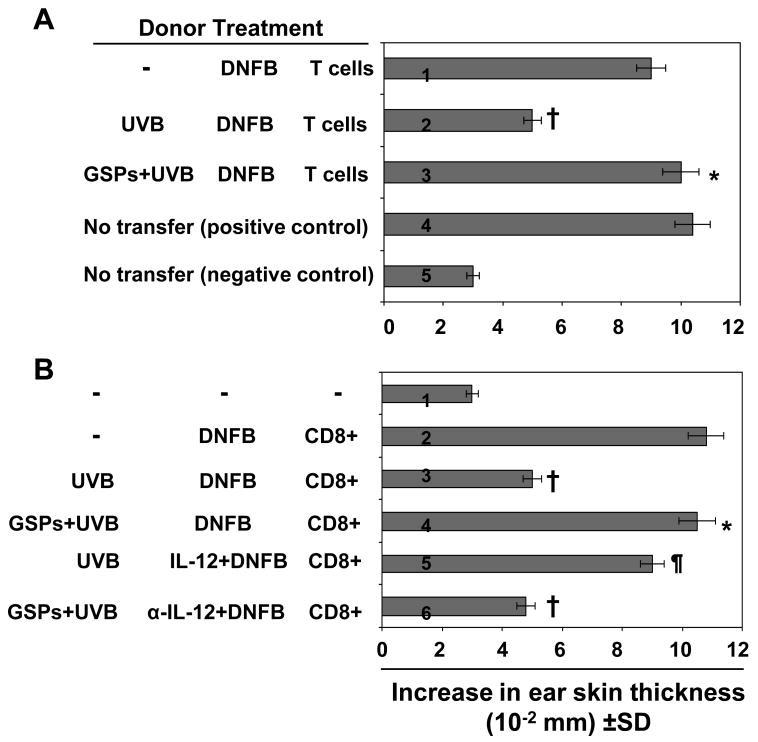
To further elucidate the mechanisms by which GSPs counteract UVB-induced immunosuppression, we first determined whether immune cells were capable of transferring this protection. The regional lymph node and spleen cells (5× 107) from C3H/HeN mice that had been sensitized 5 d earlier by topical treatment of DNFB on the UVB-exposed or UVB-unexposed skin, with or without treatment with dietary GSPs, were injected i.v. into naïve mice. The mice were challenged 24 h later by application of DNFB onto the ear skin and ear swelling was measured 24 h later (Fig. 3A). Naïve mice which received spleen and lymph node cells from UVB-exposed mice that were not treated with GSPs exhibited a lower CHS response (2nd bar) than the control group whereas naïve mice that received cells from GSPs-treated, UVB exposed donor mice showed a significant greater CHS response (P<0.001, 3rd bar) that was comparable to the response of the positive control group (Fig. 3A, 1st bar). The CHS response under 4th and 5th bar served as controls. The results from this experiment showed that the GSPs-induced prevention of immunosuppression is transferable to naïve mice.
Figure 3.
GSPs prevent transferable UVB-induced suppression through activation of T cells. A, Donor mice (C3H/HeN) that received either a standard diet or a diet supplemented with GSPs were sensitized, UVB-irradiated, sacrificed 5 days later and single-cell suspensions prepared from the regional lymph nodes and spleens, as detailed in the Materials and Methods. Syngeneic Recipient mice were injected i.v. with 5× 107 spleen and lymph node cells obtained from syngeneic donor mice. Recipient mice were sensitized with DNFB 24 h after transfer, ear challenge was performed 5 days later, and ear skin thickness was measured before and 24 h after challenge. B, The donor mice were treated as described in Panel A, except that CD8+ T cells were positively selected from the spleen and lymph nodes cell preparations. The CD8+ T cells (8× 106) were injected i.v. into naïve mice, the recipient mice were challenged immediately and the ear swelling response was measured 24 h later. In one group of mice, the donor mice were administered recombinant IL-12 (1000 ng/mouse) i.p. 3 h before sensitization. In another group, donor mice received an i.p. injection of anti-IL-12 (500 ng/mouse) 24 and 3 h before DNFB sensitization. Control mice received rat IgG1 (isotype control of anti-IL-12). The change in ear thickness is reported as the mean of millimeters (mm × 10-2) ±SD, n=5 per group. *Significantly greater CHS response versus UVB irradiation in the absence of GSPs treatment, P<0.001; ¶Significantly greater CHS response versus recipient of T cells from UVB+ DNFB treated mice, P<0.01; †Significantly lower CHS response versus the positive control (DNFB-sensitized) group, P<0.001
GSPs inhibit UVB-induced immunosuppression through the activation of CD8+ effector T cells
We then sought to identify the T-cell subpopulations responsible for the transfer of the GSPs-induced prevention of immunosuppression in the adoptive transfer model. The donor mice were treated or not with GSPs, exposed to UVB, and sensitized as shown in Figure 3B. The spleens and regional lymph nodes were obtained from C3H/HeN donor mice that had been sensitized 5 days earlier by topical treatment of the UVB-exposed skin with DNFB. Single-cell suspensions were prepared and the CD8+ T cells positively selected using the MACS system. Purified CD8+ T cells (8× 106) were injected i.v. into naïve mice, which were then challenged immediately by application of DNFB on the ear skin. Ear swelling was measured 24 h later. As shown in Figure 3B, those naïve mice which have received CD8+ effector T cells from GSPs-treated, UVB-exposed donor mice showed a greater CHS response (4th bar) than the naïve mice that received cells from UVB-exposed mice that were not treated with GSPs (3rd bar). This suggested that the prevention of UVB-induced immunosuppression by GSPs is transferable to naïve mice by CD8+ T cells, and further suggested that treatment with GSPs results in activation of the CD8+ T-cell subpopulation and that these cells play a role in the enhanced CHS response in UVB-exposed mice.
Cytokine production by the CD8+ T cells in response to UVB, GSPs or IL-12 treatments
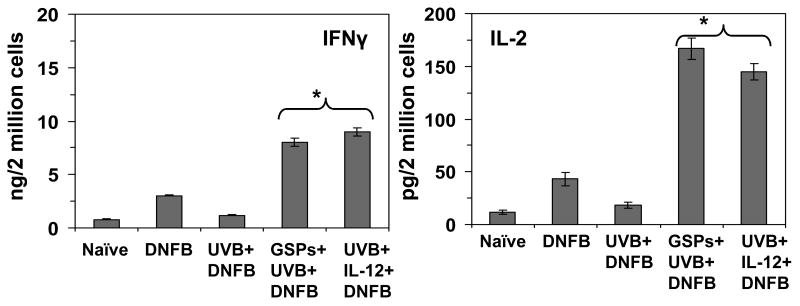
To confirm that dietary administration of GSPs activates CD8+ T cells, we determined the Th1 and Th2 cytokine profiles of CD8+ effector T cells that had been prepared from the mice in the different treatment groups and then stimulated in vitro for 48 h with DNBS-labeled BM-DC from naïve mice. The supernatants were collected and the levels of Th1 and Th2 cytokines quantified using cytokine-specific ELISAs. As shown in Figure 4, the levels of IFNγ (>5-fold, P<0.001) and IL-2 (8-fold, P<0.001) were considerably higher in the supernatants of CD8+ T cells prepared from the GSPs treated, UVB-exposed mice than in the supernatants of cells from mice that were exposed to UVB but not treated with GSPs. In contrast, the Th2 cytokines were barely detectable in the supernatants of CD8+ T cells obtained either from the GSPs-treated, UVB-exposed mice or the CD8+ T cells from the mice that were exposed to UVB but not treated with GSPs. The significantly higher levels of Th1 cytokines in the mice that were treated with GSPs suggests that the activation of CD8+ T cells by the dietary GSPs may play a role in the greater CHS response in the GSPs-treated, UVB-exposed mice.
Figure 4.
Treatment of mice with GSPs or rIL-12 enhances the levels of production of IL-2 and IFNγ by CD8+ T cells. Mice were treated and CD8+ T-cells isolated as described under Figure 3. The CD8+ T-cells were then co-cultured with DNSB-labeled BMDC for 48 h, as detailed in the Materials and Methods. The concentrations of cytokines in the cell supernatants were estimated by ELISA and are presented as the mean± SD in terms of pg or ng per 2 million cells. n=5/group. *Significant increase versus UVB+DNFB group, P<0.001
GSPs inhibit UVB-induced immunosuppression through inactivation of CD4+ suppressor T cells
There is an ongoing debate as to whether the CD8+ or the CD4+ T-cell subpopulation mediates the CHS response. Studies by some investigators (30, 31) suggest that CD4+ T cells are the critical effector cells for CHS response, while other investigators (29, 32-34) provide evidence that CD8+ T cells play a critical role in CHS response. To explain the effect of GSPs on the CD8 T cell response, spleens and regional lymph nodes were obtained from C3H/HeN donor mice sensitized 5 days earlier by topical administration of DNFB onto the UVB-exposed backs of mice that were treated with or without GSPs. Single-cell suspensions were prepared and CD4+ T cells isolated by positive selection. Naïve mice were injected i.v. with the purified CD4+ T cells (8× 106) and, in this case, the mice were sensitized 24 h later by application of DNFB onto the clipper-shaved abdominal skin prior to challenge by application of DNFB on the ear skin 5 days later and the ear swelling was measured 24 h later. As shown in Figure 5A, those naïve mice that received CD4+ T cells from UVB-irradiated and DNFB-sensitized donor mice had a significantly lower CHS response (P<0.01) (3rd bar) than those mice that were sensitized but not exposed to UVB (2nd bar). Those naïve mice that had received CD4+ T cells from the GSPs-treated, UVB-exposed donor mice had a greater CHS response (4th bar) than those naïve mice that had received CD4+ T cells from UVB-exposed donor mice that were not treated with GSPs (3rd bar), suggesting that prevention of UVB-induced immunosuppression by GSPs is transferable to naïve mice by CD4+ T cells.
Figure 5.
(A) GSPs prevent transferable suppression by UVB through the inactivation of UVB-induced CD4+ suppresser T cells. Mice were treated as described in Figure 3 and CD4+ T cells purified from the splenocytes and lymphocytes by positive selection. Twenty-four hours after i.v. injection of CD4+ T cells into naïve mice, the mice were sensitized with DNFB, and ear challenged 5 days after sensitization, as detailed in the Materials and Methods. Those naïve mice that received CD4+ T cells from UVB-exposed donor mice that were GSPs treated showed a greater CHS response than UVB-exposed mice that were not GSPs treated. Those naïve mice that received CD4+ T cells from UVB-exposed rIL-12-injected donor mice showed a significantly higher CHS response than UVB-exposed mice that were not injected with rIL-12. The change in the ear swelling response in each group is reported as mean ± SD (n=5 per group). Significantly greater CHS response versus UVB irradiation in the absence of GSPS or rIL-12 treatment, †P<0.001; Significantly lower CHS response versus positive control group (2nd bar), ¶P<0.01. (B) Treatment of mice with GSPs or rIL-12 decreases the production of IL-4 and IL-10 by CD4+ T cells. Mice were sensitized to DNFB after UVB-irradiation described in Figure 3, the CD4+ T cells isolated by positive selection and then co-cultured with DNSB-labeled BMDC for 48 h, as detailed in the Materials and Methods. The concentrations of cytokines in the cell supernatants were estimated by ELISA and are presented as the mean ±SD in terms of pg/2 million cells. Experiment was repeated once, n=5. Significant decrease versus UVB+DNFB group, †P<0.001; Significant decrease versus positive control (DNFB alone), ¶P<0.001; Significant increase versus positive control (DNFB alone), *P<0.001
Cytokine production by CD4+ T cells in response to UVB and GSPs
To confirm that the dietary administration of GSPs results in inactivation of CD4+ T cells, we compared the Th1 and Th2 cytokine profiles of CD4+ T cells from mice from the different treatment groups. As described in detail in the Materials and Methods section, purified CD4+ T cells were prepared and stimulated in vitro for 48 h with DNBS-labeled BM-DC. As shown in Figure 5B, the levels of Th2 cytokines in the supernatants of the CD4+ T cells from the GSPs-treated, UVB-exposed mice and the IL-12-treated, UVB exposed mice were significantly lower (IL-4, 80-100%; IL-10, 88-92%; P<0.001) than the levels of these cytokines in the supernatants of CD4+ T cells from UVB-exposed mice that were not treated with GSPs or IL-12. Both IFNγ and IL-2 were detectable in the supernatants of CD4+ T cells irrespective of whether the donor mice were treated with GSPs or IL-12 but the levels of these Th1 cytokines were low, particularly when compared with the levels of the Th1 cytokines in the supernatants of the CD8+ effector T cells. The low levels of the Th2 cytokines in the supernatants of the CD4+ T cells in these experiments suggest that treatment of mice with dietary GSPs suppresses the activity of CD4+ T cells.
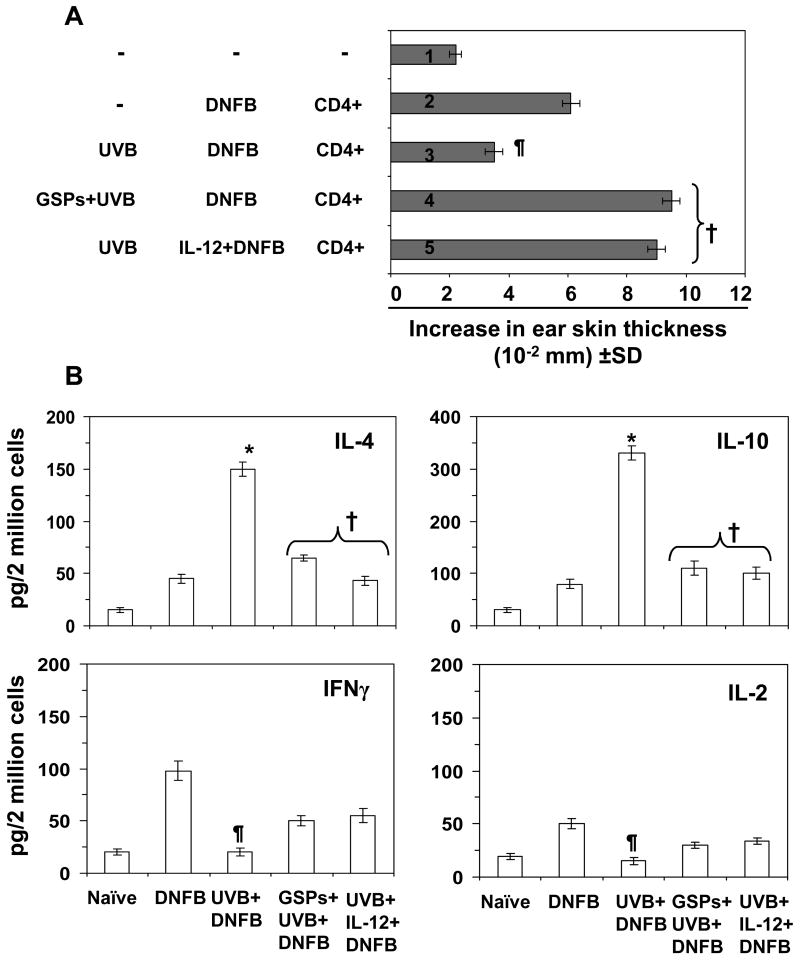
Role of IL-12 in the development or inactivation of CD8+ or CD4+ T cells and adoptive transfer of the CHS response with and without the treatment of mice with dietary GSPs
The above studies demonstrated that the chemopreventive effect of GSPs on UVB-induced suppression of CHS is mediated through the induction of IL-12 in mice, and that this chemopreventive effect is transferable to naïve mice. To examine the contribution of IL-12 to the stimulation of CD8+ T cells in the UVB-exposed GSPs-treated mice, further adoptive transfer experiments were conducted in C3H/HeN mice using cells from mice that were treated with IL-12 or anti-IL-12 antibody. The CHS responses were assessed using protocols identical to those described above. Mice that were UVB irradiated but not treated with GSPs were injected with recombinant IL-12 (Fig. 3B, 5th bar) and CD8+ T cells were isolated from the lymph nodes and spleens from the donor mice as described above. As shown in Figure 3, those naïve mice that had received CD8+ T cells from the group of mice that had been IL-12-treated and UVB-exposed showed a greater CHS response upon DNFB challenge (5th bar) than those mice that were UVB exposed but not treated with IL-12 (3rd bar). Those naïve mice that had received CD8+ T cells from donor mice that had been treated with GSPs, UVB-irradiated and treated with anti-IL-12 did not mount a significant CHS response to DNFB (6th bar) in contrast to donor mice that had been treated with GSPs and UVB-exposed, but not treated with anti-IL-12 (4th bar), suggesting a role for IL-12 in both the stimulation of CD8+ effector T cells and in the GSPs-mediated CHS response that can be transferred by adoptive transfer.
Finally, UVB-exposed donor mice were treated with rIL-12 and 5 days after sensitization CD4+ T cells were isolated from the spleens and lymph nodes. These cells were transferred to naïve mice, and the CHS response was measured after DNFB challenge. As shown in Fig. 5A (5th bar), those naïve mice that received CD4+ T cells from IL-12-treated donor mice showed a significantly greater CHS response (P<0.001) than those naïve mice that received CD4+ T cells from the UVB-irradiated donor mice that had not been treated with rIL-12 (3rd bar). The CHS response in the naïve mice in group or bar 5th was comparable to that of the naïve mice which have received CD4+ T cells from donor mice that had been treated with GSPs and UVB exposed (4th bar). These data suggest that the IL-12 might have functionally inactivated the suppressive effects of the CD4+ T cells or inhibited the development of regulatory CD4+ T cells and thus lacking to an enhanced CHS response to DNFB.
Discussion
Previously, we have shown that dietary GSPs inhibit photocarcinogenesis in mice (17). As UVB-induced immunosuppression has been implicated in the development of skin cancers, we examined the effects of dietary GSPs on UVB-induced immunosuppression and characterized the cell populations that play a role in these protective effects of the GSPs. We found that dietary GSPs prevent the UVB-induced suppression of the CHS response to a contact sensitizer (DNFB) and that this immunopreventive effect of GSPs was mediated, at least in part, through the induction of the immunoregulatory cytokine, IL-12 (18). IL-12 has been shown to have anti-tumor activity and to stimulate the immune system (19, 21, 24). The current experiments suggest that dietary GSPs fail to prevent UVB-induced immunosuppression in IL-12 KO mice but can prevent it in wild-type mice. The injection of UVB exposed IL-12 KO mice with recombinant IL-12 restored the CHS response, whether or not the mice had been treated with GSPs. Moreover, injection of wild-type mice that were given GSPs with anti-IL-12 antibody blocked the GSPs-induced prevention of the CHS response, further supporting evidence that the prevention of UVB-induced immunosuppression in mice by GSPs is mediated, at least in part, through IL-12. A similar chemopreventive effect on UVB-induced immunosuppression has been observed when mice were treated with green tea polyphenols (35), suggesting that the photoprotective effects of plant polyphenols and flavonols may be mediated by similar mechanisms. The immunostimulatory effects of IL-12 have been demonstrated using in vivo systems (36, 37) and IL-12 has been shown to play a role in vivo as a mediator and adjuvant for the induction phase of the CHS response (36). After UV exposure, the antigen presenting cells present in the skin migrate to the regional lymph nodes and initiate sensitization. We have found that dietary GSPs enhance the production of IL-12 in draining lymph node cells of mice (18). Our results also provide evidence that administration of GSPs not only prevents UVB-induced immunosuppression but also can protect the mice from UVB-induced immunosuppression for some time after the consumption of GSPs has ceased.
As the hapten-specific effects of the GSPs on UVB-induced immunosuppression could be adoptively transferred into naïve wild-type mice, we were able to use an adoptive transfer approach to characterize the cells that mediate the GSPs effects. Our experiments revealed that dietary GSPs prevent UVB-induced immunosuppression through stimulation and/or enhanced development of CD8+ effector T cells and that the dietary GSPs enhance the ability of the CD8+ T cells to secrete Tc1 cytokines. Thus, these results suggest that GSPs stimulate the development and the activity of CD8+ effector T cells, presumably by a direct effect; however, because the IL-12 was injected into donor mice we can not exclude the possibility that the IL-12 acts on the CD4+ T cells or natural killer cells in these mice and that ultimately it is these cells that enhance the development of the CD8+ T cells that can mediate the adoptive transfer of the CHS response in this system. Our data further suggest that inhibition of the development of regulatory T-cells and/or inactivation of CD4+ suppressor T cells also plays a role in the prevention of UVB-induced suppression of the CHS response by GSPs and this was borne out by the significant inhibitory effects of dietary GSPs on the ability of the CD4+ T cells to produce Th2 cytokines (IL-4 and IL-10). Thus, our results indicate that CD8+ T cells are the critical effector cells, a finding that is in accordance with the findings of other investigators (29, 33). Xu et al. (29) reported that CHS is mediated through CD8+ T cells, whereas CD4+ Th2 cells exhibit an inhibitory effect on CHS, and this observation was supported by the findings of Gocinski and Tigelaar (33) and Anderson et al. (32) who reported that depletion of CD4+ T cells before sensitization results in an enhanced ear swelling response. Thus, our results are consistent with the findings of these investigators. Moreover, our observation that transfer of CD4+ regulatory T cells from those donor mice that were treated with IL-12 into naïve mice resulted in an enhanced CHS response further suggests that IL-12 plays a role in inhibiting CD4+ T cells with this concept being supported by the inhibitory effects of IL-12 on the secretion of Th2 type cytokines, and this effect was found to be similar in magnitude to the effects of treatment of donor mice with GSPs+ UVB.
We also were interested in comparing the cytokine profile of CD8+ and CD4+ T cells obtained from mice that were exposed to UVB but not treated with GSPs and those that were GSPs treated and UVB exposed, and to delineate the relationship of these profiles with the inhibitory effect of dietary GSPs on the UVB-induced immunosuppression. It was observed that the levels of Th1 (Tc1) cytokines (IFNγ, IL-2) were much higher in CD8+ T cells from GSPs-treated mice, whereas the Th2 cytokines (IL-4 and IL-10) were hardly detectable. These alterations in cytokine profile of CD8+ T cells under the influence of GSPs may have a role in the enhancement of immune reactions. IFNγ-producing T cells are important effector cells in the CHS response and also are involved in reducing the development of UVB-induced skin tumors. Our observations also suggest that the immunopreventive effect of GSPs against UVB-induced immunosuppression are mediated, at least in part, through the inhibition of the development and/or inactivation of CD4+ regulatory T cells, which is evident in the significant reduction of Th2 cytokine (IL-4 and IL-10) levels in the cell supernatants obtained from BM-DC-stimulated CD4+ T cells as compared to the CD4+ T cells obtained from UVB irradiated mice that were not treated with GSPs. The Th2 cytokines, IL-4 and IL-10, have been implicated in the immunosuppressive effects and the development of Th2 or CD4+ cells (38). Further in this study, we measured that each mouse (mean weight 20 g) consumed approximately 13± 0.5 mg GSPs per day. Based on this information, the human equivalent dose of GSPs was calculated according to the body surface area normalization method (39). This analysis revealed that a normal person of 70 Kg body weight would require 3.8 g GSPs as a dietary supplement per day for equivalent immunological responses. This amount of GSPs seems affordable, reasonable and attainable.
In summary, the significance of our study relates to the chemopreventive effect of dietary GSPs on UVB-induced immunosuppression, which is considered to be a risk factor for skin tumor development. This property of GSPs can be used as an alternative strategy to augment the induction of CD8+ effector T cells and to diminish the development of CD4+ regulatory T cells and that may lead to the prevention of skin cancer risk in humans.
Acknowledgments
We thank Dr. Fiona Hunter for her assistance in editing the manuscript.
Financial support: This work was financially supported by the Veterans Administration Merit Review Award (S.K.K., C.A. E.), and the UAB Skin Diseases Research Center (AR050948-01).
Footnotes
Disclosure Statement: No conflict of interest
References
- 1.Toews GB, Bergstresser PR, Streilein JW, Sullivan S. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–53. [PubMed] [Google Scholar]
- 2.Cooper KD, Oberhelman L, Hamilton TA, et al. UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: relationship to dose, CD1a-DR+ epidermal macrophage induction, and Langerhans cell depletion. Proc Natl Acad Sci U S A. 1992;89:8497–501. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshikawa T, Rae V, Bruins-Slot W, vand-den-Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–6. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 4.Meunier L, Raison-Peyron N, Meynadier J. UV-induced immunosuppression and skin cancers. Rev Med Interne. 1998;19:247–54. doi: 10.1016/S0248-8663(97)89326-5. [DOI] [PubMed] [Google Scholar]
- 5.Applegate LA, Ley RD, Alcalay J, Kripke ML. Identification of molecular targets for the suppression of contact hypersensitivity by ultraviolet radiation. J Exp Med. 1989;170:1117–31. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–91. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 7.Donawho CK, Kripke ML. Evidence that the local effect of ultraviolet radiation on the growth of murine melanomas is immunologically mediated. Cancer Res. 1991;51:4176–81. [PubMed] [Google Scholar]
- 8.Sluyter R, Halliday GM. Infiltration by inflammatory cells required for solar-simulated ultraviolet radiation enhancement of skin tumor growth. Cancer Immunol Immunotherapy. 2001;50:151–6. doi: 10.1007/PL00006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullrich SE, Kripke ML. Mechanisms in the suppression of tumor rejection produced in mice by repeated UV irradiation. J Immunol. 1984;133:2786–90. [PubMed] [Google Scholar]
- 10.Beissert S, Bluestone JA, Mindt I, et al. Reduced UV-induced carcinogenesis in mice with a functional disruption in B7-mediated costimulation. J Immunol. 1999;163:6725–31. [PubMed] [Google Scholar]
- 11.Shi J, Yu J, Pohorly JE, Kakuda Y. Polyphenolics in grape seeds-biochemistry and functionality. J Med Food. 2003;6:291–9. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- 12.Prieur C, Rigaud J, Cheynier V, Moutounet M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry. 1994;36:781–9. [Google Scholar]
- 13.Silva RC, Rigaud J, Cheynier V, Chemina A. Procyanidin dimers and trimers from grape seeds. Phytochemistry. 1991;30:1259–64. [Google Scholar]
- 14.Mantena SK, Katiyar SK. Grape seed proanthocyanidins inhibit UV radiation-induced oxidative stress and activation of MAPK and NF-κB signaling in human epidermal keratinocytes. Free Rad Biol Med. 2006;40:1603–14. doi: 10.1016/j.freeradbiomed.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 15.Sharma SD, Meeran SM, Katiyar SK. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-κB signaling in in vivo SKH-1 hairless mice. Mol Cancer Ther. 2007;6:995–1005. doi: 10.1158/1535-7163.MCT-06-0661. [DOI] [PubMed] [Google Scholar]
- 16.Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Letts. 2008;269:378–87. doi: 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–88. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 18.Sharma SD, Katiyar SK. Dietary grape-seed proanthocyanidin inhibition of ultraviolet B-induced immune suppression is associated with induction of IL-12. Carcinogenesis. 2006;27:95–102. doi: 10.1093/carcin/bgi169. [DOI] [PubMed] [Google Scholar]
- 19.Trinchieri G. Interleukin-12 a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 20.Brunda MJ. Interleukin-12. J Leukocyte Biol. 1994;55:280–8. doi: 10.1002/jlb.55.2.280. [DOI] [PubMed] [Google Scholar]
- 21.Brunda MJ, Luistro L, Warrier RR, et al. Antitumor and antimetastatic activity of interleukin-12 against murine tumors. J Exp Med. 1993;178:1223–30. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson MJ, Ritz J. Interleukin-12: basic biology and potential applications in cancer treatment. Oncologist. 1996;1:88–97. [PubMed] [Google Scholar]
- 23.Zou JP, Yamatato N, Fuzii T, et al. Systemic administration of rIL-12 induces complete tumor regression and protective immunity: response is correlated with a striking reversal of suppressed IFN-γ production by anti-tumor T cells. Int Immunol. 1995;7:1135–45. doi: 10.1093/intimm/7.7.1135. [DOI] [PubMed] [Google Scholar]
- 24.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 25.Riemann H, Schwarz A, Grabbe S, et al. Neutralization of IL-12 in vivo prevents induction of contact hypersensitivity and induces hapten-specific tolerance. J Immunol. 1996;156:1799–803. [PubMed] [Google Scholar]
- 26.Meeran SM, Mantena SK, Meleth S, Elmets CA, Katiyar SK. Interleukin-12-deficient mice are at greater risk of ultraviolet Radiation-induced skin tumors and malignant transformation of papillomas to carcinomas. Mol Cancer Ther. 2006;5:825–32. doi: 10.1158/1535-7163.MCT-06-0003. [DOI] [PubMed] [Google Scholar]
- 27.Katiyar SK, Challa A, McCormick TS, Cooper KD, Mukhtar H. Prevention of UVB-induced immunosuppression in mice by green tea polyphenol (-)-epigallocatechin-3-gallate may be associated with alterations in IL-10 and IL-12 production. Carcinogenesis. 1999;20:2117–24. doi: 10.1093/carcin/20.11.2117. [DOI] [PubMed] [Google Scholar]
- 28.Meeran SM, Katiyar S, Elmets CA, Katiyar SK. Silymarin inhibits UV radiation-induced immunosuppression through augmentation of interleukin-12 in mice. Mol Cancer Ther. 2006;5:1660–8. doi: 10.1158/1535-7163.MCT-06-0095. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (IL) 4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–12. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauser C. Cultured epidermal Langerhans cells activate effector T cells for contact sensitivity. J Invest Dermatol. 1990;95:436–40. doi: 10.1111/1523-1747.ep12555587. [DOI] [PubMed] [Google Scholar]
- 31.Kondo S, Beissert S, Wang B, et al. Hyporesponsiveness in contact hypersensitivity and irritant contact dermatitis in CD4 gene targeted mouse. J Invest Dermatol. 1996;106:993–1000. doi: 10.1111/1523-1747.ep12338505. [DOI] [PubMed] [Google Scholar]
- 32.Anderson C, Hehr A, Robbins R, et al. Metabolic requirements for induction of contact hypersensitivity to immunotoxic polyaromatic hydrocarbons. J Immunol. 1995;155:3530–7. [PubMed] [Google Scholar]
- 33.Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990;144:4121–8. [PubMed] [Google Scholar]
- 34.Bour H, Peyron E, Gaucherand M, et al. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995;25:3006–10. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- 35.Meeran SM, Mantena SK, Katiyar SK. Prevention of ultraviolet radiation-induced immunosuppression by (-)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair. Clinical Cancer Res. 2006;12:2272–80. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- 36.Muller G, Saloga J, Germann T, Schuler G, Knop J, Enk AH. IL-12 as mediator and adjuvant for the induction of contact sensitivity in vivo. J Immunol. 1995;155:4661–8. [PubMed] [Google Scholar]
- 37.Trinchieri G. Interleukin-12 and its role in the generation of Th1 cells. Immunol Today. 1993;14:335–8. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 38.Mukhtar H, Elmets CA. Photocarcinogenesis: Mechanisms, models and human health implications. Photochem Photobiol. 1996;63:355–447. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 39.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]