Abstract
We describe long-term disease-free survival after unrelated donor bone marrow transplantation (BMT) for myelodysplastic syndrome (MDS) in 118 patients aged ≤18 years. Forty-six patients had refractory cytopenia (RC), 55, refractory anemia with excess blasts (RAEB) and 17, refractory anemia with excess blasts in transformation (RAEB-t). Transplant-related mortality was higher after mismatched BMT (relative risk [RR] 3.29, p=0.002). Disease recurrence was more likely with advanced stages of MDS at the time of BMT: RAEB (RR 6.50, p=0.01) or RAEB-t (RR 11.00, p=0.004). Treatment failure (recurrent disease or death from any cause; inverse of disease-free survival [DFS]) occurred in 68 patients. Treatment failure was higher after mismatched BMT (RR 2.79, p=0.001) and in those with RAEB-t (RR 2.38, p=0.02). Secondary MDS or chemotherapy prior to BMT was not associated with recurrence or treatment failure. Similarly, cytogenetic abnormalities were not associated with transplant outcomes. Eight-year DFS for patients with RC after matched and mismatched unrelated donor BMT was 65% and 40%, respectively. Corresponding DFS for patients with RAEB and RAEB-t was 48% and 28%, respectively. When a matched adult unrelated donor is available, BMT should be offered as first-line therapy and children with RC can be expected to have the best outcome.
Keywords: pediatric myelodysplastic syndrome, unrelated donor, bone marrow transplantation
INTRODUCTION
Myelodysplastic syndrome (MDS) in childhood is a rare disorder, with an estimated incidence of 3.2 per million.(1) Childhood MDS responds poorly to acute myeloid leukemia (AML)-type chemotherapy and hematopoietic cell transplantation (HCT) is the only known curative treatment option. However, apart from juvenile myelomonocytic syndrome (JMML) or MDS transformed to AML, there exists few reports for children with MDS after allogeneic HCT. Disease-free survival rates support HCT when an HLA-matched sibling donor is available (2, 3). Given that only a third of patients have an HLA-matched sibling, it is clinically relevant to understand the outcomes and risk factors associated with disease-free survival after unrelated donor HCT. Thus far, reports of unrelated donor HCT for children with MDS have been limited by sample size, heterogeneity of donor type, and the inclusion of adults with MDS (4–6). Furthermore, the standard International Prognostic Scoring System (IPSS) for adults with MDS is of little prognostic value in children (7). Therefore, we examined outcomes after unrelated donor HCT for MDS in patients aged ≤18 years after applying a standard scoring system appropriate for childhood MDS based on morphology as recommended by the European Working Group for Myelodysplasia (EWOG-MDS) (7).
PATIENTS AND METHODS
Patients
Patients with MDS aged less than 18 years at transplantation who received a bone marrow (BM) graft from an unrelated adult donor in the United States were identified from the Center for International Blood and Marrow Transplant Research (CIBMTR). The CIBMTR collects patient, disease and transplant characteristics on all unrelated donor transplantations in the United States. All patients are followed longitudinally annually. MDS subtypes included RC, RAEB or RAEB-t and were assigned retrospectively by the CIBMTR according to the European Group for childhood MDS (EWOG-MDS) criteria (7). Donor-recipient HLA-match was considered at the allele-level for HLA-A, -B, -C, -DRB1. Donor and recipients were considered to be matched when matched at HLA-A, -B, -C, -DRB1 and mismatched when a mismatch existed at one or two loci. Excluded were patients who had received prior autologous or allogeneic BMT, peripheral blood progenitor cell or cord blood transplant, those with Down syndrome, Fanconi anemia, JMML or MDS with progression to acute myeloid leukemia (AML). This study was approved by the Institutional Review Board of the Medical College of Wisconsin.
Endpoints
Neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) ≥0.5 ×109/L. Patients who failed to achieve ANC ≥0.5 ×109/L within 28 days after HCT or experienced a sustained decline in ANC (<0.5 x109/L) after initial recovery were considered to have graft failure. Platelet recovery was defined as the first of 7 consecutive days with an unsupported platelet count ≥20 ×109/L. Acute and chronic GVHD were diagnosed and classified by the transplant center according to published criteria (8, 9). Recurrent disease after BMT was defined by the evolution of RC to RAEB or for RAEB/RAEB-t by the recurrence of blasts or progression to AML. Death from any cause in the absence of recurrent disease was defined as transplant-related mortality (TRM). All surviving patients were censored at last follow-up.
Statistical Analysis
The probabilities of neutrophil and platelet recovery, acute and chronic GVHD, TRM and disease recurrence were calculated using the cumulative incidence function estimator (10). For neutrophil and platelet recovery and GVHD, death was the competing event. For TRM, recurrence was the competing event and for recurrence, TRM the competing event. The probability of disease-free survival was calculated using the Kaplan-Meier estimator (11). The 95% confidence intervals (CI) were calculated using log transformation. The following variables were considered in multivariate analysis: gender, age at transplantation (≤10 versus >10 years), disease status (RC versus RAEB versus RAEB-t), cytogenetics (monosomy 7 versus other abnormalities versus normal cytogenetics), de novo versus secondary MDS, donor-recipient HLA match (matched versus mismatched), year of transplantation (≤1994 versus >1994), and conditioning regimen (TBI-containing versus chemotherapy only regimens). Multivariate models for acute and chronic GVHD, TRM, disease recurrence and treatment failure (inverse of disease-free survival; recurrent disease or death from any cause) were built using Cox regression and a forward selection procedure (12). Only variables that attained a p-value ≤0.05 were retained in the final multivariate model. All variables tested met the proportionality assumption. All tests are two-sided. Analyses were performed with SAS version 9.1 (Cary, NC).
RESULTS
There were 118 unrelated donor bone marrow transplantations (BMT) for pediatric patients with MDS in the United States between 1990 and 2005. Patient, disease and transplant characteristics are shown in Table 1. The median age at BMT was 8 years. Approximately 40% of patients had RC and 60%, RAEB or RAEB-t at the time of BMT. For each patient, the most advanced disease stage at anytime prior to BMT was considered. Six patients with RC at diagnosis progressed to RAEB and 7 patients with RAEB at diagnosis progressed to RAEB-t before BMT. Thirty-six patients (31%, n=9 RC, n=19 RAEB and n=8 RAEB-t) received chemotherapy before BMT. The median time from diagnosis to BMT was 7 months for RC and 6 months for RAEB/RAEB-t. Thirty patients (25%; 12 with RC, 15 with RAEB and 3 with RAEB-t) had secondary MDS that resulted from chemotherapy and/or radiotherapy for a prior malignancy (n=18), or evolved from acquired or congenital bone marrow failure (n=11) or systemic lupus erythematosus (n=1). Cytogenetic abnormalities were identified in 64% of cases and the most common abnormality was monosomy 7 (33%). A third of BM grafts were T-cell depleted. Transplant conditioning regimens were exclusively myeloablative and approximately 70% of regimens contained total body irradiation (TBI). The median follow-up of surviving patients is 8 years.
Table 1.
Patient, disease and transplant characteristics
| Variable | Number (%) |
|---|---|
| Patients | 118 |
| Age at transplant, years | |
| ≤ 5 | 30 (25) |
| 6 – 10 | 38 (32) |
| 11 – 18 | 50 (43) |
| Male | 70 (59) |
| Performance score prior to transplantation | |
| < 90% | 20 (17) |
| 90% – 100% | 91 (77) |
| Not reported | 7 (6) |
| MDS classification (EWOG – MDS) | |
| Refractory cytopenia | 46 (39) |
| Refractory anemia with excess blastsa | 55 (47) |
| Refractory anemia with excess blasts in transformationa | 17 (14) |
| Interval from diagnosis to transplant, months | |
| ≤ 6 | 57 (48) |
| 7 – 12 | 31 (26) |
| > 12 | 30 (26) |
| Cytogenetics | |
| Normal karyotype | 27 (23) |
| Monosomy 7 ± other abnormalities | 39 (33) |
| Complex karyotype (≥ 3 abnormalities) | 18 (15) |
| Other karyotypes | 19 (16) |
| Not reported | 15 (13) |
| Conditioning regimen | |
| Total body irradiation + cyclophosphamide | 80 (68) |
| Total body irradiation + other | 3 (3) |
| Busulfan + cyclophosphamide | 30 (25) |
| Busulfan + other | 4 (3) |
| Melphalan + other | 1 (1) |
| Characteristics of patients | N (%) |
|---|---|
| Graft-versus-host disease prophylaxis | |
| T-cell depletion | 39 (33) |
| Cyclosporine alone | 7 (6) |
| Cyclosporine + methotrexate | 62 (52) |
| Tacrolimus + alone | 1 (1) |
| Tacrolimus + methotrexate | 9 (8) |
| Donor-recipient sex match | |
| Male donor – male recipient | 44 (37) |
| Male donor – female recipient | 17 (14) |
| Female donor – male recipient | 26 (22) |
| Female donor – female recipient | 31 (26) |
| Donor-recipient HLA match | |
| Matched at HLA A, B, C, DRB1 | 36 (31) |
| Mismatched at one or two-loci | 82 (69) |
| Donor-recipient cytomegalovirus serostatus | |
| Donor(−) - recipient(−) | 43 (36) |
| Donor(−) - recipient(+) | 19 (16) |
| Donor(+) - recipient(−) | 30 (26) |
| Donor(+) - recipient(+) | 23 (19) |
| Unknown | 3 (3) |
| Year of transplant | |
| 1990–1994 | 22 (19) |
| 1995–1999 | 63 (53) |
| 2000–2005 | 33 (28) |
| Median (range) follow-up of survivors, months | 92 (3 – 195) |
RC at diagnosis and progressed to RAEB prior to transplant: n=6; RAEB at diagnosis and progressed to RAEB-t prior to transplant: n=7
Neutrophil and Platelet Recovery
Most patients achieved hematopoietic recovery. The day-28 cumulative incidence of neutrophil recovery was 89% (95% CI 82 – 94) and the 6-month cumulative incidence of platelet recovery was 74% (95% CI 65 – 81).
Graft-versus-host Disease
Fifty-five patients developed grade 2–4 acute GVHD (n=20 grade 2, n=24 grade 3 and n=11 grade 4). Grade 2–4 acute GVHD occurred more frequently after non T-cell depleted BMT (RR 2.33, 95% CI, 1.20 – 4.52, p=0.01). The day-100 probabilities of grade 2–4 acute GVHD were 55% (95% CI 44 – 66) and 26% (95% CI 14 – 41) after non T-depleted BMT and T-cell depleted BMT, respectively. Forty-five children developed either limited (n=13) or extensive (n=32) chronic GVHD. The 8-year cumulative incidence of chronic GVHD was 40% (95% CI 31 – 50). Chronic GVHD rates were higher after conditioning that included TBI (RR 2.97, 95% CI 1.58 – 5.58, p<0.001), in patients aged >10 years (RR 3.01, 95% CI 1.49 – 6.04, p=0.002), and transplantation period 1990 – 1994 (RR 3.14, 95% CI 1.44 – 6.89, p=0.004).
Transplant-related Mortality
Forty-eight deaths occurred from transplant-related complications that occurred mostly within the first year after transplantation. In multivariate analysis, the only factor associated with TRM was donor-recipient HLA mismatch. Mismatched transplant recipients were more likely to experience TRM, RR 3.29, 95% CI 1.54 – 7.03, p=0.002. TBI containing conditioning regimens were not associated with excess TRM (RR 0.68, 95% CI 0.34 – 1.39, p=0.29). Secondary MDS was also not associated with excess TRM (RR 0.63, 95% CI 0.31 – 1.30, p=0.21). The relatively small numbers of patients in the current analysis with secondary MDS prevented us from examining for an effect by etiology of secondary MDS. It is likely that in a larger cohort, TRM rates may differ in patients with MDS secondary to a malignant disease and those who develop MDS secondary to aplastic anemia. The 1- and 8-year probabilities of TRM after HLA matched transplants were 13% and 26%, respectively. Corresponding probabilities after HLA mismatched transplants were 42% and 52%, respectively.
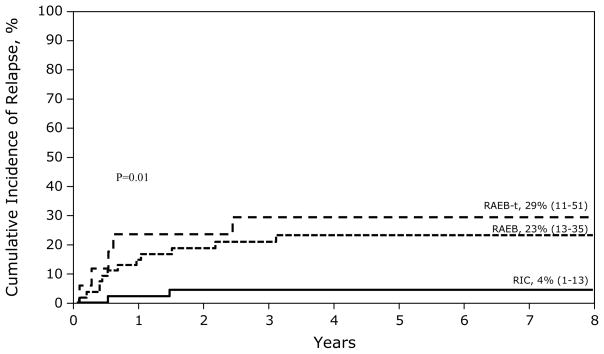
Recurrent Disease
Recurrence was defined as morphologic evidence of RAEB, RAEB-t or acute myeloid leukemia after BMT. Twenty patients had recurrent disease. Recurrent disease was more likely when patients were in RAEB (RR 6.50, 95% CI 1.45 – 29.03, p=0.01) or RAEB-t (RR 11.00, 95% CI 2.13 – 56.78, p=0.004). The 8-year probability of recurrent disease was 4% when BMT occurred for RC compared to 23% and 29% when BMT occurred for RAEB and RAEB-t, respectively (Figure 1). Risk of disease recurrence was similar in patients with primary and secondary MDS (RR 1.14, 95% CI 0.34 – 3.80, p=0.84). Treatment prior to BMT for patients with RAEB/RAEB-t was not associated with higher relapse (RR 1.85, 95% CI 0.70 – 4.88, p=0.21). However, there were only 27 patients who received prior treatment and most received low dose chemotherapy. The development of acute (RR 0.42, 95% CI 0.14 – 1.30, p=0.13) and chronic GVHD (RR 0.71, 95% CI 0.24 – 2.08, p=0.53) was not associated with disease recurrence post-transplant. Though the risk of relapse with TBI-containing conditioning regimen appears to be lower this did not reach statistical significance in this analysis (RR 0.53, 95% CI 0.18 – 1.62, p=0.27).
Figure 1.
The 8-year probabilities of recurrent disease after BMT: 4% when transplantation was performed for RC, 23% when transplantation was performed for RAEB and 29% for when transplantation was performed for RAEB-T.
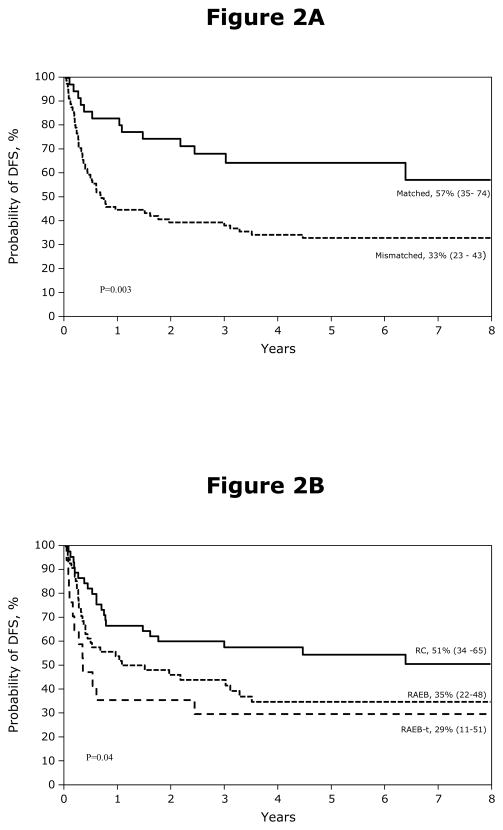
Disease-free Survival
The probability of DFS is shown in Figures 2A and 2B. Treatment failure (recurrent disease or death from any cause; inverse of DFS) occurred in 68 patients. Recipients of mismatched transplants (RR 2.79, 95% CI 1.51 – 5.14, p=0.001) and those with RAEB-t at transplantation (RR 2.38, 95% CI 1.17 – 4.84, p=0.02) were more likely to experience treatment failure. Secondary MDS was not associated with higher treatment failure (RR 0.67, 95% CI 0.36 – 1.26, p=0.22). Chemotherapy prior to transplantation was also not associated with treatment failure (RR 0.87, 95% CI 0.49 – 1.53, p=0.63). The causes of death are shown in Table 2. Early death (within 100 days after BMT) occurred in 25 patients and death beyond this period occurred in 41 patients. GVHD and veno-occlusive disease accounted for most early deaths. Beyond the early period, recurrent disease and GVHD were the most common causes. Most GVHD-related deaths occurred after mismatched BMT.
Figure 2.
Figure 2A. The 8-year probabilities of disease-free survival after BMT: 57% for patients received matched BMT (matched at HLA A, B, C, DRB1), 33% for patients received mismatched BMT.
Figure 2B. The 8-year probabilities of disease-free survival after BMT: 51% when transplantation was performed for RC, 35% when transplantation was performed for RAEB and 29% for when transplantation was performed for RAEB-T.
Table 2.
Causes of death
| Cause of death | Died within 100 days after transplantation (N=25) | Died after 100 days post transplantation (N=41) |
|---|---|---|
| Primary disease | 1 | 16 |
| Graft failure | 1 | 0 |
| Infection | 1 | 4 |
| Interstitial pneumonia | 2 | 1 |
| Adult respiratory distress syndrome | 0 | 2 |
| Veno-occlusive disease | 4 | 0 |
| EBV-PTLD | 1 | 0 |
| Hemorrhage | 2 | 1 |
| Graft-versus-host disease | 8 | 13 |
| Organ Failures | 4 | 2 |
| Other, not specified | 1 | 2 |
DISCUSSION
Sibling donor BMT has been used for many years as a primary approach to treatment of MDS, with survival rates of approximately 70%. As most children with MDS will not have a matched family donor, there is increased interest in investigating whether unrelated donor transplantation is a suitable alternative especially for children with RC. In this report we analyzed a large pediatric cohort with a median follow-up of 8 years after transplantation to determine outcomes of unrelated donor BMT facilitated by the National Marrow Donor Program in the United States, and to identify risk factors that predict for a successful outcome.
Disease status at transplantation and donor-recipient matching are important predictors for a successful outcome. Recurrent disease was more likely when disease status had advanced beyond RC. Other factors such as pre-transplant chemotherapy, secondary MDS and cytogenetic abnormality were not associated with disease recurrence TRM or disease-free survival. The relatively few patients (n=18) with complex cytogenetic abnormalities in the current analysis may have prevented us from detecting an effect of this generally considered high-risk feature on disease recurrence and disease-free survival. Similarly the relatively small numbers of patients in the current analysis may have prevented us from detecting an effect of pre-transplant chemotherapy or secondary MDS on TRM and disease-free survival.
Transplant-related mortality was higher after mismatched BMT and the effect independent of transplantation period. The transplants reported herein span over a decade and donor selection practices have evolved, the most important being the definition of an appropriately matched donor as one who is matched at HLA-A, -B, -C, -DRB1 with allele-level HLA typing (13). Most donor-recipient pairs in this analysis were mismatched with only about a third of recipients who received BM grafts from donors matched at HLA-A, -B, -C, -DRB1. Consequently, rates of acute and chronic GVHD were high and likely contributed to morbidity and subsequent mortality. Though donor-recipient HLA disparity was not associated with higher risks of acute GVHD in this analysis, larger series have shown this to be true (13). Year of transplantation, a surrogate for donor-selection practices was associated with higher chronic GVHD. We observed an effect of transplant-conditioning regimen on risks of chronic GVHD. In the current analysis TBI-containing regimens increased the risk of chronic GVHD whereas reports in adults with MDS suggest GVHD rates were comparable after TBI and non-TBI containing conditioning regimens (14, 15). While we don’t have a satisfactory explanation for this observation, TBI-containing regimens were not associated with higher risks of TRM and was suggestive for lower relapse risks though this did not reach statistical significance. Though acute GVHD was lower in recipients of T-cell depleted transplants, TRM and DFS rates were not different in recipients of T-cell depleted and non T-cell depleted transplants.
Recent advances in transplantation strategies such as use of reduced intensity conditioning regimens and cord blood grafts have been employed for children with MDS and it remains to be seen whether the observed success after myeloablative regimens and unrelated donor BMT are comparable to that after reduced intensity conditioning or cord blood transplantation. Strahm and colleagues from the EWOG-MDS recently reported 3-year event-free survival of 74% among 19 children with RC who received a uniform reduced intensity conditioning regimen and unrelated donor HCT (16). Though 3 of 19 patients experienced graft failure, 2 were rescued with CD34+ cell boosts. These results are encouraging but longer follow up of surviving patients are needed as well as validation in a larger cohort prior to widespread adoption of reduced intensity conditioning regimens for MDS in children. Umbilical cord blood grafts has also been used for MDS in children (17, 18). In the largest series describing 42 children with MDS (n=21 RC and n=21 RAEB/RAEB-t), the 2-year probabilities of TRM and DFS were 56% and 32%, respectively after umbilical cord blood transplantation 18. The current analysis suggest TRM is lower and DFS, higher after matched BMT though only a direct comparison of outcomes after transplantation of unrelated BM and umbilical cord blood will aid clinicians to select the optimal graft type in the absence of a HLA-matched sibling. Similarly, only a direct comparison of the intensity of conditioning regimens used can satisfactorily address the question of the optimal conditioning regimen. Given the rarity of this disease in children it may take several more years before sufficient numbers of patients are available for comparison of transplant conditioning regimens and/or unrelated donor graft sources.
Our data support best results after unrelated donor BMT when patients are transplanted in RC and when the donor and recipient are matched at HLA-A, -B, -C and DRB1. If a matched unrelated donor is available, waiting for disease progression before proceeding to transplantation will only increases the risk of recurrent disease. Therefore, unrelated donor BMT when a matched donor is available should be offered to children with MDS using the criteria currently used for advocating transplantation for children with a matched sibling donor. In the absence of a matched unrelated donor, transplantation of unrelated umbilical cord blood transplantation is a reasonable alternative if offered at centers experienced in unrelated cord blood transplantation.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; Canadian Blood and Marrow Transplant Group; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Eisai, Inc.; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gamida Cell, Ltd.; GE Healthcare; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Saladax Biomedical, Inc.; Schering Corporation; Society for Healthcare Epidemiology of America; Soligenix, Inc.; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; THERAKOS, Inc.; Thermogenesis Corporation; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hasle H, Kerndrup G, Jacobsen BB. Childhood myelodysplastic syndrome in Denmark: incidence and predisposing conditions. Leukemia. 1995;9:1569–1572. [PubMed] [Google Scholar]
- 2.Locatelli F, Pession A, Bonetti F, et al. Busulfan, cyclophosphamide and melphalan as conditioning regimen for bone marrow transplantation in children with myelodysplastic syndromes. Leukemia. 1994;8:844–849. [PubMed] [Google Scholar]
- 3.Woods WG, Barnard DR, Alonzo TA, et al. Prospective study of 90 children requiring treatment for juvenile myelomonocytic leukemia or myelodysplastic syndrome: a report from the Children’s Cancer Group. J Clin Oncol. 2002;20:434–440. doi: 10.1200/JCO.2002.20.2.434. [DOI] [PubMed] [Google Scholar]
- 4.Davies SM, Wagner JE, Defor T, et al. Unrelated donor bone marrow transplantation for children and adolescents with aplastic anaemia or myelodysplasia. Br J Haematol. 1997;96:749–756. doi: 10.1046/j.1365-2141.1997.d01-2087.x. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf U, Frangoul HA, Gooley TA, et al. Allogeneic bone marrow transplantation in children with myelodysplastic syndrome or juvenile myelomonocytic leukemia: the Seattle experience. Bone Marrow Transplant. 2004;33:805–814. doi: 10.1038/sj.bmt.1704438. [DOI] [PubMed] [Google Scholar]
- 6.Deeg HJ, Storer B, Slattery JT, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100:1201–1207. doi: 10.1182/blood-2002-02-0527. [DOI] [PubMed] [Google Scholar]
- 7.Hasle H, Niemeyer CM, Chessells JM, et al. A pediatric approach to the WHO classification of myelodysplastic and myeloproliferative diseases. Leukemia. 2003;17:277–282. doi: 10.1038/sj.leu.2402765. [DOI] [PubMed] [Google Scholar]
- 8.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 9.Atkinson K, Horowitz MM, Gale RP, et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood. 1990;75:2459–2464. [PubMed] [Google Scholar]
- 10.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Klein J, Moeschberger M. Survival Analysis: Techniques of censored and truncated data. New York, N.Y: Springer-Verlag; 2003. [Google Scholar]
- 12.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–202. [Google Scholar]
- 13.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 14.Kroger N, Zabelina T, Kruger W, et al. Comparison of total body irradiation vs busulfan in combination with cyclophosphamide as conditioning for unrelated stem cell transplantation in CML patients. Bone Marrow Transplant. 2001;27:349–354. doi: 10.1038/sj.bmt.1702802. [DOI] [PubMed] [Google Scholar]
- 15.Ringden O, Remberger M, Ruutu T, et al. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation: long-term results of a randomized trial in allogeneic marrow recipients with leukemia. Nordic Bone Marrow Transplantation Group. Blood. 1999;93:2196–2201. [PubMed] [Google Scholar]
- 16.Strahm B, Locatelli F, Bader P, et al. Reduced intensity conditioning in unrelated donor transplantation for refractory cytopenia in childhood. Bone Marrow Transplant. 2007;40:329–333. doi: 10.1038/sj.bmt.1705730. [DOI] [PubMed] [Google Scholar]
- 17.Parikh SH, Mendizabal A, Martin PL, et al. Unrelated donor umbilical cord blood transplantation in pediatric myelodysplastic syndrome: a single-center experience. Biol Blood Marrow Transplant. 2009;15:948–955. doi: 10.1016/j.bbmt.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 18.MorenoMadureira AB, Teira P, Locatelli F, et al. Risk Factors Analyses for Outcomes after Unrelated Cord Blood Transplantation for Children with Myelodysplastic Syndromes. An Analysis on Behalf of EWOG-MDS and Eurocord Groups. Blood. 2007;118:602a. [Google Scholar]