Abstract
Images of brain metabolism and measurements of activities of components of the electron transport chain support earlier studies that suggest that brain glucose oxidation is inherently abnormal in a significant proportion of persons with schizophrenia. Therefore, we measured activities of enzymes of the tricarboxylic (TCA) cycle in dorsolateral-prefrontal-cortex from schizophrenia patients (N=13) and non-psychiatric disease controls (N=13): the pyruvate dehydrogenase complex (PDHC), citrate synthase (CS), aconitase, isocitrate dehydrogenase (ICDH), the alpha-ketoglutarate dehydrogenase complex (KGDHC), succinate thiokinase (STH), succinate dehydrogenase (SDH), fumarase and malate dehydrogenase (MDH). Activities of aconitase (18.4%, p<0.05), KGDHC (26%) and STH (28.2%, p<0.05), enzymes in the first half of the TCA cycle, were lower, but SDH (18.3%, p<0.05) and MDH (34%, p<0.005), enzymes in the second half, were higher than controls. PDHC, CS, ICDH and fumarase activities were unchanged. There were no significant correlations between enzymes of TCA cycle and cognitive function, age or choline acetyl transferase activity, except for aconitase activity which decreased slightly with age (r=0.55, p=003). The increased activities of dehydrogenases in the second half of the TCA cycle may reflect a compensatory response to reduced activities of enzymes in the first half. Such alterations in the components of TCA cycle are adequate to alter the rate of brain metabolism. These results are consistent with the imaging studies of hypometabolism in schizophrenia. They suggest that deficiencies in mitochondrial enzymes can be associated with mental disease that takes the form of schizophrenia.
Keywords: Mitochondria, Tricarboxylic acid cycle, Energy metabolism, Postmortem interval, Schizophrenia
Introduction
Mitochondrial dysfunction and oxidative stress may underlie the pathophysiology of schizophrenia (Andreazza et al., 2010; Regenold et al., 2009; Prabakaran et al., 2004). Decreased frontal cortical glucose metabolism or blood flow is seen in schizophrenia patients while performing tasks that normally increase frontal metabolism, such as the Continuous Performance Test (Jacobsen et al., 1997) and the Wisconsin Card Sorting Test (Berman et al., 1992). Higher rates of impaired fasting glucose tolerance and insulin resistance have been seen in the schizophrenic patients (Kirkpatrick et al., 2009; Ryan et al., 2003). The prevalence of non-insulin-dependent diabetes mellitus (NIDDM) is significantly increased in schizophrenia patients. Treatment with glucose improves attention and memory in schizophrenia patients undergoing mental testing (Fucetola et al., 1999).
Brain from schizophrenia patients is under oxidative stress (Reddy and Yao, 1996), which typically accompanies mitochondrial dysfunction. Dihydrolipoyl dehydrogenase (DLD), the source of free radicals in KGDHC and PDHC has been found to be up-regulated in schizophrenia (Martins-de-Souza et al., 2009). Oxidative stress can lead to neurotransmitter abnormalities, DNA damage, protein inactivation, altered gene expression and apoptotic events. The close connection of oxidative mechanisms to neuronal plasticity may be relevant to disease pathogenesis (Ben-Shachar, 2002).
Gene expression and proteomics analysis in different brain regions of schizophrenia patients also suggest altered mitochondrial function (Andreazza et al., 2010; Clark et al., 2006; Hakak et al., 2001; Mirnics et al., 2000). Parallel transcriptomic, proteomic and metabolomic approaches were used to identify differences between controls and persons with schizophrenia. Almost half the altered proteins identified by proteomics were associated with mitochondrial function and oxidative stress responses. This was mirrored by transcriptional and metabolite perturbations (Prabakaran et al., 2004). A 20 % reduction in mitochondrial profiles in striatum from schizophrenia patients suggests a diminished capacity to respond to energy demand (Kung and Roberts, 1999). In addition, several components of the electron transport chain have been reported to be reduced in the brains of patients with schizophrenia (Rezin et al., 2009; Maurer et al., 2001).
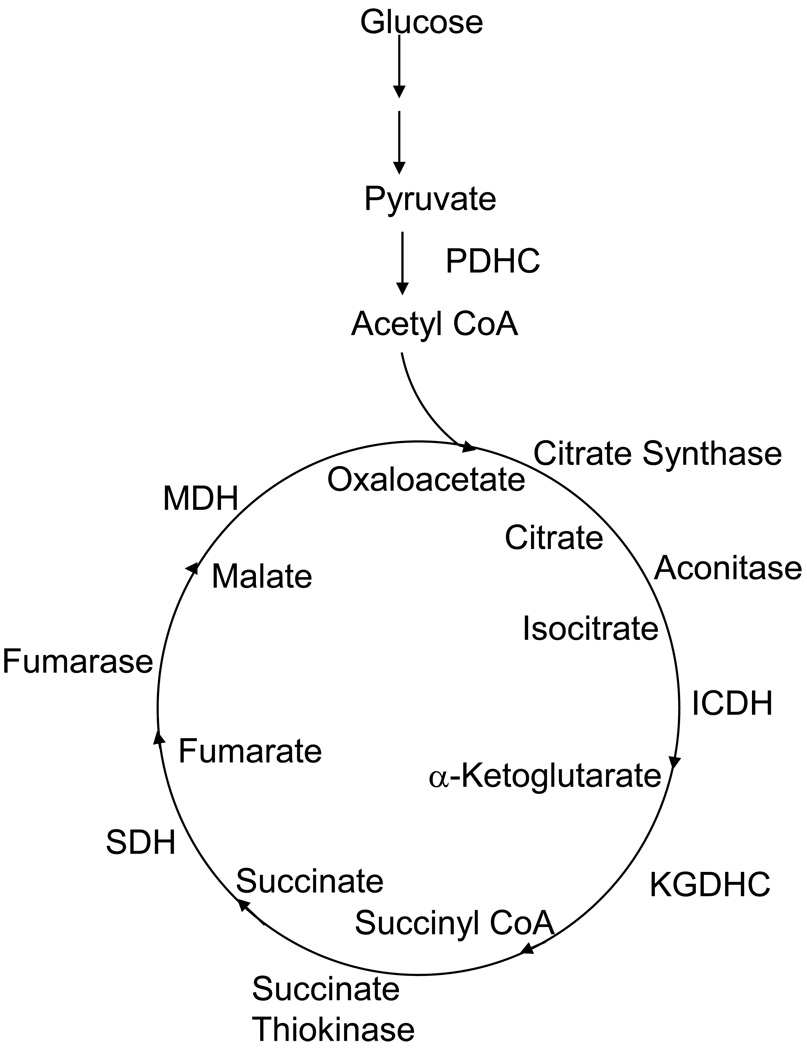
Alterations in the TCA cycle would profoundly alter the rate of brain metabolism and the production of free radicals. Pyruvate, the product of glycolysis, is decarboxylated to acetyl CoA by the pyruvate dehydrogenase complex (PDHC). The conversion of acetyl CoA to CO2 in the TCA cycle results in a large production of reducing equivalents (e.g. NADH) for the electron transport chain and subsequent production of ATP (Figure 1). The TCA cycle (Figure 1) is a supramolecular assembly (Lyubarev and Kurganov, 1989; Velot et al., 1997) of eight enzymes: citrate synthase (CS), aconitase, isocitric dehydrogenase (ICDH), the alpha-ketoglutarate dehydrogenase complex (KGDHC), succinate thiokinase (STK), succinate dehydrogenase (SDH), fumarase and malate dehydrogenase (MDH) (Lyubarev and Kurganov, 1989; Velot et al., 1997). Inactivation of any step can disrupt mitochondrial bioenergetics (Blass and Brown, 2000). Thus, we examined the activities of all mitochondrial enzymes of the TCA cycle in postmortem brain specimens of patients with the diagnosis of schizophrenia and appropriate controls. We also tested the possibility of correlation of age, cognitive function as measured by the Clinical Dementia Rating scale (CDR) and the densities of the hallmark neuropathologic feature of age-associated dementia, neuritic plaques, PDHC and each individual enzyme of TCA cycle. Measures of cognitive function and neuropathology were included because many elderly persons with schizophrenia also evidence significant cognitive impairments (Molina et al., 2009; Friedman et al., 1999). Their degree of association may hold the key for the oxidative damage and mitochondrial malfunctioning in schizophrenia.
Figure 1.
TCA Cycle
EXPERIMENTAL/MATERIALS AND METHODS
The diagnosis of schizophrenia was established by a team of research clinicians using previously published criteria and methodologies (Dracheva et al., 2001). The human brain samples were obtained from the brain bank at the Department of Psychiatry, Mount Sinai/Bronx Veterans Administration Medical Center. Normal controls had no history of any psychiatric or neurological disorders. Of the schizophrenia patients, nine had been off neuroleptic medications for at least six months prior to death. The CDR scores used in cases and controls were determined at a consensus conference after death. For each case antemortem CDR, MMSE and other measures of cognition were reviewed along with medical histories and reports and interviews of informants. The final CDR scores used here took into consideration the subject’s cognitive status during the past 6 months of life. All procedures including postmortem evaluations were approved by the Institutional review boards of Pilgrim Psychiatric Center, Mount Sinai School of Medicine and the Bronx VA Medical Center. The research was carried out in accordance with the Code of Ethics of the World Medical Association.
The mean age, postmortem interval (PMI) and gender of the patients and controls were comparable (Table 1). Although the schizophrenia patients and controls were in the geriatric age range, neuropathologic examinations ruled out specific pathologies such as Alzheimer disease and multi infarct dementia (Purohit et al., 1998). The density of neuritic plaques with amyloid cores was determined by counting the number of neuritic plaques in five high-power microscopic fields in five slides as described previously (Purohit et al., 1998). Plaque densities were determined by Bielschowsky, thioflavin-S and immunohistochemistry. The PMI of all subjects was less than 24 hours. Storage time at −80°C for the samples varied between 1–9 years, however, storage time between cases and controls did not differ significantly (p=0.38). All enzyme activity assays were performed blind to clinical information.
Table 1.
Characteristics of schizophrenic patients and controls.
| Group | Gender M: F |
Age (yrs) | Postmortem interval (hrs) |
CDR | Plaque1 | ChAT2 |
|---|---|---|---|---|---|---|
| Controls | 4:9 | 79.23 ± 3.0 | 9.16 ± 1.5 | 1.00 ± 0.45 | 1.32 ± 0.76 | 4.1 ± 0.6 |
| (66–98) | (4.75–18.5) | (0–5) | (0–7.8) | (3.4–4.7) | ||
| Schizophrenic | 9:4 | 72.15 ± 3.0 | 8.30 ± 1.4 | 2.2 ± 0.62 | 0.92 ± 0.46 | 6.0 ± 0.9 |
| Patients | (61–87) | (4.5–21.1) | (0.5–3) | (0–5.5) | (0.75–12.1) |
Values are Means ± SEM.
The number in parentheses indicate the range of values.
Mean density of neuritic plaques in 5 neocortical regions
nmol ACh/mg. protein/hour performed routinely in tissue samples from the parietal cortex (Haroutunian et al., 1994).
Gray matter (~2.0 gm wet weight) from dorsal lateral prefrontal cortex (DLPFC) (Brodmann area 46) was dissected from blocks of frozen brain (−80° C) from patients with schizophrenia (N=13) and from non-psychiatric disease controls (N=13). The tissues were pulverized in liquid nitrogen at −190°C into a fine powder, aliquoted (50 ± 5 mg) into individual Eppendorf tubes and stored at −80°C until use. Before running the assays, the brains were homogenised with a teflon-glass homogenizer in the following buffers:
50 mM Tris-HCI; 1 mM EDTA; 10% glycerol-pH 7.6 (for estimation of SDH, STH, MDH, Fumarase and CS).
50 mM Tris-HCI; 5 mM sodium citrate; 0.6 mM magnesium chloride-pH 7.4; 1 mM DTT; 0.2 mM EGTA; 0.08 % Triton X-100 and 50 µM leupeptin (for estimation of aconitase, ICDH and KGDHC)
50 mM sodium phosphate-pH 7.4; 1 mM DTT; 20% Triton X-100 and 50 µM leupeptin (for estimation of active and total PDHC).
One unit of enzyme activity was defined as the amount of enzyme catalyzing the production of 1 nmole of NADH or NADPH/min per mg protein.
Enzyme activities were measured by well standardized, published methods: PDHC (Ksiezak-Reding et al., 1982; McCormack and Denton, 1989); CS (Shepherd and Garland, 1969); aconitase (Morton et al., 1998); ICDH (Bai et al., 1999); KGDHC (Gibson et al., 1988); STH (Sungman, 1969); SDH (Veeger et al., 1969); fumarase (Hill and Bradshaw, 1969) and MDH (Kitto, 1969). Protein content was determined with a Bio-Rad 500-006 kit (Bio-Rad Laboratories, Hercules, CA, USA) based on Bradford dye binding procedure that utilizes the color change of Coomassie brilliant blue G-250 (Bradford, 1976).
Post-Mortem stability of enzymes in mice brains
NIH Swiss mice (Harlan Sprague Dawley Indianapolis, IN) were used. Upon arrival, the animals were housed in cages and were maintained under constant temperature (70°F), humidity (50%) and 12-h light-dark cycle. The animals were fed a pelleted diet (ICN Nutritional Biochemicals; Cleveland, Ohio) and provided distilled water.
Twenty four mice were divided into four equal groups. All the mice in four groups were killed by cervical dislocation and kept at 4 degrees for 0 minute (group 1), 3 (group 2), 6 (group 3) and 12 hours (group 4). Brain tissues from two mice were pulverized in liquid nitrogen into a fine powder, pooled together and aliquoted into individual Eppendorf tubes and stored at −80°C. The animal procedures were approved by the Institutional Animal Care and Use Committee of Weill Medical College of Cornell University. Estimations of enzymes of TCA cycle were carried out similarly.
Data analysis and statistics
All of the measurements of individual samples were carried out in triplicate. Comparisons of persons with schizophrenia and controls (subject characteristics and change in the TCA cycle enzyme activities) were done by Student t-test (two-way). One way analyses of variance (ANOVA) and post hoc Bonferroni test was carried out to evaluate the effect of gender differences on the level of enzyme activities. Comparisons between multiple groups (Postmortem studies) were also carried out by analysis of variance (ANOVA). Pearson correlation coefficient was used for correlation studies.
RESULTS
Characteristics of patients and controls
Age and postmortem intervals of schizophrenia patients and control subjects did not differ significantly (p>0.1) (Table 1). The age of subjects varied from 61–98 years. The mean post mortem interval (PMI) also ranged from 8–10 hours for the two groups. The small differences in CDR and choline acetyl transferase activity values between schizophrenia patients and controls were not significant (p>0.1).
TCA cycle enzyme activities in brains from humans and mice
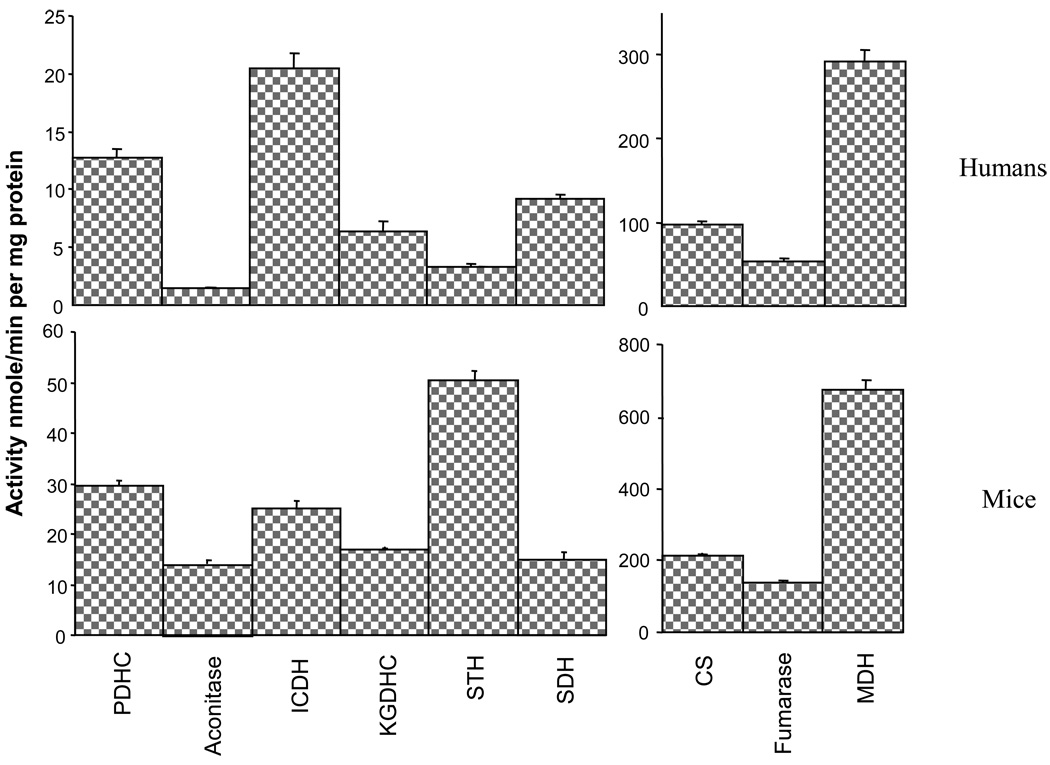
The activities of enzymes in TCA cycle varied considerably in human brain (Figure 2). MDH had the highest activity. PDHC, citrate synthase and fumarase activities were intermediate. Aconitase, STH, SDH and KGDHC activities were lowest. TCA cycle enzyme activities in mouse brains showed a similar pattern, although the activities of most of the enzymes in the brains of the smaller animals (CS, KGDHC, SDH, MDH, fumarase and PDHC) were 2–3 times higher than in human brains. Aconitase and STH activities were nine and fifteen times higher in brains from mice than humans. The activities of ICDH were similar in both species.
Figure 2.
Comparison of TCA cycle enzymes in the brains of humans and mice
Values are Means ± SEM
N1=13, N2=6
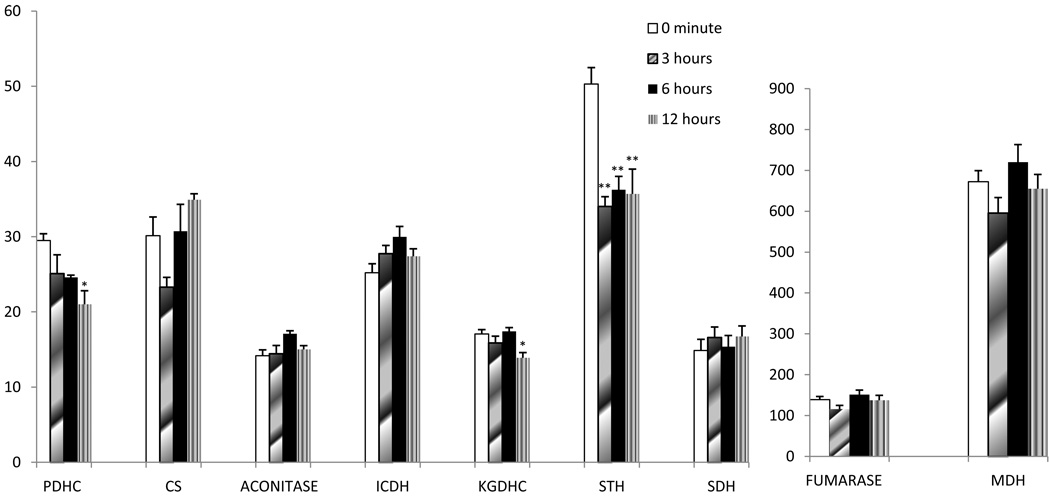
Post-mortem stability
Post-mortem stability studies of the enzymes in TCA cycle of mice brains at different post-mortem time intervals showed that most of these enzymes were stable (Figure 3). Mean activities (mU/mg protein, mean ± SEM) of enzymes at different time intervals in the brain of mice did not show significant changes except PDHC, KGDHC and STH. PDHC and KGDHC activity in mice brains decreased about 28 % and 19 % respectively after 12 hours. STH enzyme activity also decreased 30 % in mice brains after PMI interval of 3–12 hours.
Figure 3.
Post-Mortem stability of TCA cycle enzyme activities in mice brain.
Values (Means ± SEM) are specific activities (nmole/min per mg protein).
1CS activity was estimated by coupled assay.
*p<0.05
**p<0.005
NIH Swiss mice (Harlan Sprague Dawley Indianpolis, IN) were used. Twenty-four mice were divided into four equal groups. All the mice in four groups were killed by cervical dislocation and kept at 4 degrees for 0 minute, 3 hours, 6 hours and 12 hours.
Activities of TCA cycle enzymes in the brain of persons with schizophrenia
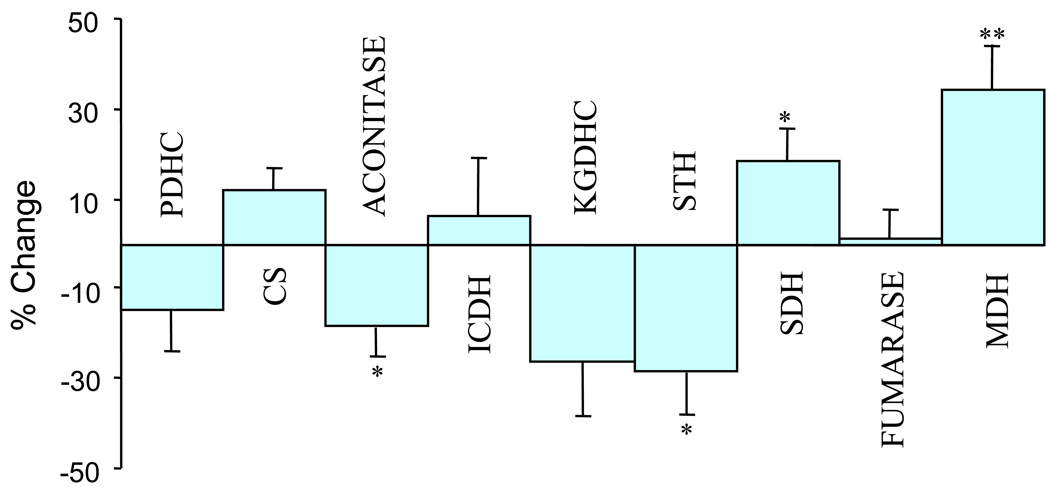
The activities of several enzymes of the TCA cycle differed significantly in schizophrenic’s brains in comparison to controls. Aconitase activity was significantly lower (18.4%, p<0.05) in schizophrenia patients than in controls as was that of KGDHC, by 26% (Figure 4) and STH, by 28.2 %, (p<0.05). SDH activities were higher in schizophrenia patients (18.3%, p<0.05) as was MDH (34%, p<0.005) (Figure 3). The activities of total PDHC, CS, ICDH, and fumarase enzyme were the same in both groups (Figure 3). One way analyses of variance (ANOVA) and post hoc Bonferroni test tested the effect of gender on the level of enzyme activities. The analysis revealed significant difference (p<0.004) in the level of MDH only in the females. However rest of the enzymes did not show any such difference. Covariates such as CDR and PMI did not show any correlation with enzyme activity (Table-3). Variable such as age showed a significant correlation only with aconitase enzyme levels.
Figure 4.
Changes in the activities of TCA cycle enzymes in brains of schizophrenia patients in comparison to controls
*p<0.05,
**p<0.005,
% Change = (Control Mean-Values)/Control Mean *100
N1=N2=13
TABLE 3.
Correlation of TCA cycle enzymes with CDR, age, mean plaques and choline acetyl transferase activity
| PMI | CDR | AGE | Mean plaque | ChAT | |
|---|---|---|---|---|---|
| PDHC | 0.193 | −0.249 | 0.025 | 0.070 | 0.082 |
| 0.344 | 0.220 | 0.902 | 0.732 | 0.780 | |
| CS | 0.153 | 0.196 | 0.001 | 0.111 | −0.006 |
| 0.454 | 0.337 | 0.994 | 0.587 | 0.984 | |
| Aconitase | 0.046 | 0.019 | 0.552 | 0.153 | 0.188 |
| 0.824 | 0.927 | 0.003 | 0.454 | 0.518 | |
| ICDH | −0.034 | 0.107 | −0.052 | −0.003 | 0.372 |
| 0.869 | 0.602 | 0.801 | 0.988 | 0.190 | |
| KGDHC | −0.030 | −0.2172 | 0.163 | 0.188 | −0.153 |
| 0.888 | 0.2865 | 0.424 | 0.357 | 0.602 | |
| STH | 0.184 | 0.1025 | 0.311 | −0.071 | −0.097 |
| 0.368 | 0.618 | 0.121 | 0.731 | 0.740 | |
| SDH | 0.313 | 0.0287 | −0.276 | −0.108 | −0.123 |
| 0.119 | 0.889 | 0.172 | 0.597 | 0.674 | |
| Fumarase | 0.081 | −0.331 | 0.334 | 0.102 | 0.489 |
| 0.694 | 0.098 | 0.095 | 0.618 | 0.076 | |
| MDH | −0.116 | 0.024 | 0.113 | 0.103 | −0.235 |
| 0.572 | 0.907 | 0.581 | 0.614 | 0.418 | |
The upper value is correlation coefficient, the lower value is significance.
N = 26
Correlation of TCA cycle enzymes with CDR, Age and Mean Plaques
There was no significant correlation between cognitive dementia rating (CDR) score, mean plaque score, choline acetyl transferase activity or PMI and activities of the enzymes of the TCA cycle. Aconitase activity fell slightly but significantly (r = 0.55, P =0.003) with age (Table-2).
TABLE 2.
Correlation of TCA cycle enzymes with CDR, age, mean plaques and choline acetyl transferase activity
| PMI | CDR | AGE | Mean plaque | ChAT | |
|---|---|---|---|---|---|
| PDHC | 0.193 | −0.249 | 0.025 | 0.070 | 0.082 |
| 0.344 | 0.220 | 0.902 | 0.732 | 0.780 | |
| CS | 0.153 | 0.196 | 0.001 | 0.111 | −0.006 |
| 0.454 | 0.337 | 0.994 | 0.587 | 0.984 | |
| Aconitase | 0.046 | 0.019 | 0.552 | 0.153 | 0.188 |
| 0.824 | 0.927 | 0.003 | 0.454 | 0.518 | |
| ICDH | −0.034 | 0.107 | −0.052 | −0.003 | 0.372 |
| 0.869 | 0.602 | 0.801 | 0.988 | 0.190 | |
| KGDHC | −0.030 | −0.2172 | 0.163 | 0.188 | −0.153 |
| 0.888 | 0.2865 | 0.424 | 0.357 | 0.602 | |
| STH | 0.184 | 0.1025 | 0.311 | −0.071 | −0.097 |
| 0.368 | 0.618 | 0.121 | 0.731 | 0.740 | |
| SDH | 0.313 | 0.0287 | −0.276 | −0.108 | −0.123 |
| 0.119 | 0.889 | 0.172 | 0.597 | 0.674 | |
| Fumarase | 0.081 | −0.331 | 0.334 | 0.102 | 0.489 |
| 0.694 | 0.098 | 0.095 | 0.618 | 0.076 | |
| MDH | −0.116 | 0.024 | 0.113 | 0.103 | −0.235 |
| 0.572 | 0.907 | 0.581 | 0.614 | 0.418 | |
The upper value is correlation coefficient, the lower value is significance.
N = 26
DISCUSSION
Our findings of altered enzyme activities of the enzymes of TCA cycle support the possibility that abnormalities in energy metabolism contribute to schizophrenia. Proteomic, metabolomic and transcriptional analysis all suggest major alterations in mitochondrial function and oxidative stress in brain of persons with schizophrenia (Prabakaran et al., 2004). The results of the present study in DLPFC agree with their findings and extend our previous reported findings (Bubber et al., 2004). Both studies reveal a non-significant decline in the activity of KGDHC. Gluck et al., (2002) also reported no change in KGDHC activity in DLPFC region of brain in comparison of schizophrenia patients and normal controls as whole groups or when they are matched for age. The current studies did demonstrate significant decreases in aconitase and STH (Bubber et al., 2004).
Even relatively small reductions in the activities of these enzymes can alter brain function. For example, even mild impairment of metabolism increases the release of dopamine and decreases the release of acetylcholine (Gibson et al., 1989, 1991; Blass et al., 2002). KGDHC can be rate controlling step of the TCA cycle. Any reduction in the activity of the TCA cycle reduces the synthesis of acetylcholine from glucose even though the acetyl group for acetylcholine synthesis is only a minor part of overall glucose metabolism (c.f. Joseph and Gibson, 2007). Reduction of KGDHC by genetic manipulation shows that even small reductions in KGDHC activities increase the GABA pathway (Santos et al., 2006; Shi et al., 2009). Thus, both in vivo and in vitro data indicate that any reduction in these enzymes will affect the brain.
There is a strong possibility that lower levels of aconitase and STH dysregulate mitochondrial metabolism in persons with schizophrenia. These enzymes participate in energy production, neurotransmitter metabolism and metabolic interaction between mitochondria and cytoplasm. Aconitase catalyses the reversible interconversion of citrate and isocitrate via the enzyme bound intermediate cis-aconitate. It belongs to the family of iron-sulfur containing dehydratases (Rose and O Connell, 1967). Inactivation of aconitase blocks NADH production (Tretter and Adam-Vizi, 2000). Aconitase is commonly used as a biomarker for oxidative stress and has been suggested to serve as an intramitochondrial sensor of redox status. The lack of correlation of the activity of aconitase with age in the current study may be attributable to the relatively advanced age of the study cohort as a whole.
Schizophrenia is accompanied by significantly lower STH activity in brain. STH catalyses the cleavage of thioester bond of succinyl COA forming succinate and CoASH. There is increased likelihood that the lower levels of KGDHC will generate lower levels of succinyl-COA and even further lower levels of STH may further lower succinate oxidation in the TCA cycle. The cellular reaction catalysed by STH enzyme is key for generation of ribonucleoside triphosphate in the TCA cycle. Lower levels of STH would diminish the metabolic energy and the ability to respond to energy demand in brains of patients with schizophrenia.
The increased activity of the TCA cycle enzymes SDH and MDH in schizophrenia contrasts with the decrease in activities of aconitase and STH. The redox coenzyme for the reaction catalyzed by SDH is FAD, rather than NAD+. FAD is a more powerful oxidizing agent than NAD+. Rapid oxidation of succinate and its non-enzymatic formation from α-ketoglutarate via transamination (Fedotcheva et al., 2006) may shunt the TCA cycle upon inactivation of KGDH under oxidative stress, which is inherent in many diseases such as schizophrenia and aging. SDH is also a component of mitochondrial electron transport chain. Its higher activities may increase electron leakage in the electron transport chain which is an important source of superoxide radicals (Bonilla et al., 1999). The high levels of MDH and SDH may be an adaptive change to deficiencies of STH and aconitase. An imbalance of dehydrogenases in the TCA cycle may favor the generation of free radicals and ROS over antioxidant defenses, leading to oxidative stress.
Since schizophrenia is a life-long disease in the chronically-ill cohort studied here, the number of defective mitochondria may accumulate with age and this could lead abnormal brain metabolism which could initiate slow degenerative processes in neurons as seen in AD (Prasad et al, 2002). Most of the TCA cycle enzymes did not show any association with age except aconitase. It showed a significant correlation with age and its activity was lower in schizophrenia patients in comparison to controls.
Future studies are warranted to determine the several links in the disease mechanism to distinguish primary from secondary disease phenomenon. Alteration in the activities of key TCA cycle enzymes seems to be the central component of schizophrenia and is unlikely to be due to the effects of medication (Stone et al., 2004). Changes in oxidative stress and mitochondrial function with schizophrenia appear to be linked to the pathology of schizophrenia (Prabakaran et al., 2004). These observations are further supported by the ability to separate schizophrenia patients from controls based on a set of genes encoding mitochondrial complexes and redox sensing proteins. Our results are significant in identifying the key altered enzymes in metabolic assembly of the TCA cycle. The changes in the enzymes may lead to an imbalance in the TCA cycle, and the resulting mitochondrial dysfunction and insufficiency could lead to predisposition to and precipitation of schizophrenia. These findings will enhance the understanding of the pathology of disease and suggest new therapeutic strategies that address the mitochondrial deficit in schizophrenia.
Acknowledgments
Role of Funding Source. This work was supported by grants MH064673 & MH066392, AG14600, AG11921, AG14930, AG11921 and Burke Medical Research Institute. The funding sources were not involved in study design, collection analysis or interpretation of the data, the writing of the report nor submission to Schizophrenia Research.
Abbreviations
- AABS
Amino Azo Benzoic acid
- AD
Alzheimer’s disease
- BSA
Bovine Serum Albumin
- CS
Citrate Synthase (EC 4.1.3.7)
- DTT
Dithiothreitol
- EGTA
Ethylene Glycol Tetra Acetic acid
- EDTA
Ethylene Diamine Tetra Acetic acid
- GTP
Guanosine Triphosphate
- KGDHC
α-Ketoglutaric Acid Dehydrogenase Complex (KGDHC; EC 1.2.4.2, EC 2.3.1.61, EC 1.6.4.3)
- ICDH
Isocitric Acid Dehydrogenase (EC 1.1.1.41)
- MDH
Malate Dehydrogenase (EC 1.1.1.37)
- PDHC
Pyruvate Dehydrogenase Complex (EC 1.2.4.1, EC 2.3.1.12, EC 1.6.4.3).
- ROS
Reactive Oxygen Species
- STH
Succinate Thiokinase (EC 6.2.1.4)
- SDH
Succinate Dehydrogenase (EC 1.3.99.1)
- TCA
Tricarboxylic Acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch. Gen. Psychiatry. 2010;67:360–368. doi: 10.1001/archgenpsychiatry.2010.22. [DOI] [PubMed] [Google Scholar]
- Bai C, Fernandez E, Yang H, Chen R. Purification and stabilization of a monomeric isocitrate dehydrogenase from Corynebacterium glutamicum. Protein. Expr. Purif. 1999;15(3):344–348. doi: 10.1006/prep.1999.1034. [DOI] [PubMed] [Google Scholar]
- Berman KF, Torrey EF, Daniel DG, Weinberger DR. Regional cerebral blood flow in monozygotic twins discordant and concordant for schizophrenia. Arch. Gen. Psychiatry. 1992;49(12):927–934. doi: 10.1001/archpsyc.1992.01820120015004. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D. Mitochondrial dysfunction in schizophrenia: a possible linkage to dopamine. J. Neurochem. 2002;83(6):1241–1251. doi: 10.1046/j.1471-4159.2002.01263.x. [DOI] [PubMed] [Google Scholar]
- Blass JP, Brown AM. Lower activity of Krebs cycle enzymes than of electron transport in human brain: disease implications. Neurobiol. Aging. 2000;21 Suppl:81. [Google Scholar]
- Blass JP, Gibson GE, Hoyer S. The role of the metabolic lesion in Alzheimer's disease. Journal of Alzheimer's Disease. 2002;4(3):225–232. doi: 10.3233/jad-2002-4312. [DOI] [PubMed] [Google Scholar]
- Bonilla E, Tanji K, Hirano M, Vu TH, DiMauro S, Schon EA. Mitochondrial involvement in Alzheimer's disease. Biochim. Biophys. Acta. 1999;1410(2):171–182. doi: 10.1016/s0005-2728(98)00165-0. [DOI] [PubMed] [Google Scholar]
- Bowling AC, Beal MF. Bioenergetic and oxidative stress in neurodegenerative diseases. Life Sci. 1995;56(14):1151–1171. doi: 10.1016/0024-3205(95)00055-b. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bubber P, Tang J, Haroutunian V, Xu H, Davis KL, Blass JP, Gibson GE. Mitochondrial enzymes in schizophrenia. J. Mol. Neurosci. 2004;24(2):315–321. doi: 10.1385/JMN:24:2:315. [DOI] [PubMed] [Google Scholar]
- Clark D, Dedova I, Cordwell S, Matsumoto I. A proteome analysis of the anterior cingulate cortex gray matter in schizophrenia. Mol. Psychiatry. 2006;11(5):459–470. doi: 10.1038/sj.mp.4001806. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Marras SA, Elhakem SL, Kramer FR, Davis KL, Haroutunian V. N-methyl-D-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am. J. Psychiatry. 2001;158(12):2107. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- Fedotcheva NI, Sokolov AP, Kondrashova MN. Nonezymatic formation of succinate in mitochondria under oxidative stress. Free. Radic. Biol. Med. 2006;41(1):56–64. doi: 10.1016/j.freeradbiomed.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Harvey PD, Kemether E, Byne W, Davis KL. Cognitive and functional changes with aging in schizophrenia. Biol. Psychiatry. 1999;46(7):921–928. doi: 10.1016/s0006-3223(99)00080-3. [DOI] [PubMed] [Google Scholar]
- Fucetola R, Newcomer JW, Craft S, Melson AK. Age- and dose-dependent glucose-induced increases in memory and attention in schizophrenia. Psychiatry Res. 1999;88(1):1–13. doi: 10.1016/s0165-1781(99)00063-3. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Blass JP, Huang H-M, Freeman GB. The cellular basis of delirium and its relevance to age related disorders including Alzheimer's disease. International Psychogeriatrics. 1991;3(2):373–395. doi: 10.1017/s1041610291000820. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Haroutunian V, Zhang H, Park LC, Shi Q, Lesser M, Mohs RC, Sheu RK, Blass JP. Mitochondrial damage in Alzheimer's disease varies with apolipoprotein E genotype. Ann. Neurol. 2000;48(3):297–303. [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J. Neural. Transm. 1998;105(8–9):855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, Blass JP, Baker A, Carlson KC, Harding B, Perrino P. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer's disease. Arch. Neurol. 1988;45(8):836–840. doi: 10.1001/archneur.1988.00520320022009. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Toral-Barza L, Manger T, Freeman G. Neurotrans mitters and calcium during hypoxia. In: Hartman A, Kuschinsky W, editors. Cerebral Ischemia and Calcium. N.Y.: Springer-Verlag; 1989. pp. 215–222. [Google Scholar]
- Gluck MR, Thomas RG, Davis KL, Haroutunian V. Increased phosphate-activated glutaminase and glutamic acid decarboxylase activities in dorsolateral prefrontal cortex of aged schizophrenics: Implications for altered glutamate and GABA metabolism in schizophrenia. Am. J. Psychiatry. 2002;159(7):1165–1173. doi: 10.1176/appi.ajp.159.7.1165. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc. Natl. Acad. Sci. USA. 2001;98(8):4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutunian V, Davidson M, Kanof PD, Perl DP, Powchik P, Losonczy M, McCrystal J, Purohit DP, Bierer LM, Davis KL. Cortical cholinergic markers in schizophrenia. Schizophr. Res. 1994;12(2):137–144. doi: 10.1016/0920-9964(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Hill RL, Bradshaw RA. Fumarase. Methods in Enzymol. 1969;13:91–92. [Google Scholar]
- Jacobsen LK, Hamburger SD, Van Horn JD, Vaituzis AC, McKenna K, Frazier JA, Gordon CT, Lenane MC, Rapoport JL, Zametkin AJ. Cerebral glucose metabolism in childhood onset schizophrenia. Psychiatry. Res. 1997;75(3):131–144. doi: 10.1016/s0925-4927(97)00050-4. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Gibson GE. Coupling of neuronal function to oxygen and glucose metabolism through changes in neurotransmitter dynamics as revealed with aging, hypoglycemia and hypoxia. In: Gibson GE, Dienel G, editors. Handbook of Neurochemistry and Molecular Biology. 3rd edition. Springer; 2007. pp. 297–320. [Google Scholar]
- Ksiezak-Reding H, Blass JP, Gibson GE. Studies on the pyruvate dehydrogenase complex in brain with the arylamine acetyltransferase-coupled assay. J. Neurochem. 1982;38:1627–1636. doi: 10.1111/j.1471-4159.1982.tb06643.x. [DOI] [PubMed] [Google Scholar]
- Kitto GB. Intra and Extramitochondrial Malate dehydrogenases from Chicken and Tuna Heart. Methods in Enzymol. 1969;13:106–116. [Google Scholar]
- Kung L, Roberts RC. Mitochondrial pathology in human schizophrenic striatum: a postmortem ultrastructural study. Synapse. 1999;31(1):67–75. doi: 10.1002/(SICI)1098-2396(199901)31:1<67::AID-SYN9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lyubarev AE, Kurganov BI. Supramolecular organization of tricarboxylic acid cycle enzymes. Biosystems. 1989;22:91–102. doi: 10.1016/0303-2647(89)90038-5. [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D, Gattaz WF, Schmitt A, Novello JC, Marangoni S, Turck CW, Dias-Neto E. Proteomic analysis of schizophrenia patients Wernicke’s area reveals an energy metabolism dysregulation. BMC. Psychiatry. 2009;30:9–17. doi: 10.1186/1471-244X-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer I, Zierz S, Möller H. Evidence for a mitochondrial oxidative phosphorylation defect in brains from patients with schizophrenia. Schizophr Res. 2001;48(1):125–136. doi: 10.1016/s0920-9964(00)00075-x. [DOI] [PubMed] [Google Scholar]
- McCormack JG, Denton RM. Influence of calcium ions on mammalian intramitochondrial dehydrogenases. Methods. Enzymol. 1989;174:95–118. doi: 10.1016/0076-6879(89)74013-1. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28(1):53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Morton RL, Ikle D, White CW. Loss of lung mitochondrial aconitase activity due to hyperoxia in bronchopulmonary dysplasia in primates. Am. J. Physiol. 1998;274(1 Pt 1):L127–L133. doi: 10.1152/ajplung.1998.274.1.L127. [DOI] [PubMed] [Google Scholar]
- Prasad KN, Cole WC, Prasad KC. Risk factors for Alzheimer's disease: role of multiple antioxidants, non-steroidal anti-inflammatory and cholinergic agents alone or in combination in prevention and treatment. J. Am. Coll. Nutr. 2002;21(6):506–522. doi: 10.1080/07315724.2002.10719249. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, Wayland M, Freeman T, Dudbridge F, Lilley KS, Karp NA, Hester S, Tkachev D, Mimmack ML, Yolken RH, Webster MJ, Torrey EF, Bahn S. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol. Psychiatry. 2004;9(7):684–697. doi: 10.1038/sj.mp.4001511. 643. [DOI] [PubMed] [Google Scholar]
- Purohit DP, Perl DP, Haroutunian V, Powchik P, Davidson M, Davis KL. Alzheimer disease and related neurodegenerative diseases in elderly patients with schizophrenia. Arch. Gen. Psychiatry. 1998;55:205–211. doi: 10.1001/archpsyc.55.3.205. [DOI] [PubMed] [Google Scholar]
- Reddy RD, Yao JK. Free radical pathology in schizophrenia: a review. Prostaglandins Leukot Essent. Fatty Acids. 1996;55(1–2):33–43. doi: 10.1016/s0952-3278(96)90143-x. [DOI] [PubMed] [Google Scholar]
- Regenold WT, Phatak P, Marano CM, Sassan A, Conley RR, Kling MA. Elevated cerebrospinal fluid lactate concentrations in patients with bipolar disorder and schizophrenia: implications for the mitochondrial dysfunction hypothesis. Biol. Psychiatry. 2009;65:489–494. doi: 10.1016/j.biopsych.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial dysfunction and psychiatric disorders. Neurochem Res. 2009;34(6):1021–1029. doi: 10.1007/s11064-008-9865-8. [DOI] [PubMed] [Google Scholar]
- Rose IA, O'Connell EL. Mechanism of aconitase action. I. The hydrogen transfer reaction. J. Biol. Chem. 1967;242(8):1870–1879. [PubMed] [Google Scholar]
- Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first episode, drug naïve patients with schizophrenia. Am. J. Psychiatry. 2003;160:284–289. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- Santos SS, Gibson GE, Cooper AJ, Denton TT, Thompson CM, Bunik VI, Alves PM, Sonnewald U. Inhibitors of the alpha-ketoglutarate dehydrogenase complex alter [1-13C]glucose and [U-13C]glutamate metabolism in cerebellar granule neurons. J Neurosci Res. 2006;83:450–458. doi: 10.1002/jnr.20749. [DOI] [PubMed] [Google Scholar]
- Shepherd D, Garland PB. Citrate Synthase from rat liver. Methods in Enzymol. 1969;13:11–13. doi: 10.1042/bj1140597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Risa O, Sonnewald U, Gibson GE. Mild reduction in the activity of the alpha-ketoglutarate dehydrogenase complex elevates GABA shunt and glycolysis. J Neurochem. 2009;109 Suppl 1:214–221. doi: 10.1111/j.1471-4159.2009.05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WS, Faraone SV, Su J, Tarbox SI, Van Eerdewegh P, Tsuang MT. Evidence for linkage between regulatory enzymes in glycolysis and schizophrenia in a multiplex sample. Am. J. Med. Genet B Neuropsychiatr Genet. 2004;127(1):5–10. doi: 10.1002/ajmg.b.20132. [DOI] [PubMed] [Google Scholar]
- Sungman Cha. Succinate thiokinase from Pig Heart. Methods in Enzymol. 1969;13:62–65. [Google Scholar]
- Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J. Neurosci. 2000;20(24):8972–8979. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeger C, Der Vartanian DV, Zeylemaker WP. Succinate Dehydrogenase. Methods in Enzymol. 1969;13:106–116. [Google Scholar]
- Velot C, Mixon MB, Teige M, Srere PA. Model of a quinary structure between Krebs TCA cycle enzymes: A model for the metabolon. Biochemistry. 1997;36(47):14271–14276. doi: 10.1021/bi972011j. [DOI] [PubMed] [Google Scholar]