Abstract
Background & Aims
Bone marrow stromal cells (MSCs) are being evaluated as a cellular therapeutic for immune-mediated diseases. We investigated the effects of MSCs in mice with chemically induced colitis and determined the effects CD11b+ cells, based on the hypothesis that MSCs increase numbers of regulatory T cells.
Methods
Colitis was induced in mice using trinitrobenzene sulfonic acid (TNBS); symptoms were monitored as function of MSC delivery. An immunomodulatory response was determined by measuring numbers of regulatory T cells in mesenteric lymph nodes. In vitro co-cultures were used to assess the interaction of MSCs with regulatory T cells and CD11b+ cells; findings were supported using near-infrared tracking of MSCs in vivo. We chemically and surgically depleted splenic CD11b+ cells before colitis was induced with TNBS to monitor the effects of MSCs. We adoptively transferred CD11b+ cells that were co-cultured with MSCs into mice with colitis.
Results
Intravenous grafts of MSCs prevented colitis and increased survival times of mice. Numbers of Foxp3+ regulatory T cells increased in mesenteric lymph nodes in mice given MSCs. MSCs increased the numbers of Foxp3+ splenocytes in a CD11b+ cell-dependent manner. Transplanted MSCs co-localized near splenic CD11b+ cells in vivo. Loss of CD11b+ cells eliminated the therapeutic effect of MSCs. MSCs increased the anti-colitis effects of CD11b+ cells in mice.
Conclusions
MSC transplants, delivered by specific parameters, reduce colitis in mice. Interactions between MSC and CD11b+ Treg cells might be used to develop potency assays for MSCs, to identify non-responders to MSC therapy, and to create new cell grafts that are composed of CD11b+ cells pre-conditioned by MSCs.
Keywords: Crohn’s Disease, Mesenchymal Stem Cells, Monocyte, Imaging
INTRODUCTION
Inflammatory bowel disease (IBD) is a group of chronic, relapsing, and remitting intestinal inflammatory conditions, which is classified into two major forms, ulcerative colitis (UC) and Crohn’s disease (CD). IBD has a prevalence of 1.2 million people in the US alone representing a significant burden to healthcare costs and patient morbidity/mortality1. Pathological hallmarks of disease distinguish UC from CD2. UC typically involves the colorectal tissue only, with restricted inflammation to the mucosa. On the other hand, CD has pan-gastrointestinal involvement and is characterized by transmural inflammation and granulomas. Thus, CD is often not correctable by surgical resection of diseased tissue and is primarily treated by medical therapy. Growing evidence has demonstrated that changes in epithelial barrier function, as well as impairments in innate and adaptive immunity increase susceptibility to the disease and therefore may be independent targets of therapy3. However, current medical standards of care do not target multiple disease pathways at once and therefore have limited effectiveness2.
In order to achieve a combinatorial approach to disease treatment, some investigators have considered the use of cells as therapeutics. In particular, academic and industrial laboratories are evaluating the intravenous use of bone marrow stromal cells (MSCs) for the treatment of inflammatory diseases. The motivation behind this choice of a cell therapy is based upon a number of studies that suggest that MSCs can modulate the immune system. Initial studies had focused on non-specific modulation of T cell effector functions after coculture with MSCs 4–7. Emerging theories of MSC-leukocyte interactions have suggested that MSCs can enhance natural suppressor populations that would, in turn, prevent self-reactivity 6, 8, 9. For example, investigators have reported an increase of Foxp3+ regulatory cells after MSC transplantation in vivo 10. These findings have spurred the use of MSCs in experimental and clinical indications of self-reactivity such as acute graft-versus host disease (GVHD)11, 12 and autoimmune diseases including rheumatoid arthritis, experimental autoimmune encephalomyelitis, and type I diabetes 13–16 to induce tolerance.
There are a number of ongoing pre-clinical and clinical studies that have explored the use of MSCs, or phenotypically similar cells derived from other tissue sources such as adipose or dental tissue, in IBD therapy17 including human tests18. These studies have demonstrated that MSCs attenuate the inflammatory insult on gut tissue by downregulating pro-inflammatory cytokines released by macrophages and increasing regulatory T cell content in local mesenteric lymph nodes 19, 20. We previously tested the efficacy of MSCs in a multi-organ autoimmunity model caused by a deficiency in regulatory T cells and found histological improvements in intestinal tissue21. Here, we evaluated MSC transplants in a well established model of colitis. Intravenous transplantation of syngeneic MSCs in trinitrobenzosulfonic acid (TNBS)-induced colitic mice resulted in a significant survival benefit and attenuation of physical symptoms of disease in a prevention trial. We observed an “amplification” of the initial immunomodulatory effects induced by the MSC graft via an increase in local Foxp3+ regulatory T cells in mesenteric lymph nodes. Using an in vitro potency assay, we observed an increase in Foxp3+ splenocytes by MSC coculture that was due to third-party interactions between MSCs and CD11b+ splenocytes. Near-infrared tracking of MSC transplants in vivo revealed that the graft is short-lived and co-localizes with CD11b+ cells in the spleen early after administration. Removal of splenic CD11b+ cells by chemical or surgical approaches resulted in a loss of MSC therapy in TNBS colitis. Moreover, CD11b+ cells after coculture with MSCs ex vivo were reprogrammed into a partially therapeutic phenotype and could be independently used as an adoptive cell therapy for colitis.
MATERIALS AND METHODS
Mice
C57Bl/6 mice (4–6 weeks, Charles River Laboratory) were maintained in a light/temperature-controlled room with chow diet and water ad libitum. All experimental procedures performed were approved by Subcommittee on Research Animal Care and Laboratory Animal Resources of Massachusetts General Hospital per guidelines set forth by the Committee on Laboratory Resources, National Institutes of Health.
Culture of Bone Marrow-Derived Stromal Cells
C57Bl/6 mouse MSCs were isolated as previously reported21 and grown in MSC expansion medium. MSC expansion medium consisted of alpha-MEM (Gibco), 10% lot selected FBS (Atlanta Biologicals), 100 U/ml penicillin (Sigma), 1 ng/ml of basic FGF (R&D Systems) and 100 µg/ml streptomycin (Sigma). NIH 3T3-J2 fibroblasts were a kind gift from Dr. Howard Green and cultured according to donor’s protocol.
Colitis Induction and Cell Transplantation
C57Bl/6 mice (male, 6–8 weeks, between 19–21g) were weighed, fasted for 24 hours, and re-weighed to document baseline data. Mice were anesthetized and colitis was induced and monitored as previously reported22. Prior to colitis induction, MSCs were infused by tail vein injection or i.p. at different cell doses. Fibroblast infusion and saline infusions served as controls. In therapeutic trials, cells were infused two days after TNBS induction and similar physical parameters were measured.
Test for Fecal Occult Blood
Fresh feces from animals was procured three days after TNBS induction and tested for fecal occult blood per vendor’s instructions (Hemoccult Sensa, Beckman Coulter). The tests were read by an independent observer and given a semi-quantitative score of 0–5 as shown by the color indicators provided by the manufacturer.
Isolation and Analysis of Lymph Node Cells
Lymph nodes of colitic mice were mechanically isolated, erythrocyte-lysed with ACK lysis buffer for 1–2, and blocked with 0.5% bovine serum albumin and antibodies to the Fc receptor CD16/32 prior to cell staining. Cells were incubated with antibodies to CD4-APC, CD25-PE, and Foxp3-FITC using a commercial kit (eBiosciences) and analyzed using flow cytometry.
Splenectomy
During isoflurane anesthesia, the abdominal cavity of mice was opened and the spleen vessels were cauterized and removed. For control experiments, the abdomen was opened, but the spleen was not removed.
Near Infrared Tracking
In order to effectively track MSC grafts in vivo, we loaded cells with a near-infrared cell tracker agent (VT680; VisEn Medical). Labeled cells were infused in TNBS mice that were maintained on a non-fluorescent diet for at least 5 days to limit autofluorescence. An Olympus OV-110 epifluorescence imager was used to take near infrared images of whole mount tissue preparations at days 1 and 3 post-transplant. The gain on the camera was set to zero and a 280 ms exposure time was used for semi-quantitative analysis.
CD11b Transfer Experiments
CD11b+ splenocytes were cocultured at a 1:1 ratio with a mesenchymal cell in IL-2 supplemented medium and were isolated after coculture using magnetic beads (Miltenyi Biotec). These CD11b+ cells were transferred into wells at different ratios (Whole splenocytes: CD11b+ cells) with whole splenocytes in IL-2 medium. After 5 days of coculture, regulatory T cell frequency was assessed. For in vivo transfer, CD11b+ splenocytes were cocultured at a 1:1 ratio with a mesenchymal cell in IL-2 supplemented medium and were isolated after coculture using magnetic bead separation. These CD11b+ cells were transferred i.v. into mice directly before they had been administered TNBS to induce colitis.
Immunofluorescence
Spleens of C57Bl/6 mice were embedded in OCT, serially sectioned to 6 µm, and incubated with anti-CD11b, M1/70 (BD Biosciences) followed by biotinylated secondary antibody and FITC-conjugated streptavidin (GE Healthcare), and counterstained with DAPI. Images were captured and processed by using a Nikon Eclipse 80i with a CCD camera.
Coculture of Splenocytes and MSCs
Splenocytes of C57Bl/6 mice (aged 12 weeks) were isolated, lysed with CK lysis buffer for 1–2 minutes and fractionated using CD11b or CD25 microbeads (Miltenyi Biotec, Auburn, CA) per vendor’s protocols. Whole or fractionated splenocytes (1×106 cells) were cultured alone or in coculture with MSCs at a 1:10 ratio of splenocyte:MSC in RPMI medium with 10% FCS, low-dose recombinant human IL-2 (10U/ml; R&D Systems), 100U/ml penicillin and 100 µg/ml streptomycin. After 5 days of coculture, splenocytes were analyzed for expression of CD4, CD25, and Foxp3 by flow cytometry.
Gross and Microscopic Histology
Lymph nodes and the colon, dissected from the ileocecal junction to the sigmoid rectum, were grossly imaged one week after treatment using a digital camera and subsequently prepared for microscopic evaluation. Intestinal tissue was fixed in 10% buffered formalin, embedded in paraffin, sectioned to 6-µm thickness, and stained with hematoylin and eosin (H&E).
CD11b Depletion Using Liposomes
Chemical depletion of CD11b+ cells was execute by intraperitoneally injections of 100 and 50 mg/kg liposomal clodronate (L-Phosphatidyl-choline/cholesterol clodronate; Encapsula NanoSciences) at 48 and 24 h prior to TNBS induction23.
Statistical Analysis
For flow cytometry data, median values ± standard deviations are reported. Results are given as a mean ± standard error of the mean and were analyzed using an unpaired Student’s t-test given an unskewed data set and assuming a normal distribution. Significance values of P<0.05 were considered statistically significant. Survival data was analyzed using a Log-Rank Test.
RESULTS
MSC Transplants Prevents Physical Evidence of TNBS-Induced Colitis
Based on our prior work21, we sought to test the efficacy of MSC grafts in a well established model of TNBS colitis24. In a prevention trial, animals were randomized and treated with saline (internal control), or unit doses of fibroblasts (cell control) or syngeneic mouse MSCs. Animals then were anesthetized and administered a 1:1 chemical mixture per rectum consisting of either ethanol:saline (sham control) or ethanol:TNBS.
Intravenous treatment with 1×106 MSCs (referred to as 1U of medicine or fraction thereof) improved all physical evidence of colitis after MSC transplantation. A significant survival benefit was observed, with 94% of animals surviving after TNBS induction when treated with MSCs (N=16), compared to 47% and 33% when treated with vehicle (N=15) or a fibroblast (N=6) control, respectively (MSC vs. Saline, P=0.003; MSC vs. Fb, P=0.001; Figure 1A). Approximately 63% of mice survived after infusion of 0.25U of MSCs, which was not statistically significant compared to saline (P=0.484) but suggested survival was a function of MSC dose. No significant improvement in survival was seen when MSCs were infused intraperitoneally compared to controls (Supplementary Figure 3A). In addition, we measured animal weight and fecal occult blood as indicators of disease. A maximal 22% reduction in body weight loss was observed post-fast in animals treated with vehicle or 1U of fibroblasts i.v. with a final 14% loss at the study endpoint (Figure 1B). MSC-treated animals had a maximal 13% loss of body weight post-fast with a 1% gain in body weight at the study endpoint. Similarly, stool guiac tests performed on day 3 post-induction showed nearly complete absence of occult blood in feces of MSC treated mice when compared to vehicle treatment (P=0.029; Figure 1C).
Figure 1. Prevention of TNBS-Induced Colitis by MSC Transplantation.
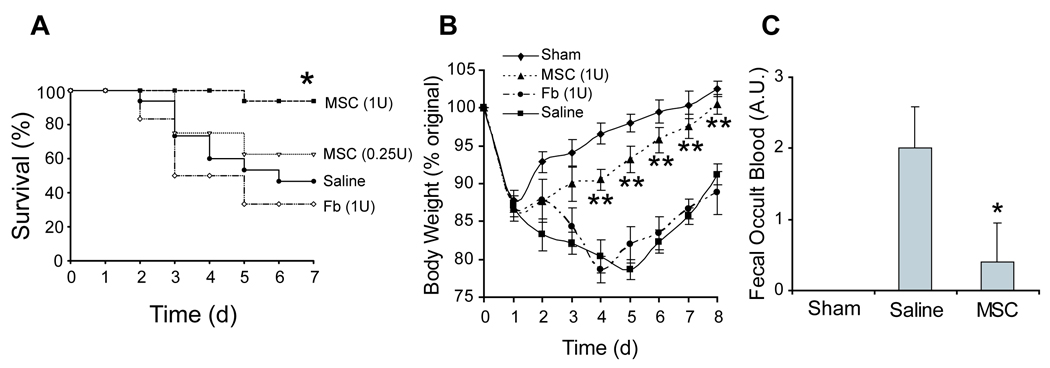
(A) Kaplan-Meier analysis of cell-based transplantation strategies with intravenous treatment. (B) Percentage of original body weight loss of mice over time. (C) Semi-quantitative fecal occult blood testing of experimental groups. TNBS, trinitrobenzylsulfonic acid; MSC, bone marrow stromal cell; Fb, fibroblast; U, unit or the equivalent of 1×106 cells. For prevention studies, the cohorts were: ethanol sham (N=4), 1U MSC (N=16), 0.25U MSC (N=8), saline (N=15), or Fb (N=6).
After identifying an optimal cell dose and route of delivery of MSC grafts in a preventative setting, we tested MSC therapy after disease onset. We first chose to infuse cells two days after TNBS induction, based on our prevention trial which showed that vehicle treated animals began to die at this time point. Intravenous infusion of 1U of MSCs led to 66% of animals surviving (P=0.33; Supplementary Figure 3B). The body weights of MSC treated animals showed a marginal 9% loss in body weight at the study endpoint compared to a 14% loss in control treatments (Supplementary Figure 3C). MSC transplants were also tried at one day after TNBS induction and failed to show a statistically significant survival benefit with 83% surviving in the MSC treatment arm (N=12) and 50% in the vehicle treated arm (N=12; P=0.15; Supplementary Figure 3D). These results trended favorably but were not significant compared to controls based on the power of the study.
MSC Prophylaxis Inhibits Histopathological Changes in Gut-Associated Tissue
At the study endpoint of the prevention trial, we examined histopathological changes in the colon and associated lymph nodes of the surviving animals with the objective of quantifying the extent of cellular inflammation in the local gut. Vehicle treated animals had macroscopically enlarged lymph nodes (Figure 2A) and thickened colonic walls (Figure 2B), which is likely due to edematous fluid, with major lesions and ulcerations found in distal half of the colon. On the other hand, mice transplanted with MSCs showed no gross signs of disease and were qualitatively similar to mice that had minor inflammation caused by the local ethanol infusion (Figure 2A–B). Mesenteric lymph node cellularity and colonic weight/length ratio were quantified and listed for each specified group. Colonic tissue was microscopically examined using conventional H&E methods. Mice treated with MSCs had few, if any, inflammatory infiltrates, crypt abscesses, goblet cell thickening, and loss of tissue architecture when compared to other treated groups (Figure 2C–F). Pathological scoring of the tissue quantitatively confirmed the significant differences in histology (shown above Figure 2C–F).
Figure 2. Histopathological Analysis of Colitic Mice after MSC Infusion.
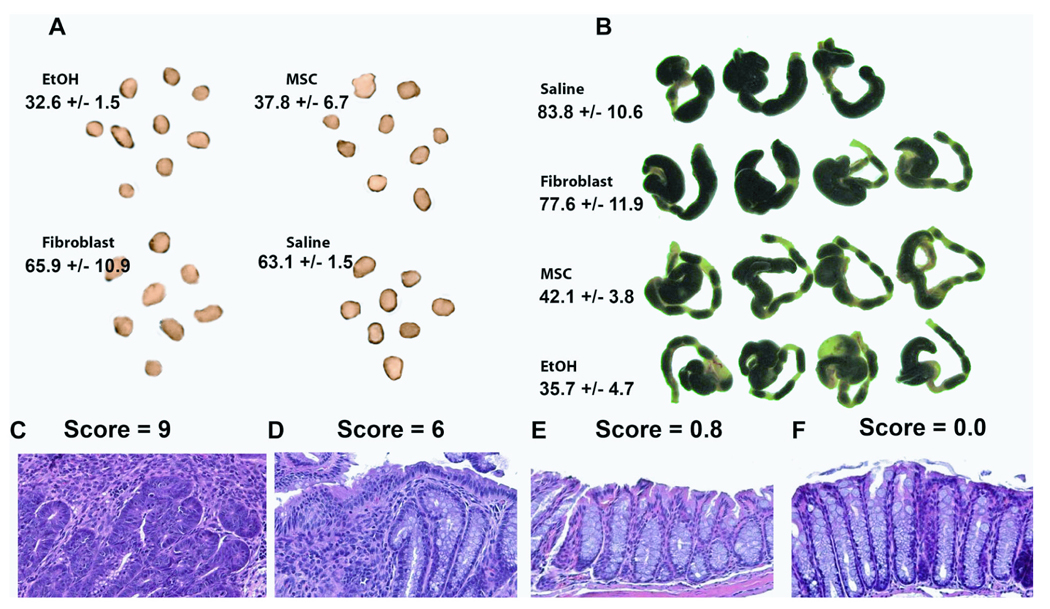
Mesenteric lymph node (A) and transverse colon (B) of colitic mice 7 days post-cell therapy in the prevention trial. Lymph node cellularity and colon weight per length ratios are stated below the group names in units of [×106 cells/node] and [mg/cm], respectively. Representative microscopic specimens are shown for (C) saline, (D) fibroblast, and (E) MSC-treated colitic mice compared to (F) ethanol sham controls. Pathology scores are stated above the images. Histopathology analysis was performed on N=4 of each group. TNBS, trinitrobenzylsulfonic acid; EtOH, ethanol (sham control); MSC, bone marrow stromal cell; Fb, fibroblast
Increase of CD4+ CD25+ Foxp3+ Cells in Mesenteric Lymph Nodes after MSC Therapy
MSCs have been shown to increase suppressor cells of both the CD4+ and CD8+ lineages in vitro 8, 9, 25 and this evidence led us to enumerate the regulatory T cell (Treg) number in colitis-induced animals at the study endpoint. Indeed, we saw a preservation of Treg frequency in the lymph nodes of MSC-treated mice consistent with a hypothesis that MSCs may “amplify” their immunosuppression by indirectly expanding endogenous suppressor T cells. When infused with 1U of MSCs, 2.3% of lymph node cells from TNBS-induced mice were CD25+ Foxp3+ compared to 0.7% and 0.9% in saline and mock cell treated animals, respectively (Figure 3). We further tallied the absolute number of Tregs given that there were quantifiable differences in lymph node cellularity. Treatment with MSCs had an approximately 2.1 fold absolute number of CD25+ Foxp3+ cells (11.35 ± 0.9 × 105 cells) when compared to saline treated animals (5.2 ± 0.8 × 105 cells).
Figure 3. Increased Regulatory T Cell Number in Mesenteric Lymph Nodes after MSC Therapy.
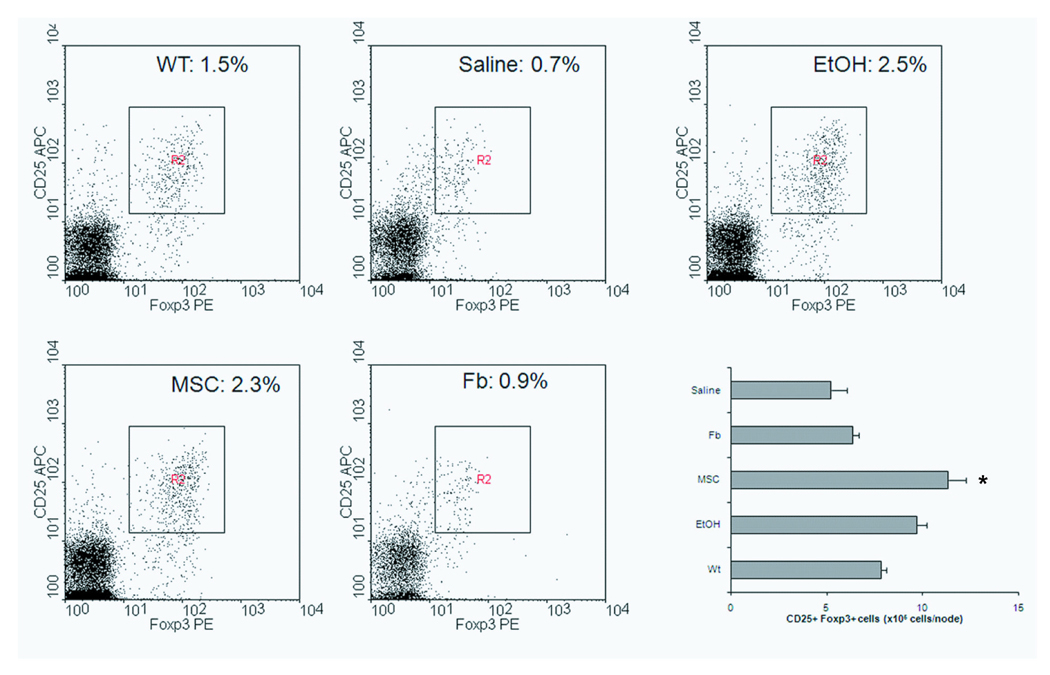
Flow cytometry of mesenteric lymph nodes for CD25 and Foxp3 expression. The graph represents one of two independent trials of N=4. WT, wild-type (healthy control); EtOH, ethanol (sham control); MSC, bone marrow stromal cell; Fb, fibroblast; U, unit or the equivalent of 1×106 cells.
MSC Coculture Increases Foxp3 + cells by Intermediate Interactions with CD11b+ Cells
We hypothesized that MSCs could directly induce the generation of regulatory T cells, which would explain the increased number of regulatory T cells observed in vivo after MSC transplantation. To this end, we cocultured MSCs with splenocytes cultured in IL-2 supplemented medium to promote regulatory T cell growth and analyzed the percentage of CD25+ Foxp3+ cells. After 5 days of MSC coculture, there was a marked increase in CD25+ Foxp3+ cells (1.28 % ± 0.28) from unfractionated splenocyte cultures compared to controls (IL-2 alone: 0.69% ± 0.16, IL-2 + fibroblasts: 0.79 ± 0.15; Figure 4A–C). However, the generation of Foxp3+ from CD4+ CD25− cells was found to be similar with or without coculture with MSCs (Supplementary Figure 4A–B). No significant change in overall cellularity of the cultures was observed. Finally, we cocultured MSCs with CD4+ CD25+ cells without IL-2 supplementation to determine if they would enhance the proliferation of existing regulatory cells. After 5 days of coculture, MSCs caused a slight increase in Foxp3+ cell number compared to no other cell (Supplementary Figure 4C). Yet, this was significantly lower than the proliferation of Foxp3+ cells when cultured with a known mitotic stimuli, such as IL-2.
Figure 4. Generation of Foxp3+ Splenocytes after MSC Coculture is Dependent on CD11b+ Cells.
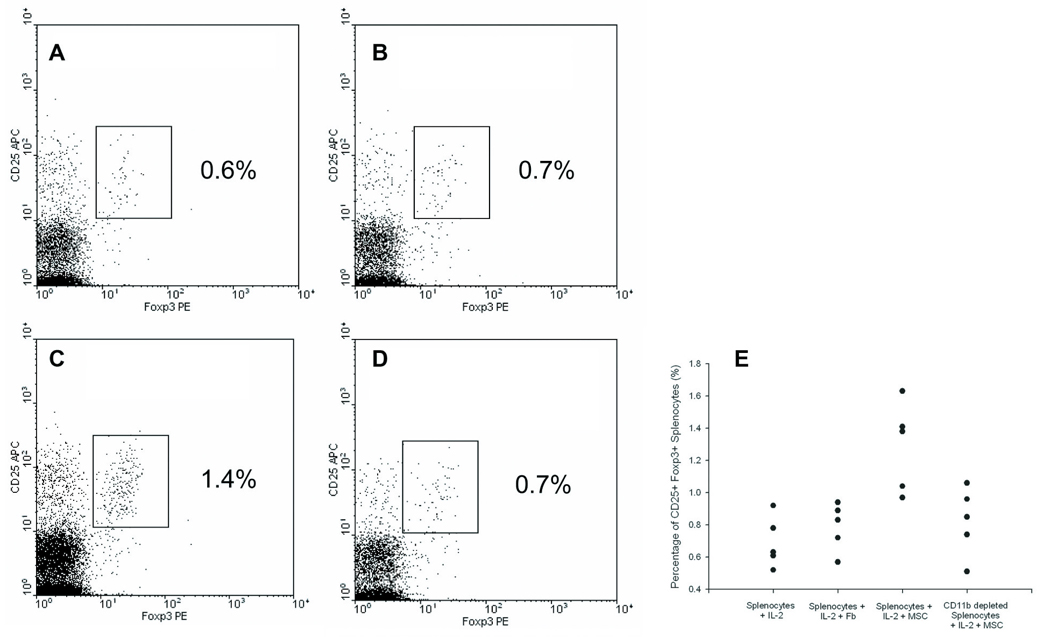
Splenocytes were cultured for 5 days with or without mesenchymal cells at a 1:10 ratio (mesenchymal cell:splenocyte) and analyzed for Foxp3 expression using flow cytometry. Dotplots gated on CD4 expression of unfractionated splenocytes cultured with (A) IL-2 alone, (B) fibroblasts and IL-2, or (C) MSCs and IL-2. The graph represents one of five independent trials. (D) Dotplot of CD11b+ depleted splenocytes cocultured with MSCs and IL-2. (E) Results of five independent trials comparing percentage of CD25+ Foxp3+ cells to coculture conditions.
Since we observed no direct influence of MSCs on suppressor cell generation or proliferation, we then tested if MSCs interacted with other cells that could affect regulatory T cell number in trans. Regulatory T cells have been demonstrated to interact with other innate immune cells in lymphoid organs to maintain peripheral tolerance 26, 27. Based on previous studies of MSC-macrophage interactions28, we depleted the CD11b+ fraction of splenocytes that includes macrophages and performed MSC cocultures. Removal of the CD11b+ fraction prior to MSC coculture reduced the percentage of CD25+ Foxp3+ cells to nearly baseline levels with IL-2 alone (0.82 % ± 0.21; Figure 4D). The replicates of these experiments on splenocytes harvested from five different mice are shown in Figure 4E. These results suggest that MSCs can increase Foxp3+ cell number through a cellular intermediate that expresses CD11b. CD11b+ cells represent a heterogeneous population of innate immune cells that include monocytes, macrophages, dendritic cells, granulocytes, and NK cells29, which we sought to globally characterize in vivo with respect to MSC therapy.
Near Infrared Tracking of Infused MSCs Shows Colocalization with Splenic CD11b+ Cells
In order to identify MSC-CD11b+ cell interactions in vivo, we used cell tracking methods to determine the major tissue reservoir for MSCs after transplantation. MSCs were labeled with a near infrared tracking dye, VT680 (see Supplementary Figure 1), which facilitated imaging in highly autofluorescent tissues such as the intestine. One day after cell transplantation, the cell graft was dispersed in the lung, colon, and spleen (Figure 5A–D). By day 3, no appreciable signal was found in any of these organs supporting a transient fate of the cells in vivo (data not shown). Given the qualitatively high signal in the spleen, we assessed if MSCs were colocalized with splenic CD11b+ cells (Figure 5A–E). We found small syncitia of MSCs that were found neighboring CD11b+ cells determined by immunohistochemistry. In the majority of sections, the two cell types were found close to each other, but not in direct cell-contact.
Figure 5. Near Infrared Tracking of MSC Transplants.
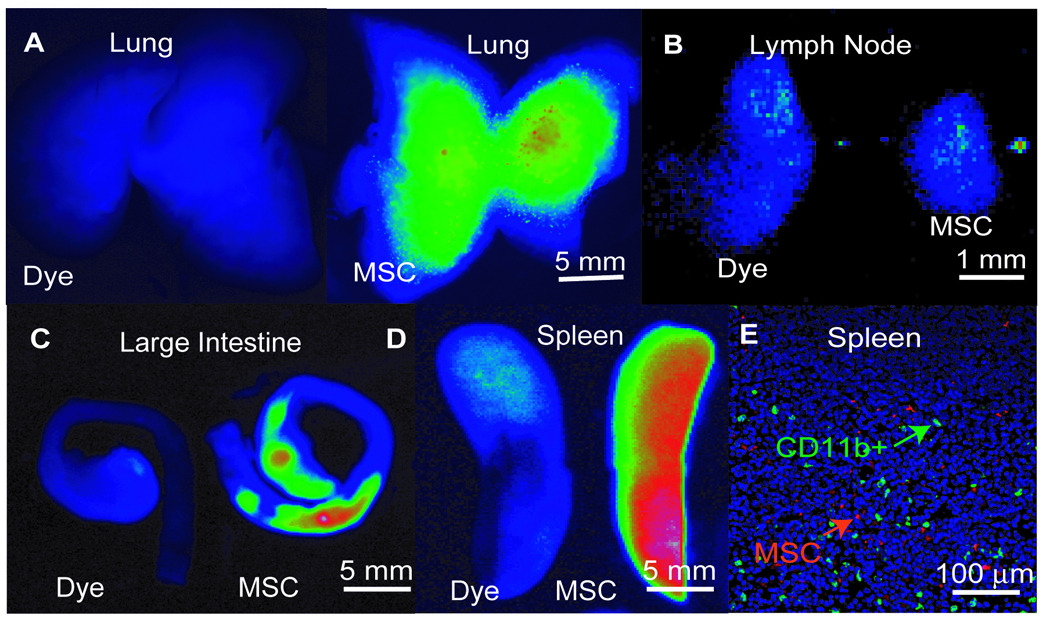
MSCs were labeled with VT680 and infused intravenously at the time of TNBS administration. Control images, labeled as ‘dye’, are injections of VT680 dye alone. Representative images from the (A) lungs, (B) mesenteric lymph nodes (MLN), (C) large intestine, and (D) spleens of mice injected with the dye alone or labeled MSCs one day post-infusion. Results of two independent imaging studies of N=3 per group. (E) Immunofluorescent micrographs of splenic tissue harvested from mice infused with VT680-labeled MSCs (red) and co-stained for CD11b (green), and DAPI (blue).
Chemical or Surgical Reduction in Splenic CD11b+ Cells Abolishes MSC Therapy
We conducted perturbation experiments with the goal of reducing splenic CD11b+ cells in order to evaluate the therapeutic relevance of MSC-CD11b+ cell interactions in vivo. Prior to TNBS induction, we infused liposomes filled with clodronate that known to specifically deplete phagocytic cells with a monocytic immunophenotype23. After TNBS induction, MSC treatment was found to be ineffective in CD11b+ depleted mice in terms of survival compared to control mice that received saline alone (Figure 6A). Furthermore, the absolute number of Foxp3+ cells in MLNs was the same in treatment groups (Figure 6B). As another approach to specifically reducing splenic CD11b+ cells, we performed splenectomies in mice prior to TNBS induction and found no improvement in survival (Figure 6C) or MLN Treg number (Figure 6D) in these mice with MSC therapy. These studies demonstrate that a therapeutic response to MSC transplantation requires the presence of splenic CD11b+ cells.
Figure 6. Depletion of CD11b+ Cells by Liposomal Cytotoxicity or Splenectomy Eliminates Therapeutic Effect of MSCs.
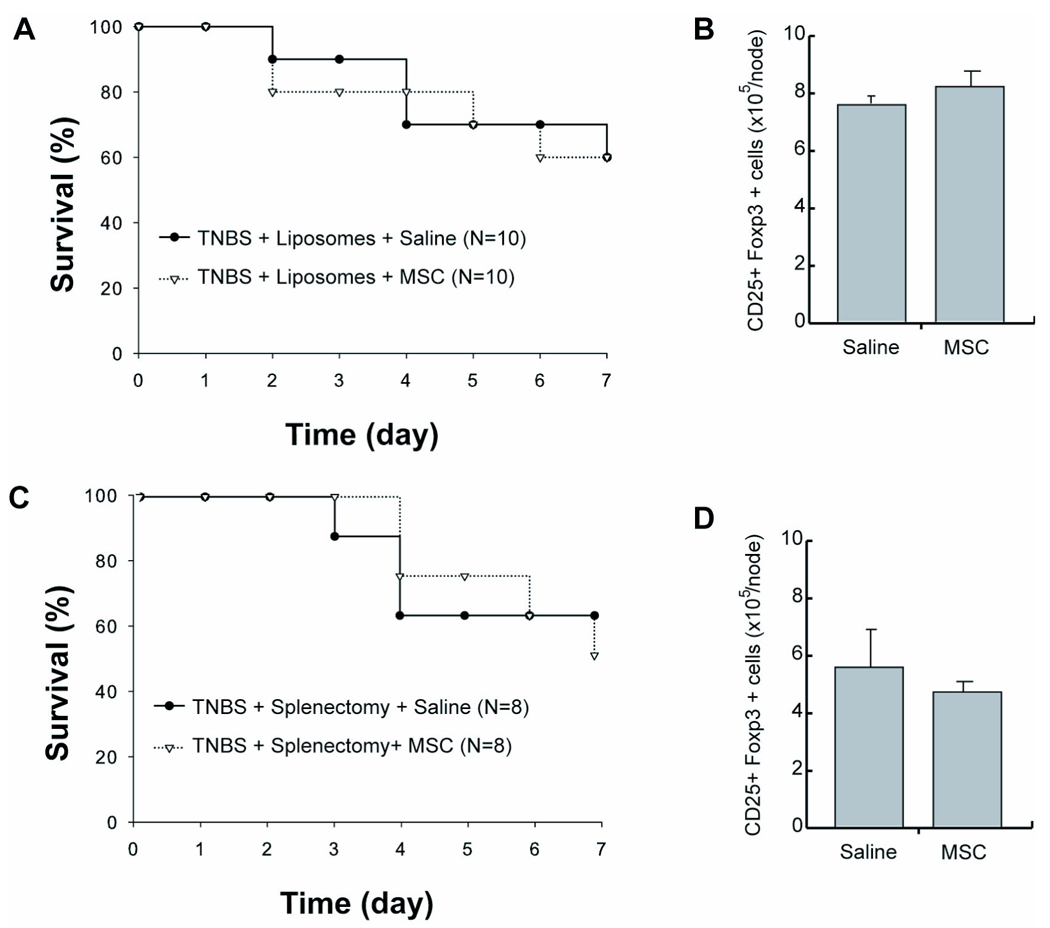
(A) Kaplan-Meier analysis of TNBS-induced mice depleted of monocytes and macrophages by liposomal clodronate and intravenously treated with saline or MSCs. (B) Total number of CD25+ Foxp3+ cells in mesenteric lymph nodes after treatment of monocytic-depleted mice. (C) Kaplan-Meier analysis of TNBS-induced mice intravenously treated with saline or MSCs that were splenectomized prior to disease induction. (D) Total number of CD25+ Foxp3+ cells in mesenteric lymph nodes after treatment of splenectomized mice. TNBS, trinitrobenzylsulfonic acid; MSC, bone marrow stromal cell.
MSCs Induce a Suppressor Phenotype in CD11b+ Cells that is Protective in Colitis
Given the importance of CD11b+ cells as an intermediate in MSC therapy, we investigated whether MSCs reprogrammed CD11b+ to a suppressive state. We first used our in vitro coculture system to determine if CD11b+ cells that were pre-conditioned by MSCs could independently increase regulatory T cells. We isolated CD11b+ cells by magnetic purification from spleens and cocultured them with MSCs or skin fibroblasts at a 1:1 ratio, prior to transferring them into a splenocyte population cultured in Treg growth conditions (Figure 7A). There was an increase in Treg populations as a function of the ratio of CD11b+ cells that were pre-incubated with MSCs (Figure 7B). These results specific and were not seen when CD11b+ cells were cocultured with a high ratio of skin fibroblasts. Finally, we adoptively transferred isolated CD11b+ cells after coculture with MSCs at the time of TNBS induction. Using purified, reprogrammed CD11b+ cells, we observed a dose-dependent survival benefit in colitic mice (Figure 7C), when compared to CD11b+ cells cocultured with skin fibroblasts (P=0.007) and trended favorably when compared to saline (P=0.13). At a dose of 1×106 purified CD11b+ cells, we observed 70% of the mice surviving compared to 20% survival if the CD11b+ cells were cocultured with skin fibroblasts or 40% with simply saline treatment and was associated with a significant increase in MLN Treg number at the study endpoint (Figure 7D). These data demonstrate that MSCs alter CD11b+ cell phenotype, as opposed to skin fibroblasts, towards a phenotype of therapeutic potential.
Figure 7. Adoptive Transfer of CD11b+ Splenocytes After MSC Coculture Confers Increase in Regulatory T Cell Number and Survival Benefit in Colitic Mice.
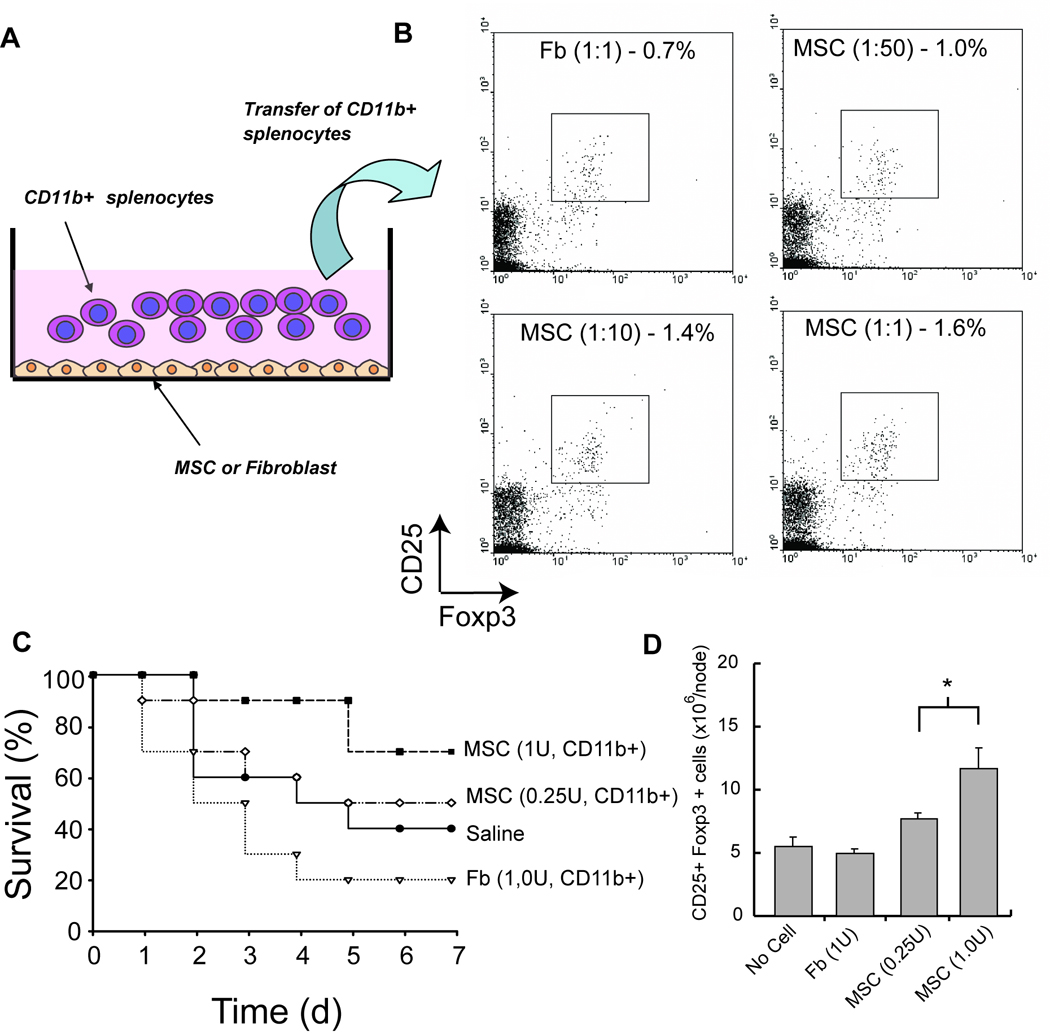
(A) Schematic of coculture composition. CD11b+ cells were isolated by MACS and directly cocultured with MSCs or fibroblasts for 5 days in vitro. Subsequently, CD11b+ cells were harvested by magnetic separation and were assessed for their immunomodulatory function in vitro and in vivo. (B) Boxplots of CD25 and Foxp3 expression of splenocytes after incubation with cocultured CD11b+ cells (ratio denotes mesenchymal cell:splenocyte) for 5 days in regulatory T cell stimulating conditions. The graph represents one of two independent trials of N=3 wells. (C) Survival of TNBS-induced mice after treatment with cocultured CD11b+ cells at a one week study endpoint. N=10 per group. (D) Total number of CD25+ Foxp3+ cells in mesenteric lymph nodes after treatment of with transferred CD11b+ cells. MSC, bone marrow stromal cell; Fb, fibroblast. U, unit or the equivalent of 1×106 cells.
Discussion
We previously demonstrated a site-specific benefit in the intestine after MSC transplantation in a multi-organ autoimmunity model. In this study, we further elaborated on these results using a chemically-induced model of colitis. MSC transplantation was found to increase survival prior to active disease and alleviate clinical symptoms after disease onset, consistent with previous studies using adipose stromal cells19, 20. In contrast, therapeutic use of MSCs after TNBS induction did not lead to a dramatic benefit in survival given the limited sample sizes that were used in this study. These results may point to the use of multiple doses of MSCs and/or a form of maintenance therapy for prevention of symptoms as a potential clinical approach. Ultimately, there may be need for large cohorts to power therapeutic studies in this indication. We also found that only intravenous treatment led to a significant survival benefit. Indeed, other reports using antigen-based tolerance strategies also confirmed that the route of drug administration was crucial to the therapeutic outcome 30. This issue of delivery may be generalized to other tolerance inductive methods and should be considered in future endeavors in the field.
In this study, we also provide evidence that infusion of MSCs can maintain levels of Tregs during disease in vivo. We did not observe an increase in the proliferation or generation of regulatory cells from naïve T splenocytes after direct MSC coculture. Given the known necessity of other splenocytes in regulatory T cell generation and function, we hypothesized that MSCs may affect T cell function in trans via innate immune cells. Depletion of CD11b+ cells prior to MSC coculture reduced the number of regulatory T cells, consistent with our hypothesis. These data demonstrate a complex interaction between MSCs, CD11b+ cells, and regulatory T cells. A recent study has illuminated the interaction of MSCs and CD11b+ tissue macrophages in the context of sepsis28. Our results confirm these interactions to be essential for MSC therapy in colitis. We also observed the subsequent differentiation of CD11b+ cells into anti-inflammatory agents that have partial therapeutic value in-and-of themselves when compared to fibroblast coculture. MSCs have been shown to cause the differentiation of lymphocyte populations in vitro to suppressor cells as well as the adoptive transfer of these cells for therapeutic use in pre-clinical models of multiple sclerosis14 and colitis19. Our study suggests that intermediary CD11b+ cells can be used as an alternative and presumably lead to a similar effect in other disease contexts.
The tissue distribution of different host species of MSCs in a variety of subjects ranging from mice to humans during health and disease has been well documented31–33, including methods to direct cell trafficking by ex vivo manipulation34. These studies have consistently shown high levels of engraftment in the lung and livers of subjects, with a lesser cell mass embedding in the spleen. These organs have a microvasculature that permits non-specific entrapment of cells in general, although the behavior of MSCs during this short period of sequestration is not well known. We show that the spleen is a critical tissue where MSCs are temporarily found after the subsequent disappearance from peripheral sites and interact with splenocytes in a therapeutic manner.
The interaction of MSCs and CD11b+ macrophages in a previous study was dependent on direct contact of the two cell types in the lung and led to a prostaglandin E2-mediated reprogramming of macrophages by MSCs28. We have found that the interaction of MSCs and CD11b+ cells to also be present in the spleen with close, but not direct, cell contact. These data would suggest that short-range secreted signals that locally increase the concentration of a paracrine mediator may also be involved in the reprogramming of CD11b+ cells. Furthermore, we’ve discovered MSC therapy to be ineffective in splenectomized hosts which may have direct relevance to clinical settings as exclusionary criteria for human trials. Recent clinical testing in patients with acute GVHD has suggested that stratification of patients based on the subclass of disease (e.g. intestinal vs. skin GVHD) may lead to different therapeutic responses and thus highlight the importance of selecting the right patients for clinical trials (www.osiris.com). Ongoing clinical testing in CD patients may benefit by identifying mechanisms of MSC action that can be used to optimize therapy as well as to include/exclude particular patients.
In conclusion, we show that MSC transplantation can be an effective means to prevent colitis in mice. This treatment correlated with a higher local regulatory T cell number in gut-associated lymph nodes indicating that the immunosuppressive signature of MSC transplantation may be amplified through the maintenance of endogenous suppressor cells in vivo. These data support the notion of MSC grafts as a cellular therapeutic for the prevention/symptomatic treatment of colitis and highlight a potential mechanism of action, whereby initial therapy is reinforced by the immune system itself to create a short-term immunosuppressive state.
ACKNOWLEDGEMENTS
The authors acknowledge the technical assistance of Dr. Ken Sugimoto for colitis work, Don Poulsen for help with illustrations, and Robert Crowther for preparation of histological sections.
FUNDING SOURCES: This work was partially supported by grants from the National Institutes of Health (5R01DK059766-06 and U24CA092782), the Broad Medical Research Foundation (BMRP498382) and the Shriners Hospitals for Children.
ABBREVIATIONS
- MSC
bone marrow stromal cell
- DC
dendritic cell
- TNBS
trinitrobenzosulfonic acid
- H&E
hematoxylin and eosin
- Tregs
T regulatory cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE: All authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS:
B.P. - study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; technical, or material support; study supervision
R.U. – acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical support
J.D. – acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical support
Y.I. – acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical support
E.M. – study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical, or material support; study supervision
A.M. – study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical, or material support; study supervision
R.W. – study concept and design; critical revision of the manuscript for important intellectual content; obtained funding; study supervision
M.L.Y. – study concept and design; critical revision of the manuscript for important intellectual content; obtained funding; study supervision
REFERENCES
- 1.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 3.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 4.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 5.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 6.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 7.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 8.Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 10.Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, Cavinato RA, Todeschini M, Solini S, Sonzogni A, Perico N, Remuzzi G, Noris M. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 2008;181:3933–3946. doi: 10.4049/jimmunol.181.6.3933. [DOI] [PubMed] [Google Scholar]
- 11.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 12.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 13.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 14.Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R, Uccelli A. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61:219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 15.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 16.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dryden GW., Jr Overview of biologic therapy for Crohn's disease. Expert Opin Biol Ther. 2009;9:967–974. doi: 10.1517/14712590903048909. [DOI] [PubMed] [Google Scholar]
- 18.Ringden O, Uzunel M, Sundberg B, Lonnies L, Nava S, Gustafsson J, Henningsohn L, Le Blanc K. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia. 2007;21:2271–2276. doi: 10.1038/sj.leu.2404833. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 21.Parekkadan B, Tilles AW, Yarmush ML. Bone marrow-derived mesenchymal stem cells ameliorate autoimmune enteropathy independently of regulatory T cells. Stem Cells. 2008;26:1913–1919. doi: 10.1634/stemcells.2007-0790. [DOI] [PubMed] [Google Scholar]
- 22.Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, Koide Y. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology. 2002;123:1912–1922. doi: 10.1053/gast.2002.37050. [DOI] [PubMed] [Google Scholar]
- 23.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 25.Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S, Frassoni F, Locatelli F. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- 26.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange C, Durr M, Doster H, Melms A, Bischof F. Dendritic cell-regulatory T-cell interactions control self-directed immunity. Immunol Cell Biol. 2007;85:575–581. doi: 10.1038/sj.icb.7100088. [DOI] [PubMed] [Google Scholar]
- 28.Németh K, Leelahavanichkul A, Yuen P, Mayer B, Parmelee A, Doi K, Robey P, Leelahavanichkul K, Koller B, Brown J. Bone marrow stromal cells attenuate sepsis via prostaglandin E2𠄣dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature Medicine. 2008;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 31.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 32.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 33.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 34.Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]


