Abstract
Rationale
In utero cocaine exposure has been associated with alterations in the dopamine (DA) system in monkeys. However, the behavioral outcomes of prenatal cocaine exposure in adulthood are poorly understood.
Objectives
To assess several behavioral measures in 14-year-old rhesus monkeys exposed to cocaine in utero and controls (n=10 per group).
Materials and methods
For these studies, two unconditioned behavioral tasks, novel object reactivity and locomotor activity, and two conditioned behavioral tasks, response extinction and delay discounting, were examined. In addition, cerebrospinal fluid (CSF) samples were analyzed for concentrations of the monoamine metabolites homovanillic acid (HVA) and 5-hydroxyindole acetic acid (5-HIAA).
Results
No differences in CSF concentrations of 5-HIAA and HVA, latencies to touch a novel object or locomotor activity measures were observed between groups or sexes. However, prenatally cocaine-exposed monkeys required a significantly greater number of sessions to reach criteria for extinction of food-reinforced behavior than control monkeys. On the delay-discounting task, male prenatally cocaine-exposed monkeys switched preference from the larger reinforcer to the smaller one at shorter delay values than male control monkeys; no differences were observed in females.
Conclusions
These findings suggest that prenatal cocaine exposure results in long-term neurobehavioral deficits that are influenced by sex of the individual.
Keywords: Animal model, Dopamine, Drug abuse, Serotonin, Sex differences, Prenatal cocaine, Impulsivity, Delay discounting, Rhesus monkey
More than 5% of pregnant women in the USA between the ages of 15 and 44 years reported past-month illicit drug use during the period of 2007–2008 (United States Department of Health and Human Services, Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies 2009). However, the long-term consequences of chronic drug exposure to the fetus are not well understood. Animal models have been used to better understand the neural and behavioral consequences of in utero cocaine exposure (e.g., Lidow 1998). Nonhuman primate models offer several advantages, including a complex frontal cortex, similar hormonal fluctuations, and a long gestational period for prenatal drug exposure (Jewitt and Dukelow 1972; Lidow 2003). Nonhuman primates have approximately 95% gene homology to humans (Hacia et al. 1998) and greater homology in dopamine (DA), serotonin (5-HT), and norepinephrine systems than rodents (Weerts et al. 2007). Additionally, nonhuman primates have similar in utero development as humans over a long (24–26 weeks) gestation period (Silk et al. 1993), making them especially valuable for prenatal cocaine exposure studies.
Several investigators have examined the physiological consequences of cocaine use throughout gestation in nonhuman primate models and have reported differences in fetal brain development (e.g., Fang et al. 1997) as well as brain weight (Lidow 1998, 2003) and survival rates of offspring (Howell et al. 2001). The present study utilized prenatal cocaine exposure in rhesus monkeys in order to evaluate the behavioral and neurochemical consequences of in utero drug exposure in adults. These animals had been exposed to cocaine throughout the 25 weeks of gestation or were controls (Morris et al. 1996, 1997) and were studied as adults (14–15 years old). From birth until the present study, prenatally cocaine-exposed monkeys and controls have had similar experimental histories with limited drug exposure, making them a unique group of monkeys. We recently examined DA receptor function in these adult monkeys (Hamilton et al. 2010) and found no differences in DA D1- and D2-like receptor function, but differences in the behavioral effects of the D3/D2 receptor agonist quinpirole such that monkeys prenatally exposed to cocaine showed greater quinpirole-elicited yawning compared with control monkeys. In the present study, we extended the examination of neurobiological characteristics to include cerebrospinal fluid (CSF) concentrations of the DA metabolite homovanillic acid (HVA) and the 5-HT metabolite 5-hydroxyindole acetic acid (5-HIAA), and extended behavioral assessments to measures thought to model aspects of human behavior related to impulsivity.
In humans, cocaine use during pregnancy has been associated with physical deficits in the offspring including reduced body weight, body length, and head circumference of the infants at birth (Nair and Watson 1991) and developmental deficits including memory (Singer et al. 2005, 2008), attention (Singer et al. 2000; Noland et al. 2005; Linares et al. 2006; Bandstra et al. 2007), cognition (Singer et al. 2001; Morrow et al. 2006), and impulse control (Savage et al. 2005; Linares et al. 2006; Accornero et al. 2007; Pulsifer et al. 2008). Investigating behaviors thought to model impulse control in a highly controlled animal model is an important complement to the human studies. However, some of the difficulty in evaluating impulsivity in humans is due to it being conceptualized as a broad spectrum of behaviors rather than a single trait (Moeller et al. 2001; Chamberlain and Sahakian 2007). While research with humans has typically assessed impulsivity using standardized questionnaires (Evenden 1999), there is no agreement on how to model this in animals. Several distinct measures have been developed to assess behaviors in animals that may be related to impulsivity, including response to novelty, locomotor activity, behavioral inhibition, and choice between an immediate, low-magnitude reward, and a delayed large-magnitude reward. In the present study, each of these dependent variables was assessed in all monkeys in order to directly compare behavioral outcomes using a within-subjects design.
The first set of behavioral measures used in the present study involved unconditioned behaviors: response to a novel object (Dellu et al. 1996; Zuckerman 1996) and locomotor activity in an open field. Reactivity to a novel object has been used to characterize behavioral phenotypes that may measure aspects of impulsivity in rodents (Hooks et al. 2001; Suto et al. 2001; Davis et al. 2008) and nonhuman primates (Bolig et al. 1992; reviewed in Clarke and Boinski 1995; Coleman et al. 2005; Czoty et al. 2010). Regarding locomotor activity, Piazza and colleagues demonstrated that responsiveness in an open field was associated with vulnerability to stimulant self-administration (Piazza et al. 1989, 1990; reviewed in Piazza and Le Moal 1998). Interestingly, Dalley et al. (2007) did not find a relationship between locomotor activity and other behaviors deemed to assess impulsivity. In the present study, we directly compared locomotor activity of adult rhesus monkeys with novel object reactivity and two conditioned behaviors in order to better determine if they are assessing similar behavioral attributes.
A third measure described by Moeller et al. (2001) of impulsivity in humans is response perseveration, which is the tendency to continue emitting a formerly reinforced response despite the response currently being either unrewarded or punished (McCleary 1966). It is thought that response perseveration is a measure of deficient behavioral inhibition because in these tasks subjects must stop their ongoing behavior (Matthys et al. 1998). In the present study, perseverative responding was assessed by examining responding during extinction of previously food-reinforced operant responding in monkeys.
Finally, we assessed food choice involving delays. Impulsive choice is most commonly assessed in human studies using a delay-discounting task in which subjects are asked to choose between a small, immediate reinforce, and a larger, delayed reinforcer. The subjective value of the larger reinforcer is decreased (i.e., discounted) as the length of time the subject must wait to receive it increases. By using a series of choices between different reinforcers varying in delay values, an indifference point can be calculated as the delay value at which the smaller, immediate reinforcer is chosen as often as the larger, delayed reinforcer. Delay discounting has been adapted for animal studies (e.g., Perry et al. 2005; Woolverton et al. 2007). Although several studies have examined delay discounting involving drug reinforcers in monkeys (Anderson and Woolverton 2003; Woolverton and Anderson 2006; Woolverton et al. 2007; Newman et al. 2008), only one study has examined discounting of a non-drug reinforcer, saccharin (Freeman et al. 2009). The present study extended this work to delay discounting involving different magnitudes of another non-drug reinforcer, banana-flavored food pellets.
Material and methods
Subjects
Twenty adult rhesus monkeys (Macaca mulatta), born between 1993 and 1995 and raised at the FDA facility in Little Rock, AR until their arrival at Wake Forest University in 2007, served as subjects. Ten monkeys (six males and four females) were prenatally exposed to cocaine and ten monkeys (five males and five females) were controls, as described previously (Morris et al. 1996, 1997). Briefly, the mothers of the cocaine-exposed monkeys used in this study received intramuscular injections of escalating doses of cocaine. Prior to mating, mothers received a dose of 1.0 mg/kg per injection cocaine which was increased throughout gestation to up to 8.5 mg/kg per injection, three times per day for the entire course of gestation, with mean cumulative cocaine intake of 1,092.3 (±59.8 SEM) mg/kg (Morris et al. 1996). When the controls and cocaine-exposed monkeys were 6 months of age, they were housed individually in the same colony room and began behavioral training involving an operant test battery (Morris et al. 1997). Other than their prenatal drug histories, all monkeys had nearly identical experimental histories (see Paule et al. 1996; Morris et al. 1996). At the start of this experiment, monkeys were individually housed in stainless-steel cages with water available ad libitum and had visual and auditory contact with each other. Since we have previously shown that monoamine function is influenced by menstrual cycle (Czoty et al. 2009), we monitored menstrual cycle phase throughout the experiment by daily vaginal swabs. Days of bleeding were recorded as indicative of menses. During quarantine, a free-feeding weight was determined and monkeys' body weights were maintained at approximately 95% of that value throughout these studies (LabDiet Monkey Chow and fresh fruit). Monkeys were fitted with an aluminum collar (Primate Products, Redwood City, CA) and trained to sit calmly in a standard primate chair (Primate Products). All manipulations were performed in accordance with the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the Wake Forest University Institutional Animal Care and Use Committee.
Apparatus
The apparatus for operant responding consisted of a ventilated, sound-attenuating chamber (1.5×0.74×0.76 m; Med Associates, East Fairfield, VT) designed to accommodate a primate chair. Two response keys (5 cm wide) were located on one side of the chamber with a horizontal row of three stimulus lights 14 cm above each response key and a food receptacle between the response keys. The receptacle was connected with tygon tubing to a pellet dispenser (Gerbarands Corp., Arlington, MA) located on the top of the chamber for delivery of 1-g banana-flavored food pellets (P.K. Noyes Co., Lancaster, NH). An infusion pump (Cole-Palmer, Inc., Chicago, IL) was located on the top of the chamber.
Surgery
Each monkey was prepared with a chronic indwelling venous catheter and subcutaneous vascular port (Access Technologies, Skokie, IL) under sterile surgical conditions. Anesthesia was induced and maintained with ketamine (15 mg/kg) and butorphanol (0.025 mg/kg). Vital signs were monitored for the duration of the surgery. Briefly, a catheter was inserted into the femoral vein to the level of the vena cava. The distal end of the catheter was passed subcutaneously to a point slightly off the midline of the back, where an incision was made. The end of the catheter was then attached to the vascular access port and placed in a pocket formed by blunt dissection. Each port and catheter was filled with heparinized saline solution (100 U/ml) after every experimental session to prolong patency. Prior to each self-administration session, the back of the animal was cleaned with betadine and 95% EtOH, and the port was connected to the infusion pump located outside the chamber via a 20-gauge Huber Point Needle (Access Technologies). The pump was operated for approximately 3 s to fill the port and catheter with saline prior to starting the session.
CSF measures of 5-HIAA and HVA
Monkeys were anesthetized with 10 mg/kg ketamine, the aluminum collar was removed and the neck and the back of the skull were shaved and cleaned with betadine and 95% EtOH. A 25-gauge, 1.5-in. needle attached to a 3-ml syringe was inserted through the cisterna magna and approximately 2 ml of CSF was removed within 10 min of induction of anesthesia. Females were studied only during the follicular phase in order to be consistent with our earlier brain imaging studies (Hamilton et al. 2010). Moreover, earlier work showed that 5-HIAA concentrations did not differ as a function of menstrual cycle phase (Riddick et al. 2009). All samples were collected in the morning, at approximately the same time of day (0900 hours). The samples were immediately transferred to vacutainer tubes on ice. Samples were centrifuged at 4°C for 30 min at 3,000 rpm and then aliquoted into microcentrifuge tubes for storage at −30 C until they were analyzed using high-pressure liquid chromatography with electrochemical detection. The mobile phase consisted of 9.6 g citric acid, 11.2 g sodium phosphate monobasic, and 0.7 g 1-octanesulfonic acid in 860 ml of ultra-pure water. One hundred microliters of 0.5 M ethylenediaminetetraacetic acid was added and the pH of the solution was adjusted to 3.0. Next, six drops of triethylamine and 140 ml acetonitrile were added, and the mobile phase was filtered twice. Mobile phase was delivered to the system at a rate of 0.2 ml/min using an ESA 582 solvent delivery module (ESA Inc., Chelmsford, MA). Three 30-μl aliquots of each sample were loaded into a ESA 542 autoinjector and 20 μl were injected. Separation was achieved with a C-18 column (150 mm length, 3.2 mm i.d., 3-μm particle size; ESA, Inc.) and 5-HIAA and HVA were detected in samples using an ESA Coulochem II detector. Concentrations of 5-HIAA and HVA were determined by interpolation using a standard curve that was generated using standard solutions containing known amounts of the metabolites. Each sample was tested in triplicate, and average values were used for data analysis.
Experiment 1: effects of prenatal cocaine exposure on unconditioned behaviors
Approximately 2–6 months after quarantine ended, monkeys were characterized using two unconditioned behaviors that have been hypothesized to model aspects of impulsivity. For the novel object reactivity test, the monkey in the cage adjacent to the subject's home cage was removed, the partition was removed from between the cages, and the subject was moved to the adjacent cage. Next, the partition was replaced and an opaque black Plexiglas box measuring 30.5×20.3×20.3 cm was placed in the monkey's empty home cage. The unbaited box was positioned so that the hole was facing the monkey. The partition was removed again and the latency to touch the object was recorded. If the monkey did not touch the object within 15 min, a score of 900 s was assigned. All sessions were videotaped and scored by an observer blind to the monkey's prenatal condition. The 900-s maximum duration was based on data from our laboratory (Riddick et al. 2009).
To assess locomotor activity, each monkey was moved to another room away from their colony room and placed in a 3.0×2.0×1.75 m enclosure with the field divided into nine equal grid zones. Over the 30-min test period, the monkeys' activity was videotaped using a camera mounted overhead. The primary dependent measure was crossings between the zones, defined as >50% of the monkey's body crossing into a new grid section, and was counted by an observer blind to the prenatal condition of the monkey.
Experiment 2: effects of prenatal cocaine exposure on response extinction
Monkeys were initially trained to respond on the left and right keys by reinforcing each response with a 1-g banana-flavored pellet; a 30-s timeout followed each food presentation. The light above the response key signaled food availability; only one key was active during a session. Over the course of 2–3 weeks, the number of responses required was increased until a fixed-ratio (FR) 30 schedule of food presentation was achieved. Sessions ended after 30 reinforcers had been delivered or 60 min had elapsed. When responding was reliably maintained (i.e., mean response rate±20% for three consecutive sessions) on both keys and maximal food reinforcement was obtained consistently, intravenous catheters were implanted.
After implantation of catheters, baseline food reinforcement rates were re-established over five sessions, but only on the response key associated with the higher rate. For control monkeys, four had higher rates on the left key and six on the right key and for prenatally cocaine-exposed monkeys the distribution was three on the left key and seven on the right key. Because future studies would involve drug self-administration, saline injections were substituted for food pellets for at least five consecutive sessions and until responding was deemed extinguished. The three criteria for extinguishing food-reinforced responding were three consecutive sessions in which response rates were reduced by at least 80% from baseline rates of food-reinforced responding, mean response rate±20% for three consecutive sessions and no trends in responding. The primary dependent measure was the number of sessions to meet these criteria.
Experiment 3: effects of prenatal cocaine exposure on delay discounting
Monkeys were re-exposed to the FR 30 schedule of food presentation, with each pellet delivery followed by a 30-s timeout (TO). At the start of the session, one light above one response key was illuminated to indicate it was active; the order in which key was active varied across sessions. Sessions ended after 30 reinforcers were received or 60 min elapsed. Subjects responded under these conditions until responding on the right and left response key was reliably maintained and was deemed stable on each lever (i.e., mean response rate±20% for three consecutive sessions). The conditions were then changed to a concurrent schedule, which served as the schedule of reinforcement for the delay-discounting procedure.
The choice was initially between one and three food pellets delivered immediately after completion of the FR 30 requirement. The 1-pellet reinforcer was contingent on responding on the key associated with the highest rates, while responding on the other key delivered three pellets. Initially, the delay value associated with both reinforcers was 0 s. The delay value associated with one pellet remained at 0 s throughout the experiment, while the delay associated with the three-pellet reinforcer varied from 5 to 300 s. Sessions began with two forced-choice (i.e., sampling) trials. During these sampling trials, only one response key was active with illumination of the light and completion of the FR 30 resulted in the reinforcer and delay. After a 30-s TO, the other response key was illuminated and completion of the FR resulted in the reinforcer and delay. During the delay, a red light above the response key flashed on and off each second for the duration of the delay. During the TO, which occurred immediately following the delay and reinforcer delivery, no lights were illuminated. Once both sampling trials were completed and following a 30-s TO, the schedule changed to a concurrent schedule with both response keys being active. To limit the influence of response perseveration, a forced choice was implemented during the session if the monkey chose the same lever five times in a row, and the session then returned to a concurrent schedule. Delay values were kept constant for at least five consecutive sessions and until the percent choice of the larger, delayed reinforcer was deemed stable (mean percent choice±20% for three consecutive sessions). Sessions terminated after 30 free choices were completed or 60 min had elapsed. Delay values were presented in a quasi-random order to determine a delay vs. percent choice of larger reinforcer curve. Based on each monkey's individual curve, the indifference point was interpolated as the delay value that engendered 50% choice of the larger, delayed reinforcer and 50% choice of the smaller, immediate reinforcer. The dependent measure for this experiment was the indifference point.
Data analysis
CSF metabolite concentrations, latency to touch a novel object, locomotor activity, responding during extinction, and indifference point (from delay discounting) were each analyzed using a two-way analysis of variance (ANOVA) using group (prenatal cocaine and saline) and sex as factors. Post hoc Bonferroni tests were conducted when significant main effects were indicated by the ANOVA. Because some monkeys received a maximum score of 900 in the novel object reactivity test, correlations involving those data were analyzed using Spearman's correlation coefficient for ranked data. In all cases, significance was accepted at the 95% level of confidence (p<0.05).
Results
Experiment 1: effects of prenatal cocaine exposure on unconditioned behaviors
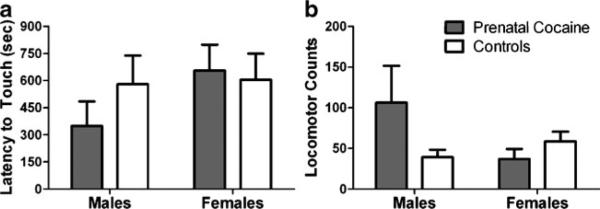
A two-way ANOVA revealed no significant main effect of prenatal cocaine exposure or sex and no significant interaction on latency to touch a novel object (Fig. 1a). Because there were no differences due to prenatal drug exposure, mean latencies for all the males were compared to mean latencies for the females and were not significantly different. Locomotor activity ranged from 5 to 316 counts over the 30-min exposure and did not differ as a function of prenatal exposure or sex and there was no significant interaction (Fig. 1b).
Fig. 1.
a Latency to touch a novel object placed in the monkey's home cage (in seconds). b Number of gridline crossings in a novel environment over 30 min. Values shown are mean±SEM for cocaine-exposed (filled bars) and control (open bars) male and female monkeys
Experiment 2: effects of prenatal cocaine exposure on response extinction
Under baseline conditions, mean response rates under the FR 30 schedule of food presentation were not different in male monkeys with mean (±SEM) values of 3.89 (±0.68) and 2.46 (±0.54) resp/s for control and prenatally cocaine-exposed monkeys, respectively. Similarly, female monkeys did not differ in mean response rates between groups (1.60±0.30 and 2.64±1.16 resp/s, for control and prenatally cocaine-exposed monkeys, respectively). Response extinction was studied by substituting saline for food presentation. A two-way ANOVA revealed a significant main effect of prenatal cocaine exposure (F(1,17)=4.78, p=0.04) but no significant effect of sex and no significant interaction on number of sessions to reach criteria for response extinction. Prenatally cocaine-exposed monkeys required a greater number of sessions to reach criteria for extinguishing food-reinforced responding than control monkeys (Fig. 2).
Fig. 2.
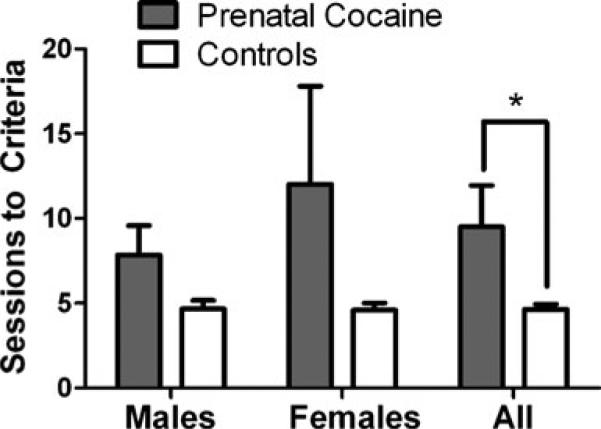
Number of sessions to extinguish previously food-reinforced responding in male and female monkeys prenatally exposed to cocaine (filled bars) and controls (open bars). Each bar represents mean±SEM values. *p<0.05
Experiment 3: effects of prenatal cocaine exposure on delay discounting
Under the concurrent schedule, when the delay was 0 s, monkeys chose the larger magnitude food reinforcer on nearly 100% of the trials. On average, response rates were higher on the key associated with the larger magnitude of food reinforcement (1.56±0.28 resps/s and 1.72±0.23 resps/s for the one-pellet- and three-pellet-associated keys, respectively). Response rates did not differ between prenatally cocaine-exposed and control monkeys nor between male and female monkeys and response rates did not change significantly from baseline at any delay value (Table 1). Increases in the delay value resulted in time-dependent reductions in the percent of trials in which the larger reinforcer was chosen; this relationship is depicted in Fig. 3 for two representative male monkeys. Indifference points were calculated as the delay value (seconds) that engendered 50% choice of the larger, delayed reinforcer and the smaller, immediate reinforcer (Fig. 4). A two-way ANOVA revealed a signficant effect of prenatal cocaine exposure (F(1, 16)=10.56, p=0.005) and a significant interaction between prenatal condition and sex (F(1, 16)=4.8, p=0.04). Post hoc Bonferroni tests indicated that male prenatally cocaine-exposed monkeys had significantly lower indifference points than male control monkeys (p=0.01), indicating that prenatally cocaine-exposed monkeys chose the smaller, immediate reinforcer over the larger reinforcer at shorter delays compared to control male monkeys (Fig. 4). There was no difference between female control and prenatally cocaine-exposed monkeys (Fig. 4).
Table 1.
Response rates (resp/s) during the delay-discounting task
| Delay value (s) | Prenatally cocaine-exposed monkeys |
Control monkeys |
||
|---|---|---|---|---|
| Immediate reinforcer key | Delayed | Immediate reinforcer key | Delayed | |
| 0 | 1.85±0.39 | 1.93±0.29 | 1.27±0.39 | 1.45±0.33 |
| 10a | 1.30±0.35 | 1.11±0.28 | 1.08±0.43 | 1.05±0.33 |
| 30 | 1.53±0.48 | 1.32±0.35 | 0.96±0.27 | 1.03±0.22 |
| 60 | 1.84±0.43 | 1.61±0.40 | 1.19±0.29 | 1.36±0.23 |
| 120b | 1.36±0.29 | 1.60±0.35 | 1.38±0.25 | 1.55±0.34 |
All points are means (±SEM) of ten monkeys, except where noted
n=10 for prenatal cocaine group and n=7 for controls
n=6 for prenatal cocaine group and n=9 for controls
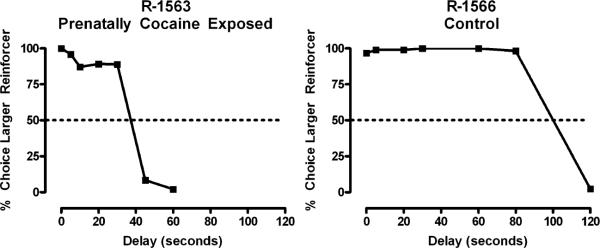
Fig. 3.
Percentage of trials in which the larger, delayed reinforcer was chosen over the smaller, immediate reinforcer as a function of delay value. Data are from two representative male monkeys, one prenatally cocaine exposed (left panel) and one control (right panel). The delay value at which the curve intersects with the dashed line (50% choice of larger reinforcer) represents the indifference point
Fig. 4.
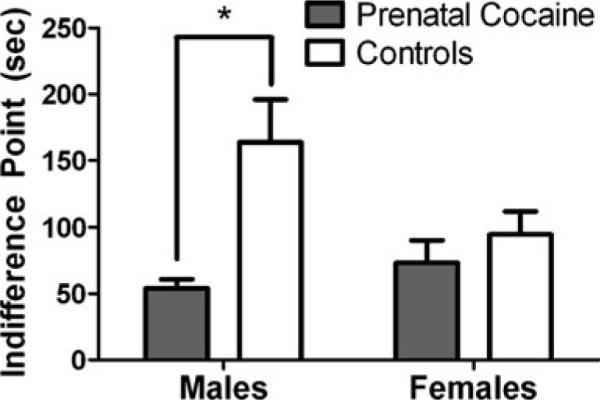
Mean indifference points calculated from delay-discounting procedures for monkeys prenatally exposed to cocaine (filled bars) and controls (open bars). Data are shown for male (left) and female (right) monkeys. Each bar represents mean±SEM values. *p<0.05
Effects of prenatal cocaine exposure on CSF measures of 5-HIAA and HVA
A two-way ANOVA indicated no significant effect of prenatal condition or sex and no interaction of these factors on CSF concentrations of 5-HIAA and HVA (Table 2). There was no significant correlation between CSF 5-HIAA or HVA and any of the dependent variables for group (prenatal cocaine-exposed vs. control) or sex (data not shown). There was a positive significant correlation between baseline concentration of 5-HIAA and HVA (r=0.63, p<0.01).
Table 2.
Comparison of CSF 5-HIAA and HVA (mean±SE) between male and female prenatally cocaine-exposed monkeys and controls
| Male prenatally cocaine exposed | Male controls | Female prenatally cocaine exposed | Female controls | |
|---|---|---|---|---|
| 5-HIAA (nM) | 148±18.1 | 153.5±4.9 | 177.1±6.4 | 156.0±24.4 |
| HVA (nM) | 614.7±68.5 | 734.2±86.8 | 802.7±113.1 | 785.1±87.1 |
Discussion
The purpose of the present studies was to extend earlier work characterizing adult rhesus monkeys prenatally exposed to cocaine and controls to include neurochemical correlates and behavioral endpoints related to measures of impulsivity. To accomplish this, CSF concentrations of the DA metabolite HVA and the 5-HT metabolite 5-HIAA were obtained from ten prenatally cocaine-exposed and ten control monkeys. In addition, several unconditioned and conditioned behaviors were examined. Finally, the interaction between prenatal drug history and sex of the monkey on these various measures was assessed. In general, there were no differences between groups or sexes in CSF concentrations of monoamine metabolites or in response to novelty or locomotor activity. In contrast, prenatally cocaine-exposed monkeys were significantly different from control monkeys on persistence of responding during extinction and prenatally cocaine-exposed male monkeys discounted food reinforcers more than male controls in a delay-discounting procedure; no differences were seen in female monkeys. These findings suggest differential effects of prenatal cocaine exposure on conditioned behaviors that have been deemed models of impulsivity and that these effects may be influenced by sex.
These monkeys represent a unique cohort of animals—adult male and female Old World macaques who had been exposed to cocaine throughout the 25 weeks of gestation (Morris et al. 1996) and grown up with nearly no exposure to drugs of abuse. Using PET imaging, we previously reported that there were no group or sex differences in DA D2-like receptor availability in these adults (Hamilton et al. 2010). Other pharmacological studies revealed no differences in D1-like receptor function, but found significant differences related to prenatal drug exposure and sensitivity to DA D3 agonist effects. Although quinpirole is considered a DA D3/D2 agonist, pharmacological studies have shown that the ascending limb of the quinpirole-elicited yawning dose–response curve is D3-mediated (Collins et al. 2005). Based on the differences in sensitivity to DA D3 agonists, we hypothesized that the prenatally cocaine-exposed monkeys would respond differently to the various behavioral manipulations utilized in the present study, although we had no precise hypotheses about which measures would be most sensitive. The present findings showing that prenatally cocaine-exposed monkeys respond more during extinction and that males have lower indifference points during delay discounting, along with our earlier findings that these monkeys are more sensitive to DA D3 agonist effects (Hamilton et al. 2010), support the idea that the compounds acting at DA D3 receptors could be a promising pharmacological target for treating behaviors related to impulsivity, including substance abuse (for reviews, see Le Foll et al. 2005; Sokoloff et al. 2006; Heidbreder 2008).
We did not find a relationship between behavior and CSF concentrations of the DA metabolite HVA or the 5-HT metabolite 5-HIAA for any unconditioned or conditioned behavior examined. This is in contrast to the extensive literature documenting an association between decreased 5-HIAA levels and increased behaviors thought to model impulsivity in monkeys (Higley et al. 1996; Westergaard et al. 1999, 2003; Fairbanks et al. 1999, 2001, 2004; Manuck et al. 2003). A likely explanation for the discrepancy between findings is the use of different behavioral measures. As described below, the construct of impulsivity is multi-faceted, such that differential contribution of 5-HT and 5-HIAA may be dependent on the experimental conditions and the dependent variable under study. Nonetheless, these data suggest that 5-HT is not necessarily a major contributor to behaviors deemed impulsive.
The present findings also extended our earlier work by showing that differential effects of prenatal cocaine exposure are influenced by the sex of the offspring. Sex-specific effects have also been found in animal studies with males more susceptible to the negative long-term effects of prenatal cocaine exposure on 5-HT receptors (Johns et al. 2002) and DA receptor binding and reactivity (Silvers et al. 2006; Dow-Edwards 2010). Additionally, recent clinical studies reported males to be more adversely affected by prenatal cocaine exposure than females, specifically increasing their risk for problems of inhibitory control (Delaney-Black et al. 2004; Bendersky et al. 2006; Dennis-Tiwary et al. 2006; Bennett et al. 2007). It has been suggested that the male fetus is more vulnerable to in utero stressors and neurotoxins than the female fetus (Kraemer 2000), which may account for the larger deficits observed in prenatally cocaine-exposed males than females. It is also possible that other changes that occur during hormonal variations of adolescence may mask effects of prenatal cocaine exposure until maturation (Cabrera-Vera et al. 2000). Our findings of lower indifference points during delay discounting in male, but not female, monkeys exposed to cocaine throughout gestation support the idea that prenatal cocaine exposure outcomes are influenced by sex.
This is the first report to investigate a wide range of behaviors hypothesized to model aspects of impulsivity in the same cohort of nonhuman primates. Behavioral outcomes from the four tasks used in this study (novel object reactivity, locomotor activity, response extinction and delay discounting) did not correlate with one another, indicating that they do not measure overlapping facets of behaviors related to impulsivity. Interestingly, differences between prenatally cocaine-exposed and control monkeys were only observed in the conditioned behavioral measures. The two unconditioned behavioral measures were tasks typically used in rodents and have been shown to correlate with drug use (Hooks et al. 2001; Klebaur et al. 2001; Piazza et al. 1989, 1990; reviewed in Piazza and Le Moal 1998). It is possible that the more complex behaviors associated with conditioned behavioral measures are necessary to unmask the subtle behavioral differences between prenatally cocaine-exposed monkeys and controls. In future studies, we will be able to establish which behavioral measures correlate with stimulant self-administration in this same cohort of monkeys in order to establish a “behavioral phenotype” predictive of substance abuse. Taken together with our earlier findings, the present results provide evidence for long-term neurobehavioral and neurobiological consequences of prenatal cocaine exposure that may be influenced by the sex of the offspring. Future research examining other behavioral endpoints, such as vulnerability to substance abuse and cognitive performance, will provide insight into the long-term consequences of early cocaine exposure.
Acknowledgements
This research was supported by the National Institute on Drug Abuse grants R01 DA25120, R37 DA10584, and K31 DA024485. The authors report no conflict of interest and would like to acknowledge the excellent technical assistance of Tonya Calhoun and Whitney Wilson. The authors also thank Dr. William Woolverton for advice regarding delay discounting, Dr. Merle Paule for providing information related to the histories of these monkeys and Dr. Peter Pierre for use of the locomotor activity field.
References
- Accornero VH, Amado AJ, Morrow CE, Xue L, Anthony JC, Bandstra ES. Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J Dev Behav Pediatr. 2007;28:195–205. doi: 10.1097/01.DBP.0000268560.72580.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Woolverton WL. Effects of dose and infusion delay on cocaine self-administration choice in rhesus monkeys. Psychopharmacology. 2003;167:424–430. doi: 10.1007/s00213-003-1435-9. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Accornero VH, Morrow CE, Xue L, Simpson GR, Glavach MK, Anthony JC. Longitudinal study of visual attention in prenatally cocaine-exposed children and adolescents. Pediatric Academic Societies' Annual Meeting; Toronto, Canada. 2007. p. 6715.3. [Google Scholar]
- Bendersky M, Bennet DS, Lewis M. Aggression at age five as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol. 2006;31:1–14. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D, Bendersky M, Lewis M. Preadolescent health risk behavior as a function of prenatal cocaine exposure and gender. J Dev Behav Pediatr. 2007;28:467–472. doi: 10.1097/DBP.0b013e31811320d8. [DOI] [PubMed] [Google Scholar]
- Bolig R, Price CS, O'Neill PL, Suomi SJ. Subjective assessment of reactivity level and personality traits of rhesus monkeys. Int J Primatol. 1992;13:287–306. [Google Scholar]
- Cabrera-Vera TM, Garcia F, Pinto W, Battaglia G. Neurochemical changes in brain serotonin neurons in immature and adult offspring prenatally exposed to cocaine. Brain Res. 2000;870:1–9. doi: 10.1016/s0006-8993(00)02382-9. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Curr Opin Psychiatry. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Boinski S. Temperament in nonhuman primates. Am J Primatol. 1995;37:103–125. doi: 10.1002/ajp.1350370205. [DOI] [PubMed] [Google Scholar]
- Coleman K, Tully LA, McMillan JL. Temperament correlates with training success in adult rhesus macaques. Am J Primatol. 2005;65:63–71. doi: 10.1002/ajp.20097. [DOI] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, Nader MA. Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Psychopharmacology. 2009;34:548–554. doi: 10.1038/npp.2008.3. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gage HD, Nader MA. Differences in D2 dopamine receptor availability and reaction to novelty in socially housed male monkeys during abstinence from cocaine. Psychopharmacology. 2010;208:585–592. doi: 10.1007/s00213-009-1756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;215:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred high-responder and low-responder rats. Pharmacol Biochem Behav. 2008;90:331–338. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Nordstrom B, et al. Prenatal cocaine: quantity of exposure and gender moderation. J Dev Behav Pediatr. 2004;25:254–263. doi: 10.1097/00004703-200408000-00005. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, LeMoal M, Simon H. Novelty-seeking in rats—biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Dennis-Tiwary T, Bendersky M, Ramsay D, et al. Reactivity and regulation in children prenatally cocaine exposed. Dev Psycho. 2006;42:688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow-Edwards D. Sex differences in the effects of cocaine abuse across the lifespan. Physiol Behav. 2010;100(3):208–215. doi: 10.1016/j.physbeh.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden J. Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol. 1999;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Fontenot MB, Phillips-Conroy JE, Jolly CJ, Kaplan JR, Mann JJ. CSF monoamines, age, and impulsivity in wild grivet monkeys (Cercopithecus aethiops) Brain Behav Evol. 1999;53:305–312. doi: 10.1159/000006601. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, McGuire MT. Neuropsychopharmacology. 2001;24:370–378. doi: 10.1016/S0893-133X(00)00211-6. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Jorgensen MJ, Huff A, Blau K, Hung YY, Mann JJ. Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. Am J Primatol. 2004;64:1–17. doi: 10.1002/ajp.20057. [DOI] [PubMed] [Google Scholar]
- Fang Y, Janowsky A, Ronnekleiv OK. Cocaine exposure in fetal rhesus monkey: consequences for dopamine D1- and D2-like receptor binding densities. Brain Res Dev Brain Res. 1997;104:163–174. doi: 10.1016/s0165-3806(97)00151-x. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Green L, Myerson J, Woolverton WL. Delay discounting of saccharin in rhesus monkeys. Behav Processes. 2009;82:214–218. doi: 10.1016/j.beproc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacia JG, Makalowski W, Edgemon K, Erdos MR, Robbins CM, Fodor SP, Brody LC, Collins FS. Evolutionary sequence comparisons using high-density oligonucleotide arrays. Nat Genet. 1998;18:155–158. doi: 10.1038/ng0298-155. [DOI] [PubMed] [Google Scholar]
- Hamilton LR, Czoty PW, Gage HD, Nader MA. Characterization of the dopamine receptor system in adult rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology. 2010;210:481–488. doi: 10.1007/s00213-010-1847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder C. Selective antagonism at dopamine D3 receptors as a target for drug addiction pharmacotherapy: a review of preclinical evidence. CNS Neurol Disord Drug Targets. 2008;7:410–421. doi: 10.2174/187152708786927822. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry. 1996;40:1067–1082. doi: 10.1016/S0006-3223(95)00675-3. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice DB., Jr Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 2001;9:121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Howell LL, Sachama KF, Ellis JE, Grrimley PJ, Kitchens AJ, Byrd LD. Fetal development in rhesus monkeys exposed prenatally to cocaine. Neurotoxicol Teratol. 2001;23:133–140. doi: 10.1016/s0892-0362(01)00121-0. [DOI] [PubMed] [Google Scholar]
- Jewitt DA, Dukelow WR. Cyclicity and gestation length of Macaca fascicularis. Primates. 1972;13:327–330. [Google Scholar]
- Johns JM, Lubin DA, Lieberman JA, Lauder JM. Developmental effects of prenatal cocaine exposure on 5-HT1A receptors in male and female rat offspring. Dev Neurosci. 2002;24:522–530. doi: 10.1159/000069363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol. 2001;12:267–275. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Kraemer S. The fragile male. Br Med J. 2000;321:1609–1612. doi: 10.1136/bmj.321.7276.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Nonhuman primate model of the effect of prenatal cocaine exposure on cerebral cortical development. Ann NY Acad Sci. 1998;846:182–193. [PubMed] [Google Scholar]
- Lidow MS. Consequences of prenatal cocaine exposure in nonhuman primates. Dev Brain Res. 2003;147:23–36. doi: 10.1016/j.devbrainres.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Linares TJ, Singer LT, Kirchner HL, Short EJ, Min MO, Hussey P, Minnes S. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006;31:85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Rymeski BA, Fairbanks LA, Wilson ME. Approach to a social stranger is associated with low central nervous system serotonergic responsivity in female cynomologus monkeys (Macaca fascicularis) Am J Primatol. 2003;61:187–194. doi: 10.1002/ajp.10118. [DOI] [PubMed] [Google Scholar]
- Matthys W, van Goozen SH, de Vries H, Cohen-Kettenis PT, van Engeland H. The dominance of behavioural activation over behavioural inhibition in conduct disordered boys with or without attention deficit hyperactivity disorder. J Child Psychol Psychiatry. 1998;39:642–651. [PubMed] [Google Scholar]
- McCleary R. Response-modulating function of the limbic system: Initiation and suppression. In: Stellar E, Sprague J, editors. Progress in physiological psychology. Vol 1. Academic; New York: 1966. pp. 209–271. [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsvity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Morris P, Binienda Z, Gillam MP, et al. The effect of chronic cocaine exposure during pregnancy on maternal and infant outcomes in the rhesus monkey. Neurotoxicol Teratol. 1996;18:147–154. doi: 10.1016/0892-0362(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Morris P, Binienda Z, Gillam MP, et al. The effect of chronic cocaine exposure throughout pregnancy on maternal and infant outcomes in the rhesus monkey. Neurotoxicol Teratol. 1997;19:47–57. doi: 10.1016/s0892-0362(96)00187-0. [DOI] [PubMed] [Google Scholar]
- Morrow CE, Culbertson JL, Accornero VH, Zue L, Anthony JC, Bandstra ES. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30:905–931. doi: 10.1207/s15326942dn3003_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair BS, Watson RR. Cocaine and the pregnant woman. J Reprod Med. 1991;36:862–867. [PubMed] [Google Scholar]
- Newman JL, Perry JL, Carrol ME. Effects of altering reinforce magnitude and reinforcement schedule on phencyclindine (PCP) self-administration in monkeys using an adjusting delay task. Pharmacol Biochem Behav. 2008;90:778–786. doi: 10.1016/j.pbb.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland JS, Singer LT, Short EJ, Minnes S, Arendt RE, Kirchner HL, Bearer C. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol. 2005;27:429–438. doi: 10.1016/j.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Paule MG, Gillam MP, Binienda Z, Morris P. Chronic cocaine exposure throughout gestation in the rhesus monkey. Pregnancy outcomes and offspring behavior. Ann NY Acad Sci. 1996;801:301–309. doi: 10.1111/j.1749-6632.1996.tb17450.x. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol. 1990;1:339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Pulsifer MB, Butz AM, O'Reilly FM, Belcher HM. Prenatal drug exposure: effects on cognitive functioning at 5 years of age. Clin Pediatr. 2008;47:58–65. doi: 10.1177/0009922807305872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick NV, Czoty PW, Gage HD, Kaplan JR, Nader SH, Icenhower M, Pierre PJ, Bennett A, Garg PK, Garg S, Nader MA. Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience. 2009;158:1257–1265. doi: 10.1016/j.neuroscience.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J, Brodsky NL, Malmud E, Giannetta JM, Hurt H. Attentional functioning and impulse control in cocaine-exposed and control children at age 10 years. J Dev Behav Pediatr. 2005;26:42–47. [PubMed] [Google Scholar]
- Silk J, Short J, Roberts J, Kusnitz J. Gestation length in rhesus macaques (Macaca mulatta) Intern J Primatol. 1993;14:95–104. [Google Scholar]
- Silvers JM, Wallace DR, Harrod SB, Mactutus CF, Booze RM. Prenatal cocaine alters dopamine and sigma receptor binding in nucleus accumbens and striatum in dams and adolescent offspring. Neurotoxicol Teratol. 2006;28:173–180. doi: 10.1016/j.ntt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Minnes S, Farkas K, Salvator A. Neurobehavioral outcomes of cocaine-exposed infants. Neurotoxicol Teratol. 2000;22:653–666. doi: 10.1016/s0892-0362(00)00092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Hawkins S, Huang J, Davillier M, Baley J. Developmental outcomes and environmental correlates of very low birthweight, cocaine-exposed infants. Early Hum Dev. 2001;64:91–103. doi: 10.1016/s0378-3782(01)00182-7. [DOI] [PubMed] [Google Scholar]
- Singer LT, Eisengart LJ, Minnes S, Noland J, Jey A, Lane C, Min MO. Prenatal cocaine exposure and infant cognition. Infant Behav Dev. 2005;28:431–444. doi: 10.1016/j.infbeh.2005.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Nelson S, Short E, Min MO, Lewis B, Russ S, Minnes S. Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr. 2008;153:105–111. doi: 10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat's propensity to self-administer nicotine. Psychopharmacology. 2001;158:175–180. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies Results from the 2008 National Survey on Drug Use and Health: National Findings. 2009 Ref Type: Report. [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol. 2007;15:309–327. doi: 10.1037/1064-1297.15.4.309. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi SJ, Higley JD, Mehlman PT. CSF 5-HIAA and aggression in female macaque monkeys: species and interindividual differences. Psychopharmacology. 1999;146:440–446. doi: 10.1007/pl00005489. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi SJ, Chavanne TJ, Houser L, Hurley A, Cleveland A, Snoy PJ, Higley JD. Physiological correlates of aggression and impulsivity in free-ranging female primates. Neuropsychopharmacology. 2003;28:1045–1055. doi: 10.1038/sj.npp.1300171. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Anderson KG. Effects of delay to reinforcement on the choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2006;186:99–106. doi: 10.1007/s00213-006-0355-x. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Myerson J, Green L. Delay discounting of cocaine by rhesus monkeys. Exp Clin Psychopharmacol. 2007;15:238–244. doi: 10.1037/1064-1297.15.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. The psychobiological model for impulsive unsocialized sensation-seeking: a comparative approach. Neuropsychobiology. 1996;34:125–129. doi: 10.1159/000119303. [DOI] [PubMed] [Google Scholar]