Abstract
Background
Traditional materials used as in vitro cell culture substrates are rigid and flat surfaces that lack the exquisite nano- and micro-scale features of the in vivo extracellular environment. While these surfaces can be coated with harvested extracellular matrix (ECM) proteins to partially recapitulate the bio-instructive nature of the ECM, these harvested proteins often exhibit large batch-to-batch variability and can be difficult to customize for specific biological studies. In contrast, recombinant protein technology can be utilized to synthesize families of protein-engineered biomaterials that are cyto-compatible, reproducible, and fully customizable.
Scope of Review
Here we describe a modular design strategy to synthesize protein-engineered biomaterials that fuse together multiple repeats of nanoscale peptide design motifs into full-length engineered ECM mimetics.
Major Conclusions
Due to the molecular-level precision of recombinant protein synthesis, these biomaterials can be tailored to include a variety of bio-instructional ligands at specified densities, to exhibit mechanical properties that match those of native tissue, and to include proteolytic target sites that enable cell-triggered scaffold remodeling. Furthermore, these biomaterials can be processed into forms that are injectable for minimally-invasive delivery or spatially patterned to enable the release of multiple drugs with distinct release kinetics.
General Significance
Given the reproducibility and flexibility of these protein-engineered biomaterials, they are ideal substrates for reductionist biological studies of cell-matrix interactions, for in vitro models of physiological processes, and for bio-instructive scaffolds in regenerative medicine therapies.
Keywords: biomaterial, protein engineering, stem cell niche, extracellular matrix, tissue engineering, regenerative medicine
1. Introduction: Moving cell culture into the third dimension
The vast majority of in vitro mammalian cell culture studies are performed on flat, rigid substrates (most often tissue culture polystyrene (TCPS) or glass in the form of Petri dishes and microscope slides) that do little to mimic the exquisite three-dimensional (3D) nano- and micro-environments found in vivo. In the past, TCPS and glass have been used because they are inexpensive, highly reproducible, cell-permissive for many cell types, and optically transparent. However, numerous recent studies have highlighted the importance of nano- and micro-scale structure [1], substrate mechanics [2], and 3D culture environments [3] in regulating cell adhesion, morphology, migration, signaling, and differentiation [4]. Therefore, in order to recreate physiologically relevant cell behavior in an artificial in vitro environment, it is imperative to design new 3D culture substrates that more accurately mimic the extracellular matrix (ECM). The ideal culture substrate would possess: (i) nano- and micro-scale reproducibility, (ii) flexibility of design, and (iii) the potential to be directly translated from lab-bench studies to clinical therapies. Here we describe recent efforts by our laboratory and others to address these goals through the molecular-level design of novel protein-based biomaterials, Table I.
Table 1.
Comparison of various cell culture substrates and matrices.
| Traditional Surfaces | Naturally-Derived Gels | Designed Gels | |
|---|---|---|---|
| Petri-dish/Coverslip | Collagen/Matrigel/Fibrin | Engineered ECM | |
| Dimensionality | 2D | 2D or 3D | 2D or 3D |
| Reproducibility | High | Variable | High |
| Mechanical Properties | Predetermined | Low, poor reproducibility | Tunable |
| Adhesion Ligands | Requires Coating | Yes, predetermined | Yes, tunable |
| Matrix Degradation | No | Yes, predetermined | Yes, tunable |
| Fibrous Network | No | Physiological | Potentially tunable |
| Commercial Availability | Yes | Yes | No |
| Clinic Translatability | No | Potential | Potential |
These materials are completely constructed from engineered recombinant proteins that are designed to mimic many of the essential properties of natural ECM. Because these materials are synthesized by host organisms through precise translation of a genetic template, the resulting materials are highly reproducible at the molecular level[5]. The genetic template is constructed in a modular fashion that enables easy customization of the engineered protein sequence and hence tailoring of matrix properties such as mechanical rigidity and cell adhesion [6] [7] Precise tuning of these matrix properties has been shown to influence cell behavior, including morphology, migration, gene regulation, intracellular signaling, and differentiation [8, 9]. Therefore, these materials have wide potential for use in reductionist in vitro studies of cell-matrix interactions, development of in vitro platforms that recapitulate complex in vivo processes, and scaffolds for potential regenerative medicine therapies. In the following five sections, we will (i) describe the design concepts used to synthesize these materials and then give specific examples of how these materials can be used to further (ii) mechanistic understanding of cell-matrix interactions, (iii) development of in vitro models of physiological processes, and (iv) advancement of regenerative medicine therapies.
2. Designing protein-engineered mimics of ECM
Traditionally, when choosing a cell-culture substrate, the scientist must decide between a natural or synthetic material, Table I. Tissue culture polystyrene, often modified by coating with natural ECM proteins, tends to be the most popular culture substrate. But as cell studies move toward 3D culture, naturally derived scaffolds are often chosen because they are commercially available in a convenient powder form that can be reconstituted into a 3D hydrogel. Since natural materials such as Matrigel and collagen are derived directly from mammalian sources [10, 11], they offer physiologically relevant chemistries and bio-functionalities. On the other hand, their biological origins also impart an inflexibility of design: natural materials act as a one-size-fits-all system that cannot be easily customized. Furthermore, the processing of commercially available natural materials often destroys higher order structures such as fibrils, can have large batch-to-batch variations, and may initiate immunogenic responses in in vivo studies [11]. It is for these reasons that synthetic polymeric hydrogels, such as poly(ethylene glycol) (PEG) and poly(acrylamide) derivatives, were introduced as 3D cell scaffolds [12]. While these materials are highly reproducible and customizable, they often lack the nano- and micro-scale biological motifs that direct cell behavior and must be carefully screened for potential cyto-toxicity. Comparing natural and synthetic materials, the former provides the advantage of a highly biomimetic structure and chemistry, while the latter supports reproducibility and customization.
In order to combine the advantages of natural and synthetic materials, engineered proteins can be designed for use as cell culture substrates that are biomimetic, reproducible, and customizable, Fig. 1. Through careful selection of the primary amino acid sequence, the resulting engineered ECM-mimetic proteins can be customized to have the desired physical structures, biomechanical properties, and biochemical properties for a particular application. In addition, since these proteins are based on the 20 canonical amino acids found in biological systems, they are inherently cyto-compatible. The primary amino acid sequence is designed by choosing shorter peptide modules that are known to elicit a specific biological response and/or fold into a specific physical structure. These peptide modules can be derived from naturally occurring protein sequences [13], selected through high-throughput screening of random sequences [6], or predicted through computational methods [14]. While engineered protein sequences can also by fabricated using solid-phase synthetic chemistry techniques, recombinant protein expression that utilizes the translational machinery of a host organism allows unparalleled molecular-level control over the primary amino acid sequence [15].
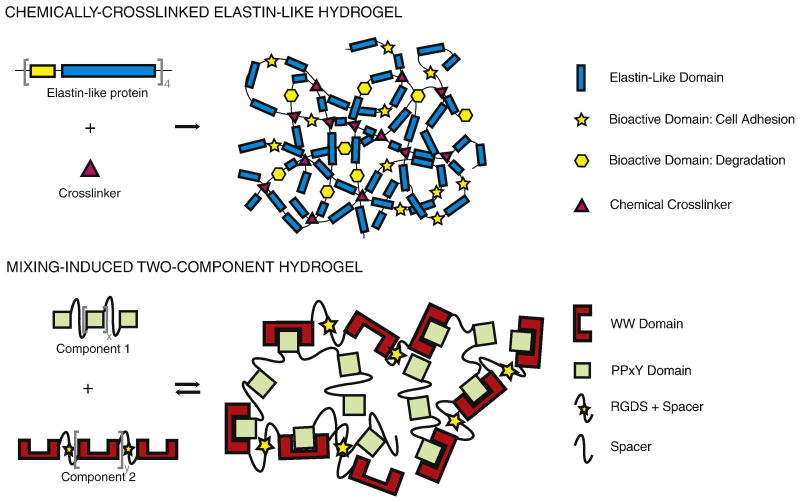
Figure 1.
Schematic of the modular design strategy used to create two families of protein-engineered biomaterials. Top: A chemically-crosslinked hydrogel fabricated from multiple repeats of elastin-like modules, cell adhesion modules, and protease degradation modules [6, 37]. The engineered proteins form a chemical hydrogel network through covalent bonding between a crosslinker and multiple lysine amino acid residues on neighboring protein chains. Bottom: A mixing-induced two-component hydrogel fabricated from two repeating peptide sequences that hetero-assemble [14]. The engineered proteins form a physical hydrogel network through transient hydrogen bonding between WW domains and PPxY domains on neighboring protein chains.
Through careful design of a flexible recombinant cloning strategy, each desired peptide module can be encoded in a specific oligonucleotide that serves as a molecular building block, Fig. 2. Oligonucleotides encoding the selected peptide modules are then spliced together to create a repetitive gene that encodes the engineered ECM-mimetic protein. Several genetic building blocks can be mixed and matched together to design multiple related recombinant genes that encode a family of customized ECM-mimetic proteins with tailored scaffold properties. Once a recombinant gene encoding the desired primary amino acid sequence is synthesized using traditional molecular biology protocols, the resulting plasmid is transformed into a carrier host. Eschirichia coli bacteria are often chosen as host organisms because they are robust, divide rapidly, and require inexpensive fermentation media. The E. coli bacteria translate the genetic template into an exact primary amino acid sequence. The newly synthesized engineered protein can be purified using a variety of methods such as affinity chromatography [14], differential solubility [16], and size-exclusion chromatography, depending on the properties of the designed sequence. These techniques can be optimized to yield significant amounts of purified engineered protein (e.g., up to 1.6 g/L) [17] with FDA-acceptable levels of bacterial contaminants (e.g., up to 0.065–0.115 endotoxin units/mg protein) [18], adequate for the formation of bulk cell culture substrates, Fig. 3.
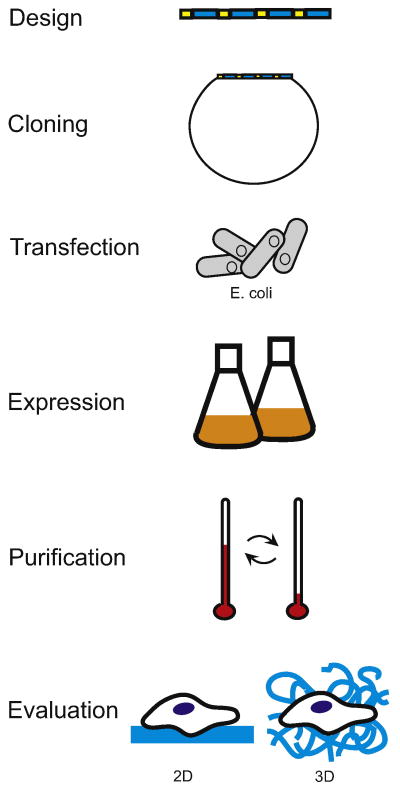
Figure 2.
Flow chart showing the sequence of experimental steps used to fabricate a protein-engineered biomaterial. Once a sequence of repetitive peptide modules is designed, the primary amino acid sequence is encoded in a recombinant gene. Solid-state oligonucleotide synthesis and molecular biology cloning are used to create a plasmid harboring the recombinant gene. The plasmid is transfected into the host of choice, often Escherichia coli. The biosynthetic machinery of the host translates the genetic message into an expressed engineered protein. The target protein is purified away from the host contaminants; for example, differential solubility induced by temperature cycling is often used to purify elastin-like proteins [6]. The proteins are processed into a suitable scaffold through chemical or physical crosslinking. The scaffolds can be used for both 2D and 3D cell culture techniques.
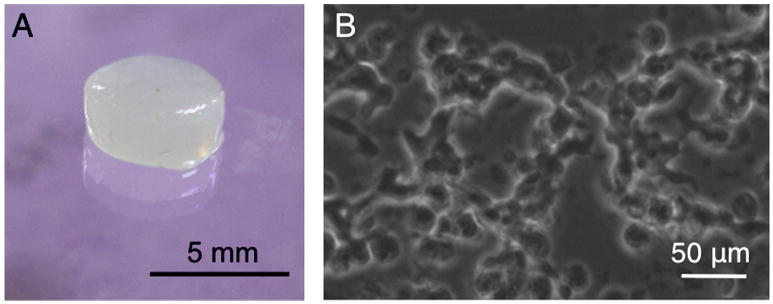
Figure 3.
Images of an elastin-like, protein-engineered biomaterial. A. Photograph of a chemically crosslinked scaffold, 5 mm in diameter, 2 mm in height. B. Phase contrast micrograph of human umbilical vein endothelial cells (HUVEC) growing within a 3D environment inside a chemically crosslinked scaffold.
Integrating concepts from biochemistry, molecular biology, and polymer physics, our group has designed two ECM-mimetic protein families using the modular protein-engineering strategy, Fig. 1. The modules chosen in our protein designs include sequences to initiate cell adhesion (e.g., integrin-binding peptides), sequences to confer resilience (e.g., elastin-like peptides), sequences to promote physical crosslinking (e.g., association peptides), and sequences to enable scaffold degradation (e.g., proteolytic target sites) [19-22]. By utilizing these nanoscale design motifs in various combinations at defined ratios, we can independently customize the properties of the resulting substrates, such as matrix stiffness, cell adhesivity, and proteolytic degradation. Within the field of engineered protein-based biomaterials, a large library of nanoscale peptide design motifs has been explored [13, 23, 24]. These include motifs to induce mineralization [25] and signaling [26] as well as structural modules such as silk [27], collagen [28], and coiled-coil [29] peptides. Despite this large body of work, the amazing diversity of evolved protein structures represents an immense potential to design new protein-engineered biomaterials with novel functionalities[5].
In the following three sections, we will describe three potential application areas where protein-engineered biomaterials are able to enhance biological and medical research: fundamental studies of cell-matrix interactions, in vitro models of complex physiological phenomena, and development of scaffolds for regenerative medicine. For each section, we provide case studies using our own protein designs; however, the reader is also directed to additional examples contained within the following recent reviews [30]. In addition to the full-length protein-engineered materials described in this review, much exciting work is also being performed in the field of small-molecular-weight, peptide-engineered materials. While this work is outside the scope of the current review, the reader is directed to the following excellent reviews of that field [31, 32].
3. Protein-engineered biomaterials in reductionist cell-matrix studies
Fundamental studies of cell-matrix interactions are often difficult to interpret due to the complex crosstalk between biochemical and biophysical factors. When performed in vitro, the ability to produce precise experimental micro-environments with single-variable control severely limits the possibility of confounding results. The use of protein-engineered biomaterials in place of traditional cell culture 2D substrates and 3D matrices confers the ability to independently manipulate biochemical and biomechanical cues to perform reductionist experiments with a series of definitive single-variable changes, thus parsing the complex crosstalk among multiple environmental factors. Furthermore, these biomaterials alleviate the potential batch-to-batch variability often exhibited by harvested, naturally occurring proteins.
Bio-instructive domain sequences incorporated into the recombinant protein system present specific biochemical cues to surrounding cells. As a first example, cell-adhesive peptide modules are commonly included in protein-engineered biomaterials to initiate cell adhesion and subsequent cell signaling via specific ligand-receptor interactions, Fig. 4. In contrast, naturally occurring ECM proteins generally contain multiple bio-instructive ligands that may be present at varying ratios depending on mRNA splice variants, sources of harvested tissue, and methods of ECM protein purification. The types of adhesive ligands included in protein-engineered biomaterials include peptide modules that are recognized by integrin cell surface receptors (e.g., the RGDS and REDV modules of fibronectin) [19, 33, 34], other ECM cell surface receptors (e.g., the YIGSR module of laminin)[35], and cell-cell adhesion receptors (e.g., cadherin modules and neural-cell-adhesion-molecule modules) [35, 36]. To verify that cell behavior is a direct consequence of a specific ligand-receptor interaction, a negative control protein-engineered biomaterial can be designed that contains a variant of the adhesive module with a scrambled amino acid sequence to disrupt ligand-recentor binding [6] Because both the test biomaterial and the negative control biomaterial have nearly identical amino acid sequences, they generally have similar isoelectric points, hydrophobicities, mechanical properties, and structural properties. Therefore, a direct comparison of cell behavior on biomaterials containing the putative ligand and the sequence-scrambled ligand allows for elimination of other potentially confounding experimental variables (e.g., non-specific binding of function-blocking antibodies or incomplete knockdown of receptor expression). For example, the adhesion of PC12 neuronal-like cells to elastin-mimetic biomaterials was prevented by replacing the putative RGDS integrin-binding sequence with the scrambled RDGS control sequence, confirming the functionality of the cell-adhesive RGDS motif [6].
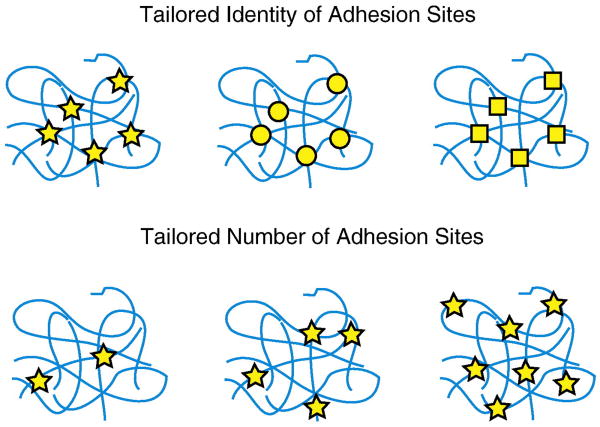
Figure 4.
Customizing the identity and density of bio-instructional ligands. Top: Encoding various nanoscale ligand modules into the primary amino acid sequence will yield protein-engineered biomaterials that elicit specific functionalities. Bottom: The density of the ligand present in the scaffold can be tailored without altering the overall protein density; therefore, these are ideal scaffolds for reductionist single-variable studies.
Beyond simply testing for the effects of the presence of a specific bio-instructive ligand, protein-engineered biomaterials also allow straight-forward manipulation of the concentration of ligand presented to each cell. For example, by simply mixing together protein-engineered family members with the putative RGDS and the scrambled RDGS sequences at varying ratios, a family of scaffolds are created with specific ligand concentrations [6]. In this example, the two protein-engineered family members are pre-mixed and then chemically crosslinked to form a single amorphous hydrogel; therefore, the RGDS ligands are assumed to be uniformly distributed throughout the scaffold. Similar to the example presented above, here the substrates have identical protein concentration, isoelectric points, hydrophobicities, mechanical properties, and structural properties; enabling single-variable studies of ligand concentration. For example, neurite extension from differentiated PC12 cells was found to be directly related to the RGD ligand density between the concentration range of 0 to 1.82 RGD/nm2 [6]. Furthermore, these types of engineered systems can often be designed to present much higher ligand concentrations than would normally be found in vivo. Previous work with an IKVAV sequence has demonstrated that high concentrations may lead to enhanced neuronal (as opposed to glial) differentiation of neural stem cells [37]. Finally, by designing modular protein-engineered scaffolds with multiple bio-instructive modules, it is possible to probe the synergistic effects of many different ligand combinations [35]. This can be achieved simply by combinatorially mixing together multiple protein-engineered family members that contain different nanoscale motifs at tailored concentrations. Alternatively, the primary amino acid sequence of a single protein-engineered family member can be designed to include multiple bio-instructive modules at pre-determined densities.
Although modular design of protein-engineered ECM mimics is a powerful tool for the simplification of complex experiments, the insertion of a specific amino acid sequence into a recombinant protein is not guaranteed to recapitulate the full activity of that sequence when present in the naturally evolved protein [38]. The identity of flanking amino acids may impact the accessibility and the secondary and tertiary structures of the target amino acid sequence, thereby altering the domain's functionality. For example, a single point mutagenesis in the primary amino acid sequence over 40 residues away from a putative minimal binding peptide motif was shown to alter the activity of the CS5 cell-binding domain in a protein-engineered ECM mimic [38]. Therefore, the tertiary structure and activity of all naturally derived sequences inserted into protein-engineered ECM mimics must be assayed to ensure that the modules are producing the intended effect. To help ensure that peptide bioactivity is retained when fused to adjacent peptide modules, many groups include flexible spacer regions between the peptide modules to encourage greater conformational flexibility [39]. Additionally, most peptide modules used to date in protein-engineered biomaterials have been based on relatively simple amino acid sequences that are known to adopt a bioactive conformation even when presented as short peptides (e.g., the RGD cell-binding domain). As the field of protein-engineered biomaterials continues to mature, new protein modules with more complicated tertiary structures and functional activities are beginning to be incorporated into modular synthetic proteins. For example, two enzymatic modules (an aldo-keto reductase domain and a polyphenol oxidase domain) have been successfully incorporated into chimeric fusion proteins with self-assembling leucine zipper domains to form catalytically active protein hydrogels. [40, 41]
In addition to the influence of bio-instructional ligands on cell behavior, the influence of matrix mechanical properties on cell adhesion, morphology, migration, gene regulation, and stem cell differentiation has been demonstrated for multiple cell types [42]. Many studies on cell-matrix mechanics interactions have utilized naturally-derived ECM proteins such as collagen [43, 44]. By altering the protein concentration (and hence density) of the matrix, the mechanical properties can be tuned to be stiffer or more compliant. However, alterations in protein concentration simultaneously change the density of bio-instructional ligands present in the matrix, making it difficult to parse apart the effects of these two variables. In response, several groups have begun to use synthetic polymeric matrices such as chemically crosslinked poly(acrylamide) gels for cell-matrix mechanics studies [45, 46]. These materials are generally not inherently cell adhesive and must be subsequently modified with natural ECM proteins or minimal cell-binding peptide modules to enhance cyto-compatibility [46]. Another alternative is the use of protein-engineered ECM mimics, which enable direct and simultaneous independent tuning of the ligand density and the mechanical properties of the cell culture matrix, Fig. 5.
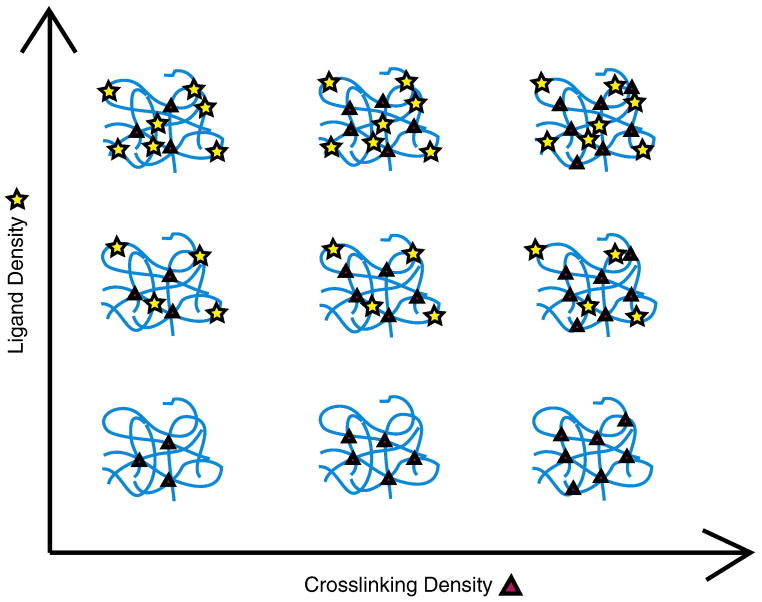
Figure 5.
Independent customization of ligand density and mechanical properties. Increasing the bio-instructional ligand density (vertical axis) without altering the overall protein density will not affect the mechanical properties of the scaffold. Increasing the crosslinking density (horizontal axis) without altering the overall protein density will increase the scaffold rigidity without affecting the ligand density. Therefore, this strategy is used to create a family of related ECM-mimetic biomaterials with customized properties.
Engineered ECM mimics are generally amorphous hydrogels that are composed of multiple protein chains crosslinked together through chemical crosslinks (e.g., covalent bonding) [6], physical crosslinks (e.g., hydrogen bonding) [14, 29], or even both [21]. Although multiple factors may be tailored to influence the mechanical properties of these ECM mimics, the most accessible option is the modulation of crosslinking density. An increase in the density of crosslinks results in stiffer scaffolds (i.e., higher elastic moduli), while a decrease in the density of crosslinks results in more compliant scaffolds (i.e., lower elastic moduli). Depending on the nature of the crosslinks in the designed ECM mimic, a variety of strategies can be utilized to alter the crosslinking density while maintaining a constant ligand density. For example, in chemically crosslinked systems, the amino acids lysine (K) and cysteine (C) are commonly used to induce site-specific crosslinks through reaction with bi- or tri-functional crosslinking molecules [6, 47]. By increasing the number of K or C residues in the primary amino acid sequence of the ECM mimic, the density of potential crosslinks that can be formed is increased [33, 38]. Similarly, simply increasing the efficiency of the crosslinking reaction (either by modulation of temperature, buffer conditions, or crosslinker concentration), the density of crosslinks can be easily tailored [6, 38]. For physically crosslinked systems, which are held together by transient physical bonds, increasing the number of physical crosslinking sites per protein chain results in stiffer matrices [14]. Another approach is to increase the association energy between the physical crosslinking sites, which also will increase the stiffness of the scaffold [14]. Finally, for both chemically and physically crosslinked systems, designing longer engineered protein sequences can be used to promote protein chain entanglements, which act like pseudo-crosslinks and stiffen the matrix [48]. For all of these strategies, careful design of the primary amino acid sequence enables tailoring of the matrix mechanics while maintaining a constant ligand density.
Although modular design of protein-engineered materials imparts combinatorial flexibility, it also places additional responsibility on the designer. Careful attention must be paid to any changes in the material's properties as a result of redesigning the protein sequence. For example, a change in sequence to include more crosslinking sites may cause the protein to form secondary structures that were not previously present, thus altering the mechanical properties in an unintended way. As discussed above, alterations in primary amino acid sequence may also affect ligand activity. Therefore, any tuning of mechanical properties must be accompanied by an analysis of how this customization may have impacted other variables.
One variable of particular importance to assess may be the ‘mesh size’ of the crosslinked scaffold (sometimes also referred to as the ‘pore size’). Similar to the recently published results relating cellular mechanotransduction behavior to matrix stiffness, the ‘mesh size’ of a crosslinked scaffold is also known to be an important variable in regulating cell proliferation and migration in 3D matrices [49, 50]. In general, stiffer scaffolds will also exhibit smaller mesh sizes. As the frequency of crosslinking is increased, the mesh size available for diffusing nutrients and soluble factors becomes smaller, resulting in a more rigid and diffusion-restrictive matrix [51]. In contrast, increasing mesh size influences the likelihood that cells seeded on a 2D scaffold will infiltrate into the matrix or that cells seeded within a 3D scaffold will have sufficient free volume to migrate [52]. Therefore, while independent customization of the scaffold elastic modulus and the scaffold ligand density can now be readily achieved, new strategies are required to begin to dissect the intertwined effects of mechanotransduction and scaffold mesh size.
4. In vitro models of physiological phenomena using protein-engineered materials
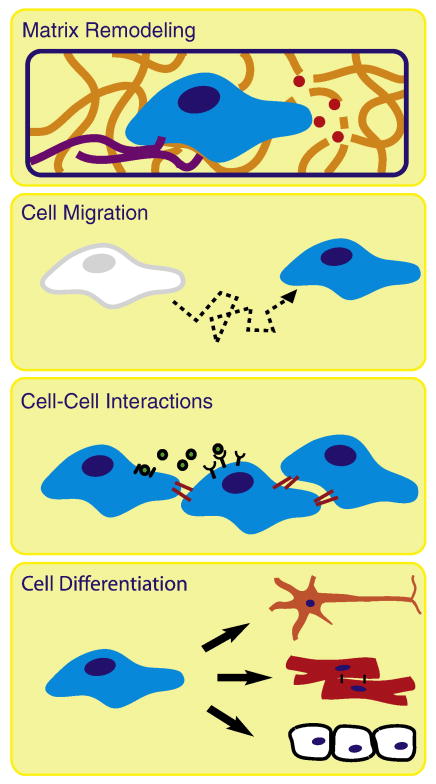
While a reductionist approach is required to parse the crosstalk among several different signaling pathways during cell-matrix interactions, studies of physiologically relevant cellular phenomena often require complex signaling micro-environments. In vivo, a multitude of 3D cues are presented to each cell in order to reinforce and refine a particular directive; however, in vivo studies have several disadvantages such as limited experimenter access to the tissue site, limited ability to perform time-lapse imaging, limited ability to quantitatively customize the micro-environment, and limited ability to perturb a specific cell phenotype without inadvertently disrupting other biological processes. As a consequence, the results of in vivo studies often can be difficult to quantitatively interpret because the effects of a single experimental variable are layered over the responses to a multitude of other cues present in the micro-environment. In addition, in vivo studies are expensive to perform, require extensive technical training, and present important ethical considerations. In response, in vitro 3D culture models are being developed to enable quantitative analyses of physiologically relevant phenomena such as matrix remodeling, cell migration, coordinated cell-cell interactions, and progenitor/stem cell differentiation, Fig. 6.
Figure 6.
Schematic of physiological processes that can be modeled in vitro using protein-engineered biomaterials. Many physiological processes occur in 3D micro-environments that are difficult to access in vivo and are difficult to accurately recreate in vitro. Protein-engineered biomaterials are suitable scaffolds to be customized for ECM-mimetic in vitro models of cell-matrix remodeling, cell migration, collective cell-cell interactions, and stem/progenitor cell differentiation.
Each of these physiologically relevant phenomena is a dynamic process that occurs over a time frame of minutes to weeks. In vivo, the micro-environment is also dynamic, with alterations in bio-instructional ligands, matrix structure, and matrix mechanics responding to the changing needs of the local cells. In contrast, a static cell culture substrate, as discussed above, has pre-designed ligand density and scaffold mechanics to appropriately interact with cells at a single specific time point that may not be appropriate at later times. Therefore, temporal control over scaffold remodeling during the time-course of an experiment is a critical additional property that the experimentalist must be able to control. One strategy to accomplish this is to design synthetic substrates that are responsive to external triggers, such as light, to induce local changes in ligand density and/or scaffold mechanics [53]. For example, this technique has been used to create in vitro microenvironments that regulate cell migration in response to dynamic changes in mechanics and regulate chondrogenic differentiation in response to dynamic changes in ligand density [54]. An alternative strategy to accomplish temporal control of substrate properties is to mimic the proteolytic degradation that occurs during matrix remodeling in vivo [22, 55-57].
For protein-engineered biomaterials, proteolytic target sites can be designed directly into the primary amino acid sequence at specified locations. During experimental studies, the researcher may choose to trigger rapid degradation with the addition of exogenous proteases [57] or to allow cell-secreted proteases to control the timing and extent of degradation [56, 58] Similarly, the extent of degradation and the size of the resulting degradation fragments can be controlled by incorporating fewer or greater numbers of proteolytic target sites into the scaffold [6]. In addition, the kinetics of the cleavage reaction can be customized by making point mutations in the primary amino acid sequence [57]. For example, three elastin-like biomaterials were designed to degrade in response to the protease urokinase plasminogen activator (uPA). By making three point mutations in the putative uPA target site, three scaffolds with 97% sequence homology exhibited customized degradation rates that spanned two orders of magnitude [6]. Depending on the local concentration of protease, this range translated to degradation on the order of minutes to weeks.
The initiation of 3D cell migration in vitro is largely dependent on the scaffold's adhesivity and the ability to locally degrade [52]. While light and laser-assisted photocleavage or scaffold ablation have been used to trigger cell migration for in vitro studies [56], these strategies rely on experimenter-controlled parameters to induce cell polarization into a leading, migrating edge. In contrast, the secretion of proteases by cells in vivo is hypothesized to be a localized, directional process that precedes directed migration and that is triggered by soluble cues such as gradients of growth factors [59]. For example, neurons are thought to selectively secrete the protease uPA directly from the growth cone, i.e., the motile process located at the tip of an extending neurite, while the stationary soma, i.e., cell body, is not believed to secrete this protease [60]. Therefore, by matching the target proteolytic sites in the scaffold to the relevant proteases involved in the cellular process of interest, it is possible to enable specific processes to occur (e.g., neurite extension) while restricting other processes (e.g., soma migration). Using similar types of strategies, synthetic matrices that degrade in response to tissue plasminogen activator (tPA) [57] and matrix metalloproteinase-2 (MMP-2) [22, 56] have also been designed. Along with computational models of cell migration [61], these engineered scaffolds are beginning to elucidate the fundamental mechanisms that govern 3D cell migration, which appear to be quite different from previously studied 2D cell migration mechanisms [62].
While the regulation of a single cell's migration is quite intriguing, it is the coordinated migration of multiple cells that ultimately results in the development or regeneration of new tissues. The coordinated movement of multiple cells is regulated through a complex interplay of cell-matrix and cell-cell interactions. Protein-engineered biomaterials can aid in the study of cell-cell interactions through two main strategies. First, peptide modules that initiate cell-cell signaling cascades, such as sequences derived from cadherin [36] and neural cell adhesion molecule (NCAM) [35] cell-cell receptors, can be directly incorporated into the scaffold. These cell-cell adhesion mimics may be able to elicit responses from a single cell that mimic cell-cell contact effects [63]. Second, ECM mimetic scaffolds can be used as in vitro platforms for time-lapse study of coordinated cell motions. For example, 3D cultures of endothelial cells within elastin-like ECM mimetic scaffolds with appropriate elastic modulus, cell-ligand density, and biodegradability undergo coordinated cell migration to form network-like structures, Fig. 3B. This in vitro network formation mimics a critical early step in in vivo angiogenesis, i.e., the formation of new blood vessels from existing conduits [64]. Building on these early successes, it may be possible to engineer scaffolds for in vitro co-culture studies of more complicated organogenesis processes. For example, the ECM-mimetic scaffold could be designed to permit local biodegradation and coordinated cell migration of a particular cell phenotype in response to protease secretion that occurs only during a specific stage of development or during a specific disease process.
In addition to studies of matrix remodeling, cell migration, and cell-cell interactions; protein-engineered ECM mimics are also ideal substrates for studies of progenitor and stem cell differentiation. Recent reports have highlighted the role that matrix elasticity [42, 45], ligand-receptor interactions [8, 65], and ligand density [66, 67] can have on stem cell differentiation. These engineered stem cell ‘niches’ enable systematic screening of multiple scaffold variables to elucidate the specific cell-microenvironment interactions that direct specific stages of differentiation [65, 68]. To date, the majority of these studies have relied on synthetic polymeric scaffolds and 2D culture environments; however, the expanded use of protein-engineered biomaterials will enable systematic scaffold perturbation in 3D micro-environments that more closely mimic physiologically relevant stem cell niches. For example, adult murine neural stem cells were observed to self-renew and differentiate into glial (GFAP-positive) and neuronal (MAP2-positive) phenotypes in 3D physically crosslinked, protein-engineered biomaterials [14]. Interestingly, even in these relatively compliant matrices with moduli in the range of 10-50 Pa (similar to the mechanical properties of Matrigel), the neurites in these 3D cultures often extended over hundreds of microns [14]. In contrast, in published reports of neural stem cell differentiation on engineered 2D substrates, scaffolds with moduli near 10 Pa were unable to support self-renewal or differentiation [69]. This discrepancy suggests that similar to the biological phenomenon of cell migration [61, [62], the optimal micro-environmental cues to induce differentiation may be different in 2D compared to 3D.
5. Use of protein-engineered biomaterials in regenerative medicine therapies
In the previous sections, we have discussed numerous approaches to studying cellular behavior, all of which involved the sequential layering of nanoscale engineered motifs in the protein-engineered biomaterial to gradually achieve higher levels of complexity. This modular design strategy also supports optimization of scaffold designs for potential clinical applications, although the scaffold requirements for translational therapies may be somewhat different than those required for in vitro biological studies. Design considerations for translational therapies can be grouped into three categories: biomimicry, therapeutic intervention, and clinical handling requirements. The first and often most apparent approach is to specify scaffold properties that mimic the ECM form and function of native biological tissue [70]. Alternatively, the therapeutic strategy might be to initiate tissue regeneration by performing drug delivery and/or redirecting the natural immune response to improve the rate of regeneration [71]. Last, any translational therapy must take into account the practical requirements that accompany its clinical application. Each of these three design considerations is discussed in more detail below.
While cell implantation studies conducted through matrix-free, direct cell injection into the site of injury have yielded encouraging results, cell survival post-injection remains a major concern [72]. Matrix-assisted cell implantation is thus viewed as a promising alternative to direct cell injection. Although regenerative therapies often are focused on in vivo environments, many of these therapies also require some form of tissue expansion or manipulation in vitro before implantation. In particular, the worldwide donor shortage makes in vitro cellular expansion a popular strategy to ensure the implantation of a requisite number of cells [73]. The choice of in vitro micro-environment for pre-implantation culture is critical, as cells will respond to the biomechanical and biochemical cues present in the culture, which may alter subsequent cell behavior upon implantation. Therefore, the reproducibility and tunability of protein-engineered biomaterials makes them ideal scaffolds for cell expansion pre-implantation. An additional potential advantage of protein-engineered biomaterials is the possibility of directly expanding cells within a specific scaffold and then transplanting the entire construct (both cells and scaffold) into the host without requiring cell-harvesting procedures.
In addition to matching of the biomechanical and biochemical properties of the scaffold to mimic the natural tissue, several research groups have also demonstrated that mimicking structural aspects of the natural tissue can also aid in promoting tissue regeneration [45, 74]. For example, aligned fibers, channels, and pores have been utilized to promote longitudinal neurite guidance in scaffolds for spinal cord repair [75-77]. Similar patterning strategies can be employed using protein-engineered biomaterials. For example, nanoscale peptide motifs can be included in the protein design to promote the self-assembly of aligned silk-like fibrils [78], photoactive chemical moieties can be included in the protein to enable photo-lithographic patterning [79], and sequential chemical crosslinking can be utilized to create composite structures of multiple protein-engineered family members [57]. In the latter example, internal pillars of a faster-degrading protein were encapsulated within a matrix of a slower-degrading protein. Because the overall pore size of the scaffold was sufficient to allow the diffusion of proteases, the internal pillars were proteolytically degraded, leaving behind an inverted replica in the form of internal channels with pre-determined dimensions within the surrounding scaffold [57]. These types of internal structures may be helpful in promoting the alignment of longitudinal structures such as nerve fiber bundles or angiogenic sprouts.
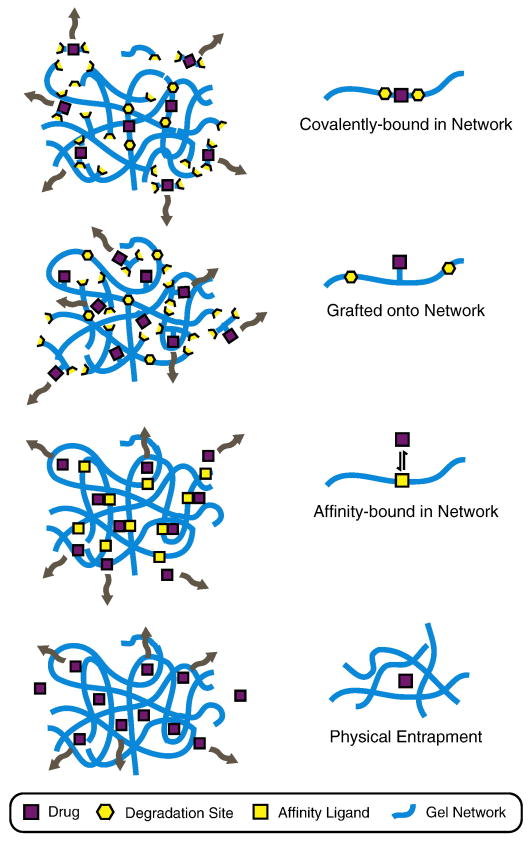
While the aim of many regenerative medicine therapies is to mimic the in vivo tissue as closely as possible, in other scenarios the goal is to provide a therapeutic intervention, such as delivery of a drug that will stimulate regeneration. These therapeutic interventions may not correlate to physiological behavior, but they often take inspiration from natural pathways in wound healing and tissue regeneration. Several strategies can be utilized to deliver pharmacological agents (including small molecules, peptides, and protein pharmaceuticals) from protein-engineered biomaterials with customized release rates, Fig. 7. Often, drug delivery from a polymeric- or protein-based scaffold can achieve more sustained release profiles compared to the burst profiles commonly associated with bolus injections [80]. The simplest strategy is to encapsulate the drug within a scaffold with a tailored mesh size that restricts the diffusion-mediated release of the drug [81]. To further slow the release, the drug can be non-covalently tethered to the protein-engineered scaffold through transient physical bonds [82]. The drug can also be directly covalently bound to the protein-engineered scaffold, either through the use of side-chain grafting [57] or direct incorporation into the primary amino acid sequence if the drug is a peptide pharmaceutical.
Figure 7.
Schematic of drug delivery strategies for protein-engineered biomaterials. The release rate of an encapsulated drug can be customized through selection of an appropriate delivery strategy. The simplest strategy is to physically entrap the drug within the pores of the network and to allow delivery to occur through diffusion. Drug release can be retarded by designing interactions between the drug and network, such as affinity binding, which decrease the effective diffusion rate. By covalently grafting the drug to the protein chain, the drug release can be targeted to occur in response to network degradation, such as protease-induced chain cleavage. Finally, if the drug is a peptide pharmaceutical, the drug can be designed directly into the primary amino acid sequence of the scaffold and released upon network degradation.
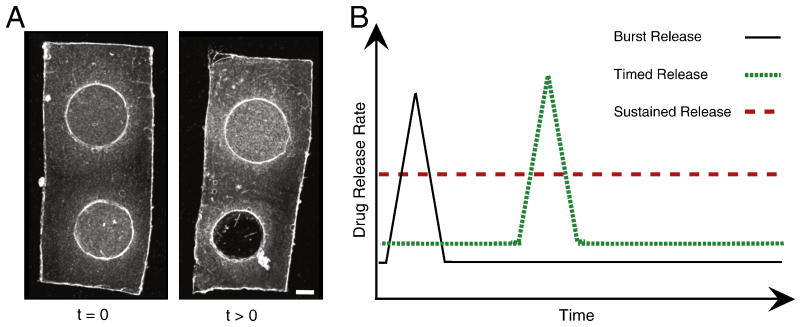
Covalent attachment of the drug within a protein-engineered biomaterial enables the triggered release in response to cell-secreted proteases, Fig. 8. Several diseases and injury states are characterized by alterations in protease secretion [83, [83]; therefore, biomaterials that encode protease target sites may be suitable for the design of disease-responsive treatment strategies that harness the degradative action of disease-regulated proteases to release pharmaceuticals on demand. Further customization of the kinetics of proteolytic degradation can be combined with scaffold patterning to enable the release of multiple drugs with distinct spatial and temporal release profiles, [57]. For example, two fluorescently-labeled model drugs were covalently attached to elastin-like proteins that are cleaved by the protease uPA at two distinct rates. Each drug/engineered-protein combination was patterned to form a disc-shaped ‘drug depot’ that was encapsulated by a third engineered-protein that was enzymatically inert. Upon exposure to the enzyme uPA, the fluorophore in the faster-degrading matrix depot was triggered to release with a burst-like profile while the fluorophore in the slower-degrading matrix depot was triggered to release with a sustained profile [57]. By combining multiple drug delivery strategies and fine-tuning the kinetics of release, future scaffold designs should enable the triggered release of multiple drugs in response to specific cell behaviors.
Figure 8.
Customization of drug release profiles from protein-engineered biomaterials. A. Phase contrast micrograph of a 3D patterned elastin-like biomaterial designed to release two model drugs with two distinct spatial and temporal delivery profiles [57]. Scale bar = 1 mm. Two fluorescently-labeled model drugs were covalently grafted to two different engineered proteins, patterned into two disc-shaped drug depots, and encapsulated within a third engineered protein by chemical crosslinking. The upper drug depot, which is fabricated from a protein designed to slowly degrade in response to the protease uPA, provides a sustained release of the model drug. The lower drug depot, which is fabricated from a protein designed to quickly degrade in response to uPA, provides a burst-like triggered release of the model drug. After several days, the upper drug depot is still present while the lower drug depot has completely disappeared. B. Schematic of three potential drug release profiles that can be designed into protein-engineered biomaterials: burst release, timed burst release (which can be designed to occur in response to a specific biochemical trigger like the protease uPA from the example in panel A), and sustained release.
Finally, for all materials development for regenerative medicine therapies, the surgical administration of the therapy in a clinical setting must be carefully considered. Regardless of the scientific complexity of the underlying regenerative mechanism, clinical administration of the cell/scaffold construct must be as straightforward and reproducible as possible. For example, this could involve designing a scaffold that initially has a more rigid structure to enable easy handling and surgical manipulation, however, after implantation, the scaffold can undergo designed proteolytic degradation either to achieve a more compliant structure that mimics the mechanics of natural tissue or to reveal 3D voids that may guide cell behavior.
For many clinical applications, minimizing the use of invasive surgical procedures through the direct injection of cell, drugs, and/or scaffolds is a key goal. However, direct injection of cells during regenerative medicine therapies often results in extremely low transplanted cell viability [72]. Pre-encapsulation of transplanted cells within hydrogel scaffolds is being explored by several groups as a means to increase transplanted cell viability, provide the transplanted cells with a hospitable micro-environment post-implantation, and ultimately enhance the effectiveness of these promising therapies. While several injectable physical hydrogels including collagen, Matrigel, fibrin, and alginate are being investigated for cell encapsulation therapies; all of these materials require that the transplanted cells be briefly exposed to non-physiological conditions during the encapsulation process such as large shifts in temperature, pH, or ionic concentration [39, 84, 85]. These encapsulation strategies may irreversibly damage the encapsulated cells and accompanying proteins and can be difficult to reproducibly control in a clinical setting. In response, a protein-engineered biomaterial termed MITCH (Mixing-Induced Two-Component Hydrogel) was recently designed to enable cell encapsulation at constant physiological conditions [14]. The two components of the hydrogel are freely-flowing liquids when kept separately; however, upon mixing the two components form physical crosslinks between hetero-association peptide modules designed into the primary amino acid sequences resulting in the formation of a hydrogel. By simply pre-suspending cells, proteins, or drugs in either of the components prior to mixing, they are uniformly encapsulated throughout the hydrogel. Because the hydrogel is held together through transient physical crosslinks, the material is shear-thinning. Upon application of shear force, such as that experienced by hand-injection through a syringe needle, the hydrogen bonds dissociate and allow the material to flow as a liquid. Upon removal of shear force, the hydrogen bonds re-form and the material self-heals to form a hydrogel with identical mechanical properties as before injection [14].
Another promising application for injectable protein materials is the delivery of therapeutic drugs for anti-cancer therapy. The precise modular design of these materials allows an environmentally responsive targeting mechanism to be built directly into the drug [13]. In one example, an inhibitory peptide that blocks cancer cell proliferation was tethered to a thermo-responsive elastin-like polypeptide carrier [86]. This thermo-responsive engineered protein enables thermal targeting of cancerous cells while protecting the stability of the inhibitory peptide. Using the design techniques described in this review, there are numerous opportunities to use protein engineering to deliver customized carrier systems for targeted therapeutics.
While recombinantly engineered protein materials provide a robust and versatile platform for cell-based studies in vitro, their use in vivo must be accompanied by stringent purification steps as well as clinical trials. The most formidable opponent to the clinical application of protein-based materials is the possibility of immunogenicity. Regardless of the intended biomimicry that a protein material may offer, there are numerous opportunities for the material to elicit an unintended biological effect in the patient. In fact, many commonly implanted synthetic biomaterials previously thought to be inert are now known to affect the immune system [87]. Due to the bacterial origin of many recombinant proteins, high levels of endotoxin, which can trigger an innate immune response, may be present in the samples. Endotoxin removal is commonly performed on commercialized recombinant protein therapeutics and can be achieved by several methods such as affinity column chromatography. Previous work with elastin-like biomaterials for potential use as small-diameter vascular grafts has demonstrated the ability to remove endotoxin below U.S. Food and Drug Administration requirements for gram-scale implantation into adult humans [38]. This work also demonstrated that the residual amounts of endotoxin present (0.065-0.115 endotoxin units/mg) were unable to elicit a response from primary human umbilical vein endothelial cells in in vitro culture [38]. As with all biomaterials including synthetic or naturally harvested materials, all post-purification handling must also minimize exposure to potential endotoxin contamination. In addition to potential innate immune responses, the non-self amino acid sequences potentially present in protein-engineered biomaterials may serve as epitopes for antibody production by the adaptive immune system. Taking inspiration from the development process for pharmaceutical vaccines, biomaterial designers may be able to screen multiple amino acid sequences in order to find protein materials that are less immunogenic [88]. As with the development of all new customized biomaterials, potential translation to a clinical setting will require thorough and independent testing of each new material candidate.
Despite the obstacles involved in preparing protein materials for therapeutic applications, protein-based biomaterial therapies are beginning to enter clinical testing. For example, a silk-elastin protein material (NuCore from Spine Wave, Inc., originally designed by Protein Polymer Technologies, Inc.) was approved to enroll patients in a pilot clinical trial to assess product safety for potential use in spinal disc arthroplasty [89]. In addition, many FDA-approved recombinant protein therapies have been developed and marketed by the pharmaceutical industry. The widespread use of these recombinantly derived drugs suggests a promising future for protein-based materials in clinical therapies.
6. Conclusion
In summary, protein-engineered biomaterials are ideal scaffolds for specific biological and medical research endeavors that require highly reproducible, cyto-compatible, customizable materials. Because protein-engineered biomaterials are synthesized using recombinant protein techniques, they require exact control over the primary amino acid sequence. A modular design strategy is used to mix and match multiple nanoscale peptide motifs into a single full-length protein that mimics many of the essential properties of natural ECM. Through careful design, these materials can be engineered to elicit specific cellular behaviors through customization of cell-ligand interactions, tailoring of scaffold mechanical properties, and temporal modulation of scaffold bio-degradation. While these biomaterials may be useful for a wide range of biological and medical research activities, they are particularly well suited to enable single-variable reductionist studies of cell-matrix interactions, to develop in vitro models of complex 3D physiological phenomena, and to facilitate direct translation between lab-bench studies and clinical studies of regenerative medicine scaffolds.
Acknowledgments
N.R. acknowledges support from a Stanford Graduate Fellowship and a National Science Foundation Graduate Fellowship. The authors acknowledge funding from NIH 1DP2 OD006477-01, NSF EFRI-CBE-0735551, NSF DMR-0846363, and a Stanford Cardiovascular Institute Seed Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Park H, Cannizzaro C, Vunjak-Novakovic G, Langer R, Vacanti CA, Farokhzad OC. Nanofabrication and microfabrication of functional materials for tissue engineering. Tissue Eng. 2007;13:1867–1877. doi: 10.1089/ten.2006.0198. [DOI] [PubMed] [Google Scholar]
- 2.Szu-Yuan C, Chao-Min C, LeDuc PR. Composite polymer systems with control of local substrate elasticity and their effect on cytoskeletal and morphological characteristics of adherent cells. Biomaterials. 2009;30:3136–3142. doi: 10.1016/j.biomaterials.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 3.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 4.Chen CS. Mechanotransduction - a field pulling together? J Cell Sci. 2008;121:3285–3292. doi: 10.1242/jcs.023507. [DOI] [PubMed] [Google Scholar]
- 5.Wong Po Foo C, Heilshorn SC. Protein-engineered biomaterials. In: Park SJ, Cochran JR, editors. Protein Engineering and Design. CRC Press; Boca Raton: 2010. [Google Scholar]
- 6.Straley KS, Heilshorn SC. Independent tuning of multiple biomaterial properties using protein engineering. Soft Matter. 2009;5:114–124. [Google Scholar]
- 7.Topp S, Prasad V, Cianci GC, Weeks ER, Gallivan JP. A genetic toolbox for creating reversible Ca2+-sensitive materials. J Am Chem Soc. 2006;128:13994–13995. doi: 10.1021/ja064546i. [DOI] [PubMed] [Google Scholar]
- 8.Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes: Control by extracellular matrix. J Cell Physiol. 1992;151:497–505. doi: 10.1002/jcp.1041510308. [DOI] [PubMed] [Google Scholar]
- 9.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 10.Gobin AS, West JL. Cell migration through defined, synthetic extracellular matrix analogues. FASEB J. 2002;16:751–753. doi: 10.1096/fj.01-0759fje. [DOI] [PubMed] [Google Scholar]
- 11.Little L, Healy KE, Schaffer D. Engineering biomaterials for synthetic neural stem cell microenvironments. Chem Rev. 2008;108:1787–1796. doi: 10.1021/cr078228t. [DOI] [PubMed] [Google Scholar]
- 12.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res Part A. 2000;51:164–171. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Megeed Z, Cappello J, Ghandehari H. Genetically engineered silk-elastinlike protein polymers for controlled drug delivery. Adv Drug Delivery Rev. 2002;54:1075–1091. doi: 10.1016/s0169-409x(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 14.Wong Po Foo C, Lee JS, Mulyasasmita W, Parisi-Amon A, Heilshorn SC. Two-component protein-engineered physical hydrogels for cell encapsulation. Proc Acad Sci USA. 2009;106:22067–22072. doi: 10.1073/pnas.0904851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer DE, Chilkoti A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: Examples from the elastin-like polypeptide system. Biomacromolecules. 2002;3:357–367. doi: 10.1021/bm015630n. [DOI] [PubMed] [Google Scholar]
- 16.Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat Biotechnol. 1999;17:1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- 17.Chow DC, Dreher MR, Trabbic-Carlson K, Chilkoti A. Ultra-high expression of a thermally responsive recombinant fusion protein in E-coli. Biotechnol Prog. 2006;22:638–646. doi: 10.1021/bp0503742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heilshorn SC, Di Zio KA, Welsh ER, Tirrell DA. Endothelial cell adhesion to the fibronectin CS5 domain in artificial extracellular matrix proteins. Biomaterials. 2003;24:4245–4252. doi: 10.1016/s0142-9612(03)00294-1. [DOI] [PubMed] [Google Scholar]
- 19.Nicol A, Gowda DC, Urry DW. Cell adhesion and growth on synthetic elastomeric matrices containing Arg-Gly-Asp-Ser-3. J Biomed Mater Res. 1992;26:393–413. doi: 10.1002/jbm.820260309. [DOI] [PubMed] [Google Scholar]
- 20.Panitch A, Yamaoka T, Fournier MJ, Mason TL, Tirrell DA. Design and biosynthesis of elastin-like artificial extracellular matrix proteins containing periodically spaced fibronectin CS5 domains. Macromolecules. 1999;32:1701–1703. [Google Scholar]
- 21.Sallach RE, Cui W, Wen J, Martinez A, Conticello VP, Chaikof EL. Elastin-mimetic protein polymers capable of physical and chemical crosslinking. Biomaterials. 2009;30:409–422. doi: 10.1016/j.biomaterials.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying C, Ying L, Cheung ACY, Nagai Y, Shuguang Z, Kobler JB, Zeitels SM, Langer R. Incorporation of a matrix metalloproteinase-sensitive substrate into self-assembling peptides - a model for biofunctional scaffolds. Biomaterials. 2008;29:1713–1719. doi: 10.1016/j.biomaterials.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 23.McHale MK, Setton LA, Chilkoti A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005;11:1768–1779. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- 24.Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, Liyou NE, Wong DCC, Merritt DJ, Dixon NE. Synthesis and properties of crosslinked recombinant pro-resilin. Nature. 2005;437:999–1002. doi: 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- 25.Wong Po Foo C, Patwardhan SV, Belton DJ, Kitchel B, Anastasiades D, Huang J, Naik RR, Perry CC, Kaplan DC. Novel nanocomposites from spider silk–silica fusion (chimeric) proteins. Proc Natl Acad Sci USA. 2006;103:9428–9433. doi: 10.1073/pnas.0601096103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu CY, Apuzzo ML, Tirrell DA. Engineering of the Extracellular Matrix: Working toward Neural Stem Cell Programming and Neurorestoration-Concept and Progress Report. Neurosurgery. 2003;52:1154–1167. [PubMed] [Google Scholar]
- 27.Kaplan DL, Yongzhong W, Kim HJ, Vunjak-Novakovic G. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27:6064–6082. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Kutschka IC, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheikh AY, Hendry SL, Pearl J, Hoyt G, Sista R, Yang PC, Blau HM, Gambhir SS, Robbins RC. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation. 2006;114:I.167–I.173. doi: 10.1161/CIRCULATIONAHA.105.001297. [DOI] [PubMed] [Google Scholar]
- 29.Tang Y, Tirrell DA. Biosynthesis of a highly stable coiled-coil protein containing hexafluoroleucine in an engineered bacterial host. J Am Chem Soc. 2001;123:11089–11090. doi: 10.1021/ja016652k. [DOI] [PubMed] [Google Scholar]
- 30.Sengupta D, Heilshorn SC. Protein-Engineered Biomaterials: Highly Tunable Tissue Engineering Scaffolds. Tissue Eng. 2010;16:285–293. doi: 10.1089/ten.teb.2009.0591. [DOI] [PubMed] [Google Scholar]
- 31.Holmes TC. Novel peptide-based biomaterial scaffolds for tissue engineering. Trends Biotechnol. 2002;20:16–21. doi: 10.1016/s0167-7799(01)01840-6. [DOI] [PubMed] [Google Scholar]
- 32.Woolfson DN, Ryadnov MG. Peptide-based fibrous biomaterials: some things old, new and borrowed. Curr Opin Chem Biol. 2006;10:559–567. doi: 10.1016/j.cbpa.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Nowatzki PJ, Tirrell DA. Physical properties of artificial extracellular matrix protein films prepared by isocyanate crosslinking. Biomaterials. 2003;25:1261–1267. doi: 10.1016/s0142-9612(03)00635-5. [DOI] [PubMed] [Google Scholar]
- 34.Girotti A, Reguera J, Rodriguez-Cabello JC, Arias FJ, Alonso M, Testera AM. Design and bioproduction of a recombinant multi(bio)functional elastin-like protein polymer containing cell adhesion sequences for tissue engineering purposes. J Mat Sci: Mater Med. 2004;15:479–484. doi: 10.1023/b:jmsm.0000021124.58688.7a. [DOI] [PubMed] [Google Scholar]
- 35.Straley KS, Heilshorn SC. Design and adsorption of modular engineered proteins to prepare customized, neuron-compatible coatings. Front Neuroeng. 2009;2 doi: 10.3389/neuro.16.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagaoka M, Ise H, Akaike T. Immobilized E-cadherin model can enhance cell attachment and differentiation of primary hepatocytes but not proliferation. Biotechnol Lett. 2002;24:1857–1862. [Google Scholar]
- 37.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 38.Heilshorn SC, DiZio KA, Welsh ER, Tirrell DA. Endothelial cell adhesion to the fibronectin CS5 domain in artificial extracellular matrix proteins. Biomaterials. 2003;24:4245–4252. doi: 10.1016/s0142-9612(03)00294-1. [DOI] [PubMed] [Google Scholar]
- 39.Petka WA, Harden JL, McGrath KP, Wirtz D, Tirrell DA. Reversible hydrogels from self-assembling artificial proteins. Science. 1998;281:389–392. doi: 10.1126/science.281.5375.389. [DOI] [PubMed] [Google Scholar]
- 40.Wheeldon IR, Campbell E, Banta S. A chimeric fusion protein engineered with disparate functionalities-enzymatic activity and self-assembly. J Mol Biol. 2009;392:129–142. doi: 10.1016/j.jmb.2009.06.075. [DOI] [PubMed] [Google Scholar]
- 41.Wheeldon IR, Gallaway JW, Barton SC, Banta S. Bioelectrocatalytic hydrogels from electron-conducting metallopolypeptides coassembled with bifunctional enzymatic building blocks. Proc Natl Acad Sci U S A. 2008;105:15275–15280. doi: 10.1073/pnas.0805249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate Modulus Directs Neural Stem Cell Behavior. Biophys J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juncosa-Melvin N, Boivin GP, Galloway MT, Gooch C, West JR, Sklenka AM, Butler DL. Effects of cell-to-collagen ratio in mesenchymal stem cell-seeded implants on tendon repair biomechanics and histology. Tissue Eng. 2005;11:448–457. doi: 10.1089/ten.2005.11.448. [DOI] [PubMed] [Google Scholar]
- 44.Levental KR, Yu HM, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 46.Reinhart-King CA, Dembo M, Hammer DA. Endothelial cell traction forces on RGD-derivatized polyacrylamide substrata. Langmuir. 2003;19:1573–1579. [Google Scholar]
- 47.Lim DW, Nettles DL, Setton LA, Chilkoti A. Rapid crosslinking of elastin-like polypeptides with hydroxymethylphosphines in aqueous solution. Biomacromolecules. 2007;8:1463–1470. doi: 10.1021/bm061059m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen W, Zhang KC, Kornfield JA, Tirrell DA. Tuning the erosion rate of artificial protein hydrogels through control of network topology. Nat Mater. 2006;5:153–158. doi: 10.1038/nmat1573. [DOI] [PubMed] [Google Scholar]
- 49.Trabbic-Carlson K, Setton LA, Chilkoti A. Swelling and mechanical behaviors of chemically cross-linked hydrogels of elastin-like polypeptides. Biomacromolecules. 2003;4:572–580. doi: 10.1021/bm025671z. [DOI] [PubMed] [Google Scholar]
- 50.Munoz-Pinto DJ, Bulick AS, Hahn MS. Uncoupled investigation of scaffold modulus and mesh size on smooth muscle cell behavior. J Biomed Mater Res Part A. 2009;90A:303–316. doi: 10.1002/jbm.a.32492. [DOI] [PubMed] [Google Scholar]
- 51.Peppas NA, Sahlin JJ. Hydrogels as mucoadhesive and bioadhesive materials: A review. Biomaterials. 1996;17:1553–1561. doi: 10.1016/0142-9612(95)00307-x. [DOI] [PubMed] [Google Scholar]
- 52.Zaman MH, Trapani LM, Siemeski A, MacKellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis (vol 103, pg 10889, 2006) Proc Natl Acad Sci U S A. 2006;103:13897–13897. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bryant SJ, Anseth KS. The effects of scaffold thickness on tissue engineered cartilage in photocrosslinked poly(ethylene oxide) hydrogels. Biomaterials. 2001;22:619–626. doi: 10.1016/s0142-9612(00)00225-8. [DOI] [PubMed] [Google Scholar]
- 54.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32:241–244. [Google Scholar]
- 56.Seliktar D, Zisch AH, Lutolf MP, Wrana JL, Hubbell JA. MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J Biomed Mater Res Part A. 2004;68A:704–716. doi: 10.1002/jbm.a.20091. [DOI] [PubMed] [Google Scholar]
- 57.Straley KS, Heilshorn SC. Dynamic, three-dimensional pattern formation within enzyme-responsive hydrogels. Advanced Materials. 2009;21:1–5. [Google Scholar]
- 58.Halstenberg S, Panitch A, Rizzi S, Hall H, Hubbell JA. Biologically engineered protein-graft-poly(ethylene glycol) hydrogels: A cell adhesive and plasmin-degradable biosynthetic material for tissue repair. Biomacromolecules. 2002;3:710–723. doi: 10.1021/bm015629o. [DOI] [PubMed] [Google Scholar]
- 59.Moscatelli D, Presta M, Rifkin DB. Purification of a factor from human-placenta that stimulates capillary endothelial-cell protease production, DNA-synthesis, and migration. Proc Natl Acad Sci U S A. 1986;83:2091–2095. doi: 10.1073/pnas.83.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seeds NW, Siconolfi LB, Haffke SP. Neuronal extracellular proteases facilitate cell migration, axonal growth, and pathfinding. Cell Tissue Res. 1997;290:367–370. doi: 10.1007/s004410050942. [DOI] [PubMed] [Google Scholar]
- 61.Zaman MH, Kamm RD, Matsudaira P, Lauffenburger DA. Computational model for cell migration in three-dimensional matrices. Biophys J. 2005;89:1389–1397. doi: 10.1529/biophysj.105.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 63.Ogiwara K, Nagaoka M, Cho CS, Akaike T. Construction of a novel extracellular matrix using a new genetically engineered epidermal growth factor fused to IgG-Fc. Biotechnol Lett. 2005;27:1633–1637. doi: 10.1007/s10529-005-2605-0. [DOI] [PubMed] [Google Scholar]
- 64.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 65.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harbers GM, Healy KE. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. J Biomed Mater Res Part A. 2005;75A:855–869. doi: 10.1002/jbm.a.30482. [DOI] [PubMed] [Google Scholar]
- 67.Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res. 2002;60:217–223. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 68.Anderson DG, Putnam D, Lavik EB, Mahmood TA, Langer R. Biomaterial microarrays: rapid, microscale screening of polymer-cell interaction. Biomaterials. 2005;26:4892–4897. doi: 10.1016/j.biomaterials.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 69.Saha K, Irwin EF, Kozhukh J, Schaffer DV, Healy KE. Biomimetic interfacial interpenetrating polymer networks control neural stem cell behavior. J Biomed Mater Res Part A. 2007;81A:240–249. doi: 10.1002/jbm.a.30986. [DOI] [PubMed] [Google Scholar]
- 70.Saha K, Pollock JF, Schaffer DV, Healy KE. Designing synthetic materials to control stem cell phenotype. Curr Opin Chem Biol. 2007;11:381–387. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8:151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 73.Data Reports: Organ Procurement and Transplantation Network. 2009 [Google Scholar]
- 74.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rajnicek AM, Britland S, McCaig CD. Contact guidance of CNS neurites on grooved quartz: influence of groove dimensions, neuronal age and cell type. J Cell Sci. 1997;110:2905–2913. doi: 10.1242/jcs.110.23.2905. [DOI] [PubMed] [Google Scholar]
- 76.Miller C, Jeftinija S, Mallapragada S. Synergistic effects of physical and chemical guidance cues on neurite alignment and outgrowth on biodegradable polymer substrates. Tissue Eng. 2002;8:367–378. doi: 10.1089/107632702760184646. [DOI] [PubMed] [Google Scholar]
- 77.Clark P, Britland S, Connolly P. Growth cone guidance and neuron morphology on micropatterned laminin surfaces. J Cell Sci. 1993;105:203–212. doi: 10.1242/jcs.105.1.203. [DOI] [PubMed] [Google Scholar]
- 78.Stark M, Grip S, Rising A, Hedhammar M, Engstrom W, Hjalm G, Johansson J. Macroscopic fibers self-assembled from recombinant miniature spider silk proteins. Biomacromolecules. 2007;8:1695–1701. doi: 10.1021/bm070049y. [DOI] [PubMed] [Google Scholar]
- 79.Carrico IS, Maskarinec SA, Heilshorn SC, Mock ML, Liu JC, Nowatzki PJ, Franck C, Ravichandran G, Tirrell DA. Lithographic patterning of photoreactive cell-adhesive proteins. J Am Chem Soc. 2007;129:4874–4875. doi: 10.1021/ja070200b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 81.Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. Poly(ethylene glycol)-containing hydrogels in drug delivery. J Control Release. 1999;62:81–87. doi: 10.1016/s0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 82.Yamaguchi N, Zhang L, Chae BS, Palla CS, Furst EM, Kiick KL. Growth factor mediated assembly of cell receptor-responsive hydrogels. J Am Chem Soc. 2007;129:3040–3041. doi: 10.1021/ja0680358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sreenan SK, Zhou YP, Otani K, Hansen PA, Currie KPM, Pan CY, Lee JP, Ostrega DM, Pugh W, Horikawa Y, Cox NJ, Hanis CL, Burant CF, Fox AP, Bell GI, Polonsky KS. Calpains play a role in insulin secretion and action. Diabetes. 2001;50:2013–2020. doi: 10.2337/diabetes.50.9.2013. [DOI] [PubMed] [Google Scholar]
- 84.Gillette BM, Jensen JA, Tang BX, Yang GJ, Bazargan-Lari A, Zhong M, Sia SK. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nat Mater. 2008;7:636–640. doi: 10.1038/nmat2203. [DOI] [PubMed] [Google Scholar]
- 85.Wang S, Nagrath D, Chen PC, Berthiaume F, Yarmush ML. Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel. Tissue Engineering Part A. 2008;14:227–236. doi: 10.1089/tea.2007.0143. [DOI] [PubMed] [Google Scholar]
- 86.Bidwell GL, Whittom AA, Thomas E, Lyons D, Hebert MD, Raucher D. A thermally targeted peptide inhibitor of symmetrical dimethylation inhibits cancer-cell proliferation. Peptides. 31:834–841. doi: 10.1016/j.peptides.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Babensee JE, Anderson JM, McIntire LV, Mikos AG. Host response to tissue engineered devices. Adv Drug Delivery Rev. 1998;33:111–139. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 88.Lee Y, Ferrari G, Lee SC. Estimating design space available for polyepitopes through consideration of major histocompatibility complex binding motifs. Biomed Microdevices. 12:207–222. doi: 10.1007/s10544-009-9376-7. [DOI] [PubMed] [Google Scholar]
- 89.Bao QB, Songer M, Pimenta L, Werner D, Reyes-Sanchez A, Balsano M, Agrillo U, Coric D, Davenport K, Yuani H. Nubac disc arthroplasty: preclinical studies and preliminary safety and efficacy evaluations. SAS Journal. 2007;1:36–46. doi: 10.1016/SASJ-2006-0007-RR. [DOI] [PMC free article] [PubMed] [Google Scholar]