Abstract
Rationale
Much evidence indicates that individuals use tobacco primarily to experience the psychopharmacological properties of nicotine. Varenicline, a partial α4β2 nicotinic acetylcholine receptor (nAChR) agonist, is effective in reducing nicotine craving and relapse in smokers, suggesting that α4β2 nAChRs may play a key role in nicotine dependence. In rats, the effect of varenicline on nicotine intake has only been studied with limited access to the drug, a model of the positive reinforcing effect of nicotine. Varenicline has not been tested on the increase in motivation to take nicotine in nicotine-dependent rats.
Objectives
The present study evaluated the effects of varenicline on nicotine intake in rats with extended access to nicotine self-administration (23 h/day), a condition leading to the development of nicotine dependence. We hypothesized that varenicline's effects on nicotine self-administration would be greater in rats with extended than limited access to the drug and after forced abstinence rather than during baseline self-administration.
Results
Varenicline dose-dependently decreased nicotine self-administration in rats with limited (1 h/day) and extended (23 h/day) access. Despite an increased sensitivity to the motivational effects of abstinence on nicotine intake compared with limited-access rats, varenicline was equally effective in decreasing nicotine intake in dependent rats with extended access to nicotine.
Conclusion
These results suggest that α4β2 nAChRs are critical in mediating the positive reinforcing effects of nicotine but may not be a key element underlying the negative reinforcement process responsible for the increased nicotine intake after abstinence in dependent subjects.
Keywords: Nicotine, α4β2 nicotinic receptor, Varenicline, Self-administration, Craving, Dependence
Introduction
Much evidence indicates that individuals use tobacco primarily to experience the psychopharmacological properties of nicotine and that a large proportion of smokers eventually become dependent on nicotine (Balfour 1984; Stolerman 1991). The main pharmacological strategy to aid smoking cessation is nicotine replacement therapy through use of a nicotine patch or nicotine-containing gum (Silagy et al. 2004). Although the positive effects of nicotine replacement therapy are clear during early withdrawal as it reduces the negative emotional states experienced during early abstinence, its effect on nicotine craving and relapse after protracted abstinence are not clearly established. Nicotine replacement therapy only slightly decreases relapse rates in smokers (Silagy et al. 2004). Bupropion, an atypical antidepressant, also reduces the severity of nicotine craving during withdrawal, but the long-term beneficial effects of bupropion on relapse are not clear (Hughes et al. 2007). A recent alternative to nicotine replacement therapies and antidepressants is the use of partial nicotinic agonists, such as varenicline. Varenicline, a partial α4β2 nicotinic acetylcholine receptor (nAChR) agonist, decreases relapse rates in humans (Gonzales et al. 2006; Jorenby et al. 2006; Tonstad et al. 2006), decreases nicotine self-administration (Rollema et al. 2007a), partially substitutes for nicotine under a progressive ratio of reinforcement (Rollema et al. 2007a), and increases nicotine-induced dopamine release in the dorsal striatum and nucleus accumbens in rats (Rollema et al. 2007a); however, the effects of varenicline on nicotine intake has only been studied in rats with limited access to the drug, a model with good validity for the positive reinforcing effect of nicotine but limited face and construct validity for the negative reinforcing effect of nicotine and dependence per se. Indeed, rats given limited access (1–3 h daily) to nicotine show very limited spontaneous signs of withdrawal and do not show increased motivation to self-administer nicotine after a prolonged period of abstinence (George et al. 2007; Paterson and Markou 2004). The objective of the present study was to evaluate the efficacy of varenicline in a more relevant animal model of nicotine dependence using extended intermittent access to nicotine self-administration in rats (George et al. 2007; Valentine et al. 1997; Fu et al. 2003; LeSage et al. 2003; Paterson and Markou 2004; O'Dell and Koob 2007). Indeed, these studies have shown that rats given 23 h daily (4 days/week) access to nicotine exhibit robust spontaneous withdrawal symptoms (O'Dell and Koob 2007).
Administration of nicotine itself decreases nicotine self-administration (LeSage et al. 2003) and mecamylamine, a nicotinic antagonist, increases nicotine intake in rats given chronic extended access to nicotine self-administration (O'Dell and Koob 2007). Moreover, blockade of the brain stress system using a corticotropin-releasing factor-1 (CRF1) receptor antagonist decreases nicotine intake in rats with extended access but not in rats with limited access (George et al. 2007), suggesting that the neurobiological mechanisms underlying the motivation for nicotine in rats given limited or extended access may differ in a way that is consistent with a dependence model. Chronic self-administration of nicotine leads to resistance to extinction and significant escalation in intake in rats given intermittent bouts of 4-day access to nicotine (O'Dell and Koob 2007). Finally, a dramatic increase in nicotine self-administration is observed in 23-h access animals after 3 days of abstinence, with daily intake exceeding 3 mg/kg/day (O'Dell and Koob 2007; George et al. 2007). The nicotine deprivation effect observed in rats with extended access to nicotine is similar to the human condition in which increased nicotine craving associated with an increase in smoking is observed after abstinence (i.e., an increase in the number and duration of puffs) followed by a titration period of nicotine intake (Benowitz and Jacob 1984; Isaac and Rand 1972). These results suggest that the comparison of the pharmacological effects of varenicline on nicotine intake in rats given limited vs. extended access to nicotine is a key step to better understand the specific role of α4β2 nAChRs in nicotine reward and nicotine dependence and to evaluate the efficacy of varenicline in a relevant model of nicotine dependence.
A prominent hypothesis in the nicotine addiction field is that upregulation of α4β2 nAChRs is a key element in nicotine dependence (Benwell et al. 1988; Breese et al. 1997; Perry et al. 1999; Marks et al. 1983, 1985, 1992; Schwartz and Kellar 1983), and that varenicline may be particularly efficient in reducing nicotine intake in dependent subjects. If this hypothesis is correct then, it is expected that varenicline would be more effective at reducing nicotine intake in rats with extended rather than limited access to nicotine self-administration. We hypothesized that varenicline would be more effective in rats with extended rather than limited access to the drug and after prolonged abstinence rather than during baseline self-administration. The results show that moderate to high-dose varenicline dose-dependently decreased nicotine self-administration in rats with limited and extended access to nicotine. Varenicline was equally effective in decreasing nicotine intake in rats with extended and limited access to nicotine despite an increased sensitivity to the motivational effects of abstinence on nicotine intake in rats with extended access compared with limited access rats. Finally, the effect of a low dose of varenicline was dependent on the baseline level of nicotine intake suggesting that the efficacy of varenicline may depend on the level of cigarette smoking in humans and not on the level of dependence per se.
Materials and methods
Animals
Male Wistar rats (250–275 g) (Charles River, Hollister, CA) were used for all experiments. The animals were group-housed and maintained on a 12-h/12-h light/dark cycle (dark at 10:00 AM) with ad libitum access to food and water. All animal procedures were approved by The Scripps Research Institute Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines.
Drugs
Nicotine hydrogen tartrate salt (Sigma, Natick, MA) was dissolved in saline at pH 7.4 and self-administered via an indwelling jugular catheter. Doses are expressed as free base. Varenicline was dissolved in saline and administered subcutaneously in a volume of 1 ml/kg 30 min before the session. Varenicline was administered using a Latin square design during baseline self-administration and after 3 days of abstinence before renewed access to nicotine.
Nicotine self-administration
The apparatus and detailed procedures for both intravenous catheterization and nicotine self-administration have been described previously (George et al. 2007). Rats were first trained to nosepoke for food and water in 23-h sessions prior to and after recovery from surgical implantation of jugular catheters but were not trained to respond to the lever that was associated with nicotine delivery. Following acquisition of these operant responses, the active and inactive levers were extended, and the rats were allowed to self-administer nicotine (0.03 mg/kg/100 μl/1 s; free base; fixed ratio, 1; time-out, 20 s) by pressing the active lever. Rats were first given access to nicotine for 1 h per day during the dark cycle (10:00 AM) for 1 week and then separated into two groups given short access (ShA, 1 h/day) and long access (LgA, 23 h/day) to nicotine for 2 weeks. After stabilization of responding, responding under a progressive ratio schedule of reinforcement was tested during baseline and after abstinence. The progression of lever presses was 1, 2, 4, 6, 9, 12, 15, etc. (Moreno et al. 2010). Break point was arbitrarily defined as the last ratio completed with no reinforced response for 60 min. FR and PR responding were measured during baseline condition and after abstinence during 2 weeks using a within-subjects design. The effects of varenicline were tested on baseline responding using a Latin square design. After completion of baseline testing, the rats were given access to nicotine 4 days per week to test the effects of varenicline on the nicotine deprivation effect (after 3 days of abstinence).
Statistical analysis
Results were analyzed with Statistica software using analysis of variance (ANOVA). In all cases, a normality test and an equal variance test were performed before the ANOVA to ensure its validity, with ShA/LgA (two levels) and active/inactive response (two levels) as between-subjects factors. The dose of varenicline (four levels) and the time of testing (baseline/nicotine deprivation effect; two levels) were used as within-subjects factors. Newman–Keuls post hoc tests and Pearson correlations were used when necessary. When assumptions in the ANOVA were violated, the nonparametric Kruskal–Wallis test was used, followed by Welch's t test. Data are expressed as mean ±SEM.
Results
Effect of abstinence from nicotine on nicotine intake and the motivation for nicotine in rats with limited and extended access to nicotine self-administration
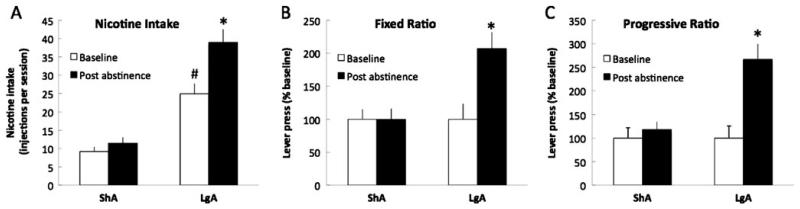
After 3 weeks of nicotine self-administration, the rats that were given short (1 h/day, ShA) or long (23 h/day, LgA) access to nicotine exhibited a very stable level of responding for the active (ShA=9.2±1.3 injection or 0.28±0.04 mg/kg; LgA=24.9±3.0 injections or 0.75±0.09 mg/kg) and inactive (ShA=4.5±1.0; LgA=9.5±3.1) levers. LgA rats exhibited higher nicotine intake per session during baseline and after abstinence compared to ShA rats (F1,16=9.7, p<0.05; Fig. 1a) and increased nicotine intake during the entire session after 3 days of abstinence (p<0.05, Fig. 1a). Moreover, after 3 days of abstinence, only LgA rats increased responding during the first hour of access to nicotine (F1,16=1.5, p<0.05; Fig. 1b) and when tested with a PR schedule of reinforcement (F1,16=2.1, p<0.05; Fig. 1c), whereas ShA rats remained stable. These results demonstrate that LgA rats exhibit increased nicotine intake and increased motivation for nicotine after 3 days of abstinence from nicotine compared to ShA rats.
Fig. 1.
Effect of abstinence from nicotine on nicotine intake and the motivation for nicotine in rats with limited and extended access to nicotine self-administration. a Total number of nicotine injection during the session in ShA (1 h, n=8) and LgA (23 h, n=10) rats during baseline and after 3 days of abstinence. b Mean active lever presses during the first hour under a fixed ratio of 1 of reinforcement in ShA (n=8) and LgA (n=10) rats represented as a percentage of baseline. *P<0.05, compared with baseline. c Mean active lever presses under a progressive ratio of reinforcement in ShA (n=8) and LgA (n=10) rats represented as a percentage of baseline, during baseline, and after 3 days of abstinence. *P<0.05, compared with baseline, # P<0.05 compared with ShA
Effects of varenicline on baseline nicotine self-administration
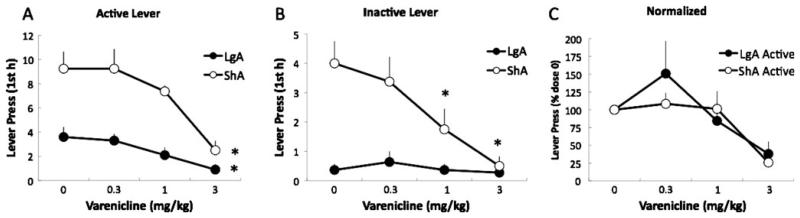
To test whether varenicline is differentially effective in reducing nicotine self-administration in nondependent and nicotine-dependent rats, we measured the effects of systemic injection of varenicline (0.3, 1, and 3 mg/kg) on nicotine self-administration in rats that were given short (1 h/day, ShA) or long (23 h/day, LgA) access to nicotine self-administration. Varenicline dose-dependently reduced nicotine intake in LgA rats, an effect mainly observed during the first 2 h of self-administration (Table 1) consistent with the pharmacokinetic properties of varenicline. In order to compare the efficiency of varenicline in ShA and LgA rats during the same period of time postadministration, we then focused our analysis on the first hour intake. Varenicline dose-dependently suppressed nicotine self-administration in ShA and LgA rats during the first hour, reaching significance at the highest dose of 3 mg/kg (F3,48=17.2, p<0.05, Fig. 2a). Varenicline also dose-dependently decreased inactive lever pressing in ShA rats (F3,48=10.4, p<0.05; Fig. 2b). LgA rats exhibited a very low level of responding on the inactive lever in the first hour of access and no effect of varenicline was observed on inactive lever pressing in this group at any time point. Comparisons between active and inactive lever pressing in ShA rats showed that inactive lever pressing was more sensitive to the effects of varenicline in the ShA group. Varenicline decreased inactive lever pressing at the 1 mg/kg dose, whereas only the 3 mg/kg dose was effective on active lever pressing in ShA and LgA rats for the first hour (Fig. 2a, b). The effects of varenicline on responding on the active lever appeared to be more effective in ShA rats than in LgA rats because of a higher responding baseline (p<0.05); however, no difference in the effect of varenicline was observed between the two groups after normalization for baseline nicotine self-administration (F2,32=0.7, p>0.05; Fig. 2c), although LgA rats exhibited a nonsignificant increase in active lever pressing at the lower dose (0.3 mg/kg). These results demonstrate that varenicline similarly reduced nicotine intake in nondependent and dependent rats during the first hour after administration.
Table 1.
Effect of varenicline administered during baseline self-administration on nicotine intake in LgA rats
| Dose (mg/kg) | 1h | 2h | 4h | 6h | 12 h | 23 h |
|---|---|---|---|---|---|---|
| 0 Mean | 3.4 | 5.5 | 6.7 | 8.9 | 16.4 | 28.7 |
| SEM | 0.9 | 1.1 | 1.3 | 1.7 | 2.0 | 2.2 |
| 0.3 Mean | 3.3 | 4.2 | 5.9 | 8.8 | 17.2 | 27.2 |
| SEM | 0.6 | 0.5 | 0.9 | 1.5 | 2.2 | 2.8 |
| 1 Mean | 2.1 | 3.9 | 6.0 | 8.1 | 18.2 | 26.6 |
| SEM | 0.6 | 1.1 | 1.1 | 1.5 | 2.6 | 2.6 |
| 3 Mean | 0.9*,*** | 1.0**,***,**** | 3.3 | 5.2 | 12.4 | 21.4 |
| SEM | 0.5 | 0.4 | 1.2 | 1.7 | 2.8 | 2.3 |
Note that the effect of varenicline significantly decreased nicotine intake during the first 2 h of self-administration
p<0.05
p<0.01 vs. dose 0
p<0.05 vs. dose 0.03
p<0.05 vs. dose 1
Fig. 2.
Effects of varenicline on baseline nicotine self-administration in ShA and LgA rats. a Mean active lever presses during the first hour in ShA (n=8) and LgA (n=10) rats. b Mean inactive lever presses during the first hour in ShA and LgA rats. *P<0.05, compared with 0 dose. c Mean active lever presses in ShA and LgA rats after normalization for baseline responding (represented as a percentage of the 0 dose). Data are expressed as mean ± SEM
To test whether varenicline would be more effective at reducing nicotine self-administration in an animal model of excessive nicotine intake, we tested the effects of varenicline on nicotine self-administration after 3 days of forced abstinence. Indeed, varenicline dose-dependently reduced nicotine intake in LgA rats, an effect that lasted for a longer period than when tested during baseline self-administration since the effect was still observed during the first 6 h of self-administration (Table 2).
Table 2.
Effect of varenicline administered after 3 days of abstinence on nicotine intake in LgA rats
| Dose (mg/kg) | 1h | 2h | 4h | 6h | 12 h | 23 h |
|---|---|---|---|---|---|---|
| 0 Mean | 6.5 | 8.2 | 10.2 | 14 | 23.8 | 33 |
| SEM | 0.9 | 1.1 | 1.6 | 2.1 | 3.4 | 3.9 |
| 0.3 Mean | 6.3 | 7.7 | 8.4 | 13 | 24.0 | 33 |
| SEM | 0.9 | 1.1 | 1.0 | 2.2 | 3.9 | 5.3 |
| 1 Mean | 4.7 | 5.9 | 7.7 | 11 | 20.2 | 31 |
| SEM | 0.6 | 1 | 1.1 | 1.8 | 3.3 | 4.8 |
| 3 Mean | 0.5**,*** | 1.1**,*** | 2.5**,*** | 5.1**,*** | 14.5 | 20 |
| SEM | 0.3***** | 0.4***** | 1.0***** | 1.3**** | 2.8 | 3.2 |
Note that the effect of varenicline significantly decreased nicotine intake during the first 6 h of self-administration
p<0.05
p<0.01 vs. dose 0
p<0.05 vs. dose 0.03
p<0.05
p<0.01 vs. dose 1
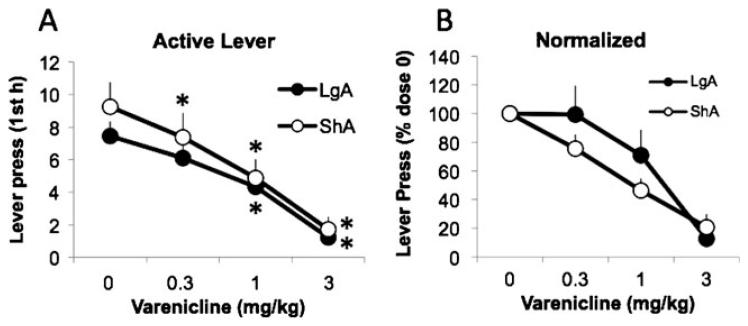
In order to compare the efficiency of varenicline in ShA and LgA rats during the same period of time postadministration, we then focused our analysis on the first hour of intake. Varenicline similarly decreased nicotine responding in ShA and LgA rats (F3,48=28.3, p<0.05; Fig. 3a), an effect still observed after normalization to baseline responding (F2,26=13.3, p<0.05; Fig. 3b); however, no significant difference was observed between ShA and LgA rats (lever press: F3,48=0.6, p>0.05; normalized: F2,26=1.3, p>0.05). Varenicline also decreased inactive lever pressing in ShA rats (data not shown). Similar to the previous experiment, LgA rats had very few inactive lever presses during the first hour, precluding any analysis of the effects of varenicline on inactive lever responding in LgA rats. Varenicline was more effective in both groups in reducing nicotine self-administration after abstinence than during baseline self-administration, despite similar levels of responding (~10 lever presses in 1 h) during the first hour. Indeed, varenicline was effective at reducing nicotine self-administration at the intermediate dose (1 mg/kg) when tested after abstinence, whereas it was only effective at the highest dose (3 mg/kg) when tested under baseline responding (Fig. 2a).
Fig. 3.
Effects of varenicline on nicotine self-administration after 3 days of abstinence in ShA and LgA rats. a Mean active lever presses during the first hour in ShA and LgA rats. *P<0.05, compared with 0 dose. b Mean lever presses after normalization for baseline responding (represented as a percentage of the 0 dose). Data are expressed as mean ± SEM
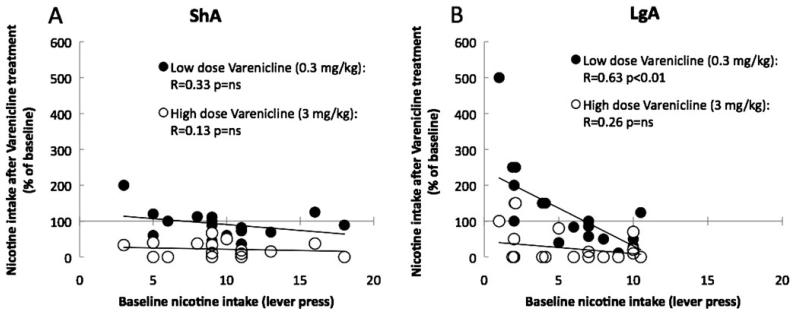
Although the effects of varenicline on nicotine intake were very robust at the intermediate and high dose, important individual variability was observed in the effects of low-dose varenicline in LgA rats during the first hour of access. To test whether this variability was related to the amount of nicotine intake during baseline, we correlated nicotine intake during baseline with nicotine intake after treatment with a low (0.3 mg/kg) or a high (3 mg/kg) dose of varenicline. As shown in Fig. 2, the high dose of varenicline dramatically reduced nicotine intake in both ShA and LgA rats, an effect that was not related to baseline nicotine intake as demonstrated by the lack of significant correlation (Fig. 4a, b); however, the effect of low-dose varenicline was highly correlated with baseline nicotine intake in LgA rats (Fig. 4b). LgA rats exhibiting low intake during the first hour of access to nicotine self-administration increased responding for nicotine while rats with high nicotine intake exhibited a decrease in nicotine intake.
Fig. 4.
Low-dose varenicline increases responding for nicotine in rats with low nicotine intake. a, b Correlation between baseline nicotine intake (after vehicle injection) and nicotine intake after varenicline treatment with a low (0.3 mg/kg) or high dose (3 mg/kg) in ShA (a) and LgA (b) rats, calculated as a percentage of baseline responding
Discussion
This report demonstrates that varenicline decreases nicotine self-administration in rats. The effect of varenicline on nicotine self-administration was similar in rats given limited or extended access to nicotine self-administration. This effect was dose-dependent but not specific to the active lever in ShA rats. Varenicline (1 and 3 mg/kg) decreased both baseline self-administration and self-administration after 3 days of forced abstinence; however, varenicline was more effective at reducing nicotine self-administration when tested after abstinence than during baseline self-administration. The effect of varenicline on nicotine intake during the first hour in rats given limited and extended access to nicotine was similar despite a dramatic increase in LgA rats in nicotine motivation after 3 days of abstinence; however, varenicline had a long duration of action in LgA rats with significant decreases at the 6-h time point. Finally, low-dose varenicline (0.3 mg/kg) increased nicotine intake in rats with low baseline responding for nicotine, whereas it decreased intake in rats with high nicotine intake.
The decrease in responding observed in rats given limited access to nicotine after varenicline administration replicates the original results observed by Rollema et al. (2007a). We extended this finding by demonstrating that this effect is not specific to the active lever. Considering the relatively high level of responding for the inactive lever during limited access (1 h) to nicotine self-administration it is possible that inactive responding was partially driven by nicotine seeking. Alternatively, varenicline may have nonspecific effects on locomotor activity, exploratory behavior, or motivation in general that may partially contribute to its effects on nicotine intake (Zaniewska et al. 2008). It is important to note that in this experiment, rats have been trained for 3 weeks before testing varenicline and exhibited very stable responding and a 2–2.6-fold discrimination ratio between active and inactive lever suggesting that rats reached a plateau in the acquisition of nicotine self-administration. The previous study investigating the effects of varenicline on nicotine self-administration (Rollema et al. 2007a) did not report any responding on the inactive lever, precluding any analysis of the specificity of the effects of varenicline on responding for nicotine.
The relative lack of effect of varenicline in LgA rats during baseline self-administration is likely attributable to a floor effect because normalization with baseline responding led to similar results in ShA and LgA rats; however, we did not observe a floor effect with ShA rats with the inactive lever, despite similar levels of responding as LgA for the active lever (4.0±0.8 vs. 3.6±0.8), suggesting that responding for nicotine under baseline conditions in LgA rats may be less sensitive to the effects of varenicline. Increased upregulation and/or desensitization of α4β 2nAChRs (Buisson and Bertrand 2002; Picciotto et al. 2008; Govind et al. 2009) in LgA rats after extended access to nicotine could contribute to this difference by slightly shifting the dose–response curve of varenicline to the right in LgA rats.
Varenicline was more effective on nicotine intake after 3 days of abstinence than during baseline self-administration in both ShA and LgA rats. Indeed, lower doses (0.3 and 1 mg/kg) decreased nicotine self-administration, after abstinence, whereas only the highest dose (3 mg/kg) decreased responding during baseline self-administration. These results suggest that after 3 days of abstinence, nicotinic receptors were more sensitive to the effects of varenicline. Converging lines of evidence suggest that chronic nicotine treatment upregulates and desensitizes nicotinic receptors in the brain and that abstinence from nicotine reverses these effects (Govind et al. 2009; Picciotto et al. 2008). The increased efficacy of varenicline in reducing nicotine self-administration after abstinence likely results from increased (or normalization) of nicotinic receptor function in ShA and LgA rats. Considering that the effect of varenicline was similar in ShA and LgA rats, these results suggest that restored sensitivity of the cholinergic system during abstinence, possibly through normalization of nicotinic receptor function, is an important mechanism underlying the motivation for nicotine but does not represent a specific neurobiological mechanism contributing to the increased motivation for nicotine in dependent rats. Indeed, we demonstrated that LgA rats, but not ShA rats, exhibit increased motivation for nicotine after abstinence when tested under a fixed ratio or a progressive ratio of reinforcement. These results, together with the fact that LgA rats show also higher somatic signs of withdrawal (Paterson and Markou 2004) and increased anxiety-like behavior (personal observation) during abstinence, suggest that the effect of varenicline on nicotine intake does not depend on dependence per se.
Varenicline is a partial agonist at α4β2 and α3β2 nAChRs, an agonist at α3β4 nAChRs and a full agonist at α7 nAChRs (Carroll et al. 2008; Mihalak et al. 2006). Modulation of these receptors is likely to mediate the observed decrease in nicotine intake in dependent and nondependent rats. Although the role of α3β2, α3β4, and α7 nAChRs in nicotine reward and nicotine withdrawal is unclear (Grottick et al. 2000; Walters et al. 2006; Fowler et al. 2008), our results confirm previous reports demonstrating a key role for α4β2 nAChRs in mediating the initial positive reinforcing effects of nicotine in nondependent rats (Corrigall and Coen 1989; Corrigall et al. 1994; Pontieri et al. 1996; Rollema et al. 2007b). Elimination of the α4or β2 nAChR subunits blocks nicotine-induced dopamine release in the nucleus accumbens and decreases nicotine self-administration (Picciotto et al. 1998; Marubio et al. 2003; Maskos et al. 2005). A similar decrease in nicotine self-administration has been observed after dopamine depletion of the nucleus accumbens using 6-hydroxydopamine lesions; however, dopamine depletion did not affect abstinence-induced increases in nicotine intake observed after 3 days of forced abstinence (Corrigall et al. 1992), suggesting again that even if activation of α4β2 nAChRs and increased dopamine release mediate the initial positive reinforcing effects of nicotine, they may not represent the only mechanism underlying the negative reinforcement process responsible for the increased motivation for nicotine during abstinence in dependent subjects.
The present report demonstrates that the α4β2 nAChR partial agonist varenicline can decrease the motivation for nicotine in different animal models of nicotine self-administration in rats. These results suggest that modulation of the α4β2 nAChR is a key element mediating the motivation for nicotine in rats; however, nicotine-dependent rats were not more sensitive than nondependent rats to the effects of varenicline despite increased sensitivity to the motivational effects of abstinence on nicotine intake. These results suggest that although α4β2 nAChRs are critical in mediating the positive reinforcing effect of nicotine, these receptors may not be a key element underlying the negative reinforcement process responsible for the increased motivation for nicotine during abstinence in dependent subjects.
Acknowledgments
This is publication number 20551 from The Scripps Research Institute. This work was supported by the Tobacco-Related Disease Research Program (TRDRP) from the State of California (grant 12RT-0099), the National Institute of Diabetes and Digestive and Kidney Diseases (grant DK26741), the Pearson Center for Alcoholism and Addiction Research, and the National Institute on Drug Abuse (DA12001). The authors thank Robert Lintz, Yanabel Grant, and Molly Brennan for their technical assistance and Taryn Grieder for helpful discussions. We also thank Michael Arends for his editorial assistance.
References
- Balfour DJ. Nicotine and the tobacco smoking habit. In: Balfour DJK, editor. Nicotine and the tobacco smoking habit (series title: international encyclopedia of pharmacology and therapeutics. Vol. 114. Pergamon; New York: 1984. pp. 61–74. [Google Scholar]
- Benowitz NL, Jacob P., 3rd Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther. 1984;35:499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50(4):1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. PubMed PMID: 3346676. [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282(1):7–13. PubMed PMID: 9223534. [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci. 2002;23:130–136. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Yokota Y, Ma W, Lee JR, Brieaddy LE, Burgess JP, Navarro HA, Damaj MI, Martin Synthesis, nicotinic acetylcholine receptor binding, and pharmacological properties of 30-(substituted phenyl)deschloroepibatidine analogs. Bioorg Med Chem. 2008;16:746–754. doi: 10.1016/j.bmc.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107(2–3):285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Fellows JL, Trosclair A, Adams EK. Annual smoking-attributable mortality, years of potential life lost, and economic costs: United States, 1995–1999. Morb Mortal Wkly Rep. 2002;51:300–303. [PubMed] [Google Scholar]
- Fowler CD, Arends MA, Kenny PJ. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav Pharmacol. 2008;19:461–484. doi: 10.1097/FBP.0b013e32830c360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Matta SG, Kane VB, Sharp BM. Norepinephrine release in amygdala of rats during chronic nicotine self-administration: an in vivo microdialysis study. Neuropharmacology. 2003;45:514–523. doi: 10.1016/s0028-3908(03)00201-6. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA. 2007;104(43):17198–17203. doi: 10.1073/pnas.0707585104. Epub 2007 Oct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. J Am Med Assoc. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol. 2009;78:756–765. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA. Evidence that nicotinic α7 receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther. 2000;294:1112–1119. [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;1:CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- Isaac PF, Rand MJ. Cigarette smoking and plasma levels of nicotine. Nature. 1972;236:308–310. doi: 10.1038/236308a0. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. J Am Med Assoc. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psycho-pharmacology (Berl) 2003;170(3):278–286. doi: 10.1007/s00213-003-1539-2. Epub 2003 Jul 25. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226(3):817–825. PubMed PMID: 6887012. [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther. 1985;235(3):619–628. PubMed PMID: 4078726. [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12(7):2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. PubMed PMID: 1613557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, McIntosh JM, Rossi F, Champtiaux N, Zoli M, Changeux JP. Effects of nicotine in the dopaminergic system of mice lacking the α4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci. 2003;17:1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at α4β2 and a full agonist at α7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- MMWR Annual smoking-attributable mortality, years of potential life lost, and economic costs: United States, 1995–1999. Morb Mort Wkly Rep. 2002;51:300–303. [PubMed] [Google Scholar]
- Moreno AY, Azar MR, Warren NA, Dickerson TJ, Koob GF, Janda KD. A critical evaluation of a nicotine vaccine within a self-administration behavioral model. Mol Pharm. 2010;7(2):431–441. doi: 10.1021/mp900213u. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Koob GF. “Nicotine deprivation effect” in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav. 2007;86:346–353. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology. 2004;173:64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Merlo-Pich E, Fuxe K, Changeux JP. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi R, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007a;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of α4β2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007b;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220(4593):214–216. doi: 10.1126/science.6828889. PubMed PMID: 6828889. [DOI] [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004;3:CD000146. doi: 10.1002/14651858.CD000146.pub2. [DOI] [PubMed] [Google Scholar]
- Stolerman IP. Behavioural pharmacology of nicotine: multiple mechanisms. Br J Addict. 1991;86:533–536. doi: 10.1111/j.1360-0443.1991.tb01803.x. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline for smoking cessation: a randomized controlled trial. J Am Med Assoc. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology. 1997;133:300–304. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The β2 but not α7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology. 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Stefański R, Przegaliński E, Filip M. Effect of varenicline on the acute and repeated locomotor responses to nicotine in rats. Synapse. 2008;62:935–939. doi: 10.1002/syn.20564. [DOI] [PubMed] [Google Scholar]