Abstract
Objective
The fetal inflammatory response syndrome (FIRS) has been described in the context of preterm labor and preterm PROM and is often associated with intra-amniotic infection/inflammation. This syndrome is characterized by systemic fetal inflammation and operationally-defined by an elevated fetal plasma interleukin (IL)-6. The objective of this study was to determine if FIRS can be found in fetuses with activation of their immune system, such as the one observed in Rh alloimmune-mediated fetal anemia.
Methods
Fetal blood sampling was performed in sensitized Rh-D negative women with suspected fetal anemia (n=16). Fetal anemia was diagnosed according to reference range nomograms established for the assessment of fetal hematologic parameters. An elevated fetal plasma IL-6 concentration was defined using a cutoff of >11 pg/mL. Concentrations of IL-6 were determined by immunoassay. Non-parametric statistics were used for analysis.
Results
1) The prevalence of an elevated fetal plasma IL-6 was 25% (4/16); 2) there was an inverse relationship between the fetal hematocrit and IL-6 concentration - the lower the hematocrit, the higher the fetal IL-6 (r= −0.68, p=0.004); 3) fetuses with anemia had a significantly higher plasma IL-6 concentration than those without anemia (3.74 pg/ml, interquartile range (IQR) 1.18–2.63 vs. 1.46 pg/ml, IQR 1.76–14.7; p=0.02); 4) interestingly, all fetuses with an elevated plasma IL-6 concentration had anemia (prevalence 40%, 4/10), while in the group without anemia, none had an elevated fetal plasma IL-6.
Conclusions
An elevation in fetal plasma IL-6 can be observed in a subset of fetuses with anemia due to Rh alloimmunization. This observation suggests that the hallmark of FIRS can be caused by non-infection-related insults. Further studies are required to determine whether the prognosis of FIRS caused by intra-amniotic infection/inflammation is different from that induced by alloimmunization.
Keywords: fetal anemia, FIRS, interleukin-6, pregnancy, Rh hemolytic disease
INTRODUCTION
The fetal inflammatory response syndrome (FIRS)[1,2] is considered the fetal counterpart of the systemic inflammatory response syndrome (SIRS) observed in adults[3]. FIRS has been described in association with intra-amniotic infection/inflammation in fetuses with preterm labor with intact membranes or preterm prelabor rupture of the membranes (PROM). [1,4] and it is an independent risk factor for perinatal morbidity and/or mortality and impending preterm labor and delivery. [1,4]
FIRS was operationally defined by an elevated fetal plasma interleukin (IL)-6 concentration[1] and or funisitis, [5,6] and is characterized by a systemic fetal inflammatory response to infectious or inflammatory insults (e.g. microbial invasion of the amniotic cavity)[7–20] that can progress toward multiple-systemic involvement, including the hematopoietic system, [7,20,21] adrenals, [22] heart, [23–25] kidneys, [26] thymus, [27–30] lung, [31–33] central nervous system, [34–36] and skin. [37,38]
In Rh-D negative women, sensitization to the D antigen will lead to production of maternal hemolytic antibodies. These antibodies (IgG) can cross the placenta and, if the fetus is Rh-D positive, attack fetal red blood cells, which are then destroyed in the fetal reticulo endothelial system, leading to fetal anemia. [39] If untreated, fetal anemia may lead to hydrops, multi-organ failure and fetal death. [40,41]
In adults, in addition to infectious insults, SIRS can be caused by non-infectious pathologic conditions such as ischemia, trauma, hemorrhage, autoimmune disorders and other mechanisms of disease. [3] In contrast, to date, intraamniotic infection/inflammation is the only pathologic condition associated with FIRS. The objective of this study was to determine if a non-infectious related pathologic fetal condition such as fetal anemia is associated with a fetal inflammatory response.
PATIENTS AND METHODS
Study groups and inclusion criteria
This retrospective cross-sectional study included Rh-D negative pregnant women who were Rh-sensitized and evaluated at the Sotero del Rio Hospital, Santiago, Chile, between June 1998 and October 2003. As part of the clinical management, patients underwent serial amniocenteses[42] and/or Doppler velocimetry of the fetal middle cerebral artery[43] and those in whom fetal anemia was suspected were offered diagnostic cordocentesis for assessment of the fetal hematocrit and intra-uterine transfusion when fetal anemia was confirmed. Women who consented for cordocentesis and in whom cordocentesis was performed for the first time during the index pregnancy were asked to donate fetal blood and amniotic fluid not required for clinical management for research purposes. Patients with one or more of the following criteria were excluded: 1) preterm labor with intact membranes or preterm PROM; 2) clinical chorioamnionitis; 3) multiple gestations; 4) fetal distress.
All participants provided written informed consent prior to the collection of fetal blood. The collection of samples and its utilization for research purposes was approved by the Institutional Review Boards of Sotero del Rio Hospital, Santiago, Chile and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS).
Clinical definitions
Fetal anemia was defined according to reference range nomograms established for the assessment of fetal hematologic parameters. [43] Fetal inflammatory response syndrome was defined as a fetal plasma IL-6 concentration >11 pg/mL, [1] and intra-amniotic inflammation was defined by an amniotic fluid IL-6 concentration > 2600 pg/ml. [44]
Fetal blood and amniotic fluid sample collection
Amniocentesis and cordocentesis procedures were performed under ultrasound guidance with the “free-hand technique” as previously described. [45] One percent lidocaine was given as a local anesthetic, but no sedative drugs were administered. A 22-gauge needle was used, and a path was chosen for needle insertion that allowed the amniocentesis and cordocentesis procedures. Gram stain and microbial cultures for aerobic and anaerobic bacteria, and mycoplasmas were performed in 4 amniotic fluid samples. The results of these tests were used for subsequent clinical management. Fetal blood was collected in ethylenediaminetetra-acetic acid (EDTA) tubes. APT tests were performed on fetal blood, and all specimens were found to be free of maternal blood. Fetal blood was analyzed for pH and gases, and complete white blood cell count, platelet count, and differential cell count were performed. Results were made available for clinical management.
Determination of IL-6 concentration in fetal plasma and amniotic fluid
Fetal plasma and amniotic fluid IL-6 concentrations were determined with commercially available enzyme-linked immunoassays (R&D Systems, Minneapolis, MN, USA). Two assays were run, one for all fetal blood samples and the other for all available amniotic fluid samples. The assays were conducted by the same person in one laboratory. For fetal plasma the sensitivity of the assay was 0.058 pg/mL (intra- and inter-assay coefficients of variation 3.3% and 8.3%, respectively). For amniotic fluid samples the sensitivity of the immunoassay was 0.5 pg/ml (intra- and inter-assay coefficients of variation 2.7% and 9.3%, respectively). The results of cytokine concentrations in amniotic fluid or fetal plasma reported herein were not available for caring physicians.
Statistical analysis
The Kolmogorov-Smirnov test was used to determine whether the data was normally distributed. A two-tailed Mann-Whitney U test was used to compare continuous nonparametric variables. Comparisons between proportions were performed using Fisher’s exact tests. Correlation between fetal plasma IL-6 concentrations and fetal hematocrit was determined using Spearman’s rank correlation test. A p-value <0.05 was considered statistically significant. Analysis was performed with SPSS, version 14 (SPSS Inc., Chicago, IL, USA)
RESULTS
Demographic and clinical characteristics
Sixteen women were included in the study. Ten fetuses were diagnosed with fetal anemia and in six cases fetal anemia was excluded based on the results of fetal blood analysis obtained by cordocentesis. Demographic and clinical characteristics of patients with and without fetal anemia are displayed in Table 1. Women with fetuses without anemia were more likely to smoke (p=0.04). There were no significant differences in any of the other characteristics between the two groups.
Table 1.
Demographic and clinical characteristics of the study population
| Variable | No fetal anemia (n=6) | Fetal anemia (n=10) | p |
|---|---|---|---|
| Maternal age (years) | 36 (31.5–39) | 35 (30.7–36.2) | 0.4 |
| Ethnic origin - Hispanic | 100 (6) | 100 (10) | 1 |
| Smoking | 50 (3) | 0 (0) | 0.04 |
| Prepregnancy BMI (kg/m2) | 26.7 (23.3–30.1) | 25.3 (22.7–27.6) | 0.5 |
| Gestational age at sampling (weeks) | 29.9 (28.2–32.7) | 30.8 (27.7–32.6) | 1.0 |
| Gestational age at delivery (weeks) | 35.8 (33.5–36.3) | 35.4 (33.5–35.9) | 0.6 |
| Birthweight (grams) | 2550 (2167–2892) | 2620 (2337–2925) | 0.9 |
Values are expressed as median (interquartile range) or percent (number)
BMI, body mass index
Among fetuses included in this study there was a negative correlation between fetal hematocrit and IL-6 concentration (Spearman’s rho −0.68, p=0.004; Figure 1). Twenty five percent of fetuses had an elevated fetal plasma IL-6 (4/16). Ten fetuses had anemia, and of those, 4 fetuses also had FIRS (40%), while none of the fetuses without anemia had FIRS. However, this difference did not reach statistical significance (4/10 vs. 0/6; p=0.1). None of the fetuses with an elevated plasma IL-6 concentration delivered within 21 days of cordocentesis. The median fetal plasma IL-6 concentration of fetuses with anemia was higher than that of fetuses without anemia (3.74 pg/ml, interquartile range (IQR) 1.18–2.63 vs. 1.46 pg/ml, IQR 1.76–14.7, p=0.02; Figure 2).
Figure 1. Correlation between fetal hematocrit and plasma IL-6 concentration among 16 fetuses with Rh alloimmunization who underwent cordocentesis for suspected anemia.
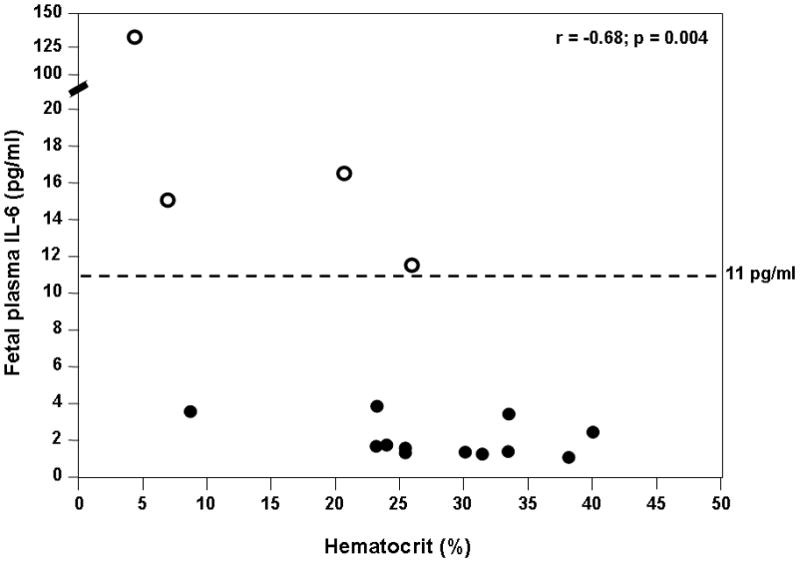
There was a significant negative correlation between the fetal hematocrit and plasma IL-6 concentration (Spearman’s rho −0.68, p=0.004). The interrupted line represents the cutoff value of IL-6 concentration for the diagnosis of FIRS. Four fetuses (“empty” dots) were diagnosed with FIRS.
Figure 2. Comparison of fetal plasma concentration of IL-6 between fetuses with anemia and those without anemia.
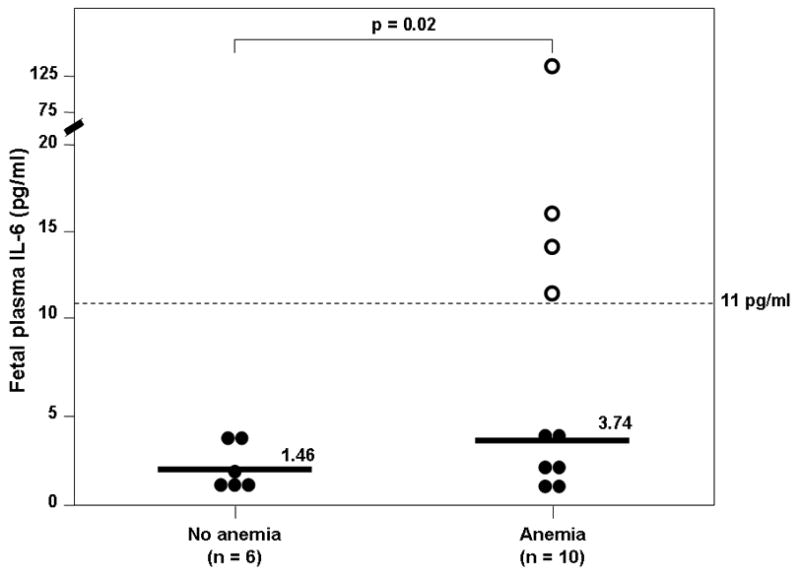
The median fetal plasma IL-6 concentration was higher in fetuses with anemia than that of those without anemia (3.74 pg/mL, interquartile range (IQR) 1.79–14.7 vs. 1.46 pg/mL, IQR 1.18–2.63; p=0.02). The interrupted line represents the cutoff value of IL-6 concentration for the diagnosis of FIRS. All fetuses with FIRS had anemia (“empty” dots).
Amniotic fluid samples were available in 15 cases (94%). All cases but one were negative for intra-amniotic inflammation. One case with FIRS (plasma IL-6 concentration 16.1 pg/ml) also had an elevated amniotic fluid IL-6 concentration (3068.1 pg/ml). The median amniotic fluid IL-6 concentration was not significantly different between women with anemic fetuses and those with fetuses without anemia (anemia: 1089 pg/mL, interquartile range (IQR) 542–2009 vs. no anemia: 471.5 pg/mL, IQR 357–693; p=0.06) (Figure 3). Although there was a trend toward a higher amniotic fluid IL-6 concentration in fetuses with anemia, the difference was marginal. Amniotic fluid Gram stain and cultures were performed in two of the four cases with FIRS, and were negative for microorganisms.
Figure 3. Comparison of amniotic fluid concentration of IL-6 between fetuses with anemia and those without anemia.
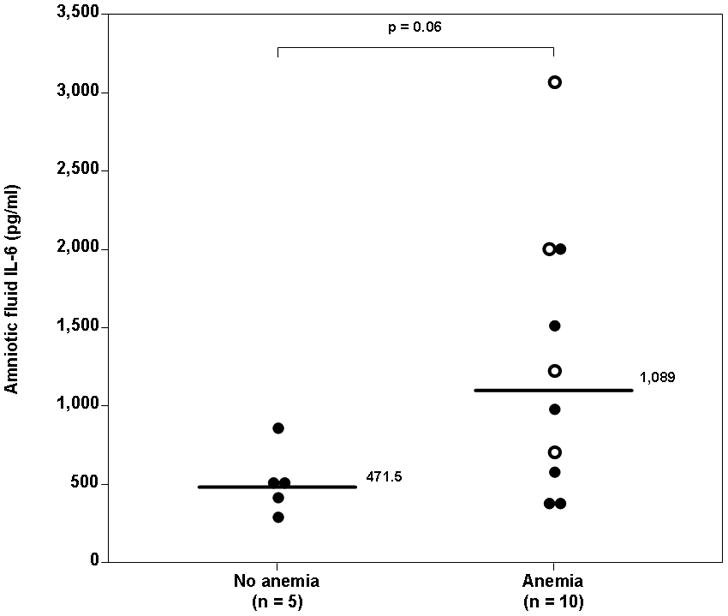
The median amniotic fluid IL-6 concentration was not significantly different between women with anemic fetuses and those with fetuses without anemia (anemia: 1089 pg/mL, interquartile range (IQR) 542–2009 vs. no anemia: 471.5 pg/mL, IQR 357–693; p=0.06). Intra-amniotic inflammation was diagnosed in only one fetus with FIRS. The interrupted line represents the cutoff value of amniotic fluid IL-6 concentration for the diagnosis of intra-amniotic inflammation. The “empty” dots represent fetuses with FIRS.
DISCUSSION
Principal findings of the study
1) Among fetuses with Rh alloimmunization, an elevated fetal plasma IL-6 concentration (>11 pg/ml) was diagnosed in 40% of fetuses with anemia and in none of those without anemia; 2) the fetal circulating IL-6 concentration was inversely correlated to the fetal hematocrit; and 3) the median fetal plasma IL-6 concentration of fetuses with anemia was significantly higher than that of fetuses without anemia.
Meaning of the Study
The fetal inflammatory response syndrome is a frequent condition in preterm labor and preterm PROM, is subclinical in nature, and is associated with an increased rate of perinatal morbidity. [1,4] FIRS is the fetal counterpart of the adult form, SIRS. The original definition of SIRS in adults was proposed in 1992 by the American College of Chest Physicians and the Society of Critical Care Medicine[3], and the rationale was to define a common clinical response to a variety of potential insults, infectious or non-infectious in origin, including ischemia, trauma, and inflammation, among others. The definition of SIRS is based on the presence of at least 2 of the following findings: (1) changes in temperature [>38°C (fever) or <36°C (hypothermia)]; (2) heart rate changes [>90 bpm (tachycardia)]; (3) respiratory rate or PaCO2 changes [>20 breaths/min (tachypnea) or PaCO2<32 mmHg (hypocapnia)]; and (4) white blood cell count changes [>12,000 cells/mm3 (leukocytosis) or <4000 cells/mm3 (leukopenia)]. However, the same definition cannot be applied to the human fetus because most of these parameters cannot be determined before birth and others (i.e. respiratory rate and PaCO2) are irrelevant. Thus, we defined FIRS as an elevation of IL-6 concentration in fetal or cord blood. [1] The original definition of FIRS was based on fetuses with preterm labor and preterm PROM, and was often (but not always) associated with microbial invasion of the amniotic cavity. To date, FIRS has been largely described in infection-related pregnancy complications. However, similar to SIRS in adults, the fetus might also be able to mount an inflammatory response to non-microbial-related insults. Currently, the indications for fetal blood sampling are limited, and cordocentesis is still performed in selected cases, mainly to gain direct access to the fetal circulation for diagnostic and therapeutic indications. Suspected fetal anemia is one of such indications. Thus, the availability of fetal blood samples from patients with complications of pregnancy associated with anemia due to alloimmunization made this investigation possible.
Fetal anemia, defined as hematocrit/hemoglobin concentration of >2 standard deviations below the mean for gestational age[46], hemoglobin concentration <0.85 times the median for gestational age, [43] or hematocrit below 30%, [47] can be the result of immune or non-immune insults. The most common immune etiology is maternal Rh disease. In this condition, women sensitized to the Rh-D antigen can produce antibodies. This occurs because of stimulation of memory B lymphocytes which produce IgG anti-D antibodies that can cross the placenta and coat D-positive fetal red blood cells. These antibody-coated cells then are destroyed in the reticuloendothelial system, leading to fetal anemia.
Using blood samples from fetuses of mothers sensitized to the Rh-D antigen, we demonstrate herein, for the first time, that the fetus is capable of mounting an inflammatory response to a non-infectious insult, namely anemia. Indeed, an elevated IL-6 concentration has been demonstrated in 4 fetuses with fetal anemia, while none of the fetuses without proven anemia had such an elevation. Moreover, there was a negative correlation between fetal hematocrit and IL-6 concentrations.
IL-6 is a cytokine with a broad range of biological activities produced by macrophages, T cells and B cells. [48,49] This cytokine is a major mediator of the host response to infection and tissue damage that plays a central role in defense mechanism regulation, acute phase reaction, and haematopoiesis. [50,51] In the immune response, IL-6 induces terminal differentiation of B cells as well as the differentiation and activation of T cells and macrophages. [52] Although IL-6 has been implicated in the pathogenesis of anemia of chronic disease in adults, [53] the cross-sectional nature of our study does not allow us to discern a cause-effect relationship between elevated fetal plasma IL-6 concentrations and severity of fetal anemia. It is conceivable that the fetal anemia-associated increase in IL-6 concentration in fetal circulation is a consequence of “tissue damage” (i.e., destruction of antibody-coated red blood cells in the reticuloendothelial system and/or tissue hypoxia) rather than the cause.
In this study, an elevation of fetal plasma IL-6 was not associated with preterm labor. In other studies, we have demonstrated that FIRS is associated with the onset of preterm labor in women with preterm PROM. [1] It is possible that FIRS not associated with infection/inflammation of the chorioamniotic membranes may not lead to the onset of labor. We propose that inflammation of the chorioamniotic membranes favors the onset of labor in the context of FIRS. Moreover, we propose that FIRS can be elicited by transplacental viral infections such as cytomegalovirus and other infections in which the fetus may be affected, but there is no evidence of chorioamnionitis. Thus, FIRS would lead to the onset of labor when there is participation of the effectors of mechanisms of labor (fetal membranes, cervix and myometrium), but is less likely to occur if the inflammatory process is limited to the fetus.
Strengths and limitations of the study
This is the first study to demonstrate an elevated fetal plasma IL-6 concentration in non-infection-related pregnancy complications, and demonstrates that an elevation of fetal plasma IL-6 can occur in the absence of infection. The main limitation of this study is the relatively small number of cases, with and without fetal anemia. Rh disease is a condition that is observed with less frequency now because of the availability of Rho(D) Immune Globulin. Yet, we were able to demonstrate a significant correlation between fetal IL-6 concentrations and fetal hematocrit, and demonstrated an elevated fetal plasma IL-6 in 40% of cases of fetal anemia. Another limitation of the study is that amniotic fluid Gram stain and cultures were not performed routinely at the time of amniocentesis; thus, intra-amniotic infection can not be excluded in all cases. It is noteworthy that in most cases of Rh disease, amniotic fluid is not subjected to a work-up for intra-amniotic infection/inflammation. Nonetheless, 15 of the 16 cases included in this study had an amniotic fluid IL-6 concentration below 2600 pg/ml (the cutoff for the definition of intra-amniotic inflammation).
In conclusion, our observations suggest that the human fetus is capable of mounting a systemic inflammatory response (defined as an elevated fetal plasma IL-6) in the context of alloimmune disease. Indeed, 40% of fetuses with anemia in this study had an elevated fetal plasma IL-6, which is the hallmark of FIRS. Further studies are needed to clarify whether, similar to FIRS in intra-amniotic infection/inflammation, [1,4] “non-infectious FIRS” is also an independent risk factor for perinatal morbidity and/or mortality.
Acknowledgments
This research was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 2.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 3.American College of Chest Physicians/Society of Critical Care Medicine. Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 4.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–193. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 5.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, Jun JK. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–1129. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 6.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 7.Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB, Bianchi DW. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol. 1995;173:1315–1320. doi: 10.1016/0002-9378(95)91378-5. [DOI] [PubMed] [Google Scholar]
- 8.Gomez R, Ghezzi F, Romero R, et al. Two thirds of human fetuses with microbial invasion of the amniotic cavity have a detectable systemic cytokine response before birth. Am J Obstet Gynecol. 1997;176:S14. [Google Scholar]
- 9.Berry SM, Gomez R, Athayde N, Ghezzi F, Mazor M, Yoon BH, Edwin S, Romero R. The role of granulocyte colony stimulating factor in the neutrophilia observed in the fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;178:S202. [Google Scholar]
- 10.Romero R, Athayde N, Gomez R, Mazor M, Yoon BH, Edwin S, Ghezzi F, Berry SM. The fetal inflammatory response syndrome is characterized by the outpouring of a potent extracellular matrix degrading enzyme into the fetal circulation. Am J Obstet Gynecol. 1998;178:S3. [Google Scholar]
- 11.Romero R, Maymon E, Pacora P, Gomez R, Mazor M, Yoon BH, Berry SM. Further observations on the fetal inflammatory response syndrome: a potential homeostatic role for the soluble receptors of tumor necrosis factor alpha. Am J Obstet Gynecol. 2000;183:1070–1077. doi: 10.1067/mob.2000.108885. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, Mazor M, Maymon E, Berry S. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol. 2002;187:1125–1130. doi: 10.1067/mob.2002.127312. [DOI] [PubMed] [Google Scholar]
- 13.Espinoza J, Chaiworapongsa T, Romero R, Gomez R, Kim JC, Yoshimatsu J, Edwin S, Rathnasabapathy C, Yoon BH. Evidence of participation of soluble CD14 in the host response to microbial invasion of the amniotic cavity and intra-amniotic inflammation in term and preterm gestations. J Matern Fetal Neonatal Med. 2002;12:304–312. doi: 10.1080/jmf.12.5.304.312. [DOI] [PubMed] [Google Scholar]
- 14.Chaiworapongsa T, Romero R, Kim JC, Kim YM, Blackwell SC, Yoon BH, Gomez R. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol. 2002;186:1178–1182. doi: 10.1067/mob.2002.124042. [DOI] [PubMed] [Google Scholar]
- 15.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294–296. doi: 10.1016/j.ajog.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Kim SK, Romero R, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, Mittal P, Erez O, Vaisbuch E, Gotsch F, Pacora P, et al. Evidence of changes in the immunophenotype and metabolic characteristics (intracellular reactive oxygen radicals) of fetal, but not maternal, monocytes and granulocytes in the fetal inflammatory response syndrome. J Perinat Med. 2009;37:543–552. doi: 10.1515/JPM.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mestan K, Yu Y, Thorsen P, Skogstrand K, Matoba N, Liu X, Kumar R, Hougaard DM, Gupta M, Pearson C, et al. Cord blood biomarkers of the fetal inflammatory response. J Matern Fetal Neonatal Med. 2009;22:379–387. doi: 10.1080/14767050802609759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth C, Manzur A, Oyarzun E, Romero R, Mor G. Viral Infection of the Placenta Leads to Fetal Inflammation and Sensitization to Bacterial Products Predisposing to Preterm Labor. J Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Digiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, et al. Prevalence and Diversity of Microbes in the Amniotic Fluid, the Fetal Inflammatory Response, and Pregnancy Outcome in Women with Preterm Pre-Labor Rupture of Membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, Kim JS, Edwin SS, Gomez R, Draghici S. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63:73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez R, Berry S, Yoon BH, Mazor M, Athayde N, Ghezzi F, Romero R. The hematologic profile of the fetus with systemic inflammatory response syndrome. Am J Obstet Gynecol. 1998;178:S202. [Google Scholar]
- 22.Yoon BH, Romero R, Jun JK, Maymon E, Gomez R, Mazor M, Park JS. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol. 1998;179:1107–1114. doi: 10.1016/s0002-9378(98)70114-0. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Berry SM, Silva M, Treadwell M, DeVore GR. A novel form of fetal cardiac dysfunction in preterm premature rupture of membranes. Am J Obstet Gynecol. 1999;180:S27. doi: 10.1080/14767050400009279. [DOI] [PubMed] [Google Scholar]
- 24.Yanowitz TD, Jordan JA, Gilmour CH, Towbin R, Bowen A, Roberts JM, Brozanski BS. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res. 2002;51:310–316. doi: 10.1203/00006450-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Romero R, Espinoza J, Goncalves LF, Gomez R, Medina L, Silva M, Chaiworapongsa T, Yoon BH, Ghezzi F, Lee W, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2004;16:146–157. doi: 10.1080/14767050400009279. [DOI] [PubMed] [Google Scholar]
- 26.Yoon BH, Kim YA, Romero R, Kim JC, Park KH, Kim MH, Park JS. Association of oligohydramnios in women with preterm premature rupture of membranes with an inflammatory response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1999;181:784–788. doi: 10.1016/s0002-9378(99)70301-7. [DOI] [PubMed] [Google Scholar]
- 27.De Felice C, Toti P, Santopietro R, Stumpo M, Pecciarini L, Bagnoli F. Small thymus in very low birth weight infants born to mothers with subclinical chorioamnionitis. J Pediatr. 1999;135:384–386. doi: 10.1016/s0022-3476(99)70140-x. [DOI] [PubMed] [Google Scholar]
- 28.Toti P, De FC, Stumpo M, Schurfeld K, Di LL, Vatti R, Bianciardi G, Buonocore G, Seemayer TA, Luzi P. Acute thymic involution in fetuses and neonates with chorioamnionitis. Hum Pathol. 2000;31:1121–1128. doi: 10.1053/hupa.2000.16676. [DOI] [PubMed] [Google Scholar]
- 29.Di Naro E, Cromi A, Ghezzi F, Raio L, Uccella S, D’Addario V, Loverro G. Fetal thymic involution: a sonographic marker of the fetal inflammatory response syndrome. Am J Obstet Gynecol. 2006;194:153–159. doi: 10.1016/j.ajog.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 30.Yinon Y, Zalel Y, Weisz B, Mazaki-Tovi S, Sivan E, Schiff E, Achiron R. Fetal thymus size as a predictor of chorioamnionitis in women with preterm premature rupture of membranes. Ultrasound Obstet Gynecol. 2007;29:639–643. doi: 10.1002/uog.4022. [DOI] [PubMed] [Google Scholar]
- 31.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- 32.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, Jun JK. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773–779. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 33.Yoon BH, Romero R, Shim JY, Lim JH, Choe G, Kadar N, Park M. “Atypical” chronic lung disease of the newborn is linked to fetal systemic inflammation. Am J Obstet” Gynecol. 2002;187:S129. [Google Scholar]
- 34.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, Syn HC. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 35.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 36.Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med. 2006;34:5–12. doi: 10.1515/JPM.2006.001. [DOI] [PubMed] [Google Scholar]
- 37.Kim YM, Kim GJ, Kim MR, Chaiworapongsa T, Espinoza J, Yoon BH, Bujold E, Romero R. Skin: An Active Component of The Fetal Innate Immune System. American Journal of Obstetrics and Gynecology. 2003;189:S74. [Google Scholar]
- 38.Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49:506–514. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moise KJ., Jr Management of rhesus alloimmunization in pregnancy. Obstet Gynecol. 2008;112:164–176. doi: 10.1097/AOG.0b013e31817d453c. [DOI] [PubMed] [Google Scholar]
- 40.Radunovic N, Lockwood CJ, Alvarez M, Plecas D, Chitkara U, Berkowitz RL. The severely anemic and hydropic isoimmune fetus: changes in fetal hematocrit associated with intrauterine death. Obstet Gynecol. 1992;79:390–393. doi: 10.1097/00006250-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 41.van Kamp IL, Klumper FJ, Bakkum RS, Oepkes D, Meerman RH, Scherjon SA, Kanhai HH. The severity of immune fetal hydrops is predictive of fetal outcome after intrauterine treatment. Am J Obstet Gynecol. 2001;185:668–673. doi: 10.1067/mob.2001.116690. [DOI] [PubMed] [Google Scholar]
- 42.LILEY AW. Liquor amnil analysis in the management of the pregnancy complicated by resus sensitization. Am J Obstet Gynecol. 1961;821359–70:1359–1370. doi: 10.1016/s0002-9378(16)36265-2. [DOI] [PubMed] [Google Scholar]
- 43.Mari G, Deter RL, Carpenter RL, Rahman F, Zimmerman R, Moise KJ, Jr, Dorman KF, Ludomirsky A, Gonzalez R, Gomez R, et al. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. N Engl J Med. 2000;342:9–14. doi: 10.1056/NEJM200001063420102. [DOI] [PubMed] [Google Scholar]
- 44.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 45.Ghezzi F, Romero R, Maymon E, Redman M, Blackwell S, Berry SM. Fetal blood sampling. In: Fleischer A, Manning F, Jeanty P, Romero R, editors. Sonography in Obstetrics and Gynecology:Principles and Practice. 6. New York: McGraw Hill; 2001. pp. 775–804. [Google Scholar]
- 46.Nicolaides KH, Rodeck CH, Millar DS, Mibashan RS. Fetal haematology in rhesus isoimmunisation. Br Med J (Clin Res Ed) 1985;290:661–663. doi: 10.1136/bmj.290.6469.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moise KJ., Jr Management of rhesus alloimmunization in pregnancy. Obstet Gynecol. 2002;100:600–611. doi: 10.1016/s0029-7844(02)02180-4. [DOI] [PubMed] [Google Scholar]
- 48.Regulation of the acute phase and immune responses: interleukin-6. Ann NY Acad Sci. 1989;557:1–583. [PubMed] [Google Scholar]
- 49.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 50.Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- 51.Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;11:443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- 52.Kishimoto T, Hibi M, Murakami M, Narazaki M, Saito M, Taga T. The molecular biology of interleukin 6 and its receptor. Ciba Found Symp. 1992;167:5–16. doi: 10.1002/9780470514269.ch2. discussion 16–23.:5–16. [DOI] [PubMed] [Google Scholar]
- 53.Raj DS. Role of interleukin-6 in the anemia of chronic disease. Semin Arthritis Rheum. 2009;38:382–388. doi: 10.1016/j.semarthrit.2008.01.006. [DOI] [PubMed] [Google Scholar]


