Abstract
Infectious entry of JC virus (JCV) into human glial cells occurs by receptor-mediated clathrin-dependent endocytosis. In this report we demonstrate that the tyrosine kinase inhibitor genistein blocks virus entry and inhibits infection. Transient expression of dominant-negative eps15 mutants, including a phosphorylation-defective mutant, inhibited both virus entry and infection. We also show that the JCV-induced signal activates the mitogen-activated protein kinases ERK1 and ERK2. These data demonstrate that JC virus binding to human glial cells induces an intracellular signal that is critical for entry and infection by a ligand-inducible clathrin-dependent mechanism.
The human polyomavirus JC virus (JCV) is the causative agent of a fatal central nervous system demyelinating disease known as progressive multifocal leukoencephalopathy (PML). This disease occurs most frequently in the setting of severe and prolonged immunosuppression and affects between 4 and 8% of patients with AIDS (8, 15). To date, there is no effective therapy for PML and, unlike many other opportunistic infections in the era of highly active antiretroviral therapy, the incidence of PML has remained constant (2). JCV, like other polyomaviruses, must deliver its double-stranded DNA genome to the nucleus of the host cell to initiate infection. Our laboratory has characterized many of the early steps involved in virus entry and trafficking to the nucleus. Our investigators have found that infectious entry of JCV occurs by receptor-mediated clathrin-dependent endocytosis (20). Our researchers have also found that infection involves elements of the cellular cytoskeleton, including both microfilaments and microtubules (3). Interestingly, two other polyomaviruses, simian virus 40 (SV40) and the mouse polyomavirus, have been shown to use clathrin-independent mechanisms to infect cells (1, 13, 14, 17-19, 22). Infection of monkey kidney cells by SV40 involves caveola-dependent endocytosis, and infection of mouse kidney cells by the mouse polyomavirus occurs either by caveola-dependent endocytosis or by an alternative pathway depending on the cell type analyzed. Thus, JCV is unique among the currently studied polyomaviruses in utilizing clathrin-dependent endocytosis to infect cells.
One difficulty in studying virus entry has been finding specific reagents to block different entry pathways. The use of drugs, such as nystatin and phorbol myristate acetate to inhibit caveola-dependent endocytosis and chlorpromazine to inhibit clathrin-dependent endocytosis, is not ideal, as these drugs may have secondary effects in the cell. Because of this, the use of dominant-negative mutants of important endocytic proteins has become the method of choice for studying mechanisms of virus entry (23). An important endocytic component of clathrin-mediated endocytosis that was first described as a substrate of the epidermal growth factor (EGF) receptor kinase is eps15 (epidermal growth factor receptor pathway substrate clone 15). Several dominant-negative mutants of eps15 have been shown to inhibit endocytosis of both receptor-mediated clathrin endocytic events, such as occurs with EGF, as well as constitutively occurring endocytic events, such as transferrin endocytosis (5, 6). Dominant-negative inhibitors of eps15 have also been used to specifically block entry of viruses that utilize clathrin-dependent endocytosis, such as Sindbis virus, and have been shown to have no effect on the entry of viruses that utilize caveolae or other modes of entry, such as SV40 and influenza virus (9, 19, 24). Recently it has been shown that posttranslational modifications of eps15 are involved in endocytosis of some but not all ligand-regulated endocytic events but are not involved in constitutively occurring clathrin-dependent endocytosis (11, 26). For example, activation of the EGF receptor by EGF or transforming growth factor α induces tyrosine phosphorylation of eps15; however, stimulation of cells with transferrin or platelet-derived growth factor does not (27). To date no one has examined whether or not viral ligands induce posttranslational modification of eps15.
In this report we examined whether JCV binding to glial cells would induce a signal necessary for facilitating entry and infection by the clathrin-mediated pathway. Recent studies with SV40 have shown that SV40 binding to the surface of cells transmits a signal that is required for entry and that this signal can be inhibited by the tyrosine kinase inhibitor genistein (12). In the presence of genistein, SV40 virions stall at the mouths of caveolae and are unable to be completely internalized (10). It is thought that genistein inhibits SV40 entry by blocking tyrosine phosphorylation of caveolin-1 (19). Our results demonstrate that JCV also induces a genistein-sensitive molecular signal that is required for virus entry and infection, albeit by the clathrin-dependent pathway. We also show that JCV, unlike SV40, activates the mitogen-activated protein kinases (MAPKs) extracellular signal-regulated kinase 1 (ERK1) and ERK2.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
SVG-A cells are a subclone of the original SVG human glial cell line established by transformation of human fetal glial cells by an origin-defective SV40 mutant (16). SVG-A cells were cultured in Eagle's minimum essential medium (EMEM; Mediatech Inc., Herndon, Va.) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and kept in a humidified 37°C 5% CO2 incubator. The generation and propagation of the Mad-1/SVEΔ strain of JCV has been described previously (25). SV40 strain 776 was propagated in the African green monkey kidney cell line CV-1. The PAB597 hybridoma produces a monoclonal antibody against the SV40 capsid protein VP1 and was a kind gift from Ed Harlow. This antibody was used to score virus-infected cells and has been previously shown to cross-react with JCV VP1. Generation of JCV and SV40 antiserum has been previously described (20). The P-ERK monoclonal antibody was from Santa Cruz Biotechnology (Santa Cruz, Ca.). The anti-tubulin and anti-FLAG antibodies were from Sigma (St. Louis, Mo.).
Inhibition of infection by genistein.
SVG-A cells were grown on coverslips to 60 to 70% confluency and pretreated for 1 h at 37°C with either genistein (100 μM in dimethyl sulfoxide) or sodium orthovanadate (50 or 100 μM in distilled water). Untreated and treated cells (5 × 105) were then infected with 514 hemagglutinin units (HA units) of JCV or 4.8 × 106 PFU of SV40 for 4 h at 37°C either in the absence or continued presence of inhibitors. This represents a multiplicity of infection of 10 for both JCV and SV40. At 4 h postinfection, cells were washed with medium and incubated with anti-JCV or anti-SV40 antibodies diluted 1:1,000 in fresh medium (with 10% FBS) to neutralize extracellular virus and left at 37°C for 72 h. Cells were then washed in phosphate-buffered saline (PBS; 137 mM NaCl, 2.682 mM KCl, 8.1 Na2HPO4, 1.47 mM KH2PO4; pH 7.4) and fixed in ice-cold acetone for 10 min. Coverslips were stained with undiluted PAB597 for 45 min at 37°C, washed, and incubated with goat anti-mouse Alexa-Fluor 488 (Molecular Probes Inc., Eugene, Oreg.) diluted 1:150 in PBS. Cells were further washed and then counterstained with 0.02% Evan's blue in PBS and mounted onto slides with fluorescence mounting medium (Vector Labs, Burlingame, Calif.). Slides were viewed using a Nikon epifluorescence microscope (Eclipse E800; Nikon, Inc., Melville, N.Y.) and scored by counting. A minimum of eight fields containing between 70 and 100 cells were counted, and all experiments were performed in triplicate.
Inhibition of entry and infection by genistein and eps15 dominant-negative mutants.
SVG-A cells grown on coverslips were treated with genistein (100 μM), sodium orthovanadate (50 or 100 μM), or chlorpromazine (25 μm) for 1 h at 37°C and then infected with 515 HA units of Alexa-Fluor 488-labeled JCV for 2 h or exposed to 35 μg of rhodamine-labeled transferrin/ml for 30 min in the continued presence of the drugs. Cells were then washed in PBS two times and fixed in 4% paraformaldehyde for 30 min. Coverslips were then mounted onto slides with mounting medium containing either propidium iodide or 4′,6′-diamidino-2-phenylindole (DAPI) and visualized by laser scanning confocal microscopy at ×63 magnification (LSM 410; Zeiss Inc., Thornwood, N.J.).
For eps15 experiments, 1 or 5 μg of dominant-negative eps15-green fluorescent protein (GFP) constructs and eps15-FLAG constructs were transfected with Lipofectamine (Gibco-BRL) for 3 h in serum-free EMEM and then washed and fed with fresh 10% medium. At 48 h posttransfection, at maximum GFP or FLAG expression, cells were infected with 514 HA units of Alexa-Fluor 594-labeled JCV or with 514 HA units of unlabeled JCV. For infection experiments, cells were fixed in acetone and V antigen was detected using an indirect immunofluorescence assay. The secondary antibody in this case was Alexa-Fluor 594 (red) antibody diluted 1:150 in PBS. For the confocal experiments, infected cells were washed in PBS and fixed with 4% paraformaldehyde for 30 min. Cells were then washed with PBS again and mounted directly or stained with a 1:100 dilution of anti-FLAG antibodies in PBS (M2; Sigma) and goat anti-rabbit Alexa-Fluor 594 secondary antibodies (Molecular Probes) diluted 1:150 in PBS and then mounted onto coverslips using fluorescent mounting medium (Vector Labs Inc.). Slides were visualized by laser scanning confocal microscopy at ×63 magnification (LSM 410; Zeiss Inc.). All images were analyzed using Adobe Photoshop.
Activation of the MAPKs ERK1 and ERK2 by Western blot analysis.
SVG-A cells grown in six-well dishes were serum starved overnight in EMEM with no FBS. Cells were then treated with 514 HA units of JCV at 4°C for 30 min and then shifted to 37°C for the indicated times to allow entry. Cells were then washed in cold PBS and lysed in ice-cold radioimmunoprecipitation assay buffer (20 mM Tris HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM EDTA) containing both protease and phosphatase inhibitors (1.5 mM phenylmethylsulfonyl fluoride), protease inhibitor cocktail (Sigma), and 1.0 mM sodium orthovanadate. Protein concentrations were determined using the Bio-Rad protein assay. Equal amounts of protein were separated on a 4-to-15% gradient or 10% Tris-HCl Ready gels from Bio-Rad. Proteins were transferred to nitrocellulose membranes using a mini-trans blot apparatus (Bio-Rad) and blocked with 1× casein blocking buffer. Blots were probed with the respective antibodies all diluted in 1× blocking buffer, washed in PBS-0.05% Tween 20, and then incubated with either IRDye 800 goat anti-mouse antibodies (Rockland Immunochemicals, Gilbertsville, Pa.) diluted 1:5,000 in blocking buffer or goat anti-rabbit Alexa-Fluor 680 antibodies also diluted 1:5,000 in blocking buffer, followed by further washes with PBS-Tween 20 and one wash in PBS. Blots were viewed using an infrared scanner from LICOR and analyzed using Odyssey software.
RESULTS
The tyrosine kinase inhibitor genistein, but not the tyrosine phosphatase inhibitor sodium orthovanadate, inhibits infection by JCV.
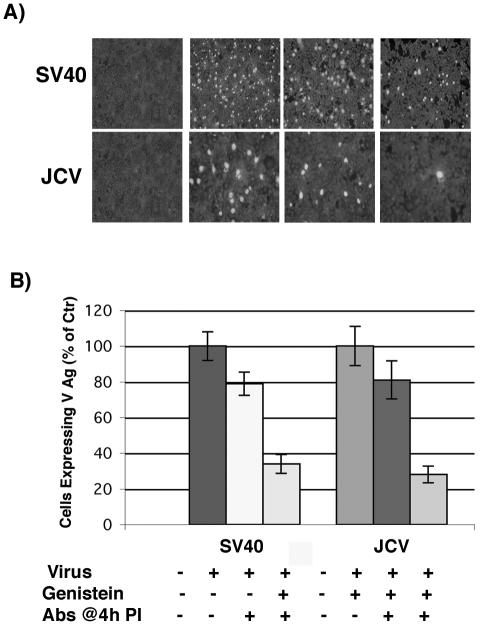
The SVG-A human glial cell line is susceptible to infection by both JCV and SV40. This allowed us to directly determine whether JCV, like SV40, induced intracellular signals important for infection. As the SV40 signal is known to be blocked by genistein, we first asked whether this drug would also block infection of glial cells by JCV. This experiment was done using an infectious entry assay, where virus is allowed to internalize in the presence or absence of drug for 4 h and then membrane-bound or extracellular virus is neutralized with antiserum against either SV40 or JCV. Infection was scored using an antibody directed against the late viral protein V antigen. As can be seen in Fig. 1, infectious entry of SV40 and JCV into SVG-A cells was significantly inhibited in the presence of genistein.
FIG. 1.
Genistein inhibits JCV and SV40 infection of glial cells. (A) SVG-A cells grown on coverslips were pretreated with 100 μM genistein for 1 h at 37°C. Cells were then infected with JCV or SV40 in the continued presence of genistein for 4 h at 37°C, and anti-JCV or anti-SV40 neutralizing antibodies were added at 4 h postinfection to neutralize any remaining extracellular virus. At 72 h postinfection the cells were washed and fixed, and infection was scored by an indirect immunofluorescence assay for the late viral protein VP1. (B) VP1-positive cells were scored by counting, and the results are graphed.
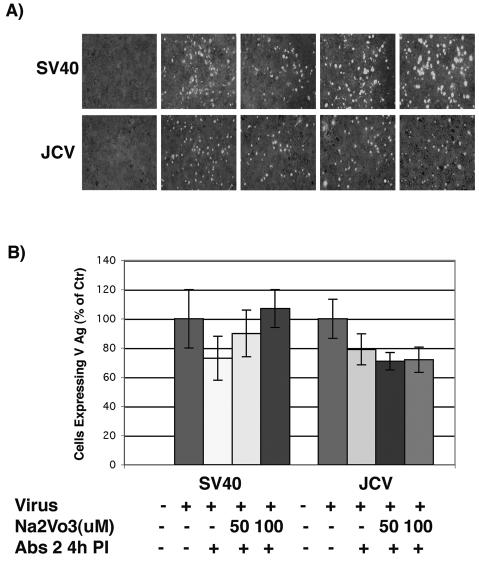
As endocytosis often involves the sequential phosphorylation and dephosphorylation of critical components of the endocytic machinery, we also asked whether inhibitors of protein phosphatases would affect infection of glial cells by JCV or SV40. In contrast to the results with genistein, inhibition of cellular phosphatases by sodium orthovanadate did not affect infection of SVG-A cells by JCV (Fig. 2). This same treatment slightly enhanced infection of SVG-A cells by SV40, which is consistent with an earlier report by Helenius et al. (19) (Fig. 2).
FIG. 2.
Sodium orthovanadate does not inhibit JCV infection of glial cells. (A) SVG-A cells grown on coverslips were pretreated with 50 or 100 μM sodium orthovanadate for 1 h at 37°C. Cells were then infected with JCV or SV40 in the continued presence of NaVO3 for 4 h at 37°C, and anti-JCV or anti-SV40 neutralizing antibodies were added at 4 h postinfection to neutralize any remaining extracellular virus. At 72 h postinfection the cells were washed and fixed, and infection was scored by an indirect immunofluorescence assay for the late viral protein VP1. (B) VP1-positive cells were scored by counting, and the results are graphed.
Genistein inhibits JCV entry into glial cells.
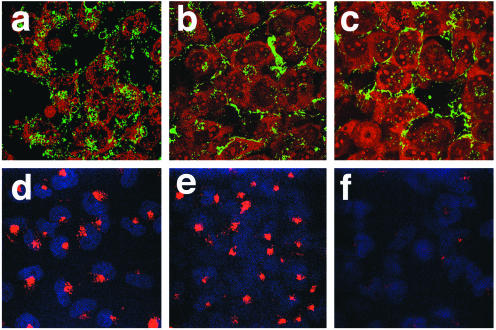
Studies with SV40 have shown that SV40 binding to the surface of the cell transmits a signal that is required for entry and that this signal can be inhibited by genistein (12). As the experiments described above used an infectious entry assay, we suspected that the effect of genistein was at virus entry, as opposed to a downstream step in the virus life cycle. To confirm this, we asked whether genistein treatment of SVG-A cells would block the entry of labeled JCV particles into cells. To do this, SVG-A cells were treated with genistein and then incubated with fluorescein isothiocyanate (FITC)-labeled JCV particles or with rhodamine-labeled transferrin. Control cells were treated with chlorpromazine, which is known to block both virus and transferrin uptake. Interestingly, genistein significantly inhibited virus entry but had no effect on transferrin uptake (Fig. 3). As transferrin is endocytosed by constitutive clathrin-dependent endocytosis, this result suggests that virus is endocytosed by a ligand-inducible mechanism.
FIG. 3.
Genistein and chlorpromazine inhibit entry of FITC-labeled JCV. SVG-A cells grown on coverslips were either untreated (control) (a and d) or pretreated with 100 μM genistein (b and e) or 25 μM chlorpromazine (c and f) for 1 h. The cells were then incubated with FITC-labeled JCV (a to c) for 4 h in the continued presence of drug or incubated with 35 μg of rhodamine-labeled transferrin/ml (d to f) for 30 min also in the continued presence of the drug. Cells were washed and fixed in 4% paraformaldehyde, and coverslips were mounted on slides with mounting medium containing propidium iodide (a to c) or DAPI (d to f). Slides were viewed using a laser scanning confocal microscope with a 63× objective.
Transient expression of dominant-negative eps15 mutants blocks virus entry and infection.
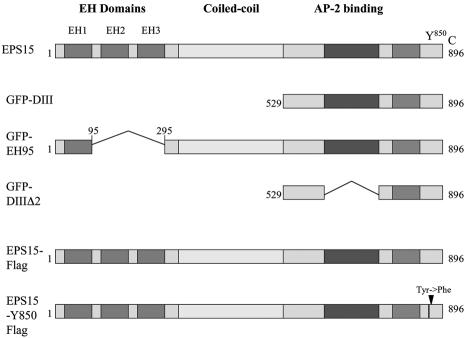
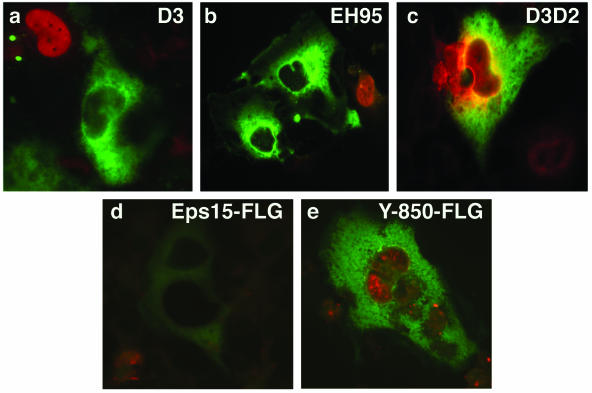
To be sure we were studying a ligand-inducible clathrin-dependent pathway, we took advantage of several dominant-negative eps15 mutants (Fig. 4) to ask whether they would interfere with virus infection. Two GFP-tagged dominant-negative mutants (GFP-D3 and GFP-EH95), a FLAG-tagged phosphorylation-defective mutant (eps15-Y850F-FLAG), and control GFP- and FLAG-tagged constructs (GFP-D3Δ2 and eps15-FLAG) were each transiently transfected into SVG-A cells. These cells were then infected with JCV at a time that corresponded to maximal expression of the transfected gene (data not shown). Single cells expressing GFP or the FLAG tag were then scored for virus infection. All three dominant-negative mutants but neither of the control constructs completely inhibited infection of SVG-A cells by JCV (Fig. 5).
FIG. 4.
Schematic representation of full-length eps15 and the dominant-negative mutant constructs used in these experiments. eps15 has an amino terminus containing three protein-protein interaction EH domains, a central coiled-coil domain thought to be involved in homodimerization, and a carboxyl terminus involved in association with the clathrin adaptor protein AP-2 during clathrin pit formation. The DIII (D3) and EH95 constructs are dominant-negative inhibitors of eps15 function and inhibit clathrin-dependent endocytosis, whereas the DIIIΔ2 (D3D2) construct serves as a negative control and has no effect on clathrin-dependent endocytosis. The FLAG constructs are either a control full-length eps15 construct or full-length eps15 with a point mutation (Tyr→Phe) in the major tyrosine phosphorylation site, Y850, which has been shown to block EGF internalization but not transferrin internalization.
FIG. 5.
Dominant-negative eps15 constructs inhibit JCV infection. SVG-A cells grown on coverslips were transfected with the indicated GFP- or FLAG-tagged dominant-negative eps15 constructs or control constructs for 24 h. Cells were then infected with JCV, and at 72 h postinfection the cells were fixed and stained with an anti-V antigen (VP1) monoclonal antibody (red). The GFP-expressing constructs were directly visualized, and the FLAG-tagged constructs were visualized using anti-FLAG antibody and an Alexa-Fluor 488-labeled secondary antibody. JCV was unable to infect any of the cells expressing a dominant-negative construct (EH95, D3, and eps15-Y850F). In contrast, JCV readily infected cells expressing control constructs (D3D2 and eps15wt).
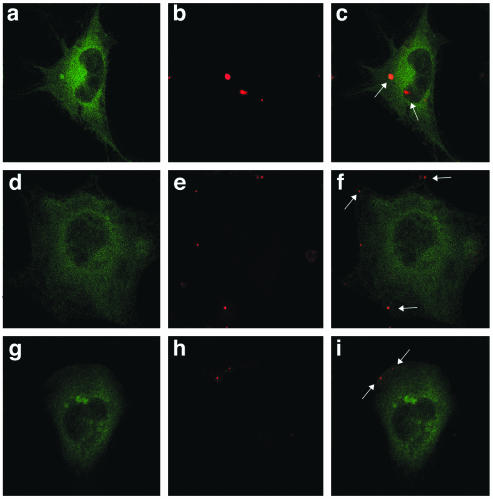
We next asked whether transient transfection of the GFP-tagged eps15 mutants or the control GFP-tagged construct could inhibit virus entry. For this experiment we used highly purified preparations of JCV that were labeled with Alexa-Fluor 594. Transiently transfected SVG-A cells were then incubated with a low multiplicity of labeled JCV, and then virus entry into GFP-expressing cells was visualized by confocal microscopy. In SVG-A cells expressing the control GFP construct (GFP-D3Δ2), virions accumulated in the cytosol by 4 h postadsorption (Fig. 6c). In contrast, labeled viral particles did not enter cells expressing either of the dominant-negative constructs but remained bound at the plasma membrane (Fig. 6f and i).
FIG. 6.
Dominant-negative eps15 constructs block JCV entry. SVG-A cells grown on coverslips were transfected with either D3 (a to c) or EH95 (d and e) dominant-negative eps15-GFP constructs or the D3Δ2 control construct (f to h) for 24 h. Twenty-four hours posttransfection, cells were exposed to Alexa-Fluor 594-labeled JCV (red) for 4 h at 37°C and then fixed in 4% paraformaldehyde for 30 min. The cells were mounted using fluorescent mounting medium and viewed using a confocal microscope. Arrows indicate the presence of internalized virus (c) or membrane-bound virus (f and i).
JCV activates the MAPKs ERK1 and ERK2.
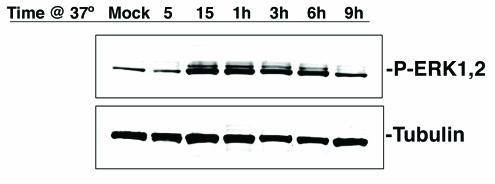
To better understand what signaling pathways were being activated in SVG-A cells upon exposure to virus, we examined the activation of the MAPKs ERK1 and ERK2, as this pathway has previously been shown to be activated in response to infection by other viruses. Highly purified preparations of unlabeled JCV virions were preadsorbed to serum-starved SVG-A cells at 4°C for 30 min to allow for virus binding but not entry. The cells were then shifted to 37°C, and whole-cell lysates were prepared at several time points between 5 min and 9 h postshift. Within 15 min of shifting the cells to 37°C, increased levels of phosphorylated ERK1 and ERK2 were observed (Fig. 7). This was not seen when cells were not exposed to virus but otherwise treated identically (Fig. 7). The increased activity was sustained for 6 h postexposure and returned to baseline levels by 9 h.
FIG. 7.
JCV activates the MAPKs ERK1 and ERK2. SVG-A cells serum starved overnight were incubated with JCV or just medium (mock) for 30 min on ice, and then cells were shifted to 37°C to allow virus internalization. Lysates were collected at the indicated time points following the shift to 37°C, and samples were run on 4- to-15% gradient gels, transferred to nitrocellulose, and blotted with antibodies recognizing the phosphorylated or active form of ERK1 and -2 or with antibodies to tubulin as a loading control.
DISCUSSION
Our results demonstrate that JCV binding to glial cells induces a signal that is required for both virus entry and infection. Additionally, these data suggest that the signal is ligand dependent, as genistein had no effect on the endocytosis of transferrin, which is a marker of constitutive endocytosis. The JCV-induced signal triggers downstream phosphorylation or activation of the MAPKs ERK1 and ERK2, and JCV entry is dependent on involvement of the endocytic protein eps15. As dominant-negative eps15 mutants specifically inhibited clathrin-dependent endocytosis, these results confirm that infectious entry of JCV is mediated by the clathrin-dependent pathway.
There are now several reports demonstrating that virus-induced signals are important early events in infection (4, 19, 21). Our results demonstrate for the first time that JCV, like SV40, induces a genistein-sensitive signal that is critical for virus entry and infection. It is interesting that both JCV and SV40 induce similar signals, despite the fact that they use dissimilar receptors to enter cells by completely opposing pathways. The SV40-induced signal leads to phosphorylation of caveolin-1, which is critical for endocytosis by the caveola-dependent pathway. Several components of the clathrin-dependent endocytic pathway, such as clathrin, adaptor protein-2 (AP-2), and eps15, are also known to be phosphorylated by extracellular ligands. As the tyrosine-to-phenylalanine phosphorylation mutant of eps15 inhibited infection by JCV, we suspect that eps15 may be one of the downstream targets of the JCV-induced signal. Our results are consistent with recent work showing that tyrosine phosphorylation of eps15 is required for ligand-induced but not constitutive clathrin-dependent endocytosis and further suggest that viral ligands may also be able to trigger phosphorylation of eps15. We made several attempts to examine the phosphorylation state of eps15 by Western blotting, but these experiments were inconclusive. This may have been due to only a small portion of total eps15 being involved in internalizing virus.
It is currently not known how eps15 phosphorylation modulates its function, as it does not alter its association with any known interacting proteins (11). It has been shown that the EH domains are required but are not sufficient for proper targeting of eps15 to clathrin-coated pits (5, 6). Additionally, eps15 mutants lacking AP-2 binding sites also fail to localize to the plasma membrane, so it is likely that interactions between multiple domains of eps15 are critical for its localization and function either during independent steps or in concert (7). Our data show that two different eps15 mutants, one full-length mutant with the second and third EH domains deleted and the other containing only the AP-2 binding site, inhibited infection. This indicates that either the second or third EH domains or more than one EH domain along with AP-2 binding sites are required for proper eps15 localization and function, further supporting the hypothesis that multiple domains of this protein play a critical role in clathrin-dependent endocytosis.
It is also interesting that JCV and SV40 differ in their ability to activate the MAPKs ERK1 and ERK2. Previous results with SV40 have shown that this pathway is not activated during early SV40 infection, and here we have shown that early infection with JCV does activate this pathway (12). These cellular signaling cascades are required to promote proper conditions for complete virus internalization, trafficking, or replication. Possibly, activation of different signaling pathways by these two viruses is due to the altered entry mechanisms, or perhaps, more likely, utilization of different cellular receptors may determine what downstream signal transduction events are triggered by both viruses. We are currently mapping the intracellular signals induced by JCV in an attempt to further understand the early events controlling infection of glial cells by this important human pathogen.
Acknowledgments
We thank all members of the Atwood laboratory for critical discussion during the course of this work. We thank Eugene Major for the Mad-1 SVEΔ strain of JCV and for the original SVG cell line. We also thank Judith Nathanson for critical help with computer graphics and Amanda Robinson and Lorie St. Pierre for administrative assistance.
Work in our laboratory was supported by a grant from the National Cancer Institute, R01 CA71878, and by a grant from the National Institute of Neurological Disorders and Stroke, R01 NS43097. W.Q. is supported by a GAANN training grant from the Department of Education, P200A030100. P.P.D.F. was supported by grants from the Italian Association of Cancer Research, the Human Science Frontier Program, the International Association for Cancer Research, the European Community (VI Framework), the Telethon Foundation, and the Italian Ministry of Health.
REFERENCES
- 1.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antinori, A., A. Ammassari, M. L. Giancola, A. Cingolani, S. Grisetti, R. Murri, L. Alba, B. Ciancio, F. Soldani, D. Larussa, G. Ippolito, and A. De Luca. 2001. Epidemiology and prognosis of AIDS-associated progressive multifocal leukoencephalopathy in the HAART era. J. Neurovirol. 7:323-328. [DOI] [PubMed] [Google Scholar]
- 3.Ashok, A., and W. J. Atwood. 2003. Contrasting roles of endosomal pH and the cytoskeleton in infection of human glial cells by JC virus and simian virus 40. J. Virol. 77:1347-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee, S., K. Narayanan, T. Mizutani, and S. Makino. 2002. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. J. Virol. 76:5937-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. [DOI] [PubMed] [Google Scholar]
- 6.Benmerah, A., C. Lamaze, B. Begue, S. L. Schmid, A. Dautry-Varsat, and N. Cerf-Bensussan. 1998. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benmerah, A., V. Poupon, N. Cerf-Bensussan, and A. Dautry-Varsat. 2000. Mapping of Eps15 domains involved in its targeting to clathrin-coated pits. J. Biol. Chem. 275:3288-3295. [DOI] [PubMed] [Google Scholar]
- 8.Berger, J. R., and E. O. Major. 1999. Progressive multifocal leukoencephalopathy. Semin. Neurol. 19:193-200. [DOI] [PubMed] [Google Scholar]
- 9.Carbone, R., S. Fre, G. Iannolo, F. Belleudi, P. Mancini, P. G. Pelicci, M. R. Torrisi, and P. P. Di Fiore. 1997. Eps15 and Eps15R are essential components of the endocytic pathway. Cancer Res. 57:5498-5504. [PubMed] [Google Scholar]
- 10.Chen, Y., and L. C. Norkin. 1999. Extracellular simian virus 40 transmits a signal that promotes virus enclosure within caveolae. Exp. Cell Res. 246:83-90. [DOI] [PubMed] [Google Scholar]
- 11.Confalonieri, S., A. E. Salcini, C. Puri, C. Tacchetti, and P. P. Di Fiore. 2000. Tyrosine phosphorylation of Eps15 is required for ligand-regulated, but not constitutive, endocytosis. J. Cell Biol. 150:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dangoria, N. S., W. C. Breau, H. A. Anderson, D. M. Cishek, and L. C. Norkin. 1996. Extracellular simian virus 40 induces an ERK/MAP kinase-independent signalling pathway that activates primary response genes and promotes virus entry. J. Gen. Virol. 77:2173-2182. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert, J. M., and T. L. Benjamin. 2000. Early steps of polyomavirus entry into cells. J. Virol. 74:8582-8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert, J. M., I. G. Goldberg, and T. L. Benjamin. 2003. Cell penetration and trafficking of polyomavirus. J. Virol. 77:2615-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou, J., and E. O. Major. 2000. Progressive multifocal leukoencephalopathy: JC virus induced demyelination in the immune compromised host. J. Neurovirol. 6(Suppl. 2):S98-S100. [PubMed] [Google Scholar]
- 16.Major, E. O., A. E. Miller, P. Mourrain, R. G. Traub, E. de Widt, and J. Sever. 1985. Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc. Natl. Acad. Sci. USA 82:1257-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mannova, P., and J. Forstova. 2003. Mouse polyomavirus utilizes recycling endosomes for a traffic pathway independent of COPI vesicle transport. J. Virol. 77:1672-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 19.Pelkmans, L., D. Puntener, and A. Helenius. 2002. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296:535-539. [DOI] [PubMed] [Google Scholar]
- 20.Pho, M. T., A. Ashok, and W. J. Atwood. 2000. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J. Virol. 74:2288-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popik, W., and P. M. Pitha. 1998. Early activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase in response to binding of simian immunodeficiency virus to Jurkat T cells expressing CCR5 receptor. Virology 252:210-217. [DOI] [PubMed] [Google Scholar]
- 22.Richterova, Z., D. Liebl, M. Horak, Z. Palkova, J. Stokrova, P. Hozak, J. Korb, and J. Forstova. 2001. Caveolae are involved in the trafficking of mouse polyomavirus virions and artificial VP1 pseudocapsids toward cell nuclei. J. Virol. 75:10880-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sieczkarski, S. B., and G. R. Whittaker. 2002. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535-1545. [DOI] [PubMed] [Google Scholar]
- 24.Sieczkarski, S. B., and G. R. Whittaker. 2002. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J. Virol. 76:10455-10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vacante, D. A., R. Traub, and E. O. Major. 1989. Extension of JC virus host range to monkey cells by insertion of a simian virus 40 enhancer into the JC virus regulatory region. Virology 170:353-361. [DOI] [PubMed] [Google Scholar]
- 26.van Delft, S., R. Govers, G. J. Strous, A. J. Verkleij, and P. M. van Bergen en Henegouwen. 1997. Epidermal growth factor induces ubiquitination of Eps15. J. Biol. Chem. 272:14013-14016. [DOI] [PubMed] [Google Scholar]
- 27.van Delft, S., C. Schumacher, W. Hage, A. J. Verkleij, and P. M. van Bergen en Henegouwen. 1997. Association and colocalization of Eps15 with adaptor protein-2 and clathrin. J. Cell Biol. 136:811-821. [DOI] [PMC free article] [PubMed] [Google Scholar]